What is Beckwith Wiedemann syndrome
Beckwith-Wiedemann syndrome is a condition that affects many parts of the body. It is classified as an overgrowth syndrome, affected infants are considerably larger than normal (macrosomia) and tend to be taller than their peers during childhood. Growth begins to slow by about age 8, and adults heights with Beckwith-Wiedemann syndrome are typically in the normal range. In some children with Beckwith-Wiedemann syndrome, specific parts of the body on one side or the other may grow abnormally large, leading to an asymmetric or uneven appearance. This unusual growth pattern, which is known as hemihyperplasia, usually becomes less apparent over time. Beckwith-Wiedemann syndrome was first recognised in 1963-64 by Dr J. Bruce Beckwith, a paediatric pathologist in America and, independently, by Dr H.E. Wiedemann, a German geneticist 1. Each had found a similar set of congenital abnormalities in children, which could not be found in any other disorders – in other words, a new syndrome.
Beckwith-Wiedemann syndrome affects an estimated 1 in 13,700 newborns worldwide 2. Beckwith-Wiedemann syndrome may actually be more common than this estimate because some people with mild symptoms are never diagnosed.
Approximately 85 percent of people with Beckwith-Wiedemann syndrome have genetic changes that appear to occur randomly (sporadically). Familial transmission occurs in approximately 10-15 percent of people with Beckwith-Wiedemann syndrome. Researchers have determined that Beckwith-Wiedemann syndrome results from various abnormalities affecting the proper expression of certain genes that control growth within a specific region of chromosome 11.
The signs and symptoms of Beckwith-Wiedemann syndrome vary among affected individuals. Some children with Beckwith-Wiedemann syndrome are born with an opening in the wall of the abdomen (an omphalocele) that allows the abdominal organs to protrude through the belly-button. Other abdominal wall defects, such as a soft out-pouching around the belly-button (an umbilical hernia), are also common. Some infants with Beckwith-Wiedemann syndrome have an abnormally large tongue (macroglossia), which may interfere with breathing, swallowing, and speaking. Other major features of this condition include abnormally large abdominal organs (organomegaly), creases or pits in the skin near the ears, low blood sugar (hypoglycemia) in infancy, and kidney abnormalities. Beckwith-Wiedemann syndrome may also be associated with other facial abnormalities, abnormal enlargement of one side or structure of the body (hemihyperplasia/hemihypertrophy) resulting in unequal (asymmetric) growth.
Children with Beckwith-Wiedemann syndrome are at an increased risk of developing several types of cancerous and noncancerous tumors, particularly a form of kidney cancer called Wilms tumor, a form of liver cancer called hepatoblastoma, adrenal carcinoma, and rhabdomyocsarcoma. Tumors develop in about 10 percent of people with Beckwith-Wiedemann syndrome and almost always appear in childhood. In infancy, Beckwith-Wiedemann syndrome has a mortality rate of approximately 20% 3.
Infants with low blood sugar may be treated with fluids given through a vein (intravenous, IV). Some infants may need medicine or other management if low blood sugar continues.
Defects in the abdominal wall may need to be repaired. If the enlarged tongue makes it hard to breathe or eat, surgery may be needed. Children with overgrowth on one side of the body should be watched for a curved spine (scoliosis). The child also must be watched closely for the development of tumors. Tumor screening includes blood tests and abdominal ultrasounds.
Beckwith-Wiedemann syndrome symptoms
Signs and symptoms of Beckwith-Wiedemann syndrome include:
- Beckwith-Wiedemann syndrome children are usually born prematurely but are larger and heavier than one would expect, given the shorter length of gestation.
- Large size for a newborn
- Red birth mark on forehead or eyelids (nevus flammeus). This usually fades in the first few years.
