What is strongyloides
Strongyloides is a nematode (roundworm) that can enter your body through exposed skin, such as bare feet to cause strongyloidiasis (Strongyloides infection). Though there are over 40 species within this genus that can infect birds, reptiles, amphibians, livestock and other primates, Strongyloides stercoralis is the primary species that accounts for human disease. The larvae are small; the longest reach about 1.5mm in length — the size of a mustard seed or a large grain of sand. Strongyloides stercoralis is endemic in humid, tropical or subtropical regions 1 including Africa, Southeast Asia, and Latin America 2. Strongyloides stercoralis is also endemic in southeastern United States and southern Europe, although most cases in the US occur in immigrants and military veterans who have lived in endemic regions 3 or more frequently found in rural areas, institutional settings, and lower socioeconomic groups. In the United States, a series of small studies in select populations have shown that between 0-6.1% of persons sampled were infected with Strongyloides stercoralis. Studies in immigrant populations have shown a much higher percentage of infected persons ranging from 0-46.1%.
A second species of Strongyloides, Strongyloides fuelleborni which infects chimpanzees and baboons and may produce limited infections in humans but is less common and mainly found in Africa and Papua New Guinea 4. Strongyloides stercoralis is unique in its ability to replicate in the human host permitting ongoing cycles of autoinfection. Strongyloidiasis can consequently persist for decades without further exposure to exogenous infection 4. The estimated prevalence of strongyloidiasis is between 50 to 100 million infections worldwide; however, the accuracy of these estimates is uncertain due to the poor sensitivity of screening methods 5.
The most common way of becoming infected with Strongyloides is by contacting soil that is contaminated with Strongyloides larvae. Therefore, activities that increase contact with the soil increase the risk of becoming infected, such as:
- walking with bare feet
- contact with human waste or sewage
- occupations that increase contact with contaminated soil such as farming and coal mining.
Furthermore, many studies have shown an association with Strongyloides and infection with Human T-Cell Lymphotropic Virus-1 (HTLV-1). These studies have shown that people infected with HTLV-1 are more likely to become infected with Strongyloides, and that once infected, are more likely to develop severe cases of strongyloidiasis. Of note, being infected with HIV/AIDS has not been shown to be a risk factor for developing Strongyloides or having a worse clinical course.
Chronic Strongyloides stercoralis infections can be asymptomatic or cause cutaneous, gastrointestinal and/or pulmonary symptoms 4. In patients with concurrent Human T-cell-lymphocytic virus 1 (HTLV-1) infection or those on corticosteroid therapy, autoinfection can go unchecked and large numbers of invasive Strongyloides larvae may disseminate widely and cause hyperinfection, which can be fatal 6. Other recognized predisposing conditions or risk factors for Strongyloides stercoralis infection include living in an endemic region, chronic malnutrition, malignancies, organ transplantation, diabetes mellitus, chronic obstructive pulmonary disease (COPD), alcoholism, chronic renal failure and breast milk from an infected mother 7.
Strongyloides stercoralis infections are frequently asymptomatic. Gastrointestinal symptoms include abdominal pain, bloating, heartburn, nausea, loss of appetite and diarrhea. Pulmonary symptoms such as dry cough and throat irritation (including Loeffler’s syndrome) can occur during pulmonary migration of the filariform larvae. Dermatologic manifestations include urticarial rashes (recurrent raised red rash) in the buttocks and waist areas, where the Strongyloides worm entered the skin. Disseminated strongyloidiasis occurs in immunosuppressed patients, can present with abdominal pain, distension, shock, pulmonary and neurologic complications and septicemia, and is potentially fatal. Blood eosinophilia is generally present during the acute and chronic stages, but may be absent with dissemination.
Strongyloides is classically diagnosed by visualization of larvae on microscopic stool examination. This may require that you provide multiple stool samples to your doctor or the laboratory. Some laboratories are capable of diagnosing Strongyloides with blood tests.
Treatment for strongyloidiasis is recommended for all persons found to be infected, whether symptomatic or not, due to the risk of developing hyperinfection syndrome and/or disseminated strongyloidiasis. Furthermore, it is recommended that patients be considered for testing prior to being initiated on any immunosuppressive therapy, particularly corticosteroids.
