Pulmonary interstitial emphysema
Pulmonary interstitial emphysema refers to the abnormal location of gas within the pulmonary interstitium and lymphatics usually due to positive pressure ventilation. Pulmonary interstitial emphysema is almost always associated with mechanical ventilation or continuous positive airway pressure in the first weeks of life. Pulmonary interstitial emphysema typically results from rupture of overdistended alveoli following barotrauma in infants with respiratory distress syndrome. Reduced lung compliance, prematurity, low birth weight, meconium aspiration syndrome and pneumonia are other risk factors. Pulmonary interstitial emphysema may also occasionally be incidentally detected in adults.
The prevalence of pulmonary interstitial emphysema widely varies with the population studied. The reported incidence of pulmonary interstitial emphysema amongst infants admitted to the neonatal intensive care unit (NICU) is 2-3% 1. The incidence rises to 20-30% among premature infants. The highest frequencies are seen in low-birth-weight infants 2. In a retrospective case-controlled study, 11 (24%) of 45 extremely low birth weight infants developed pulmonary interstitial emphysema 3. This study was done in the present era of tocolysis, antenatal steroids, and postnatal surfactant administration; however, all infants included in the study were treated with a conventional ventilator in the assist-control mode before the onset of pulmonary interstitial emphysema.
In a randomized trial of surfactant replacement therapy at birth in premature infants born at 25-29 weeks’ gestation, Kendig et al 4 reported pulmonary interstitial emphysema in 8 (26%) of 31 control neonates, and in 5 (15%) of 34 surfactant-treated neonates.
Another randomized controlled trial of prophylaxis versus treatment with bovine surfactant in neonates with respiratory distress syndrome (RDS) born at less than 30 weeks’ gestation included 2 (3%) of 62 early surfactant-treated neonates, 5 (8%) of 60 late surfactant-treated neonates, and 15 (25%) of 60 control neonates with pulmonary interstitial emphysema 5.
Kattwinkel et al 6 compared prophylactic surfactant administration versus the early treatment of respiratory distress syndrome (RDS) with calf lung surfactant in neonates born at 29-32 weeks’ gestation; 3 of 627 neonates in the prophylaxis group and 3 of 621 neonates in the early treatment group developed pulmonary interstitial emphysema. This information suggests a higher incidence of pulmonary interstitial emphysema in more immature infants as well as those administered with late surfactant therapy.
In a study by Plenat et al 7, pulmonary interstitial emphysema developed about equally in both sexes (21 males, 18 females). Although these data also included cases with intrapleural pneumatosis, no relationship between sex and type of interstitial pneumatosis was noted.
The principal therapies for pulmonary interstitial emphysema are lateral decubitus positioning, selective intubation, occlusion of the contralateral bronchus, and high-frequency ventilation. However, administration of surfactant to premature infants (< 30-32 weeks’ gestation) judged to be at risk of developing respiratory distress syndrome may reduce the risk of pulmonary interstitial emphysema development.
Pulmonary interstitial emphysema causes
Pulmonary interstitial emphysema is more common in infants of lower gestational age, and it usually occurs within the first weeks of life. That is, development of pulmonary interstitial emphysema within the first 24-48 hours after birth is often associated with extreme prematurity, very low birth weight, perinatal asphyxia, and/or neonatal sepsis and frequently indicates a grave prognosis.
Risk factors for pulmonary interstitial emphysema include the following:
- Prematurity
- Respiratory distress syndrome (RDS)
- Meconium aspiration syndrome
- Amniotic fluid aspiration
- Infection: Exposure to chorioamnionitis, neonatal sepsis, or pneumonia,
- Low Apgar score or need for positive pressure ventilation (PPV) during resuscitation at birth
- Use of high peak airway pressures on mechanical ventilation therapy in the first week of life
- Incorrect positioning of the endotracheal tube in one bronchus
- Antenatal exposure to magnesium sulfate 3. More investigation is needed
Independent risk factors for pulmonary interstitial emphysema in preterm infants include higher oxygen requirements during resuscitation as well as greater need for surfactant and higher ventilatory pressures before confirmation of the diagnosis 8.
Pulmonary interstitial emphysema prevention
Surfactant
Prophylactic surfactant administration to infants (< 30-32 weeks’ gestation) judged to be at risk of developing respiratory distress syndrome (RDS) compared with selective use of surfactant in infants with established respiratory distress syndrome (RDS) has been demonstrated to decrease the risk of pulmonary interstitial emphysema 9.
