Pneumatocele
Pneumatoceles also known as pulmonary pseudocysts, are thin-walled air-filled cysts that develop within the lung tissue that can have a variety of sizes and appearances 1. Pneumatocele may contain air-fluid levels, may be solitary or multiple lesions and are usually the result of ventilator-induced lung injury in neonates or post-infectious. Although pneumatoceles are seen in all age groups, they are most frequently encountered in infancy 2. Pneumatoceles should not be mistaken for a cavitating lung mass.
The cause of pneumatoceles includes congenital 3, traumatic 4, post-infectious 5, hydrocarbon ingestion 6 and ventilator induced 7. The most common reports in the literature are in older children and adults associated with post-infectious problems especially Staphylococcal and Klebsiella pneumonia. Neonatal pneumatoceles have mostly been described with ventilator-induced air-leak conditions during the 1970s and 1980s.
In most circumstances, pneumatoceles are asymptomatic and do not require surgical intervention 8. Treatment of the underlying pneumonia with antibiotics is the first-line therapy. Close observation in the early stages of the infection and periodic follow-up care until resolution of the pneumatocele is usually adequate treatment. The natural course of a pneumatocele is slow resolution with no further clinical complications. Invasive approaches should only be reserved for patients who develop complications.
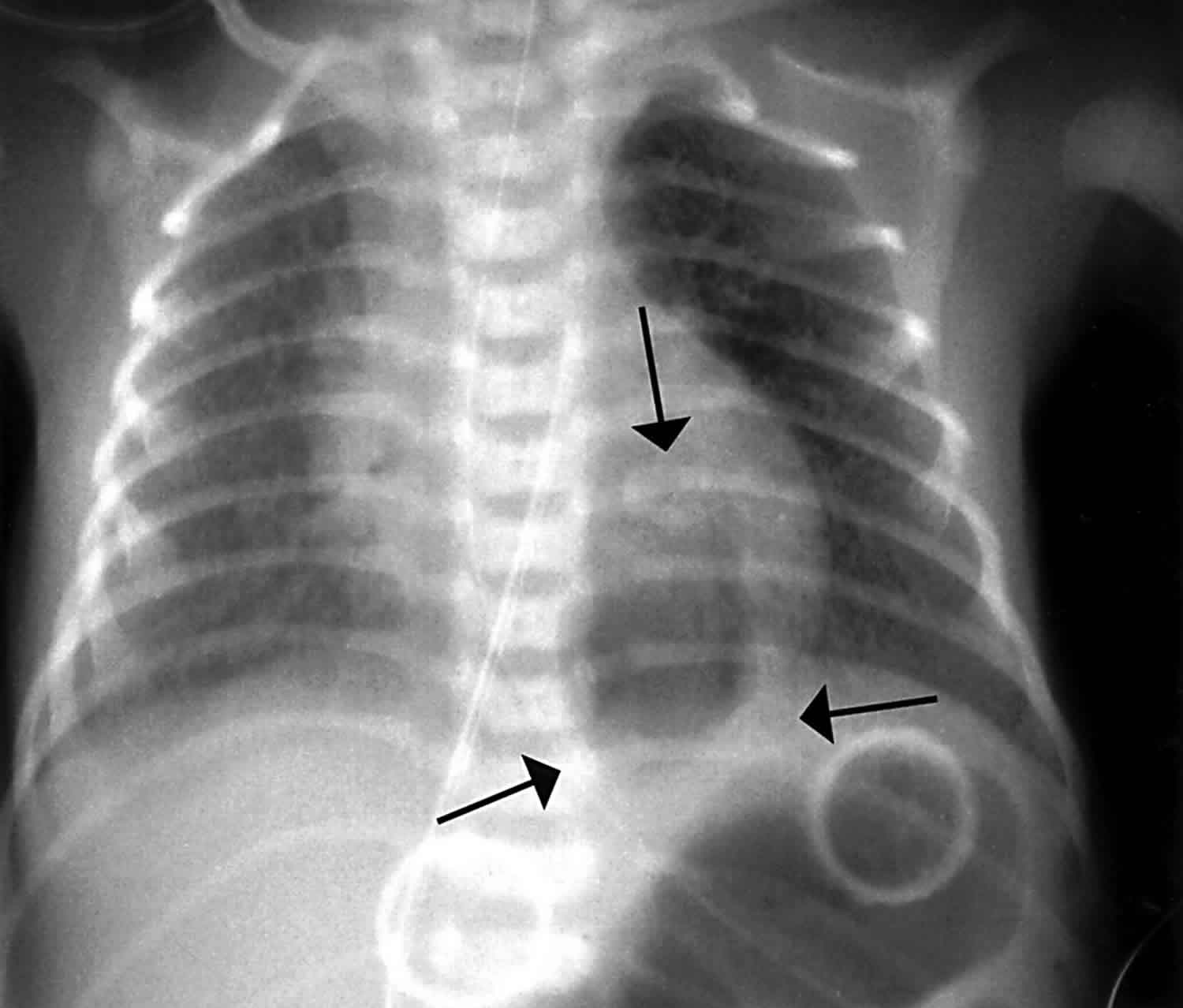
Figure 1. Pneumatocele
Footnote: 2 1/2 year old boy with recent chest infection. Now gradually improving. There is a well circumscribed rounded cystic mass in the right upper lobe. The walls are moderately thick and there is an air-fluid level.
