What is somatostatin
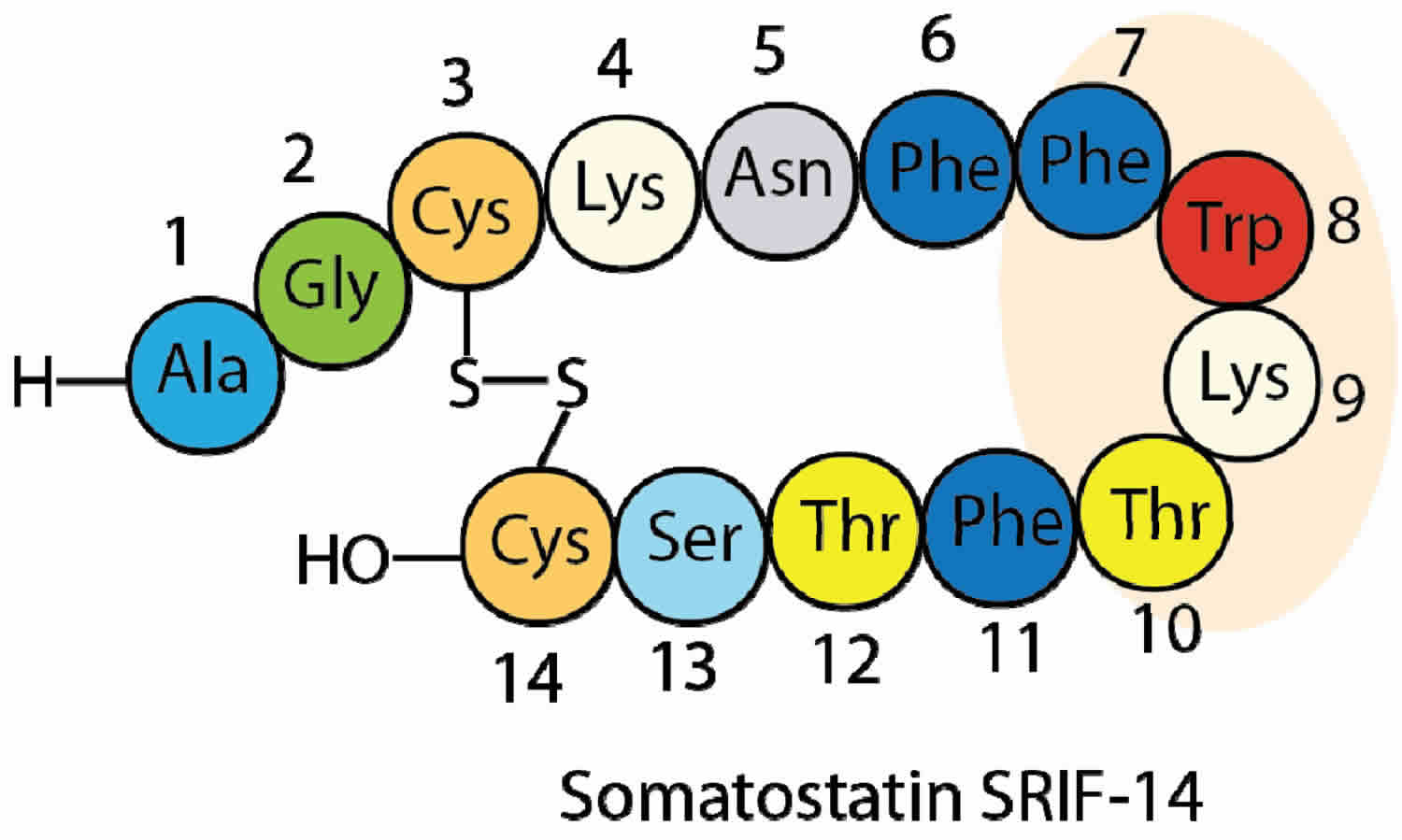
Somatostatin is also known as growth hormone release-inhibiting hormone (GHRIH) due to its ability to inhibit pituitary growth hormone release [somatotropin release-inhibiting factor (SRIF-14 & SRIF-28)]. Somatostatin is a 14-amino acid peptide that is capable of inhibiting other numerous endocrine and exocrine secretory functions. Almost all gut hormones (i.e., gastrin, cholecystokinin (CCK), ghrelin, vasoactive intestinal peptide [VIP], pancreatic polypeptide, secretin, GIP, and motilin) are inhibited by somatostatin 1. Somatostatin is a potent inhibitor of gastric acid secretion, intestinal transit time, motility and absorption of nutrients. Somatostatin also inhibits the secretion of glucagon and insulin by the nearby alpha and beta cells in the pancreas islets of Langerhans cells (see Figure 1 below) and the thyroid-stimulating hormone (TSH) by inhibiting the somatotroph cells in the anterior pituitary gland. In addition to inhibition of the endocrine secretions, somatostatin has direct effects on a number of target organs 2. For example, it is a potent inhibitor of basal and prostaglandin-stimulated gastric acid secretion.
