Monoclonal gammopathy of undetermined significance
MGUS also called monoclonal gammopathy of undetermined significance, is a medical condition in which an abnormal protein known as ‘monoclonal protein’ or ‘M protein’ is in found your blood or urine 1. The monoclonal protein or M protein, is produced by a type of blood cell known as plasma cells which are found in the blood-producing soft tissues. These tissues are present at the center of the bones, also known as the bone marrow. Monoclonal gammopathy of undetermined significance or MGUS is characterized by serum M-protein less than 30 g/L (3 g/dL), bone marrow clonal plasma cells less than 10 percent, absence of plasma cell myeloma-related end-organ damage (CRAB symptoms such as hypercalcemia, renal insufficiency, anemia, or bone lesions) and absence of B-cell lymphoma or other diseases known to produce an M-protein 2. MGUS is benign condition (not cancer), but it is sometimes called pre-malignant plasma cell disorder because each year approximately 1% of people with MGUS will eventually develop cancers such as multiple myeloma (most commonly), light chain amyloidosis, lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia), or chronic lymphocytic leukemia, which may require therapy 2, 3, 4, 5. MGUS (monoclonal gammopathy of undetermined significance) usually does not affect a person’s health or asymptomatic 6. It doesn’t cause weak bones, high calcium levels, kidney problems, or low blood counts. MGUS (monoclonal gammopathy of undetermined significance) is most often found when a routine blood test finds a high level of protein in the blood and further testing shows the protein is a monoclonal antibody or ‘M protein’. However, in some patients, MGUS may later develop a more serious condition, such as amyloidosis, or cause problems with the kidneys, heart, or nerves.
Monoclonal gammopathy of undetermined significance (MGUS) is found in approximately 2% to 3% of adults over age 50 and in 5% of adults older than the age of 70. MGUS is more common in men than in women (1.5:1) and 2 to 3-fold more common in African Americans compared to Caucasians 2.
Doctors typically estimate a person’s risk of progressing soon after MGUS is diagnosed, using a test that measures the amounts of certain markers in the blood. The risk of MGUS progression is increased when M protein greater than or equal to 15 g/L and with an abnormal serum free light chain (FLC) ratio 7, 4.
Risk factors that predict MGUS progression include the following 7, 4:
- An abnormal serum free light chain (FLC) ratio (ratio of kappa to lambda free light chains).
- Non-IgG class MGUS.
- A high level of serum M protein (≥1.5 g/dL).
A Swedish cohort study confirmed that an abnormal serum free light chain (FLC) ratio and a high level of serum monoclonal protein (M protein) are high-risk factors 8. The study described the additional risk factor of immunoparesis, which is defined as the reciprocal depression of the other immunoglobulin classes (i.e., if a patient has an IgG kappa M protein, the IgM and IgA would be below normal levels with immunoparesis). Incorporation of gene-expression profiles to better assess risk is under clinical evaluation 9.
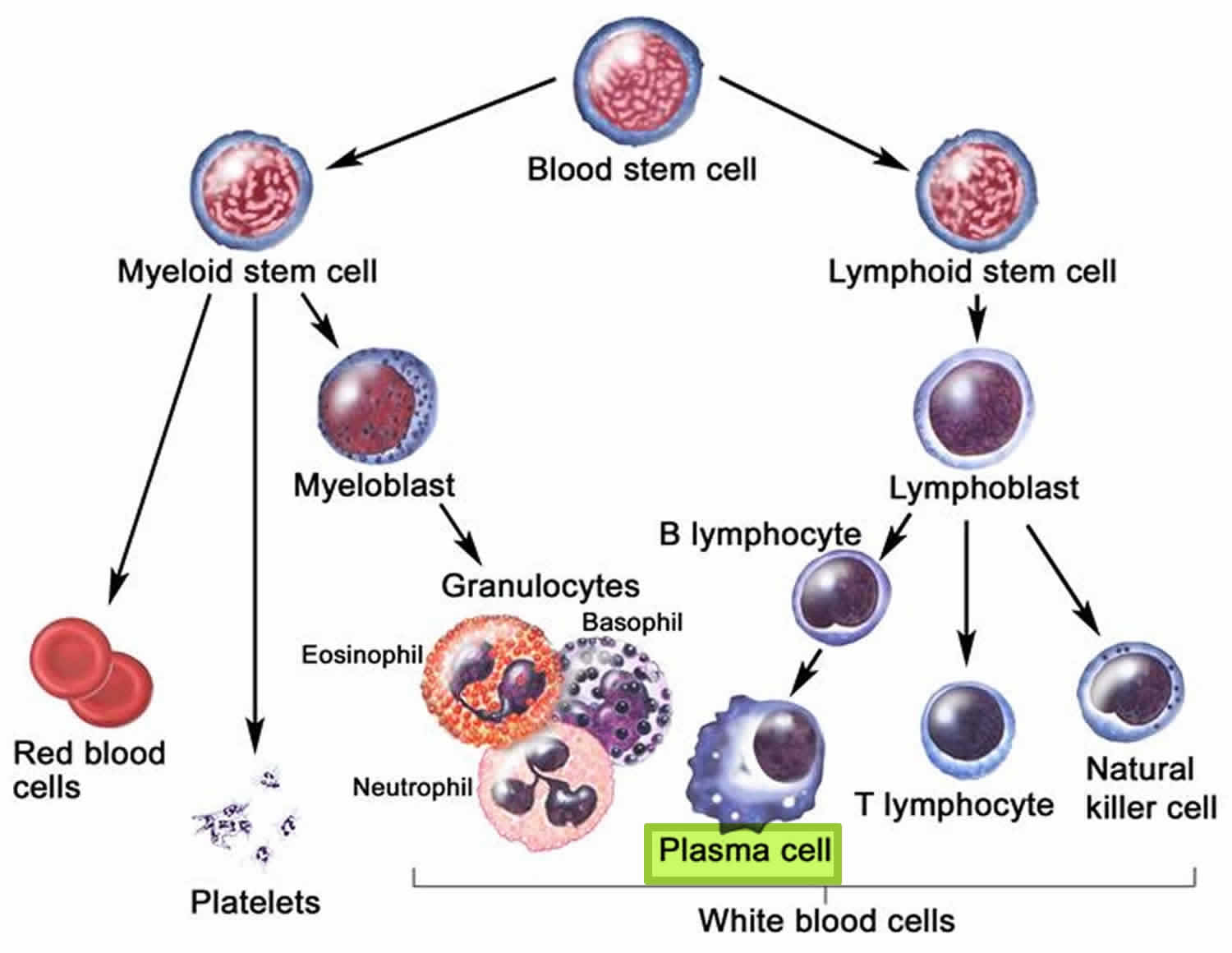
Plasma cells are part of the immune system that develop from B lymphocytes (B cells), a type of white blood cell that is made in the bone marrow. Normally, when bacteria or viruses enter your body, some of the B cells (B lymphocytes) will change into plasma cells. The normal plasma cells make antibodies (also called immunoglobulins or Ig) to fight bacteria and viruses, to stop infection and disease. Each type of plasma cell make different antibodies (immunoglobulins) for different infections. Antibodies (immunoglobulins) attack and help to kill bacteria and viruses and so protect you from infections. They work with other parts of the immune system to help protect the body from germs and other harmful substances.
