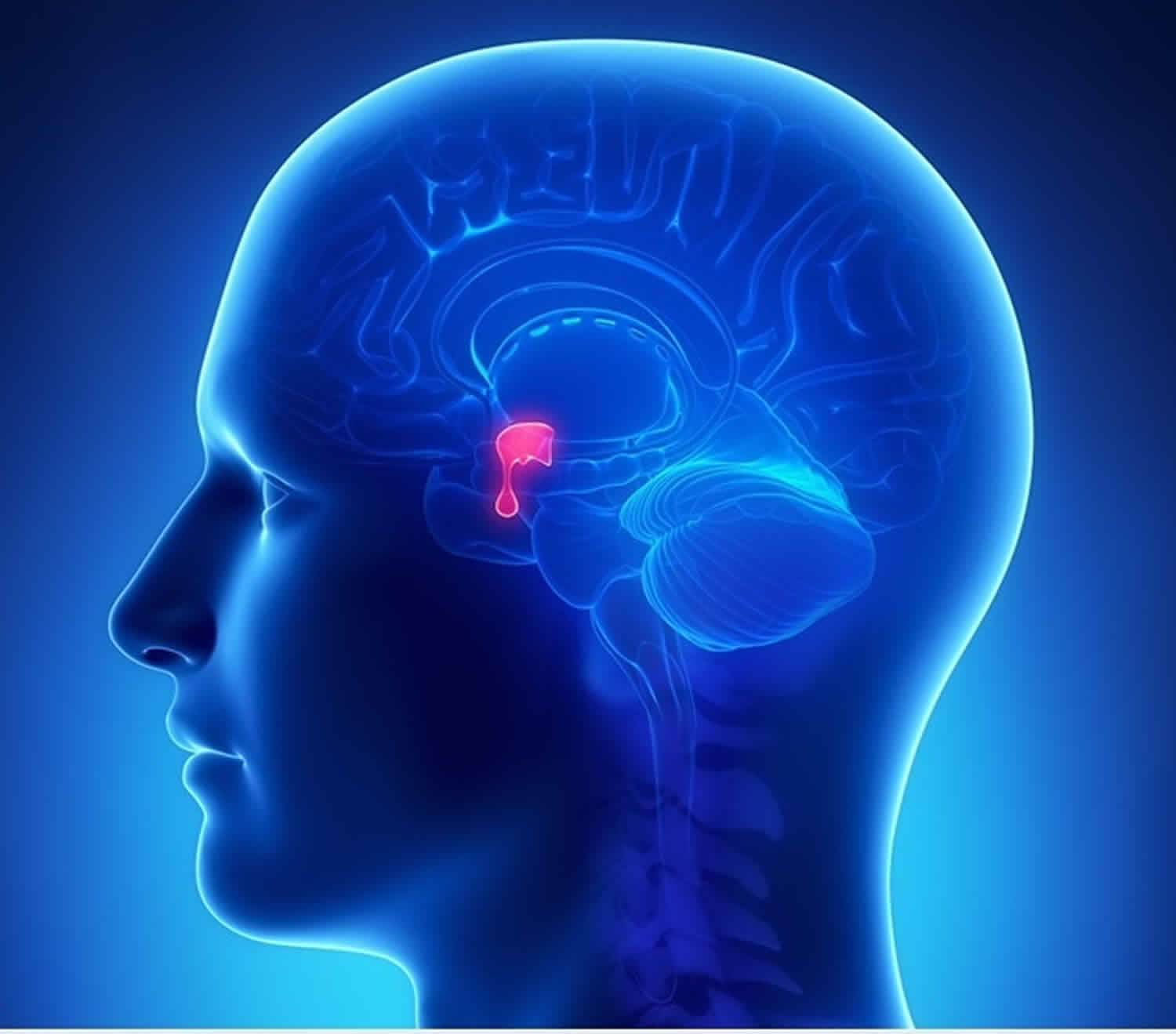
What is pituitary adenoma
Pituitary adenoma is a benign (noncancerous) tumor of the neuroendocrine epithelial cells of the pituitary gland and they do not spread to other parts of the body 1. Most pituitary adenomas are located in the anterior lobe (front portion) of the pituitary gland. The overwhelming majority of pituitary adenomas are benign and present either with characteristic syndromes of excess hormone secretion or secondary to mass effect by the growing tumor 2. The common hypersecretory syndromes include Cushing’s disease, acromegaly/gigantism, and hyperprolactinemia. As a pituitary adenoma grows beyond the confines of the sella turcica, the normal hormone-releasing cells of the pituitary may be damaged. This results in the pituitary gland not producing enough of its hormones. This condition is called hypopituitarism. Pituitary adenoma can commonly affect the visual pathways presenting with visual field deficits.
The causes of pituitary adenomas are unknown. Some pituitary adenomas are part of a hereditary disorder called multiple endocrine neoplasia I (MEN I).
The pituitary gland is part of the endocrine system and is located at the base of your brain. The pituitary gland helps control the release of hormones from other endocrine glands, such as the thyroid, sex glands (testes or ovaries), and adrenal glands. The pituitary gland also releases hormones that directly affect body tissues, such as bones and the breast milk glands. The pituitary hormones include:
- Adrenocorticotropic hormone (ACTH)
- Growth hormone (GH)
- Prolactin
- Thyroid-stimulating hormone (TSH)
- Luteinizing hormone (LH) and follicle-stimulating hormone (FSH)
Your body uses these hormones for many functions, including growth, sex, and metabolism. Metabolism is the way your body uses food for energy.
