Saliva hormone testing
Saliva hormone testing is a non-invasive collection method where patients collect their saliva in plastic tubes in order to measure hormones like cortisol, estrogens, progesterone, and androgens. Saliva hormone testing is a non-invasive saliva collection is ideal for patients because it allows them to collect their sample in the privacy of their home or office. Patients are sent a test kit with easy to follow instructions for saliva collection.
Hormone is a chemical substance produced and secreted by endocrine (ductless) glands that travels through the bloodstream and controls or regulates the activity of another organ or group of cells – its target organ. (For example, growth hormone released by the pituitary gland controls the growth of long bones of the body.) There are two main types of hormones – steroids (e.g., estrogen, testosterone, aldosterone, cortisol) and nonsteroidal. Secretion of hormones is regulated by feedback mechanisms and neurotransmitters.
As a biological medium saliva is a variable and complex fluid and is mostly produced by three pairs of salivary glands (parotid, submandibular and sublingual) with a small contribution from the buccal glands which line the mouth 1. In addition, saliva contains variable amounts of gingival crevicular fluid, which leaks from the tooth-gum margin, as well as plasma exudates, or blood, from oral abrasions or lesions. Hormones can enter saliva by a variety of mechanisms but for the neutral steroids the most common route is rapid diffusion through the acinar cells and as such their concentration is independent of the rate of saliva flow 2. For charged steroids, like DHEAS, the mode of entry is by diffusion between the tight junctions of the acinar cells and its concentration is inversely related to saliva flow rate 3. Saliva pH also alters with saliva flow rate and hence affects the partitioning of charged steroids. Steroids can also enter saliva from blood or plasma via oral abrasions or directly from foodstuffs by contamination with exogenous steroids 2.
As hormones play an important role in maintaining health, the assessment of hormone levels can help identify the cause of many health related conditions. In both men and women adequate levels of a number of hormones are necessary for optimal health and wellbeing.
The family of sex hormones, which includes the oestrogens – oestrone (E1), oestradiol (E2), oestriol (E3), progesterone, testosterone, DHEAS and cortisol, support a wide range of essential physiological functions. Variation in these hormones plays a large role in the changes seen in life cycle events such as pregnancy, menopause and ageing.
Baseline measurement of hormone levels in saliva provides an accurate assessment of menstrual irregularities for younger women, climacteric changes for the peri and postmenopausal women and andropause changes in men.
The connection between sex hormones and thyroid function, adrenal activity and liver detoxification may also be evaluated with appropriate testing and clinical history. Research shows that salivary hormone testing provides an accurate assessment of hormone status in both men and women of all ages.
Many factors contribute to altered hormone levels. These include environmental factors, such as chemical exposure and pesticides, lifestyle factors including smoking, alcohol, lack of exercise, poor diet and specific conditions such as infertility, endometriosis and enlarged prostate.
Saliva hormone testing advantages and disadvantages
The prime advantage of saliva testing for hormone is that it offers non-invasive, stress-free and real-time repeated sampling where blood collection is either undesirable or difficult. Saliva hormone testing is well suited for pediatric, time-shift and psychobiological studies. In addition, no special training or equipment is needed and subjects can conveniently collect samples themselves, if required. Salivary steroid levels can reflect the circulating level of free steroid rather than total circulating levels, which are confounded by the presence of circulating high affinity binding proteins 3. There are, however, disadvantages in the use of saliva for hormone levels testing. It can be a difficult matrix to deal with experimentally and may require physical or chemical disruption. Freeze/thaw cycles and centrifugation are often used to break up mucins and dithiothreitol treatment can facilitate filtration 4. The analysis of steroids in saliva can present analytical problems since they are present at far lower levels than in circulation. Existing assays may need to be adapted to improve sensitivity, although kits are available for measuring some steroids in saliva. Some use manual methods or automated platforms, designed for plasma, and care is required to address standardisation issues as well as the differing matrices of plasma and saliva 5. The possibility of blood contamination and its likely interference can be quantified but the presence of both sex hormone binding globulin (SHBG) and corticosteroid-binding globulin in uncontaminated saliva casts doubt on the reliability of salivary steroids to accurately reflect circulating free steroid levels 6. The presence of 11β-hydroxysteroid dehydrogenase type 2 and 17-hydroxysteroid dehydrogenase in salivary glands also complicates the relationship between salivary and plasma free steroids 7. During saliva sampling there is also the possibility of oral contamination by exogenously administered steroids. Another point, often overlooked, is that during the course of clinical investigation the physician is likely to investigate other analytes, besides salivary steroids, and more often than not a blood sample is likely to be taken.