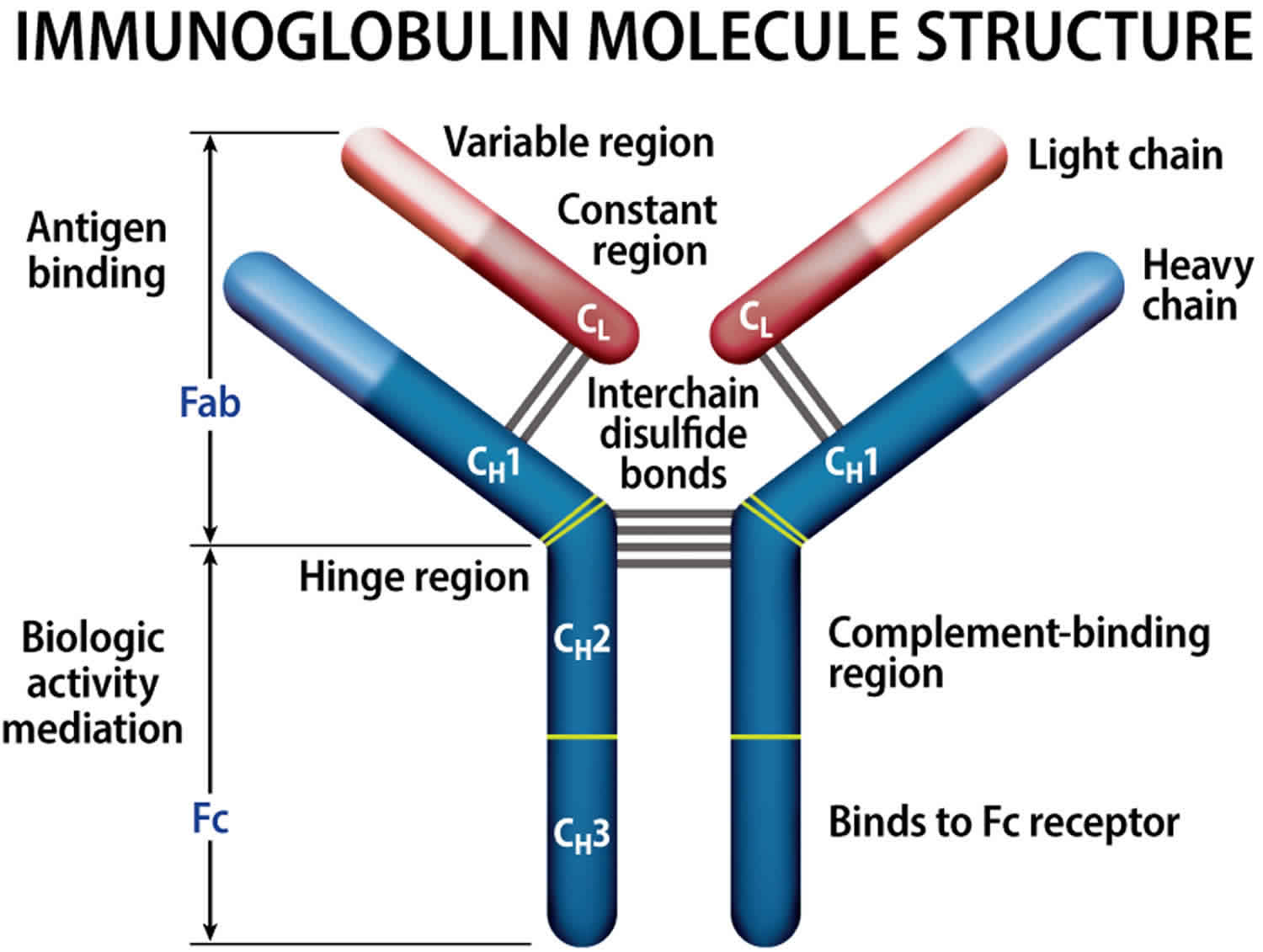
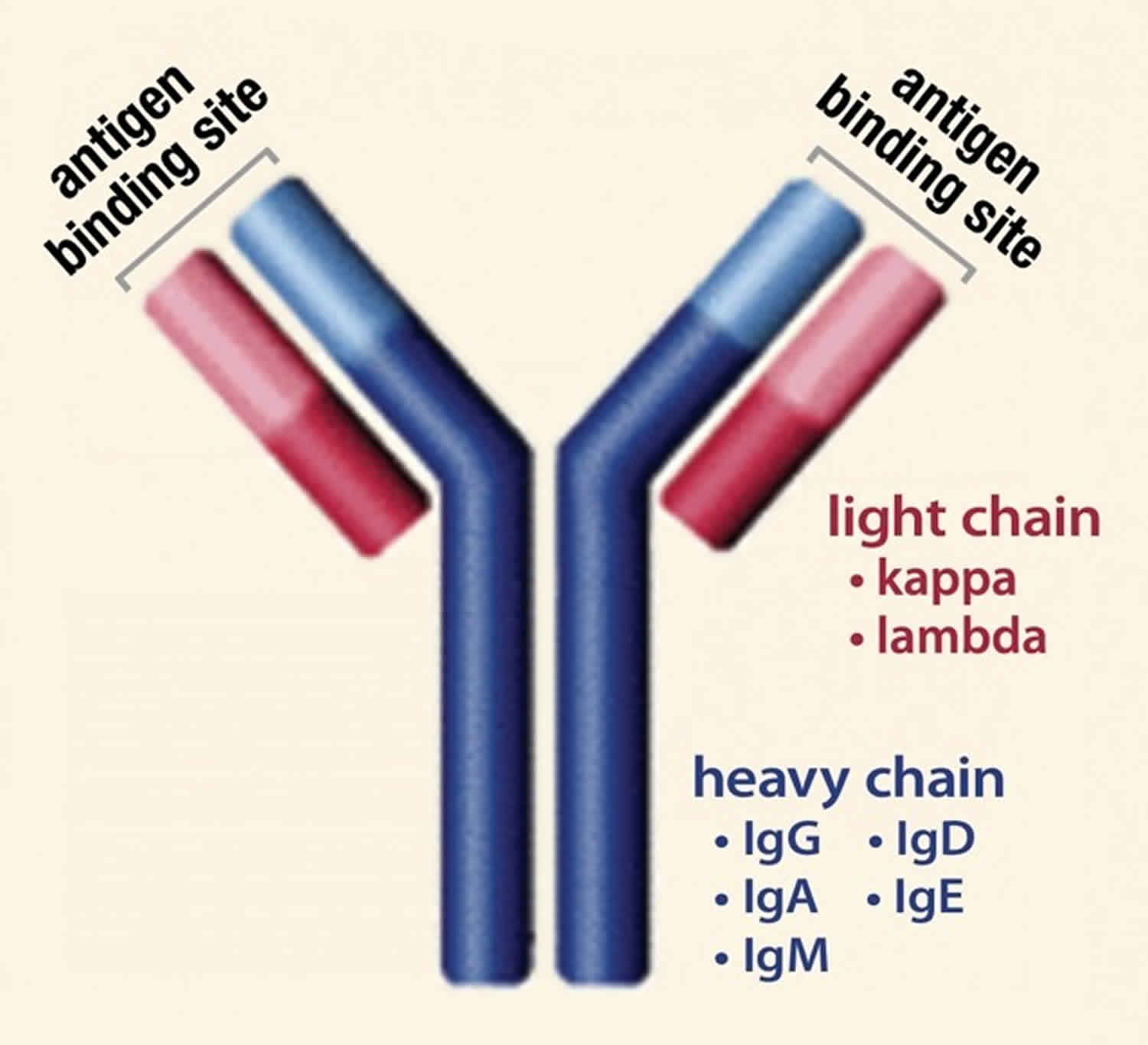
Normally, the body makes five different types of immunoglobulins – immunoglobulin G (Ig G), immunoglobulin M (Ig M), immunoglobulin A (Ig A), immunoglobulin E (Ig E) and immunoglobulin D (Ig D). Each one has slightly different immune system functions. Each type of immunoglobulin is composed of four protein chains – two identical heavy (long) protein chains and two identical light (shorter) protein chains (see Figure 4 below). The heavy chains may consist of one of five different types that correspond with the type of immunoglobulin produced: gamma (IgG), mu (IgM), alpha (IgA), epsilon (IgE) and delta (IgD). The light chains consist of one of two different types called kappa and lambda.
Within a plasma cell, two heavy chains of one type and two light chains of one type become attached to form one intact immunoglobulin molecule. Each particular plasma cell will produce only one type of immunoglobulin.
Abnormal plasma cells make many copies of the same antibody called a monoclonal protein or M protein, which is sometimes found during a routine blood or urine test. In most patients, the amount of M protein stays the same and there are no signs, symptoms, or health problems. Moreover, the abnormal plasma cells in MGUS do not form an actual tumor or mass and do not cause any of the problems seen in multiple myeloma. In MGUS, the number of plasma cells may be increased, but they still make up less than 10% of the cells in the bone marrow and there is no cancer.
Patients with MGUS don’t need treatment, but they are watched closely to see if they get a disease that does need to be treated, such as multiple myeloma, amyloidosis, lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia), or chronic lymphocytic leukemia 10, 11. Patients with MGUS usually undergo clinical follow up every 6-12 months for signs of disease progression 12, 13.