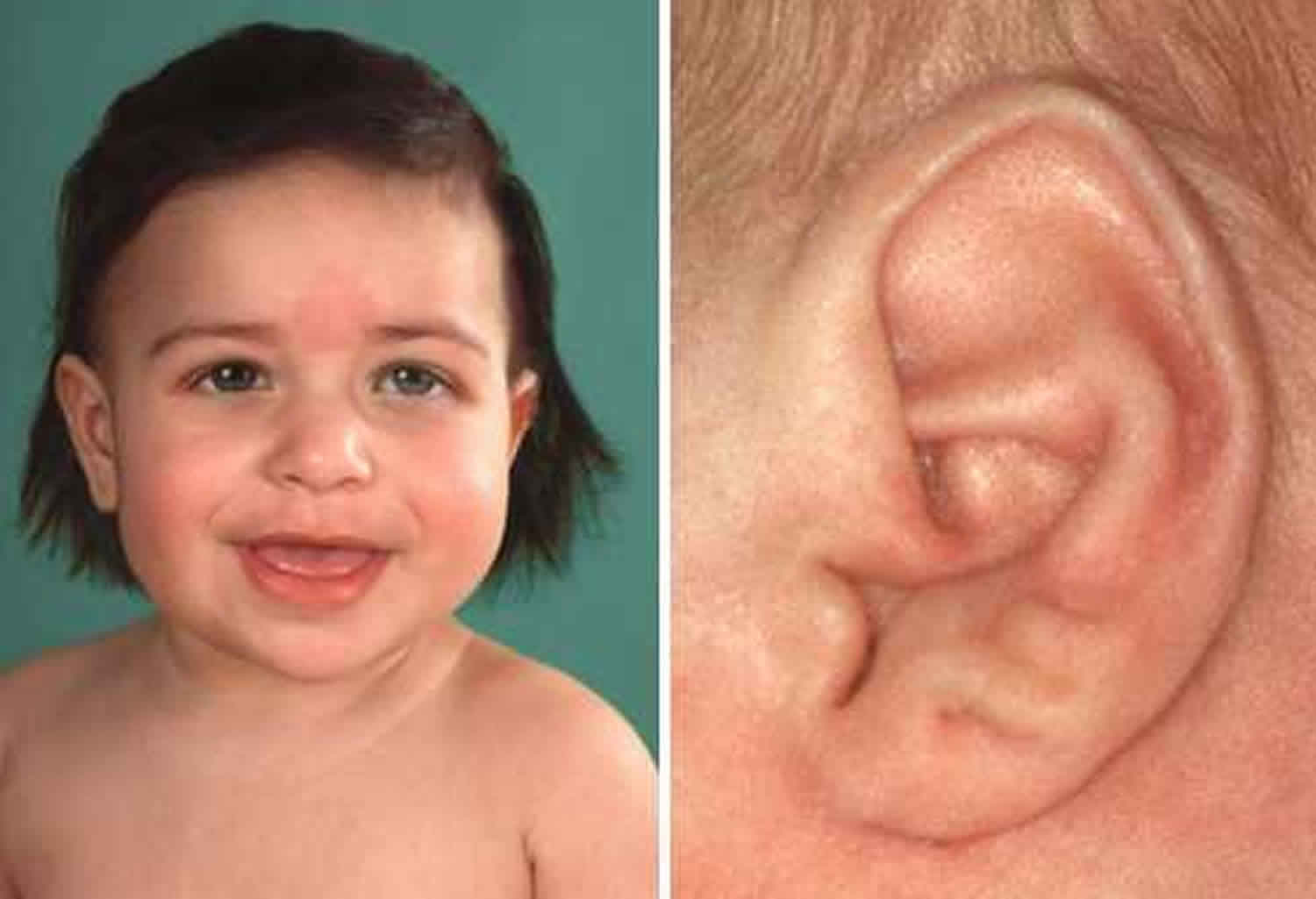
- Creases in ear lobes
- Large tongue (macroglossia)
- Low blood sugar (hypoglycemia) occurs in approximately 40% of Beckwith-Wiedemann syndrome children shortly after birth. Brain damage and other complications can result if it is not diagnosed and treated.