Saliva hormone testing accuracy
Salivary androgens
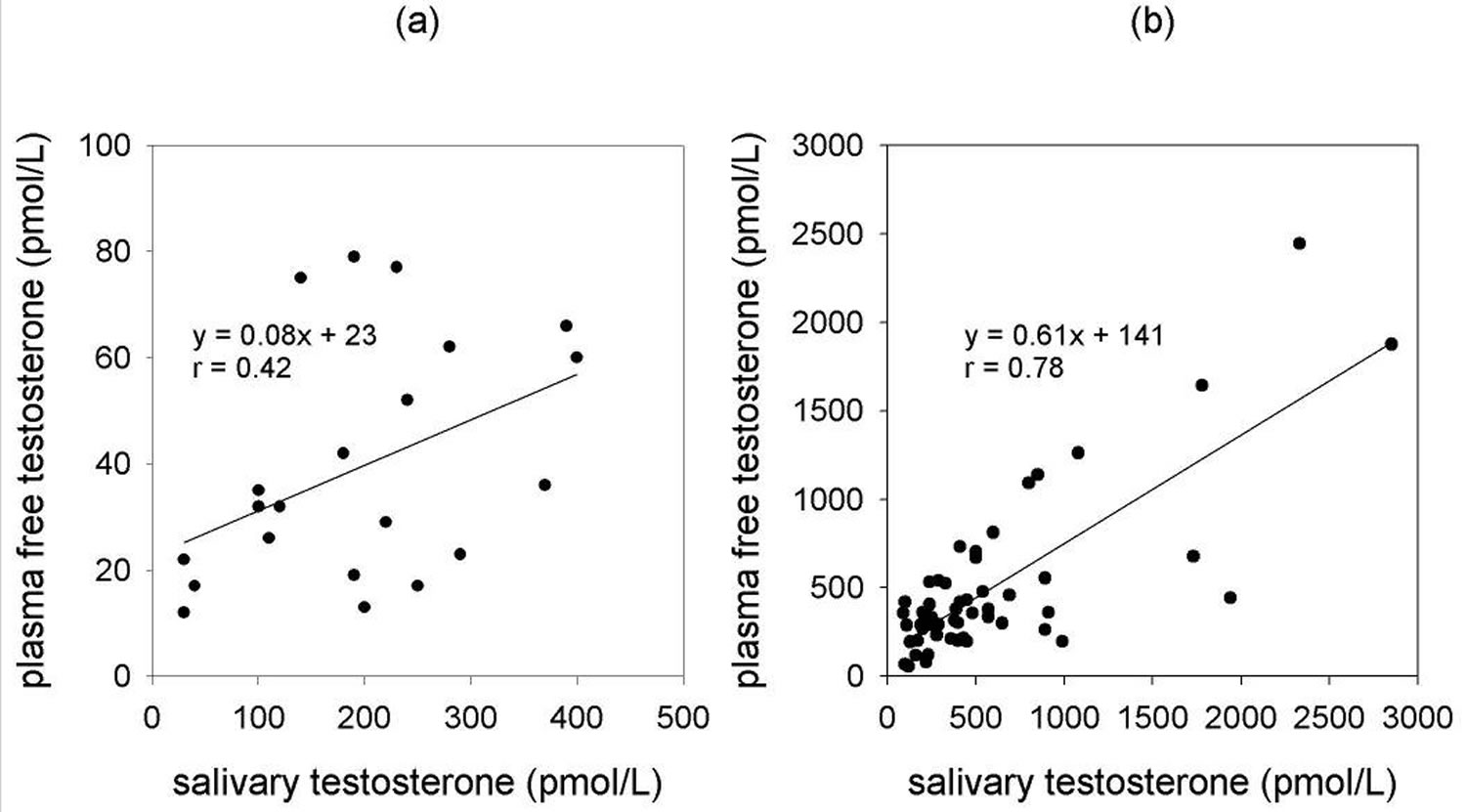
Salivary androstenedione correlates well with free plasma concentrations (r = 0.92) and has been used in paediatric studies along with DHEA and testosterone 8. However, in the clinical setting, it has been deemed necessary that salivary androstenedione determination, along with progesterone and 17α-OH progesterone determinations, are performed following extraction and chromatography. This requirement is due to the presence of unidentified cross-reacting material in unpurified extracts 9. Salivary DHEA levels, following organic extraction, are reportedly lower in adult depression but salivary DHEAS analysis is probably not as useful as its concentration is dependent on salivary flow rate 10. This suggests an alternative transport mechanism into saliva. In addition, the high concentration of DHEAS in plasma could contaminate saliva levels via gingival fluid or oral abrasions 3. There are numerous reports of testosterone analysis in saliva and in psychobiological studies it appears to correlate with psychological parameters 11. Salivary testosterone has also been used for monitoring i.v. replacement therapy with testosterone buciclate 12. However, in view of the current controversies surrounding testosterone measurement by immunoassay at low concentrations in plasma, it would seem that the even lower testosterone concentrations in saliva would likely present an analytical challenge, although it is suggested that its reliability is acceptable for research purposes 13. There are, however, real concerns regarding storage and interference 14. In males, salivary testosterone declines with age and reflects testicular production although neither salivary testosterone nor plasma testosterone appears clearly superior to the other as a measure of bioavailability 15. Extraction-based assays for salivary testosterone may mitigate some problems and improve sensitivity as well as display acceptable correlation between either total or free testosterone in male plasma and saliva levels (r = 0.71 and 0.67, respectively). However, the correlation between plasma free testosterone and saliva testosterone in females is poor (r = 0.37) 14. This is in agreement with laboratory findings using extraction-based immunoassays for testosterone. The sex-related correlation between calculated free testosterone in plasma and salivary testosterone is shown in Figure 1 16. Scientists conclude that mathematically derived indicators of bioavailable androgen status, using plasma total testosterone and sex hormone-binding globulin, are preferable as they overcome the limitations imposed by saliva.
Figure 1. Testosterone saliva test
Footnote: Correlation of calculated plasma free testosterone with salivary testosterone for females (a) and males (b). Testosterone analysis was by ELISA.
[Source 16 ]Salivary progestins and estrogens
