Hepatoblastoma
Hepatoblastoma is a very rare type of liver cancer that occurs in infants and children accounting for 80% of malignant liver tumors in childhood 1, 2. The cells of hepatoblastoma are similar to fetal liver cells. Hepatoblastoma originates from undifferentiated hepatic progenitor cells, and undergo a malignant transformation during embryogenesis 3. Hepatoblastoma is very rarely diagnosed in adolescence and is exceedingly rare in adults 4. Occasionally, nests of hepatoblastoma cells are found in hepatocellular carcinoma lesions; this is more common in adults than in children. Older children and adults tend to have a worse prognosis 4.
Hepatoblastoma typically affects children younger than 3 years of age 5. Hepatoblastoma preferentially affects boys and occurs in infants or very young children at a median age at diagnosis of 16 months 6. Hepatoblastoma incidence is estimated at 1.2–1.5 million children per year, comprising about 1% of all pediatric cancers 7. Approximately 100 cases of hepatoblastoma are reported per year 4. The annual incidence of hepatoblastoma in infants younger than 1 year is 11.2 cases per million; in 1990-1995, the annual incidence in children overall was 1.5 cases per million, which is almost double the incidence from 1975-1979 4. A significantly higher rate of hepatoblastomas is observed among low birth weight and very low birth weight infants born prematurely 8.
Although hepatoblastoma cause is unknown, its association with familial adenomatous polyposis (FAP), Beckwith-Wiedemann syndrome, and very low birth weight (less than 1000 grams) has been documented 9.
Classically, hepatoblastoma presents with abdominal mass, which can be associated with pain, distension, anorexia, and in rarer cases, acute abdomen (tumor rupture) and anemia 10. In most cases, finding a palpable abdominal mass, a liver-dependent tumor on imaging, and serum elevation of alpha-fetoprotein (AFP) is diagnostic of hepatoblastoma. Confirmation by histopathological study of a biopsy or primary tumor resection is necessary 11.
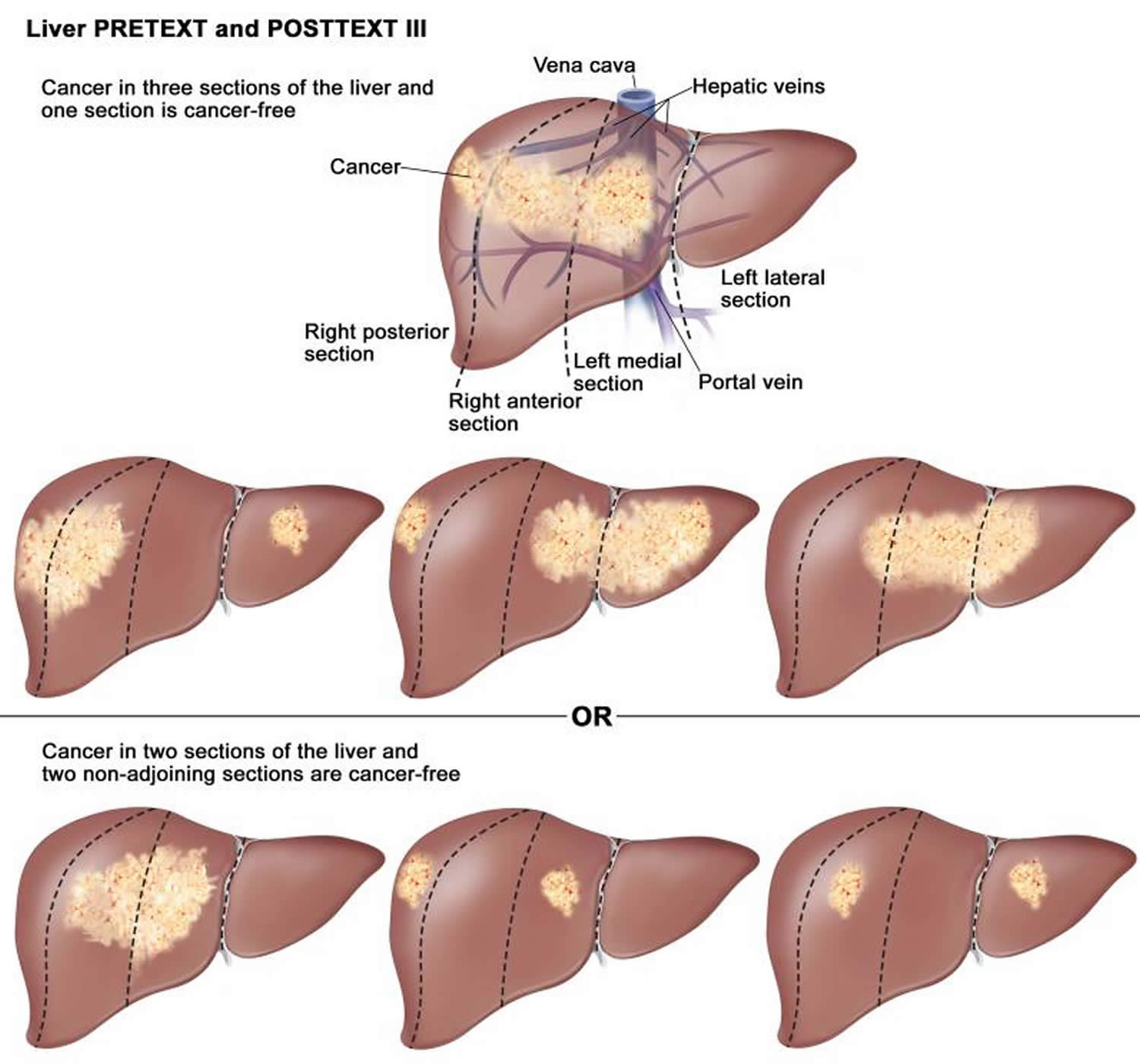
International pediatric liver tumor groups such as SIOPEL, CHIC, and COG suggest an initial approach to patients with suspected hepatoblastoma consisting of a 3-stage CT scan of the abdomen or MRI with hepatospecific contrast to obtain a PRETEXT (PRE-Treatment EXTent of tumor) staging 12. The PRETEXT group describes the tumor before the patient has any treatment. The PRETEXT group is used to describe tumor extent before any therapy, thus allowing a more effective comparison between studies conducted by different groups. The PRETEXT staging system is based on Couinaud’s segmentation of the liver (Figure 4 amd 5). The POSTTEXT (POST-Treatment EXTent of disease) group describes the tumor after the patient has had treatment such as neoadjuvant chemotherapy.
The histological tumor type, alpha-fetoprotein (AFP) level, age at the time of diagnosis, the presence of metastasis at the time of diagnosis, completeness of resection, response to chemotherapy, and the PRETEXT and POST TEXT classification (clinical stage of the disease) of the tumor significantly impact the prognosis and type of the disease 13, 14.
AFP is the tumor marker for hepatoblastoma, both diagnosis and follow-up: levels above 100 ng/mL at diagnosis are associated with 5-year survival rates below 18%. Imaging and age are also fundamental in classifying patients by risk groups and estimating their long-term survival. In addition, a biopsy is helpful for histological confirmation of the tumor 10.
Complete tumor resection is the gold standard for the treatment of patients with early-stage or low-risk diseases susceptible to surgical management. Such management has good results in terms of prognosis and recovery rates, and the 5-year survival is up to 100%. Meanwhile, tumors in more advanced or unresectable stages, such as PRETEXT III and IV, have a 5-year survival of 78 and 44%, respectively 15.
The presence of metastases at diagnosis is frequent and is an independent predictor of poor prognosis associated with lower overall survival (up to 25%). However, metastatic disease is not an absolute contraindication for surgical treatment or therapeutic liver transplantation. Therefore, evaluating the presence of poor prognostic factors, specifically unresolved pulmonary metastases, is essential to guide this therapeutic possibility 10.
The lung is the most frequent site of distant metastasis, followed by the brain, bone, and other less frequent sites 16. For example, Zhi et al. 10 found that 132 of 316 children with hepatoblastoma had distant metastases, mainly to the lung in 80%, followed by 6% with intracranial metastases, 4.5% bone, 3% diaphragmatic, 3% to the right atrium, 3% to the pleura, 1.5% intraspinal, 1.5% to the kidney and adrenal gland, and 0.75% had intestinal metastases. Likewise, Zhang et al describe, in a retrospective study, that 48% of the patients had distant metastases at diagnosis: 75% were pulmonary, 20% vascular, 34% intrahepatic, and 12% bone metastases 11, 17.
In hepatoblastoma, the histology (how the cancer cells look under a microscope) affects the way the cancer is treated. The histology for hepatoblastoma may be one of the following 18:
- Well-differentiated fetal (pure fetal) histology. Well-differentiated fetal hepatocytes morphologically indistinguishable from normal fetal liver cells. An analysis of patients with initially resected hepatoblastoma tumors (before receiving chemotherapy) has suggested that patients with well-differentiated fetal (previously termed pure fetal) histology tumors have a better prognosis than do patients with an admixture of more primitive and rapidly dividing embryonal components or other undifferentiated tissues. Studies have reported the following:
- A study of patients with hepatoblastoma and well-differentiated fetal histology tumors observed the following 19:
- The survival rate was 100% for patients who received four doses of single-agent doxorubicin. This suggested that patients with well-differentiated fetal histology tumors might not need chemotherapy after complete resection 20.
- In a Children’s Oncology Group study 21, 16 patients with well-differentiated fetal histology hepatoblastoma with two or fewer mitoses per 10 high-power fields were not treated with chemotherapy. Retrospectively, their PRETEXT (PRE-Treatment EXTent of tumor) groups were group I (n = 4), group II (n = 6), and group III (n = 2) 22.
- The survival rate was 100% for these patients who did not receive chemotherapy.
- All 16 patients were alive with no evidence of disease at a median follow-up of 4.9 years (range, 9 months to 9.2 years).
- Thus, complete resection of a well-differentiated fetal hepatoblastoma may preclude the need for chemotherapy.
- A study of patients with hepatoblastoma and well-differentiated fetal histology tumors observed the following 19:
- Non–well-differentiated fetal histology, non-small cell undifferentiated histology.
- Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver. Histologically, small cell undifferentiated hepatoblastoma is typified by a diffuse population of small cells with scant cytoplasm resembling neuroblasts 23. Small cell undifferentiated hepatoblastoma may be difficult to distinguish from malignant rhabdoid tumor of the liver, which has been conflated with small cell undifferentiated hepatoblastoma in past studies. A characteristic shared by small cell undifferentiated hepatoblastoma and malignant rhabdoid tumors is the poor prognosis associated with each 24, 25. Patients with small cell undifferentiated hepatoblastoma whose tumors are unresectable have an especially poor prognosis 25. Patients with stage 1 tumors appear to have increased risk of treatment failure when small cell elements are present 26. For this reason, completely resected tumors composed of well-differentiated fetal histology or of mixed fetal and embryonal cells must have a thorough histological examination because small foci of undifferentiated small cell histology indicates a need for aggressive chemotherapy 26. Aggressive treatment for this histology was investigated in the completed Children’s Oncology Group study 27 and all tumors were tested for SMARCB1 expression by immunohistochemistry. In this study, hepatoblastoma that would otherwise be considered very low or low risk was upgraded to intermediate risk if any small cell undifferentiated elements were found.
- Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver can be distinguished by the following characteristic abnormalities:
- Chromosomal abnormalities. These abnormalities in rhabdoid tumors include translocations involving a breakpoint on chromosome 22q11 and homozygous deletion at the chromosome 22q12 region that harbors the SMARCB1 gene 25.
- Small cell undifferentiated hepatoblastoma (SMARCB1 positive). Small cell undifferentiated hepatoblastoma (SMARCB1 positive) is an uncommon hepatoblastoma variant that represents several percent of all hepatoblastomas. It tends to occur at a younger age (6–10 months) than do other cases of hepatoblastoma 24, 25 and is associated with AFP levels that are normal for age at presentation 28, 25.
- Lack of SMARCB1 expression. Lack of detection of SMARCB1 by immunohistochemistry is characteristic of malignant rhabdoid tumors 25.
- Rhabdoid tumor of liver (SMARCB1 negative).
- Chromosomal abnormalities. These abnormalities in rhabdoid tumors include translocations involving a breakpoint on chromosome 22q11 and homozygous deletion at the chromosome 22q12 region that harbors the SMARCB1 gene 25.
- Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver can be distinguished by the following characteristic abnormalities:
Different therapeutic options are available depending on tumor stage and metastatic state, ranging from chemotherapy (neo/adjuvant), chemoembolization, tumor resection, metastasectomies, radiofrequency ablation, and liver transplant 11. The treatment has advanced with neo-adjuvant chemotherapy now the standard of care for most cases. Neo-adjuvant chemotherapy and surgical resection produce a cure rate of approximately 70%, a vast improvement over the dismal 30% cure rate in the 1970s 29. For example, in a study, Angelico et al 30 described patients with hepatoblastoma and pulmonary metastases who underwent neoadjuvant chemotherapy: 43% of the lesions disappeared, and only 27% of the cases required metastasectomy.
Within the therapeutic arsenal, liver transplantation has demonstrated high survival rates (up to 85%) in patients with locally advanced hepatoblastoma, incomplete tumor resection, or insufficient liver remnant associated with acute liver failure. However, liver transplant has serious complications that can affect patients’ quality of life 31.
The indications for surgical management of pulmonary metastases vary according to the resectability of the primary tumor 30. Surgery is indicated to remove the affected hepatic segments of patients with a good response to chemotherapy. Notwithstanding, in patients with no response to chemotherapy, pulmonary metastasectomy can be performed, and it offers a long-term survival of 88% 32, 33.
Additionally, Zsiros et al. 34 reported excellent results with the SIOPEL-4 protocol (intensive preoperative chemotherapy and radical surgery for high-risk hepatoblastoma), in which the inclusion of cisplatin in neoadjuvant chemotherapy increased the response to 97%, decreasing the requirement for metastasectomies. In contrast, children with a metastatic disease whose primary tumor is unresectable or PRETEXT stage IV, who, after receiving neoadjuvant chemotherapy, are candidates for metastasectomy or liver transplantation, have had similar results with high 5-year survival rates 30.
In patients with incomplete tumor resection or with recurrent disease, re-staging is required to determine the need for new tumor resection or liver transplantation 35. Matsunaga et al 33 reported that 20 children with distant metastases who underwent a total lumpectomy had a 2-year survival rate of 80%, while those who did not undergo complete resection died.
Liver anatomy
Your liver is the largest organ inside your body. You cannot live without your liver because your liver helps your body digest food, store energy, and remove poisons.
Liver functions:
- Your liver breaks down and stores many of the nutrients absorbed from the intestine that your body needs to function. Some nutrients must be changed (metabolized) in the liver before they can be used for energy or to build and repair body tissues.
- Your liver makes most of the clotting factors that keep you from bleeding too much when you are cut or injured.
- Your liver delivers bile into the intestines to help absorb nutrients (especially fats).
- Your liver breaks down alcohol, drugs, and toxic wastes in the blood, which then pass from the body through urine and stool
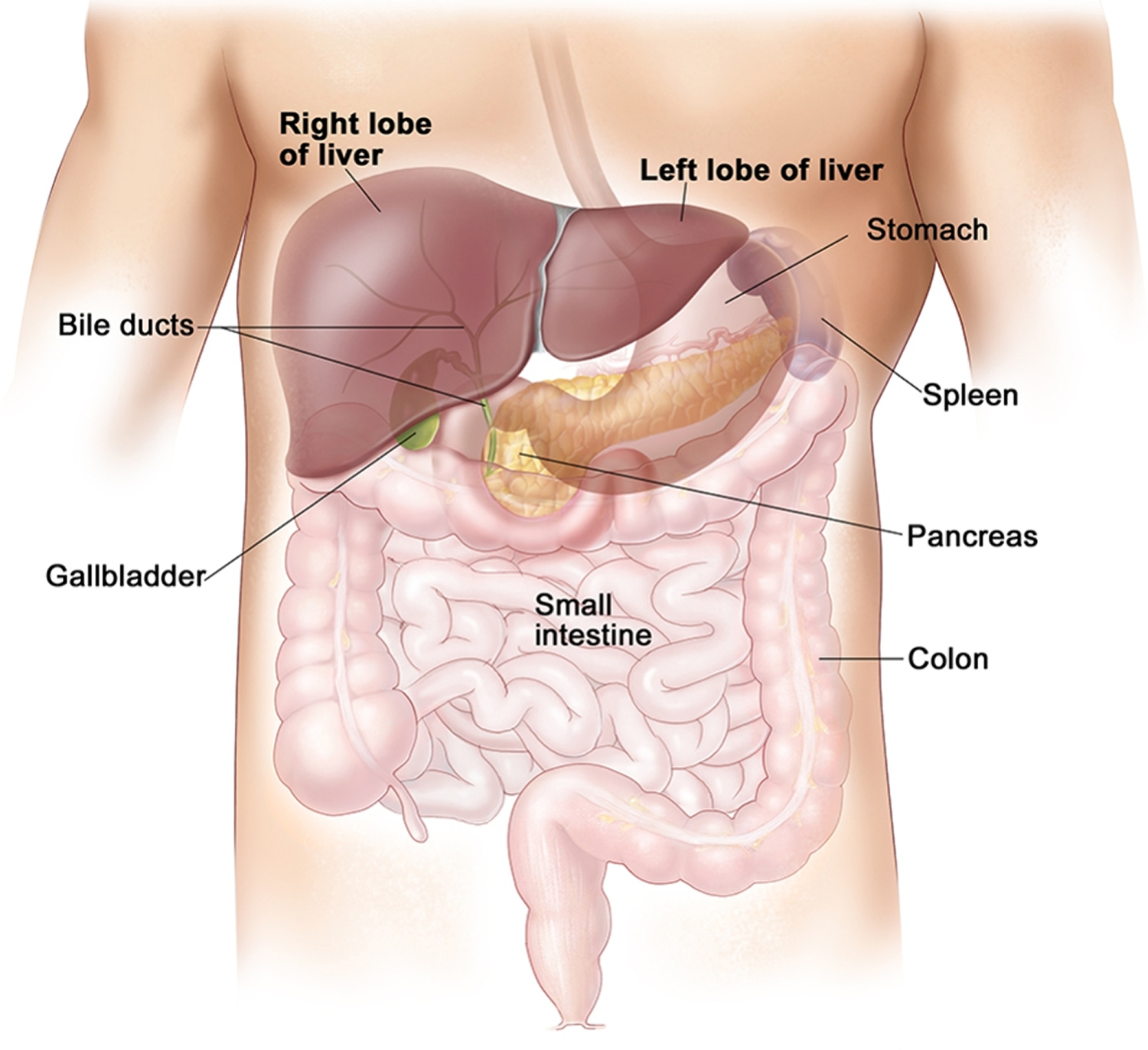
Your liver lie under your right ribs just beneath your right lung. Your liver is divided into lobes.