The clinical use of native somatostatin is limited by a very short half-life (1 to 3 min) and the broad spectrum of biological responses 3. Thus stable, receptor-selective agonists have been developed. Somatostatin and its new analogs, octreotide and lanreotide, are potent inhibitors of tumor growth including neuroendocrine tumors 4. Somatostatin agonists are established in the treatment of acromegaly (a condition in where there is oversecretion of growth hormone in an adult) with recently approved indications in the therapy of neuroendocrine tumors. Potential therapeutic uses for somatostatin analogues include diabetic complications like retinopathy, nephropathy and obesity, due to inhibition of IGF-1, VEGF together with insulin secretion and effects upon the renin–angiotensin–aldosterone system 3. Wider uses in anti-neoplastic therapy may also be considered and recent studies have further revealed anti-inflammatory and anti-nociceptive effects 3.
Glucagon stimulates somatostatin secretion via paracrine interaction between alpha cells and delta cells of the islets of Langerhans of the pancreas 5. Paracrine signaling is a form of cell-to-cell communication in which a cell produces a signal to induce changes in nearby cells, altering the behavior of those cells.
Where is somatostatin produced
Somatostatin is a hormone produced by the hypothalamus and some other tissues such as the pancreas and the gastrointestinal tract. Somatotropin release-inhibiting factor (SRIF) is ubiquitously expressed in mammalian brain. Extrahypothalamic somatotropin release-inhibiting factor (SRIF) immunoreactivity is evident in the mediobasal hypothalamus and median eminence, amygdala, preoptic area, hippocampus, striatum, cerebral cortex, olfactory regions, sensory regions and the brainstem 6. In the pancreas somatostatin is produced by the pancreatic islets of Langerhans cells called the delta (δ) cells.
Figure 1. Somatostatin is produced by the pancreatic islets of Langerhans Delta (δ) cells.
Footnote: The endocrine portion of the pancreas is arranged as discrete islets of Langerhans, which are composed of five different endocrine cell types (alpha, beta, delta, epsilon, and upsilon) secreting at least five hormones including glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide, respectively.
Somatostatin function
Somatostatin is involved in multiple biological functions, mediated by its direct interaction with at least five different G-protein coupled receptors, named called somatostatin receptor (SST)1–5 7. These five receptors share common structural features and signaling mechanisms but differ in their cellular and subcellular localization and mode of regulation. SST2 and SST5 receptors have evolved as primary targets for pharmacological treatment of pituitary adenomas and neuroendocrine tumors 8. Somatostatin inhibits the release of growth hormone from the anterior pituitary, and insulin and glucagon from the pancreas. Somatostatin also decreases the release of most gastrointestinal hormones and reduces gastric acid and pancreatic secretion. Somatostatin can reduce abdominal blood flow therefore somatostatin analogs can be used to reduce bleeding from esophageal varices.