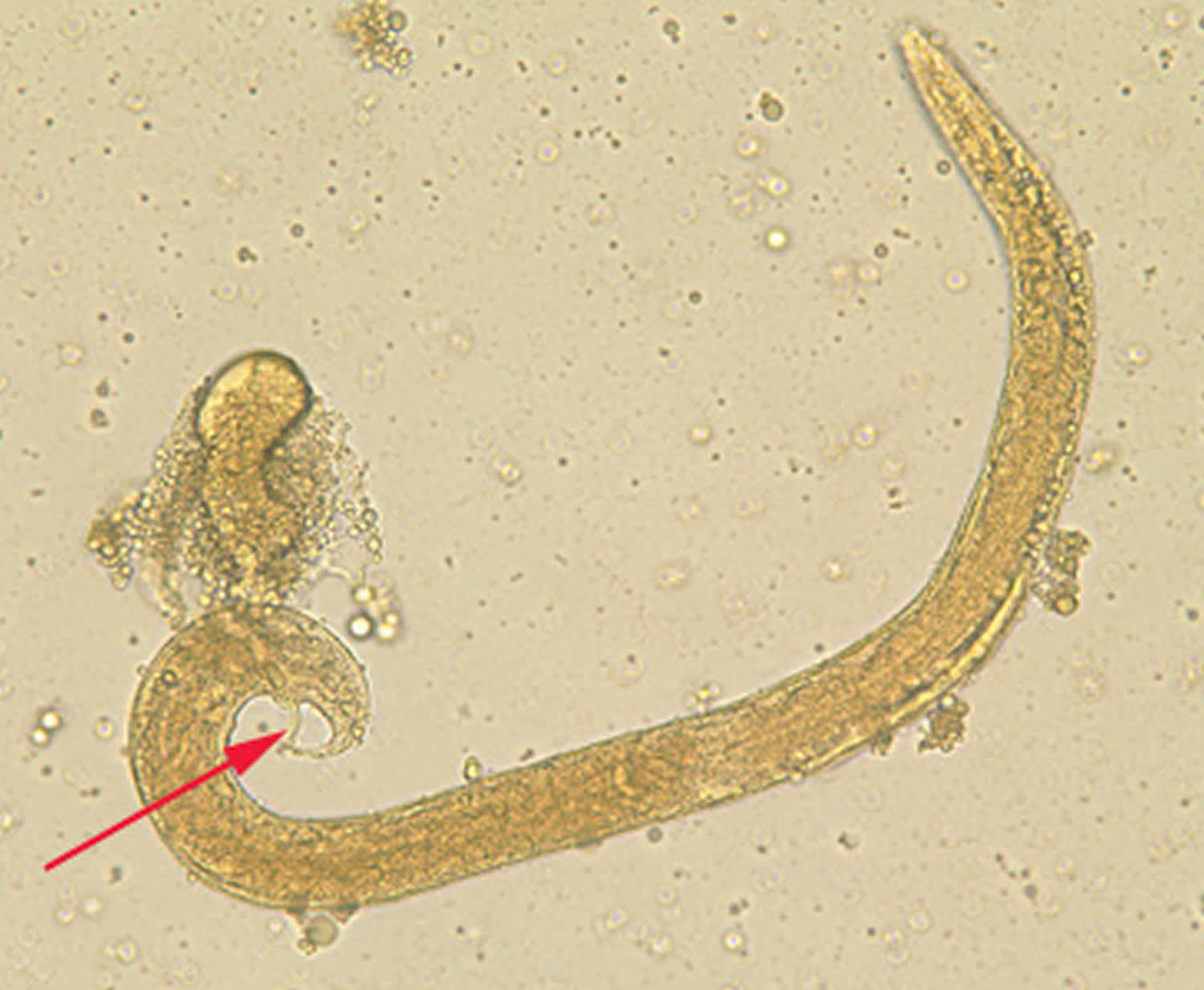
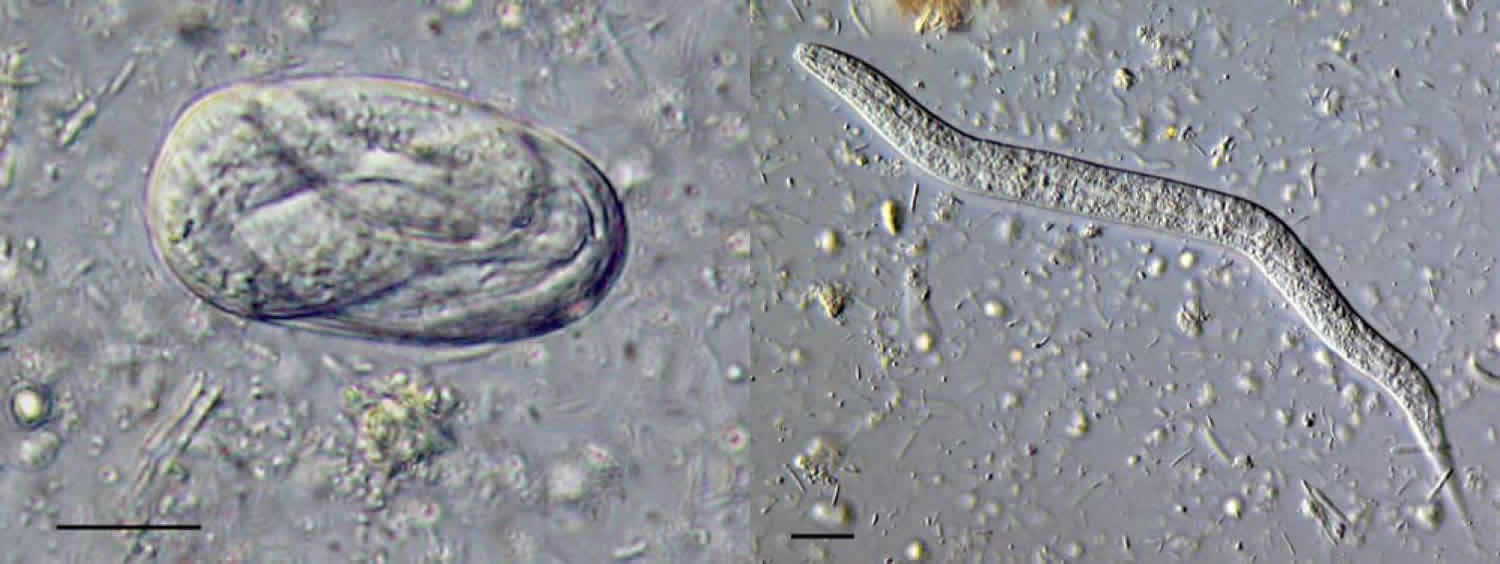
Figure 1. Strongyloides stercoralis egg and larva
How do people get infected with strongyloides?
Strongyloides is classified as a soil-transmitted helminth (worm). This means that the primary mode of infection is through contact with soil that is contaminated with free-living Strongyloides larvae. When the Strongyloides larvae come in contact with skin, they are able to penetrate it and migrate through the body, eventually finding their way to the small intestine where they burrow and lay their eggs. Unlike other soil-transmitted helminths such as hookworm and whipworm whose eggs do not hatch until they are in the environment, the eggs of Strongyloides hatch into larvae in the intestine. Most of these larvae will be excreted in the stool, but some of the larvae may molt and immediately re-infect the host either by burrowing into the intestinal wall, or by penetrating the perianal skin. This characteristic of Strongyloides is termed auto-infection. The significance of auto-infection is that unless treated for Strongyloides, persons may remain infected throughout their lifetime.
In addition to contact with soil and auto-infection, there have been rare cases of person-to-person transmission in:
- organ transplantation
- institutions for the developmentally disabled
- long-term care facilities
- daycare centers.
How can strongyloidiasis be prevented?
The best way to prevent Strongyloides infection is to wear shoes when you are walking on soil, and to avoid contact with fecal matter or sewage. Proper sewage disposal and fecal management are keys to prevention.