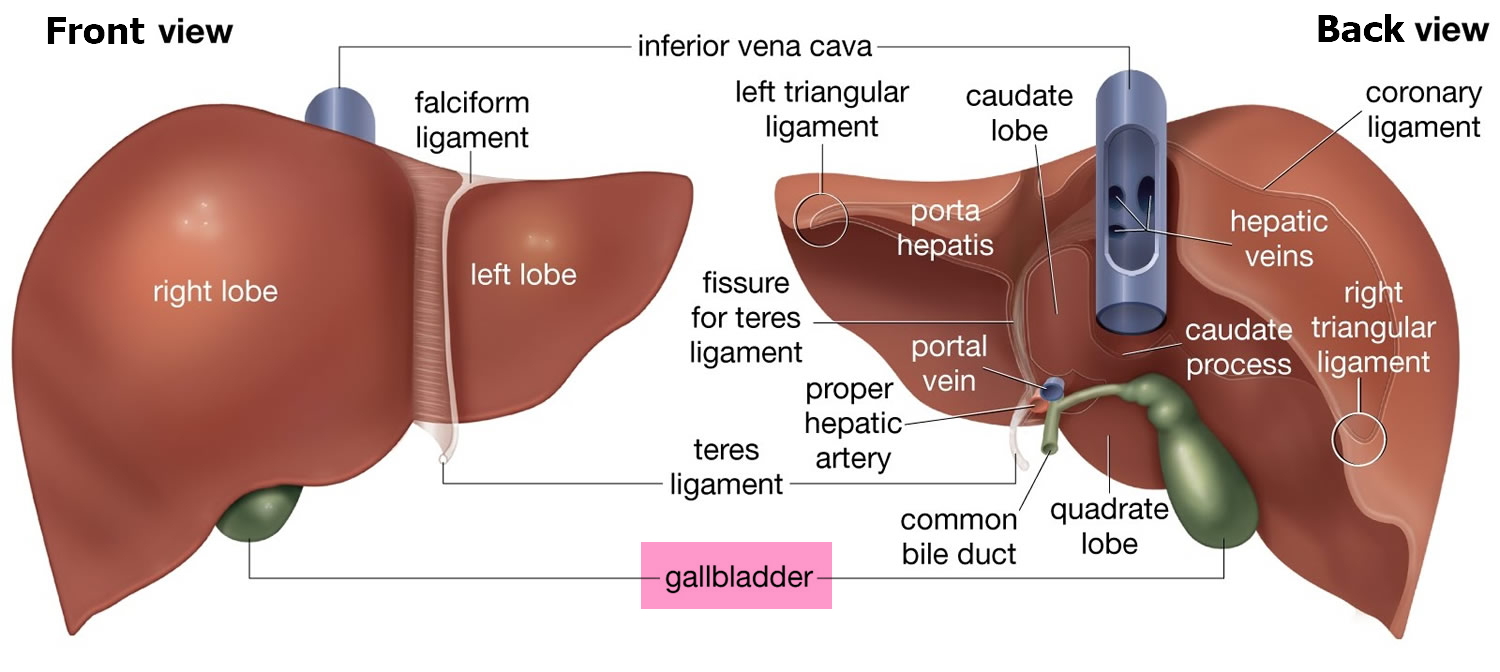
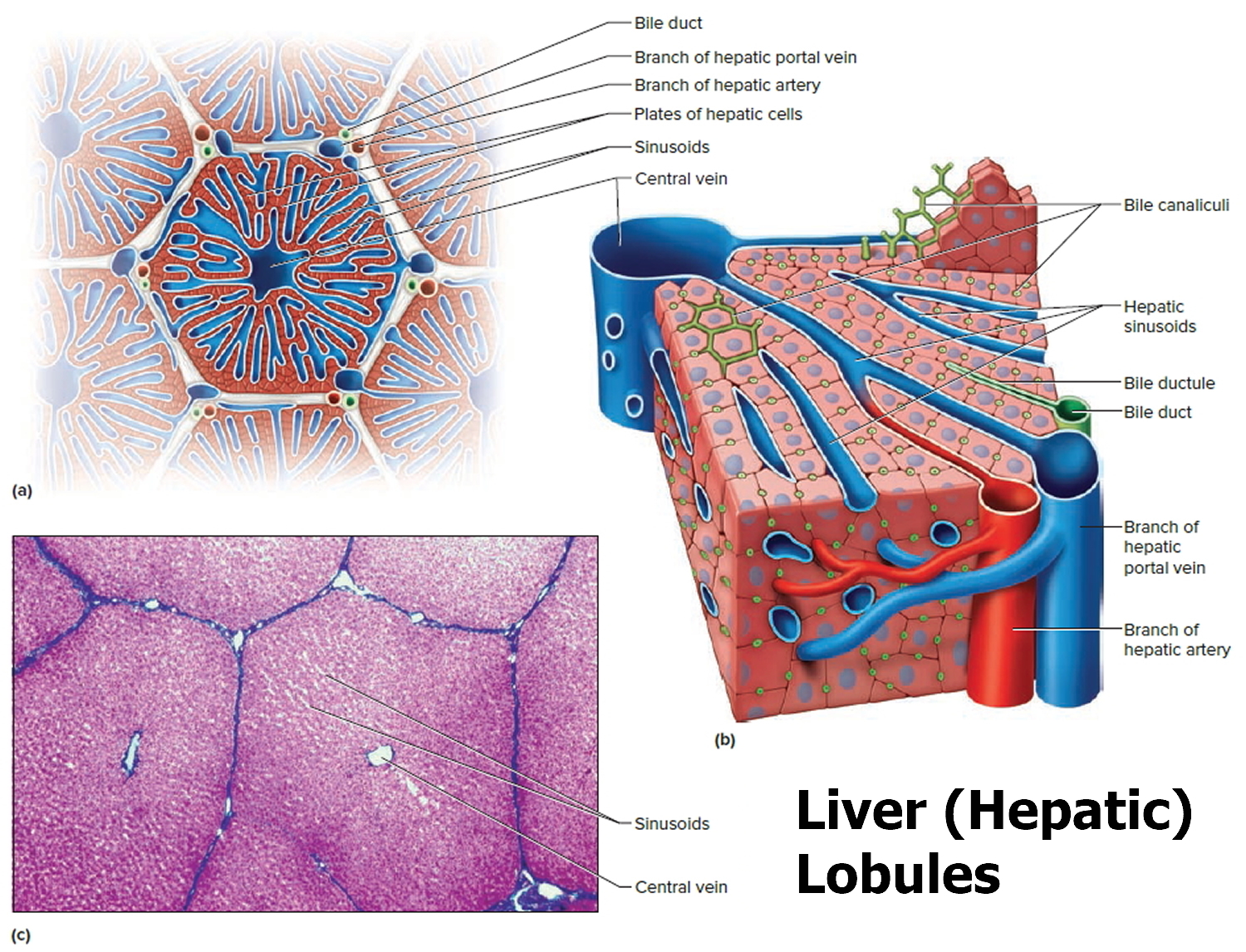
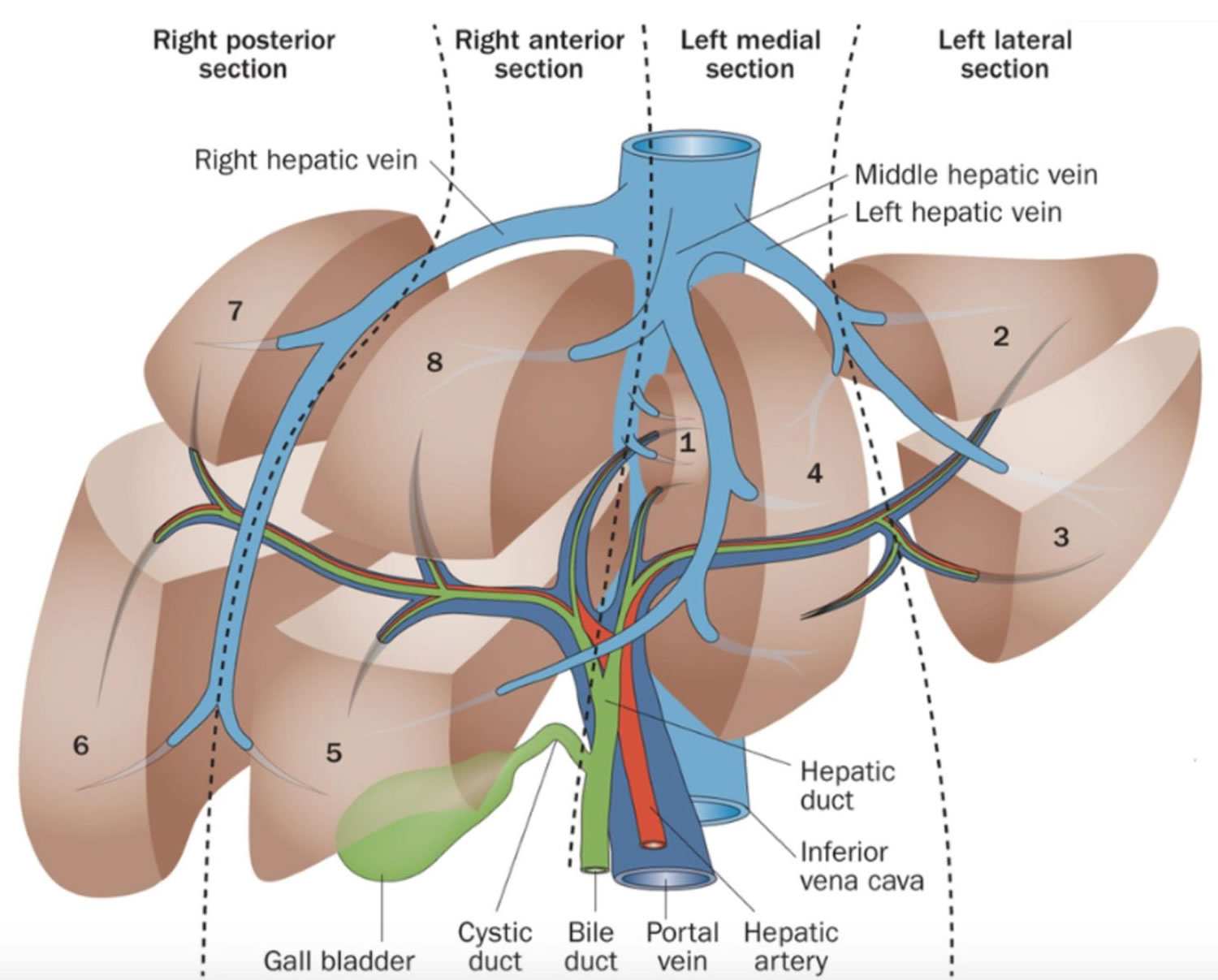
A large right lobe and a smaller left lobe and two minor lobes, the quadrate lobe and the caudate lobe (Figure 2). Each lobe is separated into many tiny hepatic lobules, the liver’s functional units (Figure 3).
The liver is made up mainly of cells called hepatocytes. It also is made up of other types of cells, including cells that line its blood vessels and cells that line small tubes in the liver called bile ducts. The bile ducts extend out of the liver and carry bile from the liver to the gallbladder or directly to the intestines.
Figure 1. Normal liver
Figure 2. Liver anatomy
Figure 3. Liver lobule
Note: (a) Cross section of a hepatic lobule. (b) Enlarged longitudinal section of a hepatic lobule. (c) Light micrograph of hepatic lobules in cross section.
Figure 4. Liver segments
[Source 36 ]Figure 5. PRETEXT (PRE-Treatment EXTent of tumor) staging hepatoblastoma
Footnotes: Schematic representations of the segmental anatomy of the liver. (a) Frontal view of the liver. The numerals label Couinaud’s segments 2 to 8. (b) The hepatic veins (black) and the intrahepatic branches of the portal veins (grey) are shown. Segment 1 (equivalent to the caudate lobe) is seen to lie between the portal vein and the inferior vena cava. (c) Exploded frontal view of the segmental anatomy of the liver (see Figure 4). The umbilical portion of the left portal vein (LPV) separates the left medial section from the left lateral section (LLS). Segment 1 is obscured in this view. Note that the term “section” has been used in preference to “segment” or “sector”. (d) Transverse section of the liver shows the planes of the major venous structures used to determine the PRETEXT number. The hepatic (blue) and portal (purple) veins define the sections of the liver (2–8). This schematic diagram shows how the right hepatic vein (RHV) and middle hepatic vein (MHV) indicate the borders of the right anterior section (RAS) with the right posterior section (RPS) and left medial section (LMS). Note that the left portal vein (LPV) actually lies caudal to the confluence of the hepatic veins and is not seen in the same transverse image. The left hepatic vein (LHV) runs between segments 2 and 3 and is not used in PRETEXT staging.
[Source 37 ]What does the liver do?
Your liver is the largest organ inside your body. You cannot live without your liver because your liver helps your body digest food, store energy, and remove poisons.
- Stores nutrients. Two blood vessels supply your liver with blood. They are the hepatic artery and the hepatic portal vein. Just before it reaches the liver, the blood in the portal vein comes through the gut (digestive system). As it flows through, it picks up the carbohydrates, proteins, fats and vitamins. These are the nutrients that the digestive system breaks down from the food that you eat. The blood then carries these nutrients to the liver.
- Converts fat to energy when the body needs it. Your liver uses chemicals to convert foods that you eat into energy. It does this with food containing carbohydrates and fat.
- Makes bile. Your liver makes bile. This is a substance that helps the digestion and absorption of food. Bile is stored in a small sack below the liver called the gallbladder. The bile passes into the bowel through the bile duct. This is a tube that goes from the liver to the first part of the small bowel (duodenum).
- Makes proteins. Your liver makes proteins including albumin. Albumin is a protein found in blood. It helps to keep the right balance of fluid between the body’s tissues and the bloodstream.
- Helps to clot the blood. Your liver makes substances that help your blood to clot. These substances help to control bleeding when you cut yourself.
- Makes substances the body needs. Your body makes substances that are essential for healthy bones and tissues. It also makes cholesterol, which is an important part of cell walls.
- Breaks down harmful substances. Your liver breaks down harmful substances so that the body can get rid of them in your pee (urine) or poop (feces). This includes alcohol, many drugs, and waste products from normal body processes. If the liver is not working properly, harmful substances can build up and cause problems.
Hepatoblastoma causes
Most cases of hepatoblastoma have unknown cause, but one-third of cases occur in association with specific predisposition syndromes in the setting of normal liver function 38. Hepatoblastoma is strongly associated with premature birth, particularly among low birth weight neonates that weigh < 1,000 g 39. Hepatoblastoma occurs in children from families affected by familial adenomatous polyposis (FAP), which is associated with an inherited germ line mutation of the adenomatous polyposis coli (APC) gene 40. This mutation is also seen in association with Beckwith-Wiedemann syndrome, with which the relative risk of hepatoblastoma is estimated to be 2,280 41. Hepatoblastoma has also been observed in patients with Li-Fraumeni syndrome, Edward syndrome (trisomy 18), nephroblastoma, neurofibromatosis type 1 (NF1) and Down syndrome (trisomy 21) 42, 43. Evidence has also shown an association with preeclampsia and parental tobacco smoking before and during pregnancy 44. Other factors thought to play a role in pathogenesis include oxygen therapy, certain medication (furosemide), radiation, plasticizers, and total parenteral nutrition (TPN) 45.
The most common genetic mutation involves the Wnt signaling pathway which results in the accumulation of beta-catenin; these mutations are present in a higher proportion of the sporadic cases 46. By immunohistochemistry, beta-catenin usually shows a membranous staining pattern in the more differentiated fetal types and nuclear staining pattern in the less differentiated histologic types 47. In aggressive cases, activation of TERT (human telomerase reverse transcriptase) and MYC signaling has been shown 45.
An American Association for Cancer Research publication suggested that all children with more than a 1% risk of developing hepatoblastoma be screened 48. This includes patients with Beckwith-Wiedemann, hemihyperplasia, Simpson-Golabi-Behmel, and trisomy 18 syndromes. Screening is by abdominal ultrasound and alpha-fetoprotein determination every 3 months from birth (or diagnosis) through the fourth birthday, which will identify 90% to 95% of hepatoblastomas that develop in these children 48.
Hepatoblastoma risk factors
Anything that increases your chance of getting a disease is called a risk factor. Conditions associated with an increased risk of hepatoblastoma are described in Table 1.
