Glomus tumor
Glomus tumor is a rare soft-tissue benign neoplasm that arises from neuromyoarterial glomus cells in the arterial portion of the glomus body (an apparatus that involves in thermoregulation of cutaneous microvasculature) or the Sucquet-Hoyer canal 1. Glomus tumors most frequently occur in areas with high concentrations of glomus bodies, including subungual regions of the fingers and the deep dermis of the hand (palm), forearm, and foot (sole). The subcutaneous nodules may be red, purple, or blue depending on their depth. Most lesions are solitary and localized to cutaneous sites. Glomus tumor is usually found on the nail bed (subungual) or palm of a young adult and can be extremely painful, particularly following change in temperature or pressure. Glomus tumor accounts for approximately 2% of all soft-tissue tumors in the hands 2. They typically present in women adults (ages 20-40 years) as small, blue-red papules or nodules of the distal extremities, with most cases involving subungual sites. Glomus tumors are characteristically painful, often causing paroxysmal pain in response to temperature changes (especially cold) or pressure 3. Its classical clinical triad consists of pain, tenderness and temperature intolerance, especially cold sensitivity. Solitary glomus tumors can be surgically excised.
While glomus tumors predominate on the hands and fingers, glomus tumors can occur in a wide anatomic distribution, including sites not known to contain glomus cells, such as deep soft tissues, nerve, bone, mediastinum, trachea, mesentery, cervix, vagina and gastrointestinal tract. In fact, gastric glomus tumors account for approximately 2% of benign gastric