Meta-analysis of early surfactant replacement therapy with brief ventilation compared with later, selective surfactant replacement and continued mechanical ventilation suggests a trend toward a reduced incidence of air leak syndromes in premature infants in the early surfactant group 10. Early surfactant treatment, less invasive ventilatory support, or both could be responsible factors for the observed beneficial trend 10.
According to one report, in infants with respiratory distress syndrome, multiple doses of animal-derived surfactant extract resulted in greater improvements in oxygenation and ventilatory requirements, a decreased risk of pneumothorax, and a trend toward improved survival 11.
High-frequency ventilation
In a study comparing high-frequency positive pressure ventilation (HFPPV) to conventional mechanical ventilation (CMV), Pohlandt et al reported a reduction in the risk of pulmonary interstitial emphysema with high-frequency positive pressure ventilation 12. A meta-analysis by Greenough et al demonstrated that, compared to conventional mechanical ventilation, high-frequency positive pressure ventilation was associated with a reduction in the risk of air leak, primarily pneumothorax, but not for pulmonary interstitial emphysema (typical relative risk for pneumothorax was 0.69) 13.
A review of different trials of elective high-frequency oscillatory ventilation (HFOV) versus conventional mechanical ventilation for acute pulmonary dysfunction in preterm infants suggested there may be an increase in the incidence of air leak syndromes, including but not limited to pulmonary interstitial emphysema in the high-frequency oscillatory ventilation group 14.
In contrast, a prospective randomized multicenter study of high-frequency oscillatory ventilation versus conventional mechanical ventilation in premature infants with RDS showed no difference in the incidence of pulmonary interstitial emphysema 15. Limited data regarding rescue high-frequency oscillatory ventilation for pulmonary dysfunction in the preterm infant also showed no difference in the rate of pulmonary interstitial emphysema 16. Similarly, Cochrane reviews of trials of elective high-frequency jet ventilation (HFJV) versus conventional mechanical ventilation for RDS demonstrated no significant difference in the incidence of air leak syndrome in the individual trials or in the overall analysis 17.
In summary, the available literature suggests elective or rescue high-frequency ventilation does not prevent the development of pulmonary interstitial emphysema.
Other considerations
Different modes of conventional mechanical ventilation do not appear to affect the risk of pulmonary interstitial emphysema. Goel at al showed that the rate of pulmonary interstitial emphysema was significantly less while delivering nasal continuous positive airway pressure (CPAP) in the mask group as compared to cannula group (4.9% vs 17.5%3) 18.
Although data are limited on the benefit of volume-targeted ventilation strategies, some data appear to be promising regarding volume-targeted ventilation to prevent pulmonary interstitial emphysema. Stefanescu et al reported that rates of pulmonary interstitial emphysema were lower among infants treated with volume guarantee pressure support ventilation versus pressure-controlled ventilation (odds ratio: 0.6) 19. However, McCallion et al 20 found no significant difference in the rate of pulmonary interstitial emphysema either in a pooled analysis within subgroups or the overall pooled analysis of trials comparing volume-targeted versus pressure-limited ventilation in the neonate.
Avoid the use of high peak inspiratory pressure (PIP). Carefully monitor the peak inspiratory pressure (watch the manometer) during manual ventilation.
Pulmonary interstitial emphysema symptoms
Pulmonary interstitial emphysema is a radiographic and pathologic diagnosis. Pulmonary interstitial emphysema is associated with few clinical signs, but a progressive, sometimes rapid, increase in oxygen (O2) requirements, carbon dioxide (CO2) retention or hypotension are suggestive of this diagnosis.
Alternatively, the infant can present with the signs of one of the complications of pulmonary interstitial emphysema, such as pneumothorax. Occasionally, pulmonary interstitial emphysema becomes apparent following reexpansion of a collapsed lung after drainage of a pneumothorax.
Physical examination
No specific signs of pulmonary interstitial emphysema are reported. There may be overinflation of the chest wall and crepitations on auscultation on the affected side. A characteristic sound of crushing styrofoam or walking on dry snow signals pulmonary interstitial emphysema.
