What is dimethylpolysiloxane
Dimethylpolysiloxane also called dimethyl polysiloxane (E900 food additive), polymethylsiloxane or dimethicone, is a silicon-based polymer used as a lubricant and conditioning agent. Dimethylpolysiloxane functions as an anti-foaming agent, skin conditioning agent, occlusive and skin protectant. Dimethylpolysiloxane is found in many cosmetic and hygiene products like nail polish, conditioners, make-up, contact lens solutions, sunscreens, deodorants, and shampoo. Examples of products that contain dimethicone include Aveeno Moisturizing Lotion and Johnson’s Baby Cream.
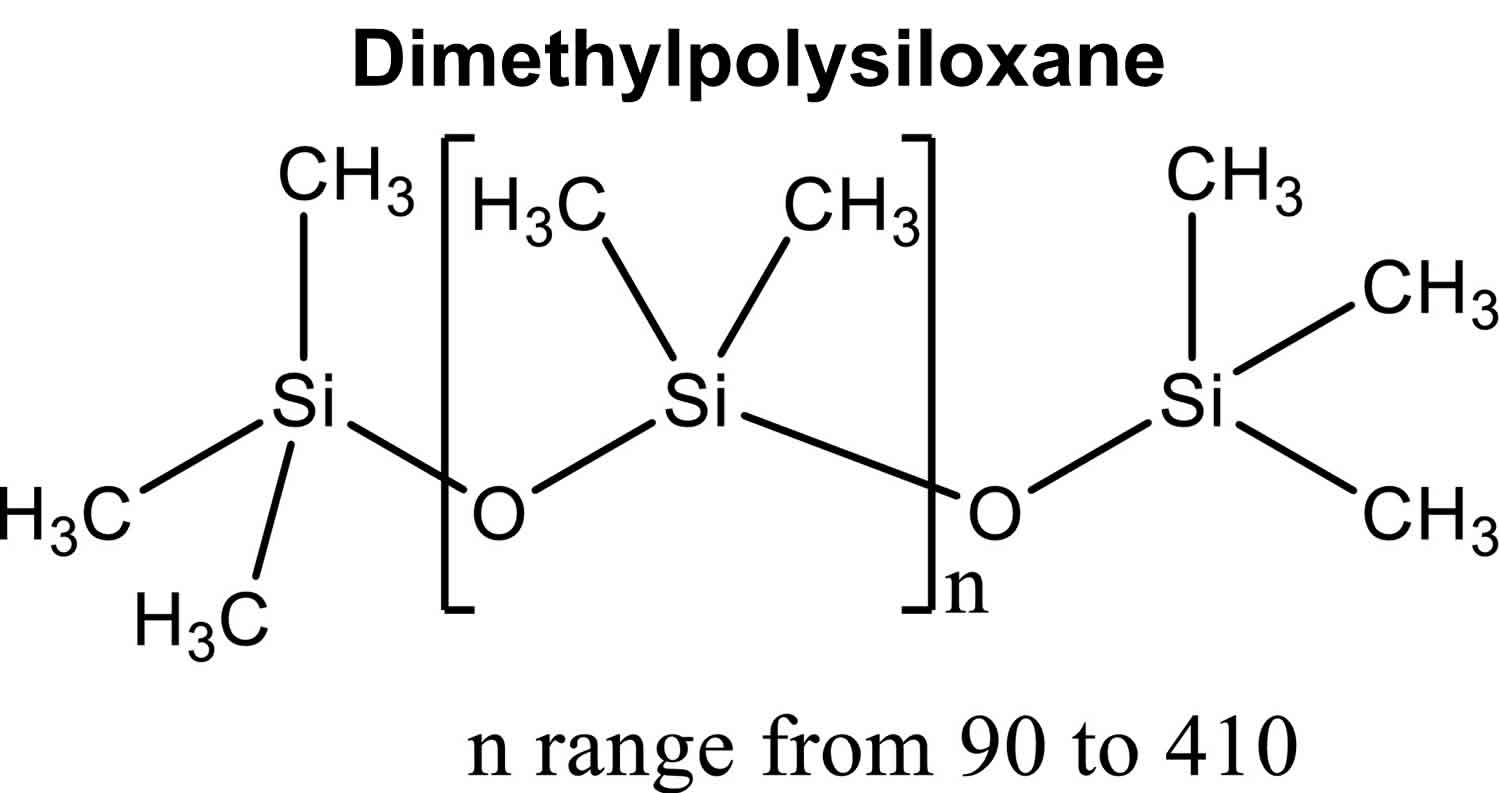
Figure 1. Dimethylpolysiloxane chemical structure
 What is dimethylpolysiloxane used for?
