Follicular hyperplasia
Follicular hyperplasia also called reactive lymphadenopathy, is a type of lymphoid hyperplasia due to stimulation of the B-cell compartment of the lymph node 1. Follicular hyperplasia is the most common pattern of reactive lymphadenopathy 2. Follicular hyperplasia is usually associated with varying degrees of paracortical and/or sinus hyperplasia. Reactive follicular hyperplasia is particularly commonly seen in children and young adults, but may be encountered in all ages, including the very elderly 3. Clinically, the lymphadenopathy is usually localized, but may be generalized. The cervical and axillary areas are most frequently involved, corresponding to the lymph-node groups most likely to drain antigens. The list below is the most common diseases that give rise to histologic findings that may suggest the specific cause of the follicular hyperplasia.
Some specific reactive lymphadenopathies with a predominantly follicular pattern 2:
- Rheumatoid arthritis
- Sjogren syndrome
- IgG4-related sclerosing disease
- Kimura disease
- Toxoplasmosis
- Syphilis
- Castleman disease
- HIV-associated lymphadenopathy
- Progressive transformation of germinal centers
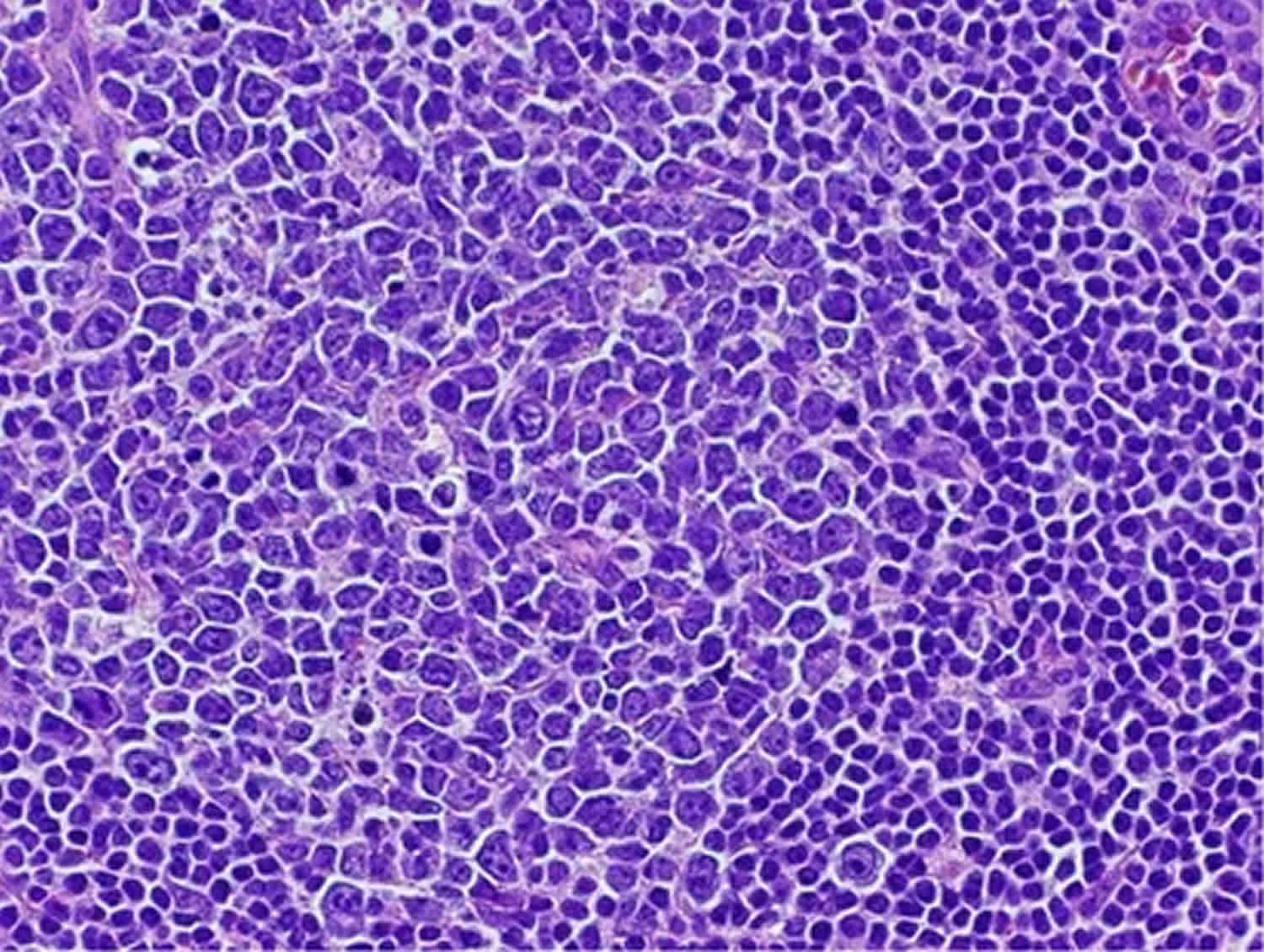
Figure 1. Reactive follicular hyperplasia
Footnote: This germinal center has a predominance of large cells along with scattered tingible-body macrophages. Cases of reactive follicular hyperplasia that feature many large cells in the germinal centers are easily mistaken for a grade 3 follicular lymphoma. Note the sharp demarcation of the mantle zone from the germinal center.