Furthermore, if you believe that you may be infected, the best way to prevent severe disease is to be tested and, if found to be positive for disease, treated.
You should discuss testing for Strongyloides infection with your doctor if you are:
- taking steroids or other immunosuppressive therapies
- about to start taking steroids or other immunosuppressive therapies
- a veteran who served in the South Pacific or southeast Asia
- infected with Human T-cell Lymphotropic Virus-1 (HTLV-1)
- diagnosed with cancer
- going to donate or receive organ transplants.
- diagnosed hematologic malignancies including leukemias and lymphomas
- diagnosed with persistent peripheral or unexplained eosinophilia
- with recent or remote travel histories to endemic areas.
Of note, though persons with HIV/AIDS can have disseminated strongyloidiasis or hyperinfection syndrome, observational studies have not shown an increased risk in this population.
Where do most cases of strongyloidiasis occur in the United States?
In the United States, Strongyloides has classically been associated with uniformed-service veterans who returned from tropical regions such as Southeast Asia and the South Pacific during World War II. Small domestic studies have shown locations of infection in rural Appalachia. The highest rates in the United States have been documented in immigrant populations.
Strongyloides is more commonly found in areas that are relatively warm and moist, in rural areas, and areas associated with agricultural activity, but it can occur anywhere. It is found more frequently in socio-economically disadvantaged persons and in institutionalized populations.
