Microcystic adnexal carcinoma
Microcystic adnexal carcinoma also called sclerosing sweat duct carcinoma, malignant syringoma, syringoid eccrine carcinoma, eccrine epithelioma, syringomatous carcinoma or sweat gland carcinoma with syringomatous features, is an uncommon, slow-growing, locally aggressive tumor of the eccrine sweat glands that typically extends far beyond the clinically evident lesion but has a low risk of regional or distant metastasis 1. Sweat gland cancers, combining all types, are particularly rare, accounting for 0.005% of all malignant epithelial neoplasms 2, with a total of ~350 cases currently described in the English literature. Clinically, microcystic adnexal carcinoma presents in the fourth to seventh decades of life, with no evident gender predilection although some literature lists a slight female predominance 3. However, microcystic adnexal carcinoma occasionally has occurred in children. It has been reported that the preferential location for the development of microcystic adnexal carcinoma is the head and neck region (85% of cases), demonstrating a preference for the periorbital skin and the centrofacial region 4.
Microcystic adnexal carcinoma or sclerosing sweat duct carcinoma is most common in Caucasians, but has been reported in African Americans, Spanish, Puerto Rican, Korean and Japanese patients 5. It also occurs in patients who previously received radiotherapy 6. Although extension to the muscle, vascular adventitia, perichondrium, periosteum and bone marrow has been reported, metastases are rare, occurring in approximately 2.1% of lesions 5. The estimated death rate is 0.2% 5.
Microcystic adnexal carcinoma often grows slowly and will present as a white to pink plaque located on the face. Often, it has been overlooked or ignored. Often it is confused with a basal cell carcinoma clinically. In such an instance, a shave biopsy is usually performed. However, in the case of microcystic adnexal carcinoma, the pathology lies deeper than a superficial shave biopsy will show. Thus, making the diagnosis more difficult. If suspected, a punch biopsy should be performed.
Microcystic adnexal carcinoma is commonly misdiagnosed about 27% of the time clinically and histologically, most commonly as desmoplastic trichoepithelioma 7, morpheaform basal cell carcinoma (BCC), or syringoma when superficial biopsy techniques are used 8. Less often, microcystic adnexal carcinoma is misdiagnosed as squamous cell carcinoma (SCC) because the superficial portion of microcystic adnexal carcinoma can have keratinaceous structures that resemble SCC 9.
Microcystic adnexal carcinoma is resistant to radiotherapy and chemotherapy, which may be predicted from its slow biological activity 10. The preferred treatment option is complete surgical excision; however, the true tumor-free margins are typically far beyond the intraoperative macroscopically established margins 11. Mohs micrographic surgery may be regarded as the gold standard treatment option, with adjuvant radiotherapy and wide local excision offering comparable efficacy 12. When performing adjuvant radiotherapy, a dose of >50 Gy should be administered with wide margins (3–5 cm), owing to the tendency of the lesion for perineural and deep invasion 13. Radiation may also be sought as treatment when standard surgical therapy may prove difficult and result in poor cosmetic outcome 14.