[Source 2 ]Atypical follicular hyperplasia
Atypical follicular hyperplasia is a descriptive term that describes a diagnostic dilemma rather than a specific diagnosis. Table 1 lists the main histologic differential diagnostic features between reactive follicular hyperplasia and follicular lymphoma 4. Note that there is no one pathognomonic histologic feature. Therefore, one should rather rely upon a constellation of characteristics. Even the history can be helpful. The older the patient, the more likely the diagnosis is follicular lymphoma. Generalized lymphadenopathy is also more commonly seen with follicular lymphoma than reactive follicular hyperplasia. The one single most useful histologic feature is the density of follicles (follicle:interfollicular ratio) on low magnification. The more the follicles and the less the interfollicular areas, the more likely is the diagnosis of malignant lymphoma. In fact, a complete back-to-back arrangement of the follicles is seen in over 75% of cases of follicular lymphoma, while only seen in the most florid cases of reactive follicular hyperplasia. The greatest exception to this rule is when there are areas of diffuse nodal effacement, another feature favoring follicular lymphoma. These areas are usually focal, but are mass forming, and not distributed evenly around individual follicles. Lymph nodes with floridly reactive follicles may also occasionally show one or more of the following features: extension of the process outside the capsule, the presence of follicles throughout the node, predominance of centroblasts, and absent or greatly diminished mantle zones (Figure 1). These latter two features are particularly common in florid reactive follicular hyperplasia occurring in childhood as well as in cases associated with human immunodeficiency virus infection.
Follicular lymphoma is one of the most common subtypes of indolent lymphoma 5. Therefore, most patients are diagnosed in an advanced stage and there is still no standard therapy fitting all patients 6. The genetic hallmark of follicular lymphoma is a t(14;18) translocation and the subsequent overexpression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl2), leading to a survival advantage of B-cells and, therefore, playing a crucial role in the pathogenesis of follicular lymphoma 7. This translocation is observed in 75–90% of follicular lymphoma patients 8. It is the result of changes in the genetic program that can lead to developmental arrest and/or uncontrolled proliferation of B-cells in a certain stage of progress. These clones are from the germinal center type, thus centrocytes and centroblasts 8. Follicular hyperplasia, on the other hand represents the most common type of reactive lymphadenopathy.
Table 1. Follicular hyperplasia versus lymphoma
| Reactive follicular hyperplasia | Follicular lymphoma |
|---|---|
| Low density of follicles | High density of follicles |
| Follicles usually limited to subcortical region | Follicles distributed evenly throughout nodal parenchyma |
| Follicles rarely extend beyond capsule | Follicles often extend beyond capsule |
| Follicles of uneven size and shape | Follicles usually of similar size and shape |
| Mixture of cell types in germinal center | Monomorphic or polymorphic population |
| Tingible-body macrophages present | Tingible-body macrophages usually rare |
| Usually moderate to high mitotic rate | Usually low to moderate mitotic rate |
| Mantle zone usually distinct | Mantle zone usually indistinct or absent |
| Cell polarization often seen | Cell polarization usually absent |
| Large interfollicular areas evident | Compressed interfollicular areas |
| Areas of nodal effacement not seen | May contain areas of nodal effacement |
Immunohistochemical and other special studies may be very helpful in distinguishing reactive follicular hyperplasia and follicular lymphoma. These studies are summarized in Table 2. Determination of bcl-2 expression in paraffin sections is the single most useful ancillary study, being consistently negative in reactive follicular hyperplasia, but positive in about 90% of cases of follicular lymphoma 9. However, one can occasionally see large numbers of bcl-2+ T-helper/inducer cells in cases of reactive follicular hyperplasia 9. Comparison with CD3 stains and the observation that the bcl-2+ cells are not centroblasts can help with this pitfall. Another pitfall is to mistake bcl-2+ primary follicles for bcl-2 positivity in follicular lymphoma. Comparison with other stains, including a germinal center marker such as bcl-6 or CD10 or a marker of primary follicle lymphoid cells such as IgD may be helpful (Figure 2).
