Temporal lobe epilepsy
Temporal lobe epilepsy is the most common form of focal epilepsy or complex partial seizures with temporal lobe origin of electrical abnormality 1. Temporal lobe epilepsy in infants and children differs from the relatively homogeneous syndrome seen in adults in several important clinical and pathological ways 2. Seizure semiology varies by age, and the ictal EEG pattern may be less clear cut than what is seen in adults. Additionally, the occurrence of intractable seizures in the developing brain may impact neurocognitive function remote from the temporal area. While many children will respond favorably to medical therapy, those with focal imaging abnormalities including cortical dysplasia, hippocampal sclerosis, or low-grade tumors are likely to be intractable. Expedient workup and surgical intervention in these medically intractable cases are needed to maximize long-term developmental outcome.
Seizures in temporal lobe epilepsy start or involve in one or both temporal lobes in the brain. Temporal lobe seizures are often associated with auras of nausea, emotions (such as déjà vu or fear), or unusual smell or taste. The seizure itself is a brief period of impaired consciousness which may appear as a staring spell, dream-like state, or repeated automatisms. Temporal lobe epilepsy often begins in childhood or teenage years. Research has shown that repeated temporal lobe seizures are often associated with shrinkage and scarring (sclerosis) of the hippocampus. The hippocampus is important for memory and learning. It is not clear whether localized asymptomatic seizure activity over years causes the hippocampal sclerosis.
In addition to seizures, temporal lobe epilepsy also presents with several varied forms of notorious clinical features. A more concerning aspect of temporal lobe epilepsy is its cognitive complications 1. Recently, several studies have shown that recurrent seizures affect all aspects of cognitive functioning including attention, language, praxis, executive function (intelligence), judgment, insight, and problem solving 3.
There are two types of temporal lobe epilepsy:
- Mesial temporal lobe epilepsy involves the medial or innermost structures of the temporal lobe. Seizures often begin in a structure of the brain called the hippocampus, parahippocampal gyrus, and amygdala 4. Mesial temporal lobe epilepsy accounts for almost 80% of all temporal lobe seizures and is usually secondary to a pathological process known as hippocampal sclerosis.
- Neocortical temporal lobe seizures or lateral temporal lobe epilepsy involves the outer part of the temporal lobe. These are very rare and most commonly secondary to genetic or acquired structural/anatomical lesions 4.
Medial temporal lobe epilepsy usually begins around age 10 or 20, but it can start at any age. Usually a person has had a seizure with fever or an injury to the brain in their early years.
There are a lot of older names for seizures that occur in temporal lobe epilepsy, including “psychomotor seizures,” “limbic seizures,” “temporal lobe seizures,” “complex partial,” and “simple partial.” The modern name for these seizures is “focal onset seizures.” Focal seizures are then described by whether a person stays awake and aware or has impaired awareness during a seizure.
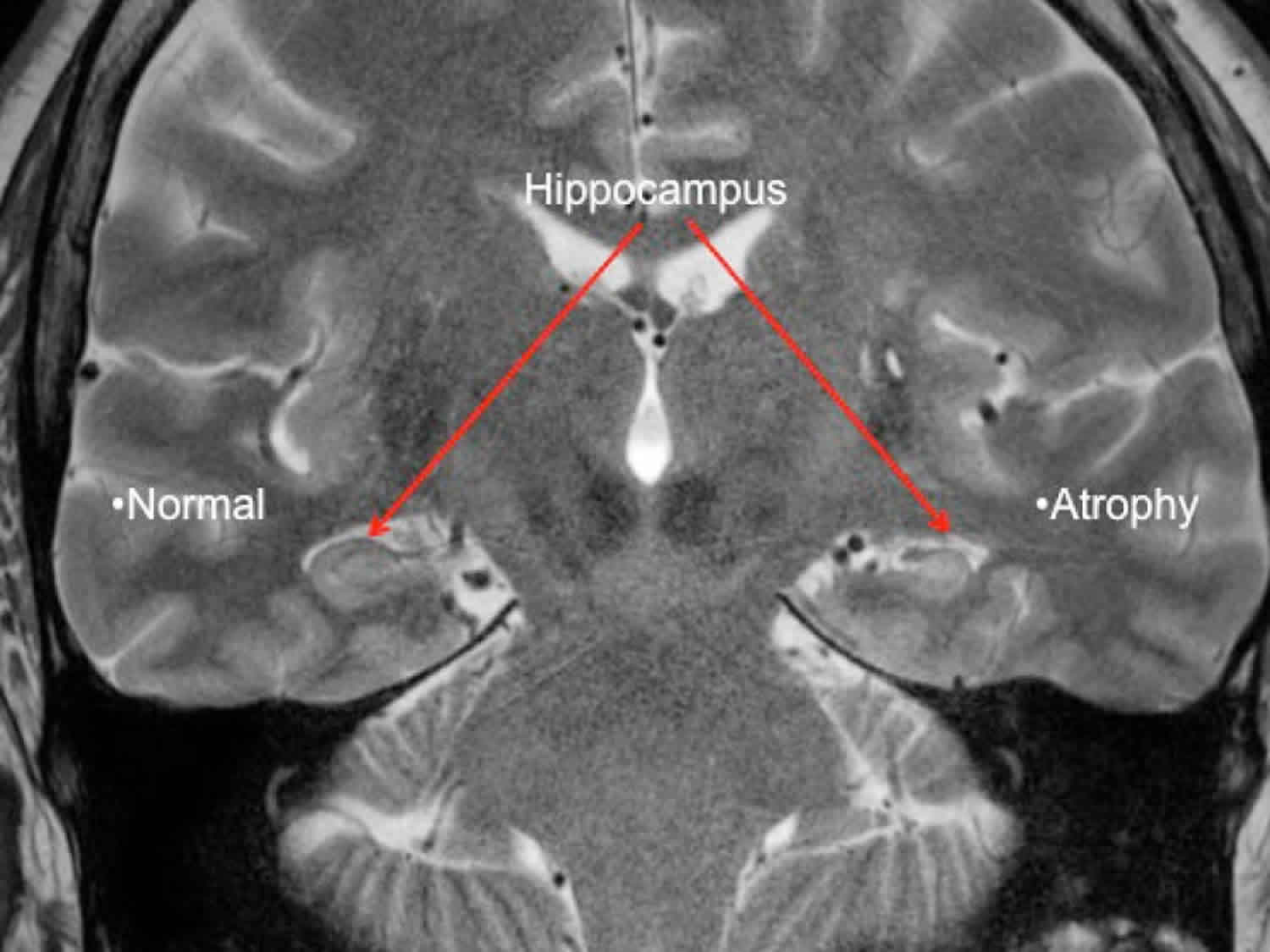
Mesial temporal lobe epilepsy is often associated with changes or abnormal findings on MRI (magnetic resonance imaging). One of the most common findings is scarring in the temporal lobe. This is called hippocampal sclerosis (sclerosis means hardening or scarring). It may look like the hippocampus on one side, or both, has shrunk or is smaller.
When the MRI is abnormal, seizures often do not stop with medication. In this case, surgery to remove the area causing the seizures is the best option for many people. This is especially true when hippocampal sclerosis is on the side of the brain that is not involved with language. This is called the non-dominant side of the brain, which for most people is the right side. Neuropsychological testing is important for any person considering epilepsy surgery. Testing helps guide doctors, people with epilepsy, and families about possible cognitive risks (attention, memory, and learning) compared to benefits of seizure control.
About 6 out of 10 people with focal epilepsy have temporal lobe epilepsy. The overall incidence of new-onset epilepsy in children ranges from 33 to 82 per 100,000 children per year, and approximately half- to two-thirds of these children have focal-onset seizures 5. However, the exact incidence of temporal lobe epilepsy is not known, as the specific lobe of onset is not specified in most incidence studies. Compared to adults, focal seizures in children are more likely to arise from extratemporal foci. Simon Harvey et al. 6 identified 63 children with new-onset temporal lobe epilepsy over a 4-year period in the state of Victoria, Australia (population 4.4 million). In a 30-year cohort of new-onset epilepsy in children 2, 276/468 (59%) had nonidiopathic focal epilepsy. Of these, 20 (7.2%) had a focal lesion on MRI in the temporal region (10: mesial temporal sclerosis, 1: malformation of cortical development, 2: ischemia/gliosis, 1: tumor, and 4: vascular malformation), while 17 (6.1%) had normal imaging and a single focus of epileptiform discharge in the temporal region. Therefore, it was determined that temporal lobe epilepsy was responsible for 8% of all pediatric epilepsy, and for 13% of all focal seizures in our cohort 5.