Congenital Adrenal Hyperplasia or CAH is an inherited genetic disorder that affects the adrenal glands. Those with Congenital Adrenal Hyperplasia lack one of the enzymes that the adrenal glands use to produce hormones that regulate metabolism, the immune system, blood pressure, and other essential functions. This can cause a number of symptoms, including accelerated puberty, infertility, and low blood pressure.
Congenital Adrenal Hyperplasia has been diagnosed by the immunoassay of 17α-OH progesterone in both plasma and saliva and an excellent correlation reported 17. An liquid chromatography/mass spectrometry method for saliva steroids has also been reported to effectively diagnose congenital adrenal hyperplasia in children 18. Latterly, commercial immunoassays for 17α-OH progesterone have been adapted and used to establish reference ranges in children. However, the use of oral collection devices for newborns induced stress responses, jeopardising both 17α-OH progesterone and cortisol determinations 19. A recent study examined the use of three different saliva collection systems, plain tube and either cotton or polyester Salivette®. Here, all saliva samples were centrifuged prior to assay, using a modified commercial assay, and no differences detected 20. However, a shortcoming was the absence of comparisons with intact extracted saliva.
Progesterone is metabolically stable in saliva and has been used in a variety of applications 21. It has been used as an index of ovulation but there was a high degree of discrepant classifications between the follicular and luteal phases 22. Similar findings were reported recently where salivary progesterone in the mid-luteal phase yielded a sensitivity and specificity of 78% and 77%, respectively 23. While suited for research and long-term clinical observation salivary progesterone determination may have a limited application for individual diagnosis 24. The parallel changes of salivary progesterone and 17α-OH progesterone over the menstrual cycle were suggested as an additional parameter to assess ovarian function 25. Similarly, paired determinations of progesterone and estradiol in saliva may be useful for fertility monitoring although wide variations in oestradiol were noted 26. Probably, the most reliable way of assessing corpus luteal function is via plasma progesterone and if noninvasive testing is required the best option is the determination of urinary pregnanediol excretion indexed to creatinine 27. Salivary progesterone determinations were used to monitor the absorption of progesterone following topical application of progesterone cream to pre- and postmenopausal women. Paradoxically, salivary progesterone levels were elevated whereas plasma and urinary progesterone and its metabolites remained low suggesting minimal benefit to target tissues using this alternative replacement therapy 28.