The types of follicular lymphoma that are most likely to be bcl-2 negative include grade 3 follicular lymphoma and follicular lymphoma arising in children. In situ involvement by follicular lymphoma may show only scattered bcl-2+ cells, but the bcl-2 expression in this process is usually much stronger than the adjacent bcl-2 staining of either T-helper/inducer cells or adjacent B-mantle cells. Ki-67 stains may also be useful; not only are higher numbers of germinal center cells usually positive in reactive follicular hyperplasia than follicular lymphoma, the stain may demonstrate a pattern of polarity that may not have been evident on routine light microscopy 10. Immunoglobulin protein studies are helpful in only a minority of cases, as is also the case for in situ hybridization studies for mRNA expression; however, they are often most helpful in grade 3 follicular lymphoma 11. Rarely, florid reactive follicular hyperplasia, particularly in younger individuals, may show light chain restriction, particularly in flow cytometry studies 12. These cases are rare and may even show monoclonal populations by molecular analysis.
Gene rearrangement studies may be helpful in the distinction of reactive vs neoplastic follicular proliferations, although one must keep in mind that PCR studies for immunoglobulin heavy and light chains may be falsely negative in up to 20% of cases of follicular lymphoma due to the process of somatic hypermutation, and that ‘pseudoclonal’ immunoglobulin gene rearrangements may be rarely seen in reactive follicular hyperplasia, with the incidence depending on the primers used, the number of B cells in the specimen, as well as the size of the aliquot (particularly when <50 ng is analyzed). Similarly, although the detection of a t(14;18) (BCL2 gene rearrangement) usually provides strong support for a diagnosis of lymphoma 13, up to 10% of cases of follicular lymphoma (usually grade 3) may be negative, and the t(14;18), as well as other cytogenetic abnormalities, may be rarely observed in reactive follicular hyperplasia 14.
Table 2. Follicular hyperplasia versus lymphoma: special studies
| Reactive follicular hyperplasia | Follicular lymphoma |
|---|---|
| Bcl-2 negative in B cells of germinal centers | Bcl-2 positive in B cells in germinal centers (90%) |
| No light chain restriction on immunostains (rare exceptions) | Light chain restriction (20% in paraffin) |
| No light chain restriction on flow cytometry (rare exceptions) | Light chain restriction or absent, when gated correctly (95%) |
| Ig rearrangements absent (rare exceptions) | Ig rearrangements usually detected (80%) |