Temporal lobe anatomy
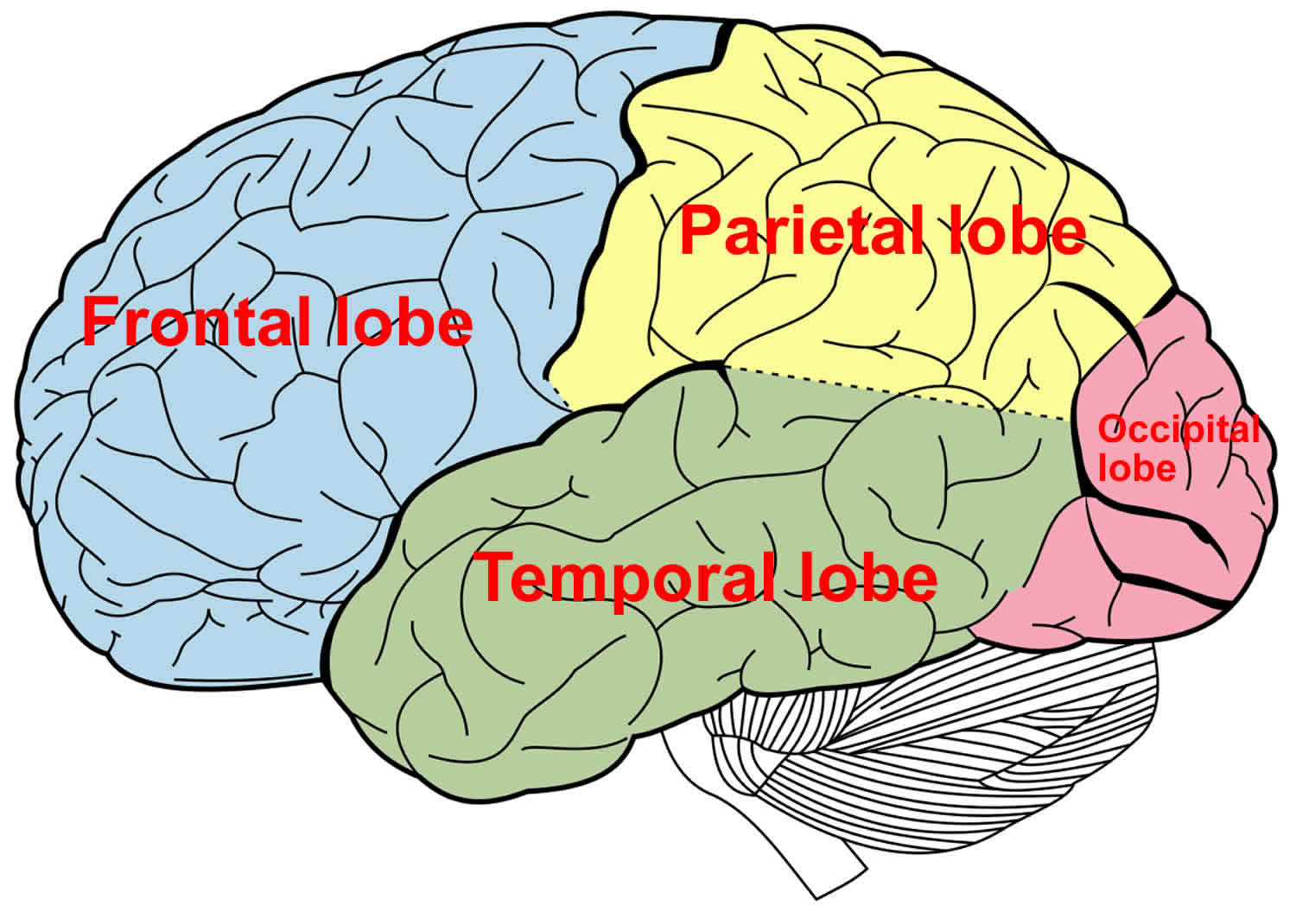
The temporal lobe of the brain is often referred to as the neocortex. The temporal lobe forms the cerebral cortex in conjunction with the occipital lobe, the parietal lobe, and the frontal lobe. Approximately 17% of the volume of the human cerebral cortex, 16% in the right and 17% in the left hemisphere, forms the surfaces of the temporal lobes 7. The temporal lobe subdivides further into the superior temporal lobe, the middle temporal lobe, and the inferior temporal lobe. The temporal lobe contains the auditory cortex and the olfactory cortex. The temporal lobe also functions in the recognition of objects, words, and faces; in language comprehension; and in emotional response and memory 8. In addition to temporal lobe cortex, the temporal lobe contains white matter, part of the lateral ventricle, the tail of the caudate nucleus, the stria terminalis, the hippocampal formation, and the amygdala. The medial side of the temporal lobe includes regions concerned with olfaction (the uncus and nearby cortex) and semantic memory (the hippocampal formation). The nearby amygdala generates responses to perceived sensory stimuli that have been partly analyzed elsewhere in the brain. Such responses include largely involuntary ones, mediated by the autonomic and somatic motor systems, and mental functions, especially those called feelings or emotions, that motivate decision and voluntary actions 9.
The temporal lobe, on the lateral side of the hemisphere, lies in the middle cranial fossa deep to the temporal bone. The temporal lobe is separated from the overlying parietal and frontal lobes by the deep lateral sulcus.
The temporal lobe is crucial in many essential activities such as processing of memory, language, and emotion.
The dominant temporal lobe, which is the left side in most people, is involved in understanding language and learning and remembering verbal information. The non-dominant lobe, which is typically the right temporal lobe, is involved in learning and remembering non-verbal information (e.g. visuo-spatial material and music).
The temporal lobe is involved in:
- Hearing (auditory cortex and association area)
- Smell (olfactory cortex)
- Object identification (posterior association area)
- Emotional response, memory (limbic association area)
The primary auditory cortex is located on the superior edge of the temporal lobe, primarily inside the lateral sulcus, functions in conscious awareness of sound. When sound waves excite the sound receptors of the inner ear, impulses are transmitted to the primary auditory cortex, where this information is related to loudness, rhythm, and pitch (high and low notes). The auditory association area lies just posterior and lateral to the primary auditory area. This area permits the evaluation of a sound as, say, a screech, thunder, or music.
The primary olfactory cortex lies on the medial aspect of the temporal lobe in a small region called the piriform cortex (“pearshaped”), which is dominated by the hooklike uncus. The olfactory nerves from the nasal cavity transmit impulses that ultimately are relayed to the olfactory cortex, resulting in conscious awareness of smells. The olfactory cortex is part of a brain area called the rhinencephalon (“nose brain”), which includes all parts of the cerebrum that directly receive olfactory signals: the piriform cortex, the olfactory tract, the olfactory bulb, and some nearby structures.
The hippocampal formation, located in the temporal lobe, consists of the hippocampus (“sea horse”) and the parahippocampal gyrus. These regions encode, consolidate, and later retrieve memories of facts and events. The hippocampal formation receives information to be remembered from the rest of the cerebral cortex; it processes these data and returns them to the cortex, where they are stored as long-term memories.
The temporal lobe can be damaged by infection, trauma, ischemia, and neoplasia. Lesions in the temporal lobe can stimulate or inhibit the functions mentioned above. The syndrome of Kluver and Bucy 10 provided an extreme example of changed behavior following bilateral temporal lobectomy in monkeys. The animals became unnaturally docile, exhibited excessive and abnormal sexual behavior, lost the ability to be trained, and had a condition that the authors termed “psychic blindness,” in which tactile exploration of objects with the mouth replaced their visual recognition. The equivalent human syndrome is rare and usually associated with pathology extending beyond the temporal lobes 11. Fragments of the classical syndrome, such as visual field defects, visual agnosia, and inability to consolidate new memories, occur more frequently, with destructive lesions in parts of one or both temporal lobes.
The temporal lobe receives oxygenated blood via two primary sources, the internal carotid system and the vertebrobasilar artery. The internal carotid system contains the anterior choroidal artery and the middle cerebral artery. The blood flow from the anterior choroidal artery supplies the uncus, amygdala, and the anterior parahippocampal gyrus. The middle cerebral artery branches into the temporopolar artery, anterior temporal artery, middle temporal artery, and posterior temporal artery. It supplies the temporal pole as well as the superior and inferior portions of the temporal gyri. Blood flow from the vertebrobasilar system supplies the inferior surface of the temporal lobe from the temper-occipital artery.
Blood is drained from the temporal lobe by veins via two major routes. One route involves blood passing from the temporal lobe anteriorly to superficial middle cerebral vein. From there, it moves into the inferior anastomotic vein, known as the vein of Labbe, which goes on to join the transverse sinus. The other route involves blood flowing from the interior temporal lobe into the posterior choroidal vein. This vessel then pairs up with the thalamostriate vein from behind the interventricular foramen to form the internal cerebral vein. The internal cerebral vein then joins the basal veins to create the great cerebral vein.
