Autoimmune progesterone dermatitis
Autoimmune progesterone dermatitis also known as progesterone dermatitis is a rare skin condition characterized by recurrent and itchy rash affecting women during their childbearing years that recurs in a cyclical manner corresponding to their menstrual cycles. Autoimmune progesterone dermatitis is a rare cyclic premenstrual reaction to progesterone produced during the luteal phase of the menstrual cycle coinciding with peak levels of endogenous progesterone 1. Skin lesions normally began a few days before menstruation and resolved a few days later. The nature of skin eruption varies widely and is diverse, and thus, its diagnosis is challenging 2. The clinical symptoms of autoimmune progesterone dermatitis overlap with other forms of dermatosis such as erythema multiforme, eczema, allergic contact dermatitis, rosacea, fixed drug eruption, urticaria, and angioedema. Because of its polymorphic characteristics, physicians can easily misdiagnose and treat this condition incorrectly 2.
- Skin lesions appear during the second half of the menstrual cycle and resolve during the menstrual period
- Urticaria, urticaria-like weals, eczema-like lesions, blisters and target lesions may occur
- Autoimmune progesterone dermatitis is prevented when ovulation is prevented by an oral contraceptive agent
Autoimmune progesterone dermatitis is thought to be a response of the skin to the hormonal changes that happen just before menses. The skin rash is an autoimmune response to endogenous or exogenous progesterone, hence its name.
Patients with autoimmune progesterone dermatitis may present with diverse unusual manifestations, resulting in a delayed diagnosis and misdiagnosis. Moreover, patients are not aware that their skin lesions are associated with their menstrual cycle before discussion with their clinicians.