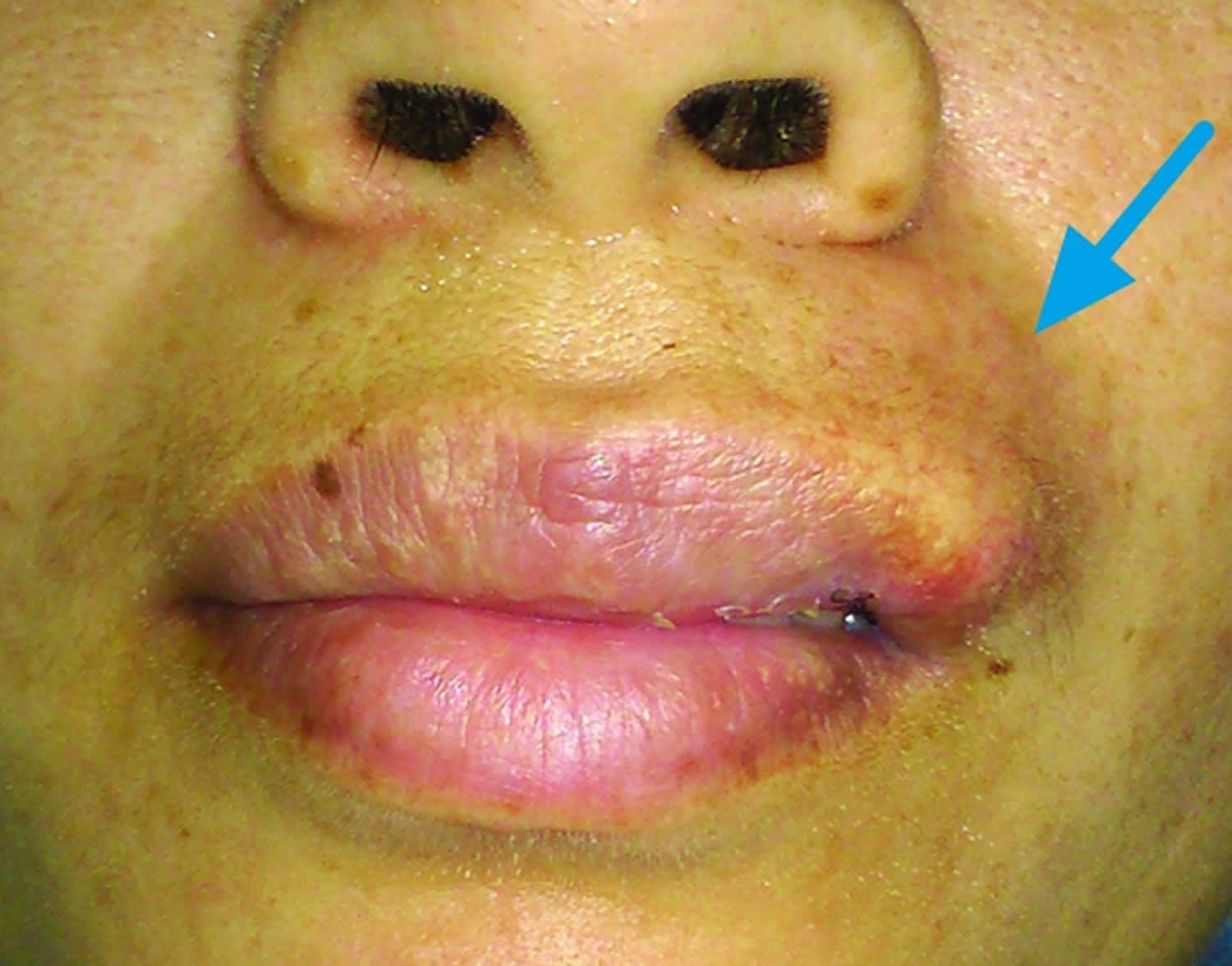
Figure 1. Microcystic adnexal carcinoma
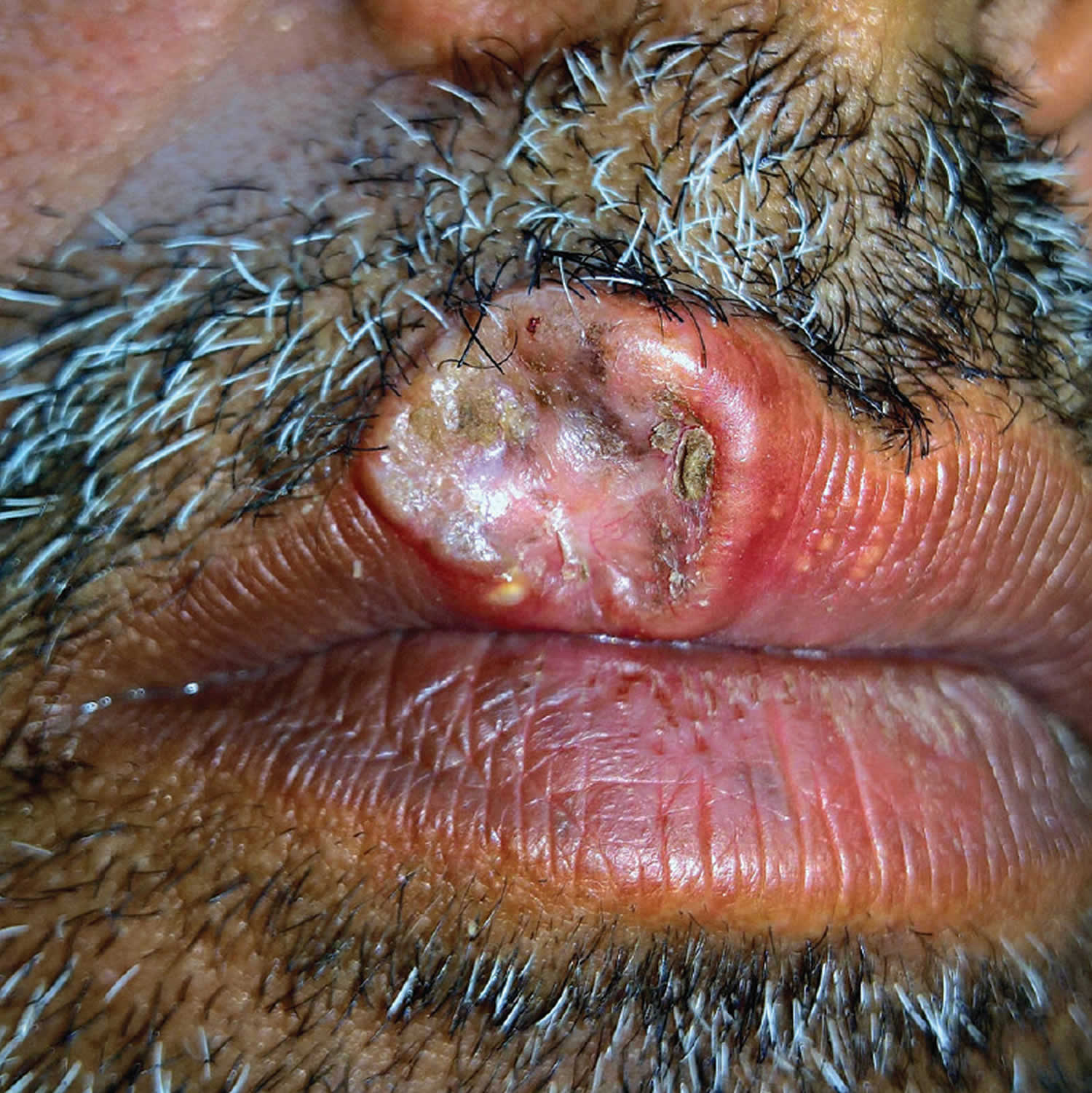
Figure 2. Microcystic adnexal carcinoma
Footnote: Indurated plaque with a central crateriform depression and elevated pearly rolled out margins on the right side of upper lip.
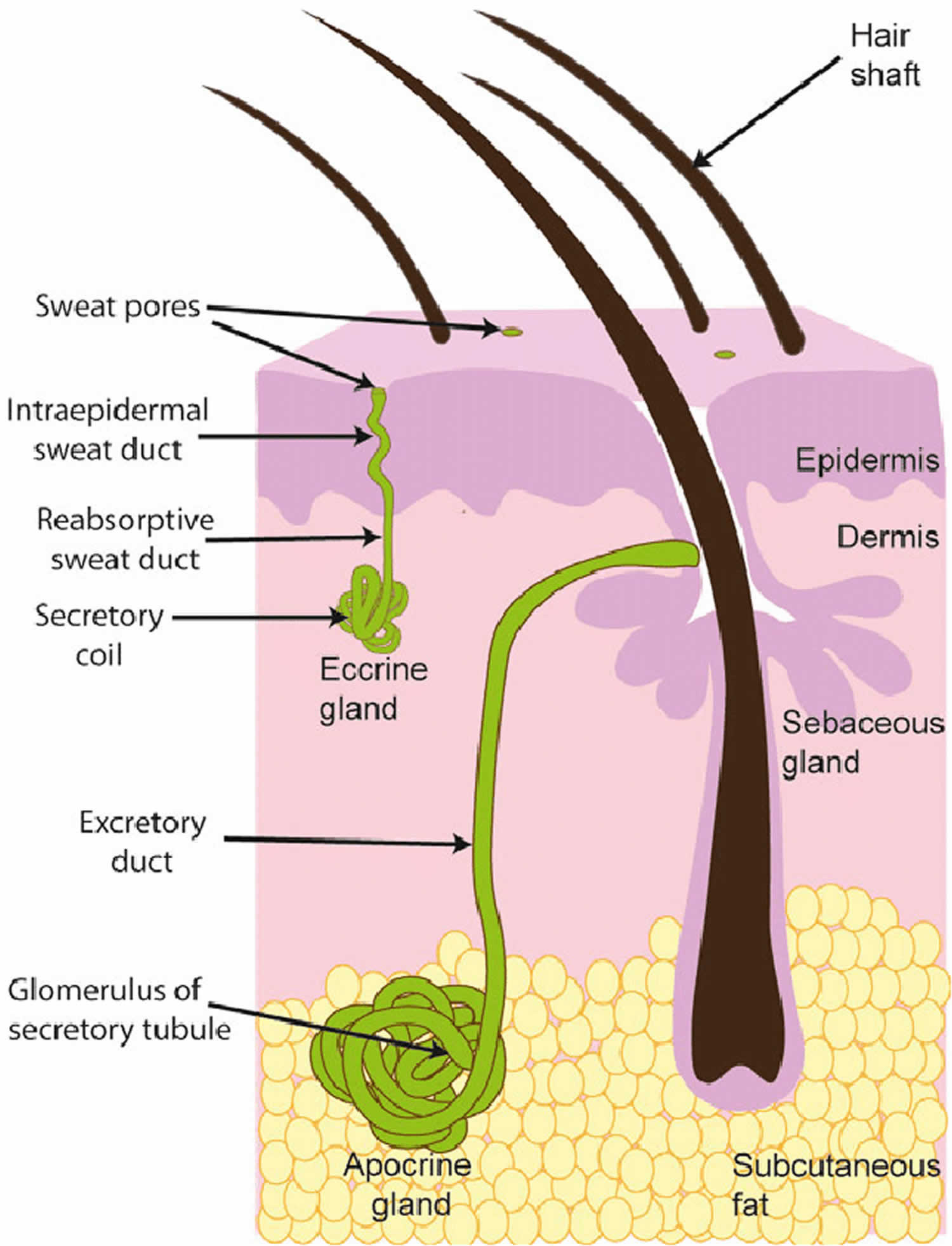
[Source 15 ]What are sweat glands?
There are two types of sweat glands: eccrine glands and apocrine glands. Eccrine and apocrine glands, hair follicles and sebaceous glands (which produce sebum) are often referred to as epidermal adnexal structures.
Sweat gland lesions generally have variable apocrine or eccrine differentiation. Traditionally these lesions have been categorized under either the apocrine or eccrine groups, but significant overlaps do exist.
Eccrine glands
Eccrine glands play an important role in the regulation of body temperature. They are distributed over the entire body skin but are highest in density on the palms and soles. Eccrine glands secrete a salty solution, which reaches the skin pores via an eccrine duct.
Eccrine sweat is a sterile electrolyte solution primarily containing sodium chloride, potassium and bicarbonate, with smaller quantities of various other components such as glucose and antimicrobial peptides. Sweat rate differs depending on the site on the body, the degree of thermal or physical stress, and between individuals. Under maximal stimulation, the body can produce 3 litres of eccrine sweat per hour.
Apocrine glands
In contrast, apocrine glands produce an oily fluid rich in triglycerides and fatty acids – subsequent colonization by anaerobic bacteria results in body odor.
Figure 3. Sweat glands
Microcystic adnexal carcinoma causes
The cause of microcystic adnexal carcinoma is not yet being fully understood, but it is believed to originate from pluripotent keratinocytes, which are capable of differentiation into hair follicles or sweat glands 16.