Pulmonary interstitial emphysema differential diagnoses
- Cystic Adenomatoid Malformation
- Diaphragmatic Hernias
- Lymphangiectasia
- Pediatric Bronchogenic Cyst
The chest x-ray appearance of pulmonary interstitial emphysema can be confused with the following 21:
- Air bronchograms in respiratory distress syndrome (RDS)
- Aspiration pneumonia
- Pulmonary edema
- Distended airways in patients on mechanical ventilatory support
Other differential diagnosis of persistent pulmonary interstitial emphysema includes the following:
- Congenital cystic adenomatoid malformation
- Lymphangiectasia
- Bronchogenic cysts
- Congenital lobar emphysema
- Cystic lymphangioma
- Sequelae of a prior infection
- Diaphragmatic hernia
Pulmonary interstitial emphysema complications
Potential complications of pulmonary interstitial emphysema include the following:
- Respiratory insufficiency
- Other air leaks (eg, pneumomediastinum, pneumothorax, pneumopericardium, pneumoperitoneum, subcutaneous emphysema [rare])
- Massive air embolism
- Chronic lung disease of prematurity
- Intraventricular hemorrhage (IVH)
- Periventricular leukomalacia
- Death
Pulmonary interstitial emphysema diagnosis
Pulmonary interstitial emphysema is a radiographic and pathologic diagnosis. However, obtain blood gases in affected infants to ensure adequate gas exchange.
The classic radiologic appearance of pulmonary interstitial emphysema often provides a clear diagnosis. pulmonary interstitial emphysema is best visualized in the anteroposterior supine projection. This condition has two basic radiographic appearances, linear or cystlike radiolucencies, although both types often appear together.
Linear radiolucencies are coarse and nonbranching, measure from 3 to 8 mm, and vary in width but rarely exceed 2 mm. Cystlike radiolucencies are small, ranging from 1 to 4 mm in diameter; although generally round, they may also appear oval or slightly lobulated.
The disorganized, haphazard distribution of pulmonary interstitial emphysema in localized areas is unlike the anatomically organized pattern of the air bronchogram. The air bronchogram is a classic radiographic sign of respiratory distress syndrome (RDS), which should not be confused with pulmonary interstitial emphysema.
In respiratory distress syndrome (RDS), long, smooth, branching, linear radiolucencies decrease in caliber from the hilum and frequently disappear at the lung periphery. pulmonary interstitial emphysema should be suspected when coarse radiolucencies appear in the lung periphery or when the lucencies do not branch in a pattern consistent with the normal bronchial tree.
In some infants receiving mechanical ventilation therapy, the distended airways and alveoli have a somewhat similar radiographic appearance to that of pulmonary interstitial emphysema. Over time, this either progresses to a classic radiographic picture of pulmonary interstitial emphysema or resolves very rapidly as the ventilator settings are lowered.