Autoimmune progesterone dermatitis treatment must be selected based on the patient’s profile and preferences. From a physiopathological perspective, ovulation must be suppressed to reduce endogenous progesterone production. Complete resolution of lesions has been achieved with the use of conjugated equine estrogens, ethinylestradiol, oral contraceptive pills (OCP) (levonorgestrel + ethinylestradiol), gonadotropin-releasing hormone analogs (GnRH) and tamoxifen 3. Variable results have been achieved with the use of systemic corticosteroids and antihistamines 4. Definitive therapy is hysterectomy with salpingo-oophorectomy, which should be reserved for severe cases, and patients with no future desire to have children 3. Desensitization with increasing doses of progesterone (intramuscular, oral or intravaginal) is also reported and has shown success in controlling symptoms and eventually achieving fertility 5.
Figure 1. Autoimmune progesterone dermatitis face
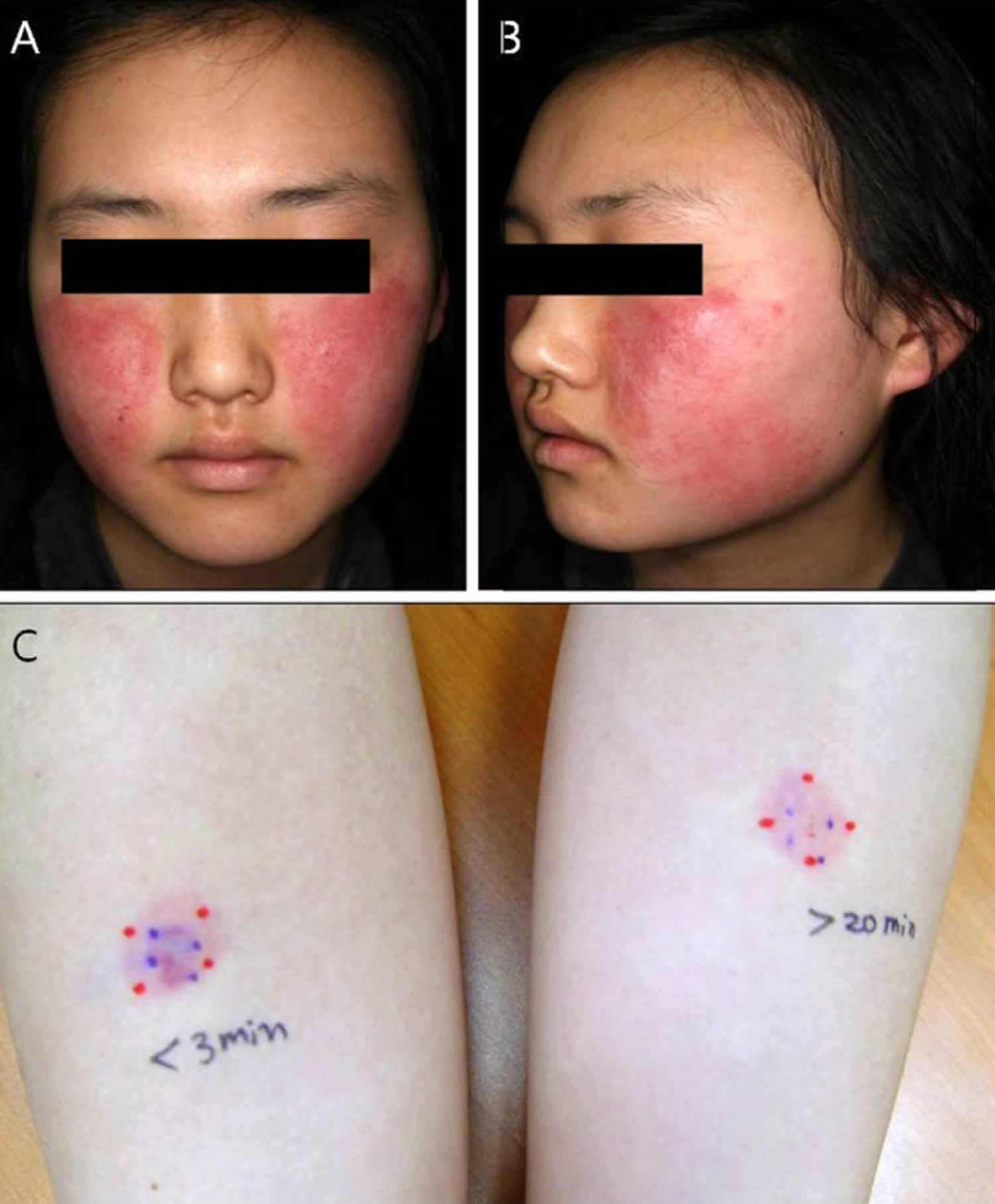
Footnote: An 18-year-old girl with no significant medical history and no prior exogenous hormone use presented with a 4-month history of skin eruption. The lesions were localized to both cheeks and appeared as well demarcated erythematous patches with heating sense and edema (Figure 1A, B). She had no history of contact, cosmetic changes or symptoms of arthritis, or photosensitivity. Routine laboratory findings including complete blood count, blood chemistry, and hormonal and immunological examinations were within the normal ranges. (A, B) Clearly marked erythematous patches with edema on both cheeks. (C) Intradermal progesterone test was performed. Within 3 minutes, a 2-cm-sized erythema at the progesterone injection site. After 20 minutes, erythema remained.