Risk factors for developing microcystic adnexal carcinoma
Risk factors have been identified for forming microcystic adnexal carcinoma:
- Ultraviolet (UV) radiation
- Previous radiation therapy
- Immunosuppressive medications
The estimate for the link to therapeutic radiation ranges from 19% to 50%, with an average latent period of 30 to 40 years 17. UV radiation is often implicated due to the fact that most lesions appear in sun-exposed areas and those with Fitzpatrick skin types 1 and 2.
Microcystic adnexal carcinoma has also been reported in younger individuals with a history of additional UV exposure, such as fighter pilots 18.
The effect of immunosuppression on the risk for the development of microcystic adnexal carcinoma is unclear at this time, although immunosuppression increases the risk of basal cell carcinoma and squamous cell carcinoma.
Microcystic adnexal carcinoma signs and symptoms
Microcystic adnexal carcinoma occurs as a slow growing, locally aggressive nodule, usually on the head or neck of an adult age 40 to 60 years 19. Typically, microcystic adnexal carcinoma appears as a solitary, smooth-surfaced, slow-growing, firm, non-ulcerated, asymptomatic skin-colored yellowish plaque, papule, nodule or cystic lesion 10. The lesion is most commonly located on the central face 5. Some studies have found microcystic adnexal carcinoma to be most often located on the left side of the face, possibly secondary to greater ultraviolet light exposure in cars 6. However, it has also been reported on the trunk 20, extremities 21, genitals 20, armpits 6 and scalp 22. Although usually asymptomatic, some lesions are associated with burning, pain, anesthesia or paresthesia, likely secondary to perineural invasion 23.
It has been reported in the literature that certain microcystic adnexal carcinoma cases have presented with tumor histories as long as 17 and 27 years 24. Notably, due to the asymptomatic presentation and extremely slow-growth of the tumors, all reported patients have been diagnosed at least 1 year after the initial appearance of the lesions.
Microcystic adnexal carcinoma complications
Complications of untreated tumors are locally aggressive invasion. Depending on location, complications can result from the invasion of vital structures. In some instances, death has resulted.
Microcystic adnexal carcinoma diagnosis
Microcystic adnexal carcinoma often may be clinically indistinguishable from other skin-colored growths on the head and neck. microcystic adnexal carcinoma may have perineural spread where patients can complain of numbness, tingling, pain, burning, and itching sensations. The key to diagnosis is taking an appropriate pathology specimen is needed. The tumor largely lies within the deep dermis. A superficial biopsy can lead to an incorrect diagnosis. If microcystic adnexal carcinoma is suspected, avoid a superficial shave biopsy. An incisional biopsy is recommended in the evaluation. However, punch biopsies and excisional biopsies are options in evaluation.
Full skin examination
If a biopsy results in a diagnosis of microcystic adnexal carcinoma, a full-body skin examination should take place.
Lymph nodes
- Lymph node examination should be performed to assess for regional metastases, albeit rare.
- Fine needle aspiration (FNA) or excisional biopsy of enlarged lymph nodes should be performed if detected on examination.
Imaging
Consider the need for imaging (CT or MRI) or fat-suppression MRI protocol for perineural invasion or intraorbital tumors 25.
Dermoscopy
Dermoscopy does not offer a definitive diagnosis of microcystic adnexal carcinoma. microcystic adnexal carcinoma is a rare tumor, so dermoscopic findings are limited to case reports with the most characteristic finding appears to be the presence of whitish clods of variable size. These whitish clods likely representing keratinous cyst 26. microcystic adnexal carcinoma can appear like a basal cell carcinoma due to the presence of arborizing vessels reported in case literature 27.
Microcystic adnexal carcinoma histopathology
Microcystic adnexal carcinoma presents histologically as a deeply-infiltrative and poorly-circumscribed tumor, consisting of solid aggregates of tumor cells in the mid dermis, superficial keratinous cysts and elongated tubular structures in the deep aspect of the lesion. These cell nests are enveloped by dense, fibrous stroma 28. Histologically, microcystic adnexal carcinoma can mimic infiltrative or morpheaform basal cell carcinoma, desmoplastic squamous cell carcinoma, desmoplastic trichoepithelioma, trichoadenoma, and syringoma.