Pituitary adenomas are common intracranial tumors and may be clinically silent, detected incidentally on MRI scans of the brain (~ 22%) 3 or found at autopsy (~ 14 to 25% of the population may harbor undiagnosed pituitary adenomas) 4. Pituitary adenomas account for approximately 10 to 15% of surgically treated primary tumors of the central nervous system (CNS) 5. The incidence appears higher in African Americans in whom pituitary adenomas account for over 20% of the non-metastatic central nervous system (CNS) tumors 6. Although the incidence varies according to age, sex, and ethnic group, between 0.5 and 8.2 per 100,000 in the population are diagnosed annually with a pituitary adenoma 7. The majority of pituitary adenomas are less than 3 to 5 mm in diameter and would not require medical or surgical intervention. More recent series using magnetic resonance imaging (MRI) of healthy subjects indicate that approximately 10% of the population harbors pituitary lesions 2. Some series report a higher rate of diagnosis among women of childbearing age. However, women may not actually have a higher incidence of pituitary adenoma 8. Because disruption of the pituitary axis affects reproductive capacity, women with pituitary adenomas may simply come to clinical attention at a higher rate than men 2.
Amongst the varying classes of pituitary adenomas, prolactinomas and non-functioning adenomas have the highest incidence, and account for nearly two-thirds of all pituitary tumors. Prolactin-secreting adenomas comprise 40 to 60% of functioning adenomas and are the most common subtype of pituitary tumor diagnosed in adolescents 9. The majority of microadenomas are found in women in their second and third decades. Men generally present later, in their fourth and fifth decades, almost always with macroadenomas.
Growth hormone (GH) secreting pituitary adenomas represent nearly 30% of all functioning tumors 2. Nearly three quarters of growth hormone (GH) secreting adenomas are macroadenomas. Approximately 40 to 60 individuals per million have acromegaly 10. Between 3 and 4 new cases per million are diagnosed annually 11. Most present in their 3rd to 5th decades after they have been developing symptoms and signs for many years 10. Acromegaly is associated with an increased incidence of cardiovascular, respiratory, cerebrovascular, and colonic cancer. Accordingly, studies report an increased risk of mortality compared to the unaffected population 12. Although some studies report a higher incidence of several cancers, others have only confirmed an increased risk of colon cancer 13. There is some evidence that mortality risk may be different between the sexes. Etxabe found a higher mortality rate in men than in women 10. Other reports find similar degrees of increased mortality in both sexes 14. Still others report increased risks of death in men from cardiovascular, respiratory, cerebrovascular, and malignant disease, but only from cerebrovascular disease in women 12.
Adrenocorticotropic hormone (ACTH) secreting pituitary adenomas account for 15 to 25% of all functioning pituitary adenomas and are the most common pituitary tumors diagnosed in pre-pubertal children 9. The majority of adrenocorticotropic hormone (ACTH) secreting pituitary adenomas, regardless of age, are microadenomas. Approximately 39 individuals per million have Cushing’s disease and the annual incidence is estimated at 2.4 per million 15. Cushing’s disease is more common in women, most of whom present in their third and fourth decades 15. There is a high incidence of hypertension and diabetes mellitus as well as higher vascular disease-related mortality 15. Nelson’s syndrome can develop after adrenalectomy in patients with Cushing’s disease, as negative feedback is then lost to a previously unrecognized intrasellar ACTH adenoma. These patients may develop hyperpigmentation and the pituitary tumors are often aggressive.
The most effective treatments for adenomas are coordinated by a multidisciplinary team that includes a neurosurgeon, otolaryngologist and/or an endocrinologist (hormone disorder specialist). Treatment may include a combination of observation, medication (including hormone therapy), radiation therapy and surgery.
Figure 1. Pituitary gland

Pituitary adenomas quick facts
- Pituitary adenomas are benign tumors of the pituitary gland. Most are located in the anterior lobe (front portion) of the pituitary gland.
- About 1 in 10 people will develop a pituitary adenoma in their lifetime.
- The pituitary gland hormones control many other glands in the body.
- Some pituitary adenomas secrete one or more hormones in excess. Even when they are small in size, these endocrine-active pituitary tumors can cause hormonal imbalances that affect body functions.
- People can develop pituitary adenomas at any age.
- Having certain genetic conditions increases the risk of developing a pituitary tumor.
- Signs of a pituitary tumor include problems with vision and certain physical changes.
- Imaging studies and tests that examine the blood and urine are used to detect (find) and diagnose a pituitary tumor.
- Certain factors affect prognosis (chance of recovery) and treatment options.
Pituitary adenoma classification
Pituitary adenomas can be classified in various ways, according to size, functional status, hormone or cytokeratin expression profile, defining somatic mutations, and histologic features (Tables 1 and 2). Pituitary adenomas that measure 10 mm or less in diameter are considered microadenomas; macroadenomas are those larger than 10 mm 2. Macroadenomas may also be sub-categorized as “giant” if their extent reaches far beyond the normal confines of the pituitary region or their greatest diameter exceeds 4 centimeters. Pituitary adenomas may also be categorized as either hypersecretory or non-functioning. The hypersecretory adenomas cause distinctive clinical syndromes that include acromegaly/gigantism (growth hormone (GH) secreting adenomas), Forbes-Albright syndrome (prolactin (PRL) secreting adenomas), thyroid-stimulating hormone (TSH)-secreting adenomas, the occasional hypersecreting luteinizing hormone (LH) or follicle-stimulating hormone (FSH) adenoma, and Cushing’s disease/Nelson’s syndrome (corticotropin (ACTH) secreting adenomas). The non-functioning adenomas have no endocrine features other than hypopituitarism (decreased pituitary hormone production) and generally present either incidentally or secondary to mass effect.