An American Association for Cancer Research publication suggested that all children with more than a 1% risk of developing hepatoblastoma undergo screening. This includes patients with Beckwith-Wiedemann syndrome, hemihyperplasia, Simpson-Golabi-Behmel syndrome, and trisomy 18 syndrome. Screening is by abdominal ultrasonography and alpha-fetoprotein (AFP) every 3 months from birth (or diagnosis) through the fourth birthday, which will identify 90% to 95% of hepatoblastomas that develop in these children 49.
Table 1. Conditions associated with hepatoblastoma
| Associated Disorder | Clinical Findings |
|---|---|
| Aicardi syndrome 50 | Refer to the Aicardi syndrome section of this summary for more information. |
| Beckwith-Wiedemann syndrome 51 | Refer to the Beckwith-Wiedemann syndrome and hemihyperplasia section of this summary for more information. |
| Familial adenomatous polyposis 52 | Refer to the Familial adenomatous polyposis section of this summary for more information. |
| Glycogen storage diseases I–IV 53 | Symptoms vary by individual disorder. |
| Low-birth-weight infants 54 | Preterm and small-for-gestation-age neonates. |
| Simpson-Golabi-Behmel syndrome 55 | Macroglossia, macrosomia, renal and skeletal abnormalities, and increased risk of Wilms tumor. |
| Trisomy 18, other trisomies 56 | Trisomy 18: Microcephaly and micrognathia, clenched fists with overlapping fingers, and failure to thrive. Most patients (>90%) die in the first year of life. |
Aicardi syndrome
Aicardi syndrome is presumed to be an X-linked condition reported exclusively in females, leading to the hypothesis that a mutated gene on the X chromosome causes lethality in males. The syndrome is classically defined as agenesis of the corpus callosum, chorioretinal lacunae, and infantile spasms, with a characteristic facies. Additional brain, eye, and costovertebral defects are often found 50.
Beckwith-Wiedemann syndrome and hemihyperplasia
The incidence of hepatoblastoma is increased 1,000-fold to 10,000-fold in infants and children with Beckwith-Wiedemann syndrome 58. The risk of hepatoblastoma is also increased in patients with hemihyperplasia, previously termed hemihypertrophy, a condition that results in asymmetry between the right and left side of the body when a body part grows faster than normal 59.
Beckwith-Wiedemann syndrome is most commonly caused by epigenetic changes and is sporadic. The syndrome may also be caused by genetic mutations and be familial. Either mechanism can be associated with an increased incidence of embryonal tumors, including Wilms tumor and hepatoblastoma 60. The expression of both IGFR2 alleles and ensuing increased expression of insulin-like growth factor 2 (IGF-2) has been implicated in the macrosomia and embryonal tumors seen in patients with Beckwith-Wiedemann syndrome 61. When sporadic, the types of embryonal tumors associated with Beckwith-Wiedemann syndrome have frequently also undergone somatic changes in the Beckwith-Wiedemann syndrome locus and IGF-2 62. The genetics of tumors in children with hemihyperplasia have not been clearly defined.
To detect abdominal malignancies at an early stage, all children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia are screened regularly for multiple tumor types by abdominal ultrasonography 63. Screening using alpha-fetoprotein (AFP) levels has also been quite helpful in the early detection of hepatoblastoma in these children 64. Because the hepatoblastomas that are discovered early are small, it has been suggested to minimize the use of adjuvant therapy after surgery 58. However, a careful compilation of published data on 1,370 children with (epi)genotyped Beckwith-Wiedemann syndrome demonstrated that the prevalence of hepatoblastoma was 4.7% in those with Beckwith-Wiedemann syndrome caused by chromosome 11p15 paternal uniparental disomy, less than 1% in the two types of alteration in imprinting control regions, and absent in CDKN1C mutation 65. The authors recommended that only children with Beckwith-Wiedemann syndrome caused by uniparental disomy be screened for hepatoblastoma using abdominal ultrasonography and AFP levels every 3 months from age 3 months to 5 years.
Familial adenomatous polyposis
There is an association between hepatoblastoma and familial adenomatous polyposis (FAP); children in families that carry the APC gene have an 800-fold increased risk of hepatoblastoma. However, hepatoblastoma has been reported to occur in less than 1% of FAP family members, so screening for hepatoblastoma in members of families with FAP using ultrasonography and AFP levels is controversial 66. However, one study of 50 consecutive children with apparent sporadic hepatoblastoma reported that five children (10%) had APC germline mutations 66.
Current evidence cannot rule out the possibility that predisposition to hepatoblastoma may be limited to a specific subset of APC mutations. Another study of children with hepatoblastoma found a predominance of the mutation in the 5′ region of the gene, but some patients had mutations closer to the 3′ region 67. This preliminary study provides some evidence that screening children with hepatoblastoma for APC mutations and colon cancer may be appropriate.
In the absence of APC germline mutations, childhood hepatoblastomas do not have somatic mutations in the APC gene; however, hepatoblastomas frequently have mutations in the beta-catenin gene, the function of which is closely related to APC 68.
Hepatoblastoma histopathology
The cells of hepatoblastoma are similar to fetal liver cells. The histologic types are subdivided into 2 broad categories: epithelial type and mixed type 29. Hepatoblastomas originate from primitive hepatic stem cells that give rise to the epithelial components of the liver. Classically, these tumors are divided into 2 broad categories: epithelial type (E-HB) and mixed epithelial and mesenchymal type (MEM-HB). Revision of this original classification system resulted in the pathology consensus of the pediatric hepatoblastoma classification system, which retained the subdivision of the histologic types into 2 broad categories, as described above. The epithelial type is subdivided into fetal, embryonal, macrotrabecular small cell undifferentiated and cholangioblastic variants, while the mixed type is subdivided into stromal derivatives and teratoid variants.
The fetal subtype is further stratified into 4 categories: well-differentiated; crowded or mitotically active; pleomorphic, poorly differentiated; and anaplastic. The well-differentiated variant is characterized by a low power view demonstrating alternating light and dark areas due to variable cytoplasmic glycogen content. Assessment at higher power reveals a uniform population of hepatocytes arranged in trabeculae that are 2 to 3 cells thick. Extramedullary hematopoiesis is a typical finding, and mitotic rate is low. Description of the other variants is beyond the scope of this article.
The embryonal subtype is the most commonly encountered subtype and consists of basophilic cells with scant cytoplasm and increased mitotic rate that is arranged in nests, trabeculae, acini, pseudorosettes, or sheets. The macrotrabecular subtype is arranged in trabeculae that are more than ten cells thick. The SCU subtype consists of dyscohesive, uniform round cells arranged in sheets with increased mitotic activity. Some cases of SCU have a loss of INI1, suggesting a possible association with primary rhabdoid tumors of the liver. The cholangioblastic variant has bile ducts, typically located at the periphery of the epithelial sheets.
The mixed subtype contains a variable combination of epithelial and mesenchymal components. Most commonly, the epithelial component is fetal or embryonal, and the mesenchymal component is osteoid. Stromal derivatives include spindle cells, osteoid, skeletal muscle, and cartilage. Teratoid features include primitive endoderm, neural derivatives, melanin, squamous and glandular elements 45.
Hepatoblastoma symptoms
Hepatoblastoma signs and symptoms are more common after the tumor gets big. Hepatoblastomas usually present with as a single, mildly painful, rapidly enlarging abdominal mass that arises in the right lobe of the liver in 55% to 60% of cases 69. Rapid enlargement of these tumors rarely results in tumor rupture and hemorrhage. Hepatoblastoma tumors may reach up to 25 cm in size. Most tumors are solitary; however, up to 15% of tumors are multifocal. Some cases are associated with non-specific symptoms such as weight loss, failure to thrive or anorexia 70. Significant elevations of alpha-fetoprotein (AFP) are observed in 90% of patients, and rarely, a paraneoplastic syndrome can occur.
Check with your child’s doctor if your child has any of the following:
- a lump in the abdomen that may be painful
- swelling in the abdomen
- weight loss for no known reason
- loss of appetite
- nausea and vomiting
Hepatoblastoma complications
Hepatoblastoma complications include:
- Intraperitoneal tumor rupture. Tumor rupture may occur at diagnosis, resulting in acute abdomen or severe hemorrhage, both of which constitute medical emergencies. Intraoperative and postoperative complications may occur as a result of resection or biopsy procedures 71.
- Complications related to chemotherapy. Complications can develop with the administration of chemotherapy. Myelosuppression and immunosuppression place the patient at risk for bleeding and infection. After several cycles of therapy, organ toxicity may occur; for example, renal function or hearing may be impaired. Particular attention must be paid to cardiac, renal, and hearing status to assess for the long-term toxic effects of anthracyclines, cisplatin, or carboplatin. One of the most important adverse effects of platinum chemotherapy is hearing loss. A Cochrane Database review of 3 studies that evaluated the use of the chemoprotective agent amifostine versus no additional treatment did not come to any definitive conclusions. Further research is needed regarding the usefulness of possible drugs to prevent hearing loss in children treated with platinum chemotherapy 72.
- Post-transplant complications. Posttransplantion complications can develop and require close long-term follow-up by the liver transplant team.
- Psychosocial effects of treatment and painful procedures. Psychosocial effects of frequent painful procedures, hospitalizations, and interference with normal childhood growth and development must be addressed, and children and families must be referred to appropriate specialists when needed. The family’s psychosocial needs are affected greatly by having a child with cancer.
Hepatoblastoma diagnosis
Ultrasound and either computed tomography (CT) or magnetic resonance imaging (MRI) are the imaging modalities used to define the extent of tumor involvement of the liver and aid in pre-surgical planning. A chest CT can help detect lung metastasis as up to 20% of cases present with metastases; the lung is the most common location of metastases 70. After imaging, a biopsy, alpha-fetoprotein (AFP) level, liver function tests, and a hepatitis panel are performed as needed.
Biopsy
During a biopsy, a doctor removes a sample of cells or tissue. A pathologist then views the cells or tissue under a microscope to look for cancer cells and find out the type of cancer. The doctor may remove as much tumor as safely possible during the same biopsy procedure. The following test may be done on the sample of tissue that is removed:
- Immunohistochemistry: This laboratory test uses antibodies to check for certain antigens (markers) in a sample of a patient’s tissue. The antibodies are usually linked to an enzyme or a fluorescent dye. After the antibodies bind to a specific antigen in the tissue sample, the enzyme or dye is activated, and the antigen can then be seen under a microscope. This type of test is used to check for a certain gene mutation, to help diagnose cancer, and to help tell one type of cancer from another type of cancer.
A biopsy is always indicated to secure the diagnosis of a pediatric liver tumor, except for the following circumstances 73:
- Infantile hepatic hemangioma. Biopsy is not indicated for patients with infantile hemangioma of the liver with classic findings on magnetic resonance imaging (MRI). If the diagnosis is in doubt after high-quality imaging, a confirmatory biopsy is done.
- Focal nodular hyperplasia. Biopsy may not be indicated or may be delayed for patients with focal nodular hyperplasia with classic features on MRI using hepatocyte-specific contrast agent. If the diagnosis is in doubt, a confirmatory biopsy is done.
- Children’s Oncology Group (COG) surgical guidelines 27 recommend tumor resection at diagnosis without preoperative chemotherapy in children with PRE-Treatment EXTent of disease (PRETEXT) group I tumors and PRETEXT group II tumors with greater than 1 cm radiographic margin on the vena cava and middle hepatic and portal veins. Therefore, biopsy is not usually recommended in this circumstance.
- Infantile hepatic choriocarcinoma. In patients with infantile hepatic choriocarcinoma, which can be diagnosed by imaging and markedly elevated beta-human chorionic gonadotropin (beta-hCG), chemotherapy without biopsy is often indicated 74.
Tumor markers
The alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (beta-hCG) tumor markers are very helpful in the diagnosis and management of liver tumors. Although AFP is elevated in most children with hepatic malignancy, it is not pathognomonic for a malignant liver tumor 75. The AFP level can be elevated with either a benign tumor or a malignant solid tumor. AFP is very high in neonates and steadily falls after birth. The half-life of AFP is 5 to 7 days, and by age 1 year, it should be less than 10 ng/mL 76.
Other tests diagnose hepatoblastoma and find out whether the cancer has spread
- Complete blood count (CBC): A sample of blood is drawn and checked for the following:
- the number of red blood cells, white blood cells, and platelets
- the amount of hemoglobin (the protein that carries oxygen) in the red blood cells
- the portion of the blood sample made up of red blood cells
- Liver function tests: These blood tests measure the amounts of certain substances released into the blood by the liver. A higher-than-normal amount of a substance can be a sign of liver damage or cancer.
- Blood chemistry studies: These blood tests measure the amounts of certain substances, such as bilirubin or lactate dehydrogenase (LDH), released into the blood by organs and tissues in the body. An unusual (higher or lower than normal) amount of a substance can be a sign of disease.
- Epstein-Barr virus (EBV) test: This blood test checks for antibodies to the EBV and DNA markers of the EBV. These are found in the blood of patients who have been infected with EBV.
- Imaging studies:
- Magnetic resonance imaging (MRI) with gadolinium: This procedure uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the liver. A substance called gadolinium is injected into a vein. The gadolinium collects around the cancer cells so they show up brighter in the picture.
- CT scan (CAT scan): This procedure uses a computer linked to an x-ray machine to make a series of detailed pictures of areas inside the body, taken from different angles. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography. In childhood liver cancer, a CT scan of the chest and abdomen is usually done.
- Ultrasound exam: This procedure uses high-energy sound waves (ultrasound) that are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. In childhood liver cancer, an ultrasound exam of the abdomen to check the large blood vessels is usually done.
- Abdominal x-ray: An x-ray of the organs in the abdomen may be done. An x-ray is a type of energy beam that can go through the body onto film, making a picture of areas inside the body.
- Currently, CT scan is the imaging of choice in preoperative diagnosis and staging 77. PET-CT with fluorodeoxyglucose (F-FDG) optimizes anatomical localization of the lesions and response to treatment, especially in the early detection of tumor recurrence or metastasis. PET-CT has a sensitivity of 98% and specificity of 56% in bone metastasis 78 and is especially useful in hepatoblastoma as it provides an accurate image of the tumor’s metabolic activity: hepatoblastoma has a high content of cellular glycogen with a great glycolytic activity 79.
Hepatoblastoma staging
There are a number of different staging systems for hepatoblastoma.
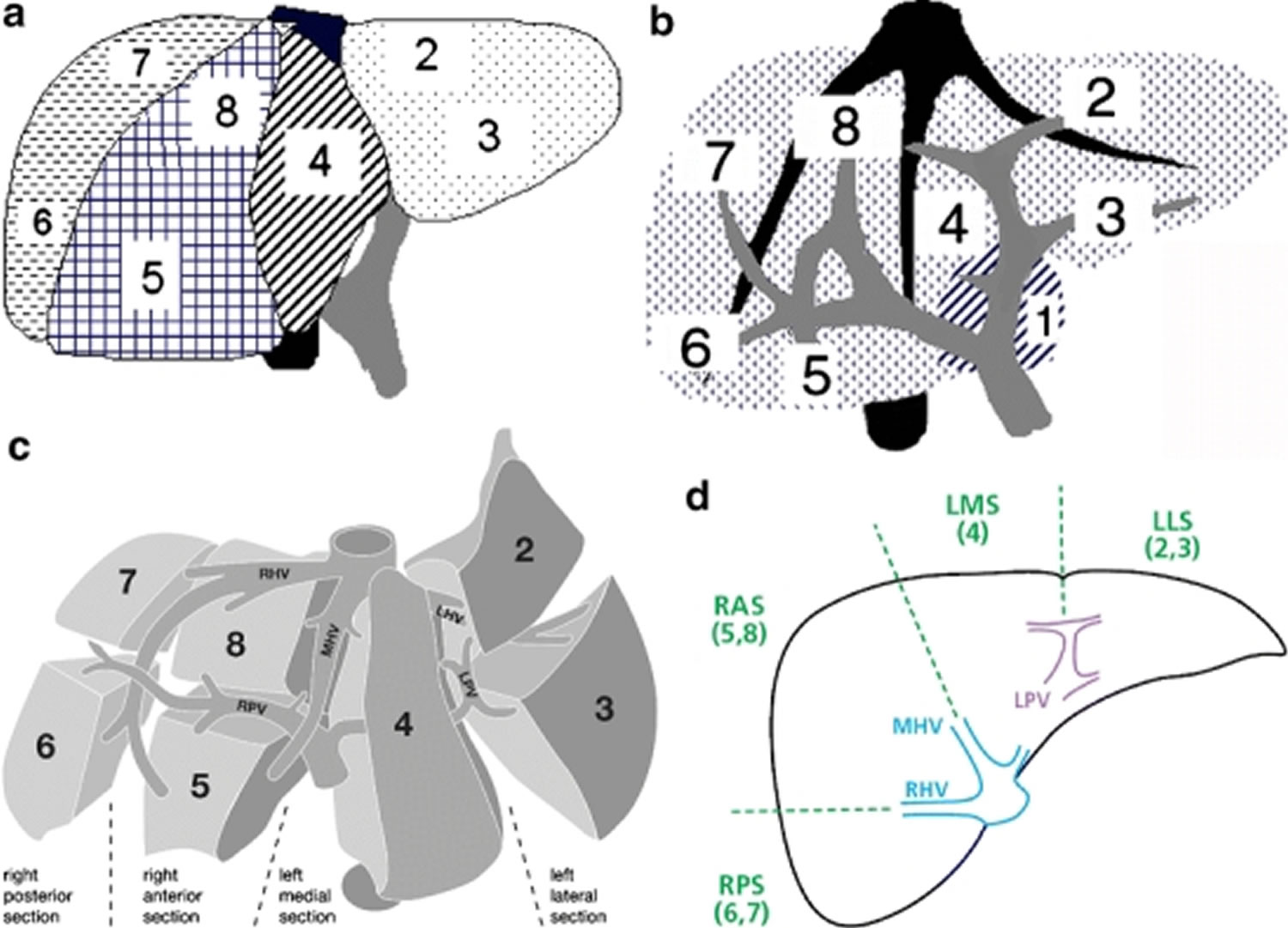
- PRETEXT grouping system of pediatric liver tumors: not specific to hepatoblastoma; used in all pediatric liver tumors. PRETEXT (PRE-Treatment EXTent of disease) staging is based on Couinaud’s system of segmentation of the liver (see Figures 4 and 5) 80.