Salivary cortisol
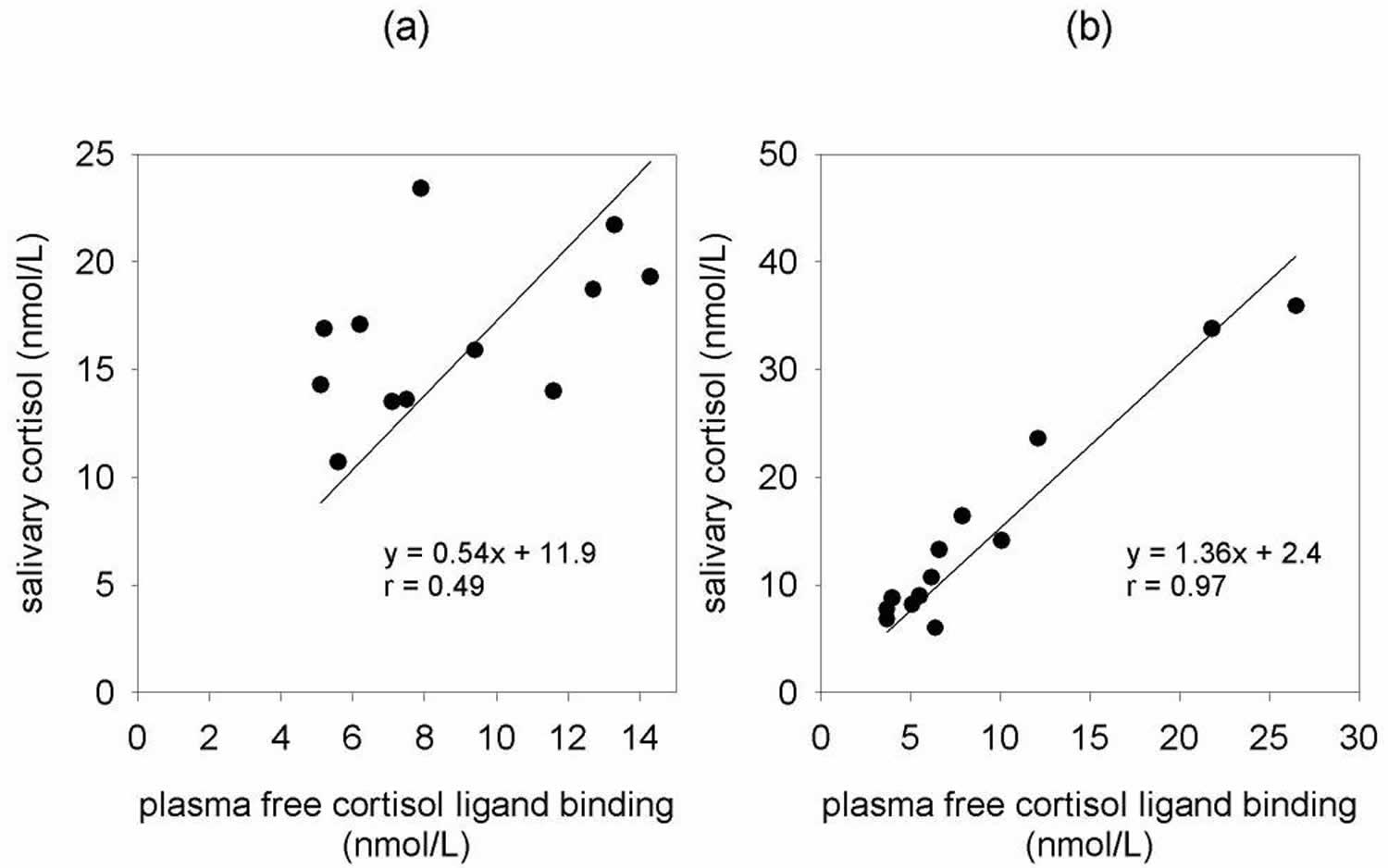
There is little information on 11-deoxycortisol in saliva, presumably due to its very low level, although it can be detected following metyrapone 29. On the other hand, salivary cortisol determinations are more promising. Since the earliest reports, there is evidence that saliva levels reflect unbound concentrations in plasma 30. Direct measurement in saliva may be comparable to extraction but extraction has the advantage of allowing the analysis of low volume saliva samples. This can avoid the use of stimulants and the loss of data due to insufficient sample volume 31. Salivary cortisol determinations have proved popular in psychobiology, stress and sports medicine studies 32. Their use is based on the assumption that salivary cortisol is a reasonable reflection of hypothalamic-pituitary- adrenal (HPA) axis function. In the diagnostic setting, salivary cortisol levels parallel those in plasma following ACTH and CRH stimulation, and following exercise induced-stress 33. However, the correlation of salivary cortisol levels with total plasma cortisol is confounded by the presence of corticosteroid-binding globulin in plasma which is largely saturated up to 500–600 nmol/L of cortisol 34. Salivary cortisol correlates better with measured plasma free cortisol than total plasma cortisol. However, it appears to be subject-specific as considerably variability is found between individuals for daily paired samples (Figure 2). Salivary cortisol determinations were used as markers of metabolic disturbances in obese and diabetic patients and used to investigate changes in glucocorticoid control of the hypothalamic-pituitary- adrenal axis following oral prednisolone 35. Conversely, salivary cortisol measurement is not a useful tool in determining dose adequacy in subjects on oral glucocorticoid replacement therapy 36. Blood spots or serum is preferable, due to contamination of saliva by oral hydrocortisone. The diurnal variation of plasma cortisol is reflected by similar changes in salivary cortisol and hence timed salivary cortisols have been used in a diagnostic setting. Early morning salivary cortisols are useful as a screening tool for adrenal suppression and salivary cortisol is helpful to investigate the control of diurnal cortisol secretion following exposure to darkness and light 37.