Figure 1. Temporal lobe
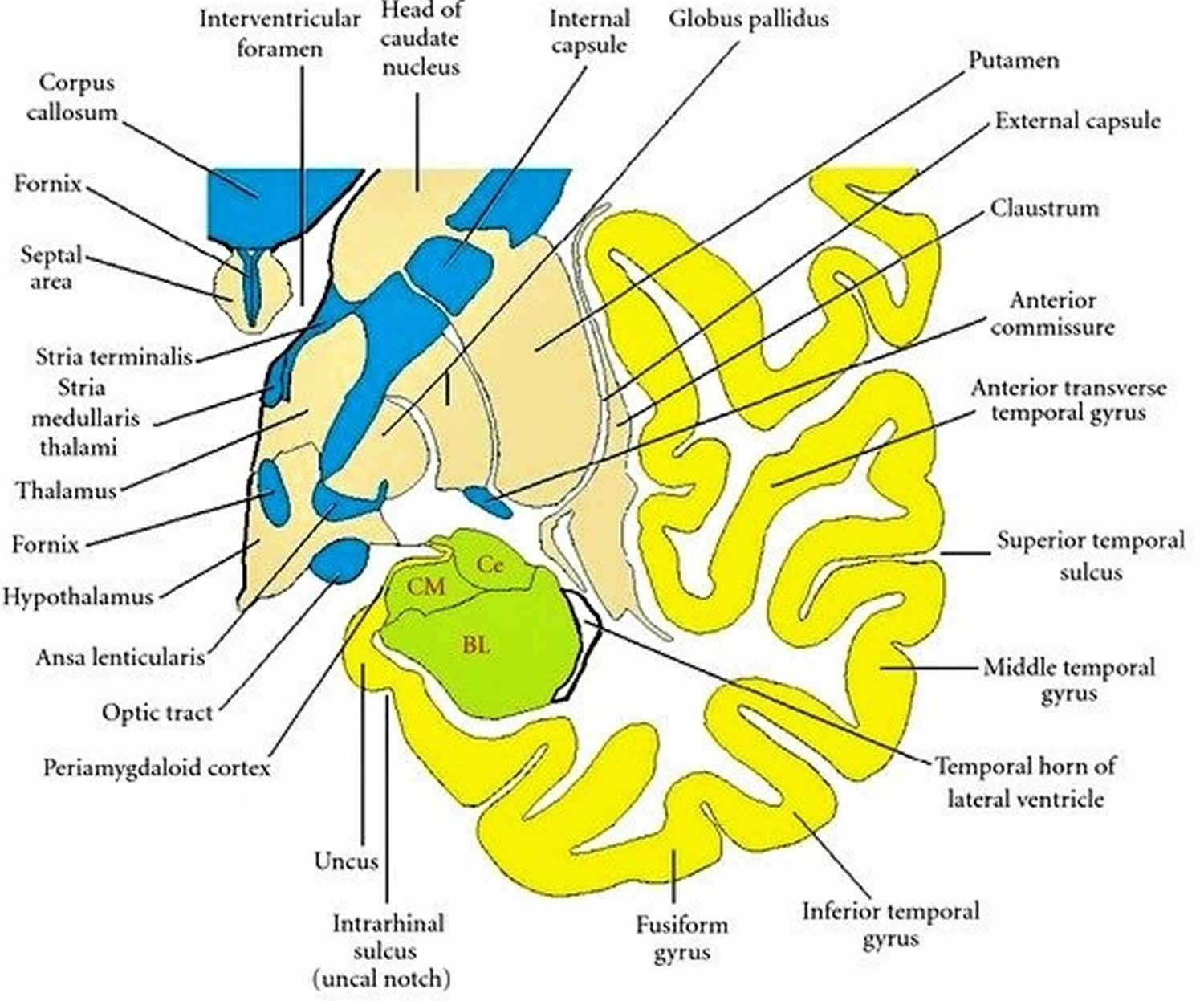
Figure 2. Coronal section through the temporal lobe
Footnote: Drawing of a coronal section through the temporal lobe and adjacent structures, at a level anterior to the hippocampal head. The amygdala is coloured green, with the positions of its three nuclear groups indicated: corticomedial (CM), basolateral (BL), and central (Ce). Selected bodies of white matter are coloured blue.
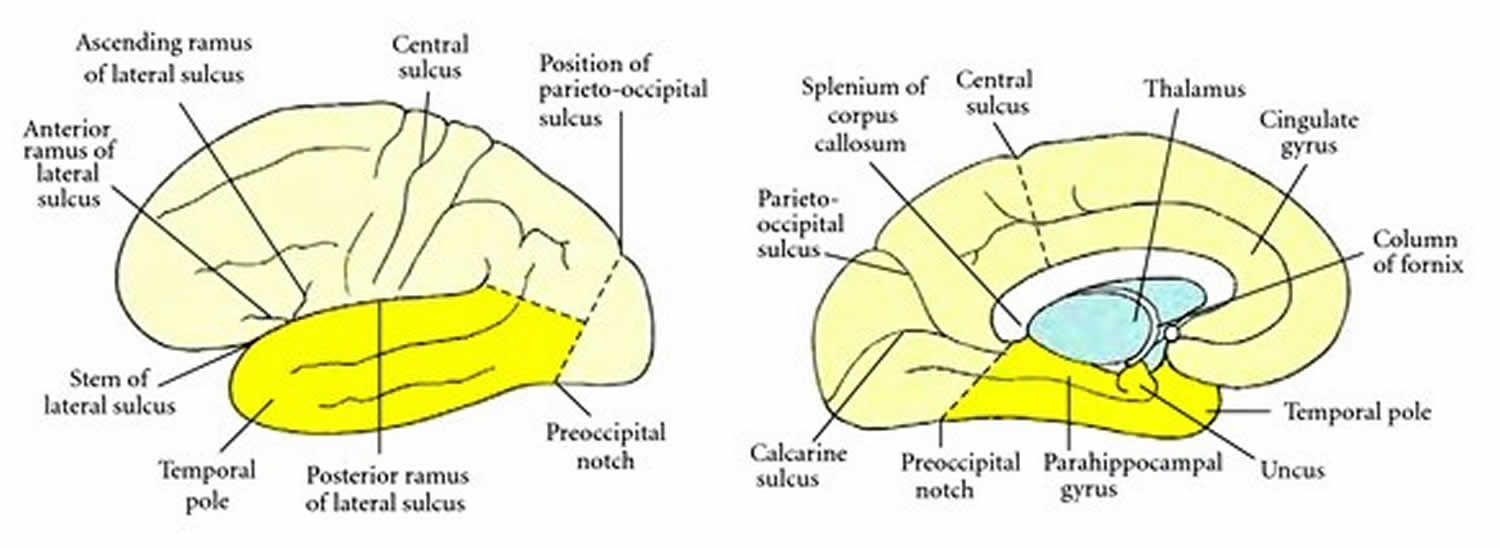
[Source 8 ]Figure 3. Boundaries of the temporal lobe
Footnote: Boundaries of the temporal lobe and positions of major sulci and gyri and other anatomical landmarks of the lateral and medial surfaces of the left cerebral hemisphere.
[Source 8 ]What does the temporal lobe do?
The primary functions of the temporal lobe are to process sensory information and derive it into meaningful memories, language, and emotions 12. The temporal lobe is responsible primarily for declarative memory, which is memory that can be said out loud, and is subdivided into episodic (life events) and semantic (fact-like) memory. Located within the middle temporal lobe are the hippocampus and the amygdala. The hippocampus manages the formation of new memories and the conversion of short-term memories into long-term ones. The hippocampus communicates closely with the amygdala, which is responsible for the processing of emotions.
The temporal lobe also plays an essential role in processing sounds. It houses the primary auditory cortex and the superior temporal gyrus. The primary auditory cortex can process input from the ears into meaningful units like words and sentences. The sounds we hear first enter the brain in an area within the superior temporal gyrus traveling from the cochlea.
Parts of the temporal lobe aid in processing visual stimuli, primarily to allow us to recognize objects. The fusiform gyrus distinguishes faces, and the parahippocampal gyrus identifies locations and landscapes.
A specialized area of the temporal lobe, known as the Wernicke area, is found on the dominant hemisphere. It is responsible for processing written and spoken language.
Mesial temporal lobe epilepsy
Mesial temporal lobe epilepsy is often discussed as a separate entity because it is quite distinct from its lateral counterpart in terms of cause, semiology, imaging, and electrophysiologic characteristics 13. Moreover, the mesial temporal lobes tend to be the site of origin of close to 80% of all temporal lobe epilepsies 14.
Most cases of mesial temporal lobe epilepsy are sporadic in occurrence, although familial forms are not uncommon 15. One study showed that as high as one-fifth of the newly-diagnosed non-lesional mesial temporal lobe epilepsy could have a familial attribute. Research has identified a genetic locus for familial mesial temporal lobe epilepsy in a large family with autosomal dominant mesial temporal lobe epilepsy phenotype 16. The familial mesial temporal lobe epilepsy cases have been shown to exhibit a complex inheritance pattern and usually do not exhibit mesial temporal sclerosis on imaging 17.
Hippocampal sclerosis is the most common histopathological abnormality found in patients with drug-resistant temporal lobe epilepsy. In a European series of 9523 patients with epilepsy undergoing surgery, HS was identified in 36.4%, long-term epilepsy-associated tumors in 23.6%, and focal cortical dysplasias in 19.8% 18. Focal cortical dysplasias classify as malformations of cortical development, which also include polymicrogyria, nodular heterotopia, and hamartomas, which are less common pathologies involved with temporal lobe epilepsy. Other less common etiologies include post-infectious (most commonly after HSV encephalitis), vascular malformations, ischemic lesions, inflammatory lesions, and old traumatic encephalomalacia 19.
There does not seem to be a specific age or sexual predominance to mesial temporal lobe epilepsy. Patients usually have normal perinatal history and have normal development. They generally have a normal neurological examination and are cognitively intact. A childhood history of febrile seizures is an important harbinger for the development of mesial temporal lobe epilepsy 20. A prospective study 21 performed on 226 children with febrile status epilepticus found evidence of acute hippocampal injury in 9.7% of patients. Subsequently, follow up MRI of the brain on 14 of these 22 patients showed hippocampal sclerosis in 10 and hippocampal volume loss in 12. Other less important risk factors include head trauma, birth trauma, childhood central nervous system (CNS) infection, and posterior cerebral artery territory infarcts.