Histopathological examination reveals small basaloid cells/keratocysts on the top, hence the term “microcystic.” This is a key feature. On higher power, one can see cords and strands of basaloid epitheloid cells displaying ductal lumina that invade the dermis and subdermis. There is also fibrotic stroma. A key feature in distinguishing microcystic adnexal carcinoma from other tumors is a zone of separation between the epidermis and tumor. Additionally, the ductal structures distinguish microcystic adnexal carcinoma from desmoplastic trichoepithelioma and trichoadenoma.
Microcystic adnexal carcinoma is distinguished from morpheaform basal cell carcinoma and desmoplastic squamous cell carcinoma on the basis of ductal differentiation.
Immunohistochemistry
Immunohistochemistry (IHC) is utilized in the pathologic evaluation of microcystic adnexal carcinoma to help support the diagnosis. In particular, the carcinoembryonic antigen stain (CEA) is positive in approximately 50% of microcystic adnexal carcinoma cases. This helps distinguish microcystic adnexal carcinoma from basal cell carcinoma and squamous cell carcinoma where CEA is negative.
Stains
Hematoxylin and eosin (H&E) stain is the standard stain used in the diagnosis of microcystic adnexal carcinoma.
Microcystic adnexal carcinoma treatment
Mohs micrographic surgery is the treatment of choice, in order to obtain margins that are tumor-free with fewer procedures and therefore decrease the likelihood of recurrence 29. In one study, the defect surface area after full extirpation following Mohs was a mean of 4 times that of the clinically apparent size, indicating that a margin cannot be safely predicted for conventional surgery; the recurrence rate was 1.98% per patient-year 20. In another study of 44 cases treated by Mohs surgery, there was 1 case of recurrence in the 20 patients who completed the 5-year follow-up 30.
In the event that Mohs micrographic surgery is not an option due to lack of procedure availability or tumor extent, intraoperative frozen sections may be useful for guiding conventional surgical excision.
Local recurrence occurs in approximately 50% of cases treated with conventional excision, likely due to involvement of deep structures, such as skeletal muscle and perineural spaces 31. Although there has been a case report of success in treating a lower lip microcystic adnexal carcinoma with radiotherapy with 6 month follow-up, radiotherapy is not currently an indicated treatment, as it may induce conversion to a more clinically extensive and histologically aggressive tumor 32.
Patients should be followed every six to twelve months following diagnosis and management for full-body skin examination and lymph node examination.
Microcystic adnexal carcinoma prognosis
The major prognostic concern for microcystic adnexal carcinoma is the recurrence of the tumor. The aggressive and locally invasive nature of microcystic adnexal carcinoma results in high recurrence rates ranging from 15–60% depending on the treatment modality, with lower rates of recurrence observed following Mohs micrographic surgery 33. However, such data may be an overestimate, with misdiagnosis often occurring at the time of excision, and positive margins can occasionally be misread. Although extension to the muscle, vascular adventitia, perichondrium, periosteum and bone marrow has been reported, metastases are rare, occurring in approximately 2.1% of lesions 5. It has been reported that tumor recurrence may occur up to 30 years after the initial surgical excision 34, highlighting the importance of regular, long-term follow-up. According to the relevant literature, only one mortality has occurred as a result of microcystic adnexal carcinoma 35. The estimated death rate is 0.2% 10.
References- Chen J, Yang S, Chen J, Liao T, Deng W, Li W. Microcystic adnexal carcinoma in a non-Caucasian patient: A case report and review of the literature. Oncol Lett. 2016;11(4):2471-2474. doi:10.3892/ol.2016.4242 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4812535
- Wick MR, Goellner JR, Wolfe JT 3rd, Su WP. Adnexal carcinomas of the skin. I. Eccrine carcinomas. Cancer. 1985 Sep 1;56(5):1147-62. doi: 10.1002/1097-0142(19850901)56:5<1147::aid-cncr2820560532>3.0.co;2-3
- Gordon S, Fischer C, Martin A, Rosman IS, Council ML. Microcystic Adnexal Carcinoma: A Review of the Literature. Dermatol Surg. 2017 Aug;43(8):1012-1016. doi: 10.1097/DSS.0000000000001142
- Yu JB, Blitzblau RC, Patel SC, Decker RH, Wilson LD. Surveillance, Epidemiology, and End Results (SEER) database analysis of microcystic adnexal carcinoma (sclerosing sweat duct carcinoma) of the skin. Am J Clin Oncol. 2010 Apr;33(2):125-7. doi: 10.1097/COC.0b013e31819791eb
- Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
- Abbate M, Zeitouni NC, Seyler M, Hicks W, Loree T, Cheney RT. Clinical course, risk factors, and treatment of microcystic adnexal carcinoma: a short series report. Dermatol Surg. 2003;29(10):1035-1038.