- Abdominal wall defect (umbilical hernia or omphalocele)
- Enlargement of some abdominal organs, usually the kidneys, liver, spleen, adrenals and pancreas (visceromegaly)
- Overgrowth of one side of the body or of one limb (hemihyperplasia/hemihypertrophy) while the rest of the body grows at a normal rate.
- Tumor growth, such as Wilms tumors (kidney cancer) and hepatoblastomas (liver tumors).
- Enlarged heart or heart defects. These are relatively uncommon and may resolve without treatment.
- Psychomotor development is usually normal except in cases of undiagnosed hypoglycemia or other complications.
The symptoms of Beckwith-Wiedemann syndrome vary greatly from person to person. Diagnosis of Beckwith-Wiedemann syndrome can be challenging because the patients are often mosaic (with the genetic changes occurring in some cells or parts of the body but not others), however external appearance is not necessarily predictive of internal effects. This results in some individuals appearing mildly affected, while others appear more significantly affected. The wide range of potential symptoms (clinical spectrum) can affect many different organs of the body. Affected individuals may not have all of the symptoms listed below. Many clinical features of Beckwith-Wiedemann syndrome become less evident with increasing age and many adults experience normal growth and appearance. Intelligence is usually unaffected in Beckwith-Wiedemann syndrome, unless associated with prolonged, untreated neonatal hypoglycemia or a chromosomal duplication.
Some infants with Beckwith-Wiedemann syndrome are born prematurely, but still have an excessive birth weight (large for gestational age). Many infants with Beckwith-Wiedemann syndrome are above the 97th percentile in weight for gestational age. Overgrowth continues throughout childhood (macrosomia) and slows around 7 or 8 years of age. Abnormal enlargement of one side or structure of the body (hemihyperplasia/hemihypertrophy) may occur, resulting in unequal (asymmetric) growth. Hemihyperplasia refers specifically to an increase in number of cells (proliferation) resulting in asymmetric overgrowth. A related term, hemihypertrophy, refers to overgrowth due to abnormally large cell size.
Abdominal wall defects include an omphalocele (also known as exomphalos), in which part of an infant’s intestines and abdominal organs protrude or stick out through the belly button. The intestines and other organs are covered by a thin membrane. Less severe defects can include protrusion of part of the intestines through an abnormal opening in the muscular wall of the abdomen near the umbilical cord (umbilical hernia), or weakness and separation of the left and right muscles (rectus muscles) of the abdominal wall (diastasis recti). The internal organs of affected individuals can become abnormally enlarged (organomegaly). Any or all of the following organs may be affected: liver, spleen, pancreas, kidneys, or adrenal glands.
Some newborns with Beckwith-Wiedemann syndrome may have low blood sugar (neonatal hypoglycemia or hyperinsulinism) due to overgrowth and excessive secretion of the hormone insulin by pancreatic islets. Insulin functions to help regulate blood glucose levels by promoting the movement of glucose into cells. Most infants with neonatal hypoglycemia associated with Beckwith-Wiedemann syndrome have mild and transient symptoms. However, without proper detection and appropriate treatment, neurological complications may result.
Macroglossia (enlarged tongue) is reported to occur in approximately 82%- 97% of cases and is one of the most common features of Beckwith-Wiedemann syndrome. Children with an enlarged tongue (macroglossia) can have difficulties in speaking, feeding, and breathing. In addition to an enlargement of the tongue (macroglossia), Beckwith-Wiedemann syndrome may be characterized by other abnormalities of the skull and facial (craniofacial) region. Such features may include distinctive slit-like linear grooves or creases in the ear lobes and indentations on the back rims of the ears (pits), prominent eyes with relative underdevelopment of the bony cavity of the eyes (intraorbital hypoplasia), and/or a prominent back region of the skull (occiput). Some infants may have flat, pale red or reddish purple facial lesions at birth, most commonly on the eyelids and forehead, which consist of abnormal clusters of small blood vessels (capillary nevus flammeus). Such lesions typically become less apparent during the first year of life. In children with hemihyperplasia/hemihypertrophy, one side of the face may appear larger than the other. Due to the mosaic nature of Beckwith-Wiedemann syndrome, some children have eyes with multiple colors. In addition, in some affected children, there may be improper contact of the teeth of the upper and lower jaws (malocclusion) and abnormal protrusion of the lower jaw (mandibular prognathism), features that may occur secondary to abnormal largeness of the tongue.