tumors 4. These tumors may arise from perivascular cells or pluripotent mesenchymal cells capable of differentiating into glomus cells.
While the vast majority of glomus tumors are benign, malignant cases have been rarely reported, with such cases typically being locally invasive. Rarely, glomus tumors can undergo malignant transformation or malignant glomus tumors can arise de novo. Malignant glomus tumors are termed glomangiosarcomas; glomangiosarcomas have a high local recurrence rate but very low rate of metastasis 5. One case report describes a glomangiosarcoma that occurred at a prior biopsy site of a lower extremity, perhaps due to local recurrence of glomangiosarcoma and/or malignant transformation 6. In one rare case of malignant glomus tumor involving the brachial plexus, excision was foregone owing to potential morbidity, and the glomus tumor was found to be sensitive to chemotherapy against the oncogenic BRAF, with resultant moderate reduction in tumor size 7. In fact, other glomus tumors have also been found to have BRAF mutations, suggesting this may be a marker of malignant potential and/or a therapeutic target, although further study is needed 8.
Malignant glomus tumors are more likely to be deep, larger than 2 cm, and have atypical features 9. Metastases are exceedingly rare 10.
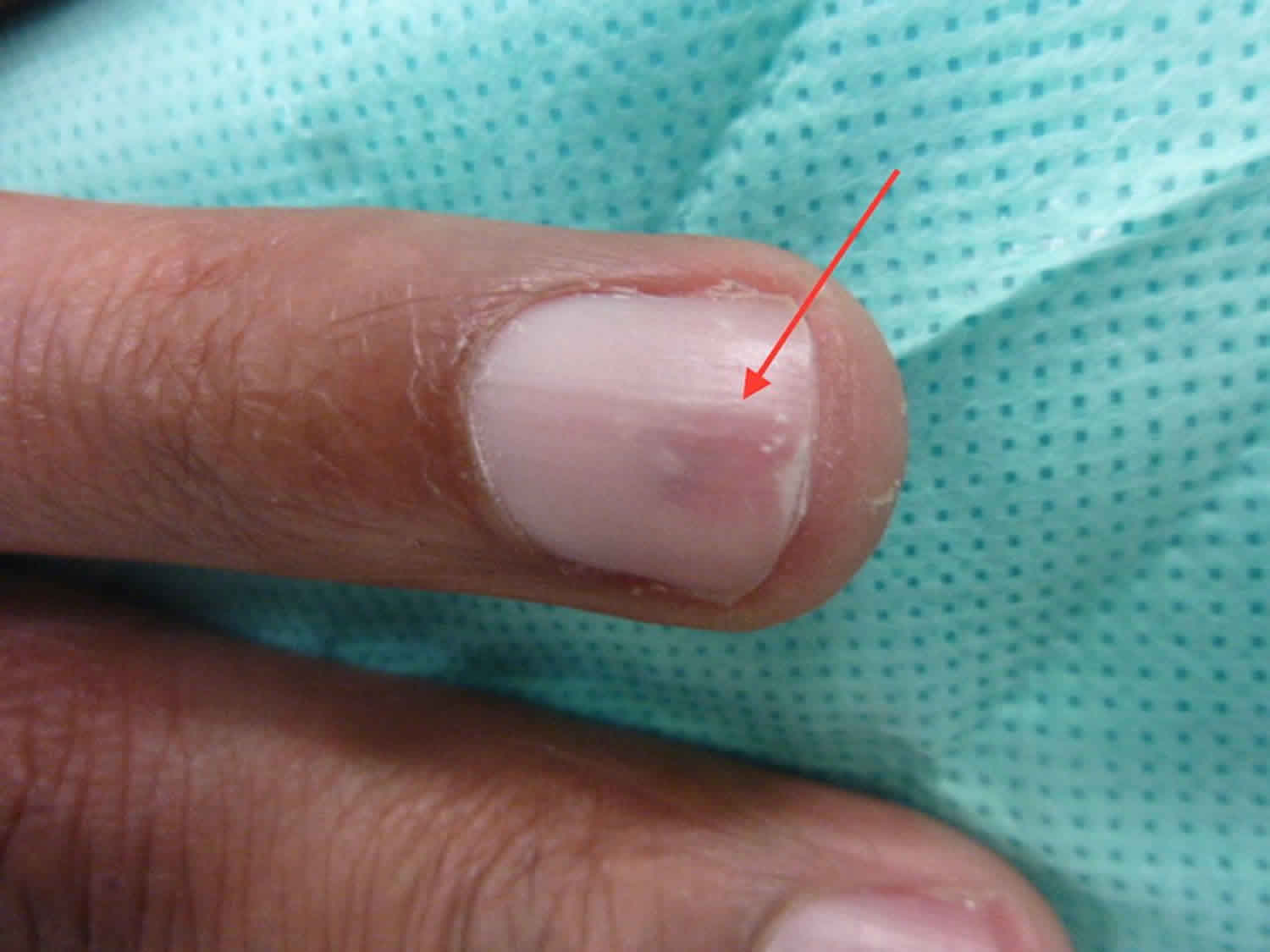
Figure 1. Glomus tumor nail
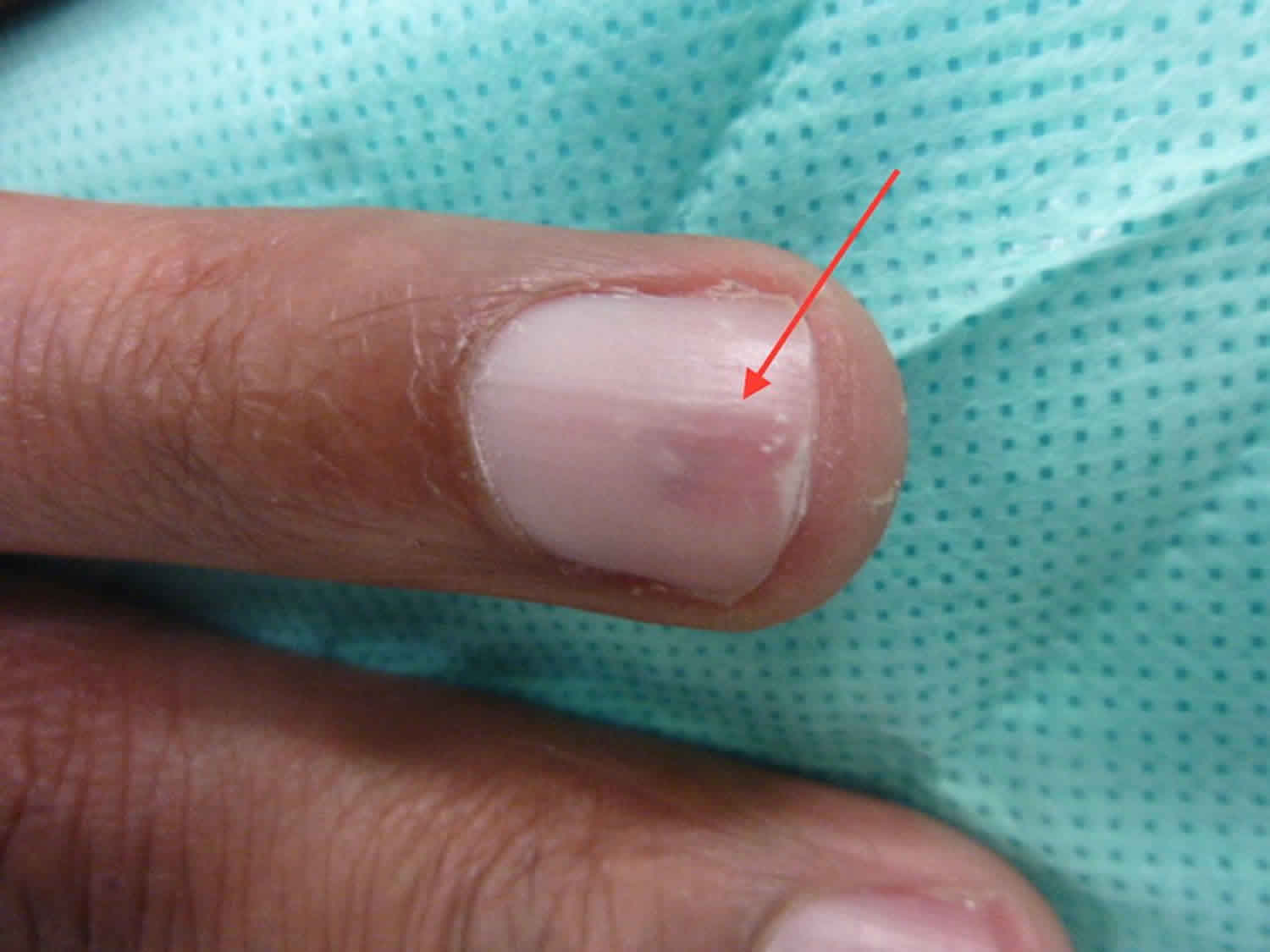
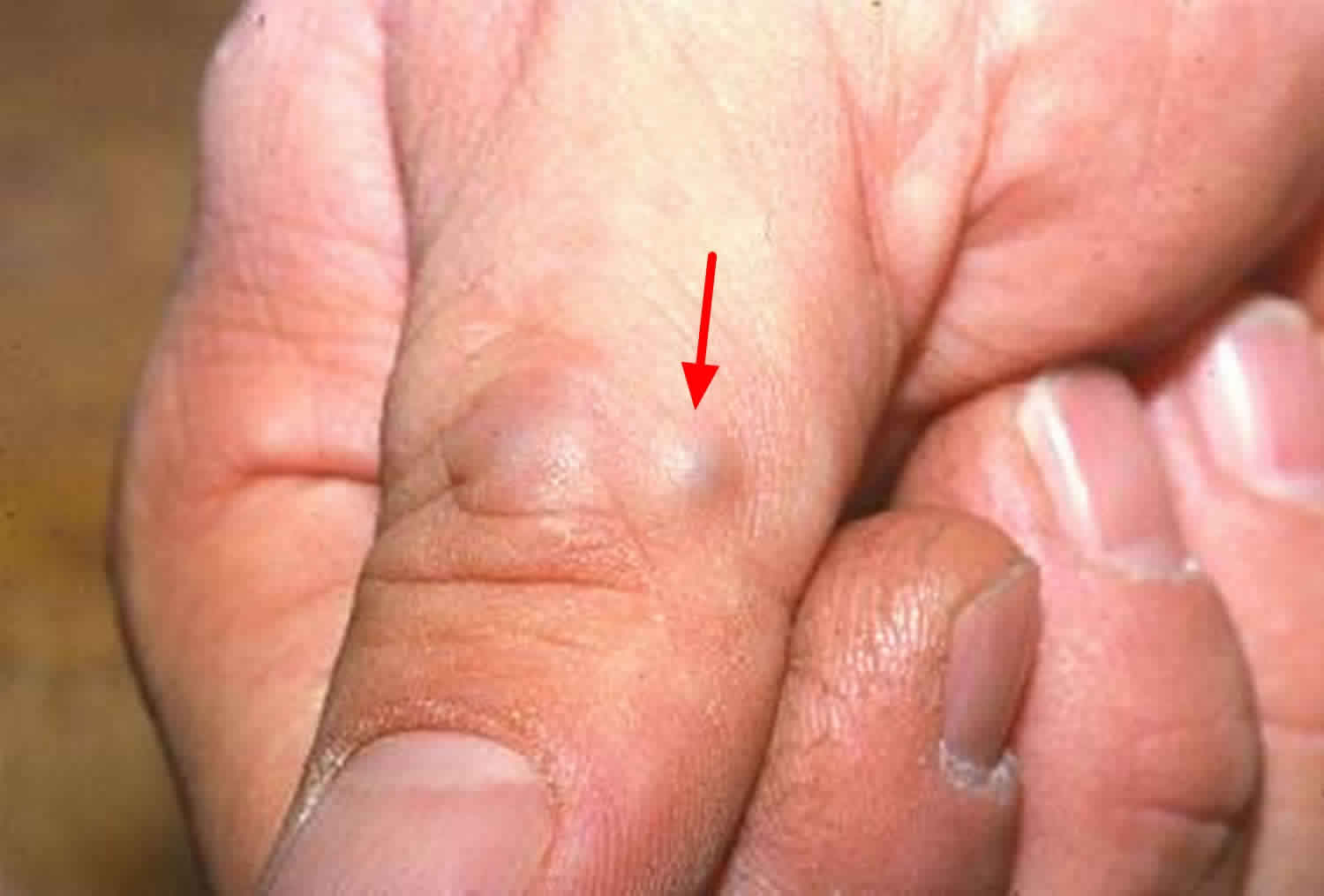
Figure 3. Glomus tumor thumb
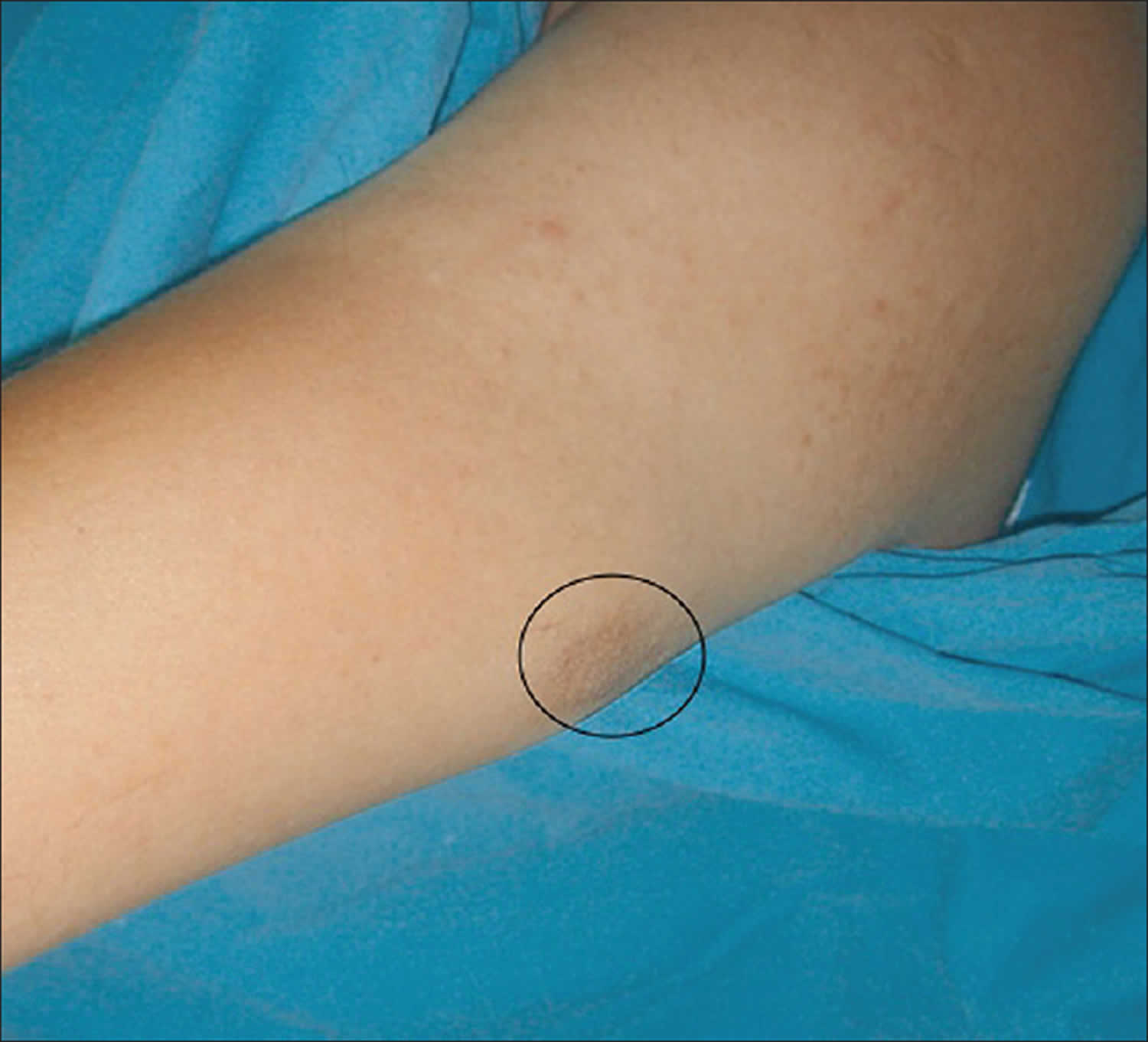
Figure 3. Glomus tumor on the forearm
Footnote: The purple reflection seen on the skin surface of a subcutaneous nodule with 2 cm diameter on medial arm.
