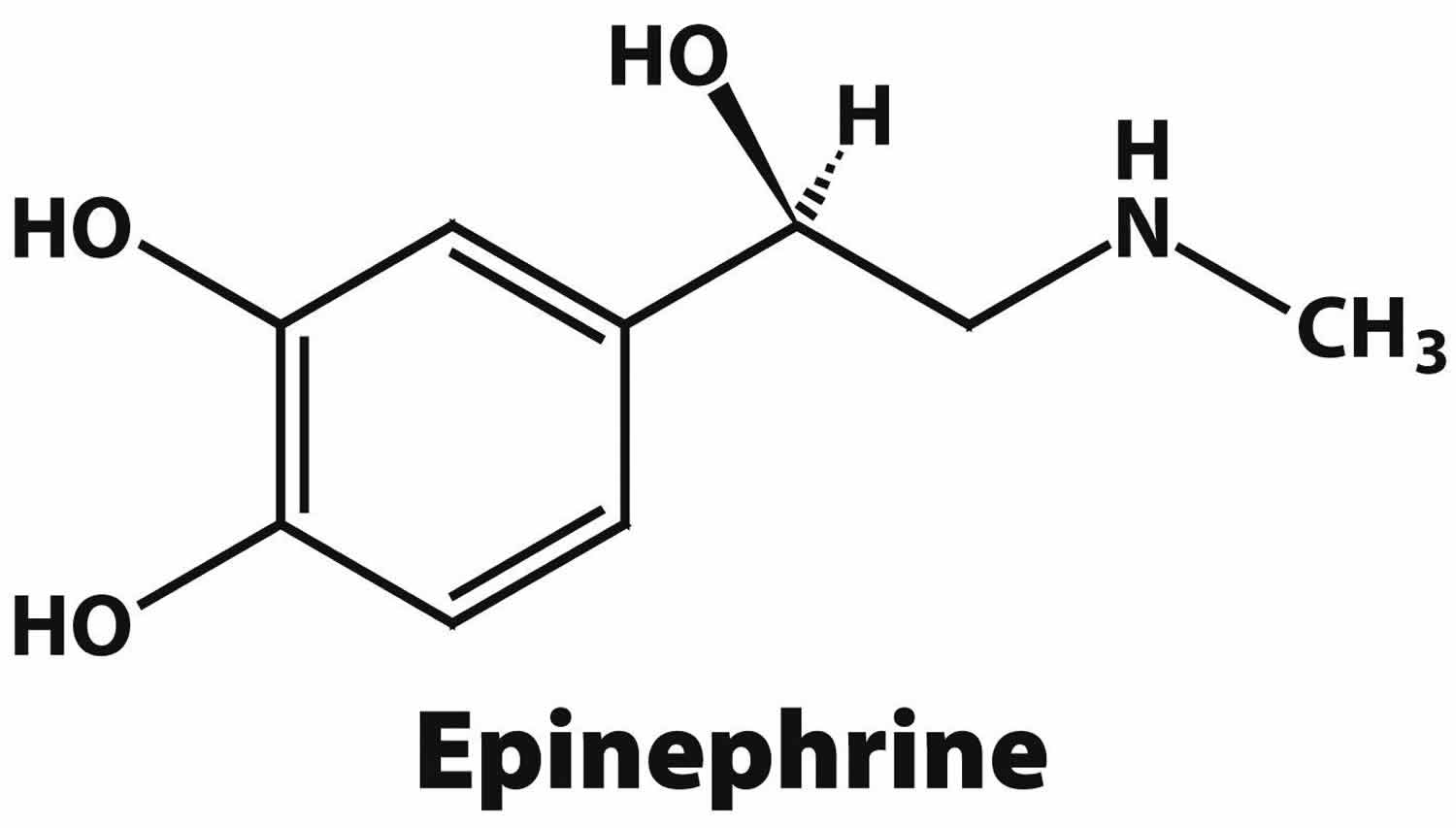
What is epinephrine
Epinephrine also known as adrenaline, is a hormone, neurotransmitter, and medication 1. Epinephrine is normally produced by both the adrenal glands and certain neurons 1. Epinephrine is one of a group of monoamines called the catecholamines. Epinephrine is produced in some neurons of the central nervous system, and in the chromaffin cells of the adrenal medulla from the amino acids phenylalanine and tyrosine 2. Epinephrine is synthesized in the medulla of the adrenal gland in an enzymatic pathway that converts the amino acid tyrosine into a series of intermediates and, ultimately, epinephrine (Figure 1). Tyrosine is first oxidized to L-DOPA, which is subsequently decarboxylated to give dopamine. Dopamine is then converted to norepinephrine by dopamine beta-hydroxylase. The final step in epinephrine biosynthesis is the methylation of the primary amine of norepinephrine. This reaction is catalyzed by the enzyme phenylethanolamine N-methyltransferase (PNMT) which utilizes S-adenosyl methionine (SAMe) as the methyl donor 3. While phenylethanolamine N-methyltransferase (PNMT) is found primarily in the cytosol of the endocrine cells of the adrenal medulla (also known as chromaffin cells), it has been detected at low levels in both the heart and brain 4.
The major physiologic triggers of epinephrine release center upon stresses, such as physical threat, excitement, noise, bright lights, and high ambient temperature. All of these stimuli are processed in the central nervous system 5. Adrenocorticotropic hormone (ACTH) and the sympathetic nervous system stimulate the synthesis of epinephrine precursors by enhancing the activity of tyrosine hydroxylase and dopamine β-hydroxylase, two key enzymes involved in catecholamine synthesis. ACTH also stimulates the adrenal cortex to release cortisol, which increases the expression of PNMT in chromaffin cells, enhancing epinephrine synthesis. This is most often done in response to stress. The sympathetic nervous system, acting via splanchnic nerves to the adrenal medulla, stimulates the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and, thus, the release of epinephrine (and norepinephrine) into the bloodstream. Unlike many other hormones epinephrine (as with other catecholamines) does not exert negative feedback to down-regulate its own synthesis. Abnormally elevated levels of adrenaline can occur in a variety of conditions, such as surreptitious epinephrine administration, pheochromocytoma, and other tumors of the sympathetic ganglia. Epinephrine action is terminated with reuptake into nerve terminal endings, some minute dilution, and metabolism by monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT).
Figure 1. Epinephrine synthesis
Epinephrine plays an important role in the fight-or-flight response by increasing blood flow to muscles, output of the heart, pupil dilation response, and blood sugar level 6. Epinephrine does this by binding to alpha and beta receptors (see Figure 2).