Virtually all cases of multiple myeloma are preceded by a gradually rising level of MGUS 14, 15.
Monoclonal gammopathies that cause organ damage, particularly to the kidney, heart, or peripheral nerves, require immediate therapy with the same strategies applied for the conventional plasma-cell dyscrasias 16. A monoclonal gammopathy causing kidney dysfunction—by direct antibody deposition or amyloidosis—is referred to as monoclonal gammopathy of renal significance 17. Rising serum creatinine, dropping glomerular filtration rates, and increasing urinary–albumin excretion are all parameters that may signify renal damage and are assessed prospectively for high-risk MGUS patients. Although the N-terminal pro-brain natriuretic peptide is a very sensitive marker for amyloid involvement in the heart, the low specificity must be noted. These extra tests are included with the M-protein level, free light chain (FLC) levels, and free light chain (FLC) ratio when following patients with MGUS 18.
In a retrospective review of 6,399 patients with newly diagnosed multiple myeloma, 44 patients were found to have a biclonal IgG or IgA MGUS. The overall response rate of the myeloma clone to induction therapy was 93%, compared with 64% for the separate-clone MGUS 19. Many MGUS plasma cell clones were unresponsive to available myeloma therapy; this result highlights the need to lower expectations for response in situations in which an MGUS may require therapy because of end-organ damage.
Figure 1. Plasma cells
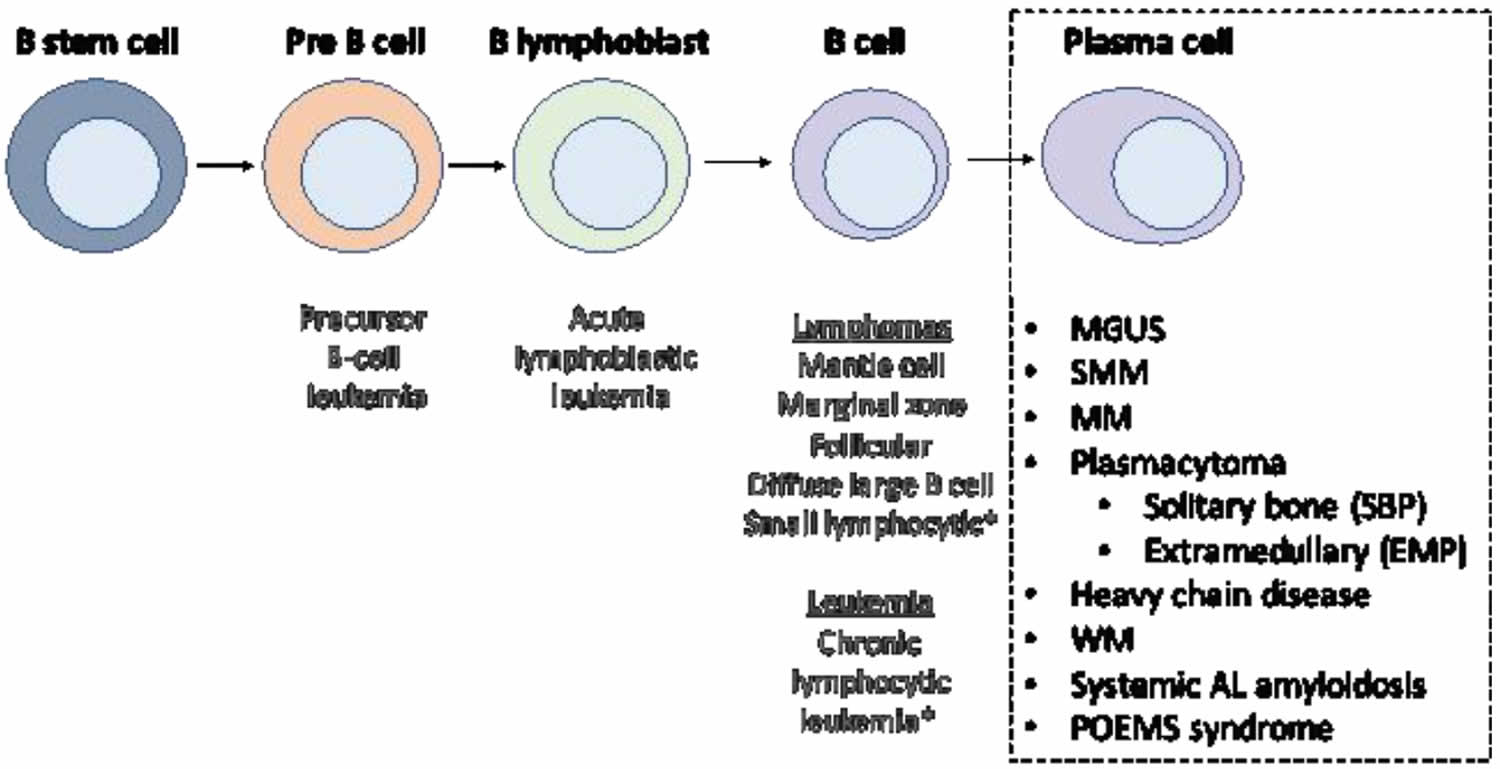
Figure 2. Stem cell origin of plasma cell neoplasms
Footnote: This figure demonstrates the origin of the monoclonal proliferation of plasma cells that characterizes plasma cell neoplasms.
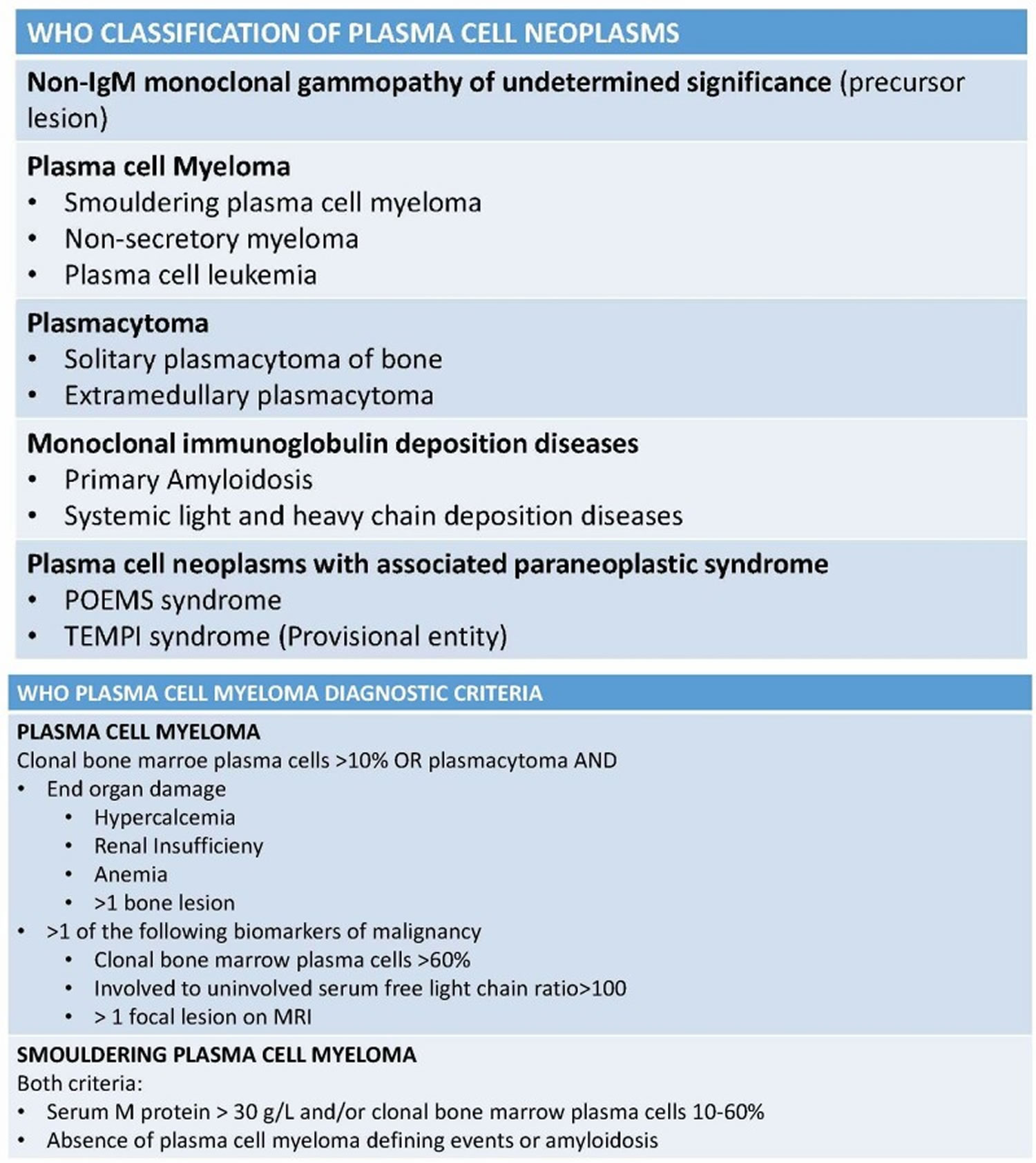
[Source 20 ]Figure 3. Plasma cell neoplasm WHO classification
[Source 21 ]Figure 4. Immunoglobulin structure
The Lymphatic System
To understand MGUS or monoclonal gammopathy of undetermined significance, it helps to know about the lymph system (also known as the lymphatic system) and the functions of lymphoid tissue in the body.