[Source 2 ]Figure 2. Autoimmune progesterone dermatitis
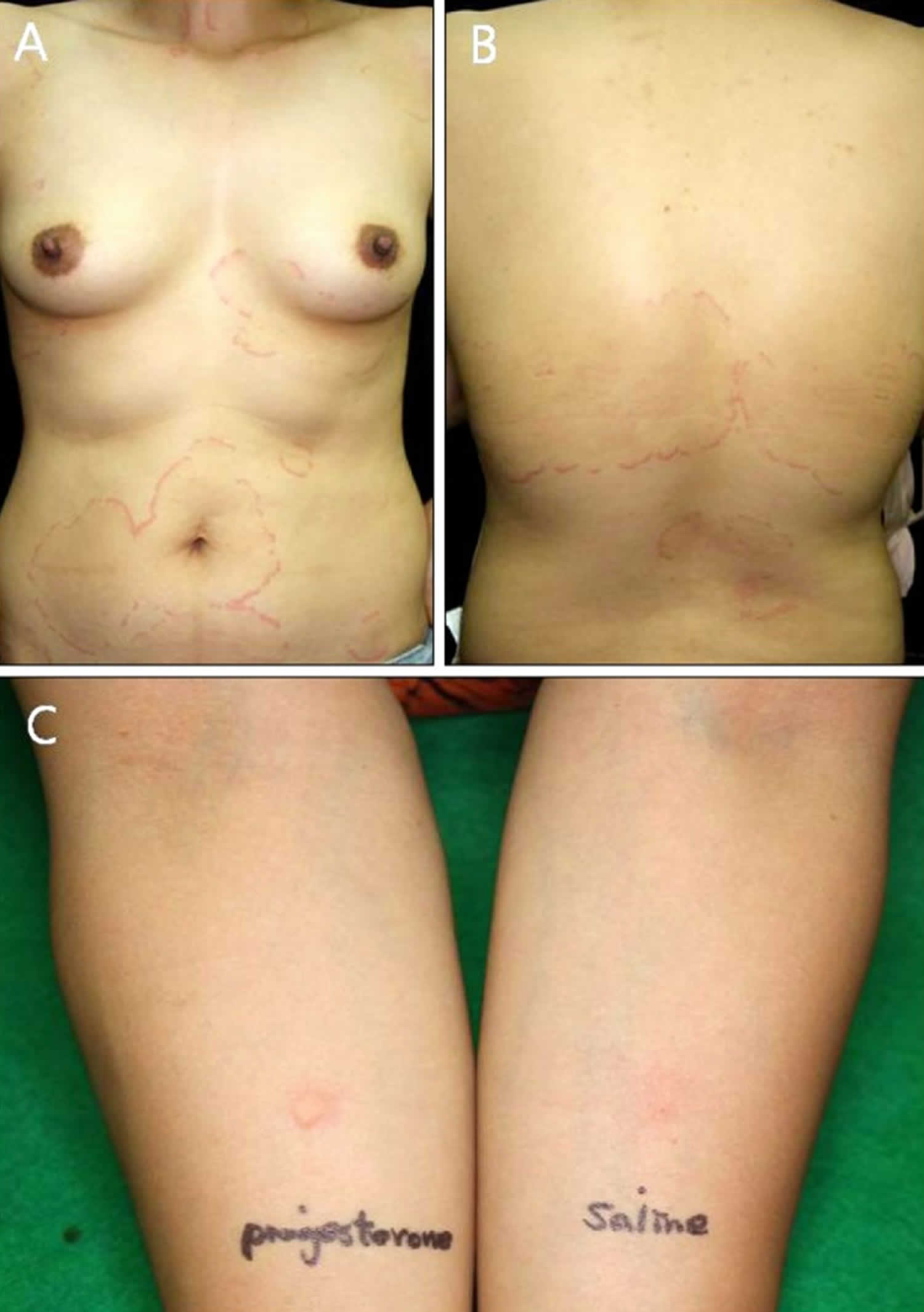
Footnote: A 29-year-old woman had pruritic erythematous patches with edema on her chest and back (Figure 2A, B). Based on her history of nickel allergy, skin lesions were noted on her bra lines, and histopathologic findings indicated dilated vessels and patchy infiltration of lymphocytes in the upper dermis (Figure 2C), and contact dermatitis or urticarial dermatitis was considered as a clinical diagnosis. (A, B) Pruritic erythematous patches with edema on the chest and back. (C) Histological examination of the chest lesion showed patchy infiltration of lymphocytes and a few eosinophils (H&E; ×40, inset: ×400). (D) Positive intradermal progesterone test. After 30 minutes, 1.5-cm sized erythema and wheal is distinctly seen (left). After 48 hours, erythema persists at the progesterone injection site right).