Table 1. Classification Schemes of Pituitary Adenomas
| Scheme | Features |
| Microadenoma OR Macroadenoma | < 10 millimeters OR > 10 millimeters |
| Non-Functioning adenoma OR Functioning adenoma | Endocrinologically inactive, patient may present with pituitary deficiency. OR Excess of pituitary hormone secreting: GH adenoma; PRL adenoma; ACTH adenoma; TSH adenoma; GH -PRL adenoma; FSH/LH adenoma (rare, most are non-functioning) |
Abbreviations:
- Adrenocorticotropic hormone = ACTH
- Growth hormone = GH
- Prolactin = PRL
- Thyroid-stimulating hormone = TSH
- Luteinizing hormone = LH
- Follicle-stimulating hormone = FSH
The 2004 edition of the World Health Organization (WHO) classification of endocrine tumors uses markers of cytodifferentiation as the principal classifier. In addition to the category of ‘typical pituitary adenoma’ and ‘pituitary carcinoma’, it also introduced the concept of ‘atypical pituitary adenoma’. However, the latter is controversial 16, as the criteria are to some degree subjective and the clinical significance of ‘atypia’ as currently defined remains to be determined in longitudinal studies 17. The current WHO classification is summarized in Table 2.
Table 2. WHO Classification of pituitary adenomas
| Pituitary adenoma type | Transcription Factors | Hormones | Cytokeratin |
| GH-producing adenomas | |||
| Densely granulated somatotroph adenoma | Pit-1 | GH, a-SU | diffuse |
| Sparsely granulated somatotroph adenoma | Pit-1 | GH | dot-like |
| Mammosomatotroph adenoma | Pit-1, ER | GH, PRL, a-SU | diffuse |
| Mixed somatotroph and lactotroph andenoma | Pit-1, ER | GH, PRL, a-SU | diffuse |
| PRL-producing adenomas | |||
| Sparsely granulated lactotroph adenoma | Pit-1, ER | PRL (Golgi) | diffuse |
| Densely granulated lactotroph adenoma | Pit-1, ER | PRL (diffuse) | diffuse |
| Acidophil stem-cell adenoma | Pit-1, ER | PRL (diffuse), GH | rare dot-like |
| TSH-producing adenoma | |||
| Thyrotroph adenoma | Pit-1, GATA-2 | b-TSH, a-SU | diffuse |
| ACTH-producing adenomas | |||
| Densely granulated corticotroph adenoma | Tpit | ACTH | diffuse |
| Sparsely granulated corticotroph adenoma | Tpit | ACTH | diffuse |
| Crooke’s cell adenoma | Tpit | ACTH | ring-like |
| Gonadotropin-producing adenoma | |||
| Gonadotroph adenoma | SF-1, GATA-2, ER | b-FSH, b-LH, a-SU | diffuse |
| Plurihormonal adenomas | |||
| Silent type III adenoma | Pit-1 (?), ER | multiple | diffuse |
| Unusual plurihormonal adenoma (NOS) | multiple | multiple | n/a |
| Hormone negative adenoma | |||
| Null cell adenoma | none | none | diffuse |
Abbreviations:
- Adrenocorticotropic hormone = ACTH
- Growth hormone = GH
- Prolactin = PRL
- Thyroid-stimulating hormone = TSH
- Luteinizing hormone = LH
- Follicle-stimulating hormone = FSH
Functioning pituitary adenomas
About 50 percent of pituitary adenomas produce excessive amounts of one or more particular hormones. These endocrine-active tumors are also known as secreting or functioning tumors. Excessive hormone secretion may cause:
- Cushing’s disease: Due to excessive corticosteroids in the body, Cushing’s syndrome can cause a number of symptoms, including:
- Upper body obesity
- Round face
- Increased fat around necK or a fatty hump between the shoulders
- Thinning arms and legs
- Fragile and thin skin
- Stretch marks on abdomen, thighs, buttocks, arms, and breasts
- Bone and muscle weakness
- Severe fatigue
- High blood pressure
- High blood sugar
- Irritability and anxiety
- Excess facial and body hair growth in women
- Irregular or stopped menstrual cycles in women
- Reduced sex drive and fertility in men
- Acromegaly: Excessive growth results in the enlargement of the extremities, face and soft tissues. Acromegaly may be associated with hypertension, diabetes mellitus and cardiovascular disease. Patients with acromegaly have decreased life expectancy.
- Galactorrhea: This condition is characterized by abnormal milk production from the mammary glands.
- Hyperprolactinemia. Prolactinoma is a type of pituitary tumor that overproduces prolactin. The prolactin hormone stimulates milk production from the breasts. Prolactin-secreting pituitary adenomas are the most common type of pituitary tumor, accounting for approximately 30 percent of all pituitary tumors.
- Reproductive problems, such as infertility
Non functioning pituitary adenomas
Nonfunctioning pituitary adenomas also called nonsecretory pituitary tumors do not produce hormones. They can press on or damage the pituitary gland and prevent it from secreting adequate levels of hormones.
Pituitary adenoma causes
The exact cause of pituitary adenomas is unknown.
In rare cases, inherited disorders may cause pituitary adenomas. These diseases include multiple endocrine neoplasia type 1 (MEN 1) syndrome, Carney complex, and isolated familial acromegaly 18.
It is likely that pituitaryadenomas are caused by abnormalities in one or more genes, by hormonal abnormalities, or by a combination of these factors 19. Scientists are still working to figure out what causes pituitary adenomas.
Pituitary adenoma symptoms
The symptoms of pituitary adenoma may include:
- Headaches
- Vision problems such as double vision, visual field loss (loss of peripheral vision), drooping eyelids or changes in color vision
- Weight gain
- Lack of energy
- Easy bleeding/bruising
- Change in bone structure, especially in the face and hands
- Menstrual irregularities
- Lactation
- Erectile dysfunction and decreased sexual function in men
- Heat intolerance
- Nasal drainage of clear fluid
- Nausea and vomiting
- Confusion
- Dizziness
- Seizure
- Problems with the sense of smell
- In rare cases, these symptoms occur suddenly and can be severe (pituitary apoplexy).