Rarely, pulmonary interstitial emphysema can be misinterpreted as a normally aerated lung surrounded by exudate, as in an aspiration syndrome or pulmonary edema 21.
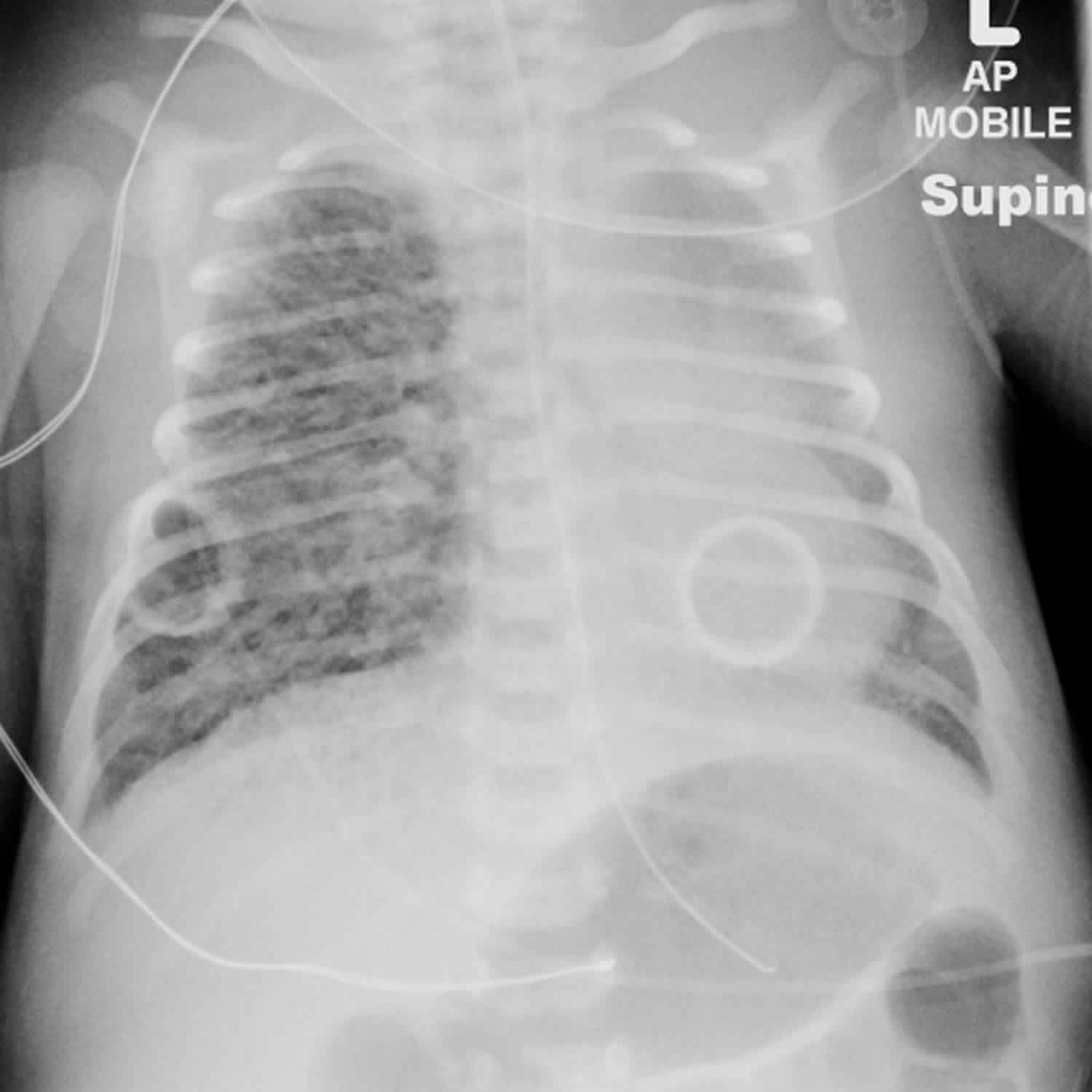
The first radiograph below shows a right-sided pneumothorax and pulmonary interstitial emphysema. Interstitial air prevents collapse of the underlying lung by a tension pneumothorax. In such cases, extreme caution is required during drainage of the pneumothorax to avoid perforation of the underlying lung.
Computed tomography (CT) scanning of the chest can be a helpful diagnostic tool if doubt about the diagnosis remains, particularly in persistent cases and if surgical interventions are being considered. A round or linear soft-tissue component seen in the wall of or within the air-containing spaces is a key to making the correct diagnosis 22. In addition, the presence of subpleural pulmonary interstitial emphysema, in which there is an interstitial air collection in the subpleural region of the lungs excluding the bronchovascular bundle, on CT scan suggests single or multiple alveolar rupture(s) as an origin of pneumomediastinal air 23.
Pulmonary interstitial emphysema treatment
Admission/transfer to a neonatal intensive care unit (NICU) is indicated for infants with pulmonary interstitial emphysema. A thoracentesis set should be readily available due to the possibility of air leak, including pneumothorax and pneumopericardium.
Different treatment modalities have been used to manage pulmonary interstitial emphysema, with variable success.
Although the primary risk factor for pulmonary interstitial emphysema, prematurity, is rarely preventable, attention should be given to the use of as little mechanical ventilatory support as is necessary for the infant’s very fragile lungs. An often-used strategy is to reduce the inspiratory time and/or decrease pressure along with adjusting the positive-end expiratory pressure (PEEP) enough to stent the airway will allow better emptying of the alveoli during expiration 24. Close clinical observation by monitoring oxygen need, work of breathing and perfusion status, as well as judicious analysis of blood gas and chest x-ray, are essential to determine an optimal PEEP for a particular infant.
Because pneumothorax is a known complication of pulmonary interstitial emphysema, anticipatory guidance for this possibility should be provided for all those caring for the infant. Appropriate personnel should be readily available to address this complication.
In addition to pulmonary treatment, the overall importance of appropriate nutritional management of these ill newborns cannot be overstressed. Most of these infants are treated with total parenteral nutrition, and their nutritional needs require diligent attention.