Although the advent of antiepileptic medications has improved the quality of life of patients with epilepsy by reducing seizure frequency, many of the patients with mesial temporal lobe epilepsy have a greater tendency to become pharmacoresistant over time 13. Studies have shown that less than 25% of patients with mesial temporal lobe epilepsy remained seizure-free for greater than one year 22. This data elucidates the importance of non-pharmacological therapies in patients having mesial temporal lobe epilepsy. However, most of these non-pharmacological modalities require accurate identification of the epileptogenic zone to provide successful seizure outcomes.
Mesial temporal lobe epilepsy treatment
The first-line therapy for mesial temporal lobe epilepsy includes the initiation of appropriately chosen antiepileptic drug treatment. For patients with mesial temporal lobe epilepsy, the most effective antiepileptic drugs are those used to treat focal epilepsies such as carbamazepine, oxcarbazepine, levetiracetam, lamotrigine, and topiramate 23. These agents can be monotherapy or, more often, in combination to achieve adequate seizure freedom. However, it is well known that patients with mesial temporal lobe epilepsy often have an inadequate response to antiepileptic drug therapy 22. Some patients who initially respond may also end up becoming medically refractory within a few years. Non-pharmacological approaches eventually play an essential role in the management of patients with medically refractory or drug-resistant mesial temporal lobe epilepsy. These include both surgical and neurostimulation approaches.
Surgical approaches for mesial temporal lobe epilepsy include open resection and other minimally invasive techniques. Standard open resective surgery is considered to be the most effective and safe treatment option for temporal lobe epilepsy with superiority to prolonged medical therapy in terms of long term outcomes 24. Several surgical procedures have been employed, including standard anterior temporal lobectomy, anteromedial temporal lobectomy, selective amygdalohippocampectomy, and temporal pole resection. Resective therapy has demonstrated an excellent outcome, especially if done early 14. Surgical resection offers postoperative seizure freedom at two years in 60% to 80% of patients with drug-resistant mesial temporal lobe epilepsy, whereas longer-term follow-ups present less favorable results 25. Anterior temporal lobectomy is generally safe, and the most common neurologic complication following such resective epilepsy surgery is a minor visual field deficit.
Advances in our understanding of epileptic networks have improved our ability to define the epileptogenic zone in patients with epilepsy better. The aim of disrupting epileptic networks with the smallest possible surgical lesion has led to the development of minimally invasive surgical techniques for epilepsy 26. Minimally invasive techniques include stereotactic radiosurgery, stereotactic radiofrequency thermocoagulation, laser interstitial thermal therapy and MRI-guided focused ultrasound ablation. Stereotactic radiosurgery using gamma knife and Cyberknife deliver ionizing radiation to a focal target of mesial temporal structures in mesial temporal lobe epilepsy and have shown comparable postoperative seizure freedom when compared to invasive surgery 27. Similarly, stereo-EEG (SEEG) guided thermocoagulation and laser interstitial thermal therapy have also shown to be promising new developments and have been employed as alternative options to standard resective surgery 28.
Neurostimulation for the treatment of epilepsy includes vagus nerve stimulation (VNS) responsive neurostimulation (RNS) and deep brain stimulation (DBS) 29. These are generally reserved for patients who are either not candidates for resective surgery or unwilling to undergo surgery. Responsive neurostimulation can be used for patients with bitemporal seizure foci or foci Involving eloquent brain regions. Neurostimulation also could be an option for patients who have seizure recurrence following surgery. In patients with bitemporal epilepsy, long term electrocochleography (ECoG) data from the responsive neurostimulation system can provide information enabling identification if one temporal lobe responsible for the majority of the seizures in certain patients; if so, resective surgery may be a consideration in such patients.
Apart from seizure management, patients with mesial temporal lobe epilepsy may have cognitive problems, psychiatric comorbidities, and psychosocial issues. A comprehensive approach to manage an individual with mesial temporal lobe epilepsy must take into account the cognitive and psychiatric comorbidities that often accompany this condition 30.
Temporal lobe epilepsy causes
The cause of temporal lobe seizures is extensive. The most common causes are 4:
- Hippocampal sclerosis
- Infections
- Tumors
- Traumatic brain injury
- Vascular anomalies
- Genetic
- Cryptogenic
Mesial temporal lobe epilepsy is the most common form of epilepsy and is most commonly due to a neurodegenerative process known as hippocampal sclerosis found in the majority of patients diagnosed with this condition, upon histological evaluation 31. This entity was first identified by Sommer and Bratz in the late 19th century and had the name Ammon’s horn sclerosis 32. Aberrant neurological conduction, primarily due to the overactivity of stimulatory neurotransmitters such as glutamate or under activity of inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA) with a primary focus in the temporal lobe results in epileptogenesis. It has been well established that the presence and degree of hippocampal sclerosis is a predictor of post-surgical outcome, with the presence of hippocampal sclerosis being a better prognostic indicator. Early works by Wyler established a grading scale based on semiquantitative measurements of from no hippocampal sclerosis (Wyler 0) to severe hippocampal sclerosis (Wyler 4). The work by Watson added one more tier to this grading system. However, the system of grading established by Wyler has limitations, as it requires analysis of all parts of the hippocampus to determine a reliable score, which is not always possible due to the resection procedure 33. The International League Against Epilepsy (ILAE) utilized a task force review to classify hippocampal sclerosis via an easily accessible, semi-quantitative, histopathological analysis of hippocampal cell loss. The International League Against Epilepsy determined three types of hippocampal sclerosis of varying severity, which should aid in determining the post-surgical outcome and the likelihood of seizure control. An additional fourth subtype with gliosis only has also been characterized 34.
The three types of hippocampal sclerosis, as classified by the International League Against Epilepsy (ILAE), are as follows:
- Hippocampal sclerosis ILAE type 1 – associated with significant neuronal cell loss and gliosis, mostly in CA1 and CA4 hippocampal regions. It has an association with a history of precipitating injuries in pediatric populations with early seizure onset. It also correlates with a better post-surgical prognosis.
- Hippocampal sclerosis ILAE type 2 – A form with more pronounced cell loss and gliosis in CA1 hippocampal regions. Less studied but may be associated with a less favorable post-surgical prognosis.
- Hippocampal sclerosis ILAE type 3 – This is with more pronounced cell loss and gliosis in CA4 hippocampal regions. As with type 2, less studied and may also be associated with a less favorable outcome.
- No-hippocampal sclerosis – with gliosis only, often associated with a less favorable prognosis.
Although about 10 to 15 percent of children who develop febrile seizures progress to a diagnosis of epilepsy, there is no compelling evidence of causality between a history of febrile seizures and the development of temporal lobe epilepsy. What is evident, however, is the association of lesions in anatomical correlates on neuroimaging, such as mesial temporal lobe sclerosis, in the pediatric population, and the development of temporal lobe epilepsy 35.
Risk factors for developing temporal lobe epilepsy
Common risk factors that lead to developing temporal lobe epilepsy include:
- Brain injury including head trauma with loss of consciousness, birth injury, infections such as encephalitis or meningitis that happen early in life
- Changes in the structure of a temporal lobe, such as brain malformations or tumors
- The most common risk factor is a prolonged or focal febrile seizure.
- About 2 out of 3 people with temporal lobe epilepsy have had a history of febrile seizures.
- Three out of 4 of these were either prolonged or had complex features.
- It is important to know that the vast majority of people with febrile seizures do not develop temporal lobe epilepsy.
Temporal lobe epilepsy pathophysiology
Being the earliest discovered, and most common pathological finding on the autopsy of patients with temporal lobe epilepsy 36, hippocampal sclerosis has been the most examined to date. As the name suggests, the primary pathology in this condition occurs in the hippocampus, with neuronal loss, or atrophy in the hilar regions, predominantly localized to the CA1, CA3, C4, and dentate gyrus, with CA2 sparing. Expansion of the normally dense granule cell layer, mossy fiber sprouting, and gliosis being some common findings on pathological specimens. It should be noted, however, that hippocampal sclerosis has a variety of pathological subtypes 37.
As noted, temporal lobe epilepsies arise through a myriad of etiologies, regardless of the focus of epileptogenic activity. For simplicity, the lateral temporal lobe epilepsies fall into categories as either lesional or non-lesional. Lesional causes are often secondary to anatomical aberrancies. However, the lateral temporal lobe epilepsies have been a poorly studied group to date. Recent studies reveal a significant number of cases clustered in families or secondary to idiopathic genetic mutations 38. There has also been evidence regarding the role of astrocytes in the pathogenesis of epilepsy as well as interconnected epileptic networks 39.
Temporal lobe epilepsy symptoms
Temporal lobe epilepsy characteristically presents with seizure activity originating in either the medial or lateral temporal lobes. The seizures can either be focal aware seizures, focal seizures with impaired awareness, and there can also be seizure activity which originates in the temporal lobe but extends to involve both cerebral hemispheres, commonly manifesting as focal to bilateral tonic-clonic seizures. Chronic memory impairment is a common finding in individuals with temporal lobe epilepsy 40.