The lymph system is part of the immune system, which helps fight infections and some other diseases. The lymphatic system plays a role in:
- fighting bacteria and other infections
- destroying old or abnormal cells, such as cancer cells
The lymphatic system also helps the flow of fluids in the body.
The lymph system is made up mainly of cells called lymphocytes, a type of white blood cell. There are 2 main types of lymphocytes:
- B lymphocytes (B cells): B lymphocytes (B cells) respond to an infection by changing into a different type of cell called a plasma cell. Plasma cells make proteins called antibodies (also called immunoglobulins) that help the body attack and kill disease-causing germs like bacteria and viruses.
- T lymphocytes (T cells): There are several types of T cells. Some T cells destroy germs or abnormal cells in the body. Other T cells help boost or slow the activity of other immune system cells.
- Natural killer (NK) cells, which attack virus-infected cells or tumor cells.
The lymphatic system is a vast collection of cells and biochemicals that travel in lymphatic vessels, and the organs and glands that produce them. The lymphatic system includes a network of vessels (like the arteries and veins that carry blood) that assist in circulating body fluids (a colorless liquid called lymph), so it is closely associated with your cardiovascular system. Lymphatic vessels transport excess fluid away from interstitial spaces in most tissues and return it to the bloodstream (Figure 5). This fluid carries food to the cells and bathes the body tissues to form tissue fluid. The fluid then collects waste products, bacteria, and damaged cells. It also collects any cancer cells if these are present. This fluid then drains into the lymph vessels. Without the lymphatic system, this fluid would accumulate in tissue spaces. Special lymphatic capillaries, called lacteals, are located in the lining of the small intestine. They absorb digested fats and transport them to the venous circulation.
The lymphatic system is a system of thin tubes and lymph nodes that run throughout the body. Lymph nodes are bean shaped glands. The thin tubes are called lymph vessels or lymphatic vessels. Tissue fluid called lymph circulates around the body in these vessels and flows through the lymph nodes.
The lymph system is an important part of your immune system. It plays a role in fighting bacteria and other infections and destroying old or abnormal cells, such as cancer cells.
The major sites of lymphoid tissue are:
- Lymph nodes: Lymph nodes are bean-sized collections of lymphocytes and other immune system cells throughout the body, including inside the chest, abdomen, and pelvis. They are connected to each other by a system of lymphatic vessels.
- Spleen: The spleen is an organ under the lower ribs on your left side. The spleen makes lymphocytes and other immune system cells. It also stores healthy blood cells and filters out damaged blood cells, bacteria, and cell waste.
- Bone marrow: The bone marrow is the spongy tissue inside certain bones. New blood cells (including some lymphocytes) are made there.
- Thymus: The thymus is a small organ behind the upper part of the breastbone and in front of the heart. Thymus is an organ in which T lymphocytes mature and multiply.
- Adenoids and tonsils: These are collections of lymphoid tissue in the back of your throat. They help make antibodies against germs that are breathed in or swallowed.
- Digestive tract: The stomach, intestines, and many other organs also have lymph tissue.
The lymphatic system has a second major function— it enables you to live in a world with different types of organisms. Some of them live in or on the human body and in some circumstances may cause infectious diseases. Cells and biochemicals of the lymphatic system launch both generalized and targeted attacks against “foreign” particles, enabling the body to destroy infectious agents. This immunity against disease also protects against toxins and cancer cells. When the immune response is abnormal, persistent infection, cancer, allergies, and autoimmune disorders may result.
The larger lymphatic vessels lead to specialized organs called lymph nodes. After leaving the lymph nodes, the vessels merge to form still larger lymphatic trunks.
Figure 5. Locations of major lymph nodes
Figure 6. Functions of lymph nodes in the lymphatic system
Figure 7. Schematic representation of lymphatic vessels transporting fluid from interstitial spaces to the bloodstream. Depending on its origin, lymph enters the right or left subclavian vein.
MGUS Types
There are 3 distinct types of MGUS 22:
- Non-IgM MGUS: Non-IgM MGUS (IgG, IgA, IgD) accounts for the majority of MGUS cases and is characterized by a monoclonal plasma cell. The clonal plasma cells producing non-IgM MGUS reside in the bone marrow. These cells harbor somatic hypermutation of the variable regions and are class-switched. The majority of non-IgM MGUS are IgG, followed by IgA, bi-clonal, and IgD 23.
- IgM MGUS
- Light-chain MGUS
Non-IgM MGUS represents up to 85% of MGUS cases; IgM MGUS represents up to 15% of MGUS cases 24, 25.
Non-IgM MGUS may progress to a malignant plasma cell neoplasm. IgM MGUS may develop into Waldenstrom macroglobulinemia, immunoglobulin light chain (AL) amyloidosis, or lymphoma. Light chain MGUS (LC-MGUS) is characterized by a monoclonal protein that lacks the immunoglobulin heavy chain component. Light chain MGUS may show progression to idiopathic Bence Jones proteinuria, light chain plasma cell myeloma (multiple myeloma), light chain (AL) amyloidosis, or light chain deposition disease 2. According to a population-based cohort study, the risk of progression to multiple myeloma in patients with light-chain MGUS is 0.3% 26, 24.
More recently, MGUS has been differentiated from monoclonal gammopathy of renal significance (MGRS), which includes kidney and sometimes systemic lesions in addition to the hematologic findings of MGUS 27. Early recognition of monoclonal gammopathy of renal significance (MGRS) is critical, as suppression of monoclonal immunoglobulin secretion by chemotherapy often improves outcomes 28. Similarly, MGUS with peripheral neuropathy has been designated as monoclonal gammopathy of neurologic significance; this condition occurs more often in IgM MGUS and typically involves slowly progressive, symmetrical, length-dependent sensory deficits 29. Recognition that monoclonal gammopathies can involve not only kidneys and nerves but skin and sometimes other organs led to coining of the term monoclonal gammopathies of clinical significance (MGCS) 30.
MGUS symptoms
People with monoclonal gammopathy of undetermined significance (MGUS) generally don’t experience signs or symptoms. Some people may experience a rash or nerve problems, such as numbness or tingling. MGUS with peripheral neuropathy has been designated as monoclonal gammopathy of neurologic significance; this condition occurs more often in IgM MGUS and typically involves slowly progressive, symmetrical, length-dependent sensory deficits 29. MGUS is usually detected by chance when you have a blood test for another condition. MGUS is usually detected when you have abnormal M-protein in your serum and/or urine. Laboratory tests called serum eletrophoresis (SPEP) and urine eletrophoresis (UPEP) indicate the amount of M-protein. The type of M-protein for each patient is determined by a laboratory test called immunofixation eletrophoresis (IFE).
Each year about 1% of people with MGUS go on to develop certain types of blood cancers or other serious diseases such as:
- Multiple myeloma
- Light chain amyloidosis
- Waldenstrom macroglobulinemia
- Lymphoma
Other complications associated with MGUS include bone fractures, blood clots and kidney problems.