| Cytogenetic abnormalities infrequent, but have been described; t(14;18) absent (rare exceptions) | t(14;18) usually present (90%) |
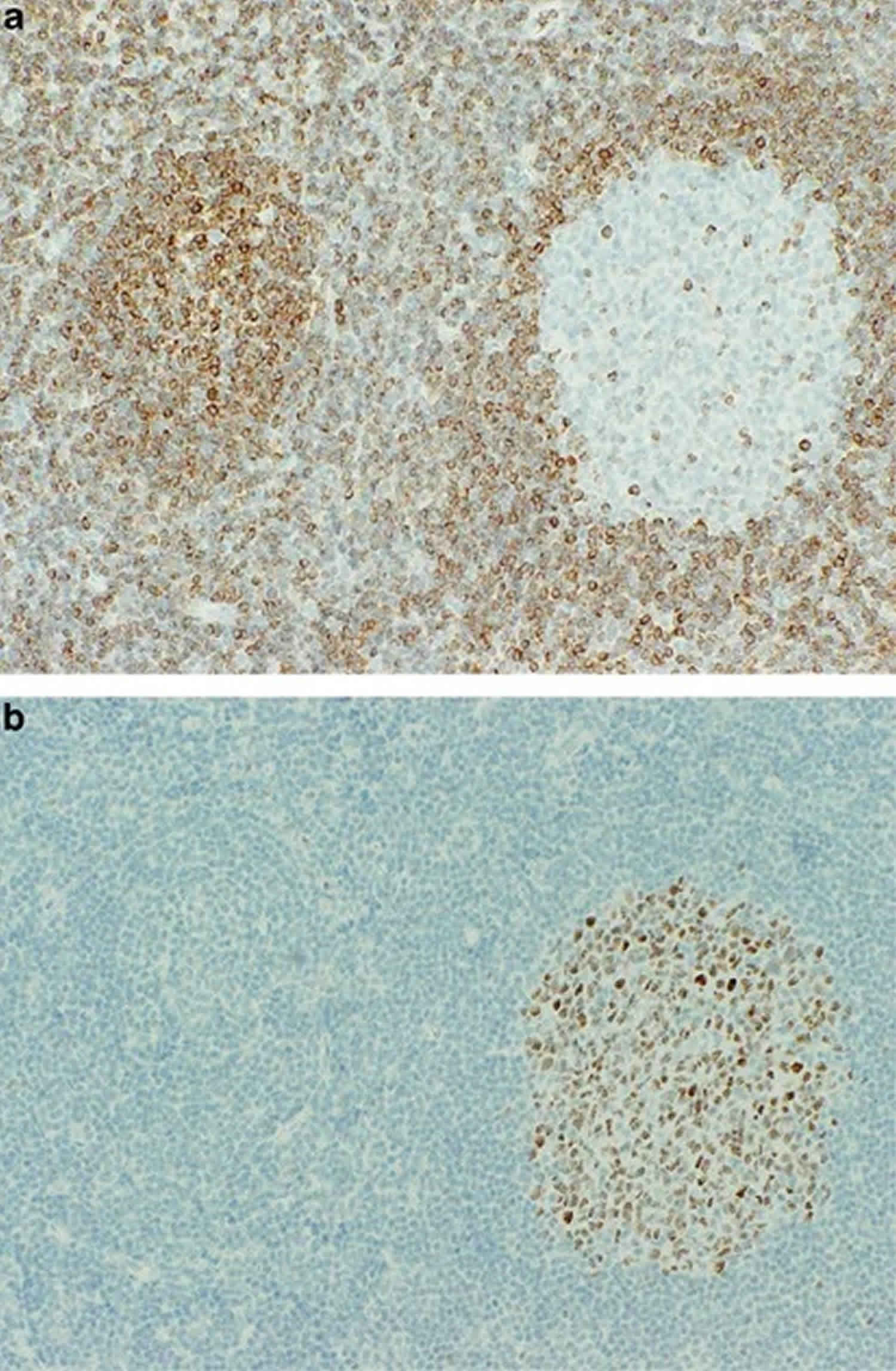
Figure 2. Follicular hyperplasia
Footnote: At left is a bcl-2 stain. The bcl-2-positive primary follicle may easily be mistaken for follicular lymphoma. At right is a serial section stained for bcl-6. The bcl-6 negativity is consistent with a primary folllicle.
[Source 2 ] References- Modern Surgical Pathology 2nd Edition, 2009. ISBN 978-1-4160-3966-2 https://doi.org/10.1016/B978-1-4160-3966-2.X0001-X
- Benign lymphadenopathies. Mod Pathol. 2013 Jan;26 Suppl 1:S88-96. doi: 10.1038/modpathol.2012.176. https://www.nature.com/articles/modpathol2012176.pdf
- Osborne BM, Butler JJ . Clinical implications of nodal reactive follicular hyperplasia in elderly patients with enlarged lymph nodes. Mod Pathol 1991;4:24–30.
- Good DJ, Gascoyne RD . Atypical lymphoid hyperplasia mimicking lymphoma. Hematol Oncol Clin North Am 2009;4:729–745.
- Woess C, Drach M, Villunger A, Tappert R, Stalder R, Pallua JD. Application of mid-infrared (MIR) microscopy imaging for discrimination between follicular hyperplasia and follicular lymphoma in transgenic mice. Analyst. 2015;140(18):6363–6372. doi:10.1039/c5an01072a https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4562367
- Izutsu K. J. Clin. Exp. Hematopathol. 2014;54:31–37
- Egle A, Harris AW, Bath ML, O’Reilly L, Cory S. Blood. 2004;103:2276–2283.
- Kishimoto W, Nishikori M. J. Clin. Exp. Hematopathol. 2014;54:23–30.
- Lai R, Arber DA, Chang KL et al Frequency of bcl-2 expression in non-Hodgkin’s lymphoma. A study of 798 cases with comparison of marginal zone lymphoma and monocytoid B cell hyperplasia. Mod Pathol 1998;11:864–869.
- Wood BL, Bacchi MM, Bacchi CE et al Immunocytochemical differentiation of reactive hyperplasia from follicular lymphoma using monoclonal antibodies to cell surface and proliferation-related markers. Appl Immunohistochem 1994;2:48–53.
- Weiss LM, Loera S, Bacchi CE . Immuoglobulin light chain immunohistochemistry revisited, with emphasis on reactive follicular hyperplasia versus follicular lymphoma. Appl Immunohistochem Mol Morphol 2010;18:199–205.
- Nam-Cha SH, San-Millan B, Mollejo M et al Light-chain-restricted germinal centres in reactive lymphadenitis: report of eight cases. Histopatology 2008;52:436–444.
- Segal GH, Scott M, Jorgensen T et al Standard polymerase chain reaction analysis does not detect t(14;18) in reactive lymphoid hyperplasia. Arch Pathol Lab Med 1994;118:791–794.
- Sevilla DW, Murty W, Sun XL et al Cytogenetic abnormalities in reactive lymphoid hyperplasia: byproducts of the germinal center reaction or indicators of lymphoma? Hematol Oncol 2011;29:81–20.