Epinephrine is the treatment of choice and the first drug administered for acute anaphylaxis, as confirmed internationally by most consensus anaphylaxis guidelines published in the English language over the past 30 years 7. Therapeutic recommendations for epinephrine use in anaphylaxis are largely based on clinical pharmacology studies, clinical observation, and animal models.
Epinephrine mechanism of action
Epinephrine is a sympathomimetic catecholamine that exerts its pharmacologic effects on both alpha and beta-adrenergic receptors using a G protein-linked second messenger system 8. Epinephrine’s actions vary by tissue type and tissue expression of adrenergic receptors. Epinephrine has a greater affinity for beta receptors in small doses. However, large doses produce selective action on alpha receptors. Through its action on alpha-1 receptors, epinephrine induces increased vascular smooth muscle contraction, pupillary dilator muscle contraction, and intestinal sphincter muscle contraction. For example, high levels of epinephrine causes smooth muscle relaxation in the airways but causes contraction of the smooth muscle that lines most arterioles. Other important effects of epinephrine include increasing heart rate, myocardial contractility, and renin release via beta-1 receptors. Beta-2 effects produce bronchodilation which may be useful as an adjunct treatment of asthma exacerbations as well as vasodilation, tocolysis, and increased aqueous humor
Epinephrine is a nonselective agonist of all adrenergic receptors, including the major subtypes α1, α2, β1, β2, and β3 9. Epinephrine’s binding to these receptors triggers a number of metabolic changes. Binding to α-adrenergic receptors inhibits insulin secretion by the pancreas, stimulates glycogenolysis in the liver and muscle 10, and stimulates glycolysis and inhibits insulin-mediated glycogenesis in muscle 11. β adrenergic receptor binding triggers glucagon secretion in the pancreas, increased adrenocorticotropic hormone (ACTH) secretion by the pituitary gland, and increased lipolysis by adipose tissue. Together, these effects lead to increased blood glucose and fatty acids, providing substrates for energy production within cells throughout the body 12.
Epinephrine actions are to increase peripheral resistance via α1 receptor-dependent vasoconstriction and to increase cardiac output via its binding to β1 receptors. The goal of reducing peripheral circulation is to increase coronary and cerebral perfusion pressures and therefore increase oxygen exchange at the cellular level 13. While epinephrine does increase aortic, cerebral, and carotid circulation pressure, it lowers carotid blood flow and end-tidal CO2 or ETCO2 levels. It appears that epinephrine may be improving macrocirculation at the expense of the capillary beds where actual perfusion is taking place 14.
Table 1. Physiologic responses to epinephrine by organ
| Organ | Effects |
|---|---|
| Heart | Increases heart rate; contractility; conduction across AV node |
| Lungs | Increases respiratory rate; bronchodilation |
| Systemic | Vasoconstriction and vasodilation |
| Liver | Stimulates glycogenolysis |
| Systemic | Triggers lipolysis |
| Systemic | Muscle contraction |
Figure 2. Epinephrine mechanism of action
Epinephrine uses
Epinephrine is one of the most commonly used agents in a variety of clinical settings as it functions as medication and hormone. Epinephrine is currently FDA-approved for various situations, including emergency treatment of type 1 hypersensitivity reactions including anaphylaxis, induction, and maintenance of mydriasis during intraocular surgeries, and hypotension due to septic shock 8. Off-label uses of epinephrine include, but are not limited to, ventricular fibrillation, pulseless ventricular tachycardia, asystole, pulseless electrical activity, croup, and severe asthma exacerbations unresponsive to standard treatment. In the operating room setting, epinephrine is used as a local anesthetic block as well. Produced by the adrenal medulla, epinephrine plays a vital role in the body’s acute stress response by stimulating the sympathetic nervous system.
Epinephrine at recommended dosages and routes of administration, the α-adrenergic vasoconstrictive effects reverse peripheral vasodilation, which alleviates hypotension and also reduces erythema, urticaria, and angioedema. Local injection of epinephrine may also minimize further absorption of antigen from a sting or injection, but this has not been studied systematically. The β-adrenergic properties of epinephrine cause bronchodilation, increase myocardial output and contractility, and suppress further mediator release from mast cells and basophils 15. Epinephrine administered in low concentrations (e.g., 0.1 μg/kg) paradoxically can produce vasodilation, hypotension, and increased release of inflammatory mediators 16.
Epinephrine administration enhances coronary blood flow. Two mechanisms are probably responsible: an increased duration of diastole compared with systole and a vasodilator effect caused by increased myocardial contractility. These actions usually offset the vasoconstrictor effects of epinephrine on the coronary arteries 15.
Rapid achievement of peak plasma and tissue epinephrine levels seems to optimize survival because retrospective human studies demonstrate that delayed administration is associated with poor outcomes 17. However, epinephrine administration during anaphylaxis is not always effective, and patients may still die 18. Reasons may be multifactorial and include delayed administration, inadequate doses, inappropriate route of administration, use of epinephrine that has passed its expiration date, leading to inadvertent administration of an inadequate dose, or an underlying disease, such as poorly controlled asthma, cardiovascular disease, mastocytosis, and perhaps other serious systemic disorders 19. A study done in a canine model also demonstrates that achievement of peak epinephrine plasma levels and hemodynamic recovery is not as effective when epinephrine administration is delayed until hypotension has developed 20.
Figure 3. Epinephrine therapeutic window
[Source 7 ]Epinephrine has a relatively narrow therapeutic window (relative benefit vs risk; Figure 3). Common pharmacological effects that occur at recommended doses via any route of administration include agitation, anxiety, tremulousness, headache, dizziness, pallor, or palpitations 16. Rarely, and usually associated with overdosage or overly rapid rate of intravenous infusion, epinephrine administration might contribute to or cause myocardial ischemia or infarction 21, pulmonary edema 22, prolonged QTc (QTc = QT interval divided by the square root of the RR interval [in seconds] of the electrocardiogram) interval 23, ventricular arrhythmias, accelerated hypertension, and intracranial hemorrhage in adults and children alike 24. Nonetheless, some patients have survived massive overdoses of epinephrine, with no evidence of myocardial ischemia 25. Particularly vulnerable populations are those individuals at the extremes of age and those with hypertension, peripheral vascular disease, ischemic heart disease, or untreated hyperthyroidism (increased number of β-adrenergic receptors in the vasculature of these individuals render the myocardium more sensitive to β-adrenergic effects of epinephrine) 26. Certain medications might also increase the risk of adverse events from drug interactions 26. Some medications decrease the effectiveness of endogenous catecholamine stores or exogenously administered epinephrine (β-adrenergic blockers), interfere with intrinsic compensatory responses to hypotension (angiotensin-converting enzyme inhibitors and possibly angiotensin II receptor blockers), or impede epinephrine metabolism and lead to increased plasma and tissue concentrations (tricyclic antidepressants and monoamine oxidase inhibitors). The β-adrenergic antagonists and α-adrenergic antagonists can also potentially exaggerate pharmacological effects of epinephrine by permitting unopposed α-adrenergic (vasoconstrictor) and β-adrenergic (vasodilator) effects, respectively. Cocaine and amphetamines sensitize the myocardium to effects of epinephrine, thus increasing the risk of toxicity.