- Intergroup staging system: specific for hepatoblastoma
Historically, the four major study groups—International Childhood Liver Tumors Strategy Group (previously known as Société Internationale d’Oncologie Pédiatrique–Epithelial Liver Tumor Study Group [SIOPEL]), Children’s Oncology Group (COG), Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology), and Japanese Study Group for Pediatric Liver Tumors—have had disparate risk stratification categories, making it difficult to compare outcomes across continents. All groups are now using the PRE-Treatment EXTent of tumor (PRETEXT) grouping system as part of the risk stratification 81.
The imaging grouping systems used to radiologically define the extent of liver involvement by the tumor are designated as the following:
- PRETEXT (PRE-Treatment EXTent of disease): The extent of liver involvement is defined before therapy.
- POSTTEXT (POST-Treatment EXTent of disease): The extent of liver involvement is defined in response to therapy.
In hepatoblastoma, the PRETEXT and POSTTEXT groups are used for hepatoblastoma to decide whether the tumor can be removed by surgery 81.
Although PRETEXT can be used to predict tumor resectability, there are limitations. The distinction between real invasion beyond the anatomical border of a given hepatic section and the compression and displacement by the tumor can be difficult, especially at diagnosis. Additionally, it can be difficult to distinguish between vessel encroachment and involvement, particularly if imaging is inadequate. The PRETEXT group assignment has a moderate degree of interobserver variability. In a report published in 2005 using data from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study, the preoperative PRETEXT group aligned with postoperative pathological findings only 51% of the time, with overstaging in 37% of patients and understaging in 12% of patients 82.
Because distinguishing PRETEXT group assignment is difficult, central review of imaging is critical and is generally performed in all major clinical trials. For patients not enrolled in clinical trials, expert radiological review should be considered in questionable cases in which the PRETEXT group assignment affects choice of treatment.
PRETEXT classification hepatoblastoma
The Children’s Hepatic Tumors International Collaboration constructed a staging and risk stratification system, the PRETEXT (PRE-Treatment EXTent of tumor) system, intended to standardize the assessment of hepatoblastoma cancer across the world. The PRETEXT grouping system is used to describe tumor extent before any therapy, thus allowing a more effective comparison between studies conducted by different groups.
PRETEXT system is based on the Couinaud eight-segment anatomic structure of the liver using cross-sectional imaging (see Figures 4 and 5). The PRETEXT system divides the liver into four parts, called sections. The left lobe of the liver consists of a lateral section (Couinaud segments I, II, and III) and a medial section (segment IV), whereas the right lobe consists of an anterior section (segments V and VIII) and a posterior section (segments VI and VII) (see Figures 4 and 5).
The original PRETEXT system was created to classify hepatoblastomas, but after its revision in 2005 it can be used for any tumor lesion of the liver in children 37.
The PRETEXT system is made of two components: the PRETEXT group and the annotation factors. The PRETEXT group (I, II, III, or IV) describes the extent of tumor within the liver while the annotation factors help to describe associated features such as vascular involvement (either portal vein or hepatic vein/inferior vena cava), extrahepatic disease, multifocality, tumor rupture and metastatic disease (to both the lungs and lymph nodes) 83. PRETEXT annotation factor is determined to be positive if at least 1 of the following 5 factors are present: involvement of the vena cava or all 3 hepatic veins, or both (V); involvement of portal bifurcation or both right and left portal veins, or both (P); extrahepatic contiguous tumor extension (E); multifocal liver tumor (F); tumor rupture at diagnosis (R).
This new staging system is called the Children’s Hepatic Tumors International Collaboration – Hepatoblastoma Stratification and incorporates confirmed prognostic factors from prior risk stratification systems with new additional factors to stratify patients into 4 risk groups.
Found to be most predictive are alpha-fetoprotein (AFP) levels, patient age, Pretreatment Extent of Disease (PRETEXT) group (I, II, III, or IV), the presence of metastases, and PRETEXT annotation factor.
Gender, low birth weight, prematurity, and Beckwith-Wiedemann syndrome were not found to be significant. Of note, the histologic type was not included in this risk stratification system but may be incorporated at a future date. Validation of these risk groups is in progress 84.
The PRETEXT system has good interobserver reproducibility 82 and good prognostic value in children with hepatoblastoma 82 and is the basis of risk stratification in current Children’s Hepatic Tumors International Collaboration hepatoblastoma studies. Most other study groups now use the PRETEXT system to describe imaging findings at diagnosis, even if this is not their main staging system.
The liver tumors could be staged by interpretation of CT or ultrasound with or without additional imaging by MRI.
Table 2. Definitions of PRETEXT/POST-TEXT group (I, II, II, IV) and PRETEXT grouping system annotations V,P,E,M,C,F,N,R.
| PRETEXT/POST-text group | Definition |
|---|---|
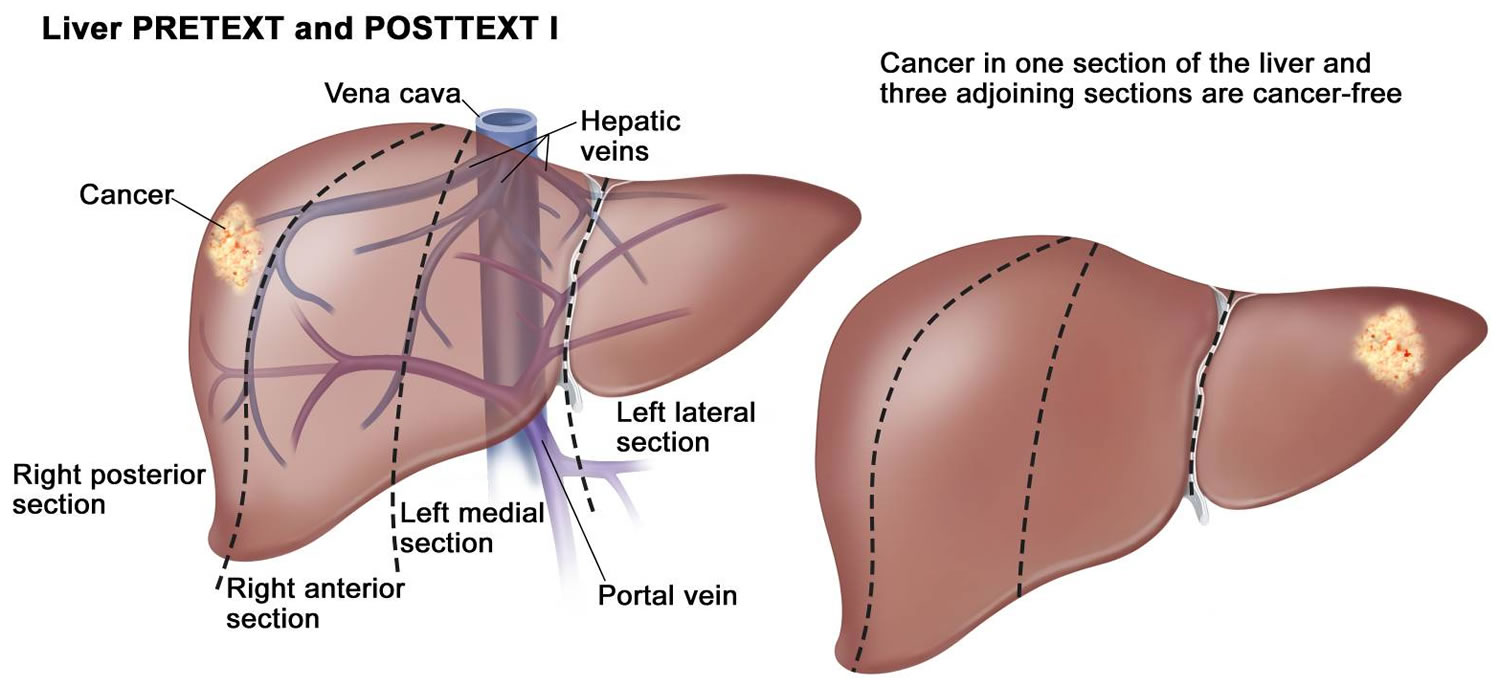
| I | One liver section involved; three adjoining sections are tumor free. |
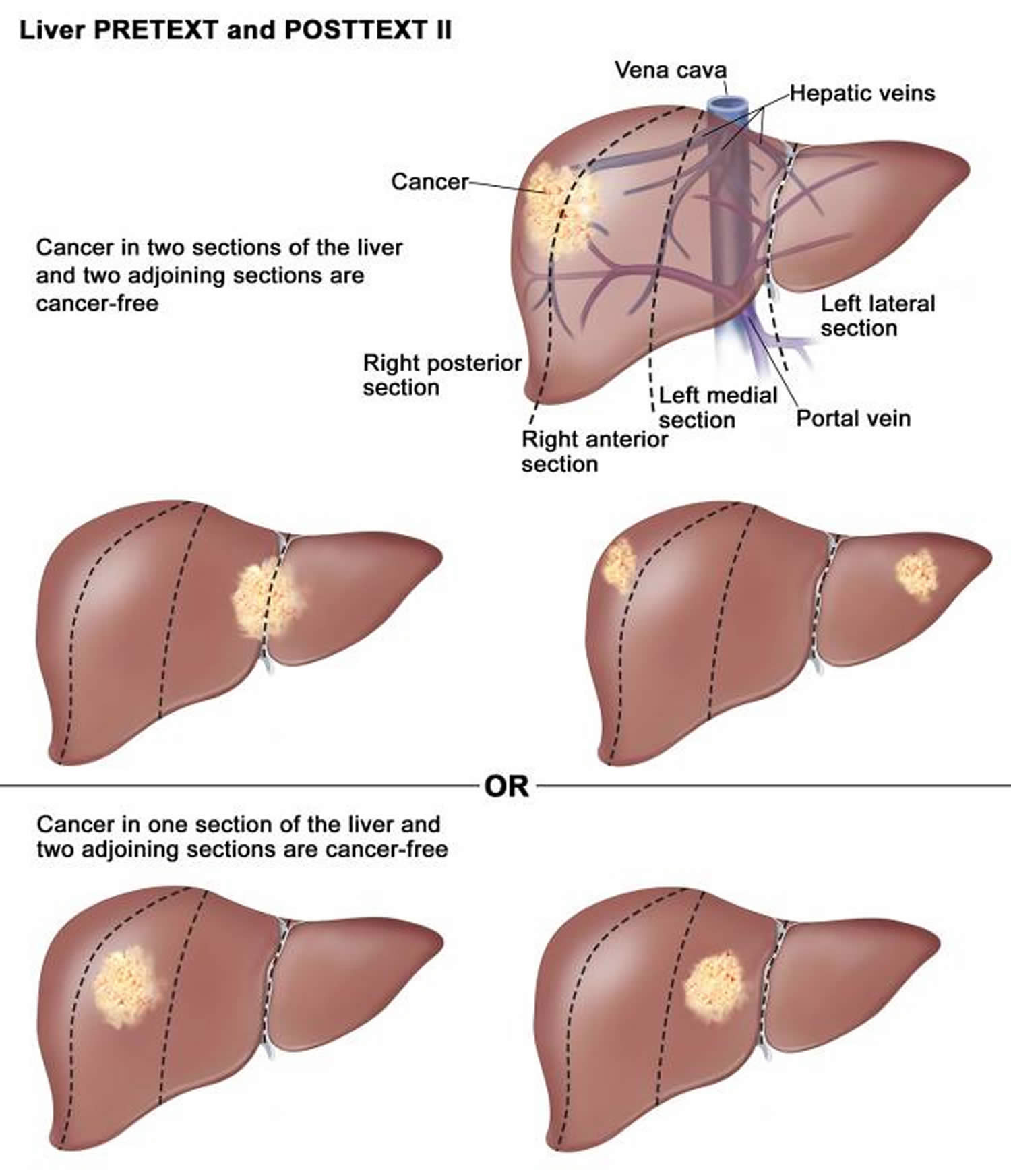
| II | One or two liver sections involved; two adjoining sections are tumor free. |
| III | Two or three liver sections involved; one adjoining section is tumor free. |
| IV | Four liver sections involved |
| Annotation: | |
| V | Venous involvement, V, denotes vascular involvement of the retrohepatic vena cava or involvement of ALL THREE major hepatic veins (right, middle, and left) |
| P | Portal involvement, P, denotes vascular involvement of the main portal vein and/or BOTH right and left portal veins |
| E | Extrahepatic involvement of a contiguous structure such as the diaphragm, abdominal wall, stomach, colon, etc. |
| M | Distant metastatic disease (usually lungs, occasionally bone or brain) |
| C | Caudate lobe |
| F | Multifocal tumor nodules |
| N | Lymph node involvement |
| R | Tumor rupture |
Figure 6. PRETEXT 1 hepatoblastoma
Figure 7. PRETEXT 2 hepatoblastoma
Figure 8. PRETEXT 3 hepatoblastoma
Figure 9. PRETEXT 4 hepatoblastoma
Intergroup staging system
- Stage 1
- complete surgical resection
- Stage 2
- Stage 2a
- complete macroscopic surgical resection
- intrahepatic residual microscopic disease
- Stage 2b
- complete macroscopic surgical resection
- extrahepatic residual microscopic disease
- Stage 2a
- Stage 3
- Stage 3a
- incomplete surgical resection with macroscopic residual AND / OR
- significant tumor spill AND / OR
- positive lymph node disease
- Stage 3b
- tumor not resected
- Stage 3a
- Stage 4
- Stage 4a
- distant metastatic disease
- primary tumor completely resected
- Stage 4b
- distant metastatic disease
- primary tumor incompletely resected
- Stage 4a
Hepatoblastoma prognostic factors
Individual childhood cancer study groups have attempted to define the relative importance of a variety of prognostic factors present at diagnosis and in response to therapy 86. A collaborative group consisting of four study groups (International Childhood Liver Tumors Strategy Group [SIOPEL], Children’s Oncology Group, Gesellschaft für Pädiatrische Onkologie und Hämatologie [GPOH], and Japanese Study Group for Pediatric Liver Tumor [JPLT]), termed Childhood Hepatic tumor International Collaboration (CHIC), have retrospectively combined data from eight clinical trials (N = 1,605) conducted between 1988 and 2010. The CHIC published a univariate analysis of the effect of clinical prognostic factors present at the time of diagnosis on event-free survival (EFS) 87. The analysis confirmed many of the findings described below. The statistically significant adverse factors included the following 88:
- Higher PRETEXT group.
- Positive PRETEXT annotation factors:
- V: Involvement all three hepatic veins and/or intrahepatic inferior vena cava.
- P: Involvement of both left and right portal veins.
- E: Contiguous extrahepatic tumor extensions (e.g., diaphragm, adjacent organs).
- F: Multifocal tumors.
- R: Tumor rupture.
- M: Distant metastases, usually lung.
- Low AFP level (<100 ng/mL or 100–1,000 ng/mL to account for infants with elevated AFP levels) 87.
- Older age. Patients aged 3 to 7 years have a worse outcome in the PRETEXT IV group 88. Patients aged 8 years and older have a worse outcome than do younger patients in all PRETEXT groups.
In contrast, in the SIOPEL-2 and -3 studies, infants younger than 6 months had PRETEXT, annotation factors, and outcomes similar to that of older children undergoing the same treatment 89.
In contrast, sex, prematurity, birth weight, and Beckwith-Wiedemann syndrome had no effect on event-free survival 88.
A multivariate analysis of these prognostic factors has been published to help develop a new risk group classification for hepatoblastoma 87. This classification was used to generate a risk stratification schema to be used in international clinical trials.
Other studies of factors affecting prognosis observed the following:
- PRETEXT group: In SIOPEL studies, having a low PRETEXT group at diagnosis (PRETEXT I, II, and III tumors) is a good prognostic factor, whereas PRETEXT IV is a poor prognostic factor.
- Tumor stage: In Children’s Oncology Group studies, stage I tumors that were resected at diagnosis and tumors with well-differentiated fetal histology have a good prognosis. These tumors are treated differently than tumors of other stages and histologies 88.
- Treatment-related factors:
- Chemotherapy: Chemotherapy often decreases the size and extent of hepatoblastoma, allowing complete resection.[45-49] Favorable response of the primary tumor to chemotherapy, defined as either a 30% decrease in tumor size by Response Evaluation Criteria In Solid Tumors (RECIST) or 90% or greater decrease in AFP levels, predicted the resectability of the tumor; in turn, this favorable response predicted overall survival among all CHIC risk groups treated with neoadjuvant chemotherapy on the JPLT-2 Japanese national clinical trial.[50][Level of evidence: 2A]
- Surgery: Cure of hepatoblastoma requires gross tumor resection. Hepatoblastoma is most often unifocal and thus, resection may be possible. If a hepatoblastoma is completely removed, most patients survive, but because of vascular or other involvement, less than one-third of patients have lesions amenable to complete resection at diagnosis 88. Thus, it is critically important that a child with probable hepatoblastoma be evaluated by a pediatric surgeon; the surgeon should be experienced in the techniques of extreme liver resection with vascular reconstruction and have access to a liver transplant program. In advanced tumors, surgical treatment of hepatoblastoma is a demanding procedure. Postoperative complications in high-risk patients decrease the rate of overall survival 90.