A variety of kidney (renal) abnormalities have occurred in individuals with Beckwith-Wiedemann syndrome, including abnormally large kidneys (nephromegaly), improper development of the innermost tissues of the kidney (renal medullary dysplasia), and the formation of calcium deposits in the kidney (nephrocalcinosis), which could potentially impair kidney function. Additional abnormalities include duplication of the series of tubes and ducts through which the kidneys reabsorb water and sodium (duplicated collecting system), widening of some of the small tubes and collecting ducts (medullary sponge kidney), and the presence of small pouches (diverticula) on the kidneys.
Children with Beckwith-Wiedemann syndrome may have an increased risk of developing certain childhood cancers, particularly Wilms tumor (nephroblastoma), which is a malignancy of the kidney, and tumors involving the liver (hepatoblastoma). Less commonly, other malignancies have been reported (e.g., neuroblastoma, rhabdomyosarcoma). The risk of malignancy is greatest before the age of 8. Around 7.5% of Beckwith-Wiedemann syndrome children will develop Wilms Tumour. Because of the aggressiveness of these tumors, abdominal ultrasound scans should take place every three months up to the age of 7 or 8 years. A baseline MRI scan may also be performed. The susceptibility to these tumors diminishes and is not usually a problem after the age of 8.
Liver tumors (hepatoblastoma) risk diminishes after the age of 3 years. Liver tumors (hepatoblastoma) can also be detected by abdominal ultrasound but, as not all the liver can be viewed, alpha-fetoprotein (AFP) levels in the blood may also be monitored 3 monthly. Screening is recommended by alpha-fetoprotein (AFP) every six weeks – three months until age 4 years and abdominal ultrasounds every 3 months until age 8 years.
Beckwith Wiedemann syndrome life expectancy
Most children and adults with Beckwith-Wiedemann syndrome do not have serious medical problems associated with the condition. Their life expectancy is usually normal. Children with Beckwith-Wiedemann syndrome are at an increased risk of developing several types of cancerous and noncancerous tumors, particularly a form of kidney cancer called Wilms tumor, a form of liver cancer called hepatoblastoma, adrenal carcinoma, and rhabdomyocsarcoma. Tumors develop in about 10 percent of people with Beckwith-Wiedemann syndrome and almost always appear in childhood. In infancy, Beckwith-Wiedemann syndrome has a mortality rate of approximately 20% 3. Because of the aggressiveness of these tumors, abdominal ultrasound scans should take place every three months up to the age of 7 or 8 years. A baseline MRI scan may also be performed. The susceptibility to these tumors diminishes and is not usually a problem after the age of 8.
Beckwith-Wiedemann syndrome causes
The genetic causes of Beckwith-Wiedemann syndrome are complex. The condition usually results from the abnormal regulation of genes in a particular region of chromosome 11 2. People normally inherit one copy of this chromosome from each parent. For most genes on chromosome 11, both copies of the gene are expressed, or “turned on,” in cells. For some genes, however, only the copy inherited from a person’s father (the paternally inherited copy) is expressed. For other genes, only the copy inherited from a person’s mother (the maternally inherited copy) is expressed. These parent-specific differences in gene expression are caused by a phenomenon called genomic imprinting. Abnormalities involving genes on chromosome 11 that undergo genomic imprinting are responsible for most cases of Beckwith-Wiedemann syndrome.