MGUS complications
Monoclonal gammopathy of undetermined significance (MGUS) can progress to develop myeloproliferative disorders or other serious conditions such as:
- Multiple myeloma
- Light chain amyloidosis
- Waldenstrom macroglobulinemia (lymphoplasmacytic lymphoma)
- Lymphoma
- Osteoporosis
- Venous thromboembolism
- Infection
The risk of venous thromboembolism (VTE) is increased in patients with MGUS 31. In a study by Srkalovic et al 32, 13% of patients with MGUS developed venous thromboembolism. Univariate correlates of venous thromboembolism in patients with MGUS included the following:
- Family or past history of venous thromboembolism
- Immobility
- Low serum albumin level
- High leukocyte count
Osteoporosis
Using population-based data from Sweden, Kristinsson et al 33 compared the risks of fractures in 5,326 patients with MGUS diagnosed from 1958-2006 with 20,161 matched controls. Patients with MGUS had an increased risk of any fracture at 5 years and 10 years. The risk was significantly higher for axial (skull, vertebral/pelvis, and sternum/costae) compared with distal (arm and leg) fractures 33.
A French study found that, in addition to low bone density, patients with MGUS who experienced nontraumatic vertebral fractures were more likely to have a lambda light chain isotype 34. In this prospective study of 201 patients with incidentally discovered MGUS and no known history of osteoporosis, nontraumatic vertebral fracture was discovered in 8.4% of the patients, with equal distribution between men and women. Patients with lambda light chain had an odds ratio of 4.32 for fracture, compared with patients with kappa light chain 34.
Infection
Kristinsson et al reported that patients with MGUS had a 2-fold increased risk of developing any infection 6. The risk extended to both bacterial and viral infections, and the following specific infections were noted:
- Pneumonia
- Osteomyelitis
- Septicemia
- Pyelonephritis
- Cellulitis
- Endocarditis
- Meningitis
- Influenza
- Herpes zoster
Infection risk was highest in patients with M-protein concentrations over 2.5 g/dL, but was also increased in those with concentrations below 0.5 g/dL. Patients with MGUS who developed infections had no excess risk of progression to related malignancy.
MGUS causes
The precise cause of MGUS isn’t known. Genetic changes and environmental triggers appear to play a role. Nagoshi et al 35 identified a possible secondary genetic change involving MGUS; they found transcriptional dysregulation of the deleted in colorectal carcinoma (DCC) gene in 25% of MGUS cases studied, and in 57% of multiple myeloma. The DCC gene encodes a tumor suppressor that prevents cell growth; allele loss or decreased expression of DCC has been associated with the progression of solid tumors and hematologic malignancies 35.
MGUS may be associated with some non-malignant disorders such as connective tissue disorders, peripheral neuropathies, dermatological diseases such as acquired C1 esterase inhibitor deficiency (angioedema), endocrine diseases, and liver infections such as hepatitis C virus infection and HIV liver disease 24, 25, 23. Population-based studies from northern Europe and the United States show increased risk of MGUS among first-degree relatives of those with MGUS or myeloma, supporting a role for germline susceptibility genes, shared environmental influences, or an interaction between both 36, 37.
Risk factors for developing MGUS
Factors that increase your risk of developing MGUS include:
- Age. The average age at diagnosis is 70 years.
- Race. Africans and African Americans are more likely to experience MGUS than are white people.
- Sex. MGUS is more common in men.
- Family history. You may have a higher risk of MGUS if other people in your family have the condition.
- Autoimmune disorders. Some autoimmune disorders, including pernicious anemia and lupus, can increase the risk of MGUS.
- Specific prior infections (eg, pneumonia, hepatitis, meningitis, HIV) 38
- Inflammatory disorders
- Smoking
- Pesticide exposure (eg, aldrin, dieldrin, permethrin) 39
- Overweight or obesity has been inconsistently linked to the development of MGUS and its transition to multiple myeloma (plasma cell myeloma) 40, 41
MGUS diagnosis
Because MGUS usually causes no symptoms, it’s usually detected by chance during blood tests for other conditions. Afterwards, your doctor may recommend:
- More-detailed blood tests. These can help rule out other causes of elevated protein levels and can check for kidney damage.
- Urine tests. A 24-hour urine collection can help determine if abnormal protein is being released into your urine. It can also assess any resulting kidney damage.
- Imaging tests. If you are experiencing bone pain, your doctor might recommend an MRI or positron emission tomography (PET) scan. The images can help your doctor find bone abnormalities related to MGUS. Your bone density also might need to be checked.
- Bone marrow test. A hollow needle can remove a portion of your bone marrow from the back of one of your hipbones. Bone marrow analysis is generally done only when you’re at risk of developing a more serious disease or if you have unexplained anemia, kidney failure, bone lesions or high calcium levels.
Based on the International Myeloma Working Group consensus 42, a formal diagnosis of MGUS is established when a serum M protein is detected and measured at a concentration less than 3g/dL on serum protein electrophoresis along with less than 10 percent clonal plasma cells in the bone marrow, the absence of myeloma-related organ damage — particularly CRAB symptoms osteolytic bone lesions, anemia, otherwise unexplained renal failure and hypercalcemia and absence of B-cell lymphoma or other diseases known to produce an M-protein — is fundamental and necessary for a diagnosis of MGUS.
Investigations should include complete blood count (CBC), bone marrow aspirate or bone marrow biopsy, immunohistochemical analysis (IHC), serum calcium and creatinine, serum protein electrophoresis and immunofixation, urine protein electrophoresis and immunofixation, serum-free light chains (FLC) assay, quantification of immunoglobulins, immunophenotyping utilizing flow cytometry and cross-sectional imaging studies 9, 22.
Complete blood count (CBC) and peripheral smear is usually normal. However, some cases may show rouleaux formation. Bone marrow biopsy usually shows 3% to 5% mature plasma cells (less than 10%) evenly scattered or in occasional small clusters. CD138 is useful in highlighting these plasma cells in the bone marrow biopsy. Immunohistochemical analysis (IHC) of the plasma cells for kappa and lambda will demonstrate monoclonal restriction. MGUS is characterized by the presence of a monoclonal (M) protein which is produced by clonal plasma cells and detected by serum and urine protein electrophoresis, and immunofixation of the serum and urine. Immunophenotyping shows monoclonal plasma cells that are CD38+ (bright) cells with an aberrant CD56 population (may also be negative). Molecular testing for non-IgM MGUS has shown that this disease entity usually shows a normal karyotype because of the relatively small number of plasma cells. Some patients show chromosomal alterations of plasma cell myeloma that include: t(11;14), t(4;14), t(14;16), deletions of 13q and hyperdiploid. No clinical correlation for these genetic alterations has been found in non-IgM MGUS 4.
The 2019 International myeloma working group consensus recommends the following approach for cross-sectional imaging in MGUS 43:
- Whole-body imaging is recommended only in high-risk MGUS. Since IgM-MGUS usually progresses to Waldenstrom macroglobulinemia and not multiple myeloma, routine bone imaging is not recommended.
- In suspected high-risk non-IgM MGUS, a whole-body CT to rule out multiple myeloma is recommended. CT scan has superior sensitivity compared with a skeletal survey for the detection of osteolytic lesions in patients with multiple myeloma. If whole-body CT is not available, conventional skeletal survey or whole-body MRI are alternatives.
In patients with equivocal findings on whole-body CT (or conventional skeletal survey) in whom there is a concern for myeloma development, a whole-body MRI is recommended (or MRI of the spine and pelvis if whole-body MRI is not available).
If whole-body CT is positive, a PET/CT should be done. A follow-up bone imaging is not recommended unless there are signs of progression to symptomatic disease (e.g., pain or increase in serological parameters).
In patients with MGUS with positive imaging findings for focal and osteolytic lesions, other malignancies should be ruled out as well. If needed, a biopsy of such a lesion should be performed.
MGUS differential diagnosis
It is crucial to distinguish non-IgM MGUS from other advanced plasma cell neoplasms since these diseases will require a different management approach. The differential diagnosis of non-IgM MGUS include:
- Plasma cell myeloma (smoldering or symptomatic): Any patient with a non-IgM serum monoclonal protein greater than or equal to 30 g/L or with greater than or equal to 10% clonal plasma cells in the bone marrow should not be diagnosed as non-IgM MGUS. Many providers consider smoldering plasma cell myeloma as a transition stage between non-IgM MGUS and symptomatic PCM. Smoldering plasma cell myeloma is distinguished from MGUS based on the size of the M protein and the percentage of clonal plasma cells in the bone marrow. Smoldering PCM is distinguished from symptomatic plasma cell myeloma by the presence of CRAB symptoms 23.