Recently, there has been considerable interest in the use of night time salivary cortisols for the initial screening for Cushing’s syndrome. However, despite the optimism, an element of caution is required. Reported cut-off values differ considerably. Papanicolaou et al. 38 determined a normal night time (2330–2400 hour) saliva cut-off of 15 nmol/L with values above this threshold suggestive of Cushing’s. They reported 100% specificity and 91% sensitivity for saliva, similar to a late night serum cortisol measurement cut-off value exceeding 240 nmol/L 38. Both were reportedly clearly superior to urinary free cortisol excretion, contrasting with other recent reports. Yaneva et al. 39 determined a lower saliva cut-off value of 5.7 nmol/L, with 100% sensitivity and 96% specificity which effectively separated Cushing’s from obese subjects 40. The corresponding excretion of urinary free cortisol determined a cut-off value of 248 nmol/day, with similar sensitivity and specificity to saliva. Viardot et al. 40. reported a similar night time saliva cut-off value of 6.1 nmol/L and view salivary cortisol as a reliable first line alternative to urinary free cortisol, the urinary free cortisol:creatinine ratio, and the 1mg overnight dexamethasone suppression test. However, their cut-off value for urinary free cortisol was considerably higher at 504 nmol/day compared to the 248 nmol/day reported by the Yaneva group 39. Both groups used extraction based assays for urinary free cortisol which, as an aside, highlights the importance of well characterised methods and reagents for the determination of urinary free cortisol.
Late night salivary cortisol measurement is nevertheless very promising for the diagnosis of Cushing’s although elevation above threshold values can occur in the elderly, diabetics and women in late pregnancy 41. The variations in reported late night salivary cortisol cut-off values could result from the relatively small numbers in each of the study control groups, which may have varying degrees of obesity, non-adrenal disorders and pseudo-Cushing states, which themselves may be influenced by periodic hypercortisolism 38. Methodological and standardisation issues are also likely contributors to differences in reported cut-off values but a major factor is probably differing specificities of cortisol antibodies towards cortisone. The salivary gland has abundant 11 β-hydroxysteroid dehydrogenase type 2 activity and as a consequence, saliva, unlike plasma, has up to three times the level of cortisone compared to cortisol 42. Depending on the relative cross-reactivity of cortisol antibodies towards cortisone, there could be quite different values of salivary cortisol measured by different immunoassays. Conversely, differences in plasma would be expected to be minimal as cortisone levels are normally only 10% of circulating cortisol levels 42. It is therefore desirable that laboratories establish their own method-specific reference ranges before using salivary cortisol for diagnostic purposes.