At least half of all cases result from changes in a process called methylation. Methylation is a chemical reaction that attaches small molecules called methyl groups to certain segments of DNA. In genes that undergo genomic imprinting, methylation is one way that a gene’s parent of origin is marked during the formation of egg and sperm cells. Beckwith-Wiedemann syndrome is often associated with changes in regions of DNA on chromosome 11 called imprinting centers. Imprinting centers control the methylation of several genes that are involved in normal growth, including the CDKN1C, H19, IGF2, and KCNQ1OT1 genes. Abnormal methylation disrupts the regulation of these genes, which leads to overgrowth and the other characteristic features of Beckwith-Wiedemann syndrome.
About twenty percent of cases of Beckwith-Wiedemann syndrome are caused by a genetic change known as paternal uniparental disomy. Paternal uniparental disomy causes people to have two active copies of paternally inherited genes rather than one active copy from the father and one inactive copy from the mother. People with paternal uniparental disomy are also missing genes that are active only on the maternally inherited copy of the chromosome. In Beckwith-Wiedemann syndrome, paternal uniparental disomy usually occurs early in embryonic development and affects only some of the body’s cells. This phenomenon is called mosaicism. Mosaic paternal uniparental disomy leads to an imbalance in active paternal and maternal genes on chromosome 11, which underlies the signs and symptoms of the disorder.
Less commonly, mutations in the CDKN1C gene cause Beckwith-Wiedemann syndrome. This gene provides instructions for making a protein that helps control growth before birth. Mutations in the CDKN1C gene prevent this protein from restraining growth, which leads to the abnormalities characteristic of Beckwith-Wiedemann syndrome.
About 1 percent of all people with Beckwith-Wiedemann syndrome have a chromosomal abnormality such as a rearrangement (translocation), abnormal copying (duplication), or loss (deletion) of genetic material from chromosome 11. Like the other genetic changes responsible for Beckwith-Wiedemann syndrome, these abnormalities disrupt the normal regulation of certain genes on this chromosome.
Beckwith-Wiedemann syndrome inheritance pattern
In about 85 percent of cases of Beckwith-Wiedemann syndrome, only one person in a family has been diagnosed with the condition. However, parents of one child with Beckwith-Wiedemann syndrome may be at risk of having other children with the disorder. This risk depends on the genetic cause of the condition.
Another 10 to 15 percent of people with Beckwith-Wiedemann syndrome are part of families with more than one affected family member. In most of these families, the condition appears to have an autosomal dominant pattern of inheritance. Autosomal dominant inheritance means that one copy of an altered gene in each cell is typically sufficient to cause the disorder. In most of these cases, individuals with Beckwith-Wiedemann syndrome inherit the genetic change from their mothers. Occasionally, a person who inherits the altered gene will not have any of the characteristic signs and symptoms of the condition.
Rarely, Beckwith-Wiedemann syndrome results from changes in the structure of chromosome 11. Some of these chromosomal abnormalities are inherited from a parent, while others occur as random events during the formation of reproductive cells (eggs and sperm) or in the earliest stages of development before birth.
Adults with Beckwith-Wiedemann syndrome must ensure they have had genetic testing to confirm their molecular subtype of the condition before they have children. This will allow them to receive the correct advice on the risk of the condition occurring in their children. The risk is low for most cases but is higher in some (see above). If you have not been tested or have not received recent advice, your should ask your family doctor to refer you to your local Clinical Genetics Service.
Genetic counseling may help you understand the risks of passing Beckwith-Wiedemann syndrome on to any children you have.
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Beckwith-Wiedemann syndrome diagnosis
Beckwith-Wiedemann syndrome may be diagnosed or confirmed shortly after birth based on a thorough clinical evaluation, detection of characteristic physical findings (e.g., increased weight and length, macroglossia, abdominal wall defects and careful methylation testing and chromosomal (cytogenetic) analysis of the Beckwith-Wiedemann syndrome region (i.e., chromosome 11p15)). Note that in around 20% of Beckwith-Wiedemann syndrome children there is no detectable molecular cause i.e. the blood test will not confirm Beckwith-Wiedemann syndrome.