What is dimethylpolysiloxane used for?
Dimethylpolysiloxane functions as an anti-foaming agent in foods, glazing agents for fruit, food additive in color preparations of E 160a carotenes, E 160b annatto, bixin, norbixin, E 160c Paprika extract, capsanthin, capsorubin, E 160d lycopene and E 160e beta‐apo‐8′‐carotenal, skin conditioning agent, occlusive and skin protectant. Dimethylpolysiloxane is found in many cosmetic and hygiene products like nail polish, conditioners, make-up, contact lens solutions, sunscreens, deodorants, and shampoo. Examples of products that contain dimethicone include Aveeno Moisturizing Lotion and Johnson’s Baby Cream.
Currently, dimethylpolysiloxane (E 900) is an authorized food additive, used as an antifoaming agent in foods:
- Fats and oils essentially free from water (excluding anhydrous milk fat)
- Other fat and oil emulsions including spreads and liquid emulsions
- Canned or bottled fruit and vegetables
- Jam, jellies and marmalades and sweetened chestnut purée. Other similar fruit or vegetable spreads
- Other confectionery including breath freshening microsweets
- Chewing gum
- Decorations, coatings and fillings, except fruit‐based fillings
- Batters
- Soups and broths
- Fruit juices and vegetable juices
- Flavored drinks
- Cider and perry
- Food supplements supplied in a solid form, excluding food supplements for infants and young children.
Dimethylpolysiloxane fluids of various viscosities also called silicone oils, are used as an intraocular tamponade during retinal detachment repairs. Retinal and corneal lesions (keratopathy and degeneration of the corneal endothelium) have been reported as a complication of their use 1.
Several short‐term studies (ranging from a single oral dose to up to four consecutive oral doses) were performed to determine the suitability of dimethylpolysiloxane (dimethicone) as an agent to reduce gastrointestinal gas and improve the quality of ultrasound investigations of the abdomen. The studies recruited 20 patients 2 and 28 patients 3. Jacyna et al. 4 also tested dimethyl polysiloxane when combined with antacids in 60 subjects. In an 8‐week study, Smart and Atkinson 5. compared the efficacy of a dimethylpolysiloxane (dimethicone) antacid containing preparation (n = 28) vs. an alginate antacid containing preparation (n = 25) in patients with reflux oesophagitis. None of the studies indicated any adverse effects from the administration of dimethylpolysiloxane or any of the dimethylpolysiloxane‐containing preparations.
Dimethylpolysiloxane in food
Dimethylpolysiloxane also known as E900 authorized food additive was evaluated by the Scientific Committee on Food in 1990 and agreed with the Acceptable Daily Intake (ADI) of 1.5 mg/kg body weight per day previously established by Joint FAO /WHO Expert Committee on Food Additives (JECFA ) in 1974 6 based on a No Observed Adverse Effect Level (NOAEL) of 150 mg/kg body weight per day from a long‐term toxicity rat study performed in 1959 7. The Scientific Committee on Food re‐established an ADI of 0–1.5 mg/kg body weight per day and withdrew the temporary ADI of 0–0.8 mg/kg body weight established in 2008 8.