- Orthotopic liver transplant: Orthotopic liver transplant is an additional treatment option for patients whose tumor remains unresectable after preoperative chemotherapy 91; however, the presence of microscopic residual tumor at the surgical margin does not preclude a favorable outcome 34. This may result from the additional courses of chemotherapy that are administered before or after resection 92.
- Tumor marker–related factors: Ninety percent of children with hepatoblastoma and two-thirds of children with hepatocellular carcinoma exhibit the serum tumor marker AFP, which parallels disease activity. The level of AFP at diagnosis and rate of decrease in AFP levels during treatment are compared with the age-adjusted normal range. Lack of a significant decrease in AFP levels with treatment may predict a poor response to therapy 93. Absence of elevated AFP levels at diagnosis (AFP <100 ng/mL) occurs in a small percentage of children with hepatoblastoma and appears to be associated with very poor prognosis, as well as with the small cell undifferentiated variant of hepatoblastoma. Some of these variants do not express INI1 and may be considered rhabdoid tumors of the liver; all small cell undifferentiated hepatoblastomas are tested for loss of INI1 expression by immunohistochemistry 94. Beta-hCG levels may also be elevated in children with hepatoblastoma or hepatocellular carcinoma, which may result in isosexual precocity in boys 95.
- Tumor histology 96:
- Well-differentiated fetal (pure fetal) histology. Well-differentiated fetal hepatocytes morphologically indistinguishable from normal fetal liver cells. An analysis of patients with initially resected hepatoblastoma tumors (before receiving chemotherapy) has suggested that patients with well-differentiated fetal (previously termed pure fetal) histology tumors have a better prognosis than do patients with an admixture of more primitive and rapidly dividing embryonal components or other undifferentiated tissues. Studies have reported the following:
- A study of patients with hepatoblastoma and well-differentiated fetal histology tumors observed the following 19:
- The survival rate was 100% for patients who received four doses of single-agent doxorubicin. This suggested that patients with well-differentiated fetal histology tumors might not need chemotherapy after complete resection 20.
- In a Children’s Oncology Group study 21, 16 patients with well-differentiated fetal histology hepatoblastoma with two or fewer mitoses per 10 high-power fields were not treated with chemotherapy. Retrospectively, their PRETEXT (PRE-Treatment EXTent of tumor) groups were group I (n = 4), group II (n = 6), and group III (n = 2) 22.
- The survival rate was 100% for these patients who did not receive chemotherapy.
- All 16 patients were alive with no evidence of disease at a median follow-up of 4.9 years (range, 9 months to 9.2 years).
- Thus, complete resection of a well-differentiated fetal hepatoblastoma may preclude the need for chemotherapy.
- A study of patients with hepatoblastoma and well-differentiated fetal histology tumors observed the following 19:
- Non–well-differentiated fetal histology, non-small cell undifferentiated histology.
- Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver. Histologically, small cell undifferentiated hepatoblastoma is typified by a diffuse population of small cells with scant cytoplasm resembling neuroblasts 23. Small cell undifferentiated hepatoblastoma may be difficult to distinguish from malignant rhabdoid tumor of the liver, which has been conflated with small cell undifferentiated hepatoblastoma in past studies. A characteristic shared by small cell undifferentiated hepatoblastoma and malignant rhabdoid tumors is the poor prognosis associated with each 24, 25. Patients with small cell undifferentiated hepatoblastoma whose tumors are unresectable have an especially poor prognosis 25. Patients with stage 1 tumors appear to have increased risk of treatment failure when small cell elements are present 26. For this reason, completely resected tumors composed of well-differentiated fetal histology or of mixed fetal and embryonal cells must have a thorough histological examination because small foci of undifferentiated small cell histology indicates a need for aggressive chemotherapy 26. Aggressive treatment for this histology was investigated in the completed Children’s Oncology Group study 27 and all tumors were tested for SMARCB1 expression by immunohistochemistry. In this study, hepatoblastoma that would otherwise be considered very low or low risk was upgraded to intermediate risk if any small cell undifferentiated elements were found.
- Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver can be distinguished by the following characteristic abnormalities:
- Chromosomal abnormalities. These abnormalities in rhabdoid tumors include translocations involving a breakpoint on chromosome 22q11 and homozygous deletion at the chromosome 22q12 region that harbors the SMARCB1 gene 25.
- Small cell undifferentiated hepatoblastoma (SMARCB1 positive). Small cell undifferentiated hepatoblastoma (SMARCB1 positive) is an uncommon hepatoblastoma variant that represents several percent of all hepatoblastomas. It tends to occur at a younger age (6–10 months) than do other cases of hepatoblastoma 24, 25 and is associated with AFP levels that are normal for age at presentation 28, 25.
- Lack of SMARCB1 expression. Lack of detection of SMARCB1 by immunohistochemistry is characteristic of malignant rhabdoid tumors 25.
- Rhabdoid tumor of liver (SMARCB1 negative).
- Chromosomal abnormalities. These abnormalities in rhabdoid tumors include translocations involving a breakpoint on chromosome 22q11 and homozygous deletion at the chromosome 22q12 region that harbors the SMARCB1 gene 25.
- Published studies of prognostic features related to small cell undifferentiated histology include the following:
- In 2009, the results of a study of 11 young children with low AFP levels and small cell morphology were reported. Ten children died of disease progression, and one child died of complications. Six of six children tested were SMARCB1 negative, but only one child had any rhabdoid morphology. This finding suggests that many or all liver tumors with small cell morphology and very low AFP levels in young children may be rhabdoid tumors of the liver. These tumors have a poor prognosis that is associated with the driver mutation 25.
- A single-institution study of seven children with small cell morphology liver tumors found that all retained expression of SMARCB1. Six children survived, and one child died of complications from liver transplant 97.
- A study of 23 liver tumors from the Kiel tumor bank found 12 tumors with small cell morphology. Nine tumors had malignant rhabdoid tumor classic histology, and two tumors had mixed small cell and rhabdoid histologies. Outcomes were not provided, but it was noted that rhabdoid brain tumors had small cell, not classic, rhabdoid histology 98.
- In a single-institution study of six children with SMARCB1-negative liver tumors, two children with small cell morphology died. The remaining four children with classic rhabdoid histology were not treated with cisplatin-based therapy; three children survived, and one child died of complications from transplant 99.
- Patients with small cell undifferentiated hepatoblastoma whose tumors are unresectable have an especially poor prognosis 25. Patients with stage 1 tumors appear to have increased risk of treatment failure when small cell elements are present.[90] For this reason, completely resected tumors composed of well-differentiated fetal histology or of mixed fetal and embryonal cells must have a thorough histological examination because small foci of undifferentiated small cell histology indicates a need for aggressive chemotherapy 26. Aggressive treatment for this histology was investigated in the completed Children’s Oncology Group study 27, and all tumors were tested for SMARCB1 expression by immunohistochemistry. In this study, hepatoblastoma that would otherwise be considered very low or low risk was upgraded to intermediate risk if any small cell undifferentiated elements were found.
- Small cell undifferentiated histology hepatoblastoma and rhabdoid tumors of the liver can be distinguished by the following characteristic abnormalities:
- Well-differentiated fetal (pure fetal) histology. Well-differentiated fetal hepatocytes morphologically indistinguishable from normal fetal liver cells. An analysis of patients with initially resected hepatoblastoma tumors (before receiving chemotherapy) has suggested that patients with well-differentiated fetal (previously termed pure fetal) histology tumors have a better prognosis than do patients with an admixture of more primitive and rapidly dividing embryonal components or other undifferentiated tissues. Studies have reported the following:
Other variables have been suggested as poor prognostic factors, but the relative importance of their prognostic significance has been difficult to define. In the SIOPEL-1 study, a multivariate analysis of prognosis after positive response to chemotherapy showed that only one variable, PRETEXT, predicted overall survival (OS), while metastasis and PRETEXT predicted event-free survival (EFS) 100. In an analysis of the intergroup U.S. study from the time of diagnosis, well-differentiated fetal histology, small cell undifferentiated histology, and AFP less than 100 ng/mL were prognostic in a log rank analysis. PRETEXT was prognostic among patients designated group III, but not group IV 101.
Hepatoblastoma treatment
Treatment options for newly diagnosed hepatoblastoma depend on the following 81:
- Whether the cancer is resectable at diagnosis.
- The tumor histology.
- How the cancer responds to chemotherapy.
- Whether the cancer has metastasized.
Surgical resection is the mainstay of treatment with resectability of the tumor determining the need for neo-adjuvant or adjuvant chemotherapy 102. However, at presentation, approximately 60% of tumors are unresectable 103. If unresectable and chemotherapy fails to shrink the tumor to a resectable size, a liver transplant can be done and has a good long-term survival rate 104. The benefit of radiation therapy is unclear, with some unresectable cases responding well. Alpha-fetoprotein levels are useful for tracking surgical success and whether the tumor has metastasized 105. An increased risk of post-transplant lymphoproliferative disorder after immunosuppression for liver transplant has been suggested in some publications 47.
Cisplatin-based chemotherapy has resulted in a survival rate of more than 90% for children with PRETEXT and POST-Treatment EXTent (POSTTEXT) I and II resectable disease before or after chemotherapy 106.
Chemotherapy regimens used in the treatment of hepatoblastoma and their respective outcomes are described in Table 3.
Table 3. Outcomes for Hepatoblastoma Multicenter Trials
| Study | Chemotherapy Regimen | Number of Patients | Outcomes |
|---|---|---|---|
| INT0098 (CCG/POG) 1989–1992 | C5V vs. CDDP/DOXO | Stage I/II: 50 | 4-Year Event-Free Survival/Overall Survival: |
| I/II = 88%/100% vs. 96%/96% | |||
| Stage III: 83 | III = 60%/68% vs. 68%/71% | ||
| Stage IV: 40 | IV = 14%/33% vs. 37%/42% | ||
| P9645 (COG)b 1999–2002 | C5V vs. CDDP/CARBO | Stage I/II: Pending publication | 1-Year Event-Free Survival: |
| I/II: Pending publication | |||
| Stage III: 38 | III/IV: C5V = 51%; CDDP/CARBO = 37% | ||
| Stage IV: 50 | |||
| HB 94 (GPOH) 1994–1997 | I/II: IFOS/CDDP/DOXO | Stage I: 27 | 4-Year Event-Free Survival/Overall Survival: |
| I = 89%/96% | |||
| Stage II: 3 | II = 100%/100% | ||
| III/IV: IFOS/CDDP/DOXO + VP/CARBO | Stage III: 25 | III = 68%/76% | |
| Stage IV: 14 | IV = 21%/36% | ||
| HB 99 (GPOH) 1999–2004 | SR: IPA | SR: 58 | 3-Year Event-Free Survival/Overall Survival: |
| SR = 90%/88% | |||
| HR: CARBO/VP16 | HR: 42 | HR = 52%/55% | |
| SIOPEL-2 1994–1998 | SR: PLADO | PRETEXT I: 6 | 3-Year Event-Free Survival/Overall Survival: |
| SR: 73%/91% | |||
| PRETEXT II: 36 | |||
| PRETEXT III: 25 | |||
| HR: CDDP/CARBO/DOXO | PRETEXT IV: 21 | HR: IV = 48%/61% | |
| Metastases: 25 | HR: metastases = 36%/44% | ||
| SIOPEL-3 1998–2006 | SR: CDDP vs. PLADO | SR: PRETEXT I: 18 | 3-Year Event-Free Survival/Overall Survival: |
| SR: CDDP = 83%/95%; PLADO = 85%/93% | |||
| PRETEXT II: 133 | |||
| PRETEXT III: 104 | |||
| HR: SUPERPLADO | HR: PRETEXT IV: 74 | HR: Overall = 65%/69% | |
| VPE+: 70 | |||
| Metastases: 70 | Metastases = 57%/63% | ||
| AFP <100 ng/mL: 12 | |||
| SIOPEL-4 2005–2009 | HR: Block A: Weekly; CDDP/3 weekly DOXO; Block B: CARBO/DOXO | PRETEXT I: 2 | 3-Year Event-Free Survival/OS: |
| All HR = 76%/83% | |||
| PRETEXT II: 17 | |||
| PRETEXT III: 27 | |||
| PRETEXT IV: 16 | HR: IV = 75%/88% | ||
| Metastases: 39 | HR: Metastases = 77%/79% | ||
| JPLT-1 1991–1999 | I/II: CDDP(30)/THP-DOXO | Stage I: 9 | 5-Year Event-Free Survival/Overall Survival: |
| I = NR/100% | |||
| Stage II: 32 | II = NR/76% | ||
| III/IV: CDDP(60)/THP-DOXO | Stage IIIa: 48 | IIIa = NR/50% | |
| Stage IIIb: 25 | IIIb = NR/64% | ||
| Stage IV: 20 | IV = NR/77% | ||
| JPLT-2 1999–2010 | I: Low-dose CDDP-pirarubicin | PRETEXT I–IV: 212 | 5-Year Event-Free Survival/Overall Survival: |
| I = NR/100% | |||
| II–IV: CITA | II = NR/89% | ||
| III = NR/93% | |||
| IV = NR/63% | |||
| Metastases: High dose chemotherapy + stem cell transplant | Metastases = 32% |
Abbreviations: AFP = alpha-fetoprotein; C5V = cisplatin, 5-fluorouracil (5FU), and vincristine; CARBO = carboplatin; CCG = Children’s Cancer Group; CDDP = cisplatin; CITA = pirarubicin-cisplatin; COG = Children’s Oncology Group; DOXO = doxorubicin; EFS = event-free survival; GPOH = Gesellschaft für Pädiatrische Onkologie und Hämatologie (Society for Paediatric Oncology and Haematology); HR = high risk; IFOS = ifosfamide; IPA = ifosfamide, cisplatin, and doxorubicin; JPLT = Japanese Study Group for Pediatric Liver Tumor; NR = not reported; OS = overall survival; PLADO = cisplatin and doxorubicin; POG = Pediatric Oncology Group; PRETEXT = PRE-Treatment EXTent of disease; SIOPEL = International Childhood Liver Tumors Strategy Group; SR = standard risk; SUPERPLADO = cisplatin, doxorubicin, and carboplatin; THP = tetrahydropyranyl-adriamycin (pirarubicin); VP = vinorelbine and cisplatin; VPE+ = venous, portal, and extrahepatic involvement; VP16 = etoposide.
Footnote: (a) Adapted from Czauderna et al. 101 and Meyers et al. 107; (b) Study closed early because of inferior results in the CDDP/CARBO arm.
Treatment options for hepatoblastoma that is resectable at diagnosis
Approximately 20% to 30% of children with hepatoblastoma have resectable disease at diagnosis. The Children’s Oncology Group surgical guidelines 27 recommend tumor resection at diagnosis without preoperative chemotherapy in children with PRETEXT 1 tumors and PRETEXT 2 tumors with greater than 1 cm radiographic margin on the vena cava and middle hepatic and portal veins. Outcomes for patients after undergoing a complete resection at diagnosis, compared with patients who had positive microscopic margins found at resection, are similar after receiving chemotherapy 108, 109.
Prognosis varies depending on the histological subtype, as follows:
- Patients with well-differentiated fetal histology (4% of hepatoblastomas) have a 3- to 5-year overall survival (OS) rate of 100% with minimal or no adjuvant chemotherapy 24, 22.
- Patients with non–well-differentiated fetal histology, non–small cell undifferentiated hepatoblastomas have a 3- to 4-year overall survival (OS) rate of 90% to 100% with adjuvant chemotherapy 24, 110.
- If any small cell undifferentiated elements are present, the 3-year survival rate is 40% to 70% 24, 28.
Treatment options for hepatoblastoma resectable at diagnosis showing non–well-differentiated fetal histology include the following:
- Resection followed by two to four cycles of chemotherapy 111.
Re-resection of positive microscopic margins may not be necessary. Conclusive evidence is lacking for tumors with resection at diagnosis compared with those with positive microscopic margins resected after preoperative chemotherapy.
Evidence (gross surgical resection, with or without microscopic margins, and postoperative chemotherapy):
- Gross surgical excision with or without microscopic margins has been followed by four courses of combination chemotherapy with cisplatin, vincristine, and fluorouracil or cisplatin and doxorubicin or cisplatin alone 112, 113. Second resection of positive margins and/or radiation therapy may not be necessary in patients with incompletely resected hepatoblastoma whose residual tumor is microscopic and who receive subsequent chemotherapy 109, 92.
- In the Children’s Oncology Group trial, 49 of 51 patients with stage 1 or stage 2 hepatoblastoma (without pure fetal histology) received two cycles of adjuvant chemotherapy consisting of cisplatin, fluorouracil, and vincristine 114.
- The 5-year event-free survival (EFS) rate was 88%, and the 5-year overall survival (OS) rate was 91%.
- This outcome is comparable to the outcomes for children treated with four cycles after initial resection, and to the outcomes for children treated with two cycles of neoadjuvant chemotherapy before resection followed by two cycles of chemotherapy after resection.
- There is no reliable data for local recurrence risk in patients with a positive microscopic margin status who underwent resection at diagnosis 115. SIOPEL studies suggest that in patients who received preoperative chemotherapy, positive microscopic margin did not increase risk of local recurrence 108.