Abbreviations: P = progesterone, S = saline.
[Source 2 ]Figure 3. Progesterone dermatitis
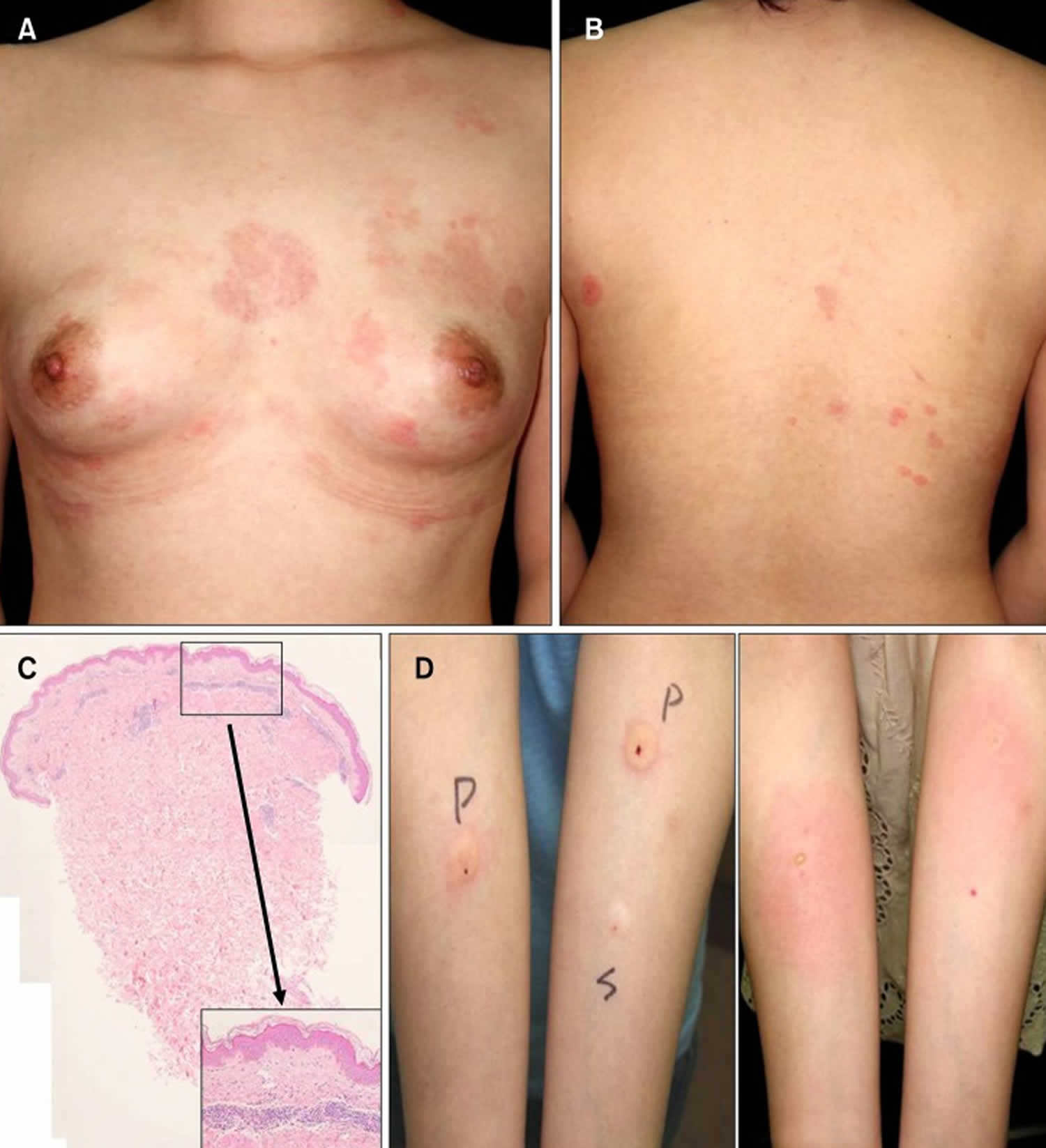
Footnote: A 38-year-old woman who complained of pruritic and erythematous polycyclic patches on her trunk, arms, and face that caused a stinging sensation (Figure 3A, B). Clinically, erythema annulare centrifugum or tinea corporis or urticaria was considered. (A, B) Pruritic and stingy erythematous polycyclic patches on the trunk. (C) A 0.5-cm-sized wheal is observed after 30 minutes. Further, gyrated patches on the face, neck, hands appeared after 24 hours.