- Waldenström macroglobulinemia (smoldering or symptomatic): Waldenstrom macroglobulinemia is a distinct clinicopathologic disorder that shows lymphoplasmacytic lymphoma (LPL) in the bone marrow and an IgM monoclonal gammopathy. Symptoms include blood hyperviscosity, lymphadenopathy, or splenomegaly. The WHO (2017) diagnostic criteria of Waldenström macroglobulinemia include 23:
- IgM monoclonal gammopathy
- Clonal plasma cells less than 10%
- Absence of end-organ damage (CRAB symptoms)
- Absence of symptoms or signs of amyloidosis
- Monoclonal gammopathy of renal significance (MGRS): This disease entity is diagnosed when the patient has diagnostic criteria for MGUS as well as renal insufficiency and monoclonal immunoglobulin deposits in the kidney by immunofluorescence 27. Kidney biopsy is indicated in most cases of monoclonal gammopathy of renal significance (MGRS), to identify the exact lesion and determine its severity 28. Treatment to eradicate the underlying clone is indicated; the proteasome inhibitor bortezomib is the preferred therapy in monoclonal gammopathy of renal significance (MGRS) but a number of other agents have been used in some settings, including rituximab, cytotoxic chemotherapy, and immunomodulatory agents.
- Light chain smoldering multiple myeloma (idiopathic Bence Jones proteinuria): The diagnostic criteria of light chain smoldering multiple myeloma include:
- Monoclonal light chains in the urine (Bence Jones proteinuria)
- No immunoglobulin heavy chain expression in the serum or urine
- No symptoms of plasma cell myeloma, Waldenstrom macroglobulinemia or light chain amyloidosis
- Primary (amyloid light chain) amyloidosis and light chain deposition disease: This entity of plasma cell neoplasms is associated with the pathologic deposition of monoclonal light chains in tissue. The diagnostic criteria include the presence of amyloid in tissue and evidence of plasma cell neoplasm.
- B cell lymphoproliferative disorder
MGUS treatment
Treatment is usually not recommended for patients with MGUS 44. But your doctor (hematologist) is likely to recommend periodic checkups to monitor your health, probably starting 3 to 6 months after your diagnosis. Your serum eletrophoresis (SPEP) should be repeated 3 to 6 months after your MGUS diagnosis. If your level of M-protein remains stable and there are no other health problems, the time between visits to your doctor can be extended to 1 to 2 years barring any change in your health or any suggestion of symptoms.
The intensity of follow-up in patients with MGUS is guided by risk stratification. Initial follow-up at six months is recommended, with subsequent visits scheduled according to the level of risk 2. If clinical trials of preventive strategies are available, patients at high risk for progression should be encouraged to participate.
The International Myeloma Working Group recommends followup serum protein electrophoresis for patients with MGUS 6 months after diagnosis, with subsequent followup depending on risk 44. The International Myeloma Working Group considers patients with IgG MGUS who have an M-protein level below 1.5 g/dL and a normal FLC ratio to be at low risk; if findings at 6 months are stable, subsequent follow-up can be every 2 to 3 years thereafter or when symptoms suggestive of a plasma cell malignancy arise 44. For patients with intermediate and high-risk MGUS, the International Myeloma Working Group recommends annual follow-up 44.
With IgM MGUS, which poses a high risk for malignant progression, some experts recommend more intensive follow-up, with twice-annual visits that include the following 45:
- Clinical assessment
- Complete blood count (CBC)
- Comprehensive metabolic panel
- Serum protein electrophoresis
- Serum FLC assay
- Quantitative immunoglobulin serum electrophoresis
The European Myeloma Network advises that for low-risk MGUS, follow-up at 6 months and every 1–2 years thereafter can be justified 46. Alternatively, follow-up for low-risk MGUS may be limited to performing laboratory studies or bone marrow analysis when patients develop symptoms suggestive of MM or related diseases. In patients who are elderly or have significant morbidity with a short life expectancy, it may be reasonable to forgo follow-up 46.
If a patient has an IgM M-protein, bone marrow biopsy and computed tomography (CT) scanning of the abdomen may be helpful in detecting Waldenström macroglobulinemia or other lymphoproliferative disorders 2.
MGUS-associated neuropathies are usually not treated, except in the case of a disabling IgM monoclonal gammopathy or IgG/IgA MGUS associated with chronic inflammatory demyelinating neuropathy (CIDP) 2. About 80% of patients with IgG/IgA MGUS chronic inflammatory demyelinating neuropathy (CIDP) respond to one of the typical chronic inflammatory demyelinating neuropathy (CIDP) treatments and some patients stabilize without therapy. MGUS patients with associated osteopenia or osteoporosis may benefit from treatment with intravenous bisphosphonates.
Watchful waiting
If you are at high risk of MGUS developing into a more serious condition, your doctor may recommend more frequent checkups so that any progression can be diagnosed and treatment started as soon as possible.
Your doctor is likely to watch for signs and symptoms such as:
- Bone pain
- Fatigue or weakness
- Unintentional weight loss
- Fever or night sweats
- Headache, dizziness, nerve pain, or changes in vision or hearing
- Bleeding
- Anemia or other blood abnormalities
- Swollen lymph nodes, liver or spleen
Medications
If you have osteoporosis, your doctor might recommend a medication to increase bone density. Examples include alendronate (Fosamax), risedronate (Actonel, Atelvia), ibandronate (Boniva) and zoledronic acid (Reclast, Zometa).
Polyneuropathy in IgM monoclonal gammopathy can be a disabling disorder. A prospective open-label trial by Niermeijer et al 47 in 17 patients with IgM MGUS polyneuropathy found that treatment with rituximab (a chimeric anti-CD-20 monoclonal antibody) therapy was associated with improvement on three impairment measures and the presence of CD20 B-cell depletion in the bone marrow of all patients, and no serious adverse events.
Niermeijer et al 47 also reported that the response percentage rate with rituximab was comparable to that seen with intermittent cyclophosphamide/prednisone or fludarabine therapy, but with fewer side effects, and suggested that the presence of anti-MAG antibodies and a disease duration shorter than 10 years may predict treatment response.
MGUS prognosis
MGUS is considered a pre-malignant condition with an annual risk of progression into multiple myeloma (most commonly), light chain amyloidosis, lymphoplasmacytic lymphoma (Waldenstrom macroglobulinemia), or chronic lymphocytic leukemia of approximately 1% 2. The risk of progression is increased when M protein greater than or equal to 15 g/L and with an abnormal serum free light chain (FLC) ratio 7, 4. MGUS does not require treatment. However, the management of patients with non-IgM MGUS requires an understanding of the risk of progression of the disease. Generally, close follow-up is recommended for these patients. Most experts believe that all MGUS patients should undergo a clinical examination and laboratory evaluation for disease progression annually 9. The patients with MGUS who are at risk for progressing to advanced disease can be risk-stratified based on the following criteria:
- Serum monoclonal protein (M protein) level greater than or equal to 1.5 g/dL
- Non-IgG MGUS (i.e., IgA, IgM, and IgD MGUS)
- Abnormal serum free light chain (FLC) ratio (ratio of kappa to lambda free light chains)
Patients with three risk factors are categorized as high-risk MGUS; patients with two risk factors are categorized as high-intermediate risk MGUS. Patients with one risk factor are categorized as low-intermediate risk MGUS. Patients with no risk factors are categorized as low-risk MGUS. Despite close observation, some non-IgM MGUS patients may show progression to multiple myeloma abruptly 4, 23.
An abnormal serum free light-chain (FLC) ratio (ratio of kappa to lambda free light chains) and a high serum monoclonal protein (M protein) level (≥1.5 g/dL) are risk factors for progression. In IgM MGUS, Kyle et al 4 reported a risk of progression at 20 years of 55% in patients with both risk factors, compared with 41% in those with one risk factor and 19% in patients with neither risk factor. In patients with non-IgM MGUS, the risk of progression at 20 years was 30% in those with both risk factors, 20% in those iwth one risk factor, and 7% in those with neither risk factor.
Rajkumar et al 7 found that the risk of progression in patients with an abnormal serum free light chain (FLC) ratio (kappa-lambda ratio < 0.26 or > 1.65) is independent of the size and type of the serum M protein, and that the relative risk of progression is related to the extent to which the ratio is abnormal. These authors proposed a risk-stratification model for the progression of MGUS, using the combination of the size and type of the M protein and the serum FLC ratio.