However, none of these circumstances pose an absolute contraindication to epinephrine administration for anaphylaxis 27.
Epinephrine administration
Depending on the diagnosis, epinephrine can be given in various forms. For the treatment of anaphylaxis, epinephrine is preferably injected intramuscularly into the anterolateral aspect of the thigh due to rapid absorption or subcutaneously as well. For advanced cardiovascular life support, epinephrine may be given intravenously or intraosseous if needed. Another route of administration is through an endotracheal tube often used in neonatal resuscitation.
Monitoring
When administered parenterally, epinephrine has a rapid onset, but short duration of action. When given intravenously, it has a half-life of fewer than 5 minutes. It is primarily metabolized in the liver along with various other locations such as the kidneys, skeletal muscle, and mesenteric organs. It is degraded into an inactive metabolite named vanillylmandelic acid by monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT) and excreted into the urine. However, a small amount of the drug is excreted unchanged as well.
Epinephrine is a hormone that produces widespread effects. Certain effects need to be monitored. Tachycardia and hypertension are expected when epinephrine is given intravenously, so it is important to titrate carefully while monitoring hemodynamics. Epinephrine is also used with anesthetic agents to provide analgesia. In locations where extravasation of epinephrine has occurred, prevention and treatment of ischemia-induced necrosis are necessary. The infiltrated area should be treated with 10 mL to 15 mL saline solution containing 5 mL to 10 mg of phentolamine, an alpha-adrenergic blocking agent.
Renal impairment must be monitored as epinephrine causes renal blood vessel constriction and can decrease urine impairment. In patients with chronic kidney disease and various other renal pathologies, clinical judgment should be exercised. During intraocular use, epinephrine must be diluted otherwise corneal endothelial damage can result if undiluted concentrations of sodium bisulfite are administered.
Epinephrine auto injector
Epinephrine is an injection-based medication used to treat life-threatening allergic reactions called anaphylaxis or severe allergic reaction. These very serious reactions are most commonly caused by foods, drugs, stinging insects, latex, medications or other allergens. Most fatalities occur due to delay and delivery of the needed medication. Although many medications may be used for treating anaphylaxis, epinephrine is the life-saving medication that must be given immediately to avoid death. People with severe allergy or a history of anaphylaxis should carry an epinephrine auto-injector with them at all times. Epinephrine typically comes as a single-dose pre-filled automatic injection device to be injected into the thigh. After you use the epinephrine autoinjector, go the closest emergency room immediately.
Epinephrine autoinjectors, which are easy to use and will inject through clothing, are currently available in 2 fixed doses: 0.15 mg and 0.3 mg 7. The potential exists for overdosage in infants receiving the 0.15 mg, overdosage in some small children receiving the 0.3 mg dose, and for underdosage in many adolescents receiving the 0.15 mg dose 28. The relative benefits and risks of dosage might vary with each individual, but autoinjectors with 0.15 mg of epinephrine are recommended for otherwise healthy children who weigh 10 to 25 kg (22-55 lb) and autoinjectors with 0.3 mg of epinephrine for children who weigh approximately 25 kg (55 lb) or more 28. Providing parents with an epinephrine ampule, syringe, and needle is not an appropriate option unless autoinjectors are not available for prescription 29.
There are several readily available brands of epinephrine autoinjectors: EpiPen®, Auvi-Q® and Adrenaclick®. There is also an authorized generic of Adrenaclick® called epinephrine injection, USP auto-injector. As generics are sometimes substituted for brand names, it is possible that a generic epinephrine autoinjector would be substituted at the pharmacy for the brand your physician prescribed. Although the medication is the same, the method for injecting it is different for each brand. Your physician or his/her staff will provide training on how to use the specific brand prescribed.
The next time you pick up your prescription, be sure to compare the brand and dose you received with the brand you have been trained to use. If the epinephrine medication appears to be different than what you expected, find out why a substitution was made. Also, be sure to get a demonstration on how to use the product.
Your pharmacist and your doctor’s office want to make sure you know how to use the epinephrine autoinjector in an emergency, so ask as many questions as you need to feel comfortable. If you have additional concerns, contact your allergist for more information.
What is the recommendation regarding use of epinephrine in patients with a known food allergy who have ingested the allergenic food but have no symptoms?
There is no “official recommendation” regarding the issue you present. There is no consensus of opinion in this regard, and different physicians utilize different strategies. The mean time to respiratory or cardiovascular arrest after the ingestion of a food to which a patient is allergic is 30 minutes 30. Thus there is very little time for you to act after you exprience even the mildest symptom of an anaphylactic event. If the situation occurs in the school, it’s recommended you administer epinephrine immediately, regardless of whether or not any symptoms are present. However, this is a philosophical decision and varies amongst allergists. It is not one which guidelines have addressed definitively to date.
Experts may differ on how they define the clinical threshold by which they define and treat anaphylaxis. However, they have no disagreement whatsoever that appropriate doses of intramuscular epinephrine should be administered rapidly once that threshold is reached. There is no absolute contraindication to epinephrine administration in anaphylaxis, and all subsequent therapeutic interventions depend on the initial response to epinephrine 31.
Based on available evidence, the benefit of using appropriate doses of intramuscular epinephrine in anaphylaxis far exceeds the risk 31. Consensus experts opinion and anecdotal evidence recommend epinephrine administration sooner rather than later, that is, when the initial signs and symptoms of anaphylaxis occur, regardless of their severity, because fatalities in anaphylaxis usually result from delayed or inadequate administration of epinephrine 31.
In July 2008, the World Allergy Organization published the following statements 7:
“Anaphylaxis is an acute and potentially lethal multisystem allergic reaction. Most consensus guidelines for the past 30 years have held that epinephrine is the drug of choice and the first drug that should be administered in acute anaphylaxis. Some state that properly administered epinephrine has no absolute contraindication in this clinical setting. A committee of anaphylaxis experts assembled by the World Allergy Organization has examined the evidence from the medical literature concerning the appropriate use of epinephrine for anaphylaxis. The committee strongly believes that epinephrine is currently underused and often dosed suboptimally to treat anaphylaxis, is underprescribed for potential future self-administration, that most of the reasons proposed to withhold its clinical use are flawed, and that the therapeutic benefits of epinephrine exceed the risk when given in appropriate intramuscular doses.”