Within the hypothalamus–pituitary system, somatostatin is the main regulatory element exerting inhibitory control on both basal and stimulated GH secretion and reduces prolactin and thyroid-stimulating hormone (TSH) secretion in normal subjects 9. Somatostatin can also suppress release of adrenocorticotropic hormone (ACTH) from tumor cells 10. Somatostatin brain actions are mediated by presynaptic or postsynaptic mechanisms. somatostatin modulates neuronal excitability, and in the hippocampus, cortex, and hypothalamus it also induces presynaptic inhibition of excitatory neurotransmission 11. In other brain areas, somatostatin also decreases GABA release. Postsynaptic mechanisms of action include membrane hyperpolarization via activation of potassium ion currents (K+ currents), in particular voltage-gated K+ currents, noninactivating potassium currents (M currents), and voltage-insensitive leak currents 12. Somatostatin is co-released with GABA from hippocampal neurons and from axonal terminals in other brain areas 13. Somatostatin inhibits dopamine release from the midbrain as well as hypothalamic release of noradrenaline, thyrotropin-releasing hormone, and corticotropin-releasing hormone (CRH) 14. Activation of brain somatostatin signaling may alleviate endocrine, autonomic, and behavioral responses to stress mediated by central corticotropin-releasing hormone (CRH) and CRH receptors 15. Somatostatin has a role in cognitive functions, learning and memory processes, control of locomotor activity, control of food intake, nociception, and autonomic functions 8. Somatostatin is highly expressed in brain regions associated with seizures and has been suggested as an endogenous antiepileptic 16.
Peripheral somatostatin actions include inhibition of hormone secretion, exocrine secretion, and cell proliferation. In the gastrointestinal (GI) tract (GIT), somatostatin exerts a generalized inhibitory effect on release of gut hormones [including gastrin, CCK, gastric inhibitory polypeptide, vasoactive intestinal peptide, enteroglucagon, motilin], gastric acid, digestive enzymes, bile, and colonic fluid. somatostatin also negatively affects gallbladder contraction, small intestinal segmentation, and gastric emptying. In pancreatic islets, release of somatostatin from δ-cells inhibits secretion of insulin, glucagon, and other peptides from neighboring cells. somatostatin reduces TSH-induced release of triiodothyronine (T3) and thyroxine as well as calcitonin release. In the adrenals, somatostatin inhibits angiotensin II–stimulated aldosterone secretion and acetylcholine-stimulated medullary catecholamine secretion. somatostatin reduces release of kidney-derived renin caused by hypovolemia and vasopressin-mediated water absorption. In addition to nervous system functions and regulation of endocrine and GI functions, somatostatin also may affect key cellular processes in diverse tissues by regulating the release of both growth factors and cytokines as well as cellular responses to these stimuli. somatostatin can contribute to control of smooth muscle cell contractility, lymphocyte and inflammatory cell proliferation and activity, tumor cell growth, and normal tissue plasticity 17. In human skin, somatostatin has been suggested as a negative regulator of epidermal wound healing 18. Finally, at variance with its nearly universal inhibitory actions, low (pM) concentrations of somatostatin stimulate in vitro GH release on cultured pituitary cells derived from pigs 19 and nonhuman primates 20 and from human somatotroph adenomas 21.
Somatostatin medication
Somatostatin analogs are used for treatment of tumors secreting vasoactive intestinal peptide, carcinoid tumors, glucagonomas and various pituitary adenomas. It is also used to treat acromegaly (a condition in where there is oversecretion of growth hormone in an adult) 22.