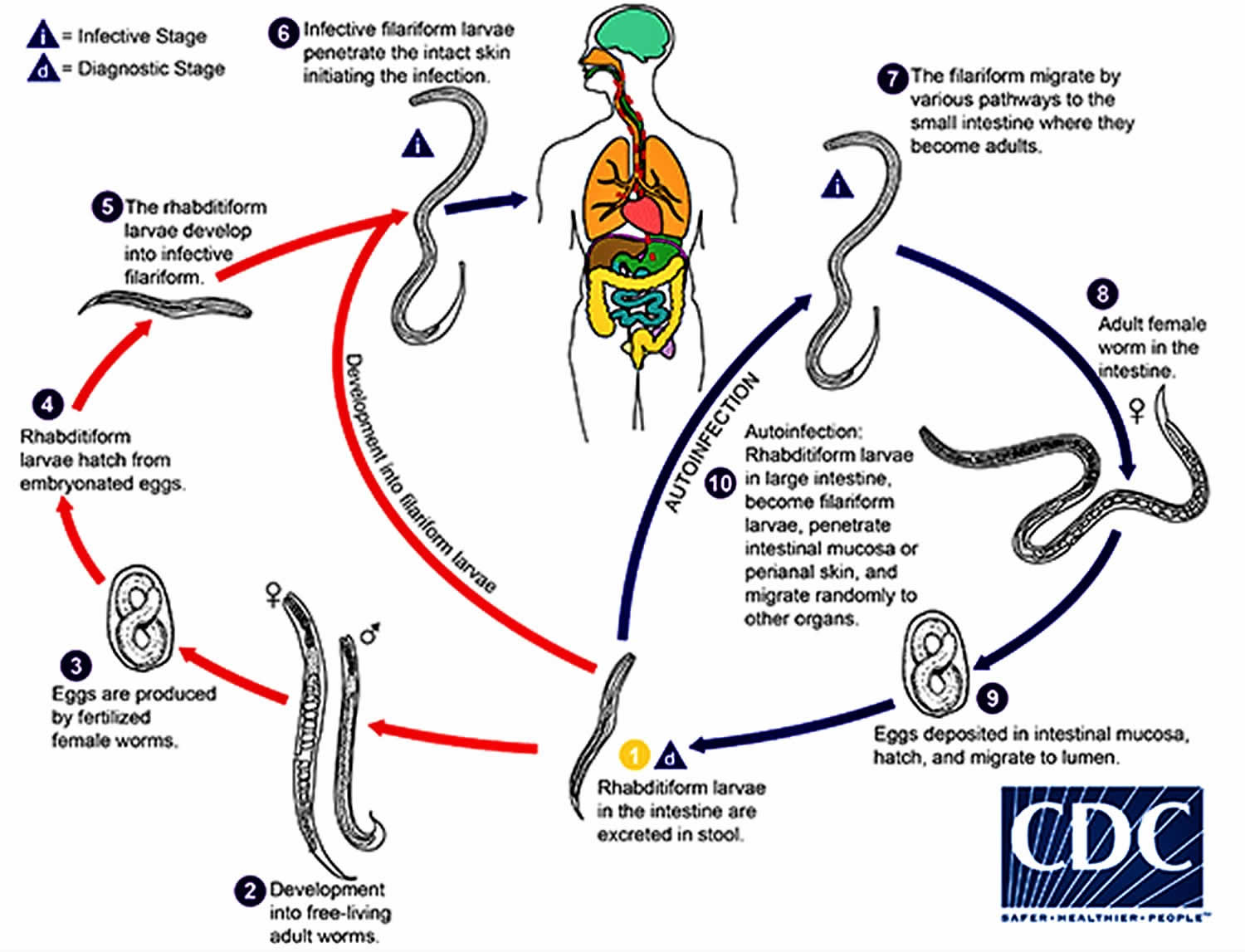
Strongyloides life cycle
The Strongyloides life cycle is more complex than that of most nematodes (roundworm) with its alternation between free-living and parasitic cycles, and its potential for autoinfection and multiplication within the host. Two types of cycles exist:
- Free-living cycle: The rhabditiform larvae passed in the stool (1) can either become infective filariform larvae (direct development) (6), or free-living adult males and females (2) that mate and produce eggs (3) from which rhabditiform larvae hatch (4) and eventually become infective filariform larvae (5). The filariform larvae penetrate the human host skin to initiate the parasitic cycle (see below) (6).
- Parasitic cycle: Filariform larvae in contaminated soil penetrate the human skin (6), and by various, often random routes, migrate to the small intestine (7). Historically it was believed that the L3 larvae migrate via the bloodstream to the lungs, where they are eventually coughed up and swallowed. However, there is also evidence that L3 larvae can migrate directly to the intestine via connective tissues. In the small intestine they molt twice and become adult female worms (8). The females live threaded in the epithelium of the small intestine and by parthenogenesis produce eggs (9), which yield rhabditiform larvae. The rhabditiform larvae can either be passed in the stool (1) (see “Free-living cycle” above), or can cause autoinfection (10). In autoinfection, the rhabditiform larvae become infective filariform larvae, which can penetrate either the intestinal mucosa (internal autoinfection) or the skin of the perianal area (external autoinfection); in either case, the filariform larvae may disseminate throughout the body. To date, occurrence of autoinfection in humans with helminthic infections is recognized only in Strongyloides stercoralis and Capillaria philippinensis infections. In the case of Strongyloides, autoinfection may explain the possibility of persistent infections for many years in persons who have not been in an endemic area and of hyperinfections in immunodepressed individuals.