Pneumatocele causes
The majority of pneumatoceles occur as a result of pneumonia (post-infectious pneumatocele). The causative agents include:
- Staphylococcus aureus (most common)
- Streptococcus pneumoniae
- Haemophilus influenzae
- Escherichia coli (E. coli)
- group A streptococci
- Klebsiella pneumoniae
- Adenovirus
- Primary pulmonary tuberculosis
Incidence of postinfectious pneumatocele formation ranges from 2-8% of all cases of pneumonia in children 9. However, the frequency can be as high as 85% in staphylococcal pneumonias. Infants younger than 1 year account for three fourths of the cases of staphylococcal pneumonia. Because pneumatoceles commonly develop as a complication of staphylococcal pneumonia, pneumatoceles are found more frequently in infants and young children. One study reported that 70% of pneumatoceles occurred in children younger than 3 years 10.
In addition to infection, pneumatoceles are also seen in a number of other settings, including:
- Trauma: usually blunt trauma
- Positive pressure ventilation, especially in preterm neonates 1
- Hydrocarbon inhalation 11
- Although no particular genetic predisposition is recognized, pneumatocele formation is associated with hyperimmunoglobulin E (IgE) syndrome (Buckley-Job syndrome) 12. Because of immunodeficiency, individuals with Buckley-Job syndrome are predisposed to infection with staphylococcal pneumonia, with the known complications of abscess and pneumatocele formation.
Three main theories have been put forward to explain the formation of pneumatoceles 2:
- Pulmonary overinflation caused by transient bronchial/bronchiolar obstruction and a ball-valve effect.
- Drainage of necrotic lung parenchyma with subsequent enlargement secondary to a ball-valve effect.
- Focal collections of air within the pulmonary interstitium following inflammation and necrosis of the airway wall and fistula formation with the pleura.
Carrey 13 suggested that the initial event is inflammation and narrowing of the bronchus, leading to the formation of an endobronchial ball valve. Ultimately, this bronchial obstruction leads to distal dilatation of the bronchi and alveoli. In 1951, Conway 14 proposed that a peribronchial abscess forms and subsequently ruptures its contents into the bronchial lumen. This also acts similarly to a ball-valve obstruction in the bronchus and leads to distal dilatation. In 1972, Boisset 15 concluded that pneumatoceles are caused by bronchial inflammation that ruptures the bronchiolar walls and causes the formation of “air corridors.” Air dissects down these corridors to the pleura and forms pneumatoceles, a form of subpleural emphysema.
Traumatic pneumatocele has a different pathophysiology from the infectious type 16, developing in a 2-step process. Initially, the lung is compressed by the external force of the trauma, followed by rapid decompression from increased negative intrathoracic pressure. A “bursting lesion” of the lung occurs and leads to pneumatocele formation.
Pneumatocele symptoms
Children present with typical features of pneumonia, including cough, fever, and respiratory distress. No clinical findings differentiate pneumonia with or without pneumatocele formation. The most significant feature in patients with pulmonary pneumatoceles is the paucity or absence of symptoms after the pneumonitis resolves and the pneumatocele remains 2.
Mild, moderate, or severe respiratory distress may be present, with tachypnea, retractions, grunting, and nasal flaring. Fever is almost always present and may be as high as 104-105.8 °F (40-41°C).
Lung examination findings vary depending on the stage of the pneumonia. Auscultation of the chest reveals focal or bilateral decreased breath sounds. Inspiratory crackles are frequently heard. As the pneumonia resolves and the pneumatocele persists, the lung examination findings can be normal or focal decreases in breath sounds can be present, depending on the size of the pneumatocele.
In most children admitted to the hospital, the average time from admission to the development of the pneumatocele is 4-7 days. Occasionally, pneumatoceles are present on the initial radiograph.
Pneumatocele diagnosis
The diagnosis is typically made with chest imaging, including chest X-ray and CT, though use of lung ultrasound has also been shown to be effective in the diagnosis of pneumatoceles in the pediatric population 17.
If findings are positive, blood culture helps to guide antibiotic therapy in patients with pneumatocele. If sputum is available, this is a good noninvasive method to discover potential pathogens. If effusion is present, culturing pleural fluid from thoracentesis can be a direct method to identify the causative organism. Tests for bacterial antigen detection can be performed on blood, urine and pleural fluid.