- In a European study conducted between 1990 and 1994, 11 patients had tumor found at the surgical margins after hepatic resection and two patients died, neither of whom had a local recurrence. None of the 11 patients underwent a second resection, and only one patient received radiation therapy postoperatively. All of the patients were treated with four courses of cisplatin and doxorubicin before surgery and received two courses of postoperative chemotherapy 92.
- In another European study of high-risk hepatoblastoma, 11 patients had microscopic residual tumor remaining after initial surgery and received two to four postoperative cycles of chemotherapy with no additional surgery. Of these 11 patients, 9 survived 109.
- In the SIOPEL-2 study, 13 of 13 patients with microscopic positive resection margins survived 112.
- An unplanned retrospective study of the SIOPEL-2 and SIOPEL-3 trials found that after four courses of cisplatin for standard-risk patients and seven courses of cisplatin alternating with doxorubicin/carboplatin for high-risk patients, resection was performed where imaging suggested it would be safe. Of the 431 children treated in these trials, 58 patients had positive microscopic tumor margins, and 371 patients were in complete remission. There were no statistically significant differences in the rates of local recurrence, EFS, or OS between the two groups 108.
- A randomized clinical trial demonstrated comparable efficacy with postoperative cisplatin/vincristine/fluorouracil and cisplatin/doxorubicin in the treatment of patients with hepatoblastoma 19.
- Although survival outcomes were nominally higher for the children who received cisplatin/doxorubicin, this difference was not statistically significant.
- The combination of cisplatin/vincristine/fluorouracil was significantly less toxic than were the doses of cisplatin/doxorubicin.
Results of chemotherapy clinical trials are described in Table 3 above.
Treatment options for hepatoblastoma of well-differentiated fetal (pure fetal) histology resectable at diagnosis include the following:
- Complete surgical resection followed by watchful waiting or chemotherapy 22.
Evidence (complete surgical resection followed by watchful waiting or chemotherapy):
- In a Children’s Cancer Group prospective clinical trial (INT0098), nine children with stage I (completely resected) well-differentiated fetal histology and fewer than two mitoses per high-power field were treated with four cycles of adjuvant doxorubicin 19.
- At a median follow-up of 5.1 years, the event-free survival (EFS) and overall survival (OS) rates were 100% for all nine children.
- In the Children’s Cancer Group P9645 study, 16 patients with stage I (completely resected) tumors had well-differentiated fetal histology and received no adjuvant chemotherapy. In a retrospective PRETEXT classification of 21 of these 25 patients with adequate data, PRETEXT I, II, and III tumors were found in 7, 10, and 4 patients, respectively 22.
- The event-free survival (EFS) and overall survival (OS) rates were 100% for patients with stage 1 well-differentiated fetal histology, including one patient who had a second surgery to address a positive tumor margin.
- Treatment of a small focus of undifferentiated small cell histology within an otherwise well-differentiated fetal histology tumor with aggressive chemotherapy has been reported in the following small series, suggesting the importance of a thorough histological examination of apparent well-differentiated fetal histology 26. A retrospective study of 16 patients with well-differentiated fetal histology treated at multiple institutions had complete surgical resections, but also had elements of (or, in some case, predominance of) small cell histology found in the resected tumor 26.
- Despite receiving postoperative chemotherapy, hepatoblastoma recurred in 10 of 16 patients, and 5 of these patients died.
Treatment options for hepatoblastoma that is not resectable or not resected at diagnosis
Approximately 70% to 80% of children with hepatoblastoma have tumors that are not resected at diagnosis. Children’s Oncology Group (COG) surgical guidelines recommend biopsy without an attempt to resect the tumor at diagnosis in children with PRETEXT II tumors with less than 1 cm radiographic margin on the vena cava and middle hepatic vein and in all children with PRETEXT III and IV tumors.
Tumor rupture at presentation, resulting in major hemorrhage that can be controlled by transcatheter arterial embolization or partial resection to stabilize the patient, does not preclude a favorable outcome when followed by chemotherapy and definitive surgery 116.
Treatment options for hepatoblastoma that is not resectable or is not resected at diagnosis include the following:
- Chemotherapy followed by reassessment of surgical resectability and complete surgical resection.
- Chemotherapy followed by reassessment of surgical resectability and orthotopic liver transplant 117.
- Transarterial chemoembolization (TACE) and transarterial radioembolization (TARE). TACE and TARE may be used to improve resectability before definitive surgical approaches 118, 119.
In recent years, almost all children with hepatoblastoma have been treated with chemotherapy, and in European centers, children with resectable hepatoblastoma are treated with preoperative chemotherapy, which may reduce the incidence of surgical complications at the time of resection 106. Treatment with preoperative chemotherapy has been shown to benefit children with hepatoblastoma. In contrast, an American intergroup study of treatment of children with hepatoblastoma encouraged resection at the time of diagnosis for all tumors amenable to resection without undue risk. The study (COG-P9645) did not treat children with stage I tumors of well-differentiated fetal histology with preoperative or postoperative chemotherapy unless they developed progressive disease 120. In this study, most patients with PRETEXT III and all PRETEXT IV tumors were treated with chemotherapy before resection or transplant.
Patients whose tumors remain unresectable after chemotherapy should be considered for liver transplant 121. In the presence of features predicting unresectability, early coordination with a pediatric liver transplant service is critical 122. In the COG AHEP0731 (NCT00980460) study, early referral (i.e., based on imaging done after the second cycle of chemotherapy) to a liver specialty center with liver transplant capability was recommended for patients with POSTTEXT III tumors with positive V or P and POSTTEXT IV tumors with positive F.
Evidence (chemotherapy followed by reassessment of surgical resectability and complete surgical resection):
- In the SIOPEL-1 study, preoperative chemotherapy (doxorubicin and cisplatin) was given to all children with hepatoblastoma with or without metastases. After chemotherapy, and excluding those who underwent a liver transplant (<5% of patients), complete resection was performed 123.
- The chemotherapy was well tolerated.
- Complete resection was obtained in 87% of children.
- This strategy resulted in an overall survival rate of 75% at 5 years after diagnosis.
- Identical results were seen in a follow-up international study (SIOPEL-2) 106.
- The SIOPEL-3 study compared cisplatin alone with cisplatin and doxorubicin in patients with preoperative standard-risk hepatoblastoma. Standard risk was defined as tumor confined to the liver and not involving more than three sectors 124.
- The rates of resection were similar for the cisplatin (95%) and cisplatin/doxorubicin (93%) groups.
- The overall survival rates were also similar for the cisplatin (95%) and cisplatin/doxorubicin (93%) groups.
- In a pilot study, SIOPEL-3HR, cisplatin alternating with carboplatin/doxorubicin was administered in a dose-intensive fashion to high-risk patients with hepatoblastoma 94.
- In 74 patients with PRETEXT IV tumors, 22 of whom also had metastases, 31 became resectable and 26 underwent transplant. The 3-year overall survival of this group was 69% (± 11%).
- Of the 70 patients with metastases enrolled in the trial, the 3-year event-free survival rate was 56% and the overall survival rate was 62%. Of patients with lung metastases, 50% were able to achieve complete remission of metastases with chemotherapy alone (without lung surgery).
- SIOPEL-4 (NCT00077389) was a multinational feasibility trial of dose-dense cisplatin/doxorubicin chemotherapy and radical surgery for a group of children with high-risk hepatoblastoma. Surgical removal of all remaining tumor lesions after chemotherapy was performed if feasible (including liver transplant and metastasectomy, if needed). Patients whose tumors were resected or whose livers were transplanted after three cycles of chemotherapy subsequently received two postoperative cycles of carboplatin and doxorubicin. Patients whose tumors remained unresectable after three cycles of chemotherapy received two cycles of very intensive carboplatin and doxorubicin before surgery. The primary tumor masses were identified as PRETEXT II (27%), III (44%), and IV (26%) 125.
- Ninety-seven percent of patients (60 of 61) had a partial response with chemotherapy.
- Eighty-five percent of patients (53) underwent complete macroscopic resection; tumor was microscopically present in five patients, all of whom are disease-free survivors.
- Two patients died postoperatively.
- There were 37 partial hepatectomies and 16 liver transplants.
- The study had a total of 62 high-risk patients; 74% of patients (62%–84%) underwent resection. The 3-year disease-free survival (DFS) was 76% and the 3-year overall survival was 83%.
- Of the 16 PRETEXT IV patients, 11 were downstaged after chemotherapy—6 patients to PRETEXT III, 4 patients to PRETEXT II, and 1 patient to PRETEXT I. Twelve tumors became resectable; of these, four patients underwent a partial hepatectomy and eight patients underwent a liver transplant. For patients who presented with PRETEXT IV disease, the 3-year disease-free survival (DFS) was 73% and the 3-year overall survival was 80%.
- In approximately 75% of children and adolescents with initially unresectable hepatoblastoma, tumors can be rendered resectable with cisplatin-based preoperative chemotherapy, and 60% to 65% will survive disease-free 126.
- A combination of ifosfamide, cisplatin, and doxorubicin followed by postinduction resection has also been used in the treatment of advanced-stage disease 127.
In the United States, unresectable tumors have been treated with chemotherapy before resection or transplant 120. On the basis of radiographic imaging, most stage III and IV hepatoblastomas are rendered resectable after two cycles of chemotherapy 128. Some European centers have also used extended resection of selected POSTTEXT III and IV tumors rather than liver transplant 129.
Chemotherapy followed by transarterial chemoembolization (TACE) followed by high-intensity focused ultrasound showed promising results in China for PRETEXT III and IV patients with hepatoblastoma, some of whom were resectable but did not undergo surgical resection because of parent refusal 130.
Treatment options for hepatoblastoma with metastases at diagnosis
The outcomes of patients with metastatic hepatoblastoma at diagnosis are poor, but long-term survival and cure is possible 131. Survival rates at 3 to 5 years range from 20% to 79% 125. To date, the best outcomes for children with metastatic hepatoblastoma resulted from treatment with dose-dense cisplatin and doxorubicin, although significant toxicity was also noted (SIOPEL-4 [NCT00077389] trial) 125.
Treatment options for hepatoblastoma with metastases at diagnosis include the following:
- Chemotherapy followed by reassessment of surgical resectability.
- If the primary tumor and extrahepatic disease (usually pulmonary nodules) are resectable after chemotherapy, surgical resection followed by additional chemotherapy.
- If extrahepatic metastatic disease is in complete remission after chemotherapy and/or surgical resection of lung nodule but the primary tumor remains unresectable, orthotopic liver transplant.
- If extrahepatic metastatic disease is not resectable or the patient is not a transplant candidate, additional chemotherapy, transarterial chemoembolization (TACE), or radiation therapy.
The standard combination chemotherapy regimen in North America is four courses of cisplatin/vincristine/fluorouracil 131 or doxorubicin/cisplatin 120 followed by attempted complete tumor resection. If the tumor is completely removed, two postoperative courses of the same chemotherapy are usually given. Study results for different chemotherapy regimens have been reported.
High-dose chemotherapy with stem cell rescue does not appear to be more effective than standard multiagent chemotherapy 132.
Evidence (chemotherapy to treat metastatic disease at diagnosis):
- A subset of 39 patients presenting with metastases were entered on the SIOPEL-4 (NCT00077389) trial, a multinational feasibility trial of dose-dense cisplatin/doxorubicin chemotherapy and radical surgery for a group of children with high-risk hepatoblastoma. Patients whose tumors were resected or livers transplanted after three cycles of chemotherapy subsequently received two postoperative cycles of carboplatin and doxorubicin. Patients whose tumors were unresectable after three cycles of chemotherapy received two additional cycles of very intensive carboplatin and doxorubicin before surgery 125.
- After three cycles of chemotherapy, there was a complete response (only in the metastases) in 20 of 39 patients and a partial response in 18 of 39 patients. Nineteen of the patients who achieved a complete response were alive without disease 3 years after diagnosis.
- Of the patients who achieved a partial response, seven patients underwent metastasectomy near the time of resection or liver transplant, with an OS of 100%. An additional seven patients with residual small pulmonary nodules underwent resection without metastasectomy; of those, six patients did well and one patient recurred.
- Two patients with initial metastases eventually recurred.
- Liver transplant, rather than resection alone, was needed to treat 7 of the 39 patients who presented with metastases.
- For the subset of 39 patients presenting with metastases, the 3-year disease-free survival was 77% and the overall survival was 79%.
In patients with resected primary tumors, any remaining pulmonary metastasis is surgically removed, if possible 133. A review of patients treated on a U.S. intergroup trial suggested that resection of metastasis may be done at the time of resection of the primary tumor 134.
If extrahepatic disease is in complete remission after chemotherapy, and the primary tumor remains unresectable, an orthotopic liver transplant may be performed 125.
The outcome results are discrepant for patients with lung metastases at diagnosis who undergo orthotopic liver transplant after complete resolution of lung disease in response to pretransplant chemotherapy. Some studies have reported favorable outcomes for these groups 125, while others have noted high rates of hepatoblastoma recurrence 135. All of these studies are limited by small patient numbers; additional study is needed to better define outcomes for this subset of patients. Recent clinical trials have resulted in few pulmonary recurrences in children who underwent liver transplants and presented with metastatic disease 125.
If extrahepatic disease is not resectable after chemotherapy or the patient is not a transplant candidate, alternative treatment approaches include the following:
- Nonstandard chemotherapy agents. Nonstandard chemotherapy agents such as irinotecan, high-dose cisplatin/etoposide, or continuous-infusion doxorubicin have been used 136.
- Hepatic arterial chemoembolization (HACE) or transarterial chemoembolization (TACE) 137.
- Radiation therapy 138.
Treatment options for progressive or recurrent hepatoblastoma
The prognosis for a patient with progressive or recurrent hepatoblastoma depends on several factors, including the following 139:
- Site of recurrence.
- Previous treatment.
- Individual patient considerations.
Treatment options for progressive or recurrent hepatoblastoma include the following:
- Surgical resection. In patients with hepatoblastoma that is completely resected at initial diagnosis, aggressive surgical treatment of isolated pulmonary metastases that develop in the course of the disease, in conjunction with an overall strategy that includes appropriate chemotherapy, may make extended disease-free survival possible 140. If possible, isolated metastases are resected completely in patients whose primary tumor is controlled.[108] A retrospective study of patients in SIOPEL studies 1, 2, and 3 showed a 12% incidence of recurrence after complete remission by imaging and AFP. Outcome after recurrence was best if the tumor was amenable to surgery. Of patients who underwent chemotherapy and surgery, the 3-year Event-Free Survival was 34%, and the Overall Survival was 43% 139. Percutaneous radiofrequency ablation has been used as an alternative to surgical resection of oligometastatic hepatoblastoma 141. Enrollment in a clinical trial should be considered if all of the recurrent disease cannot be surgically removed. Phase I and phase II clinical trials may be appropriate and should be considered.
- Chemotherapy. Analysis of survival after recurrence demonstrated that some patients treated with cisplatin/vincristine/fluorouracil could be salvaged with doxorubicin-containing regimens, but patients treated with doxorubicin/cisplatin could not be salvaged with vincristine/fluorouracil 142. Addition of doxorubicin to vincristine/fluorouracil/cisplatin is under clinical evaluation in the COG study AHEP0731 [NCT00980460]. Combined vincristine/irinotecan and single-agent irinotecan have been used with some success 136. A review of Children’s Oncology Group (COG) phase I and II studies found no promising agents for relapsed hepatoblastoma 143.
- Liver transplant. Liver transplant should be considered for patients with nonmetastatic disease recurrence in the liver that is not amenable to resection 135.
- Percutaneous ablation. Percutaneous ablation techniques may also be considered for palliation 144 or, in some cases, for curative therapy of oligometastatic disease 141.
Hepatoblastoma prognosis
The prognosis (chance of recovery) for hepatoblastoma is based on numerous factors including age of diagnosis, PRETEXT group, metastases, alfa fetal protein (AFP) levels, histologic subtype, completeness of resection, and clinical stage of the disease. With certainty, the well-differentiated fetal subtype is associated with a better prognosis compared with small cell undifferentiated and macrotrabecular, which are linked to unfavorable prognosis 84. The 5-year overall survival (OS) rate for children with hepatoblastoma is 70% 145, 146. Neonates with hepatoblastoma have outcomes comparable to older children up to age 5 years 147.
Alfa-fetoprotein is typically high upon diagnosis, but a significant drop after neo-adjuvant chemotherapy portends a better response to treatment. Younger age of diagnosis has historically been a poor prognostic factor; however, recent studies have called this into question, providing evidence that these younger patients do just as well as older children 148. Specifically, children younger than 1 year of age have a better prognosis and children greater than 6 years of age have a worse prognosis 45. Tumor presence at the resection margin, multifocality, and metastases have been shown to be poor prognostic factors. Beta-catenin expression has been shown to be associated with a lower period of event-free survival, while EpCAM expression has been associated with higher tumor viability and a poorer response to neo-adjuvant chemotherapy 149.
A large multicenter Children’s Oncology Group study included 182 children with hepatoblastoma diagnosed between August 1989 and December 1992 19. Of these, 9 had stage 1 disease with favorable histology, 43 had stage 1 with unfavorable histology, 7 had stage 2, 83 had stage 3, and 40 had stage 4 disease (see hepatoblastoma staging).
All 9 patients with stage 1 and favorable histology received treatment with low-dose doxorubicin and were alive and free of disease. Overall survival rates for all stages 5 years after treatment were 57-69%. The 5-year event-free survival rates were 91% for patients with stage 1 with unfavorable histology, 100% for stage 2, 64% for stage 3, and 25% for stage 4 19.
In general, patients who undergo complete resection of the tumor and adjuvant chemotherapy have a survival rate of 100%. Patients with favorable histology and low mitotic rate with complete resection may not require chemotherapy; for this reason, US protocols have advocated for formal staging and pathologic diagnosis prior to administering any adjuvant chemotherapy.