Figure 2. Strongyloides life cycle
 [Source 8 ]
[Source 8 ]Strongyloides symptoms
Most people who are infected with Strongyloides do not know they are infected and have no symptoms. Others may develop a severe form and, if untreated, become critically ill and potentially die.
Those who do develop symptoms tend to have non-specific, or generalized complaints. Some people develop abdominal pain, bloating, heartburn, intermittent episodes of diarrhea and constipation, a dry cough, and rashes. Rarely people will develop arthritis, kidney problems, and heart conditions.
Strongyloidiasis can be severe and life-threatening in persons who:
- are on oral or intravenous steroids — such as those with asthma or chronic obstructive pulmonary disease (COPD) exacerbations, lupus, gout, or in persons using steroids for immunosuppression or symptomatic relief
- are infected with the virus HTLV-1
- have hematologic malignancies such as leukemia or lymphoma
- are transplant recipients.
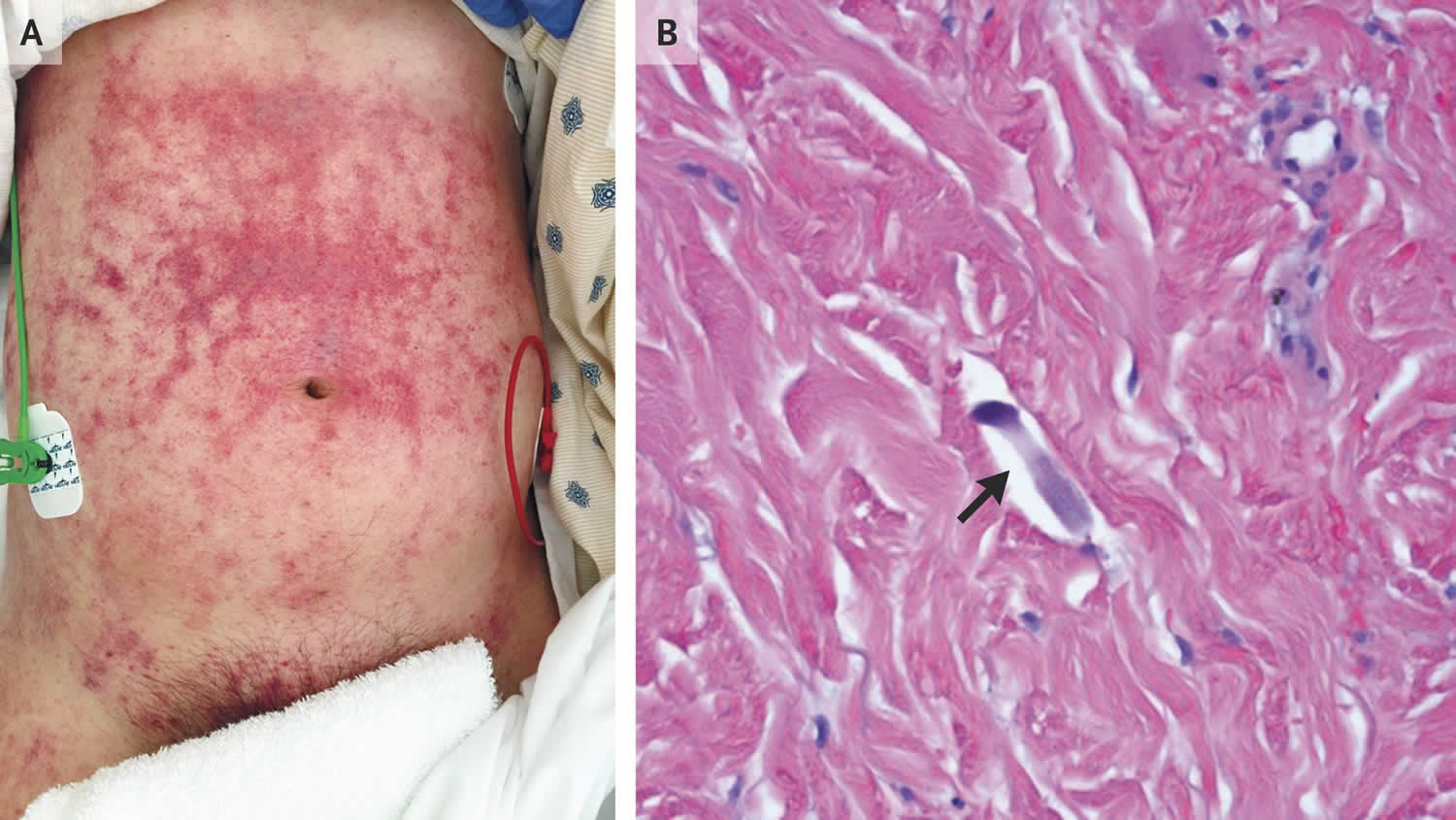
Figure 3. Strongyloides rash
Footnotes: A 47-year-old man who had lived in Laos and was taking prednisone for dermatomyositis presented with a 4-day history of abdominal pain, fever, melena, and hemoptysis and a 2-week history of rash. One month before presentation, he had been hospitalized and treated with high-dose glucocorticoids for diffuse alveolar hemorrhage. At that time, the cause of the hemorrhage was not identified, but the patient’s condition improved with glucocorticoid treatment. At the time of the current presentation, he had hypotension and tachycardia; physical examination revealed reticulated purpuric patches with dusky centers on the abdomen, extending to the flanks, groin, penis, and thighs (Panel A). Progressive respiratory distress developed, and the patient underwent endotracheal intubation. Bronchoscopy revealed diffuse alveolar hemorrhage and Strongyloides stercoralis larvae. Punch biopsy of the rash revealed filariform larvae (Panel B, arrow) and parasites dispersed between collagen bundles in the dermis. Blood cultures grew Enterococcus faecalis and Klebsiella pneumoniae. Although infestation with the intestinal nematode Strongyloides stercoralis may remain quiescent indefinitely, immunosuppression can lead to the hyperinfection syndrome, which is characterized by a large increase in worm burden, with organ invasion. Invasion of the intestinal wall can facilitate the translocation of bacteria. For people who are from or have traveled to an area where the organism is endemic, testing and empirical treatment for Strongyloides stercoralis should be considered before immunosuppression in order to reduce the risk of the hyperinfection syndrome. In addition to receiving antibiotic treatment for polymicrobial bacteremia, the patient was treated with ivermectin and albendazole, and he made a full recovery.