Percutaneous catheter drainage should only be considered for a significant tension pneumatocele or a secondarily infected pneumatocele. In these rare situations, drainage has been reported to dramatically improve the patient’s cardiovascular status 18.
Pneumatocele treatment
Pneumatocele treatment is treatment of the underlying condition. In most circumstances, this involves administration of broad-spectrum antibiotics to treat the pneumonia. Therapy should be directed against the most common bacterial organisms, including Staphylococcus aureus and Streptococcus pneumoniae 17. Conservative management alone is unlikely to benefit the patient in situations of cardiorespiratory instability or extensive disease, as death has been reported 19.
Positive pressure ventilation can result in a sudden increase in size and tension of a pneumatocele. Therefore, careful monitoring is essential in patients receiving positive pressure ventilation when pneumatoceles have been documented.
Pneumatoceles almost never require surgical resection. Percutaneous catheter drainage of a pneumatocele that involves more than 50% of hemithorax with severe atelectasis, tension pneumatocele, bronchopleural fistula, or an infected pneumatocele is rarely required. Recently, video-assisted thoracoscopy has been used successfully to treat enlarging multicystic pneumatoceles 20.
Traumatic pneumatoceles commonly resolve with observation without additional therapy. Indications for surgical intervention with a traumatic pneumatocele are similar to those of a postinfectious pneumatocele (ie, development of tension pneumatoceles, a secondary infection of the pneumatocele, and cardiovascular compromise).
Case reports have suggested that treatment can be successful with observation, unilateral ventilation, ventilation with high-frequency oscillation, percutaneous drainage, video-assisted thoracoscopic surgery, lobectomy, and injection of intrapleural fibrin sealant 21. Algorithms have been proposed to aid clinician decision making, which suggest percutaneous needle decompression when the pneumatocele has the following characteristics: (1) occupying greater than 50% of the hemithorax; (2) creating significant atelectasis; (3) bronchopleural fistulae development; (4) tension pneumatocele; or (5) “when follow-up cannot be certain” 22. This algorithm would likely benefit most patients; however, percutaneous drainage many not benefit all patients. Percutaneous needle decompression is not entirely without risk, as this treatment can lead to the development of bronchopleural fistulae 22. Additionally, needle decompression may not be successful, thereby necessitating the patient proceed to surgical intervention 22. Lobectomy is considered a form of definitive management and has been shown to quickly improve the clinical stability of some patients though mortality following this procedure has been reported 23.
Further outpatient care
Most pneumatoceles resolve completely in a few weeks to months. However, in some healthy children, pneumatoceles persist as long as 16 months. Therefore, intermittent outpatient monitoring of chest radiographs is appropriate until resolution. Some recommend chest CT imaging after the findings on plain radiography are clear to ensure complete resolution. However, no clearly recognized radiological or clinical signs help to predict progression of the pneumatocele.
Findings on pulmonary function studies frequently are abnormal initially because of a restrictive defect and, at times, an obstructive defect. Over time, these abnormalities improve and, most often, return to normal predicted ranges. These should not be routinely performed during the acute stages. The increased pressures in spirometry may increase the risk of rupture.
Pneumatocele prognosis
Although mortality from the initial pneumonia can be significant, mortality associated with pneumatoceles is quite low. Complete resolution without long-term sequelae is typical; however, rare complications can occur, including the following 24:
- Tension pneumatocele. A tension pneumatocele can develop if airtrapping continues and the pneumatocele expands. This complication occurs most frequently with positive pressure ventilation. If severe, the lesion can cause compression of adjacent structures, with hemodynamic instability and severe airway obstruction. If unrecognized and untreated, this can result in respiratory failure and death.
- Pneumothorax. Pneumothorax can occur from a pneumatocele rupturing into the pleural space. This can lead to collapse of the lung, requiring evacuation of the pleural air to reexpand the lung. A bronchopleural fistula can result as a complication of the pneumothorax.
- Secondarily infected pneumatocele. A pneumatocele can become secondarily infected, usually by a different bacterium from the one that caused the primary pneumonia. Some advocate percutaneous drainage of infected pneumatoceles, especially if fluid- or pus-filled to prevent the development of severe lung abscess that may require surgical excision. Drainage can be both diagnostic and therapeutic. If drained, the fluid should be cultured for bacteria and fungus.
Several small studies suggest resolution of simple pneumatoceles by 6 months in most cases although other cases suggest that some pneumatoceles can persist for greater than 12 months without intervention 25. It is unclear whether coinfection with multiple organisms confers increased risk of developing pneumatocele, or if the clinical presentation of these patients is more severe.