- Nausea and vomiting
Nonfunctioning tumors press on or damage the pituitary and prevent it from secreting enough hormones. If there is too little of a particular hormone, the gland or organ it normally controls will not function correctly. Symptoms of nonfunctioning pituitary adenomas are:
- Headache
- Some loss of vision
- Loss of body hair
- In women, less frequent menstrual periods or no periods at all, or no milk from the breasts
- In men, loss of facial hair, growth of breast tissue, and impotence
- In women and men, lower sex drive
- In children, slowed growth and sexual development
Hypersecretion
- Growth hormone (GH)-secreting adenoma:
- Gigantism (abnormal growth due to higher than normal level of growth hormone during childhood) or
- Acromegaly (higher than normal level of growth hormone in adults)
- Adrenocorticotropic hormone (ACTH)-secreting adenoma: Cushing’s disease (body has a higher than normal level of the hormone cortisol)/Nelson’s syndrome
- Prolactin (PRL)-secreting adenoma: Amenorrhea-galactorrhea (nipple discharge and irregular or absent menstrual periods in women)
- Thyroid-stimulating hormone (TSH)-secreting adenoma: Secondary hyperthyroidism (thyroid gland makes too much of its hormones; this is an extremely rare condition of pituitary tumors)
Prolactin
A pituitary tumor that produces too much prolactin may cause:
- Headache
- Some loss of vision
- Less frequent or no menstrual periods or menstrual periods with a very light flow
- Difficulty getting pregnant
- Impotence in men
- Lower sex drive
- The flow of breast milk in a woman who is not pregnant or breastfeeding
Adrenocorticotropic Hormone (ACTH)
A pituitary tumor that produces too much adrenocorticotropic hormone (ACTH) may cause:
- Headache
- Some loss of vision
- Weight gain reflected in the face, neck, and trunk of the body, but thin arms and legs
- A lump of fat on the back of the neck
- Thin skin that may include purple or pink stretch marks on the chest or abdomen
- Easy bruising
- Growth of fine hair on the face, upper back, or arms
- Bones that break easily
- Anxiety, irritability, depression
- Growth deceleration with weight gain in children
- Irregular menses
Growth Hormone
A pituitary tumor that produces too much growth hormone may cause:
- Headache
- Some loss of vision
- In adults, growth of the bones in the face, hands, and feet
- In children, excessive growth of the whole body
- Tingling or numbness in the hands and fingers
- Snoring or pauses in breathing during sleep
- Joint pain
- Sweating more than usual
- Extreme dislike of or concern about one or more parts of the body
Thyroid-Stimulating Hormone (TSH)
A pituitary tumor that produces too much TSH (through high T4) may cause:
- Irregular heartbeat
- Shakiness
- Weight loss
- Trouble sleeping
- Frequent bowel movements
- Sweating
Pituitary insufficiency (hypopituitarism)
- Symptoms: diminished libido, fatigue, weakness
- Gonadal dysfunction, Hypothyroidism, Adrenal Insufficiency, Somatotroph Insufficiency
- Mass Effect (symptomsrelated to compressed adjacent structures)
- Optic chiasm: bitemporal visual field deficit and diminished visual acuity
- Cavernous sinus: trigeminal nerve, facial pain; cranial nerves 3, 4, 6, diplopia, ptosis, mydriasis, anisocoria
- Pressure on dura or diaphragma sellae: headache
- Hypothalamus: behavior, eating, and vigilance disturbances (somnolence)
- Temporal lobe: complex partial seizures, memory and cognitive disturbances
Incidental
Discovered during the evaluation for headaches, trauma, nasal sinus disorders, dizziness
Hypersecretory syndromes
Acromegaly (growth hormone secreting pituitary adenoma) induces characteristic growth hormone-induced structural changes in physiognomy. There is an insidious coarsening of facial features with an enlarged forehead, enlarged tongue, malocclusion of the teeth, and prognathism. Patients’ hands and feet also enlarge. Many patients also report excessive sweating. The external hypertrophy of tissue is paralleled within the body. Patients suffer enlarged organs (visceromegaly) and overgrowth of joints and cartilage, along with high blood pressure, congestive heart failure, sleep apnea, spinal canal narrowing (facet hypertrophy), and carpal tunnel syndrome. Significant numbers of patients with acromegaly also have impaired glucose metabolism and diabetes mellitus.
Cushing’s disease (adrenocorticotropic hormone or ACTH secreting pituitary adenoma) causes changes in body habitus with characteristic increased weight gain, truncal obesity, “buffalo hump”, and moon facies. Skin changes are also common and include purple striae, easy bruisability, ruddy complexion, and increased body and facial hair. Patients suffer from fatigue, proximal muscle weakness, osteoporosis, psychological disorders, high blood pressure, and impaired glucose metabolism. They often have headache, menstrual disorders, and cognitive dysfunction.
Patients with prolactinomas (prolactin hormone secreting pituitary adenoma) classically present with amenorrhea or oligomenorrhea and galactorrhea. Most are women in their childbearing years and are more likely to pursue medical attention for infertility and menstrual irregularity. Men, and women beyond their reproductive years, more often have headache, visual symptoms, sexual dysfunction and signs of decreased pituitary function.
Amenorrhea and galactorrhea are not specific to prolactinomas, however. Prolactin secretion is under constant inhibitory control from the hypothalamus. Any lesion that imposes pressure upon the portal venous connection of the stalk (infundibulum) connecting the brain and pituitary gland can interrupt these inhibitory dopaminergic signals. This, in turn, causes an increase in serum prolactin levels, and mimics a prolactinoma, i.e. a ‘pseudo-prolactinoma’. In such cases serum prolactin levels are usually only moderately elevated. As a general rule, serum prolactin levels over 200 ng/ml (3600mU/L) are indicative of prolactinomas.
Hypopituitarism
Tumor growth impairs the normal secretory function of the anterior pituitary and causes hypopituitarism. Common complaints include diminished sex drive, fatigue, weakness, and hypothyroidism. Pituitary insufficiency generally develops slowly over time. However, acute pituitary insufficiency may occur in the setting of pituitary apoplexy, a condition in which the tumor infarcts or has internal bleeding. Apoplexy can be particularly devastating because it combines acute hypopituitarism with a rapidly expanding intracranial mass, and often causes visual loss or even sudden blindness..