Symptoms of temporal lobe epilepsy include the following:
- Focal aware seizures classically referred to as “auras” these phenomena include special sensory symptoms such as occurring in auditory, olfactory, gustatory, and visceral systems. They may also manifest with autonomic, somatosensory, and cognitive events such as “deja vu,” and “jamais vu.” Emotional symptoms, such as fear or anxiety, are also possible.
- Auras are the same as focal aware seizures. They used to be called simple partial seizures. They are the first symptoms of a seizure.
- The most common auras are feelings of déjà-vu or some stomach upset.
- Feelings of fear, panic, anxiety, a rising sensation coming from the stomach to the chest or throat, or butterflies with nausea are other common auras.
- Some people may sense an unusual smell. This symptom may raise the possibility of a lesion or tumor in the hippocampus of the temporal lobe.
- Sometimes the auras can be very hard to describe.
- Focal impaired awareness seizures used to be called complex partial seizures: sometimes, a focal aware seizure may progress to a loss of consciousness. During this progression, the patient may display a motionless stare, dilated pupils, and automatisms such as in the facial-oral musculature or unilateral dystonic limb posturing may present.
- During this type of seizure, a person may have a fixed stare, be unaware or confused about what is going on around them, have fumbling with their fingers, or lip-smacking movements. The seizures last 30 seconds to a couple of minutes.
- There can be unusual posturing (movement or positioning) in an arm. This can help identify where seizures start in the brain.
- Some people also speak gibberish or lose their ability to speak in a sensible manner. Language problems are more common if the seizures are coming from the dominant temporal lobe.
- The focal seizure can go into generalized tonic-clonic jerking. The person may be weak after the seizure has stopped.
- Some people can also have prolonged seizures. Rarely, repeated or long seizures called status epilepticus may develop.
- Seizures in neocortical or lateral temporal lobe epilepsy often start with an auditory aura, such as buzzing or hearing a specific sound.
- Focal seizures may extend to involve both cerebral hemispheres and frequently manifest with bilateral tonic-clonic convulsions.
- Both (2) and (3) above may be associated with a postictal period, which may manifest with confusion, aphasia, and/or amnesia.
Inter-ictal symptoms are non-specific in the majority of cases. Occasionally, patients may manifest with a change in the prosody of speech and flattening of the contralateral nasolabial fold with emotional incitement 41.
A typical patient story: “The whole world suddenly seems more real at first. It’s as though everything becomes crystal clear. Then I feel as if I’m here but not here, kind of like being in a dream. It’s as if I’ve lived through this exact moment many times before. I hear what people say, but they don’t make sense. I know not to talk during the episode, since I just say foolish things. Sometimes I think I’m talking but later people tell me that I didn’t say anything. The whole thing lasts a minute or two.”
Temporal lobe epilepsy complications
The risk of irreversible neurocognitive decline increases with the duration and frequency of epilepsy. Quality of life and other psychosocial domains are affected for these individuals 42. Depression, anxiety, memory impairment, and other neurocognitive disorders are resultant comorbidities in this population 40. Also of concern in the epileptic patient is the adverse side-effects of many antiepileptic drugs, including hepatotoxicity, teratogenicity, and toxic dermatoses, among others, and surgical complications in those undergoing these procedures.
Sudden unexplained death in epilepsy (SUDEP) is sudden death in an epileptic patient occurring in the absence of status epilepticus and with unknown causes 43. It has a yearly incidence of around 0.1 percent annually in the epileptic population and is the leading cause of death individuals who are unable to achieve seizure control 44. Cardio-autonomic or respiratory dysfunction is likely central to the pathogenesis of SUDEP 45.
Temporal lobe epilepsy diagnosis
Medial temporal lobe epilepsy is a clinical diagnosis. This means that a number of factors are put together. There isn’t one test for temporal lobe epilepsy.
- It’s important to listen to a person describe their seizures in as much detail as possible or by hearing observations of a witness.
- An MRI of the brain should be done to look for changes in the temporal lobe.
- An EEG (electroencephalogram) should be done and often shows spike or sharp waves in the tip or front of the temporal lobe. These can be seen when a person is awake or asleep.
- When seizures arise in more mesial (middle) temporal lobe areas, the EEG may only show rhythmic slowing during seizures. These may be hard to diagnose unless a typical seizure is recorded on the EEG.
Neuro-imaging and EEG are valuable in the evaluation of the patient with suspected temporal lobe epilepsy. Since seizures are a relatively transient and rare event for the majority of individuals affected by epilepsy, the ability to perform inter-ictal diagnostic assessments is vital.
EEG should be performed in all individuals with suspected temporal lobe epilepsy, as it can assist in the localization of epileptic focus, and potentially elucidate possible epileptic networks 46.
An ictal EEG recording of a rhythmic 5 to 7 Hz theta-wave frequency, with peak recordings in sphenoidal and basal temporal electrodes on the ipsilateral side to epileptic focus, is diagnostic. Interictal EEG assessment may be remarkable for spike-and-wave or sharp and slow complexes, usually located in the anterior temporal region, or basal temporal electrodes. Differentiating between mesial temporal lobe epilepsy and lateral temporal lobe epilepsy by EEG is difficult as the waveforms are similar.
Sometimes a patient with temporal lobe epilepsy may have a normal initial EEG; tools such as sleep-deprivation and video EEG telemetry may help in ceasing the diagnosis. Also, if there is discordance between the scalp EEG, and clinical or other data, the placement of intracranial electrodes could assist in identifying epileptogenic focus before surgery.
Neuroimaging is vital for the identification of organic or structural anomalies, which may precipitate temporal lobe seizures, such as vascular malformations, tumors, and hippocampal sclerosis. Computed tomogram (CT) scan is routinely used in the ambulatory evaluation but provides limited sensitivity when compared to higher-resolution imaging modalities, such as magnetic resonance imaging (MRI), which is the primary method of choice. MRI is also vital in the pre-surgical assessment of individuals with refractory temporal lobe epilepsy; with mesial temporal lobe epilepsy with hippocampal sclerosis being the most common finding in these cases, key findings on MRI include a reduction in hippocampal volume and an increased signal intensity on T2 imaging in the hippocampus-FLAIR may be used to enhance imaging. T1 imaging may visualize grey-white matter contrast and provide enhanced neuroanatomical detail of the hippocampus. The majority of cases of hippocampal sclerosis are bilateral in nature, which may lead to difficulty with diagnosis by conventional imaging modalities; volumetric analysis may help to identify these occurrences 32. There are some limitations to the use of MRI, such as being subjective to the interpreter’s expertise. Approximately 57 percent of focal epileptogenic lesions are missed on standard MRI, making a referral to specialty epileptic clinic beneficial, for evaluation and accessibility to functional neuroimaging modalities 47.
A functional imaging positron emission tomography (PET) scan or magnetic resonance spectroscopy (MRS) may also be an option in select unconfirmed or inconclusive cases 32.
For individuals under evaluation for surgical intervention, adequate localization of epileptic focus with the preservation of unaffected areas is essential for optimizing post-surgical outcomes. Sometimes epileptic foci may not be apparent on MRI, and additional imaging modalities may be necessary interventions. The PET scan is a useful intervention in such cases, as it can be to identify epileptogenic zones interictally, independently, or in conjunction with CT/MRI for localization. An interictal PET scan with FDG labeling may reveal hypoperfusion in epileptogenic zones.
SPECT scanning is also a useful modality as it can identify epileptic focus in approximately 80 to 90% of cases evaluated; however, diagnostic accuracy is limited in interictal evaluations. Ictal findings with the use of 99mTc-HMPAO (hexamethyl propylene amine oxime) (within 30 seconds of seizure onset) show hyper-perfusion of the medial and/or lateral temporal lobe. Interictal-ictal SPECT subtraction may add enhanced localization value.
Magnetic encephalography (MEG) usually measures the magnetic field generated from interictal spikes and may be used in conjunction with MRI to obtain 3-dimensional magnetic source imaging, and useful in patients who require structural evaluation without the exposure to contrast agents. There are limitations, with this modality as it relies on interictal characterization, and is less informative than ictal EEG recordings.
Traditionally, the intra-carotid amobarbital test (WADA test or IAP) has been used in the pre-surgical evaluation of temporal lobe epilepsy to localize the verbal and visuospatial memory centers in the temporal lobe, as well as extra-temporally, and to assist with selective resection. This test is, however, an invasive procedure, and recently, researchers have made functional MRI (fMRI) comparisons to the WADA test as a less invasive measure. Limited class I and class II evidence shows significant concordance between IAP and WADA test findings and supports the ability of fMRI to assess post-surgical outcomes in language centers.
Presurgical evaluation for epilepsy
The basis of presurgical evaluation for epilepsy is to identify the epileptogenic zone, which is defined as the minimum amount of cortex that needs to be inactivated/resected/disconnected to render the patient seizure-free 48. However, the epileptogenic zone is a theoretical construct, and its identification is a matter of careful approximation of all available information sources. These sources include electrophysiologic data obtained from electroencephalography (EEG) and magnetoencephalography (MEG) that have a good temporal resolution and various neuroimaging modalities such as magnetic resonance imaging (MRI,) interictal positron emission tomography (PET), ictal single-photon emission tomography (SPECT), subtracted ictal SPECT co-registered to MRI (SISCOM), and functional MRI that have a good spatial resolution. Neuropsychological assessment is also employed as a part of a presurgical evaluation to evaluate for functional characteristics of the affected epileptogenic region. Such an extensive evaluation can be performed most effectively at a comprehensive epilepsy center with a cohesive team of specialists with training in neurology, neurosurgery, neuroradiology, neuropsychology, neuropathology, and psychiatry liaison services.