Figure 2. Cortisol saliva test
Footnote: Correlation of salivary cortisol and plasma free cortisol in paired samples from two normal individuals (a and b). Salivary cortisol was measured by ELISA and plasma free cortisol by ligand binding/ultrafiltration.
Summary
Salivary steroid testing has a recognised place in research and diagnostic medicine although its limitations must be acknowledged. It is clearly not desirable for androgen assays as well as assays to assess ovarian function and the monitoring of absorption of steroids from transdermal creams. Caution must be exercised for these applications. The use of salivary cortisol for measuring endogenous cortisol is the most encouraging. It can be successfully applied to research studies, adrenal stimulation tests, investigating diurnal variation as well as night time samples as a screening test for Cushing’s syndrome. However, there is a need to establish methodspecific reference ranges and sample stimulation, collection and storage should remain consistent across study groups with a preference for the collection of whole unstimulated saliva, if possible.
References- Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev. 2006;27(3):139–146. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1579286
- Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29:1752–6.
- Vining RF, McGinley RA. The measurement of hormones in saliva: possibilities and pitfalls. J Steroid Biochem. 1987;27:81–94.
- Kalfas S, Rundegren J. Biological qualities of saliva sterilized by filtration or ethylene oxide treatment. Oral Microbiol Immuno. 1991;6:182–6.
- Raff H, Homar PJ, Skoner DP. New Enzyme immunoassay for salivary cortisol. Clin Chem. 2003;49:203–4.
- Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M, Shirtcliff EA. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Horm Behav. 2004;46:39–46.
- Stewart PM, Whorwood CB, Mason JI. Type 2 11β – hydroxysteroid dehydrogenase in foetal and adult life. J Steroid Biochem Mol Bio. 1995;55:465–71.
- Azurmendi A, Braza F, Garcia A, Braza P, Munoz JM, Sanchez-Martin JR. Aggression, dominance, and affilliation: their relationships with androgen levels and intelligence in 5-year-old children. Horm Behav. 2006;50:132–40.
- Stikkelbroeck NM, Sweep CG, Braat DD, Hermus AR, Otten BJ. Monitoring of menstrual cycles, ovulation, and adrenal suppression by saliva sampling in female patients with 21-hydroxylase deficiency. Fertil Steril. 2003;80:1030–6.
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–95.
- Edwards DA, Wetzel K, Wyner DR. Intercollegiate soccer: saliva cortisol and testosterone are elevated during competition, and testosterone is related to status and social connectedness with teammates. Physiol Behav. 2006;87:135–43.
- Tschop M, Behre HM, Nieschlag E, Dressendorfer RA, Strasburger CJ. A time-resolved fluorescence immunoassay for the measurement of testosterone in saliva: monitoring of testosterone replacement therapy with testosterone buciclate. Clin Chem Lab Med. 1998;36:223–30.
- Herold DA, Fitzgerald RL. Immunoassays for testosterone in women: better than a guess? Clin Chem. 2003;49:1250–1.
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–40.
- Ellison PT, Bribiescas RG, Bentley GR, et al. Population variation in age-related decline in male salivary testosterone. Hum Reprod. 2002;17:3251–3.
- Broadbent JL. Salivary testosterone: is it an indicator of free testosterone concentration in plasma? NZ J Med Lab Science. 2002;56:85–9.
- Ueshiba H, Zerah M, New MI. Enzyme-linked immunosorbent assay (ELISA) method for screening of non-classical steroid 21-hydroxylase deficiency. Horm Metab Res. 1994;26:43–5.
- Shindo N, Yamauchi N, Murayama K, Fairbrother A, Korlik S. Identification of 17-hydroxyprogesterone and other steroid hormones in saliva from a normal child and patients with congenital adrenal hyperplasia by plasmaspray liquid chromatography/mass spectrometry. Biomed Chromatogr. 1990;4:171–4.
- Gröschl M, Rauh M, Dörr HG. Circadian rhythm of salivary cortisol, 17alpha–hydroxyprogesterone, and progesterone in healthy children. Clin Chem. 2003;49:1688–91.