Neurological Dysfunction
Neurologic signs and symptoms develop as pituitary adenomas grow beyond the confines of the sella turcica and exert pressure upon adjacent brain structures. As tumors enlarge, they compress the optic nerves and optic chiasm and patients experience visual deficits and diminished visual acuity. Classically this causes a bitemporal hemianopia, i.e. visual loss in the temporal fields of each eye. Tumor growth may also affect other nerves (such as the 3rd, 4th, 5th, or 6th cranial nerves) and cause facial pain and/or double vision or drooping of the eyelid. Headache, although a non-specific complaint, can occur when a tumor stretches the dural sac that surrounds the pituitary gland. Headache from pituitary lesions is usually frontal or retro-orbital – it may be bitemporal or radiate to the occipito-cervical region. Many patients will have been previously diagnosed with “migraine”.
Pituitary adenoma possible complications
The most serious complication is blindness. This can occur if the optic nerve is seriously damaged.
The tumor or its removal may cause lifelong hormone imbalances. The affected hormones may need to be replaced, and you may need to take medicine for the rest of your life.
Tumors and surgery can sometimes damage the posterior pituitary (back part of the gland). This can lead to diabetes insipidus, a condition with symptoms of frequent urination and extreme thirst.
Pituitary adenoma diagnosis
Your doctor will usually begin by giving you a physical exam and asking about your medical history. She or he will check your general health and examine your body for unusual things like lumps.
You might be given tests or procedures such as 20:
- Eye and visual field exam
- Neurological exam: During this exam, the doctor gives you a series of tests and questions to check your coordination, mental status, reflexes, and muscle function.
- Magnetic resonance imaging (MRI) or computed tomography (CT) scan: MRI uses magnetic waves to produce detailed images of the inside of the body, while a CT scan uses X-rays to produce these pictures. These machines create images of the inside of the brain and spinal cord.
- Blood tests to check levels of hormones, blood sugar, and other substances
- Urine tests to determine levels of certain hormones
- Venous sampling: In this type of test, a sample of blood is taken from veins coming from the pituitary gland. Levels of certain hormones are measured in the blood sample.
- Biopsy: Cells or tissues are removed from the pituitary gland. They are then examined under a microscope to check for signs of cancer.
Your doctor may use blood tests, urine tests and imaging to diagnose a pituitary adenoma. Blood and urine tests can detect abnormal levels of hormones such as plasma prolactin (PRL), growth hormone (GH), insulin-like growth factor-1 (IGF-1), free thyroxine, cortisol, and testosterone. Abnormal amounts of certain hormones may indicate a specific pituitary-related syndrome.
Tests to check endocrine function may be ordered, including:
- Cortisol levels: dexamethasone suppression test, urine cortisol test
- FSH level
- Insulin growth factor-1 (IGF-1) level
- LH level
- Prolactin level
- Testosterone/estradiol levels
- Thyroid hormone levels: free T4 test, TSH test
Your physician may also use enhanced, high-resolution MRI technology to identify unique characteristics of a pituitary adenoma.
Advances in neuroimaging, namely CT, CT angiography and particularly magnetic resonance imaging (MRI) have improved the visualization of the pituitary region. Increasing numbers of pituitary adenomas are diagnosed incidentally during the evaluation of sinus disorders, trauma, cervical spine disease and headache 2. These “incidentalomas” are not necessarily asymptomatic. Although visual deficits are discovered in fewer than 5%, some degree of pituitary dysfunction is found in up to 15% 21. More than one third are macroadenomas and, of these, approximately 30% will show significant enlargement over time 22. Asymptomatic incidental microadenomas are less likely to have clinically significant growth and often can be followed over time with repeated MRIs.
Although increasing numbers of tumors are diagnosed incidentally, pituitary adenomas more often present secondary to hypersecretion, hypopituitarism, or mass effect.
Figure 2. Pituitary adenoma MRI

Footnote: MRI centered on the pituitary demonstrates a large mass which involves the clivus, pituitary fossa and extends both anteriorly into the sphenoid sinus and superiorly into the suprasellar cistern, elevating and distorting the chiasm and floor of the third ventricle. No normal pituitary can be identified. It is isodense on T1 with moderate heterogeneous enhancement. Many small areas of high T2 signal as scattered throughout the mass. Microscopic Description:
Sections show multiple pieces of pituitary adenoma, associated with nasopharyngeal mucosa, and thin portions of bone. Extremely sparse cells are immunoreactive for ACTH. There is negative immunoreactivity for growth hormone, prolactin, FSH, TSH and LH.
[Source 23 ]Pituitary adenoma treatment
Although some incidentally discovered microadenomas that do not cause symptoms may be followed clinically and with repeated MRI, patients with macroadenomas generally need medical or surgical intervention. Therapeutic goals are improved quality of life and survival; elimination of mass effect and reversal of related signs and symptoms; normalization of hormonal hypersecretion; preservation or recovery of normal pituitary function; and prevention of recurrence of the pituitary tumor.
The most common treatments for pituitary adenomas are:
- Drug therapy. Drugs can be given to treat the abnormal hormone levels caused by functioning pituitary tumors. The drug given depends on the hormone that is affected by the tumor.
- Surgery. The tumor is removed by performing an operation. The surgeon may reach the pituitary gland through a cut made under the upper lip or at the bottom of the nose between the nostrils. In other cases, the surgeon may cut through the skull to reach the pituitary tumor.
- Radiation therapy. Radiation therapy involves targeting a tumor with high-energy X-rays that kill tumor cells or keep them from growing.
The primary approach for treatment will depend on the type of pituitary tumor.