High-resolution 3T/7T MRI of the brain with thin cuts obtained through the temporal lobes is a powerful tool to assess subtle structural abnormalities involving the mesial temporal structures. Additional information regarding hippocampal pathology is obtainable with the use of multiple MR modalities such as volumetry, spectroscopy, and Diffusion Tensor Imaging (DTI) 49. Interictal PET looks at hypometabolism and can identify the epileptogenic temporal lobe in up to 70% to 90% of patients with mesial temporal lobe epilepsy 50. On the other hand, ictal SPECT/SISCOM looks at hyperperfusion and is also a useful tool, especially while looking at the origin and spread along the epileptogenic network 51.
The goal of presurgical evaluation for epilepsy surgery is to lateralize and localize the seizure focus accurately; this includes phase I and phase II evaluation:
- Phase I evaluation includes the use of non-invasive modalities to determine where the seizure starts; this includes techniques such as video-EEG, MEG, MRI, interictal PET, ictal SPECT/ SISCOM, and neuropsychological assessment. Patients with temporal lobe epilepsy involving the dominant temporal lobe also need functional MRI and/or intracarotid amobarbital/methohexital (Wada) test for language and memory lateralization.
- Phase II evaluation includes the use of surgically placed electrodes directly over the brain parenchyma to determine where exactly the seizure is originating. This phase involves the use of invasive techniques such as placement of subdural grids/strips and/or depth electrode placement for electrocorticography (ECoG) and stereo-electroencephalography (SEEG).
Scalp electroencephalogram
The EEG background in patients with mesial temporal lobe epilepsy is usually normal. There may be periods of intermittent slowing noted in the anterior temporal EEG derivations that become prominent during sleep and hyperventilation and are suggestive of focal cerebral dysfunction. Sometimes the focal slowing can be more robust and manifests as temporal intermittent rhythmic delta activity (TIRDA) 14.
In addition to slowing, the classic interictal EEG abnormality in mesial temporal lobe epilepsy are spikes or sharp waves which phase reverse over the anterior temporal regions. The dipole orientation of these sharp waves seems to have maximum electronegativity and voltage in the basal temporal derivations (T8/T9; FT8/FT9), and electropositivity distributed widely in the contralateral centro-parietal derivations (C3/C4; P3/P4) 52. The sharp waves in the anterior temporal region present in the majority of patients with mesial temporal lobe epilepsy 53. They tend to occur more frequently during drowsiness and early stages of sleep 54. They become less frequent during REM sleep and are somewhat similar in frequency to that seen during awake periods.
Ictal EEG findings in patients with mesial temporal lobe epilepsy are unique when compared to neocortical epilepsy because of its gradual, rhythmic build-up and delayed spread to neighboring brain regions. The seizures that arise from the hippocampus usually spread to the basal temporal regions. Therefore, the use of sphenoidal electrodes can be very useful in picking up the ictal onset in many of the cases with this seizure type. The characteristic pattern seen at the onset of an mesial temporal lobe seizure is a rhythmic theta activity starting in the anterior/anterior-inferior temporal or sphenoidal electrode contacts with gradual spread to the lateral temporal, insular, and frontal regions 52. An important localizing feature that can sometimes present is the occurrence of diffuse EEG attenuation and cessation of interictal epileptiform discharges (IEDs) at the onset of the seizure 55. Shortly after the onset, a slower rhythmic theta build-up or organized spiking is noted, which gradually evolves in frequency and amplitude until the seizure spreads to neighboring brain regions followed by spread to the contralateral hemisphere. When the EEG onset precedes the clinical onset, the localization of the seizure onset to the ipsilateral hemisphere is close to 95% 56. Finally, studies have found that postictal slowing is an important lateralizing feature in up to 70% of the cases 52.
In contrast, an ictal onset with unilateral delta slowing and repetitive interictal spiking is less likely to be arising from the mesial temporal region 57. Also, seizure onset with bilateral rhythmic activity and delayed evolution into a temporal pattern are a poor indicator of seizures arising from the mesial temporal region 56.
Invasive electroencephalogram
The use of invasive intracranial recordings using subdural grids or intracerebral depth electrodes has improved our diagnostic precision in the identification of the seizure focus. Although mesial temporal lobe epilepsy primarily involves the temporal lobes, the abnormal network is known to have widespread extra-temporal connectivity. It is essential to rule out other potential nodes in the network that can be independently epileptogenic. Stereo-electroencephalography (SEEG) is an important tool that registers electrical activity from very confined deep-seated brain regions that usually escape detection by usual surface recording modalities 58. Complex signal processing techniques have been employed to understand the intrinsic properties of epileptogenic networks from electrophysiologic signals obtained from SEEG data 59.
Unlike in scalp recording, the ictal activity recorded from intracranial electrodes detects a largely focal or regional fast beta or gamma rhythm. The focality of the rhythm on depth recording is directly proportional to the degree of hippocampal pathology 60.
Temporal lobe epilepsy treatment
Since cumulative seizure time is directly related to neurocognitive decline, reducing seizure frequency, and ideally, achieving seizure control is essential. The impact of epilepsy, as well as its management, have serious implications on the quality of life of the patient and should be taken into consideration.
Upon diagnosis of temporal lobe epilepsy, initial management should be pharmacological intervention with one of a variety of antiepileptic drugs. Older antiepileptic drugs such as phenytoin, valproate, carbamazepine, and phenobarbital, are equal in efficacy to newer antiepileptic drugs like lamotrigine, gabapentin, or levetiracetam, but correlate with a higher rate of adverse effects such as hepatotoxicity. Clinicians should avoid valproate and topiramate if possible, and lamotrigine and may consider levetiracetam, due to clinical evidence of higher rates of favorable outcomes in pregnancy 61. Women of childbearing age should also understand the potential teratogenicity with the use of antiepileptic drugs in the first trimester.
About one-third of patients with temporal lobe epilepsy do not have a resolution of seizures after initiation of antiepileptic drugs 62. The definition of refractory epilepsy has no objective definition but gets determined through the assessment of various domains that influence the prognosis of the condition such a frequency and severity of seizures, the number of antiepileptic drug failures and adverse effects of antiepileptic drugs used, and the subsequent impact on the livelihood of the individual with epilepsy 63. Seizure remission, or tractable seizures, may be considered as a period of greater than 6 months to 2 years of seizure freedom 63. Surgery is one option for individuals with refractory temporal lobe epilepsy and may provide up to an 80 percent remission rate in individuals with hippocampal sclerosis 31. Evidence suggests the superiority of the addition of temporal lobe surgery over antiepileptic drugs alone, for seizure control in refractory cases 64. Early surgical evaluation and intervention, when indicated, may be beneficial through improvements in quality of life indices as well as evidence suggesting an improvement in intelligence quotient (IQ) scores, and overall lifetime medical cost with pediatric surgical interventions 65.
The two most commonly performed surgical interventions anterior temporal lobectomy, involving the resection of the anterior temporal lobe, amygdala, hippocampus, and parahippocampal gyrus, as well as selective amygdalohippocampectomy, which targets the mesial structures specifically, preserving much of the cortical anatomy 66. A meta-analysis comparing the one-year seizure freedom for anterior temporal lobectomy and amygdalohippocampectomy, as well as comparing each of these interventions to antiepileptic drugs revealed no significant difference between the two procedures, but significant improvement in seizure freedom when compared to antiepileptic drugs 64. With regards to anterior temporal lobectomy, there are two general techniques employed: the traditional, or standard anterior temporal lobectomy, as well as anteromedial temporal resection.
In the standard anterior temporal lobectomy, a posterior cortical incision is made at the level of the lateral temporal gyri beginning around 5.5 cm from the temporal tip in the nondominant hemisphere and 4.5 cm from the temporal tip in the dominant hemisphere at the level of second temporal gyrus. This incision is slanted to avoid the primary auditory cortex in the first temporal gyrus. In comparison, the anteromedial temporal resection technique was developed to preserve more function of the lateral temporal lobe, in addition to aid in the access of mesial temporal structures. This procedure removes around 5 to 6 cm of the temporal lobe. The cortical incision is initiated at about 3 to 3.5 cm from the temporal tip and continued inferiorly towards the third temporal gyrus, with sparing of the auditory cortex in the first temporal gyrus. The mesial structures are subsequently removed using an ultrasound aspirator.
There is inconclusive evidence that a more selective approach may improve neurocognitive outcomes 66. The choice of open surgery versus selective interventions should be under the guidance by the challenges provided by the lesion, either locational or intrinsic characteristics. Included in more selective and less invasive approaches to anatomical correction for temporal lobe epilepsy are stereotactic radiosurgery as well as stereotactic laser ablation. These interventions are also valuable in lesional sites that are difficult or too risky to access via open surgical procedures. Recent evidence suggests that stereotactic radiosurgery provided similar results as open surgery with regards to seizure remission rates and neuropsychological prognosis, but has an extensive latency period until maximum therapeutic benefits, needed for corrective radio-surgical lesion formation. It also appears to have limited benefits in individuals with lesions secondary to arteriovenous malformations, as it may be associated with rebleeding. Stereotactic laser ablation is a more recent intervention but has shown promising results with regards to therapeutic outcomes. The use of MRI thermometry minimizes thermal damage to unaffected structures adjacent to the lesion as compared to radiofrequency ablation.