Case reports and/or case series describe a variety of other approaches for the management of pulmonary interstitial emphysema, including the following:
- A 3-day course of dexamethasone (0.5 mg/kg/d) 25 or hydrocortisone (2 mg/kg/day) 26
- Chest physiotherapy with intermittent 100% oxygen in localized and persistent compressive pulmonary interstitial emphysema 27
- Artificial pneumothorax 28
- Multiple pleurotomies 29
- Heliox with inhaled nitric oxide 30
- Percutaneous catheter insertion 31
Despite the success claimed by the authors of these reports, the efficacy of the treatment modalities they discussed seem questionable. With advancements in respiratory care, these treatment modalities are rarely used.
All infants with pulmonary interstitial emphysema need to be under the care of a neonatologist. In some cases, pediatric pulmonology and pediatric surgery consultations are appropriate.
Lateral decubitus positioning
Lateral decubitus positioning is a conservative approach that has been used successfully in infants with pulmonary interstitial emphysema, and it is most effective in infants with unilateral pulmonary interstitial emphysema. In different case studies of lateral decubitus positioning as a treatment of unilateral pulmonary interstitial emphysema in infants, pulmonary interstitial emphysema resolved in 48 hours to 6 days with minimal recurrence and a low failure rate. Thus, lateral decubitus positioning should be considered as an early first-line therapy in the management of unilateral pulmonary interstitial emphysema; it has also been used successfully for patients with bilateral pulmonary interstitial emphysema when one side is more significantly affected.
Place the infant in the lateral decubitus position, with the affected lung dependent. This therapy can result in plugging of dependent airways and improved oxygenation of the nondependent lung. The latter allows for an overall decrease in ventilatory settings. The combination of the above factors helps in resolving pulmonary interstitial emphysema 32.
Selective main bronchial intubation and occlusion
Many case reports detail successful treatment of infants with severe localized pulmonary interstitial emphysema by selective intubation of the contralateral bronchus 33. This maneuver decompresses the overdistended lung tissue and avoids exposing it to high positive inflationary pressures. Selective bronchial intubation of the right main bronchus is not a difficult procedure; the left side may be more difficult.
This procedure uses an endotracheal tube of the same diameter as for a regular intubation. However, the tube is inserted 2-4 cm beyond its usual position. It is introduced with the bevel on the end of the tube positioned so that the long part of the tube is toward the bronchus to be intubated. This increases the chance of the tube entering the correct bronchus as it is advanced into the airway. Turning the infant’s head to the left or right moves the tip of the endotracheal tube to the contralateral side of the trachea and may help in selective tube placement.
Weintraub et al 34 described a method for left selective bronchus intubation using a regular Portex endotracheal tube in which an elliptical hole 1 cm in length has been cut through half the circumference about 0.5 cm above the tip of the oblique distal end. By directing the side with the elliptical hole to the left lung, left selective bronchus intubation can be easily and repeatedly accomplished.
Another method of selective intubation is the use of a small fiberoptic bronchoscope to direct the endotracheal tube tip into the desired bronchus. Selective intubation under fluoroscopy can also be considered.
Potential complications of selective intubation/ventilation include the following:
- Atelectasis in the affected lung
- Injury to the bronchial mucosa, with subsequent scarring and stenosis
- Acute hypoventilation or hypoxemia if ventilating one lung is inadequate
- Excessive secretions
- Hyperinflation of the intubated (nonoccluded) lung
- Upper lobe collapse when intubating the right lung
- Bradycardia
Despite the potential risks, selective bronchial intubation is a desirable alternative to lobectomy in a patient with persistent, severe localized pulmonary interstitial emphysema that is causing a mediastinal shift and compression atelectasis that is refractory to conservative management. This procedure should be attempted before any surgical intervention.
High frequency ventilation
Keszler et al 35 found that high-frequency ventilation was safe and more effective than rapid-rate conventional ventilation in the treatment of newborns with pulmonary interstitial emphysema (pulmonary interstitial emphysema). In their study of 144 newborns with pulmonary interstitial emphysema, the use of high-frequency ventilation resulted in similar oxygenation and ventilation obtained at lower peak and mean airway pressures. These results suggested that less air would leak into the interstitial spaces in these infants.
Similar effects can be achieved by use of high-frequency oscillatory ventilation (HFOV). A study by Clark et al 36 demonstrated the efficacy of high-frequency oscillatory ventilation in 27 low birth weight infants who developed pulmonary interstitial emphysema and respiratory failure while on conventional ventilation. Overall survival in nonseptic patients was 80%. Surviving patients showed continued improvement in oxygenation and ventilation at an increasingly lower fraction of inspired oxygen (FiO2) and proximal airway pressure with resolution of pulmonary interstitial emphysema, whereas nonsurvivors progressively developed chronic respiratory insufficiency with continued pulmonary interstitial emphysema from which recovery was not possible 36.
