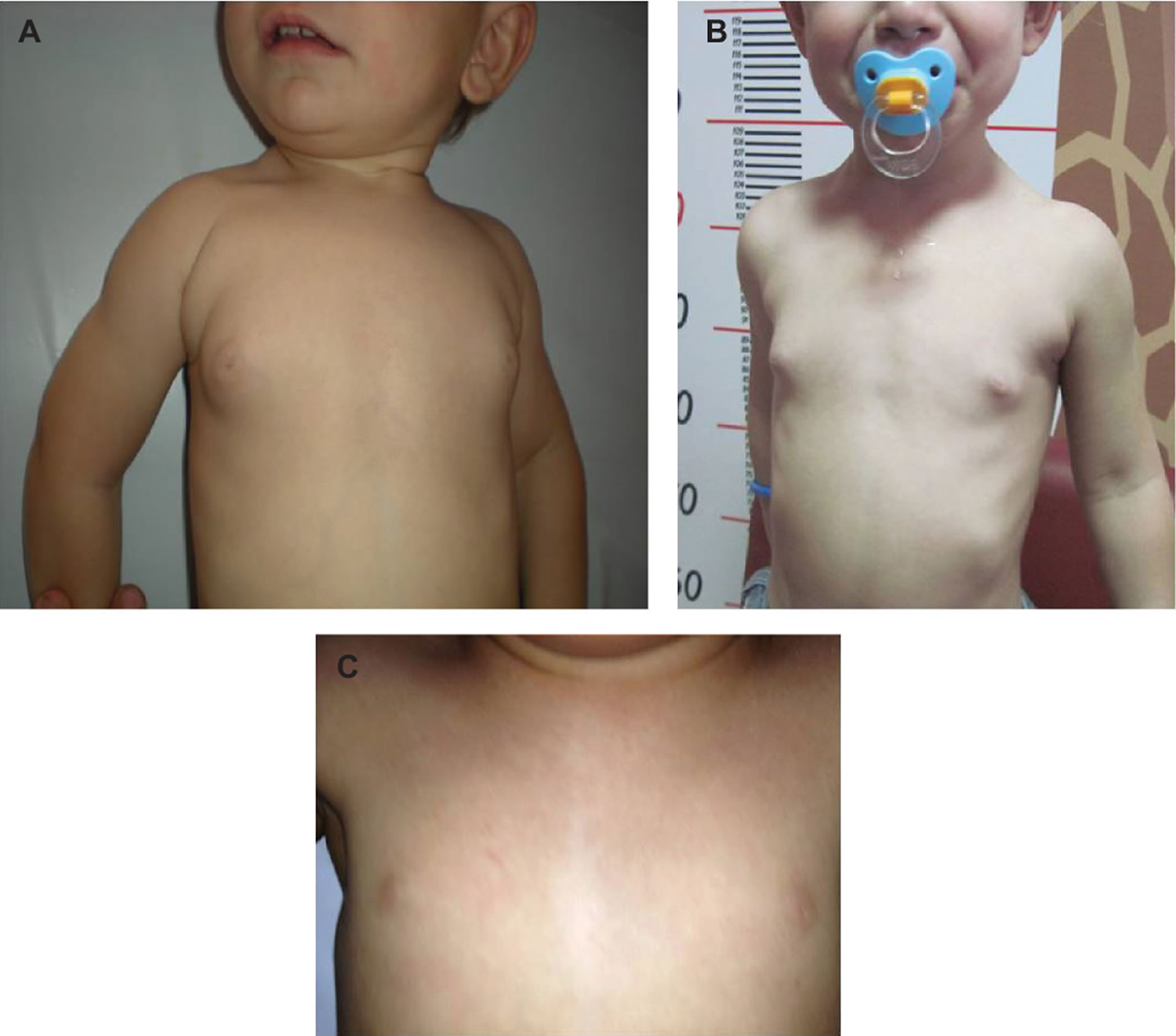
Premature thelarche
Thelarche is a medical term for breast development in young girls, is typically the first sign of female puberty 1. Breast development (thelarche) typically occurs between 8 to 12 years old with a mean age of 10 years old 2. Menarche (a girl’s first period) follows shortly after thelarche about 2.5 years later with a mean age of 12.5 years but can range from 9 to 15 years old 3. Pubic hair development usually follows along with breast development and occurs due to the production of adrenal androgens. Some very young girls (usually from 6 months to 3 years old) may show breast development that later disappears or may last but without other physical changes of puberty. This is called premature thelarche and usually doesn’t cause lasting problems.