In some cases, certain procedures may be performed before birth (prenatally). For example, ultrasound imaging may allow assessment of organ size and overall size of the developing fetus and potentially reveal other findings that may be suggestive of Beckwith-Wiedemann syndrome, such as increased amniotic fluid surrounding the fetus (hydramnios), enlarged placenta, omphalocele, enlarged abdominal circumference, and/or other abnormalities. If Beckwith-Wiedemann syndrome is suspected, prenatal testing is available.
Beckwith Wiedemann syndrome treatment
The treatment of Beckwith-Wiedemann syndrome is directed toward the specific symptoms that are apparent in each individual. Treatment may require the coordinated efforts of a team of specialists. Geneticists, pediatricians, plastic surgeons, kidney specialists, dental specialists, speech pathologists, pediatric oncologists, and other healthcare professionals may need to systematically and comprehensively plan an affected child’s treatment.
In newborns with Beckwith-Wiedemann syndrome, regular monitoring of blood glucose levels should be performed to ensure prompt detection and treatment of hypoglycemia. Although neonatal hypoglycemia is usually mild and temporary, its prompt detection and treatment is essential in preventing associated neurologic complications. Treatment measures may include the administration of intravenous glucose, frequent feedings, certain medications (e.g., diazoxide or octreotide), and/or surgical intervention in some cases.
In many infants with umbilical hernia, the defect may spontaneously disappear by the age of approximately one year. Surgery usually is not required unless an umbilical hernia becomes progressively larger, does not spontaneously resolve (e.g., by about three or four years of age), and/or is associated with certain complications. However, in newborns with omphalocele, surgical repair of the defect is typically required shortly after birth.
Children with macroglossia should undergo feeding evaluation and sleep studies in addition to consultations with plastic surgeons and pulmonologists if needed. Feeding difficulties caused by an abnormally large tongue (macroglossia) may be treated by the use of specialized nipples or the temporary insertion of a nasogastric tube. Some affected children may undergo tongue reduction surgery. Such surgery is performed if macroglossia causes dentoskeletal defects, psychosocial problems, upper airway obstruction, or difficulties swallowing, feeding or speaking. Macroglossia may also correct itself without medical intervention.
Orthopedic evaluation is recommended for patients with hemihyperplasia or hemihypertrophy.
In addition, infants and children with Beckwith-Wiedemann syndrome should undergo regular abdominal and kidney (renal) ultrasounds, and serum alpha-fetoprotein (AFP) levels as recommended to enable early detection and treatment of certain malignancies that may occur in association with Beckwith-Wiedemann syndrome (e.g., Wilms tumor, hepatoblastoma). Alpha-fetoprotein (AFP) is a protein produced by the liver. Alpha-fetoprotein (AFP) levels typically decline during infancy; however, alpha-fetoprotein (AFP) may be abnormally elevated in blood serum if certain malignancies are present. The trend in alpha-fetoprotein (AFP) levels over time should be followed in children with Beckwith-Wiedemann syndrome. Screening is recommended by AFPs every six weeks – three months until age 4 years and abdominal ultrasounds every 3 months until age 8 years.
If malignancies develop in association with Beckwith-Wiedemann syndrome (e.g., Wilms tumor, hepatoblastoma), the appropriate treatment measures vary depending upon the specific malignancy present, grade and/or extent of disease, and/or other factors. Treatment methods may include surgery, use of certain anticancer drugs (chemotherapy), radiation therapy, and/or other measures.
Children with cardiac, gastrointestinal, and renal abnormalities may require certain medications, surgery, or other medical interventions. These children should be referred to appropriate specialists. Genetic counseling may be of benefit for affected individuals and their families. Other treatment is symptomatic and supportive.
References