References- Hussain N, Noce T, Sharma P et-al. Pneumatoceles in preterm infants-incidence and outcome in the post-surfactant era. J Perinatol. 2010;30 (5): 330-6. https://www.nature.com/articles/jp2009162
- Post-Pneumonic Pulmonary Pneumatoceles. Flaherty RA, Keegan JM, Sturtevant HN. Radiology 1960 74:1, 50-53 https://doi.org/10.1148/74.1.50
- Seo T, Ando H, Watanabe Y, Harada T, Ito F, Kaneko K et al. Acute respiratory failure associated with intrathoracic masses in neonates. J Pediatr Surg 1999; 34 (11): 1633–1637.
- Albertini M, Larrede N, Coussement A, Berard E, Mariani R . Post-traumatic lung pneumatocele. A case in an infant. Pediatrie 1992; 47 (2): 113–116.
- Caksen H, Ozturk MK, Uzum K, Yuksel S, Ustunbas HB . Pulmonary complications in patients with staphylococcal sepsis. Pediatr Int 2000; 42 (3): 268–271.
- Campbell JB . Pneumatocele formation following hydrocarbon ingestion. Am Rev Respir Dis 1970; 101 (3): 414–418.
- Williams DW, Merten DF, Effmann EL, Scatliff JH . Ventilator-induced pulmonary pseudocysts in preterm neonates. AJR Am J Roentgenol 1988; 150 (4): 885–887.
- Imamoglu M, Cay A, Kosucu P, et al. Pneumatoceles in postpneumonic empyema: an algorithmic approach. J Pediatr Surg. 2005 Jul. 40(7):1111-7.
- Amitai I, Mogle P, Godfrey S, Aviad I. Pneumatocele in infants and children. Report of 12 cases. Clin Pediatr (Phila). 1983 Jun. 22(6):420-2.
- Kunyoshi V, Cataneo DC, Cataneo AJ. Complicated pneumonias with empyema and/or pneumatocele in children. Pediatr Surg Int. 2006 Feb. 22(2):186-90.
- Brown KW, Armstrong TJ. Hydrocarbon Inhalation. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470289
- Schimke LF, Sawalle-Belohradsky J, Roesler J, Wollenberg A, Rack A, Borte M, et al. Diagnostic approach to the hyper-IgE syndromes: immunologic and clinical key findings to differentiate hyper-IgE syndromes from atopic dermatitis. J Allergy Clin Immunol. 2010 Sep. 126(3):611-7.e1.
- Carrey J. On the natural regression of pulmonary cysts during early infancy. Pediatr. 1953. 11:48-64.
- Conway DJ. The origin of lung cysts in childhood. Arch Dis Child. 1951. 26:504-529.
- Boisset GF. Subpleural emphysema complicating staphylococcal and other pneumonias. J Pediatr. 1972 Aug. 81(2):259-66.
- Galea MH, Williams N, Mayell MJ. Traumatic pneumatocele. J Pediatr Surg. 1992 Dec. 27(12):1523-4.
- Marostica P. J. C., Stein R. T. Community-acquired bacterial pneumonia. In: Wilmott R. W., Boat T. F., Bush A., Chernick V., Deterding R. R., Ratjen F., editors. Kendig & Chernick’s Disorders of the Respiratory Tract in Children. 8th. Philadelphia, PA, USA: W. B. Saunders; 2012. pp. 461–472.
- Park TH, Kim JK. Nonsurgical management of an enlarging pneumatocele by fibrin sealant injection via pigtail catheter. Pediatr Pulmonol. 2016 Feb. 51 (2):E5-7.
- Amitai I., Mogle P., Godfrey S., Aviad I. Pneumatocele in infants and children. Clinical Pediatrics. 1983;22(6):420–422. doi: 10.1177/000992288302200605
- Fujii AM, Moulton S. VATS management of an enlarging multicystic pneumatocele. J Perinatol. 2008 Jun. 28(6):445-7.
- Park T. H., Kim J. K. Nonsurgical management of an enlarging pneumatocele by fibrin sealant injection via pigtail catheter. Pediatric Pulmonology. 2016;51(2):E5–E7. doi: 10.1002/ppul.23311
- İmamoğlu M., Çay A., Koşucu P., et al. Pneumatoceles in postpneumonic empyema: an algorithmic approach. Journal of Pediatric Surgery. 2005;40(7):1111–1117. doi: 10.1016/j.jpedsurg.2005.03.048
- Salahuddin N., Baig-Ansari N., Fatimi S. H. Unusual case of non-resolving necrotizing pneumonia: a last resort measure for cure. JPMA Journal of Pakistan Medical Association. 2016;66(6):754–756.
- Pneumatocele. https://emedicine.medscape.com/article/1003289-overview
- İmamoğlu M., Çay A., Koşucu P., et al. Pneumatoceles in postpneumonic empyema: an algorithmic approach. Journal of Pediatric Surgery. 2005;40(7):1111–1117. doi: 10.1016/j.jpedsurg.2005.03.048.