More recently, data from the International Childhood Liver Tumour Strategy Group (SIOPEL), which used preoperative adjuvant chemotherapy, demonstrated overall survival rates as high as 89% and event-free survival rates as high as 80% 82. Australian investigators demonstrated that patients treated between 1984 and 2004 also had excellent outcomes, provided surgical margins were clear 150. No correlation between alpha-fetoprotein (AFP) levels and outcome was reported in the study.
Other morbidity can result from precancerous medical conditions, operative complications, or toxic effects of chemotherapy.
Hepatoblastoma survival rate
The 5-year overall survival (OS) rate for children with hepatoblastoma is 70% 145, 146. Neonates with hepatoblastoma have outcomes comparable to older children up to age 5 years 147.
A collaborative group consisting of four study groups (International Childhood Liver Tumors Strategy Group [SIOPEL], COG, Gesellschaft für Pädiatrische Onkologie und Hämatologie, and Japanese Study Group for Pediatric Liver Tumor [JPLT]) was named the Children’s Hepatic Tumor International Collaboration (CHIC). The CHIC group analyzed survival in a collaborative database of 1,605 patients with hepatoblastoma treated in eight separate multicenter clinical trials, with central review of all tumor imaging and histological details 111. Patients who underwent orthotopic liver transplant are included in all of the international study results 151.
Survival rates at 5 years, unrelated to PRETEXT/POST-TEXT annotation factors, were found to be the following 81:
- 90% for patients with PRETEXT I group tumors.
- 83% for patients with PRETEXT II group tumors.
- 73% for patients with PRETEXT III group tumors.
- 52% for patients with PRETEXT IV group tumors.
When each annotation factor was examined separately, regardless of the PRETEXT group or other annotation factors present in each patient, the 5-year overall survival (OS) rates were found to be the following 81:
- 51% for patients with positive V (involvement of all three hepatic veins and/or inferior vena cava).
- 49% for patients with positive P (involvement of both right and left portal veins).
- 53% for patients with positive E (contiguous extrahepatic tumor).
- 52% for patients with positive F (multifocal).
- 51% for patients with positive R (tumor rupture).
- 41% for patients with positive M (distant metastasis).
- Maternal and infant birth characteristics and hepatoblastoma. McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Am J Epidemiol. 2006 May 1; 163(9):818-28.
- Liu L, Wang J, Sun G, Wu Q, Ma J, Zhang X, Huang N, Bian Z, Gu S, Xu M, Yin M, Sun F, Pan Q. m6A mRNA methylation regulates CTNNB1 to promote the proliferation of hepatoblastoma. Mol Cancer. 2019 Dec 23;18(1):188. doi: 10.1186/s12943-019-1119-7. Erratum in: Mol Cancer. 2020 Feb 4;19(1):24.
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34:192–200. doi: 10.1053/j.semdp.2016.12.015
- Pediatric Hepatoblastoma. https://emedicine.medscape.com/article/986802-overview#a2
- Bell D, Ranganathan S, Tao J, Monga SP. Novel Advances in Understanding of Molecular Pathogenesis of Hepatoblastoma: A Wnt/β-Catenin Perspective. Gene Expr. 2017;17(2):141–154. doi:10.3727/105221616X693639 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5311458
- Pritchard J , Brown J , Shafford E . et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach—results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–28.
- Epidemiology of primary hepatic malignancies in U.S. children. Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Hepatology. 2003 Sep; 38(3):560-6.
- Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M, Yokoyama M, Kaneko M. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998 Jul 15;58(14):3032-5.
- Towbin AJ, Braojos Braga FDC, Zhang B, Geller JI, Tiao GM, Podberesky DJ. Fractures in children with newly diagnosed hepatoblastoma. Pediatr Radiol. 2018;48(4):581–585. doi: 10.1007/s00247-017-4050-3
- Zhi T, Zhang WL, Zhang Y, Hu HM, Huang DS. Clinical characteristics and prognostic factors of hepatoblastoma in 316 children aged under 3 years—a 14-year retrospective single-center study. BMC Pediatr. 2021;21(1):1–11. doi: 10.1186/s12887-021-02630-2
- Hu HM, Zhang WL, Wang YZ, Zhang Y, You Y, Fan L, et al. Treatment outcomes for hepatoblastoma children with pulmonary metastasis and extrapulmonary involvement: Experience of 36 cases at a single institution. Transl Cancer Res. 2020;9(10):6402–6411. doi: 10.21037/tcr-20-1876
- Zhang Y, Zhang W, Tang S, Chen L, Yi Y, Zhang P, et al. A single-center retrospective study of pediatric hepatoblastoma. Oncol Lett. 2016;12(5):3919–3925. doi: 10.3892/ol.2016.5195
- Barragan V, Escudero MC, Jimenez IC, Correa C, Luengas JP. Bone metastases in hepatoblastoma, an unusual presentation. Case report and review of the literature. Radiol Case Rep. 2022 Sep 13;17(11):4272-4275. doi: 10.1016/j.radcr.2022.08.025
- Czauderna P, Lopez-Terrada D, Hiyama E, Häberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014 Feb;26(1):19-28. doi: 10.1097/MOP.0000000000000046
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79–95. doi: 10.1177/1093526619875228
- Begemann M, Trippett TM, Lis E, Antunes NL. Brain metastases in hepatoblastoma. Pediatr Neurol. 2004;30(4):295–297. doi: 10.1016/j.pediatrneurol.2003.10.014
- Yevich S, Calandri M, Gravel G, Fresneau B, Brugières L, Valteau-Couanet D, et al. Reiterative radiofrequency ablation in the management of pediatric patients with hepatoblastoma metastases to the lung, liver, or bone. Cardiovasc Intervent Radiol. 2019;42(1):41–47. doi: 10.1007/s00270-018-2097-7
- PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Childhood Liver Cancer Treatment: Patient Version. 2022 May 18. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65879
- Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, Haas JE, King DR, Liu-Mares W, Sensel MG, Krailo MD. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000 Jul;18(14):2665-75. doi: 10.1200/JCO.2000.18.14.2665
- Haas JE, Muczynski KA, Krailo M, Ablin A, Land V, Vietti TJ, Hammond GD. Histopathology and prognosis in childhood hepatoblastoma and hepatocarcinoma. Cancer. 1989 Sep 1;64(5):1082-95. doi: 10.1002/1097-0142(19890901)64:5<1082::aid-cncr2820640520>3.0.co;2-g
- Combination Chemotherapy With or Without Amifostine in Treating Young Patients With Liver Cancer. https://clinicaltrials.gov/ct2/show/NCT00003994
- Malogolowkin MH, Katzenstein HM, Meyers RL, Krailo MD, Rowland JM, Haas J, Finegold MJ. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children’s Oncology Group. J Clin Oncol. 2011 Aug 20;29(24):3301-6. doi: 10.1200/JCO.2010.29.3837
- Rowland JM. Hepatoblastoma: assessment of criteria for histologic classification. Med Pediatr Oncol. 2002 Nov;39(5):478-83. doi: 10.1002/mpo.10171
- Meyers RL, Rowland JR, Krailo M, Chen Z, Katzenstein HM, Malogolowkin MH. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009 Dec;53(6):1016-22. doi: 10.1002/pbc.22088
- Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, Gore L, Feusner JH. Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer. 2009 Mar;52(3):328-34. doi: 10.1002/pbc.21834
- Haas JE, Feusner JH, Finegold MJ. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer. 2001 Dec 15;92(12):3130-4. doi: 10.1002/1097-0142(20011215)92:12<3130::aid-cncr10115>3.0.co;2-#
- Risk-Based Therapy in Treating Younger Patients With Newly Diagnosed Liver Cancer. https://clinicaltrials.gov/ct2/show/NCT00980460
- De Ioris M, Brugieres L, Zimmermann A, Keeling J, Brock P, Maibach R, Pritchard J, Shafford L, Zsiros J, Czaudzerna P, Perilongo G. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer. 2008 Mar;44(4):545-50. doi: 10.1016/j.ejca.2007.11.022
- Musick SR, Babiker HM. Cancer, Hepatoblastoma. [Updated 2018 Dec 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534795
- Angelico R, Grimaldi C, Gazia C, Saffioti M, Manzia T, Castellano A, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel) 2019;11(11) doi: 10.3390/cancers11111693
- Lake CM, Tiao GM, Bondoc AJ. Surgical management of locally-advanced and metastatic hepatoblastoma. Semin Pediatr Surg. 2019;28(6) doi: 10.1016/j.sempedsurg.2019.150856
- Wanaguru D, Shun A, Price N, Karpelowsky J. Outcomes of pulmonary metastases in hepatoblastoma—is the prognosis always poor? J Pediatr Surg. 2013;48(12):2474–2478. doi: 10.1016/j.jpedsurg.2013.08.023
- Matsunaga T, Sasaki F, Ohira M, Kohei H, Hayashi A, Mugishima H, et al. Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int. 2003;19(3):142–146. doi: 10.1007/s00383-002-0906-0
- Zsiros J, Brugieres L, Brock P, Roebuck D, Maibach R, Zimmermann A, Childs M, Pariente D, Laithier V, Otte JB, Branchereau S, Aronson D, Rangaswami A, Ronghe M, Casanova M, Sullivan M, Morland B, Czauderna P, Perilongo G; International Childhood Liver Tumours Strategy Group (SIOPEL). Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol. 2013 Aug;14(9):834-42. doi: 10.1016/S1470-2045(13)70272-9
- Kitagawa N, Shinkai M, Mochizuki K, Usuj H, Miyagi H, Nakamura K, et al. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr Surg Int. 2015;31(4):407–411. doi: 10.1007/s00383-015-3679-y
- López-Terrada, D., Alaggio, R., de Dávila, M. et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 27, 472–491 (2014). https://doi.org/10.1038/modpathol.2013.80
- Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F, Mackinlay G, Maibach R, McHugh K, Olsen OE, Otte JB, Pariente D, Plaschkes J, Childs M, Perilongo G; International Childrhood Liver Tumor Strategy Group. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007 Feb;37(2):123-32; quiz 249-50. doi: 10.1007/s00247-006-0361-5
- Rodriguez-Galindo C, Pappo AS. Tumors of the Liver. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13085
- Increased risk of hepatoblastoma among immature children with a lower birth weight. Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M, Yokoyama M, Kaneko M. Cancer Res. 1998 Jul 15; 58(14):3032-5.
- Garber JE , Li FP , Kingston JE . et al. Hepatoblastoma and familial adenomatous polyposis. J Natl Cancer Inst. 1998;80:1626–8.
- Debaun MR , Tucker MA . Risk of cancer during the first four years of life in children from the Beckwith-Wiedemann syndrome registry. J Pediatr. 1998;132(3 Pt 1):398–400. https://www.ncbi.nlm.nih.gov/pubmed/9544889
- Finegold MJ, Lopez-Terrada DH, Bowen J, Washington MK, Qualman SJ., College of American Pathologists. Protocol for the examination of specimens from pediatric patients with hepatoblastoma. Arch. Pathol. Lab. Med. 2007 Apr;131(4):520-9.
- Uçar, C., Çalışkan, Ü., Toy, H. and Günel, E. (2007), Hepatoblastoma in a child with neurofibromatosis type I. Pediatr. Blood Cancer, 49: 357-359. https://doi.org/10.1002/pbc.20663
- Heck JE, Meyers TJ, Lombardi C, Park AS, Cockburn M, Reynolds P, Ritz B. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013 Aug;37(4):390-5
- Czauderna P, Lopez-Terrada D, Hiyama E, Häberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr. Opin. Pediatr. 2014 Feb;26(1):19-28.
- Curia MC, Zuckermann M, De Lellis L, Catalano T, Lattanzio R, Aceto G, Veschi S, Cama A, Otte JB, Piantelli M, Mariani-Costantini R, Cetta F, Battista P. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod. Pathol. 2008 Jan;21(1):7-14.
- Ng K, Rana A, Masand P, Patel K, Heczey A, Goss J, Himes R. Fatal Central Nervous System Post-Transplant Lymphoproliferative Disease in a Patient Who Underwent Liver Transplantation for Hepatoblastoma. J. Pediatr. Gastroenterol. Nutr. 2018 Jan;66(1):e21-e23
- Kalish JM, Doros L, Helman LJ, et al.: Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin Cancer Res 23 (13): e115-e122, 2017
- Kalish JM, Doros L, Helman LJ, Hennekam RC, Kuiper RP, Maas SM, Maher ER, Nichols KE, Plon SE, Porter CC, Rednam S, Schultz KAP, States LJ, Tomlinson GE, Zelley K, Druley TE. Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin Cancer Res. 2017 Jul 1;23(13):e115-e122. doi: 10.1158/1078-0432.CCR-17-0710
- Kamien BA, Gabbett MT: Aicardi syndrome associated with hepatoblastoma and pulmonary sequestration. Am J Med Genet A 149A (8): 1850-2, 2009
- Weksberg R, Shuman C, Smith AC: Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 137C (1): 12-23, 2005.
- Giardiello FM, Petersen GM, Brensinger JD, et al.: Hepatoblastoma and APC gene mutation in familial adenomatous polyposis. Gut 39 (96): 867-9, 1996.
- Ito E, Sato Y, Kawauchi K, et al.: Type 1a glycogen storage disease with hepatoblastoma in siblings. Cancer 59 (10): 1776-80, 1987
- Turcotte LM, Georgieff MK, Ross JA, et al.: Neonatal medical exposures and characteristics of low birth weight hepatoblastoma cases: a report from the Children’s Oncology Group. Pediatr Blood Cancer 61 (11): 2018-23, 2014.
- Buonuomo PS, Ruggiero A, Vasta I, et al.: Second case of hepatoblastoma in a young patient with Simpson-Golabi-Behmel syndrome. Pediatr Hematol Oncol 22 (7): 623-8, 2005 Oct-Nov.
- Tan ZH, Lai A, Chen CK, et al.: Association of trisomy 18 with hepatoblastoma and its implications. Eur J Pediatr 173 (12): 1595-8, 2014
- PDQ Pediatric Treatment Editorial Board. Childhood Liver Cancer Treatment (PDQ®): Health Professional Version. 2019 Jun 13. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65790
- Trobaugh-Lotrario AD, Venkatramani R, Feusner JH: Hepatoblastoma in children with Beckwith-Wiedemann syndrome: does it warrant different treatment? J Pediatr Hematol Oncol 36 (5): 369-73, 2014
- Clericuzio CL, Martin RA: Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med 11 (3): 220-2, 2009.
- Weksberg R, Shuman C, Smith AC: Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 137C (1): 12-23, 2005
- Algar EM, St Heaps L, Darmanian A, et al.: Paternally inherited submicroscopic duplication at 11p15.5 implicates insulin-like growth factor II in overgrowth and Wilms’ tumorigenesis. Cancer Res 67 (5): 2360-5, 2007
- Albrecht S, Hartmann W, Houshdaran F, et al.: Allelic loss but absence of mutations in the polyspecific transporter gene BWR1A on 11p15.5 in hepatoblastoma. Int J Cancer 111 (4): 627-32, 2004
- Clericuzio CL, Martin RA: Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med 11 (3): 220-2, 2009
- Clericuzio CL, Chen E, McNeil DE, et al.: Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J Pediatr 143 (2): 270-2, 2003
- Mussa A, Molinatto C, Baldassarre G, et al.: Cancer Risk in Beckwith-Wiedemann Syndrome: A Systematic Review and Meta-Analysis Outlining a Novel (Epi)Genotype Specific Histotype Targeted Screening Protocol. J Pediatr 176: 142-149.e1, 2016
- Aretz S, Koch A, Uhlhaas S, et al.: Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr Blood Cancer 47 (6): 811-8, 2006
- Hirschman BA, Pollock BH, Tomlinson GE: The spectrum of APC mutations in children with hepatoblastoma from familial adenomatous polyposis kindreds. J Pediatr 147 (2): 263-6, 2005
- Koch A, Denkhaus D, Albrecht S, et al.: Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res 59 (2): 269-73, 1999.
- Hiyama E. Pediatric hepatoblastoma: diagnosis and treatment. Transl Pediatr. 2014 Oct;3(4):293-9.
- Zhong S, Zhao Y, Fan C. Hepatoblastoma with pure fetal epithelial differentiation in a 10-year-old boy: A rare case report and review of the literature. Medicine (Baltimore). 2018 Jan;97(2):e9647
- Pediatric Hepatoblastoma. https://emedicine.medscape.com/article/986802-overview#a3
- van As JW, van den Berg H, van Dalen EC. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst Rev. 2012 May 16;(5):CD009219. doi: 10.1002/14651858.CD009219.pub2. Update in: Cochrane Database Syst Rev. 2014;(7):CD009219
- Childhood Liver Cancer Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/liver/hp/child-liver-treatment-pdq
- Yoon JM, Burns RC, Malogolowkin MH, Mascarenhas L. Treatment of infantile choriocarcinoma of the liver. Pediatr Blood Cancer. 2007 Jul;49(1):99-102. doi: 10.1002/pbc.20623
- Boman F, Bossard C, Fabre M, et al.: Mesenchymal hamartomas of the liver may be associated with increased serum alpha foetoprotein concentrations and mimic hepatoblastomas. Eur J Pediatr Surg 14 (1): 63-6, 2004
- Blohm ME, Vesterling-Hörner D, Calaminus G, et al.: Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol 15 (2): 135-42, 1998 Mar-Apr.