Dimethylpolysiloxane was only absorbed to a very limited extent from the gastrointestinal tract following oral administration and the vast majority was excreted unchanged in the feces. Corneal opacities and other effects on cornea were observed in studies in rats. These effects are considered to be caused by direct contact with the test substance in the feed and/or with the test substance in the feces and not due to systemic exposure 7. The European Food Safety Authority Panel considered that oral exposure of dimethylpolysiloxane did not result in any systemic adverse effects in any species and dose tested and there is no concern with respect to genotoxicity of dimethyl polysiloxane (E 900) 7. From a 26‐month toxicity study in rats, a No Observed Adverse Effect Level (NOAEL ) of 1,742 and 2,055 mg dimethylpolysiloxane/kg body weight per day for female and male, respectively, was identified. Using the NOAEL 1,742 mg/kg bw per day, the Panel established an ADI of 17 mg/kg bw per day for E 900 by applying an uncertainty factor of 100. Accordingly, the Acceptable Daily Intake (ADI) for dimethyl polysiloxane (E 900) of 1.5 mg/kg body weight per day, established by Scientific Committee on Food in 1990, is withdrawn. The exposure estimates for the different population groups of all exposure scenarios did not exceed the Acceptable Daily Intake (ADI ) of 17 mg/kg body weight per day for E 900. The European Food Safety Authority Panel concluded that there is not a safety concern at the reported uses and use levels for dimethylpolysiloxane (E 900).
Dimethylpolysiloxane (various compositions and different viscosities – 10, 350 and 1,000 centistokes) was only absorbed to a very limited extent from the gastrointestinal tract following oral administration to mice, rats, monkeys and humans. The vast majority (more than 99.9%) of the orally administered dimethylpolysiloxane was excreted unchanged in the feces.
The acute toxicity of dimethylpolysiloxane is low. There is no concern with respect to genotoxicity of dimethylpolysiloxane (E 900) 7.
Dimethylpolysiloxane toxicity
The acute toxicity of dimethyl polysiloxane is low. There is no concern with respect to genotoxicity of dimethyl polysiloxane (E 900) 7.
In short‐term and subchronic toxicity studies in mice, rats and dogs, anal leakage and matting in the urogenital area were reported. Corneal opacities and other effects on cornea such as inflammation and vascularisation have been observed in some rat studies at doses ranging from 900 to 100,000 mg/kg body weight per day. Similarly, in chronic toxicity and carcinogenicity studies in mice and rats matting of the fur (predominantly in the urogenital and/or anogenital areas) along with effects on the cornea and inflammation of the nasolacrimal duct were observed.
The European Food Safety Authority Panel agrees with the suggestion of the authors that these effects were caused by direct contact with the test substance in the feed, or with the test substance in the faeces and not due to systemic exposure. A local effect is supported by the following:
- The vast majority of dimethyl polysiloxane is not absorbed and thus excreted via feces, consequently contaminating beddings and cages.
- Additional contamination of beddings and cages would occur from feed spillage.
- Dimethylpolysiloxane is a surfactant and has ocular irritating effects. It is expected that due to this local irritative effect the rats would have scratched their eyes.
- The cornea is not directly vascularised and, therefore, it is not expected to be a target through systemic exposure.
- Dimethylpolysiloxane has been demonstrated to damage the cornea in humans and rabbits following intra‐ocular treatment only.
Overall, the European Food Safety Authority Panel considered that oral exposure of dimethylpolysiloxane did not result in any systemic adverse effects in any species and dose tested 7. From a 26‐month toxicity study in rats 9, an No Observed Adverse Effect Level (NOAEL) of 1,742 and 2,055 mg dimethylpolysiloxane/kg body weight per day for females and males, respectively, was identified.
The NOAEL identified from the study of Kawabe et al. 9 is supported by the combined 24 months chronic oral toxicity and carcinogenicity study in rats (WIL‐Research‐Laboratories‐Inc., 2003) in which no adverse effects were seen even at the highest dose tested of 1,000 mg/kg body weight per day. The latter study used a low viscosity test item of 10 centistokes, which is outside the viscosity range given in the EU specifications for E 900. Since the low viscosity implies a low molecular weight distribution, compared to E 900, the test item at 10 centistokes can be considered to be a worse case regarding the extent of the absorption.