[Source 2 ]Figure 4. Autoimmune progesterone dermatitis
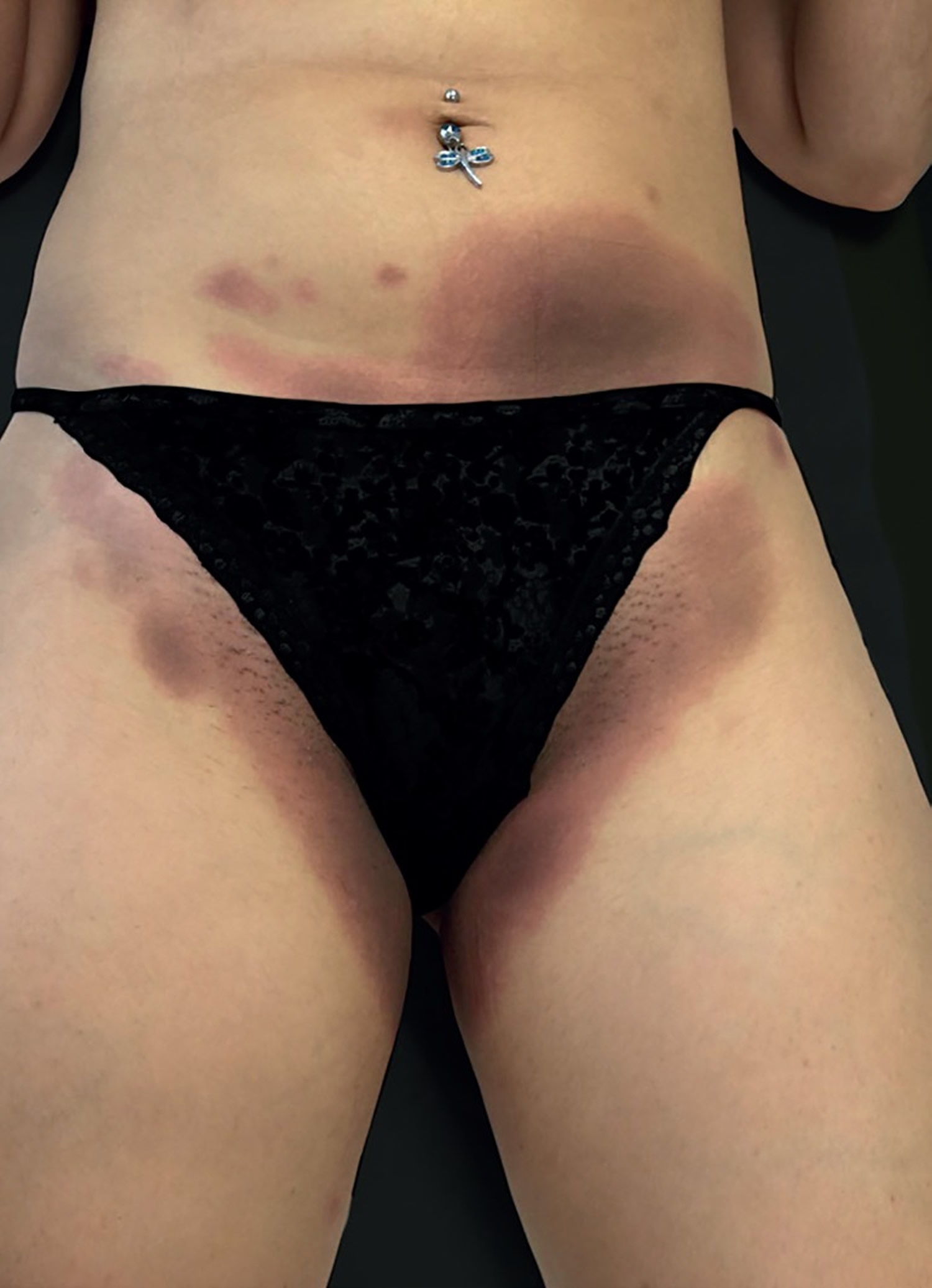
Footnote: A 26-year-old female patient presented with a 5-month history of recurring skin lesions associated with itching and burning sensations. Symptoms first began 4 days after uterine curettage following a missed abortion. The patient’s history was otherwise clear, except for irregular use of combined oral contraceptive pills (OCP) (levonorgestrel; ethinylestradiol). The lesions recurred once monthly at the same sites, beginning one day prior to menstruation and resolving 3 days after the end of the menstrual cycle, leaving residual hyperpigmentation. They were initially treated with oral antihistamines and clobetasol cream, with no response. Physical examination revealed sharply demarcated erythematous or violaceous plaques located at the armpits, groin, labia majora, intergluteal cleft, and abdomen.
[Source 6 ]Autoimmune progesterone dermatitis causes
The cause of autoimmune progesterone dermatitis is not entirely understood. Autoimmune progesterone dermatitis is a rare autoimmune response to endogenous or exogenous progesterone 2 and that autoimmune progesterone dermatitis could be part of a broader syndrome of hypersensitivity to some or all sex steroids 6. Some patients have had previous exposure to external progesterone in the form of oral contraceptive pills. This is thought to pre-sensitize patients to react against their internal progesterone. However, not all patients with autoimmune progesterone dermatitis are exposed to previous hormone therapy. It has been postulated that these patients produce an altered form of progesterone that incites an immunologic response against it. In another theory, progesterone is thought to heighten a patient’s hypersensitivity response to another allergen.