Observation
Observation involves seeing a neurosurgeon or endocrinologist who can prescribe a regular schedule of imaging tests to check the status of the tumor. If the pituitary tumor grows or if symptoms worsen, you may need to pursue further treatment.
Although some incidentally discovered microadenomas that do not cause symptoms may be followed clinically and with repeated MRI, patients with macroadenomas generally need medical or surgical intervention.
Medication
Medication (drug therapy) can be very effective in treating some hormone-producing pituitary tumors. It may be used to:
- Stop a tumor from producing excess hormones.
- Shrink the tumor to stop it from pressing on the pituitary gland or other parts of the nervous system.
- Treat a pituitary tumor or control hormones after surgery or radiation therapy.
- Substitute missing hormones if a pituitary tumor has decreased the body’s ability to produce the necessary hormones or if hormone production is too low after surgery (also known as hormone replacement therapy).
Medical therapy is available for some hypersecretory tumors 24. Most prolactin-secreting adenomas are effectively treated with dopamine agonists (eg. bromocriptine, but usually cabergoline). Surgical intervention is reserved for those who are intolerant of medical therapy, whose prolactin levels remain elevated or whose tumors continue to grow despite maximal medical treatment.
Medical treatment using somatostatin analogues or dopamine agonists has varying degrees of efficacy for treating growth hormone (GH) adenomas. The growth hormone receptor antagonist, pegvisamont, may prove more effective and can be used in combination with other agents 25. Although medical therapy is most often reserved for those patients awaiting surgery or those with persistent disease postoperatively, some advocate primary medical therapy, particularly for invasive tumors 26. There is some evidence that pre-surgical medical therapy may improve surgical outcome 27.
Ketoconazole and/or metyrapone therapy can normalize serum cortisol levels in patients with Cushing’s disease preoperatively. Like acromegaly, surgery remains the first-line therapy. Clinical trials have demonstrated some role for medical therapy with cabergoline or pasireotide, and with mifepristone in selected cases 28. The disadvantage of medical treatment of hypersecretory syndromes is that it is suppressive in nature. Tumors often recur when medications are discontinued.
Pituitary adenoma surgery
For most pituitary tumors, surgery remains the first-line treatment of symptomatic pituitary adenomas. Surgery is also chosen secondarily when medical treatment or radiotherapy fails; particularly for prolactin and growth hormone-secreting adenomas. Surgery provides prompt relief from excess hormone secretion and mass effect. There is evidence to suggest that debulking of medically refractory prolactinomas and growth hormone (GH) adenomas can return these tumors to a responsive state 29. Surgery may also be indicated in pituitary apoplexy with progressive compressive signs regardless of the tumor type. However, some patients can be successfully treated without operative intervention; the ideal patient has not been conclusively established for operative versus non-operative treatment 30.
The minimally invasive transsphenoidal approach can be used effectively for 95% of pituitary tumors. Exceptions are those large tumors with significant temporal or anterior cranial fossa extension. In such circumstances, transcranial approaches are often more appropriate. Occasionally, combined transsphenoidal and transcranial approaches are used. Nevertheless, some surgeons extend the basic transsphenoidal exposure in order to remove some of these tumors and avoid a craniotomy (Figure 3) 31.
The transsphenoidal approach is a versatile method for treating pituitary tumors. Endoscopic approaches may be used in isolation or as an adjunct to the other transsphenoidal approaches 32. Computer-guided neuronavigational techniques are occasionally used in lieu of traditional fluoroscopic guidance 33. The role of neuronavigation is most pertinent in recurrent adenomas in which the midline anatomy has been distorted by previous transsphenoidal surgery. Intraoperative MRI is increasingly available and appears to be most applicable for large tumors 34. There are three basic variations of the transsphenoidal approach.
Transsphenoidal surgery: A type of surgery in which the instruments are inserted into part of the brain by going through an incision (cut) made under the upper lip or at the bottom of the nose between the nostrils and then through the sphenoid bone (a butterfly-shaped bone at the base of the skull) to reach the pituitary gland. The pituitary gland lies just above the sphenoid bone.
Figure 3. Transsphenoidal surgery

You will be asleep during the surgery. You will not feel pain. The doctor can get to your pituitary gland in one of three ways.
- The doctor makes a cut under your upper lip. This is called an incision. Then he or she puts a thin, flexible tube called a scope through the incision. The tube has a small camera on the end. The camera helps the doctor find the gland. Next, the doctor uses special tools to cut out the growth and remove it through the incision. Then he or she stitches up the incision.
- The doctor makes an incision in the back of your nose. You may get another one under your upper lip. Then the doctor puts a thin, flexible tube called a scope through one of these incisions. After the tube reaches the pituitary gland, the doctor uses special tools to remove the growth through your nose. After this surgery, you may not need stitches. The doctor may use a small piece of fat from your belly or thigh to plug up the hole in your nose. This helps prevent spinal fluid from leaking out of your nose. If this is done, you will have a small scar on your belly. It will fade with time. You will not have a scar on your face.
- In rare cases, the doctor makes an incision near the top of your head (craniotomy). He or she uses special tools to remove part of your skull. Your skull is the bone that surrounds your brain. Then the doctor gently moves your brain out of the way to get to your pituitary gland. Next, he or she cuts out the growth. Then the doctor puts your skull back in place with metal plates and clamps.
This surgery usually takes about 2 to 3 hours. If the doctor goes under your lip or through your nose, you will probably leave the hospital in 1 to 3 days. You will probably be able to return to work or your normal routine in 1 to 2 weeks. If your doctor goes through your skull, you will probably leave the hospital in 3 to 9 days. But it may take 4 to 6 weeks to fully recover.
After surgery, your symptoms may go away. For example, your vision may improve. Or your headaches may go away. If the growth comes back, or if the doctor could not remove the whole growth, you may need other treatment. This may include radiation.
After the surgery, you may need to take medicines to replace the hormones made by the pituitary gland.