Alternative treatments – such as antihistamines, sublingual isoproterenol, inhaled epinephrine, and corticosteroids without epinephrine – have failed to prevent or relieve severe anaphylactic reactions. It is therefore inappropriate to use them for the first-line treatment or prevention of anaphylaxis.
Epinephrine dose for anaphylaxis
No controlled trials have been performed with epinephrine therapy to answer dose response questions. There is toxicity with increasing dose and the 0.01 mg/kg is based partially on the lack of significant side effect and clinical experience indicating this dose is effective 32.
Also, it is important to note that the optimal dose of epinephrine is unknown. There have been no published dose response studies documenting that the suggested dose of 0.01 mg/kg is indeed the “correct dose,” and the origin of this suggested dosage regimen could not be found 33. In fact, before the advent of currently available automatic epinephrine injectors, the recommended doses of epinephrine varied considerably. In the early epinephrine literature, asthma was treated in adults with 1-mg doses and in infants weighing 25 pounds with 1/16th of the adult dose. Variations of this dose ranging from 0.2 to 0.5 mg were recommended for the treatment of anaphylaxis as late as 1978. There have been commercially available preloaded epinephrine injectors filled with a dose of 0.5 mg for the administration to adults, and this dose was well accepted as optimal for the treatment of anaphylaxis until the advent of automatic injectors. Thus, the actual optimal dosing regimen is unknown.
Expert consensus and anecdotal evidence indicate aqueous epinephrine 1:1000 dilution (1 mg in 1 mL), 0.2 to 0.5 mg (0.01 mg/kg in children; maximum dose, 0.3 mg) administered intramuscularly every 5 to 15 minutes or as necessary, depending on the severity of the anaphylaxis, should be used to control symptoms and sustain or increase blood pressure 34. Efficacy comparisons of intramuscular injections to subcutaneous injections have not been done during acute anaphylaxis. However, absorption is complete and more rapid and plasma levels are higher in asymptomatic adults and children who receive epinephrine intramuscularly in the anterolateral thigh (vastus lateralis) 35. In overweight and obese individuals, the thickness of the subcutaneous fat pad may preclude intramuscular access 36.
The dose of 0.01 mg/kg to a maximum dose of 0.3-0.5 mg is based upon extensive experience and some evidence that higher doses have resulted in adverse effects 17. A published guideline on the treatment of anaphylaxis concluded that “despite previous guidelines, there is still confusion about the indications, dose and route of adrenaline” and “early treatment with intramuscular adrenaline is the treatment of choice for patients having an anaphylactic reaction” 37. That is also the conclusion of a literature review published by Lieberman and Simons focusing on the risk of epinephrine in subjects with heart disease 32.. The 1mg IV dose of epinephrine for cardiovascular arrest is based on maximum stimulation of cardiac adrenergic receptors in the setting of ventricular fibrillation or electromechanical disassociation and is not related to the effects specific for anaphylaxis.
The physiologic effects of epinephrine that are important in anaphylaxis include alpha-1 adrenergic receptor stimulation resulting in vasoconstriction and increased blood pressure and cardiac venous return improving shock and edema of the larynx, direct cardiac effects via ionotropic and chronotropic β1-adrenergic receptors, bronchodilation via β2-adrenergic receptors and decreased mast cell/basophil mediator release. The earlier the use of epinephrine the better the effect as there is less benefit and greater side effect after third space fluid loss occurs with vascular leakage. However, there is no “preemptive” role for epinephrine. That is, epinephrine is not used for someone at risk of anaphylaxis but rather after symptoms and signs develop. There is a potential that early use reduces mediator release and minimizes severity of anaphylaxis and possibly reduces biphasic anaphylaxis.
In summary, epinephrine by the IM route is the only effective therapy for the life-threatening aspects of anaphylaxis, i.e. shock, bronchospasm and laryngeal edema. Early use in the time course of anaphylaxis is more effective with repeated dosing during on-going anaphylaxis with worsening symptoms up to a total of 1-1.5 mg. Other measures including administration of intravenous fluid, nebulized bronchodilators and oxygen are also important. The dose of epinephrine is not based on rigorous evidence but is supported by clinical experience, the medical literature, practice parameters and expert opinion. Antihistamine therapy helps with symptoms of itching but is not life-saving. There is no role for epinephrine in the prevention of anaphylaxis but early administration may modify the severity by blocking the mediator release of basophils and mast cells.
What is epinephrine used for and epinephrine dosage?
Adult Dose for Asystole
Use: For prophylaxis and treatment of cardiac arrest and attacks of transitory atrioventricular heart block with syncopal seizures (Stokes-Adams Syndrome)
Injectable Solution of 0.1 mg/mL (1:10,000):
- IV: 0.5 to 1 mg (5 to 10 mL) IV once; during resuscitation effort, 0.5 mg (5 mL) should be given IV every 5 minutes
- Intracardiac: 0.3 to 0.5 mg (3 to 5 mL) via intracardiac injection into left ventricular chamber once
- Endotracheal: 0.5 to 1 mg (5 mL to 10 mL) via endotracheal tube directly into bronchial tree once
Comments:
Intracardiac injection should only be administered by personnel well trained in this technique and only if there has not been sufficient time to establish an IV route.
The American Heart Association (AHA) recommends
Use: For administration during cardiac arrest
- IV or intraosseous: 1 mg IV or intraosseous every 3 to 5 minutes during cardiac arrest
- Endotracheal: 2 to 2.5 mg endotracheally every 3 to 5 minutes during cardiac arrest if IV or intraosseous route cannot be established
Adult Dose for Ventricular Fibrillation
Use: For prophylaxis and treatment of cardiac arrest and attacks of transitory atrioventricular heart block with syncopal seizures (Stokes-Adams Syndrome)
Injectable Solution of 0.1 mg/mL (1:10,000):
- IV: 0.5 to 1 mg (5 to 10 mL) IV once; during resuscitation effort, 0.5 mg (5 mL) should be given IV every 5 minutes
- Intracardiac: 0.3 to 0.5 mg (3 to 5 mL) via intracardiac injection into left ventricular chamber once
- Endotracheal: 0.5 to 1 mg (5 mL to 10 mL) via endotracheal tube directly into bronchial tree once
Comments:
Intracardiac injection should only be administered by personnel well trained in this technique and only if there has not been sufficient time to establish an IV route.