[Source 11 ]Glomus tumor causes
Glomus tumors are thought to arise from the glomus body or Sucquet-Hoyer canal, a thermoregulatory arteriovenous shunt composed of modified smooth muscle cells 1. Glomus tumors are neoplasms caused by a proliferation of glomus cells, which make up a portion of the glomus body. The initiating event for glomus cell proliferation is unknown. Some authors have postulated that trauma induces solitary subungual glomus tumors, although this theory is not well studied.
Most glomuvenous malformations are inherited in an autosomal dominant pattern with incomplete penetrance. Most hereditary glomuvenous malformations are associated with defects in the glomulin gene (GLMN) located on chromosome 1 12.
Glomus tumor pathophysiology
Glomus bodies play an important role in thermoregulation via arteriovenous shunting. The glomus body is composed of an afferent arteriole, anastomotic vessel (termed Sucquet-Hoyer canal), primary collecting vein, intraglomerular reticulum, and a capsular portion. These specialized arteriovenous anastomoses are particularly concentrated in the reticular dermis of the fingers.
Glomus tumors are thought to represent hamartomatous proliferations of modified smooth muscle cells originating from preexisting normal glomus cell populations. The three components of most glomus tumors include glomus cells, vasculature, and smooth muscle cells. The most common type of glomus tumor is the solid glomus tumor, characterized by a prominent smooth muscle cell component 13.
Most glomus tumors are solitary and sporadic. An epidemiologic relationship may exist between glomus tumors and neurofibromatosis, which most often produces subungual glomus tumors 14. A distinct but related condition known as glomuvenous malformations differ clinically from glomus tumors in that they occur more often in children and adolescents, are typically multifocal, and do not have a predilection for subungual sites. Glomuvenous malformations are more likely to be hereditary and painless.
It is thought that glomuvenous malformations and glomus tumors have different causes, with glomuvenous malformations resembling venous malformations and containing more dilated venous channels than glomus tumors.
Note that most cases of glomuvenous malformation are sporadic; however, familial cases with autosomal dominant inheritance patterns have also been described. Such familial glomuvenous malformations have been mapped to 1p21-22 and are thought to result from loss-of-function mutations in the cytoplasmic protein glomulin 12.
Glomus tumor symptoms
Patients with solitary glomus tumors usually have paroxysmal pain, which can be severe and can be exacerbated by pressure or temperature changes, especially cold. The classic triad of symptoms includes severe pain, with pinpoint localization, and cold hypersensitivity. While glomus tumors are classically red, blue, or purple, skin-colored glomus tumors have been reported and may delay diagnosis and complicate excision. Glomuvenous malformations are typically less painful than glomus tumors; however, they may become more painful with menstruation or pregnancy. While glomus tumors are most often found on the hand, the presence of characteristic symptoms should raise suspicion for glomus tumor in other locations, for example in the thigh or lower limb 15. In the absence of pain, glomus tumor should still be considered in the differential diagnosis of nodules, even in uncommon locations such as the mouth 16. Additionally, subungual glomus tumors have been reported to rupture 17 and have also been reported to result in various nail changes, including color change, erythronychia, splitting, and thickening of the nail bed 18. Therefore, any subungual nodule causing color and/or nail change should raise suspicion for glomus tumor.
Patients with multiple lesions often seek medical attention because they are worried or have cosmetic concerns. Because multiple glomus tumors are inherited as an autosomal dominant condition, a family history of similar lesions may be helpful for diagnosis.