Be sure to make and go to all appointments, and call your doctor or nurse call line if you are having problems. It’s also a good idea to know your test results and keep a list of the medicines you take.
Figure 4. Craniotomy

Complications of Transsphenoidal Surgery
The overall mortality rate for transsphenoidal surgery is less than 0.5% 2. Major morbidity (cerebrospinal fluid leak, meningitis, stroke, intracranial hemorrhage, and visual loss) occurs in between 1 and 2% of cases. Less serious complications (sinus disease, nasal septal perforations, and wound issues) occur in approximately 6.5%. Larger invasive tumors and giant adenomas are associated with a higher morbidity.
Pituitary adenoma surgery recovery
Pituitary surgery removes an abnormal growth on your pituitary gland. Your pituitary gland is at the base of your brain. It makes important chemicals called hormones. These hormones are involved in many of your body’s functions, including growth, sex, and your metabolism (the way your body uses food for energy).
If you had surgery under your lip or through your nose (transsphenoidal surgery), you may have a headache and a slight runny nose after surgery. This will get better in 1 to 2 weeks. Your doctor may recommend pain or decongestant medicines to help with these symptoms.
There are other less common symptoms after this type of surgery. You will feel tired, your front teeth or upper lip may feel numb, and you may gain weight. You may also have trouble breathing through your nose, you may have bruises under your eyes or on the side of your nose, and you may not be able to smell as well as usual.
If the doctor used a small piece of fat from your belly or thigh to plug up the hole in your nose, you will have a small scar on your belly or thigh that will fade over time.
You will probably be able to return to work or your normal routine in 1 to 2 weeks. If you had stitches, they will disappear on their own in 7 to 10 days.
If you had surgery through your skull (craniotomy), you will probably feel very tired for several weeks after surgery. You may also have headaches or problems concentrating. It can take up to 6 weeks to fully recover.
The cuts the doctor made (incisions) may be sore for about 5 days after surgery. You may also have numbness and shooting pains near your wound, or swelling and bruising around your eyes. As your wound starts to heal, it may begin to itch. Medicines and ice packs can help with headaches, pain, swelling, and itching.
It is common for your scalp to swell with fluid. After the swelling goes down, you may have a dent in your scalp.
This care sheet gives you a general idea about how long it will take for you to recover. But each person recovers at a different pace. Follow the steps below to get better as quickly as possible.
Radiation Therapy
Radiation therapy for pituitary tumors includes fractionated external beam radiation therapy and stereotactic radiosurgery. It can take several months for these treatments to improve symptoms and conditions related to pituitary adenoma.
- External radiation therapy uses a machine outside the body to send radiation toward the cancer. Certain ways of giving radiation therapy can help keep radiation from damaging nearby healthy tissue. This type of radiation therapy may include the following:
- Stereotactic radiosurgery: A rigid head frame is attached to the skull to keep the head still during the radiation treatment. A machine aims a single large dose of radiation directly at the tumor. This procedure does not involve surgery. It is also called stereotaxic radiosurgery, radiosurgery, and radiation surgery.
Fractionated external beam radiation therapy can reduce excessive hormone production and can reduce the incidence of tumor recurrence 35;however, it can be replaced by stereotactic radiotherapy with focal conformal fractionated delivery. Gamma knife, Cyberknife, proton beam or linear accelerator stereotactic radiosurgery is increasingly applied to pituitary tumors and is also effective in normalizing hormonal hypersecretion and preventing recurrence 36. Whether by fractionated external beam or radiosurgery, the effects of radiotherapy are delayed. Patients require continued suppressive medical therapy during the period between treatment and effect. There is also a significant incidence of radiation-induced delayed hypopituitarism 35. There is no evidence to date that one of these various modalities is superior to another in efficacy, risks of complications, recurrence rates or incidence of hypopituitarism.
Radiation treatment might be appropriate for pituitary adenomas that:
- Are located in areas of the brain where surgery is too risky.
- Cannot be completely removed during surgery.
- Grow quickly.
- Do no shrink with medication.
- Recur after surgery.
Sometimes radiation therapy can cause the pituitary gland to stop working, even years after treatment. In that case, individuals may need to take hormone supplements.
Pituitary adenoma prognosis
If the tumor can be surgically removed, the outlook is fair to good, depending on whether the entire tumor is removed.
Visual deficits in patients with non-functioning pituitary adenomas are improved in approximately 85-90% 2. Some visual deterioration may occur in 4%. Most patients with intact pituitary function preoperatively retain their normal function. Those with preoperative pituitary deficiency regain function in 27% of the cases. The remaining patients are managed with oral hormone replacement therapy. Ten-year recurrence rates are approximately 16%, although only 6% require additional treatment. On long-term follow-up, 83% of patients are alive and well without evidence of disease.
Currently, using strict criteria for remission and in expert hands, transsphenoidal surgery obtains remission in 85-90% of patients with acromegaly with microadenomas and 65% of those harboring macroadenomas. In our hands, acromegalic symptoms are improved in 95% and recurrence is less than 2 percent at ten years. Ninety seven percent of patients have preserved normal pituitary function 37. Seventy-two percent of patients with greater than ten year follow-up, including those with adjunctive therapy, are alive and well without evidence of active disease.
Patients with prolactinomas who present for surgery are most often those who have failed medical management. Prolactin levels are normalized in 87% of microadenomas and 56% of macroadenomas. The recurrence rate among those patients who are normalized after a transsphenoidal operation is 13% at ten years. Preserved pituitary function occurs in all but 3%.
Surgical management of Cushing’s disease achieves a 91% remission rate for microadenomas, but falls to 65% for those with macroadenomas. Some 10-20% of adults experience recurrence after ten years Postoperative stereotactic radiosurgery has achieved remission in approximately 68% of patients whose disease either did not remit following surgery or recurred.