The pathogenic mechanisms of autoimmune progesterone dermatitis may be diverse and they are probably similar to hypersensitivity reactions to drugs-with the participation of IgE antibodies, T cells, or proinflammatory cytokines 7. A significant percentage of patients with autoimmune progesterone dermatitis show immediate or delayed positive reactions to intradermal tests with progesterone, suggesting that type 1 and 4 hypersensitivity reactions may be involved. Given that immunological mechanisms have not been confirmed in all patients, Foer et al 5 recently suggested that this pathology should be renamed as hypersensitivity to progesterone instead of autoimmune progesterone dermatitis, as this feature is indeed shared by all patients. They also recommend a new classification, in which patients exhibit 2 phenotypes: 1) those in whom it is triggered by endogenous progesterone (situations such as menstruation and pregnancy) with symptoms emerging during the luteal phase, and 2) those who had previously tolerated menstruation and in some cases even pregnancy, but symptoms emerged following exposure to exogenous progesterone. This latter group has been considered to have a mixed phenotype, as following sensitization they suffer exacerbation from endogenous triggers 5.
It has been shown that 14% of cases of autoimmune progesterone dermatitis are triggered by pregnancy, with symptoms usually beginning during interpartum or postpartum 3. The alleviation of autoimmune progesterone dermatitis experienced by some patients during pregnancy may relate to a reduction in natural cell-mediated immunity, allowing the fetus to be tolerated. Nonetheless, case studies show that not all patients respond in the same way to hypersensitivity to progesterone. Indeed, a case of miscarriage potentially caused by hypersensitivity to progesterone has been reported 8. Furthermore, it has been seen that implantation and success of pregnancy depend on suitable immune responses. Cases of recurring miscarriages have been associated with an excess of Th1 proinflammatory cytokines compared to levels of type Th2/3 7. Although miscarriages are caused by multiple factors, the time course of the miscarriage and the emergence of autoimmune progesterone dermatitis in a patient suggests a possible role of autoimmunity and a potential shared etiopathology 6.