A myriad of somatostatin analogs have been synthesized over the past few decades introducing modifications such as exchange and deletion of amino acids, ring size reduction, disulfide bridge modification, multiple N-methylation and site-specific PEGylation 23. Several of those analogs displayed an improvement of different “drug-like” properties in comparison to somatostatin. A few synthetic analogs have reached the market: octreotide (Sandostatin®) 24, lanreotide (Somatuline®), vapreotide (Sanvar®) and pasireotide (Signifor®) (Figure 2) 25. These are octa- or hexapeptides, thus having a shorter and consequently less flexible ring than that of somatostatin. They are long-acting analogs with increased plasma stability and are highly selective against receptor SSTR2. To date, most of the research efforts in this field have focused on the design of new more conformationally restricted analogs with shorter rings (octreotide analogs, in fact), and on the improvement of the methodology to prepare them in an efficient and simple manner.
Figure 2. Somatostatin analogs
Somatostatin octreotide
Octreotide immediate-release injection is a man-made protein that is similar to a hormone in the body called somatostatin. Octreotide lowers many substances in the body such as insulin and glucagon (involved in regulating blood sugar), growth hormone, and chemicals that affect digestion. Octreotide immediate-release injection is used to decrease the amount of growth hormone produced by people with acromegaly (condition in which the body produces too much growth hormone, causing enlargement of the hands, feet, and facial features; joint pain; and other symptoms) who cannot be treated with surgery, radiation, or another medication. Octreotide immediate-release injection is also used to control diarrhea and flushing caused by carcinoid tumors (slow-growing tumors that release natural substances that can cause symptoms) and vasoactive intestinal peptide secreting adenomas (VIP-omas; tumors that form in the pancreas and release natural substances that can cause symptoms). Octreotide long-acting injection is used to control acromegaly, carcinoid tumors, and VIP-omas in people who have been successfully treated with otreotide injection but prefer to receive injections less often. Octreotide injection is in a class of medications called octapeptides. It works by decreasing the amounts of certain natural substances produced by the body.
Octreotide comes as an immediate-release injection to be injected subcutaneously (under the skin) or intravenously (into a vein) and as a long-acting injection to be injected into the muscles of the buttocks by a doctor or nurse. Octreotide immediate-release injection is usually injected 2 to 4 times a day. Octreotide long-acting injection is usually injected once every 4 weeks. Inject octreotide immediate-release injection at around the same times every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Inject octreotide injection exactly as directed. Do not inject more or less of it or inject it more often than prescribed by your doctor.
If you are using the immediate-release injection, you may be able to inject the medication yourself at home or have a friend or relative perform the injections. Ask your doctor to show you or the person who will be performing the injections how to inject the medication. Also talk to your doctor about where on your body you should inject the medication and how you should rotate injection spots so that you do not inject in the same spot too often. Before you inject your medication, always look at the liquid and do not use it if it is cloudy or contains particles. See your pharmacist for new medication.
Octreotide is injected under the skin, or into a vein through an IV. You may be shown how to use injections at home. Do not self-inject this medicine if you do not fully understand how to give the injection and properly dispose of used needles, IV tubing, and other items used to inject the medicine. Be sure to follow the instructions for the exact type of octreotide your doctor has prescribed for you.
Octreotide should be at room temperature when you inject it. Take the medicine out of the refrigerator 30 to 60 minutes before preparing your dose. Do not heat the medicine. After mixing your dose, give the injection right away. Do not save it for later use.
If you are not already being treated with octreotide injection, you will begin your treatment with immediate-release octreotide injection. You will be treated with the immediate-release injection for 2 weeks, and your doctor may gradually increase your dose during that time. If the medication works for you and does not cause severe side effects, your doctor may give you the long-acting injection after 2 weeks. In order to control your condition, you may need to continue to receive the immediate-release injection for 2 weeks or longer after you receive your first dose of the long-acting injection. Your doctor may increase or decrease your dose of the long-acting injection 2 or 3 months after you first receive it.
If you are being treated for a carcinoid tumor or VIP-oma, you may experience worsening of your symptoms from time to time during your treatment. If this happens, your doctor may tell you to use the immediate-release injection for a few days until your symptoms are controlled.