For individuals in whom surgery is contraindicated, other options are available for consideration 67. Neurostimulation is one such option, with vagus nerve stimulation (VNS) and responsive neurostimulation (RNS) being some modalities employed in the United States 68. Deep brain stimulation has been a new tool used to treat a variety of neurocognitive and neuromotor disorders but is not FDA approved at the time of this article, for the management of refractory epilepsy in the United States 69.
The use of ketogenic dieting may also be beneficial at reducing ictal frequency 70.
Seizure medications
Many people with temporal lobe epilepsy achieve full seizure control with anti-seizure drugs. But almost a third of people may not respond to drug therapy.
Uncontrolled seizures may cause a number of problems. For example, people often report problems with memory, socialization, and a fear of leaving their home. They may restrict their daily activities, which leads to a decrease in quality of life.
Surgery
If seizures fail to respond to medication, then epilepsy surgery may be an option. When an MRI shows hippocampal sclerosis in the medial temporal lobe and EEGs show seizures starting in that same area, seizures may be cured by surgery. In some cases, up to 7 out of 10 people can be seizure-free after surgery with few problems afterwards.
Devices
If surgery is not possible or doesn’t work, devices such as vagus nerve stimulation (VNS) or responsive neurostimulation (RNS) may help.
Temporal lobe epilepsy prognosis
Two out of 3 people with temporal lobe epilepsy achieve good seizure control with one or a combination of antiepileptic drugs, temporal lobe epilepsy is often refractory to neuroprotective agents 62. Seizures may also go away in some children with temporal lobe epilepsy. A good outcome is most often seen in people with normal MRI scans.
If the MRI is abnormal, there is a much higher risk that seizures will not respond to medicines called drug-resistant epilepsy.
About 75 percent of patients with mesial temporal lobe epilepsy do not achieve significant seizure control with medical treatment, with about 75 percent obtaining seizure freedom following surgical intervention 71. Of those refractory to antiepileptic drugs, temporal lobe surgery has been shown to improve the quality of life, mortality, and overall healthcare cost in successful procedural outcomes 65.
Overall, the prognosis for people with drug-resistant medial temporal lobe epilepsy includes a higher risk for memory and mood problems, lower quality of life, and an increased risk for sudden unexpected death in epilepsy (SUDEP). If surgery can be done to control seizures, these risks and problems can be improved.
References- Zhao F, Kang H, You L, Rastogi P, Venkatesh D, Chandra M. Neuropsychological deficits in temporal lobe epilepsy: A comprehensive review. Ann Indian Acad Neurol. 2014;17(4):374–382. doi:10.4103/0972-2327.144003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4251008
- Nickels KC, Wong-Kisiel LC, Moseley BD, Wirrell EC. Temporal lobe epilepsy in children. Epilepsy Res Treat. 2012;2012:849540. doi:10.1155/2012/849540 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3420576
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13:12–20.
- McIntosh WC, M Das J. Temporal Seizure. [Updated 2019 Oct 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549852
- Wirrell EC, Grossardt BR, Wong-Kisiel LCL, Nickels KC. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted county, Minnesota from 1980 to 2004: a population-based study. Epilepsy Research. 2011;95(1-2):110–118.
- Simon Harvey A, Berkovic SF, Wrennall JA, Hopkins LJ. Temporal lobe epilepsy in childhood: clinical, EEG, and neuroimaging findings and syndrome classification in a cohort with new-onset seizures. Neurology. 1997;49(4):960–968.
- Mai JG, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edition. Amsterdam, The Netherlands: Elsevier; 2008
- Kiernan JA. Anatomy of the temporal lobe. Epilepsy Res Treat. 2012;2012:176157. doi:10.1155/2012/176157 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3420617
- Baloh RW, Kerber KA. Neurophysiology of the Vestibular System. 4th edition. New York, NY, USA: Oxford University Press; 2011
- Kluver H, Bucy PC. Preliminary analysis of functions of temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000
- Jha S, Patel R. Kluver-Bucy syndrome—an experience with six cases. Neurology India. 2004;52(3):369–371
- Patel A, Fowler JB. Neuroanatomy, Temporal Lobe. [Updated 2019 Jan 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519512
- Nayak CS, Bandyopadhyay S. Mesial Temporal Lobe Epilepsy. [Updated 2020 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554432
- Tatum WO. Mesial temporal lobe epilepsy. J Clin Neurophysiol. 2012 Oct;29(5):356-65.
- Berkovic SF, McIntosh A, Howell RA, Mitchell A, Sheffield LJ, Hopper JL. Familial temporal lobe epilepsy: a common disorder identified in twins. Ann. Neurol. 1996 Aug;40(2):227-35.
- Hedera P, Blair MA, Andermann E, Andermann F, D’Agostino D, Taylor KA, Chahine L, Pandolfo M, Bradford Y, Haines JL, Abou-Khalil B. Familial mesial temporal lobe epilepsy maps to chromosome 4q13.2-q21.3. Neurology. 2007 Jun 12;68(24):2107-12.
- Crompton DE, Scheffer IE, Taylor I, Cook MJ, McKelvie PA, Vears DF, Lawrence KM, McMahon JM, Grinton BE, McIntosh AM, Berkovic SF. Familial mesial temporal lobe epilepsy: a benign epilepsy syndrome showing complex inheritance. Brain. 2010 Nov;133(11):3221-31.
- Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, Pfäfflin M, Elger C, Widman G, Schramm J, Becker A, Braun KP, Leijten F, Baayen JC, Aronica E, Chassoux F, Hamer H, Stefan H, Rössler K, Thom M, Walker MC, Sisodiya SM, Duncan JS, McEvoy AW, Pieper T, Holthausen H, Kudernatsch M, Meencke HJ, Kahane P, Schulze-Bonhage A, Zentner J, Heiland DH, Urbach H, Steinhoff BJ, Bast T, Tassi L, Lo Russo G, Özkara C, Oz B, Krsek P, Vogelgesang S, Runge U, Lerche H, Weber Y, Honavar M, Pimentel J, Arzimanoglou A, Ulate-Campos A, Noachtar S, Hartl E, Schijns O, Guerrini R, Barba C, Jacques TS, Cross JH, Feucht M, Mühlebner A, Grunwald T, Trinka E, Winkler PA, Gil-Nagel A, Toledano Delgado R, Mayer T, Lutz M, Zountsas B, Garganis K, Rosenow F, Hermsen A, von Oertzen TJ, Diepgen TL, Avanzini G., EEBB Consortium. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017 Oct 26;377(17):1648-1656.
- Al Sufiani F, Ang LC. Neuropathology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:624519.
- Harvey AS, Grattan-Smith JD, Desmond PM, Chow CW, Berkovic SF. Febrile seizures and hippocampal sclerosis: frequent and related findings in intractable temporal lobe epilepsy of childhood. Pediatr. Neurol. 1995 Apr;12(3):201-6.
- Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, Xu Y, MacFall J, Gomes WA, Moshé SL, Mathern GW, Pellock JM, Nordli DR, Frank LM, Provenzale J, Shinnar RC, Epstein LG, Masur D, Litherland C, Sun S., FEBSTAT Study Team. Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann. Neurol. 2014 Feb;75(2):178-85.
- Pohlen MS, Jin J, Tobias RS, Maheshwari A. Pharmacoresistance with newer anti-epileptic drugs in mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2017 Nov;137:56-60.
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper PN, Doughty J, Eaton B, Gamble C, Goulding PJ, Howell SJ, Hughes A, Jackson M, Jacoby A, Kellett M, Lawson GR, Leach JP, Nicolaides P, Roberts R, Shackley P, Shen J, Smith DF, Smith PE, Smith CT, Vanoli A, Williamson PR., SANAD Study group. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007 Mar 24;369(9566):1000-15.
- Wiebe S, Blume WT, Girvin JP, Eliasziw M., Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N. Engl. J. Med. 2001 Aug 02;345(5):311-8.
- de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harkness WF, Sander JW, Duncan JS. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011 Oct 15;378(9800):1388-95.
- Quigg M, Harden C. Minimally invasive techniques for epilepsy surgery: stereotactic radiosurgery and other technologies. J. Neurosurg. 2014 Dec;121 Suppl:232-40.
- Feng ES, Sui CB, Wang TX, Sun GL. Stereotactic radiosurgery for the treatment of mesial temporal lobe epilepsy. Acta Neurol. Scand. 2016 Dec;134(6):442-451.
- Fan X, Shan Y, Lu C, An Y, Wang Y, Du J, Wang D, Wei P, Fisher RS, Wang Y, Ren L, Zhao G. Optimized SEEG-guided radiofrequency thermocoagulation for mesial temporal lobe epilepsy with hippocampal sclerosis. Seizure. 2019 Oct;71:304-311.
- Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK, Boggs JG, Cash SS, Cole AJ, Duchowny MS, Duckrow RB, Edwards JC, Eisenschenk S, Fessler AJ, Fountain NB, Geller EB, Goldman AM, Goodman RR, Gross RE, Gwinn RP, Heck C, Herekar AA, Hirsch LJ, King-Stephens D, Labar DR, Marsh WR, Meador KJ, Miller I, Mizrahi EM, Murro AM, Nair DR, Noe KH, Olejniczak PW, Park YD, Rutecki P, Salanova V, Sheth RD, Skidmore C, Smith MC, Spencer DC, Srinivasan S, Tatum W, Van Ness P, Vossler DG, Wharen RE, Worrell GA, Yoshor D, Zimmerman RS, Skarpaas TL, Morrell MJ. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017 Jun;58(6):1005-1014.
- Mula M, Sander JW. Psychosocial aspects of epilepsy: a wider approach. BJPsych Open. 2016 Jul;2(4):270-274.
- Kurita T, Sakurai K, Takeda Y, Horinouchi T, Kusumi I. Very Long-Term Outcome of Non-Surgically Treated Patients with Temporal Lobe Epilepsy with Hippocampal Sclerosis: A Retrospective Study. PLoS ONE. 2016;11(7):e0159464.
- Malmgren K, Thom M. Hippocampal sclerosis–origins and imaging. Epilepsia. 2012 Sep;53 Suppl 4:19-33.
- Proper EA, Jansen GH, van Veelen CW, van Rijen PC, Gispen WH, de Graan PN. A grading system for hippocampal sclerosis based on the degree of hippocampal mossy fiber sprouting. Acta Neuropathol. 2001 Apr;101(4):405-9.
- Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Bernasconi N, Bien CG, Cendes F, Coras R, Cross JH, Jacques TS, Kahane P, Mathern GW, Miyata H, Moshé SL, Oz B, Özkara Ç, Perucca E, Sisodiya S, Wiebe S, Spreafico R. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013 Jul;54(7):1315-29.
- Mühlebner A, Breu M, Kasprian G, Schmook MT, Stefanits H, Scholl T, Samueli S, Gröppel G, Dressler A, Prayer D, Czech T, Hainfellner JA, Feucht M. Childhood onset temporal lobe epilepsy: Beyond hippocampal sclerosis. Eur. J. Paediatr. Neurol. 2016 Mar;20(2):228-235.
- Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009 Oct;19(4):565-72.
- Blümcke I, Coras R, Miyata H, Ozkara C. Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathol. 2012 May;22(3):402-11.
- Michelucci R, Pasini E, Nobile C. Lateral temporal lobe epilepsies: clinical and genetic features. Epilepsia. 2009 May;50 Suppl 5:52-4.
- Jessberger S, Parent JM. Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol. 2015 Nov 09;7(12).
- Tramoni-Negre E, Lambert I, Bartolomei F, Felician O. Long-term memory deficits in temporal lobe epilepsy. Rev. Neurol. (Paris). 2017 Jul – Aug;173(7-8):490-497.
- Berberian AP, Hopker C, Mazzarotto I, Cunha J, Guarinello AC, Massi G, Crippa A. Aspects of Oral Language, Speech, and Written Language in Subjects with Temporal Lobe Epilepsy of Difficult Control. Int Arch Otorhinolaryngol. 2015 Oct;19(4):302-8.
- Kobau R, Cui W, Zack MM. Adults with an epilepsy history fare significantly worse on positive mental and physical health than adults with other common chronic conditions-Estimates from the 2010 National Health Interview Survey and Patient Reported Outcome Measurement System (PROMIS) Global Health Scale. Epilepsy Behav. 2017 Jul;72:182-184.
- Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012 Feb;53(2):227-33.
- Tomson T, Walczak T, Sillanpaa M, Sander JW. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia. 2005;46 Suppl 11:54-61.
- Goldman AM. Mechanisms of sudden unexplained death in epilepsy. Curr. Opin. Neurol. 2015 Apr;28(2):166-74.
- Holmes MD, Tucker DM. Identifying the epileptic network. Front Neurol. 2013;4:84.
- Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernández G, Elger CE. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J. Neurol. Neurosurg. Psychiatry. 2002 Dec;73(6):643-7.
- Panzica F, Varotto G, Rotondi F, Spreafico R, Franceschetti S. Identification of the Epileptogenic Zone from Stereo-EEG Signals: A Connectivity-Graph Theory Approach. Front Neurol. 2013 Nov 06;4:175.
- Ercan K, Gunbey HP, Bilir E, Zan E, Arslan H. Comparative Lateralizing Ability of Multimodality MRI in Temporal Lobe Epilepsy. Dis. Markers. 2016;2016:5923243.
- Peter J, Houshmand S, Werner TJ, Rubello D, Alavi A. Novel assessment of global metabolism by 18F-FDG-PET for localizing affected lobe in temporal lobe epilepsy. Nucl Med Commun. 2016 Aug;37(8):882-7.
- Amorim BJ, Ramos CD, dos Santos AO, de Lima Mda C, Min LL, Camargo EE, Cendes F, Etchebehere EC. Brain SPECT in mesial temporal lobe epilepsy: comparison between visual analysis and SPM. Arq Neuropsiquiatr. 2010 Apr;68(2):153-60.
- Ebersole JS, Pacia SV. Localization of temporal lobe foci by ictal EEG patterns. Epilepsia. 1996 Apr;37(4):386-99.
- Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann. Neurol. 1993 Dec;34(6):781-7.
- Yu-Dan L, Zan W, Ma DH, Meng HM, Cui L. Association between epileptiform discharges and the sleep cycle in 200 epileptic patients. Int. J. Neurosci. 2013 Mar;123(3):196-203.
- Murro AM, Park YD, King DW, Gallagher BB, Smith JR, Yaghmai F, Toro V, Figueroa RE, Loring DW, Littleton W. Seizure localization in temporal lobe epilepsy: a comparison of scalp-sphenoidal EEG and volumetric MRI. Neurology. 1993 Dec;43(12):2531-3.
- Risinger MW, Engel J, Van Ness PC, Henry TR, Crandall PH. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology. 1989 Oct;39(10):1288-93.
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005 May;46(5):669-76.
- Gonzalez-Martinez J, Mullin J, Vadera S, Bulacio J, Hughes G, Jones S, Enatsu R, Najm I. Stereotactic placement of depth electrodes in medically intractable epilepsy. J. Neurosurg. 2014 Mar;120(3):639-44.
- Wang MY, Wang J, Zhou J, Guan YG, Zhai F, Liu CQ, Xu FF, Han YX, Yan ZF, Luan GM. Identification of the epileptogenic zone of temporal lobe epilepsy from stereo-electroencephalography signals: A phase transfer entropy and graph theory approach. Neuroimage Clin. 2017;16:184-195.
- Vossler DG, Kraemer DL, Haltiner AM, Rostad SW, Kjos BO, Davis BJ, Morgan JD, Caylor LM. Intracranial EEG in temporal lobe epilepsy: location of seizure onset relates to degree of hippocampal pathology. Epilepsia. 2004 May;45(5):497-503.
- Pennell PB. Use of Antiepileptic Drugs During Pregnancy: Evolving Concepts. Neurotherapeutics. 2016 Oct;13(4):811-820.
- Fernandes MJ, Carneiro JE, Amorim RP, Araujo MG, Nehlig A. Neuroprotective agents and modulation of temporal lobe epilepsy. Front Biosci (Elite Ed). 2015 Jan 01;7:79-93.
- Lee SK. Treatment strategy for the patient with hippocampal sclerosis who failed to the first antiepileptic drug. J Epilepsy Res. 2014 Jun;4(1):1-6.
- Jain P, Tomlinson G, Snead C, Sander B, Widjaja E. Systematic review and network meta-analysis of resective surgery for mesial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry. 2018 Nov;89(11):1138-1144.
- Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K., Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012 Mar 07;307(9):922-30.
- Hoyt AT, Smith KA. Selective Amygdalohippocampectomy. Neurosurg. Clin. N. Am. 2016 Jan;27(1):1-17.
- Gross RE, Mahmoudi B, Riley JP. Less is more: novel less-invasive surgical techniques for mesial temporal lobe epilepsy that minimize cognitive impairment. Curr. Opin. Neurol. 2015 Apr;28(2):182-91.
- Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW, Berg MJ, Bergey GK, Cash SS, Cole AJ, Duckrow RB, Edwards JC, Eisenschenk S, Fessler J, Fountain NB, Goldman AM, Gwinn RP, Heck C, Herekar A, Hirsch LJ, Jobst BC, King-Stephens D, Labar DR, Leiphart JW, Marsh WR, Meador KJ, Mizrahi EM, Murro AM, Nair DR, Noe KH, Park YD, Rutecki PA, Salanova V, Sheth RD, Shields DC, Skidmore C, Smith MC, Spencer DC, Srinivasan S, Tatum W, Van Ness PC, Vossler DG, Wharen RE, Worrell GA, Yoshor D, Zimmerman RS, Cicora K, Sun FT, Morrell MJ. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017 Jun;58(6):994-1004.
- Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018 Feb;59(2):273-290.
- Elia M, Klepper J, Leiendecker B, Hartmann H. Ketogenic Diets in the Treatment of Epilepsy. Curr. Pharm. Des. 2017;23(37):5691-5701.
- Spencer SS. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002 Oct;1(6):375-82.