Autoimmune progesterone dermatitis symptoms
Autoimmune progesterone dermatitis is characterized by skin eruptions occurring during the luteal phase or the late pre-menstrual phase of the menstrual cycle. This is when the blood level of the sex-hormone progesterone rises. Within a few days of menstruation when progesterone level falls, there is partial to complete resolution of the rash. On average, the skin rash happens seven days before the onset of menstruation and lasts for 1–3 days after menstruation. Autoimmune progesterone dermatitis will recur during the next cycle. The age of onset is variable, the youngest case occurred at menarche, and autoimmune progesterone dermatitis can begin as late as 48 years of age.
The clinical features of autoimmune progesterone dermatitis dermatitis vary and a variety of rashes has been described. The most common are urticaria 9, angioedema and erythema multiforme 10. Other presentations include:
- Papulovesicles (eczema-like)
- Annular erythema
- Mouth erosions (stomatitis and aphthous ulcers)
- Itch (the most common complaint)
In addition to these, eczema, Steven-Johnson syndrome, fixed drug eruption 11 and erythema annulare centrifugum like lesions 12 have also been observed.
Several other skin conditions may be more severe during the perimenstrual period, but these are not classified as autoimmune progesterone dermatitis. These include:
- Herpes simplex infection (cold sores)
- Acne and seborrhea
- Rosacea
- Atopic dermatitis
- Contact allergy to nickel
- Lupus erythematosus
- Psoriasis
Autoimmune progesterone dermatitis diagnosis
Autoimmune progesterone dermatitis diagnosis is usually made from the characteristic cyclical presentation.
A skin prick test with intradermal progesterone is helpful. Positive tests with progesterone can be fairly rapid, usually developing as urticaria within 30 minutes of inoculation, or delayed with rashes peaking at 24–48 hours.
Provocative testing with intramuscular or oral progesterone can be performed as an alternative.
Skin biopsy alone is seldom diagnostic. A variety of histological features have been described. Superficial perivascular mixed inflammation is the most consistent finding.
The diagnostic criteria for autoimmune progesterone dermatitis proposed by Warin include 13:
- Skin lesions associated with menstrual cycle (premenstrual flare);
- A positive response to the progesterone intradermal test or reproducibility of the rash with the intramuscular test. However, to date there is no standardization regarding the dose, environmental conditions, or the technique to use when performing this test on patients suspected to have autoimmune progesterone dermatitis. Therefore, the test is not a reliable predictor of the phenomenon of autoimmune progesterone dermatitis 14. In a series of 24 cases of autoimmune progesterone dermatitis, only 50% of patients showed a positive result to this test 5.
- Symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.
In particular, skin lesions of autoimmune progesterone dermatitis develop 3~10 days before menstruation and persist up to 1~2 days after cessation of menses, and correlate with serum progesterone levels 15. Autoimmune estrogen dermatitis also presents as a cyclic skin disorder but the hallmark of estrogen dermatitis is the cyclic premenstrual flare. This hormone-induced dermatosis may appear as variable clinical manifestations. However, the lesions tend to involute during pregnancy and at menopause 16.
Autoimmune progesterone dermatitis treatment
Autoimmune progesterone dermatitis can be treated or controlled mainly by suppressing ovulation. If the skin problem is mild, it may improve with topical steroids (for eczema) and antihistamines (for urticaria). Severe disease may be treated with systemic corticosteroids.
The production of progesterone can be suppressed with hormone-based therapy. This includes the use of conjugated estrogen, ethinylestradiol, tamoxifen and danazol.
Women with autoimmune progesterone dermatitis should try to avoid medications containing progesterone including the combined oral contraceptive pill, minipill, and depo injections. The specific drugs to avoid include norethindrone, norgestrel, levonorgestrel.
Surgical removal of ovaries or bilateral oophorectomy is curative in refractory cases 17.