[Source 9 ]The symptomatic spectrum of Strongyloides ranges from subclinical in acute and chronic infection to severe and fatal in hyperinfection syndrome and disseminated strongyloidiasis, which have case-fatality rates that approach 90%. In either case, patients’ symptoms are a result of the parasite’s larval form migrating through various organs of the body.
Acute strongyloidiasis
The initial sign of acute strongyloidiasis, if noticed at all, is a localized pruritic, erythematous rash at the site of skin penetration. Patients may then develop tracheal irritation and a dry cough as the larvae migrate from the lungs up through the trachea. After the larvae are swallowed into the gastrointestinal tract, patients may experience diarrhea, constipation, abdominal pain, and anorexia.
Chronic strongyloidiasis
Chronic strongyloidiasis is generally asymptomatic, but in patients with clinical disease gastrointestinal and cutaneous manifestations are the most common. Of the gastrointestinal complaints, epigastric pain, postprandial fullness, heartburn, and brief episodes of intermittent diarrhea and constipation are the most frequent. Less commonly, patients may present with fecal occult blood, or massive colonic and gastric hemorrhage. Presentations resembling inflammatory bowel disease, specifically ulcerative colitis, are rare. Also rare, but documented, are endoscopic exams revealing pathology similar to pseudopolyposis.
Cutaneous symptoms include chronic urticaria and the pathognomonic larva currens- a recurrent serpiginous maculopapular or urticarial rash along the buttocks, perineum, and thighs due to repeated auto-infection. It has been described as advancing as rapidly as 10cm/hr.
Rarely, patients with chronic strongyloidiasis have complained of arthritis, cardiac arrhythmias, and signs and symptoms consistent with chronic malabsorption, duodenal obstruction, nephrotic syndrome, and recurrent asthma.
Up to 75% of people with chronic strongyloidiasis have mild peripheral eosinophilia or elevated IgE levels.
Hyperinfection syndrome and disseminated strongyloidiasis
Hyperinfection syndrome and disseminated strongyloidiasis are most frequently associated with subclinical infection in patients receiving high-dose corticosteroids for the treatment of asthma or chronic obstructive pulmonary disease (COPD) exacerbations (see Figure 3). Subsequent impaired host immunity leads to accelerated autoinfection and an overwhelming number of migrating larvae. Whereas in chronic strongyloidiasis and in hyperinfection syndrome the larvae are limited to the gastrointestinal tract and the lungs, in disseminated strongyloidiasis the larvae invade numerous organs. Left untreated, the mortality rates of hyperinfection syndrome and disseminated strongyloidiasis can approach 90%.