- Mylonas PG, Makri M, Georgopoulos NA, et al. Adequacy of saliva 17-hydroxyprogesterone determination using various collection methods. Steroids. 2006;71:273–6.
- Laine MA, Ojanotko AO. Progesterone metabolism in human saliva in vitro. J Steroid Biochem Mol Biol. 1999;70:109–13.
- Metcalf MG, Evans JJ, Mackenzie JA. Indices of ovulation: comparison of plasma and salivary levels of progesterone with urinary pregnanediol. J Endocrinol. 1984;100:75–80.
- Ishikawa M, Sengoku K, Tamate K, Takaoka Y, Kane M, Fottrell PF. The clinical usefulness of salivary progesterone measurement for the evaluation of the corpus luteum function. Gynecol Obstet Invest 144 I Clin Biochem Rev Vol 27 August 2006 Lewis JG Salivary Steroids. 2002;53:32–7.
- Lipson SF, Ellison PT. Reference values for luteal progesterone measured by salivary radioimmunoassay. Fertil Steril. 1994;61:448–54.
- Gröschl M, Rauh M, Schmid P, Dörr HG. Relationship between salivary progesterone, 17-hydroxyprogesterone, and cortisol levels throughout the normal menstrual cycle of healthy postmenarcheal girls. Fertil Steril. 2001;76:615–7.
- Tamate K, Charleton M, Gosling JP, et al. Direct colorimetric monoclonal antibody enzyme immunoassay for estradiol–17β in saliva. Clin Chem. 1997;43:1159–64.
- Lewis JG, Manley L, Whitlow JC, Elder PA. Reexamining steroid hormone metabolites as ovulation markers using monoclonal antibodies. Steroids. 1994;59:288–91.
- Lewis JG, McGill H, Patton VM, Elder PA. Caution on the use of saliva measurements to monitor absorption of progesterone from transdermal creams in postmenopausal women. Maturitas. 2002;41:1–6.
- Al-Ansari AA, Massoud M, Perry LA, Smith DS. Polarization. fluoroimmunoassay of 11-deoxycortisol in serum and saliva. Clin Chem. 1983;29:1803–5.
- Umeda T, Hiramatsu R, Iwaoka T, Shimada T, Miura F, Sato T. Use of saliva for monitoring unbound free cortisol levels in serum. Clin Chim Acta. 1981;110:245–53.
- de Weerth C, Graat G, Buitelaar JK, Thijssen JH. Measurement of cortisol in small quantities of saliva. Clin Chem. 2003;49:658–60.
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–96.
- Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic-pituitary-adrenal axis activity. Clin Endocrinol (Oxf) 2005;63:336–41.
- Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20:329–35.
- Jerjes WK, Cleare AJ, Wood PJ, Taylor NF. Assessment of subtle changes in glucocorticoid negative feedback using prednisolone: comparison of salivary free cortisol and urinary cortisol metabolites as endpoints. Clin Chim Acta. 2006;364:279–86.
- Wong V, Yan T, Donald A, McLean M. Saliva and bloodspot cortisol: novel sampling methods to assess hydrocortisone replacement therapy in hypoadrenal patients. Clin Endocrinol (Oxf) 2004;61:131–7.
- Patel RS, Shaw SR, McIntyre HE, McGarry GW, Wallace AM. Morning salivary cortisol versus short synacthen test as a test of adrenal suppression. Ann Clin Biochem. 2004;41:408–10.
- Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing’s Syndrome. J Clin Endocrinol Metab. 2002;87:4515–21.
- Yaneva M, Mosnier-Pudar H, Dugue M-A, Grabar S, Fulla Y, Bertagna X. Midnight salivary cortisol for the Clin Biochem Rev Vol 27 August 2006 I 145 initial diagnosis of Cushing’s Syndrome of various causes. J Clin Endocrinol Metab. 2004;89:3345–51.
- Viardot A, Huber P, Puder J, Zulewski H, Keller U, Müller B. Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism as compared to urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab. 2005;90:5730–6.
- Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf) 2005;63:642–9.
- Morineau G, Boudi A, Barka A, et al. Radioimmunoassay of cortisone in serum, urine, and saliva to assess the status of the cortisol-cortisone shuttle. Clin Chem. 1997;43:1397–407.