References- Herzberg AJ, Strohmeyer CR, Cirillo-Hyland VA. Autoimmune progesterone dermatitis. J Am Acad Dermatol. 1995 Feb;32(2 Pt 2):333-8. doi: 10.1016/0190-9622(95)90398-4
- You HR, Yun SJ, Kim SJ, Lee SC, Won YH, Lee JB. Three Cases of Autoimmune Progesterone Dermatitis. Ann Dermatol. 2017;29(4):479-482. doi:10.5021/ad.2017.29.4.479 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5500715
- Nguyen T, Razzaque Ahmed A. Autoimmune progesterone dermatitis: Update and insights. Autoimmun Rev. 2016 Feb;15(2):191-7. doi: 10.1016/j.autrev.2015.11.003
- Prieto-Garcia A, Sloane DE, Gargiulo AR, Feldweg AM, Castells M. Autoimmune progesterone dermatitis: clinical presentation and management with progesterone desensitization for successful in vitro fertilization. Fertil Steril. 2011;95:1121.e9–1121.13.
- Foer D, Buchheit KM, Gargiulo AR, Lynch DM, Castells M, Wickner PG. Progestogen Hypersensitivity in 24 Cases: Diagnosis, Management, and Proposed Renaming and Classification. J Allergy Clin Immunol Pract. 2016 Jul-Aug;4(4):723-9. doi: 10.1016/j.jaip.2016.03.003
- Carrasco-Zuber JE, Álvarez-Véliz S, Moll-Manzur C, González-Bombardiere S. Autoimmune progesterone dermatitis manifesting as generalized fixed drug eruption. An Bras Dermatol. 2018;93(6):874-877. doi:10.1590/abd1806-4841.20187290 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6256208
- Itsekson AM, Seidman DS, Zolti M, Alesker M, Carp HJ. Steroid hormone hypersensitivity: clinical presentation and management. Fertil Steril. 2011 Jun 30;95(8):2571-3. doi: 10.1016/j.fertnstert.2011.05.025
- Calapai G, Imbesi S, Miroddi M, Isola S, Venuto L, Navarra M, et al. Adverse reaction after administration of progesterone. Allergol Immunopathol (Madr) 2014;42:377–379.
- Walling HW, Scupham RK. Autoimmune progesterone dermatitis. Case report with histologic overlap of erythema multiforme and urticaria. Int J Dermatol. 2008;47:380–382.
- Wojnarowska F, Greaves MW, Peachey RD, Drury PL, Besser GM. Progesterone-induced erythema multiforme. J R Soc Med. 1985;78:407–408.
- Asai J, Katoh N, Nakano M, Wada M, Kishimoto S. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643–645.
- Halevy S, Cohen AD, Lunenfeld E, Grossman N. Autoimmune progesterone dermatitis manifested as erythema annulare centrifugum: confirmation of progesterone sensitivity by in vitro interferon-gamma release. J Am Acad Dermatol. 2002;47:311–313.
- Warin AP. Case 2. Diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001 Jan;26(1):107-8. doi: 10.1046/j.1365-2230.2001.00747.x
- Bernstein IL, Bernstein DI, Lummus ZL, Bernstein JA. A case of progesterone-induced anaphylaxis, cyclic urticaria/angioedema, and autoimmune dermatitis. J Womens Health (Larchmt). 2011 Apr;20(4):643-8. doi: 10.1089/jwh.2010.2468
- Burstein M, Rubinow A, Shalit M. Cyclic anaphylaxis associated with menstruation. Ann Allergy. 1991 Jan;66(1):36-8.
- Chung HS, Shin HK, Lee KH. A case of estrogen dermatitis. Ann Dermatol. 1997;9:231–235.
- Ródenas JM, Herranz MT, Tercedor J. Autoimmune progesterone dermatitis: treatment with oophorectomy. Br J Dermatol. 1998 Sep;139(3):508-11. doi: 10.1046/j.1365-2133.1998.02420.x