The American Heart Association (AHA) recommends
Use: For administration during cardiac arrest
- IV or intraosseous: 1 mg IV or intraosseous every 3 to 5 minutes during cardiac arrest
- Endotracheal: 2 to 2.5 mg endotracheally every 3 to 5 minutes during cardiac arrest if IV or intraosseous route cannot be established
Adult Dose for Ventricular Tachycardia
Use: For prophylaxis and treatment of cardiac arrest and attacks of transitory atrioventricular heart block with syncopal seizures (Stokes-Adams Syndrome)
Injectable Solution of 0.1 mg/mL (1:10,000):
- IV: 0.5 to 1 mg (5 to 10 mL) IV once; during resuscitation effort, 0.5 mg (5 mL) should be given IV every 5 minutes
- Intracardiac: 0.3 to 0.5 mg (3 to 5 mL) via intracardiac injection into left ventricular chamber once
- Endotracheal: 0.5 to 1 mg (5 mL to 10 mL) via endotracheal tube directly into bronchial tree once
Comments:
Intracardiac injection should only be administered by personnel well trained in this technique and only if there has not been sufficient time to establish an IV route.
The American Heart Association (AHA) recommends
Use: For administration during cardiac arrest
- IV or intraosseous: 1 mg IV or intraosseous every 3 to 5 minutes during cardiac arrest
- Endotracheal: 2 to 2.5 mg endotracheally every 3 to 5 minutes during cardiac arrest if IV or intraosseous route cannot be established
Adult Dose for Cardiac Arrest
Use: For prophylaxis and treatment of cardiac arrest and attacks of transitory atrioventricular heart block with syncopal seizures (Stokes-Adams Syndrome)
Injectable Solution of 0.1 mg/mL (1:10,000):
- IV: 0.5 to 1 mg (5 to 10 mL) IV once; during resuscitation effort, 0.5 mg (5 mL) should be given IV every 5 minutes
- Intracardiac: 0.3 to 0.5 mg (3 to 5 mL) via intracardiac injection into left ventricular chamber once
- Endotracheal: 0.5 to 1 mg (5 mL to 10 mL) via endotracheal tube directly into bronchial tree once
Comments:
Intracardiac injection should only be administered by personnel well trained in this technique and only if there has not been sufficient time to establish an IV route.
The American Heart Association (AHA) recommends
Use: For administration during cardiac arrest
- IV or intraosseous: 1 mg IV or intraosseous every 3 to 5 minutes during cardiac arrest
- Endotracheal: 2 to 2.5 mg endotracheally every 3 to 5 minutes during cardiac arrest if IV or intraosseous route cannot be established
Adult Dose for Asthma – Acute
Use: For the treatment of acute asthmatic attacks to relieve bronchospasm not controlled by inhalation or subcutaneous administration of other solutions of the drug
- Injectable Solution of 0.1 mg/mL (1:10,000): 0.1 to 0.25 mg (1 to 2.5 mL) IV slowly once
Adult Dose for Allergic Reaction
Uses: For the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging or biting insects, allergen immunotherapy, foods, drugs, diagnostic testing substances, and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis; and for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including those with a history of anaphylactic reactions
Auto-Injector:
- 30 kg or greater: 0.3 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
Comments:
- The manufacturer product information for the specific auto-injector being used should be consulted for administration instructions.
- More than 2 sequential doses should only be administered under direct medical supervision.
- The auto-injectors are intended for immediate administration as emergency supportive therapy only and not as a replacement or substitute for immediate medical care.
Injectable Solution of 1 mg/mL (1:1000):
- 30 kg or greater: 0.3 to 0.5 mg (0.3 to 0.5 mL) of undiluted drug IM or subcutaneously into anterolateral aspect of the thigh; repeat every 5 to 10 minutes as needed
- Maximum dose per injection: 0.5 mg (0.5 mL)
Comments:
- For IM administration, use a long enough needle (at least 1/2 inch to 5/8 inch) to ensure injection into the muscle.
- Repeated injections should not be administered at the same site as resulting vasoconstriction may cause tissue necrosis.
- The patient should be monitored clinically for reaction severity and cardiac effects with repeat doses titrated to effect.
Injectable Solution of 0.1 mg/mL (1:10,000):
- 0.1 to 0.25 mg (1 to 2.5 mL) IV slowly once
Convenience Kit 1 mg/mL (1:1000):
- 0.2 to 1 mg IM or subcutaneous
Adult Dose for Anaphylaxis
Uses: For the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging or biting insects, allergen immunotherapy, foods, drugs, diagnostic testing substances, and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis; and for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including those with a history of anaphylactic reactions
Auto-Injector:
- 30 kg or greater: 0.3 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
Comments:
- The manufacturer product information for the specific auto-injector being used should be consulted for administration instructions.
- More than 2 sequential doses should only be administered under direct medical supervision.
- The auto-injectors are intended for immediate administration as emergency supportive therapy only and not as a replacement or substitute for immediate medical care.
Injectable Solution of 1 mg/mL (1:1000):
- 30 kg or greater: 0.3 to 0.5 mg (0.3 to 0.5 mL) of undiluted drug IM or subcutaneously into anterolateral aspect of the thigh; repeat every 5 to 10 minutes as needed
- Maximum dose per injection: 0.5 mg (0.5 mL)
Comments:
- For IM administration, use a long enough needle (at least 1/2 inch to 5/8 inch) to ensure injection into the muscle.
- Repeated injections should not be administered at the same site as resulting vasoconstriction may cause tissue necrosis.
- The patient should be monitored clinically for reaction severity and cardiac effects with repeat doses titrated to effect.
Injectable Solution of 0.1 mg/mL (1:10,000):
- 0.1 to 0.25 mg (1 to 2.5 mL) IV slowly once
Convenience Kit 1 mg/mL (1:1000):
- 0.2 to 1 mg IM or subcutaneous
Adult Dose for Pupillary Dilation
Use: For the induction and maintenance of mydriasis during intraocular surgery
Injectable Solution of 1 mg/mL (1:1000):
- Intraocular: Dilute 1 mL of the 1 mg/mL single-use vial (1:1000) in 100 to 1000 mL of an ophthalmic irrigation fluid to a concentration of 1:100,000 to 1:1,000,000 (10 mcg/mL to 1 mcg/mL) and use the irrigating solution as needed for the surgical procedure
- Intracameral: Following dilution in an ophthalmic irrigating fluid, the solution may also be injected intracamerally as a bolus dose of 0.1 mL at a dilution of 1:100,000 to 1:400,000 (10 mcg/mL to 2.5 mcg/mL)
Comments:
- The Adrenalin(R) formulation is not for ophthalmic use.