- Rustemeyer J, Zwerger S, Pörksen M, Junker K. Microcystic adnexal carcinoma of the upper lip misdiagnosed benign desmoplastic trichoepithelioma. Oral Maxillofac Surg. 2013;17:141–144. doi: 10.1007/s10006-012-0341-x
- Worley B, Owen JL, Barker CA, et al. Evidence-Based Clinical Practice Guidelines for Microcystic Adnexal Carcinoma: Informed by a Systematic Review. JAMA Dermatol. 2019;155(9):1059–1068. doi:10.1001/jamadermatol.2019.1251
- Hoang MP, Dresser KA, Kapur P, High WA, Mahalingam M. Microcystic adnexal carcinoma: an immunohistochemical reappraisal. Mod Pathol. 2008 Feb;21(2):178-85. doi: 10.1038/modpathol.3801000
- Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008 Nov-Dec;21(6):452-8. doi: 10.1111/j.1529-8019.2008.00246.x
- Mayer MH, Winton GB, Smith AC, Lupton GP, Parry EL, Shagets FW. Microcystic adnexal carcinoma (sclerosing sweat duct carcinoma). Plast Reconstr Surg. 1989 Dec;84(6):970-5. doi: 10.1097/00006534-198912000-00018
- Chaudhari SP, Mortazie MB, Blattner CM, Garelik J, Wolff M, Daulat J, Chaudhari PJ. Treatments for microcystic adnexal carcinoma–A review. J Dermatolog Treat. 2016;27(3):278-84. doi: 10.3109/09546634.2015.1089351
- Baxi S, Deb S, Weedon D, Baumann K, Poulsen M. Microcystic adnexal carcinoma of the skin: the role of adjuvant radiotherapy. J Med Imaging Radiat Oncol. 2010 Oct;54(5):477-82. doi: 10.1111/j.1754-9485.2010.02200.x
- Hinther K, Henni J, Asiniwasis RN. An atypical presentation of extensive centrofacial microcystic adnexal carcinoma responding to radiation: A case report and review of the literature. SAGE Open Med Case Rep. 2020;8:2050313X20953114. Published 2020 Sep 26. doi:10.1177/2050313X20953114 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7534061
- Gupta V, Kakkar A, Agarwal S, Sulaiman M, Ramam M. Dermoscopic pitfall: Microcystic adnexal carcinoma mimicking basal cell carcinoma. Indian J Dermatol Venereol Leprol 2020;86:202-5. https://www.ijdvl.com/text.asp?2020/86/2/202/277252
- LeBoit PE, Sexton M. Microcystic adnexal carcinoma of the skin. A reappraisal of the differentiation and differential diagnosis of an underrecognized neoplasm. J Am Acad Dermatol. 1993 Oct;29(4):609-18. doi: 10.1016/0190-9622(93)70228-l
- Zito PM, Mazzoni T. Microcystic Adnexal Carcinoma. [Updated 2020 Sep 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557857
- Gerall CD, Sippel MR, Yracheta JL, Hogan FS. Microcystic Adnexal Carcinoma: A Rare, Commonly Misdiagnosed Malignancy. Mil Med. 2019 Dec 01;184(11-12):948-950.
- Ongenae KC, Verhaegh ME, Vermeulen AH, Naeyaert JM. Microcystic adnexal carcinoma: an uncommon tumor with debatable origin. Dermatol Surg. 2001;27(11):979-984.
- Chiller K, Passaro D, Scheuller M, Singer M, McCalmont T, Grekin RC. Microcystic adnexal carcinoma: forty-eight cases, their treatment, and their outcome. Arch Dermatol. 2000;136(11):1355-1359.