If you have acromegaly and have been treated with radiation therapy, your doctor will probably tell you not to use octreotide immediate-release injection for 4 weeks every year or not to receive the octreotide long-acting injection for 8 weeks every year. This will allow your doctor to see how the radiation therapy has affected your condition and decide whether you should still be treated with octreotide.
Octreotide injection may control your symptoms, but it will not cure your condition. Continue to use octreotide injection even if you feel well. Do not stop using octreotide injection without talking to your doctor. If you stop using octreotide injection, your symptoms may return.
Before using octreotide injection
- tell your doctor and pharmacist if you are allergic to octreotide injection, any other medications, or any of the ingredients in octreotide injection. Ask your pharmacist for a list of the ingredients. If you will be using the long-acting injection, also tell your doctor if you are allergic to latex.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: beta blockers such as atenolol (Tenormin), labetalol (Normodyne), metoprolol (Lopressor, Toprol XL), nadolol (Corgard), and propranolol (Inderal); bromocriptine (Cycloset, Parlodel); calcium channel blockers such as amlodipine (Norvasc), diltiazem (Cardizem, Dilacor, Tiazac, others), felodipine (Plendil), nifedipine (Adalat, Procardia), nisoldipine (Sular), and verapamil (Calan, Isoptin, Verelan); cyclosporine (Gengraf, Neoral, Sandimmune); insulin and oral medications for diabetes; quinidine; and terfenadine (Seldane) (not available in the U.S.). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you are being fed by total parenteral nutrition (TPN; feeding by giving a fluid containing nutrients directly into a vein) and if you have or have ever had diabetes or heart, liver, or kidney disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. You may be able to become pregnant during your treatment with octreotide even if you were not able to become pregnant before your treatment because you have acromegaly. Talk to your doctor about methods of birth control that will work for you. If you become pregnant while receiving octreotide injection, call your doctor.
Somatostatin octreotide side effects
Octreotide injection may cause changes in your blood sugar. You should know the symptoms of high and low blood sugar and what to do if you have these symptoms.
Octreotide injection may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- diarrhea
- constipation
- pale, bulky, foul-smelling stools
- constantly feeling the need to empty the bowels
- gas
- stomach pain
- nausea
- heartburn
- headache
- dizziness
- tiredness
- back, muscle, or joint pain
- nosebleed
- hair loss
- pain in the area where the medication was injected
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately:
- pain in the upper right part of the stomach, center of the stomach, back, or shoulder
- yellowing of the skin or eyes
- slowed or irregular heartbeat
- sluggishness
- sensitivity to cold
- pale, dry skin
- brittle fingernails and hair
- puffy face
- hoarse voice
- depression
- heavy menstrual periods
- swelling at the base of the neck
- tightness in the throat
- difficulty breathing and swallowing
- rash
- itching
Octreotide injection may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
Symptoms of overdose may include:
- slowed or irregular heartbeat
- dizziness
- fainting
- flushing
- diarrhea
- weakness
- weight loss
- Vinik A, Pacak K, Feliberti E, et al. Somatostatinoma. [Updated 2017 Jun 14]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279034/
- Creutzfeldt W, Arnold R. Somatostatin and the stomach: exocrine and endocrine aspects. Metabolism 1978; 27(9 Suppl 1):1309-1315.
- Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacology & Therapeutics Volume 152, August 2015, Pages 98-110. https://doi.org/10.1016/j.pharmthera.2015.05.007
- Caplin ME, Pavel M, Cwikla JB et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer 2016; 23(3):191-199.
- ElSayed SA, Bhimji SS. Physiology, Pancreas. [Updated 2018 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459261
- Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Johansson O, Hökfelt T, Elde RP. Neuroscience. 1984 Oct; 13(2):265-339
- Hoyer, D.; Bell, G.I.; Berelowitz, M.; Epelbaum, J.; Feniuk, W.; Humphrey, P.P.A.; O’Carroll, A.-M.; Patel, Y.C.; Schonbrunn, A.; Taylor, J.E.; et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol. Sci. 1995, 16, 86–88.