Extracutaneous sites have been reported, including involvement of the gastrointestinal tract, trachea, nerve, bone, mediastinum, liver, pancreas, kidney, and ovary 19. Cases of benign and malignant glomus tumors of the kidney have been reported 20. Pulmonary involvement was described in a case report of a primary pulmonary glomus tumor in a segmental bronchus manifesting as obstructive pneumonia 21. More recently, a case of glomus tumor was reported to cause a liver mass 22 and another case of a gastric glomus tumor was reported to cause upper gastrointestingal bleeding 23.
Glomus tumor diagnosis
Glomus tumor may be suspected clinically, as it is typically a solitary, painful, 1–2 cm reddish-blue papule or nodule on or around a nail bed. It usually undergoes biopsy. The histology of a glomus tumor reveals solid sheets of glomus cells around small blood vessels. Immunohistochemical studies may be helpful in diagnosis. Glomus cells are immunoreactive with markers for a-smooth muscle actin (aSMA), muscle-specific actin (MSA), and h-caldesmon. Glomus tumors also have abundant type IV collagen. These markers can be useful in distinguishing glomus tumors from hemangiomas. It is also important to note that glomus tumors are distinct from paragangliomas, which are positive for S100 24.
Solitary glomus tumors have the following characteristics:
- Blue or red blanchable papules or nodules in deep dermis or subcutis
- Acral location, most commonly, especially subungual
- Usually smaller than 2 cm
Glomuvenous malformation is subdivided into regional or localized, disseminated, and congenital plaquelike forms, as follows:
- Regional variant – Consists of blue-to-purple partially compressible papules or nodules that are grouped with a cobblestonelike appearance and limited to a specific area, most commonly to an extremity
- Disseminated type – Consists of multiple lesions distributed over the body with no specific grouping; less common than the regional variant
- Congenital plaquelike glomus tumors – Consist of either grouped papules that coalesce to form indurated plaques or clusters of discrete nodules; rarest variant
Three useful findings for diagnosing glomus tumors, particularly solitary painful glomus tumors (especially those under a nail), are the following 25:
- Love test (>90% sensitivity) – Eliciting exquisite localized pain by applying pressure to the suspected areas with a pencil tip or pinhead 26
- Hildreth sign (>90% sensitivity) – Reduction of pain and tenderness and reduction of tenderness with the Love test by inducing transient ischemia with a tourniquet 26
- Cold sensitivity test – Considered positive when immersing the affected area in cold water elicits severe pain around the lesion
Features of malignant glomus tumors, or glomangiosarcomas, may include the following 9:
- Larger than 2 cm
- Rapid growth
- Deep soft-tissue involvement
Glomus tumor treatment
The treatment of choice for symptomatic solitary glomus tumors is total surgical excision 27, which is curative. While various treatment modalities have been reported, to include laser and sclerotherapy, in the case of solitary glomus tumors, complete removal of the tumor capsule is recommended to relieve pain and minimize risk for recurrence.
Most subungual lesions are treated with total nail avulsion followed by excision, although several additional techniques have been described to include a straightforward excision using a nail bed margin approach 28, a trap-door technique 29 and a technique described by Lee et al designed to conserve the nail plate itself 30. In the transungual approach, the nail plate is removed, the tumor excised, and the nail bed repaired. A lateral subperiosteal approach has also been described, but it may have a higher risk of incomplete excision. Removal of subungual glomus tumors has been reported to have recurrence rates of 2-13% (highest reported at 50%) and nail bed deformity rates of 0-19% 31. Recurrence can be due to incomplete excision or development of a new lesion, with the probability of recurrence of glomus tumors in general being highest for subungual glomus tumors.
Glomus tumors that are skin-colored or located in the nail matrix have a higher incidence of recurrence. However, the use of preoperative MRI or ultrasound studies in preoperative planning is associated with a lower incidence of recurrence and may be helpful in these cases 32.
For multiple glomus tumors, excision may be more difficult because of their poor circumscription and the large number of lesions. Other reported treatment modalities, more useful in treating multiple lesions, include argon, carbon dioxide, or Nd:YAG laser therapy, as well as sclerotherapy with hypertonic saline or sodium tetradecyl sulfate 33.
The treatment recommendations for glomangiosarcoma are based on a few case reports. Wide local excision remains the treatment of choice. Follow-up is important, especially for malignant glomus tumors; one case report highlighted a patient with multiple episodes of local recurrence of malignant glomus tumor treated with excision over a period of 40 years 34.