- Do not use if the solution is colored, cloudy, or contains particulate matter.
Adult Dose for Hypotension
Use: To increase mean arterial blood pressure in patients with hypotension associated with septic shock
- Injectable Solution of 1 mg/mL (1:1000): 0.05 to 2 mcg/kg/min IV and titrate to achieve desired mean arterial pressure (MAP)
- Dosage may be adjusted periodically, such as every 10 to 15 minutes in increments of 0.05 to 0.2 mcg/kg/min to achieve desired blood pressure goal
Comments:
- Must be diluted prior to use; consult manufacturer product information for appropriate dilution instructions.
- Correct blood volume depletion as fully as possible prior to administration; may be administered before and concurrently with blood volume replacement as an emergency measure.
- Continuous infusion is generally required over several hours or days until patient’s hemodynamic status improves; the duration of perfusion or total cumulative dose cannot be known.
- Following hemodynamic stabilization, may wean incrementally over time, such as decreasing doses every 30 minutes over a 12 to 24 hour period.
Adult Dose for Shock
Use: To increase mean arterial blood pressure in patients with hypotension associated with septic shock
- Injectable Solution of 1 mg/mL (1:1000): 0.05 to 2 mcg/kg/min IV and titrate to achieve desired mean arterial pressure (MAP)
- Dosage may be adjusted periodically, such as every 10 to 15 minutes in increments of 0.05 to 0.2 mcg/kg/min to achieve desired blood pressure goal
Comments:
- Must be diluted prior to use; consult manufacturer product information for appropriate dilution instructions.
- Correct blood volume depletion as fully as possible prior to administration; may be administered before and concurrently with blood volume replacement as an emergency measure.
- Continuous infusion is generally required over several hours or days until patient’s hemodynamic status improves; the duration of perfusion or total cumulative dose cannot be known.
- Following hemodynamic stabilization, may wean incrementally over time, such as decreasing doses every 30 minutes over a 12 to 24 hour period.
Adult Dose for Bradyarrhythmia
Use: For patients with symptomatic bradycardia, particularly if associated with hypotension, for whom atropine may be inappropriate or after atropine fails
The manufacturer gives no specific dosing instructions.
The American Heart Association (AHA) recommends:
- 2 to 10 mcg/min IV and titrate to patient response
- Alternate dose: 0.1 to 0.5 mcg/kg/min (in a 70 kg patient, 7 to 35 mcg/min) IV; titrate to effect
Pediatric Dose for Cardiac Arrest
Use: For resuscitation in the pediatric patient
The manufacturer gives no specific dosing instructions.
The American Heart Association (AHA) recommends:
- Neonates:
- IV: 0.01 to 0.03 mg/kg (1:10,000 injectable solution) IV once
- Endotracheal: 0.05 to 0.1 mg/kg (1:10,000 injectable solution) via endotracheal route once may be reasonable while attempting to gain IV access
Comments:
Due to lack of data to support endotracheal use, it is reasonable to provide medications via IV route as soon as venous access is established.
- Infants and Children:
- IV or intraosseous: 0.01 mg/kg (0.1 mL/kg of 1:10,000 injectable solution) IV or intraosseous once; may repeat every 3 to 5 minutes
- Maximum dose: 1 mg
- Endotracheal: 0.1 mg/kg (0.1 mL/kg of 1:1000 injectable solution) via endotracheal tube once, flush with 5 mL normal saline and follow with 5 ventilations; may repeat every 3 to 5 minutes
- Maximum dose: 2.5 mg
- IV or intraosseous: 0.01 mg/kg (0.1 mL/kg of 1:10,000 injectable solution) IV or intraosseous once; may repeat every 3 to 5 minutes
Pediatric Dose for Allergic Reaction
Uses: For the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging or biting insects, allergen immunotherapy, foods, drugs, diagnostic testing substances, and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis; and for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including those with a history of anaphylactic reactions
Auto-Injector:
- 7.5 to 15 kg: 0.1 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
- 15 to 30 kg: 0.15 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
- 30 kg or greater: 0.3 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
Comments:
- Since the lowest dose of the auto-injector is 0.1 mg, consider using other injectable forms of this drug if doses lower than 0.1 mg are necessary.
- More than 2 sequential doses should only be administered under direct medical supervision.
- The manufacturer product information for the specific auto-injector being used should be consulted for administration instructions.
Injectable Solution of 1 mg/mL (1:1000):
- Less than 30 kg: 0.01 mg/kg (0.01 mL/kg) of undiluted drug IM or subcutaneously into anterolateral aspect of thigh; repeat every 5 to 10 minutes as needed. Maximum dose per injection: 0.3 mg (0.3 mL)
- 30 kg or greater: 0.3 to 0.5 mg (0.3 to 0.5 mL) of undiluted drug IM or subcutaneously into anterolateral aspect of the thigh; repeat every 5 to 10 minutes as needed. Maximum dose per injection: 0.5 mg (0.5 mL)
Comments:
- For IM administration, use a long enough needle (at least 1/2 inch to 5/8 inch) to ensure injection into the muscle.
- Repeated injections should not be administered at the same site as resulting vasoconstriction may cause tissue necrosis.
- The patient should be monitored clinically for reaction severity and cardiac effects with repeat doses titrated to effect.
Injectable Solution of 0.1 mg/mL (1:10,000):
- Neonate: 0.01 mg/kg IV slowly once
- Infant: 0.05 mg IV slowly once; may repeat at 20 to 30 minute intervals as needed
Pediatric Dose for Anaphylaxis
Uses: For the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging or biting insects, allergen immunotherapy, foods, drugs, diagnostic testing substances, and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis; and for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including those with a history of anaphylactic reactions
Auto-Injector:
- 7.5 to 15 kg: 0.1 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
- 15 to 30 kg: 0.15 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
- 30 kg or greater: 0.3 mg IM or subcutaneously into anterolateral aspect of thigh; repeat as needed
Comments:
- Since the lowest dose of the auto-injector is 0.1 mg, consider using other injectable forms of this drug if doses lower than 0.1 mg are necessary.
- More than 2 sequential doses should only be administered under direct medical supervision.
- The manufacturer product information for the specific auto-injector being used should be consulted for administration instructions.