Pelzer et al 48 concluded that light-chain MGUS—defined as an abnormal FLC ratio, increase of involved FLC with complete loss of immunoglobulin heavy chain, and absence of a history of lymphoproliferative disease—is a relatively benign condition, and that the monoclonal protein often diisappears over time. In their longitudinal analysis of 75 German patients with light-chain MGUS, after a median observation time of 11.5 years, none of the cases had progressed to light-chain multiple myeloma or other lymphoproliferative disorders. On serial analysis, light-chain MGUS could not be confirmed in 17 of 31 cases (55%), and disappearance of the monoclonal protein was associated with low concentrations of the involved FLC. Although patients with light-chain MGUS had a 1.5-fold increased risk of cancer, overall survival and renal function were not different than in patients with normal FLC.
In a study of 728 Swedish MGUS patients followed for up to 30 years, 84 patients developed a lymphoid disorder, representing a cumulative risk of 15.4% 8. The 30-year cumulative risk for myeloid malignancies was less than 2%. The 30-year cumulative risk for MM, which occurred in 53 patients, was 10.6%, with an approximately 0.5% annual risk. The following factors were significantly associated with progression 8:
- An abnormal FLC ratio of less than 0.26 or more than 1.65
- An M-protein concentration of 1.5 g/dL or more
- Reduction of one or two noninvolved immunoglobulin isotype levels
Although autoimmune disease is a well-described risk factor for the development of MGUS, a Swedish population-based study determined that patients with a history of autoimmune disease have a significantly lower risk of progression from MGUS to multiple myeloma or other lymphoproliferative diseases. The study included 19,303 MGUS patients, 5612 (29.1%) of whom had preceding autoimmune diseases 49. Similarly, a Mayo Clinic study of 249 young patients with MGUS (age < 40 years), 135 of whom had immune-related conditions, reported a trend toward higher risk of progression in patients without immune-related conditions 50.
References- Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013:478-87. doi: 10.1182/asheducation-2013.1.478
- Kaseb H, Annamaraju P, Babiker HM. Monoclonal Gammopathy Of Undetermined Significance. [Updated 2022 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507880
- Zingone A, Kuehl WM. Pathogenesis of monoclonal gammopathy of undetermined significance and progression to multiple myeloma. Semin Hematol. 2011 Jan;48(1):4-12. doi: 10.1053/j.seminhematol.2010.11.003
- Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2018 Jan 18;378(3):241-249. doi: 10.1056/NEJMoa1709974
- Bird, J., Behrens, J., Westin, J., Turesson, I., Drayson, M., Beetham, R., D’Sa, S., Soutar, R., Waage, A., Gulbrandsen, N., Gregersen, H., Low, E. and (2009), UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). British Journal of Haematology, 147: 22-42. https://doi.org/10.1111/j.1365-2141.2009.07807.x
- Kristinsson SY, Tang M, Pfeiffer RM, Björkholm M, Goldin LR, Blimark C, Mellqvist UH, Wahlin A, Turesson I, Landgren O. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012 Jun;97(6):854-8. doi: 10.3324/haematol.2011.054015
- Rajkumar SV, Kyle RA, Therneau TM, Melton LJ 3rd, Bradwell AR, Clark RJ, Larson DR, Plevak MF, Dispenzieri A, Katzmann JA. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005 Aug 1;106(3):812-7. doi: 10.1182/blood-2005-03-1038
- Turesson I, Kovalchik SA, Pfeiffer RM, Kristinsson SY, Goldin LR, Drayson MT, Landgren O. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014 Jan 16;123(3):338-45. doi: 10.1182/blood-2013-05-505487
- Dhodapkar MV, Sexton R, Waheed S, Usmani S, Papanikolaou X, Nair B, Petty N, Shaughnessy JD Jr, Hoering A, Crowley J, Orlowski RZ, Barlogie B. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood. 2014 Jan 2;123(1):78-85. doi: 10.1182/blood-2013-07-515239
- Go RS, Gundrum JD, Neuner JM. Determining the clinical significance of monoclonal gammopathy of undetermined significance: a SEER-Medicare population analysis. Clin Lymphoma Myeloma Leuk. 2015 Mar;15(3):177-186.e4. doi: 10.1016/j.clml.2014.09.004
- Sigurdardottir EE, Turesson I, Lund SH, et al. The Role of Diagnosis and Clinical Follow-up of Monoclonal Gammopathy of Undetermined Significance on Survival in Multiple Myeloma. JAMA Oncol. 2015;1(2):168–174. doi:10.1001/jamaoncol.2015.23
- McLellan L, Pohlman B, Rybicki L, et al. Distress screening scores of malignant and benign hematology patients: Results of a pilot project. Blood. 2012;120.
- Khimani F, Curley B, Almubarak M. Survey of Patients Referred to a University Cancer Center for Benign Hematology: Quality Measures and Patient Understanding. J Oncol Pract. 2015 Jan;11(1):26-9. doi: 10.1200/JOP.2014.001543
- Bladé J, Rosiñol L, Cibeira MT. Are all myelomas preceded by MGUS? Blood. 2009 May 28;113(22):5370. doi: 10.1182/blood-2009-03-207241
- Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009 May 28;113(22):5418-22. doi: 10.1182/blood-2008-12-195008
- Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, Merlini G. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018 Oct 4;132(14):1478-1485. doi: 10.1182/blood-2018-04-839480
- Leung N, Bridoux F, Nasr SH. Monoclonal Gammopathy of Renal Significance. N Engl J Med. 2021 May 20;384(20):1931-1941. doi: 10.1056/NEJMra1810907
- Merlini G. Determining the significance of MGUS. Blood. 2014 Jan 16;123(3):305-7. doi: 10.1182/blood-2013-12-539940
- Campbell JP, Heaney JLJ, Pandya S, Afzal Z, Kaiser M, Owen R, Child JA, Cairns DA, Gregory W, Morgan GJ, Jackson GH, Bunce CM, Drayson MT. Response comparison of multiple myeloma and monoclonal gammopathy of undetermined significance to the same anti-myeloma therapy: a retrospective cohort study. Lancet Haematol. 2017 Dec;4(12):e584-e594. doi: 10.1016/S2352-3026(17)30209-0
- Rowell, Sean & Ho, Matthew & Anderson, Kenneth. (2019). Plasmacytoma (Solitary bone plasmacytoma, extramedullary plasmacytoma). Atlas of Genetics and Cytogenetics in Oncology and Haematology. 23. 10.4267/2042/69762
- Kaseb H, Durer C, Fazal S, et al. Plasma Cell Cancer. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [Figure, Plasma Cancer Cell. Hatem Kaseb, MD, Ph.D., M.P.H.] Available from: https://www.ncbi.nlm.nih.gov/books/NBK507913/figure/article-41398.image.f1
- Rajkumar, V., Dimopoulos, M., Palumbo, A., Blade, J., Merlini, G., Mateos, M.-V., Kumar, S., Hillengass, J., Kastritis, E., Richardson, P., Landgren, O., Paiva, B., Dispenzieri, A., Weiss, B., Leleu, X., Zweegman, S., Lonial, S., Rosinol, L., Zamagni, E., & San Miguel, J. (2014). International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncology, 538-548. doi:10.1016/S1470-2045(14)70442-5
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375-90. doi: 10.1182/blood-2016-01-643569
- Kyle, R.A. and Vincent Rajkumar, S. (2006), Monoclonal gammopathy of undetermined significance. British Journal of Haematology, 134: 573-589. https://doi.org/10.1111/j.1365-2141.2006.06235.x