The investigators hypothesized that interstitial air leak is decreased during high-frequency oscillatory ventilation because adequate ventilation is provided at lower peak distal airway pressures 36. Although this mode of ventilation has inherent risks, it can be a very effective tool for experienced clinicians to treat severe diffuse pulmonary interstitial emphysema. Note that care must be taken in smaller infants who require a high amplitude to ventilate, because the active exhalation during high-frequency oscillatory ventilation may cause small airway collapse and exacerbate gas trapping.
Squires et al also found that low oscillatory frequency of high-frequency oscillatory ventilation had some benefits for preterm infants with severe pulmonary interstitial emphysema 37. After transition to low-frequency high-frequency oscillatory ventilation, physiologic responses were seen in both unilateral and bilateral pulmonary interstitial emphysema, in particular a rapid and sustained improvement in oxygenation in the bilateral group 37.
Lobectomy
Lobectomy is indicated in a small number of patients with localized pulmonary interstitial emphysema in whom spontaneous regression is not occurring and medical management has failed 38. However, a case report exists of the spontaneous resolution of diffuse persistent pulmonary interstitial emphysema with pneumomediastinum, supporting the consideration of a nonsurgical approach in a stable infant with persistent pulmonary interstitial emphysema 39. Thus, clear guidelines for surgical intervention in pulmonary interstitial emphysema are difficult to establish. In general, lobectomy should be reserved for infants in whom the risks of recurring complications outweigh those of surgery. Lobectomy seems most helpful in infants who develop severe lobar emphysema.
Pulmonary interstitial emphysema prognosis
Pulmonary interstitial emphysema can predispose an infant to other air leaks. In a study by Greenough et al 40, 31 of 41 infants with pulmonary interstitial emphysema developed pneumothorax, compared to 41 of 169 infants without pulmonary interstitial emphysema. In addition, 21 of 41 babies with pulmonary interstitial emphysema developed intraventricular hemorrhage (IVH), compared to 39 of 169 among infants without pulmonary interstitial emphysema.
Pulmonary interstitial emphysema may not resolve for 2-3 weeks; therefore, it can increase the duration of mechanical ventilation support as well as the incidence of bronchopulmonary dysplasia. Some infants may develop chronic lobar emphysema, which may require surgical lobectomies 41.
In a study in the postsurfactant era, 4 of 11 infants with pulmonary interstitial emphysema developed severe intraventricular hemorrhage (grade 2 or higher) compared to 4 of 34 infants without pulmonary interstitial emphysema. Additionally, pulmonary interstitial emphysema remained significantly associated with death (odds ratio, 14.4) 3.
Long-term follow-up data are scarce. Gaylord et al 42 demonstrated a high (54%) incidence of chronic lung disease in survivors of pulmonary interstitial emphysema compared to their nursery’s overall incidence of 32%. In addition, 19% of the infants with pulmonary interstitial emphysema developed chronic lobar emphysema; of these babies, 50% received surgical lobectomies.
The mortality rate associated with pulmonary interstitial emphysema is reported to be as high as 53-67% 42. Lower mortality rates of 24% and 38% reported in other studies could result from differences in population selection 43. Morisot et al 44 reported an 80% mortality rate with pulmonary interstitial emphysema in infants with a birth weight of fewer than 1600 g and severe respiratory distress syndrome.
The early appearance of pulmonary interstitial emphysema (< 48 hours after birth) is associated with increased mortality. However, this may reflect the severity of the underlying parenchymal disease 44.
Long term monitoring
Monitoring for physical and psychomotor development in a neonatal follow-up care program or equivalent program is important, because most infants with pulmonary interstitial emphysema (pulmonary interstitial emphysema) are premature and are at risk for developmental delay. In addition, pulmonary interstitial emphysema has been associated with increased risks of intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL), which also raise the risks of developmental delay in these infants.
Patients with chronic lung disease may need pediatric pulmonology follow-up care. Note that the available literature remains unclear regarding the role of bronchodilator agents in preventing or treating chronic lung disease in preterm infants; in addition, no specific trials appear to have studied the use of these agents for managing chronic lung disease in this population 45.