Injectable Solution of 1 mg/mL (1:1000):
- Less than 30 kg: 0.01 mg/kg (0.01 mL/kg) of undiluted drug IM or subcutaneously into anterolateral aspect of thigh; repeat every 5 to 10 minutes as needed. Maximum dose per injection: 0.3 mg (0.3 mL)
- 30 kg or greater: 0.3 to 0.5 mg (0.3 to 0.5 mL) of undiluted drug IM or subcutaneously into anterolateral aspect of the thigh; repeat every 5 to 10 minutes as needed. Maximum dose per injection: 0.5 mg (0.5 mL)
Comments:
- For IM administration, use a long enough needle (at least 1/2 inch to 5/8 inch) to ensure injection into the muscle.
- Repeated injections should not be administered at the same site as resulting vasoconstriction may cause tissue necrosis.
- The patient should be monitored clinically for reaction severity and cardiac effects with repeat doses titrated to effect.
Injectable Solution of 0.1 mg/mL (1:10,000):
- Neonate: 0.01 mg/kg IV slowly once
- Infant: 0.05 mg IV slowly once; may repeat at 20 to 30 minute intervals as needed
Pediatric Dose for Asthma – Acute
Use: For the treatment of acute asthmatic attacks to relieve bronchospasm not controlled by inhalation or subcutaneous administration of other solutions of the drug
Injectable Solution of 0.1 mg/mL (1:10,000):
-Neonate: 0.01 mg/kg IV slowly once
-Infant: 0.05 mg IV slowly once; may repeat at 20 to 30 minute intervals as needed
Pediatric Dose for Pupillary Dilation
Use: For the induction and maintenance of mydriasis during intraocular surgery
Injectable Solution of 1 mg/mL (1:1000):
- Intraocular: Dilute 1 mL of the 1 mg/mL single-use vial (1:1000) in 100 to 1000 mL of an ophthalmic irrigation fluid to a concentration of 1:100,000 to 1:1,000,000 (10 mcg/mL to 1 mcg/mL) and use the irrigating solution as needed for the surgical procedure
- Intracameral: Following dilution in an ophthalmic irrigating fluid, the solution may also be injected intracamerally as a bolus dose of 0.1 mL at a dilution of 1:100,000 to 1:400,000 (10 mcg/mL to 2.5 mcg/mL)
Comments:
- The Adrenalin(R) formulation is not for ophthalmic use.
- Do not use if the solution is colored, cloudy, or contains particulate matter.
Renal Dose Adjustments
- Data not available
Liver Dose Adjustments
- Data not available
Dose Adjustments
- Elderly patients may be particularly sensitive to the effects of this drug. Infuse slowly as there is an increased risk for adverse reactions in this patient population; consider lower starting doses for the treatment of anaphylaxis.
Epinephrine side effects
The more common side effects include tachycardia, hypertension, headache, anxiety, apprehension, palpitations, diaphoresis, nausea, vomiting, weakness, and tremors.
Epinephrine side effects listed by system
- Central nervous system (CNS): Anxiety, dizziness, nervousness, agitation, headache, Parkinson’s disease exacerbation
- Cardiovascular: Arrhythmias, chest pain, hypertension, palpitations, tachycardia, cerebrovascular accidents, ventricular ectopy, vasospasm, tissue ischemia
- Dermatologic: Gangrene at injection site (especially in buttocks), skin necrosis with extravasation
- Endocrine: Hyperglycemia, hypokalemia, lactic acidosis
- Gastrointestinal: Nausea, vomiting, increase in AST and ALT
- Neuromuscular: Tremors, weakness
- Renal: Decreased renal perfusion
- Respiratory: Dyspnea, pulmonary edema
Toxicity
Administration of excess epinephrine that leads to supra-therapeutic levels may cause predictable adverse effects that warrant supportive treatment. Overdose may cause elevated arterial pressures leading to cerebrovascular accidents. Pressor effects can be minimized by the usage of an alpha-adrenergic blocker or by the usage of vasodilators such as nitrites. Pulmonary edema has also been noted due to the underlying mechanism of peripheral vasoconstriction along with myocardial stimulation. Respiratory support may be needed alongside an alpha-adrenergic blocking drug to decrease vasoconstriction and enhance vascular flow. Due to strong beta-1 adrenergic effects on cardiac tissue, epinephrine toxicity may lead to potentially fatal cardiac arrhythmias or ischemia. Treatment involves administration of beta-adrenergic blocking agents such as metoprolol.
Drug Interactions
- Alpha-adrenergic blockers: Antagonizes pressor effects
- Antihypertensives: Antagonizes pressor effects
- Vasodilators: Antagonizes pressor effects
- Diuretics: Antagonizes pressor effects
- Beta-adrenergic blockers: Potentiates pressor effects
- Monoamine oxidase (MAO) inhibitors: Potentiates pressor effects
- Catechol-o-methyltransferase (COMT) inhibitors: Potentiates pressor effects
Careful monitoring of vital signs is crucial especially in patients with polypharmacy.
Epinephrine Contraindications
There are no absolute contraindications against using epinephrine 8. Some relative contraindications include hypersensitivity to sympathomimetic drugs, closed-angle glaucoma, anesthesia with halothane. As is the case with prescribing any medication, all practitioners should use clinical judgment and evaluate the benefits versus risk with epinephrine.
The use of epinephrine in select circumstances must be assessed as discussed below.
Pregnancy
Epinephrine is considered a pregnancy Category C medication.
- Category C: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
There are no well-controlled studies in humans, although animal studies have shown a teratogenic risk during organogenesis. It is capable of crossing the placenta. Gastroschisis has been demonstrated when epinephrine has been administered subcutaneously in rabbits at 15 times the maximum dosage. Epinephrine should be used cautiously when maternal blood pressure is 130/80 mm Hg and greater.
Labor and Delivery
Due to its effect on beta-2 adrenergic receptors causing tocolysis, epinephrine opposes the actions of oxytocin on the uterus and may delay labor. It must also be used with caution during anaphylaxis induced hypotension in pregnancy as it may lead to uterine vasoconstriction, thus decreasing oxygen delivery to the fetus.
Breastfeeding
More clinical studies are needed to determine if epinephrine is excreted through breast milk.
Pediatrics
Epinephrine is effective at a dilution of 1:100,000 to 1:400,000 for mydriasis induction and maintenance in pediatric intraocular surgeries.
Geriatrics
Due to the expected decrease in renal, hepatic, and cardiac function of geriatric patients, epinephrine should be started at the lower end of the dosing regime and titrated appropriately for clinical effect.
Location
Several locations should be avoided when injecting epinephrine specifically the digits, nose, penis, and toes as these areas are more prone to ischemia. Epinephrine should be avoided in tissues supplied by end arteries.
References- Lieberman M, Marks A, Peet A (2013). Marks’ Basic Medical Biochemistry: A Clinical Approach (4th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 175. ISBN 9781608315727.