References- Larkin S, Ansorge O. Pathology And Pathogenesis Of Pituitary Adenomas And Other Sellar Lesions. [Updated 2017 Feb 15]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425704
- Jane JA Jr., Laws ER Jr.. Surgical Treatment of Pituitary Adenomas. [Updated 2016 Dec 5]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278983
- Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer 2004; 101:613-619
- Tomita, T. and E. Gates, Pituitary adenomas and granular cell tumors. Incidence, cell type, and location of tumor in 100 pituitary glands at autopsy. American Journal of Clinical Pathology, 1999. 111(6): p. 817-25
- Monson, J.P., The epidemiology of endocrine tumours. Endocrine-Related Cancer, 2000. 7(1): p. 29-36.
- Fan, K.J. and G.H. Pezeshkpour, Ethnic distribution of primary central nervous system tumors in Washington, DC, 1971 to 1985. Journal of the National Medical Association, 1992. 84(10): p. 858-63.
- Robinson, N., V. Beral, and J.S. Ashley, Incidence of pituitary adenoma in women. Lancet, 1979. 2(8143): p. 630.
- Annegers, J.F., et al., Pituitary adenoma in Olmsted County, Minnesota, 1935–1977. A report of an increasing incidence of diagnosis in women of childbearing age. Mayo Clinic Proceedings, 1978. 53(10): p. 641-3.
- Clayton, R.N., Sporadic pituitary tumours: from epidemiology to use of databases. Best Practice & Research Clinical Endocrinology & Metabolism, 1999. 13(3): p. 451-60.
- Etxabe, J., et al., Acromegaly: an epidemiological study. Journal of Endocrinological Investigation, 1993. 16(3): p. 181-7.
- Bengtsson, B.A., et al., Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Medica Scandinavica, 1988. 223(4): p. 327-35.
- Alexander, L., et al., Epidemiology of acromegaly in the Newcastle region. Clinical Endocrinology, 1980. 12(1): p. 71-9.
- Popovic, V., et al., Increased incidence of neoplasia in patients with pituitary adenomas. The Pituitary Study Group. Clinical Endocrinology, 1998. 49(4): p. 441-5.
- Nabarro, J.D., Acromegaly. Clinical Endocrinology, 1987. 26(4): p. 481-512.
- Etxabe, J. and J.A. Vazquez, Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clinical Endocrinology, 1994. 40(4): p. 479-84.
- Laws ER, Jr., Lopes MB. The new WHO classification of pituitary tumors: highlights and areas of controversy. Acta neuropathologica 2006; 111:80-81
- Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER, Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. Journal of neurosurgery 2011; 114:336-344
- Asa, S. L., & Ezzat, S. (2002). The pathogenesis of pituitary tumours. Nature Reviews Cancer, 2, 836−849.
- Melmed, S. (2011). Pathogenesis of pituitary tumors. Nature Reviews Endocrinology, 7, 257−266
- Pituitary Tumors Symptoms, Tests, Prognosis, and Stages (PDQ®)–Patient Version. https://www.cancer.gov/types/pituitary/patient/about-pituitary-tumors-pdq
- Feldkamp, J., et al., Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas – results of a prospective study. Clinical Endocrinology, 1999. 51(1): p. 109-13.
- Molitch, M.E., Pituitary incidentalomas. Endocrinology & Metabolism Clinics of North America, 1997. 26(4): p. 725-40.
- Pituitary adenoma – involving clivus. https://radiopaedia.org/cases/pituitary-adenoma-involving-clivus?lang=us
- Orrego, J.J. and A.L. Barkan, Pituitary disorders. Drug treatment options. Drugs, 2000. 59(1): p. 93-106.
- Higham, C.E., et al., Long-term experience of pegvisomant therapy as a treatment for acromegaly. Clinical Endocrinology, 2009. 71(1): p. 86-91
- Bush, Z.M. and M.L. Vance, Management of acromegaly: is there a role for primary medical therapy? Reviews in Endocrine & Metabolic Disorders, 2008. 9(1): p. 83-94.
- Losa, M., P. Mortini, and M. Giovanelli, Is presurgical treatment with somatostatin analogs necessary in acromegalic patients? Journal of Endocrinological Investigation, 1999. 22(11): p. 871-3.
- Fleseriu, M., Medical treatment of Cushing disease: new targets, new hope. Endocrinology & Metabolism Clinics of North America, 2015. 44(1): p. 51-70
- Colao, A., et al., Partial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. Journal of Clinical Endocrinology & Metabolism, 2006. 91(1): p. 85-92.
- Briet, C., et al., Pituitary Apoplexy. Endocrine Reviews, 2015. 36(6): p. 622-45.
- Kaptain, G.J., et al., Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions.[Reprint in Neurosurgery. 2008 Jun;62(6 Suppl 3):1264-71; PMID: 18695546]. Neurosurgery, 2001. 49(1): p. 94-100; discussion 100-1.
- Jane, J.A., Jr., et al., Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. Journal of Clinical Endocrinology & Metabolism, 2011. 96(9): p. 2732-40.
- Jane, J.A., Jr., et al., Fluoroscopic frameless stereotaxy for transsphenoidal surgery. Neurosurgery, 2001. 48(6): p. 1302-7; discussion 1307-8.
- Nimsky, C., et al., Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery, 2006. 59(1): p. 105-14; discussion 105-14.
- Zaugg, M., et al., External irradiation of macroinvasive pituitary adenomas with telecobalt: a retrospective study with long-term follow-up in patients irradiated with doses mostly of between 40-45 Gy. International Journal of Radiation Oncology, Biology, Physics, 1995. 32(3): p. 671-80.
- Sheehan, J.P., et al., Gamma Knife surgery for pituitary adenomas: factors related to radiological and endocrine outcomes. Journal of Neurosurgery, 2011. 114(2): p. 303-9
- Starke, R.M., et al., Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. Journal of Clinical Endocrinology & Metabolism, 2013. 98(8): p. 3190-8.