- Günther T, Tulipano G, Dournaud P, et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol Rev. 2018;70(4):763-835. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6148080/
- Neuroendocrine control of growth hormone secretion. Müller EE, Locatelli V, Cocchi D. Physiol Rev. 1999 Apr; 79(2):511-607.
- Role of somatostatin receptors in normal and tumoral pituitary corticotropic cells. Hofland LJ, Lamberts SW, Feelders RA. Neuroendocrinology. 2010; 92 Suppl 1():11-6.
- AMPA-sst2 somatostatin receptor interaction in rat hypothalamus requires activation of NMDA and/or metabotropic glutamate receptors and depends on intracellular calcium. Peineau S, Potier B, Petit F, Dournaud P, Epelbaum J, Gardette R. J Physiol. 2003 Jan 1; 546(Pt 1):101-17.
- Somatostatin receptor subtype 4 couples to the M-current to regulate seizures. Qiu C, Zeyda T, Johnson B, Hochgeschwender U, de Lecea L, Tallent MK. J Neurosci. 2008 Apr 2; 28(14):3567-76.
- Regulation and function of somatostatin receptors. Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. J Neurochem. 2004 Jun; 89(5):1057-91.
- Somatostatin and its receptor family. Patel YC. Front Neuroendocrinol. 1999 Jul; 20(3):157-98.
- Activation of Brain Somatostatin Signaling Suppresses CRF Receptor-Mediated Stress Response. Stengel A, Taché YF. Front Neurosci. 2017; 11():231.
- The role of brain somatostatin receptor 2 in the regulation of feeding and drinking behavior. Stengel A, Karasawa H, Taché Y. Horm Behav. 2015 Jul; 73():15-22.
- Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Rai U, Thrimawithana TR, Valery C, Young SA. Pharmacol Ther. 2015 Aug; 152():98-110.
- Somatostatin inhibits cell migration and reduces cell counts of human keratinocytes and delays epidermal wound healing in an ex vivo wound model. Vockel M, Pollok S, Breitenbach U, Ridderbusch I, Kreienkamp HJ, Brandner JM. PLoS One. 2011 May 11; 6(5):e19740.
- Identification of the somatostatin receptor subtypes (sst) mediating the divergent, stimulatory/inhibitory actions of somatostatin on growth hormone secretion. Luque RM, Durán-Prado M, García-Navarro S, Gracia-Navarro F, Kineman RD, Malagón MM, Castaño JP. Endocrinology. 2006 Jun; 147(6):2902-8.
- Somatostatin dramatically stimulates growth hormone release from primate somatotrophs acting at low doses via somatostatin receptor 5 and cyclic AMP. Córdoba-Chacón J, Gahete MD, Culler MD, Castaño JP, Kineman RD, Luque RM. J Neuroendocrinol. 2012 Mar; 24(3):453-63.
- Expression and function of somatostatin receptor subtype 1 in human growth hormone secreting pituitary tumors deriving from patients partially responsive or resistant to long-term treatment with somatostatin analogs. Matrone C, Pivonello R, Colao A, Cappabianca P, Cavallo LM, Del Basso De Caro ML, Taylor JE, Culler MD, Lombardi G, Di Renzo GF, Annunziato L. Neuroendocrinology. 2004 Mar; 79(3):142-8.
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938.
- Chatterjee, J.; Laufer, B.; Beck, J.G.; Helyes, Z.; Pintér, E.; Szolcsányi, J.; Horvath, A.; Mandl, J.; Reubi, J.C.; Kéri, G.; et al. N -methylated sst 2 selective somatostatin cyclic peptide analogue as a potent candidate for treating neurogenic inflammation. ACS Med. Chem. Lett. 2011, 2, 509–514.
- Van der Lely, A.J.; de Herder, W.W.; Krenning, E.P.; Kwekkeboom, D.J. Octreoscan radioreceptor imaging. Endocrine 2003, 20, 307–311.
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716.