The following are signs and symptoms that can be seen with hyperinfection syndrome and disseminated strongyloidiasis:
Gastrointestinal manifestations
- abdominal pain, nausea, vomiting, diarrhea
- ileus, bowel edema, intestinal obstruction
- mucosal ulceration, massive hemorrhage, and subsequent peritonitis or bacterial sepsis
Pulmonary manifestations and findings
- cough, wheezing, dyspnea, hoarseness
- pneumonitis
- hemoptysis
- respiratory failure
- diffuse interstitial infiltrates or consolidation on chest radiographs
Neurologic findings
- aseptic or gram-negative meningitis
- larvae have been reported in the CSF, meningeal vessels, dura, epidural, subdural, and subarachnoid spaces
Systemic signs and symptoms
- peripheral edema and ascites secondary to hypoalbuminemia from protein losing enteropathy
- recurrent gram negative bacteremia/sepsis from larvae carrying bacteria that penetrate mucosal walls
- syndrome of inappropriate secretion of anti-diuretic hormone (SIADH)
- peripheral eosinophilia is frequently absent
Cutaneous manifestations
- recurrent maculopapular or urticarial rash most commonly found along the buttocks, perineum, and thighs due to repeated auto-infection, but can be found anywhere on the skin
- larva currens – pathognomonic serpiginous or urticarial rash that advances as rapidly as 10cm/hr.
How soon after the exposure do symptoms develop?
Most people do not know when their exposure occurred. For those who do, a local rash can occur immediately. The cough usually occurs several days later. Abdominal symptoms typically occur approximately 2 weeks later, and larvae can be found in the stool about 3 to 4 weeks later.
Strongyloides diagnosis
The gold standard for the diagnosis of Strongyloides is serial stool examination. However, traditional stool examinations are insensitive and require up to seven stool exams to reach a sensitivity of 100%. Specialized stool exams include Baermann concentration, Horadi-Mori filter paper culture, quantitative acetate concentration technique, and nutrient agar plate cultures. Duodenal aspirate is more sensitive than stool examination, and duodenal biopsy may reveal parasites in the gastric crypts, in the duodenal glands, or eosinophilic infiltration in the lamina propria. Frequently, larvae can be seen by a simple wet-mount in fluid from a bronchoalveolar lavage (BAL).
Many of the serologic tests that are available are quite sensitive, but cross-react with other filarial parasites, schistosomes, and Ascaris lumbricoides, decreasing the specificity of the tests. Furthermore, it can be difficult to distinguish between active cases and historical cases as traditional antibodies can persist for some time. More sensitive and specific serologic tests using recombinant antigens have been, and are being developed, and are available at specific laboratories. An additional advantage to these serologic tests is that there is typically a significant drop in titer by 6 months after parasite eradication, which may make it possible to use these tests as a “test of cure.”
Laboratory diagnosis
Diagnosis rests on the microscopic identification of Strongyloides larvae (rhabditiform and occasionally filariform) in the stool or duodenal fluid. Examination of serial samples may be necessary, and not always sufficient, because stool examination is relatively insensitive.
The stool can be examined in wet mounts:
- directly
- after concentration (formalin-ethyl acetate)
- after recovery of the larvae by the Baermann funnel technique
- after culture by the Harada-Mori filter paper technique
- after culture in agar plates
The duodenal fluid can be examined using techniques such as the Enterotest string or duodenal aspiration. Larvae may be detected in sputum from patients with disseminated strongyloidiasis.
Antibody Detection
Immunodiagnostic tests for strongyloidiasis are indicated when the infection is suspected and the organism cannot be demonstrated by duodenal aspiration, string tests, or by repeated examinations of stool. Antibody detection tests should use antigens derived from Strongyloides stercoralis filariform larvae for the highest sensitivity and specificity. Although indirect fluorescent antibody (IFA) and indirect hemagglutination (IHA) tests have been used, enzyme immunoassay (EIA) is currently recommended because of its greater sensitivity (90%). Immunocompromised persons with disseminated strongyloidiasis usually have detectable IgG antibodies despite their immunodepression. Cross-reactions in patients with filariasis and some other nematode infections may occur. Antibody test results cannot be used to differentiate between past and current infection. A positive test warrants continuing efforts to establish a parasitological diagnosis followed by antihelminthic treatment. Serologic monitoring may be useful in the follow-up of immunocompetent treated patients: antibody levels decrease markedly within 6 months after successful chemotherapy.
Strongyloides treatment
Acute and chronic strongyloidiasis
First line therapy
- Ivermectin, in a single dose, 200 µg/kg orally for 1-2 days
Relative contraindications:
- confirmed or suspected concomitant Loa loa infection
- persons weighing less than 15kg
- pregnant or lactating women
Oral ivermectin is available for human use in the United States.