The European Food Safety Authority Panel considered that the NOAEL of 1,742 mg/kg body weight per day could be used to derive an Acceptable Daily Intake (ADI) for dimethylpolysiloxane (E 900). Using an uncertainty factor of 100, the European Food Safety Authority Panel derived an Acceptable Daily Intake (ADI) of 17 mg/kg body weight per day for dimethylpolysiloxane (E 900).
Considering that dimethylpolysiloxane (E 900) is authorised in flavored drinks and changes the organoleptic properties of these drinks, the European Food Safety Authority Panel selected the brand‐loyal refined exposure assessment scenario as the most relevant scenario for risk characterisation. In this scenario, mean exposure to dimethylpolysiloxane (E 900) from its use as a food additive ranged from 0.01 mg/kg body weight per day in adults and the elderly to 0.16 mg/kg body weight per day in children. The 95th percentile of exposure to dimethylpolysiloxane (E 900) ranged from 0.02 mg/kg body weight per day in the elderly to 0.49 mg/kg body weight per day in toddlers.
The exposure estimates for the different population groups of all exposure scenarios did not exceed the ADI of 17 mg/kg body weight per day for dimethylpolysiloxane (E 900). The European Food Safety Authority Panel concluded that there is no safety concern at the reported uses and use levels for dimethylpolysiloxane (E 900) 7.
References- Choi WC, Choi SK and Lee JH, 1993. Silicone oil keratopathy. Korean Journal of Ophthalmology, 7, 65–69.
- Gladisch R, Elfner R, Massner B and Ulrich H, 1985. Premedication for abdominal sonography–comparison of the efficacy of 2 dimethicone preparations. Ultraschall in der Medizin, 2, 114–117.
- Sommer G, Filly RA and Laing FC, 1977. Use of simethicone as a patient preparation for abdominal sonography. Radiology, 125, 219–221.
- Jacyna MR, Boyd EJ and Wormsley KG, 1984. Comparative study of four antacids. Postgraduate Medical Journal, 707, 592–596.
- Smart HL and Atkinson M, 1990. Comparison of a dimethicone/antacid (Asilone gel) with an alginate/antacid (Gaviscon liquid) in the management of reflux oesophagitis. Journal of the Royal Society of Medicine, 83, 554–556.
- JECFA (Joint FAO/WHO Expert Comittee on Food Additives), 1974. Evaluation of Certain Food Additives, Eighteenth Report, TRS 557 report 18th meeting.
- EFSA Panel on Food Additives and Flavourings (FAF), Younes, M, Aquilina, G, Castle, L, Engel, K‐H, Fowler, P, Jose Frutos Fernandez, M, Fürst, P, Gürtler, R, Gundert‐Remy, U, Husøy, T, Manco, M, Mennes, W, Passamonti, S, Shah, R, Waalkens‐Berendsen, DH, Wölfle, D, Wright, M, Boon, P, Tobback, P, Giarola, A, Rincon, AM, Tard, A and Moldeus, P, 2020. Scientific Opinion on the re‐evaluation of dimethyl polysiloxane (E 900) as a food additive. EFSA Journal 2020;18(5):6107, 47 pp. https://doi.org/10.2903/j.efsa.2020.6107
- JECFA (Joint FAO/WHO Expert Committee on Food Additive), 2011a. Evaluation of Certain Food Additives and Contaminants. Seventy‐fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No., 966, 27–32.
- Kawabe M, Ichihara T, Sano M, Hagiwara A, Tarnano S, Ogawa K and Shirai T, 2005. Lack of carcinogenicity of silicone resin (KS66) in F344 rats. Food and Chemical Toxicology, 43, 1065–1071.