References- Moriette G, Paris-Llado J, Walti H, et al. Prospective randomized multicenter comparison of high-frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics. 2001;107(2):363-372. https://doi.org/10.1542/peds.107.2.363
- Hart SM, McNair M, Gamsu HR, Price JF. Pulmonary interstitial emphysema in very low birthweight infants. Arch Dis Child. 1983;58(8):612-615. doi:10.1136/adc.58.8.612 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1628320/pdf/archdisch00743-0044.pdf
- Verma RP, Chandra S, Niwas R, Komaroff E. Risk factors and clinical outcomes of pulmonary interstitial emphysema in extremely low birth weight infants. J Perinatol. 2006 Mar. 26(3):197-200.
- Kendig JW, Notter RH, Cox C, et al. Surfactant replacement therapy at birth: final analysis of a clinical trial and comparisons with similar trials. Pediatrics. 1988 Nov. 82(5):756-62.
- Dunn MS, Shennan AT, Zayack D, Possmayer F. Bovine surfactant replacement therapy in neonates of less than 30 weeks’ gestation: a randomized controlled trial of prophylaxis versus treatment. Pediatrics. 1991 Mar. 87(3):377-86.
- Kattwinkel J, Bloom BT, Delmore P, et al. Prophylactic administration of calf lung surfactant extract is more effective than early treatment of respiratory distress syndrome in neonates of 29 through 32 weeks’ gestation. Pediatrics. 1993 Jul. 92(1):90-8.
- Plenat F, Vert P, Didier F, Andre M. Pulmonary interstitial emphysema. Clin Perinatol. 1978 Sep. 5(2):351-75.
- Nunez-Ramiro A, Aguar M, Cernada M, Parra-Llorca A, Vento M. Oxygen needs during resuscitation and surfactant to achieve stabilisation were independent risks factors for pulmonary interstitial emphysema in preterm infants. Acta Paediatr. 2018 Jan. 107(1):28-32.
- Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001. CD000510
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007 Oct 17. CD003063
- Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009 Jan 21. CD000141
- Pohlandt F, Saule H, Schroder H, et al. Decreased incidence of extra-alveolar air leakage or death prior to air leakage in high versus low rate positive pressure ventilation: results of a randomised seven-centre trial in preterm infants. Eur J Pediatr. 1992 Dec. 151(12):904-9.
- Greenough A, Murthy V, Milner AD, Rossor TE, Sundaresan A. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst Rev. 2016 Aug 19. CD000456
- Henderson-Smart DJ, Cools F, Bhuta T, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2007 Jul 18. CD000104
- Moriette G, Paris-Llado J, Walti H, et al. Prospective randomized multicenter comparison of high-frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics. 2001 Feb. 107(2):363-72.
- Bhuta T, Henderson-Smart DJ. Rescue high frequency oscillatory ventilation versus conventional ventilation for pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2000. (2):CD000438
- Bhuta T, Henderson-Smart DJ. Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2000. (2):CD000328
- Goel S, Mondkar J, Panchal H, Hegde D, Utture A, Manerkar S. Nasal mask versus nasal prongs for delivering nasal continuous positive airway pressure in preterm infants with respiratory distress: a randomized controlled trial. Indian Pediatr. 2015 Dec. 52(12):1035-40.
- Stefanescu BM, Frewan N, Slaughter JC, O’Shea TM. Volume guarantee pressure support ventilation in extremely preterm infants and neurodevelopmental outcome at 18 months. J Perinatol. 2015 Jun. 35(6):419-23.
- McCallion N, Davis PG, Morley CJ. Volume-targeted versus pressure-limited ventilation in the neonate. Cochrane Database Syst Rev. 2005 Jul 20. CD003666
- Campbell RE. Intrapulmonary interstitial emphysema: a complication of hyaline membrane disease. Am J Roentgenol Radium Ther Nucl Med. 1970 Nov. 110(3):449-56.
- Jabra AA, Fishman EK, Shehata BM, Perlman EJ. Localized persistent pulmonary interstitial emphysema: CT findings with radiographic-pathologic correlation. AJR Am J Roentgenol. 1997 Nov. 169(5):1381-4.
- Kim HR, Yoo SM, Lee HY, Han JH, Frazier AA, White CS. Presence of subpleural pulmonary interstitial emphysema as an indication of single or multiple alveolar ruptures on CT in patients with spontaneous pneumomediastinum. Acta Radiol. 2016 Dec. 57(12):1483-1489.
- Donn SM. Assisted ventilation and its complications. Martin RJ, Fanaroff AA, Walsh MC, eds. Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. 10th ed. Philadelphia: Elsevier Saunders; 2015. Vol 2: 1107.