Glomus tumor prognosis
The prognosis for patients with glomus tumors is excellent. Excision of painful lesions most often results in cure, with a low recurrence rate for solitary lesions 35. With subungual glomus tumors, the most important complications are recurrence and nail deformity; recurrence requires repeat wide excision 1. Additionally, one case report describes infection due to rupture of a subungual glomus tumor 17.
Malignant glomus tumors are usually locally infiltrative and aggressive. Their overall prognosis is good if they are treated with wide excision; otherwise, there is a risk of local recurrence. For example, a 2017 study of malignant glomus tumors of the head and neck reports recurrence in 45% of patients 24. While extremely rare, metastases have been described and are associated with a poor prognosis 36.
Patients with glomus tumors classically present with paroxysmal severe pain, often precipitated by cold, pressure, or dependency. Pain is more common in solitary lesions. The multiple tumors of glomuvenous malformations arise in younger patients and tend to be asymptomatic.
Systemic effects of glomus tumors are rare; however, in one report, a patient with more than 400 glomus tumors developed thrombocytopenia as a result of platelet sequestration 37.
References- Chou T, Pan SC, Shieh SJ, Lee JW, Chiu HY, Ho CL. Glomus Tumor: Twenty-Year Experience and Literature Review. Ann Plast Surg. 2016 Mar. 76 Suppl 1:S35-40.
- Rao AG, Indira D, Kamal J. Extra digital glomangioma. Indian J Dermatol. 2010 Oct. 55 (4):397-8.
- Dermatologic Manifestations of Glomus Tumor. https://emedicine.medscape.com/article/1083405-overview
- Fabiani P, Benizri E, Michiels JF, Gugenheim J, Saint-Paul MC, Mouiel J. [A new case of gastric glomangioma]. Gastroenterol Clin Biol. 1993. 17 (12):974-5.
- Toti L, Manzia TM, Roma S, Meucci R, Blasi F, Ferlosio A, et al. Rare malignant glomus tumor of the stomach with liver metastases. Radiol Case Rep. 2019 Apr. 14 (4):463-467.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma Arising from a Prior Biopsy Site. Plast Reconstr Surg Glob Open. 2017 Jan. 5 (1):e1219.
- Cuviello A, Goyal A, Zick A, Ahlawat S, Rodriguez FJ, Belzberg AJ, et al. Sporadic Malignant Glomus Tumor of the Brachial Plexus With Response to Targeted Therapy Directed Against Oncogenic BRAF. JCO Precis Oncol. 2018.
- Karamzadeh Dashti N, Bahrami A, Lee SJ, Jenkins SM, Rodriguez FJ, Folpe AL, et al. BRAF V600E Mutations Occur in a Subset of Glomus Tumors, and Are Associated With Malignant Histologic Characteristics. Am J Surg Pathol. 2017 Nov. 41 (11):1532-1541.
- Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001 Jan. 25(1):1-12.
- Zhang Y, Li H, Zhang WQ. Malignant glomus tumor of the esophagus with mediastinal lymph node metastases. Ann Thorac Surg. 2013 Oct. 96(4):1464-6.
- Temiz G, Sirinoglu H, Demirel H, Yesiloglu N, Sarici M, Filinte GT. Extradigital glomus tumor revisited: Painful subcutaneous nodules located in various parts of the body. Indian J Dermatol 2016;61:118 http://www.e-ijd.org/text.asp?2016/61/1/118/174080
- Brauer JA, Anolik R, Tzu J, Meehan S, Lieber CD, Geronemus RG. Glomuvenous Malformations (Familial generalized multiple glomangiomas). Dermatol Online J. October 2011. 17 (10): 9
- Drapé JL, Idy-Peretti I, Goettmann S, Wolfram-Gabel R, Dion E, Grossin M, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995 May. 195 (2):507-15.
- Aqil N, Gallouj S, Moustaide K, Mernissi FZ. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018 Oct 19. 12 (1):319.
- Sbai MA, Benzarti S, Gharbi W, Maalla R. A Rare Case of Glomus Tumor of the Thigh with Literature Review. J Orthop Case Rep. 2018 Sep-Oct. 8 (5):22-24.
- Smith MH, Bhattacharyya I, Cohen DM, Hinze SR, Islam MN. Glomus tumor: a comprehensive review of the clinical and histopathologic features with report of two intraoral cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019 Jan. 127 (1):62-70.