- Niels Weinhold, David C. Johnson, Andrew C. Rawstron, Asta Försti, Chi Doughty, Jayaram Vijayakrishnan, Peter Broderick, Nasrin B. Dahir, Dil B. Begum, Fay J. Hosking, Kwee Yong, Brian A. Walker, Per Hoffmann, Thomas W. Mühleisen, Christian Langer, Elisabeth Dörner, Karl-Heinz Jöckel, Lewin Eisele, Markus M. Nöthen, Dirk Hose, Faith E. Davies, Hartmut Goldschmidt, Gareth J. Morgan, Kari Hemminki, Richard S. Houlston; Inherited genetic susceptibility to monoclonal gammopathy of unknown significance. Blood 2014; 123 (16): 2513–2517. doi: https://doi.org/10.1182/blood-2013-10-532283
- Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ 3rd, Colby CL, Therneau TM, Clark R, Kumar SK, Bradwell A, Fonseca R, Jelinek DF, Rajkumar SV. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010 May 15;375(9727):1721-8. doi: 10.1016/S0140-6736(10)60482-5. Erratum in: Lancet. 2010 Jul 31;376(9738):332
- Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA; International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012 Nov 22;120(22):4292-5. doi: 10.1182/blood-2012-07-445304
- Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, Picken MM, Herrera GA, Kastritis E, Merlini G, Roussel M, Fervenza FC, Dispenzieri A, Kyle RA, Nasr SH; International Kidney and Monoclonal Gammopathy Research Group. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015 Apr;87(4):698-711. doi: 10.1038/ki.2014.408
- Castillo JJ, Callander NS, Baljevic M, Sborov DW, Kumar S. The evaluation and management of monoclonal gammopathy of renal significance and monoclonal gammopathy of neurological significance. Am J Hematol. 2021 Jul 1;96(7):846-853. doi: 10.1002/ajh.26155
- Dispenzieri A. Monoclonal gammopathies of clinical significance. Hematology Am Soc Hematol Educ Program. 2020 Dec 4;2020(1):380-388. doi: 10.1182/hematology.2020000122
- Cohen AL, Sarid R. The relationship between monoclonal gammopathy of undetermined significance and venous thromboembolic disease. Thromb Res. 2010 Mar;125(3):216-9. doi: 10.1016/j.thromres.2009.01.004
- Srkalovic, G., Cameron, M.G., Rybicki, L., Deitcher, S.R., Kattke-Marchant, K. and Hussein, M.A. (2004), Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer, 101: 558-566. https://doi.org/10.1002/cncr.20405
- Kristinsson SY, Tang M, Pfeiffer RM, Björkholm M, Blimark C, Mellqvist UH, Wahlin A, Turesson I, Landgren O. Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood. 2010 Oct 14;116(15):2651-5. doi: 10.1182/blood-2010-04-282848
- Piot JM, Royer M, Schmidt-Tanguy A, Hoppé E, Gardembas M, Bourrée T, Hunault M, François S, Boyer F, Ifrah N, Renier G, Chevailler A, Audran M, Chappard D, Libouban H, Mabilleau G, Legrand E, Bouvard B. Factors associated with an increased risk of vertebral fracture in monoclonal gammopathies of undetermined significance. Blood Cancer J. 2015 Aug 28;5(8):e345. doi: 10.1038/bcj.2015.71
- Nagoshi, H., Taki, T., Chinen, Y., Tatekawa, S., Tsukamoto, T., Maegawa, S., Yamamoto-Sugitani, M., Tsutsumi, Y., Kobayashi, T., Matsumoto, Y., Horiike, S., Okuno, Y., Fujiwara, S., Hata, H., Kuroda, J. and Taniwaki, M. (2015), Transcriptional dysregulation of the deleted in colorectal carcinoma gene in multiple myeloma and monoclonal gammopathy of undetermined significance. Genes Chromosomes Cancer, 54: 788-795. https://doi.org/10.1002/gcc.22290
- Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH, Wahlin A, Bjorkholm M, Turesson I. Risk of plasma cell and lymphoproliferative disorders among 14621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood. 2009 Jul 23;114(4):791-5. doi: 10.1182/blood-2008-12-191676
- Vachon CM, Kyle RA, Therneau TM, Foreman BJ, Larson DR, Colby CL, Phelps TK, Dispenzieri A, Kumar SK, Katzmann JA, Rajkumar SV. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009 Jul 23;114(4):785-90. doi: 10.1182/blood-2008-12-192575
- Genet P, Sutton L, Chaoui D, Al Jijakli A, Gerbe J, Masse V, Wifaq B. Prevalence of monoclonal gammopathy in HIV patients in 2014. J Int AIDS Soc. 2014 Nov 2;17(4 Suppl 3):19649. doi: 10.7448/IAS.17.4.19649
- Hofmann JN, Beane Freeman LE, Murata K, Andreotti G, Shearer JJ, Thoren K, Ramanathan L, Parks CG, Koutros S, Lerro CC, Liu D, Rothman N, Lynch CF, Graubard BI, Sandler DP, Alavanja MC, Landgren O. Lifetime Pesticide Use and Monoclonal Gammopathy of Undetermined Significance in a Prospective Cohort of Male Farmers. Environ Health Perspect. 2021 Jan;129(1):17003. doi: 10.1289/EHP6960
- Castaneda-Avila MA, Ulbricht CM, Epstein MM. Risk factors for monoclonal gammopathy of undetermined significance: a systematic review. Ann Hematol. 2021 Apr;100(4):855-863. doi: 10.1007/s00277-021-04400-7
- Georgakopoulou R, Andrikopoulou A, Sergentanis TN, Fiste O, Zagouri F, Gavriatopoulou M, Psaltopoulou T, Kastritis E, Terpos E, Dimopoulos MA. Overweight/Obesity and Monoclonal Gammopathy of Undetermined Significance. Clin Lymphoma Myeloma Leuk. 2021 Jun;21(6):361-367. doi: 10.1016/j.clml.2021.01.008
- Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. British Journal of Haematology, 121: 749-757. https://doi.org/10.1046/j.1365-2141.2003.04355.x
- Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos MV, Lonial S, Joao C, Anderson KC, García-Sanz R, Riva E, Du J, van de Donk N, Berdeja JG, Terpos E, Zamagni E, Kyle RA, San Miguel J, Goldschmidt H, Giralt S, Kumar S, Raje N, Ludwig H, Ocio E, Schots R, Einsele H, Schjesvold F, Chen WM, Abildgaard N, Lipe BC, Dytfeld D, Wirk BM, Drake M, Cavo M, Lahuerta JJ, Lentzsch S. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019 Jun;20(6):e302-e312. doi: 10.1016/S1470-2045(19)30309-2. Erratum in: Lancet Oncol. 2019 Jul;20(7):e346.
- Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010 Jun;24(6):1121-7. doi: 10.1038/leu.2010.60
- Monoclonal Gammopathies of Undetermined Significance (MGUS) Treatment & Management. https://emedicine.medscape.com/article/204297-treatment#d9
- van de Donk NW, Palumbo A, Johnsen HE, Engelhardt M, Gay F, Gregersen H, Hajek R, Kleber M, Ludwig H, Morgan G, Musto P, Plesner T, Sezer O, Terpos E, Waage A, Zweegman S, Einsele H, Sonneveld P, Lokhorst HM; European Myeloma Network. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014 Jun;99(6):984-96. doi: 10.3324/haematol.2013.100552
- Niermeijer JM, Eurelings M, Lokhorst HL, van der Pol WL, Franssen H, Wokke JH, Notermans NC. Rituximab for polyneuropathy with IgM monoclonal gammopathy. J Neurol Neurosurg Psychiatry. 2009 Sep;80(9):1036-9. doi: 10.1136/jnnp.2008.155325
- Pelzer BW, Arendt M, Moebus S, Eisele L, Jöckel KH, Dührsen U, Dürig J; Heinz Nixdorf Recall Study Investigative Group. Light chain monoclonal gammopathy of undetermined significance is characterized by a high disappearance rate and low risk of progression on longitudinal analysis. Ann Hematol. 2018 Aug;97(8):1463-1469. doi: 10.1007/s00277-018-3305-x
- Baldursdóttir TR, Löve ÞJ, Gíslason GK, Björkholm M, Mellqvist UH, Lund SH, Blimark CH, Turesson I, Hultcrantz M, Landgren O, Kristinsson SY. Autoimmune disease is associated with a lower risk of progression in monoclonal gammopathy of undetermined significance. Eur J Haematol. 2021 Mar;106(3):380-388. doi: 10.1111/ejh.13563
- Pang L, Rajkumar SV, Kapoor P, Buadi F, Dispenzieri A, Gertz M, Lacy M, Kyle R, Kumar S. Prognosis of young patients with monoclonal gammopathy of undetermined significance (MGUS). Blood Cancer J. 2021 Feb 1;11(2):26. doi: 10.1038/s41408-021-00406-6