- Fitzgerald D, Willis D, Usher R, Outerbridge E, Davis GM. Dexamethasone for pulmonary interstitial emphysema in preterm infants. Biol Neonate. 1998. 73(1):34-9.
- Mahapatra S, Scottoline B. Steroid-induced resolution of refractory pulmonary interstitial emphysema. J Matern Fetal Neonatal Med. 2016 Dec. 29(24):4092-5.
- Leonidas JC, Hall RT, Rhodes PG. Conservative management of unilateral pulmonary interstitial emphysema under tension. J Pediatr. 1975 Nov. 87(5):776-8.
- Dordelmann M, Schirg E, Poets CF, Ure B, Gluer S, Bohnhorst B. Therapeutic lung puncture for diffuse unilateral pulmonary interstitial emphysema in preterm infants. Eur J Pediatr Surg. 2008 Aug. 18(4):233-6.
- Levine DH, Trump DS, Waterkotte G. Unilateral pulmonary interstitial emphysema: a surgical approach to treatment. Pediatrics. 1981 Oct. 68(4):510-4.
- Phatak RS, Pairaudeau CF, Smith CJ, Pairaudeau PW, Klonin H. Heliox with inhaled nitric oxide: a novel strategy for severe localized interstitial pulmonary emphysema in preterm neonatal ventilation. Respir Care. 2008 Dec. 53(12):1731-8.
- Kim C, Shin JE, Lee SM, et al. A case of pulmonary interstitial emphysema treated by percutaneous catheter insertion in extremely low birth weight infant. Yonsei Med J. 2016 Nov. 57(6):1523-6.
- Schwartz AN, Graham CB. Neonatal tension pulmonary interstitial emphysema in bronchopulmonary dysplasia: treatment with lateral decubitus positioning. Radiology. 1986 Nov. 161(2):351-4.
- Chalak LF, Kaiser JR, Arrington RW. Resolution of pulmonary interstitial emphysema following selective left main stem intubation in a premature newborn: an old procedure revisited. Paediatr Anaesth. 2007 Feb. 17(2):183-6.
- Weintraub Z, Oliven A, Weissman D, Sonis Z. A new method for selective left main bronchus intubation in premature infants. J Pediatr Surg. 1990 Jun. 25(6):604-6.
- Keszler M, Donn SM, Bucciarelli RL, et al. Multicenter controlled trial comparing high-frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema. J Pediatr. 1991 Jul. 119(1 Pt 1):85-93.
- Clark RH, Gerstmann DR, Null DM, et al. Pulmonary interstitial emphysema treated by high-frequency oscillatory ventilation. Crit Care Med. 1986 Nov. 14(11):926-30.
- Squires KA, De Paoli AG, Williams C, Dargaville PA. High-frequency oscillatory ventilation with low oscillatory frequency in pulmonary interstitial emphysema. Neonatology. 2013. 104(4):243-9.
- Gessler P, Toenz M, Gugger M, Pfenninger J. Lobar pulmonary interstitial emphysema in a premature infant on continuous positive airway pressure using nasal prongs. Eur J Pediatr. 2001 Apr. 160(4):263-4.
- Jassal MS, Benson JE, Mogayzel PJ Jr. Spontaneous resolution of diffuse persistent pulmonary interstitial emphysema. Pediatr Pulmonol. 2008 Jun. 43(6):615-9.
- Greenough A, Dixon AK, Roberton NR. Pulmonary interstitial emphysema. Arch Dis Child. 1984 Nov. 59(11):1046-51.
- Perea L, Blinman T, Piccione J, Laje P. Bilateral congenital lobar emphysema: staged management. J Pediatr Surg. 2017 Sep. 52(9):1442-5.
- Gaylord MS, Thieme RE, Woodall DL, Quissell BJ. Predicting mortality in low-birth-weight infants with pulmonary interstitial emphysema. Pediatrics. 1985 Aug. 76(2):219-24.
- Heneghan MA, Sosulski R, Alarcon MB. Early pulmonary interstitial emphysema in the newborn: a grave prognostic sign. Clin Pediatr (Phila). 1987 Jul. 26(7):361-5.
- Morisot C, Kacet N, Bouchez MC, et al. Risk factors for fatal pulmonary interstitial emphysema in neonates. Eur J Pediatr. 1990 Apr. 149(7):493-5.
- Ng G, da Silva O, Ohlsson A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2016 Dec 14. 12:CD003214