- Lu H, Chen LF, Chen Q. Rupture of a subungual glomus tumor of the finger. BMC Cancer. 2018 May 2. 18 (1):505.
- Jayasree P, Kaliyadan F, Elizabeth MJ, Bhaskara KGR. Subungual glomus tumour showing an unusual presentation. Australas J Dermatol. 2019 Mar 8.
- Kang G, Park HJ, Kim JY, Choi D, Min BH, Lee JH, et al. Glomus tumor of the stomach: a clinicopathologic analysis of 10 cases and review of the literature. Gut Liver. 2012 Jan. 6(1):52-7.
- Almaghrabi A, Almaghrabi N, Al-Maghrabi H. Glomangioma of the Kidney: A Rare Case of Glomus Tumor and Review of the Literature. Case Rep Pathol. 2017. 2017:7423642.
- Oide T, Yasufuku K, Shibuya K, Yoshino I, Nakatani Y, Hiroshima K. Primary pulmonary glomus tumor of uncertain malignant potential: A case report with literature review focusing on current concepts of malignancy grade estimation. Respir Med Case Rep. 2016. 19:143-149.
- Li L, Xu QX, Zhang XY, Han CH. Unusual location of the glomus tumour in the liver: A case report and literature review. Medicine (Baltimore). 2018 Jun. 97 (26):e11294.
- Morte D, Bingham J, Sohn V. Gastric Glomus Tumor: An Uncommon Source for an Acute Upper GI Bleed. Case Rep Gastrointest Med. 2018. 2018:7961981
- Wolter NE, Adil E, Irace AL, Werger A, Perez-Atayde AR, Weldon C, et al. Malignant glomus tumors of the head and neck in children and adults: Evaluation and management. Laryngoscope. 2017 Dec. 127 (12):2873-2882.
- Sethu C, Sethu AU. Glomus tumour. Ann R Coll Surg Engl. 2016 Jan. 98 (1):e1-2.
- Tang CY, Tipoe T, Fung B. Where is the Lesion? Glomus Tumours of the Hand. Arch Plast Surg. 2013 Sep. 40(5):492-495.
- Lee SH, Roh MR, Chung KY. Subungual glomus tumors: surgical approach and outcome based on tumor location. Dermatol Surg. 2013 Jul. 39(7):1017-22.
- Wang PJ, Zhang Y, Zhao JJ. Treatment of Subungual Glomus Tumors Using the Nail Bed Margin Approach. Dermatol Surg. 2013 Oct 9.
- Pahwa M, Pahwa P, Kathuria S. Glomus tumour of the nail bed treated with the ‘trap door’ technique: A report of two patients. J Dermatolog Treat. 2010 May 4.
- Lee HJ, Kim PT, Kyung HS, Kim HS, Jeon IH. Nail-preserving excision for subungual glomus tumour of the hand. J Plast Surg Hand Surg. 2013 Nov 21.
- Kim YJ, Kim DH, Park JS, Baek JH, Kim KJ, Lee JH. Factors affecting surgical outcomes of digital glomus tumour: a multicentre study. J Hand Surg Eur Vol. 2018 Jul. 43 (6):652-658.
- Lin YC, Hsiao PF, Wu YH, Sun FJ, Scher RK. Recurrent Digital Glomus Tumor: Analysis of 75 Cases. Dermatol Surg. 2010 Sept 2010 [ePub 2010 Jul 9].
- Moreno-Arrones OM, Jimenez N, Alegre-Sanchez A, Fonda P, Boixeda P. Glomuvenous malformations: dual PDL-Nd:YAG laser approach. Lasers Med Sci. 2018 Dec. 33 (9):2007-2010.
- Nagata K, Hashizume H, Yamada H, Yoshida M. Long-term survival case of malignant glomus tumor mimicking “dumbbell-shaped” neurogenic tumor. Eur Spine J. 2017 May. 26 (Suppl 1):42-46.
- Gandhi J, Yang SS, Hurd J. The anatomic location of digital glomus tumor recurrences. J Hand Surg Am. 2010 Jun. 35(6):986-9.
- Lamba G, Rafiyath SM, Kaur H, Khan S, Singh P, Hamilton AM, et al. Malignant glomus tumor of kidney: the first reported case and review of literature. Hum Pathol. 2011 Aug. 42(8):1200-3.
- McEvoy BF, Waldman PM, Tye MJ. Multiple hamartomatous glomus tumors of the skin. Arch Dermatol. 1971 Aug. 104 (2):188-91.