Alternative
- Albendazole, 400 mg orally two times a day for 7 days.
Relative contraindications:
- hypersensitivity to benzimidazole compounds or any component of product
- use should be avoided in the 1st trimester of pregnancy
Oral albendazole is available for human use in the United States.
In patients with positive stool examination for Strongyloides and persistent symptoms, follow-up stool exams should be performed 2-4 weeks after treatment to confirm clearance of infection. If renewed outbreak of larvae is observed, retreatment is indicated.
Ivermectin
- Treatment in Pregnancy
- Ivermectin is pregnancy category C.
- Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
- Data on the use of ivermectin in pregnant women are limited, though the available evidence suggests no difference in congenital abnormalities in the children of women who were accidentally treated during mass prevention campaigns with ivermectin compared with those who were not. The World Health Organization (WHO) excludes pregnant women from mass prevention campaigns that use ivermectin. However, the risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
- Ivermectin is pregnancy category C.
- Treatment during breastfeeding: Ivermectin is excreted in low concentrations in human milk. Ivermectin should be used in breast-feeding women only when the risk to the infant is outweighed by the risk of disease progress in the mother in the absence of treatment.
- Treatment in Pediatric Patients: The safety of ivermectin in children who weigh less than 15kg has not been demonstrated. According to the WHO guidelines for mass prevention campaigns, children who are at least 90 cm tall can be treated safely with ivermectin. The WHO growth standard curves show that this height is reached by 50% of boys by the time they are 28 months old and by 50% of girls by the time they are 30 months old, many children less than 3 years old been safely treated with ivermectin in mass prevention campaigns, albeit at a reduced dose.
Albendazole
- Treatment in Pregnancy
- Albendazole is pregnancy category C.
- Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
- Data on the use of albendazole in pregnant women are limited, though the available evidence suggests no difference in congenital abnormalities in the children of women who were accidentally treated with albendazole during mass prevention campaigns compared with those who were not. In mass prevention campaigns for which the World Health Organization (WHO) has determined that the benefit of treatment outweighs the risk, WHO allows use of albendazole in the 2nd and 3rd trimesters of pregnancy. However, the risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
- Albendazole is pregnancy category C.
- Treatment during breastfeeding: It is not known whether albendazole is excreted in human milk. Albendazole should be used with caution in breastfeeding women.
- Treatment in Pediatric Patients: The safety of albendazole in children less than 6 years old is not certain. Studies of the use of albendazole in children as young as one year old suggest that its use is safe. According to WHO (World Health Organization) guidelines for mass prevention campaigns, albendazole can be used in children as young as 1 year old. Many children less than 6 years old have been treated in these campaigns with albendazole, albeit at a reduced dose.
Hyperinfection syndrome/Disseminated strongyloidiasis
If possible, immunosuppressive therapy should be stopped or reduced, and:
- Ivermectin, 200 µg/kg per day orally until stool and/or sputum exams are negative for 2 weeks.
For patients unable to tolerate oral therapy, such as those with ileus, obstruction, or known or suspected malabsorption, published case reports have demonstrated efficacy with rectal administration.
If oral and/or rectal administrations are not possible, there have been instances where Investigational New Drug (IND) exemptions for the veterinary subcutaneous formulation of ivermectin have been granted by the FDA.
References- Abrescia F, Falda A, Caramaschi G, Scalzini A, Gobbi F, Angheben A, et al. Reemergence of strongyloidiasis, northern Italy. Emerg Infect Dis. 2009 Sep;15(9):1531–3.
- Agrawal V, Agarwal T, Ghoshal U. Intestinal strongyloidiasis: a diagnosis frequently missed in the tropics. Trans R Soc Trop Med Hyg. 2009 Mar;103(3):242–6.
- Weller PF, Nutman TB. Harrison’s Principles of Internal Medicine: The McGraw-Hill Companies. 2010.
- Siddiqui AA, Berk, Steven L. Diagnosis of Strongyloides stercoralis Infection. Clinical Infectious Diseases. 2001;33(7):1040–7.
- Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis–the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009 Oct;103(10):967–72.
- Mirdha B. Human strongyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009 Jan-Mar;30(1):1–4. 2009
- Iriemenam N, Sanyaolu A, Oyibo W, Fagbenro-Beyioku A. Strongyloides stercoralis and the immune response. Parasitol Int. 2010 Mar;59(1):9–14.
- Strongyloidiasis. https://www.cdc.gov/dpdx/strongyloidiasis/index.html
- Strongyloides stercoralis Hyperinfection. N Engl J Med 2017; 376:2376. https://www.nejm.org/doi/full/10.1056/NEJMicm1612018