- Chen CY, Wu K, Lin WH, Lan TY, Wang SY, Sun JS, et al. High false negative rate of Tc-99m MDP whole-body bone scintigraphy in detecting skeletal metastases for patients with hepatoma. J Formos Med Assoc. 2012;111(3):140–146. doi: 10.1016/j.jfma.2011.01.005
- O’Sullivan GJ. Imaging of bone metastasis: an update. World J Radiol. 2015;7(8):202. doi: 10.4329/wjr.v7.i8.202
- Cistaro A, Treglia G, Pagano M, Fania P, Bova V, Basso ME, et al. A comparison between 18F-FDG PET/CT imaging and biological and radiological findings in restaging of hepatoblastoma patients. Biomed Res Int. 2013;2013 doi: 10.1155/2013/709037
- Couinaud C (1994) The paracaval segments of the liver. J Hepatobiliary Pancreat Surg 2:145–151
- PDQ Pediatric Treatment Editorial Board. Childhood Liver Cancer Treatment (PDQ®): Health Professional Version. 2022 Sep 9. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65790
- Aronson DC, Schnater JM, Staalman CR, Weverling GJ, Plaschkes J, Perilongo G, Brown J, Phillips A, Otte JB, Czauderna P, MacKinlay G, Vos A. Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol. 2005 Feb 20;23(6):1245-52. doi: 10.1200/JCO.2005.07.145
- Towbin, A.J., Meyers, R.L., Woodley, H. et al. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol 48, 536–554 (2018). https://doi.org/10.1007/s00247-018-4078-z
- Meyers RL, Maibach R, Hiyama E, Häberle B, Krailo M, Rangaswami A, Aronson DC, Malogolowkin MH, Perilongo G, von Schweinitz D, Ansari M, Lopez-Terrada D, Tanaka Y, Alaggio R, Leuschner I, Hishiki T, Schmid I, Watanabe K, Yoshimura K, Feng Y, Rinaldi E, Saraceno D, Derosa M, Czauderna P. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017 Jan;18(1):122-131
- Czauderna P, Haeberle B, Hiyama E, et al. The Children’s Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer. 2016;52:92–101. doi:10.1016/j.ejca.2015.09.023 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5141607
- Aronson DC, Schnater JM, Staalman CR, et al.: Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. J Clin Oncol 23 (6): 1245-52, 2005.
- Meyers RL, Maibach R, Hiyama E, et al.: Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol 18 (1): 122-131, 2017
- Czauderna P, Haeberle B, Hiyama E, et al.: The Children’s Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer 52: 92-101, 2016
- Dall’Igna P, Brugieres L, Christin AS, et al.: Hepatoblastoma in children aged less than six months at diagnosis: A report from the SIOPEL group. Pediatr Blood Cancer 65 (1): , 2018
- Becker K, Furch C, Schmid I, et al.: Impact of postoperative complications on overall survival of patients with hepatoblastoma. Pediatr Blood Cancer 62 (1): 24-8, 2015.
- McAteer JP, Goldin AB, Healey PJ, Gow KW. Surgical treatment of primary liver tumors in children: outcomes analysis of resection and transplantation in the SEER database. Pediatr Transplant. 2013 Dec;17(8):744-50. doi: 10.1111/petr.12144
- Schnater, J.M., Aronson, D.C., Plaschkes, J., Perilongo, G., Brown, J., Otte, J.-B., Brugieres, L., Czauderna, P., MacKinlay, G. and Vos, A. (2002), Surgical view of the treatment of patients with hepatoblastoma. Cancer, 94: 1111-1120. https://doi.org/10.1002/cncr.10282
- Van Tornout JM, Buckley JD, Quinn JJ, et al.: Timing and magnitude of decline in alpha-fetoprotein levels in treated children with unresectable or metastatic hepatoblastoma are predictors of outcome: a report from the Children’s Cancer Group. J Clin Oncol 15 (3): 1190-7, 1997
- Zsíros J, Maibach R, Shafford E, et al.: Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 28 (15): 2584-90, 2010
- Schneider DT, Calaminus G, Göbel U: Diagnostic value of alpha 1-fetoprotein and beta-human chorionic gonadotropin in infancy and childhood. Pediatr Hematol Oncol 18 (1): 11-26, 2001 Jan-Feb.
- López-Terrada D, Alaggio R, de Dávila MT, Czauderna P, Hiyama E, Katzenstein H, Leuschner I, Malogolowkin M, Meyers R, Ranganathan S, Tanaka Y, Tomlinson G, Fabrè M, Zimmermann A, Finegold MJ; Children’s Oncology Group Liver Tumor Committee. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014 Mar;27(3):472-91. doi: 10.1038/modpathol.2013.80
- Zhou S, Gomulia E, Mascarenhas L, Wang L. Is INI1-retained small cell undifferentiated histology in hepatoblastoma unfavorable? Hum Pathol. 2015 Apr;46(4):620-4. doi: 10.1016/j.humpath.2014.12.013
- Vokuhl C, Oyen F, Häberle B, von Schweinitz D, Schneppenheim R, Leuschner I. Small cell undifferentiated (SCUD) hepatoblastomas: All malignant rhabdoid tumors? Genes Chromosomes Cancer. 2016 Dec;55(12):925-931. doi: 10.1002/gcc.22390
- Cornet M, De Lambert G, Pariente D, Planchon JM, Guettier C, Martelli H, Guérin F, Branchereau S. Rhabdoid tumor of the liver: Report of 6 pediatric cases treated at a single institute. J Pediatr Surg. 2018 Mar;53(3):567-571. doi: 10.1016/j.jpedsurg.2017.09.005
- Brown J, Perilongo G, Shafford E, et al.: Pretreatment prognostic factors for children with hepatoblastoma– results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 36 (11): 1418-25, 2000
- Czauderna P, Lopez-Terrada D, Hiyama E, et al.: Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 26 (1): 19-28, 2014
- Xing H, Yang C, Tan B, Zhang M. Competitive risk analysis of the therapeutic value of liver transplantation for liver cancer in children: A population-based study. Front Surg. 2022 Aug 31;9:938254. doi: 10.3389/fsurg.2022.938254
- Meyers RL, Tiao G, de Ville de Goyet J, Superina R, Aronson DC. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr. Opin. Pediatr. 2014 Feb;26(1):29-36
- Uchida H, Sakamoto S, Sasaki K, Takeda M, Hirata Y, Fukuda A, Hishiki T, Irie R, Nakazawa A, Miyazaki O, Nosaka S, Kasahara M. Surgical treatment strategy for advanced hepatoblastoma: Resection versus transplantation. Pediatr Blood Cancer. 2018 Dec;65(12):e27383
- Hiyama E. Pediatric hepatoblastoma: diagnosis and treatment. Transl Pediatr. 2014 Oct;3(4):293-9
- Perilongo G, Shafford E, Maibach R, et al.: Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology–SIOPEL 2. Eur J Cancer 40 (3): 411-21, 2004
- Meyers RL, Tiao G, de Ville de Goyet J, et al.: Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 26 (1): 29-36, 2014
- Aronson DC, Weeda VB, Maibach R, Czauderna P, Dall’Igna P, de Ville de Goyet J, Branchereau S, Perilongo G, Brock P, Zsiros J, Semeraro M, Chardot C, Wildhaber B, Morland B, Brugières L; Childhood Liver Tumour Strategy Group (SIOPEL). Microscopically positive resection margin after hepatoblastoma resection: what is the impact on prognosis? A Childhood Liver Tumours Strategy Group (SIOPEL) report. Eur J Cancer. 2019 Jan;106:126-132. doi: 10.1016/j.ejca.2018.10.013
- Zsíros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A, Laithier V, Otte JB, de Camargo B, MacKinlay G, Scopinaro M, Aronson D, Plaschkes J, Perilongo G. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010 May 20;28(15):2584-90. doi: 10.1200/JCO.2009.22.4857
- Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, de Camargo B, Zsiros J, Roebuck D, Zimmermann A, Aronson D, Childs M, Widing E, Laithier V, Plaschkes J, Pritchard J, Scopinaro M, MacKinlay G, Czauderna P. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009 Oct 22;361(17):1662-70. doi: 10.1056/NEJMoa0810613
- Czauderna P, Haeberle B, Hiyama E, Rangaswami A, Krailo M, Maibach R, Rinaldi E, Feng Y, Aronson D, Malogolowkin M, Yoshimura K, Leuschner I, Lopez-Terrada D, Hishiki T, Perilongo G, von Schweinitz D, Schmid I, Watanabe K, Derosa M, Meyers R. The Children’s Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer. 2016 Jan;52:92-101. doi: 10.1016/j.ejca.2015.09.023
- Perilongo G, Shafford E, Maibach R, Aronson D, Brugières L, Brock P, Childs M, Czauderna P, MacKinlay G, Otte JB, Pritchard J, Rondelli R, Scopinaro M, Staalman C, Plaschkes J; International Society of Paediatric Oncology-SIOPEL 2. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology–SIOPEL 2. Eur J Cancer. 2004 Feb;40(3):411-21. doi: 10.1016/j.ejca.2003.06.003
- Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks-Mireaux C, Keeling J, Phillips A, Vos A, Plaschkes J. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach–results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000 Nov 15;18(22):3819-28. doi: 10.1200/JCO.2000.18.22.3819
- Katzenstein HM, Langham MR, Malogolowkin MH, Krailo MD, Towbin AJ, McCarville MB, Finegold MJ, Ranganathan S, Dunn S, McGahren ED, Tiao GM, O’Neill AF, Qayed M, Furman WL, Xia C, Rodriguez-Galindo C, Meyers RL. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children’s Oncology Group, multicentre, phase 3 trial. Lancet Oncol. 2019 May;20(5):719-727. doi: 10.1016/S1470-2045(18)30895-7. Epub 2019 Apr 8. Erratum in: Lancet Oncol. 2019 May;20(5):e243.
- Meyers RL, Czauderna P, Otte JB. Surgical treatment of hepatoblastoma. Pediatr Blood Cancer. 2012 Nov;59(5):800-8. doi: 10.1002/pbc.24220
- Madanur MA, Battula N, Davenport M, et al.: Staged resection for a ruptured hepatoblastoma: a 6-year follow-up. Pediatr Surg Int 23 (6): 609-11, 2007
- Khan AS, Brecklin B, Vachharajani N, et al.: Liver Transplantation for Malignant Primary Pediatric Hepatic Tumors. J Am Coll Surg 225 (1): 103-113, 2017
- Xianliang H, Jianhong L, Xuewu J, Zhongxian C. Cure of hepatoblastoma with transcatheter arterial chemoembolization. J Pediatr Hematol Oncol. 2004 Jan;26(1):60-3. doi: 10.1097/00043426-200401000-00018
- Aguado A, Dunn SP, Averill LW, Chikwava KR, Gresh R, Rabinowitz D, Katzenstein HM. Successful use of transarterial radioembolization with yttrium-90 (TARE-Y90) in two children with hepatoblastoma. Pediatr Blood Cancer. 2020 Sep;67(9):e28421. doi: 10.1002/pbc.28421
- Malogolowkin MH, Katzenstein HM, Meyers RL, et al.: Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children’s Oncology Group. J Clin Oncol 29 (24): 3301-6, 2011
- Pham TA, Gallo AM, Concepcion W, et al.: Effect of Liver Transplant on Long-term Disease-Free Survival in Children With Hepatoblastoma and Hepatocellular Cancer. JAMA Surg 150 (12): 1150-8, 2015
- D’Antiga L, Vallortigara F, Cillo U, et al.: Features predicting unresectability in hepatoblastoma. Cancer 110 (5): 1050-8, 2007
- Pritchard J, Brown J, Shafford E, et al.: Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach–results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 18 (22): 3819-28, 2000
- Perilongo G, Maibach R, Shafford E, et al.: Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 361 (17): 1662-70, 2009
- Zsiros J, Brugieres L, Brock P, et al.: Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 14 (9): 834-42, 2013
- Aronson DC, Meyers RL: Malignant tumors of the liver in children. Semin Pediatr Surg 25 (5): 265-275, 2016
- von Schweinitz D, Hecker H, Harms D, et al.: Complete resection before development of drug resistance is essential for survival from advanced hepatoblastoma–a report from the German Cooperative Pediatric Liver Tumor Study HB-89. J Pediatr Surg 30 (6): 845-52, 1995
- Venkatramani R, Stein JE, Sapra A, et al.: Effect of neoadjuvant chemotherapy on resectability of stage III and IV hepatoblastoma. Br J Surg 102 (1): 108-13, 2015
- Fonseca A, Gupta A, Shaikh F, et al.: Extreme hepatic resections for the treatment of advanced hepatoblastoma: Are planned close margins an acceptable approach? Pediatr Blood Cancer 65 (2): , 2018
- Wang S, Yang C, Zhang J, et al.: First experience of high-intensity focused ultrasound combined with transcatheter arterial embolization as local control for hepatoblastoma. Hepatology 59 (1): 170-7, 2014
- Ortega JA, Douglass EC, Feusner JH, et al.: Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol 18 (14): 2665-75, 2000
- Karski EE, Dvorak CC, Leung W, et al.: Treatment of hepatoblastoma with high-dose chemotherapy and stem cell rescue: the pediatric blood and marrow transplant consortium experience and review of the literature. J Pediatr Hematol Oncol 36 (5): 362-8, 2014
- Perilongo G, Brown J, Shafford E, et al.: Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 89 (8): 1845-53, 2000
- Meyers RL, Katzenstein HM, Krailo M, et al.: Surgical resection of pulmonary metastatic lesions in children with hepatoblastoma. J Pediatr Surg 42 (12): 2050-6, 2007
- Austin MT, Leys CM, Feurer ID, et al.: Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg 41 (1): 182-6, 2006
- Zsíros J, Brugières L, Brock P, et al.: Efficacy of irinotecan single drug treatment in children with refractory or recurrent hepatoblastoma–a phase II trial of the childhood liver tumour strategy group (SIOPEL). Eur J Cancer 48 (18): 3456-64, 2012
- Malogolowkin MH, Stanley P, Steele DA, et al.: Feasibility and toxicity of chemoembolization for children with liver tumors. J Clin Oncol 18 (6): 1279-84, 2000
- Habrand JL, Nehme D, Kalifa C, et al.: Is there a place for radiation therapy in the management of hepatoblastomas and hepatocellular carcinomas in children? Int J Radiat Oncol Biol Phys 23 (3): 525-31, 1992
- Semeraro M, Branchereau S, Maibach R, et al.: Relapses in hepatoblastoma patients: clinical characteristics and outcome–experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur J Cancer 49 (4): 915-22, 2013
- Shi Y, Geller JI, Ma IT, et al.: Relapsed hepatoblastoma confined to the lung is effectively treated with pulmonary metastasectomy. J Pediatr Surg 51 (4): 525-9, 2016
- Yevich S, Calandri M, Gravel G, et al.: Reiterative Radiofrequency Ablation in the Management of Pediatric Patients with Hepatoblastoma Metastases to the Lung, Liver, or Bone. Cardiovasc Intervent Radiol 42 (1): 41-47, 2019
- Malogolowkin MH, Katzenstein HM, Krailo M, et al.: Redefining the role of doxorubicin for the treatment of children with hepatoblastoma. J Clin Oncol 26 (14): 2379-83, 2008
- Trobaugh-Lotrario AD, Meyers RL, Feusner JH: Outcomes of Patients With Relapsed Hepatoblastoma Enrolled on Children’s Oncology Group (COG) Phase I and II Studies. J Pediatr Hematol Oncol 38 (3): 187-90, 2016
- Liu B, Zhou L, Huang G, et al.: First Experience of Ultrasound-guided Percutaneous Ablation for Recurrent Hepatoblastoma after Liver Resection in Children. Sci Rep 5: 16805, 2015
- Perilongo, G., Malogolowkin, M. and Feusner, J. (2012), Hepatoblastoma clinical research: Lessons learned and future challenges. Pediatr. Blood Cancer, 59: 818-821. https://doi.org/10.1002/pbc.24217
- Trobaugh-Lotrario, A.D. and Katzenstein, H.M. (2012), Chemotherapeutic approaches for newly diagnosed hepatoblastoma: Past, present, and future strategies. Pediatr. Blood Cancer, 59: 809-812. https://doi.org/10.1002/pbc.24219
- Trobaugh-Lotrario, A.D., Chaiyachati, B.H., Meyers, R.L., Häberle, B., Tomlinson, G.E., Katzenstein, H.M., Malogolowkin, M.H., von Schweinitz, D., Krailo, M. and Feusner, J.H. (2013), Outcomes for patients with congenital hepatoblastoma. Pediatr Blood Cancer, 60: 1817-1825. https://doi.org/10.1002/pbc.24655
- Dall’Igna P, Brugieres L, Christin AS, Maibach R, Casanova M, Alaggio R, de Goyet JV, Zsiros J, Morland B, Czauderna P, Childs M, Aronson DC, Branchereau S, Brock P, Perilongo G. Hepatoblastoma in children aged less than six months at diagnosis: A report from the SIOPEL group. Pediatr Blood Cancer. 2018 Jan;65, 1
- Kiruthiga KG, Ramakrishna B, Saha S, Sen S. Histological and immunohistochemical study of hepatoblastoma: correlation with tumour behaviour and survival. J Gastrointest Oncol. 2018 Apr;9(2):326-337
- Ang JP, Heath JA, Donath S, Khurana S, Auldist A. Treatment outcomes for hepatoblastoma: an institution’s experience over two decades. Pediatr Surg Int. 2007 Feb;23(2):103-9. doi: 10.1007/s00383-006-1834-1
- Otte JB, Pritchard J, Aronson DC, Brown J, Czauderna P, Maibach R, Perilongo G, Shafford E, Plaschkes J; International Society of Pediatric Oncology (SIOP). Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer. 2004 Jan;42(1):74-83. doi: 10.1002/pbc.10376