- von Bohlen und Haibach O, Dermietzel R (2006). Neurotransmitters and Neuromodulators: Handbook of Receptors and Biological Effects. Wiley-VCH. p. 125. ISBN 978-3-527-31307-5.
- The formation of adrenaline from noradrenaline. Biochim Biophys Acta. 1957 Jun;24(3):658-9. https://www.ncbi.nlm.nih.gov/pubmed/13436503
- Axelrod J (May 1962). “Purification and Properties of Phenylethanolamine-N-methyl Transferase”. The Journal of Biological Chemistry. 237 (5): 1657–1660.
- Nelson L, Cox M (2004). Lehninger Principles of Biochemistry (4th ed.). New York: Freeman. p. 908. ISBN 0-7167-4339-6.
- Bell DR (2009). Medical physiology : principles for clinical medicine (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 312. ISBN 9780781768528
- Kemp SF, Lockey RF, Simons FE, World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis-a statement of the world allergy organization. World Allergy Organ J. 2008;1(7 Suppl):S18-26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3666145/
- Dalal R, Grujic D. Epinephrine. [Updated 2018 Jan 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482160
- Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 4. ISBN 1-59541-101-1.
- Effect of infusing epinephrine on liver and muscle glycogenolysis during exercise in rats. Am J Physiol. 1986 Jun;250(6 Pt 1):E641-9. https://www.physiology.org/doi/pdf/10.1152/ajpendo.1986.250.6.E641
- Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle. Am J Physiol. 1991 Mar;260(3 Pt 1):E430-5.https://www.physiology.org/doi/pdf/10.1152/ajpendo.1991.260.3.E430
- Sabyasachi Sircar (2007). Medical Physiology. Thieme Publishing Group. p. 536. ISBN 3-13-144061-9.
- Potential negative effects of epinephrine on carotid blood flow and ETCO2 during active compression-decompression CPR utilizing an impedance threshold device. Resuscitation. 2012 Aug;83(8):1021-4. doi: 10.1016/j.resuscitation.2012.03.018. Epub 2012 Mar 20 https://www.ncbi.nlm.nih.gov/pubmed/22445865
- ANZCOR Guideline 11.5 – Medications in Adult Cardiac Arrest. resus.org.au/?wpfb_dl=55
- Lin RY, Curry A, Pitsios VI, Morgan JP. et al. Cardiovascular responses in patients with acute allergic reactions treated with parenteral epinephrine. Am J Emerg Med. 2005;1:266–272. doi: 10.1016/j.ajem.2005.02.030. III
- Westfall TC. , Westfall DP. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9. Brunton LL, editor. New York, NY: McGraw-Hill; 2006. Adrenergic agonists and antagonists; pp. 215–268. IV.
- Pumphrey RSH. Lessons for the management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;1:1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. III
- Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;1:1016–1018. doi: 10.1016/j.jaci.2006.12.622. III
- Simons FER. First-aid treatment of anaphylaxis to food: focus on epinephrine. J Allergy Clin Immunol. 2004;1:837–844. IV
- Bautista E, Simons FE, Simons KJ. et al. Epinephrine fails to hasten the hemodynamic recovery in fully developed canine anaphylactic shock. Int Arch Allergy Immunol. 2002;1:151–164. doi: 10.1159/000059406. III
- Goldhaver-Fiebert S, Grecu L. Postoperative ST-segment elevation: was vasospasm caused by anaphylaxis or by its treatment with epinephrine? Ann Allergy Asthma Immunol. 2006;1:449–453. doi: 10.1016/S1081-1206(10)60933-7. IV
- Ersoz N, Firestone SC. Adrenaline-induced pulmonary edema and its treatment: a report of two cases. Br J Anaesth. 1971;1:709–712. doi: 10.1093/bja/43.7.709. IV
- Ackerman MJ, Khositseth A, Tester DJ, Hejlik JB, Shen WK, Porter CB. Epinephrine induced QT interval prolongation: a gene specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc. 2002;1:413–421. IV.
- Horowitz BZ, Jadallah S, Derlet RW. Fatal intracranial bleeding associated with prehospital use of epinephrine. Ann Emerg Med. 1996;1:725–727. doi: 10.1016/S0196-0644(96)70100-2. IV
- Novey HS, Meleyco LN. Alarming reaction after intravenous administration of 30 ml of epinephrine. JAMA. 1969;1:2435–2436. doi: 10.1001/jama.1969.03150260095018. IV
- McLean-Tooke APC, Bethune CA, Fay AC, Spickett GP. Adrenaline in the treatment of anaphylaxis: what is the evidence? BMJ. 2003;1:1332–1335. doi: 10.1136/bmj.327.7427.1332. IV
- Lieberman P, Kemp SF, Oppenheimer J, Lang DM, Bernstein IL, Nicklas RA. Joint Task Force on Practice Parameters. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;1:S483–S523. doi: 10.1016/j.jaci.2005.01.010. IV.
- Self-injectable epinephrine for first-aid management of anaphylaxis. Sicherer SH, Simons FE, Section on Allergy and Immunology, American Academy of Pediatrics. Pediatrics. 2007 Mar; 119(3):638-46.
- Simons FER, Chan ES, Gu X, Simons KJ. Epinephrine for the out-of-hospital (first aid) treatment of anaphylaxis in infants: is the ampule/syringe/needle method practical? J Allergy Clin Immunol. 2001;1:1040–1044. doi: 10.1067/mai.2001.119916. III
- Pumphrey RS, Clinical and Experimental Allergy 2000; 30(8):1144-1150
- Although there is little debate about using epinephrine to treat a SCIT SR, there is a lack of consensus about when it should be first used. The Journal of Allergy and Clinical Immunology. Volume 125, Issue 3 , Pages 569-574.e7, March 2010
- Lieberman P, Simons FER. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin & Exp Allergy 2015;45:1288-95.
- Epinephrine dosing. https://www.aaaai.org/ask-the-expert/epinephrine-dosing
- American Heart Association in collaboration with International Liaison Committee on Resuscitation. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Anaphylaxis. Circulation. 2005;1(suppl IV):IV143–IV145. IIb.
- Simons FER, Gu X, Simons KJ. Epinephrine absorption in adults: Intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;1:871–873. doi: 10.1067/mai.2001.119409. IIa.
- Song TT, Nelson MR, Chang JH. et al. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol. 2005;1:539–542. doi: 10.1016/S1081-1206(10)61130-1. III
- Soar J, Deakin CD, Nolan JP. et al. European Resuscitation Council guidelines for resuscitation 2005. Section 7. Cardiac arrest in special circumstances. Resuscitation. 2005;1(suppl 1):S135–S170.