- Sabhikhi AK, Rao CR, Kumar RV, Hazarika D. Microcystic adnexal carcinoma. Int J Dermatol. 1997;36(2):134-136.
- Chow WC, Cockerell CJ, Geronemus RG. Microcystic adnexal carcinoma of the scalp. J Dermatol Surg Oncol. 1989;15(7):768-771.
- Sebastien TS, Nelson BR, Lowe L, Baker S, Johnson TM. Microcystic adnexal carcinoma. J Am Acad Dermatol. 1993;29(5 Pt 2):840-845.
- Miyamoto T, Kambe N, Nishiura S, Mihara M, Shimao S. Microcystic adnexal carcinoma. Electron microscopic and immunohistochemical study. Dermatologica. 1990;180(1):40-3.
- Worley B, Owen JL, Barker CA, Behshad R, Bichakjian CK, Bolotin D, Bordeaux JS, Bradshaw S, Cartee TV, Chandra S, Cho N, Choi J, Council ML, Eisen DB, Golda N, Huang CC, Ibrahim SF, Jiang SIB, Kim J, Lacutoure M, Lawrence N, Lee EH, Leitenberger JJ, Maher IA, Mann M, Minkis K, Mittal B, Nehal KS, Neuhaus I, Ozog DM, Petersen B, Samie F, Shin TM, Sobanko JF, Somani AK, Stebbins WG, Thomas JR, Thomas V, Tse D, Waldman A, Xu YG, Yu SS, Zeitouni NC, Ramsay T, Poon E, Murad A. Evidence-Based Clinical Practice Guidelines for Microcystic Adnexal Carcinoma: Informed by a Systematic Review. JAMA Dermatol. 2019 Jul 3. doi: 10.1001/jamadermatol.2019.1251
- Calderón-Castrat X, Román-Curto C, Santos-Briz A, Fernández-López E. Microcystic adnexal carcinoma mimicking basal cell carcinoma. JAAD Case Rep. 2017;3(6):492-494. Published 2017 Oct 9. doi:10.1016/j.jdcr.2017.07.010 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5635954
- Gupta V, Kakkar A, Agarwal S, Sulaiman M, Ramam M. Dermoscopic pitfall: Microcystic adnexal carcinoma mimicking basal cell carcinoma. Indian J Dermatol Venereol Leprol. 2020 Mar-Apr;86(2):202-205. doi: 10.4103/ijdvl.IJDVL_209_19
- Klein W, Chan E, Seykora JT. Tumors of the epidermal appendages. In: Elder DE, editor. Lever’s Histopathology of the Skin. 9th. Lippincott Williams & Wilkins; Edinburgh: 2005. pp. 897–898.
- Khachemoune A, Olbricht SM, Johnson DS. Microcystic adnexal carcinoma: report of four cases treated with Mohs’ micrographic surgical technique. Int J Dermatol. 2005;44(6):507-512.
- Leibovitch I, Huilgol SC, Selva D, Lun K, Richards S, Paver R. Microcystic adnexal carcinoma: treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2005:52(2):295-300.
- Cooper PH, Headington JT, Mills SE, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9(6):422-433.
- Stein JM, Ormsby A, Esclamado R, Bailin P. The effect of radiation therapy on microcystic adnexal carcinoma: a case report. Head Neck. 2003;25(3):251-254.
- Nelson PS, Bourgeois KM, Nicotri T Jr, Chiu ES, Poole JC. Sclerosing sweat duct carcinoma in a 6-year-old African American child. Pediatr Dermatol. 2008 Jan-Feb;25(1):38-42. doi: 10.1111/j.1525-1470.2007.00579.x
- Burns MK, Chen SP, Goldberg LH. Microcystic adnexal carcinoma. Ten cases treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1994 Jul;20(7):429-34. doi: 10.1111/j.1524-4725.1994.tb03212.x
- Peterson CM, Ratz JL, Sangueza OP. Microcystic adnexal carcinoma: First reported case in an African American man. J Am Acad Dermatol. 2001 Aug;45(2):283-5. doi: 10.1067/mjd.2001.114298