Premature thelarche refers to the precocious appearance of breast tissue in either one or both breasts in girls before 8 years of age with no other signs of sexual maturation, accelerated growth velocity, or bone age advancement 4. Premature thelarche is most common during the first 2 years of life and its incidence in nursery children from Northern Italy was reported as high as 36.6% 4. The cause of premature telarche is still unknown, although different pathogenetic mechanisms have been suggested. Some authors postulated that an increase in breast sensitivity to estrogen might be responsible for the premature development of breast tissue. Others, using an ultrasensitive recombinant cell bioassay, showed that girls with premature thelarche exhibit higher estradiol levels than those of normal pre-pubertal girls 5. Transient estrogen secretion from ovarian follicular cysts 6, increased production of estrogens from adrenal precursors, and transient partial activation of the hypothalamic-pituitary-gonadal axis with increased secretion of follicle-stimulating hormone (FSH), were also claimed as possible causes of premature telarche 7. An increased prevalence of detectable ovarian microcysts at ultrasound was also reported, but the presence or absence of these cysts did not correlate with basal gonadotropins or estradiol levels 8. Recent studies identified activating mutations of GNAS1 gene in some patients with chronic fluctuating and/or exaggerated thelarche, without other classic signs of McCune-Albright syndrome 9. Body mass index (BMI) was also shown to be associated with occurrence of premature thelarche in 4- to 8-year old girls 10, supporting a previous observation that the prevalence of premature thelarche can be affected by BMI 11.
In the last years great attention was paid to the effects on pubertal development of the so-called endocrine disruptors. A growing list of chemicals were shown to influence the endocrine system either in vitro or in vivo, but only a few were associated with altered pubertal development. The outbreak of epidemics of premature telarche in some geographical areas was suggested to be linked to exposure to endocrine disruptors 4. Phthalates were suspected in Puerto Rico, whereas in Michigan polybrominated biphenyls were associated with advanced breast development in children of exposed mothers. Thus, possible exposure to endocrine disruptors should be borne in mind in the diagnostic work-up of premature telarche 12.
Premature telarche is usually a self-limited condition that undergoes spontaneous regression during the first 2-3 years of life 4. However, in some cases the outcome of premature thelarche is not always entirely benign. It has been observed that when its onset occurs after 2-3 years of age, a certain percentage of patients develop central precocious puberty 13, but onset of thelarche under 2 years of age does not assure a transient course 14. In fact, progression to precocious puberty is not associated with age at presentation of thelarche or clinical course, meaning that girls presenting with thelarche at birth have the same risk of developing true precocious puberty as girls presenting at an older age 15. In an initial Italian series, 14% of girls diagnosed with premature thelarche at a mean age of 5.1 years, progressed to precocious or early puberty 13. In a following retrospective multi-center study on 119 girls 16, only 60% of the patients who presented with premature breast development before 2 year of age showed a complete regression during the follow-up period. In 40% of these girls the breast size was unmodified at a follow-up period of 134 months, and 7/38 (18.4%) patients developed central precocious puberty. Furthermore, another subgroup was identified (28.5%) which included patients showing an accelerated height velocity and/or bone maturation at diagnosis but did not develop precocious puberty 16. In premature thelarche patients who developed precocious or early puberty, final height was unaffected and normal for mid-parental height, so that the sexual precocity was interpreted as a reflection of early maternal menarcheal age 17.
The observation of IGF-1 serum concentrations and IGF-1/IGFBP-3 values intermediate between those detected in prepubertal children and in central precocious puberty suggests that premature thelarche could be considered a very early stage of puberty 18. Also, children developing premature thelarche in late childhood exhibit a pubertal response to gonadotropin-releasing hormone (GnRH) stimulation test one year after breast development 19, supporting the hypothesis that precocious thelarche may represent a decreased juvenile inhibition of the hypothalamic-gonadotropic-gonadal axis.
Identifying clinical or laboratory parameters predictive for progression into precocious puberty is a crucial process. For example, in premature thelarche girls younger than 2 years, growth velocity value of >1 standard deviation or a basal luteinizing hormone (LH) level ≥0.3 IU/L have been suggested as indicators for close follow-up 14. Conversely, in girls younger than 3 years of age, no laboratory parameters were identified able to predict progression into precocious puberty 20. Elevated Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) peak responses to gonadotropin-releasing hormone (GnRH) are frequently observed in infancy and early childhood, and they are not related with the clinical progression to true precocious puberty. Even though a peak LH/FSH ratio >1 could represent a good marker of progression, its sensitivity is low in the early phases of pubertal activation. The combined measurement of both basal LH levels and longitudinal diameter of the uterus may represent a reliable screening approach to identify the subjects at risk of true precocious puberty who should undergo GnRH testing 20.
In addition to clinical and hormonal assessments, pelvic ultrasound might be useful to distinguish premature thelarche from precocious puberty. In fact, while no significant differences in uterine and ovarian ultrasound measurements were detected between children with premature thelarche and controls, significant differences in pelvic ultrasound parameters were reported between healthy girls and age-matched girls with central precocious puberty 4. Uterine transverse diameter (“‘width”‘), uterine length, fundal anteroposterior diameter, uterine volume, ovarian length, ovarian circumference, and mean ovarian volume are all increased in girls with central precocious puberty. The calculated cut-off values to predict precocious puberty by ultrasound vary among studies. Haber et al. found that a uterine volume >1.8 ml, uterine length >3.6 cm, and ovarian volume >1.2 ml were highly predictive for precocious puberty 21. Herter et al. 22 reported that the best cut-off points were uterine length 4.0 cm, uterine cross-sectional area 4.5 cm², uterine volume 3.0 cm³, and ovarian volume 1.0 cm³. Comparing 30 girls with precocious puberty and 21 with premature thelarche in whom peak luteinizing hormone was <5 mIU/ml on the GnRH stimulation test, De Vries et al. 23 found significant differences in uterine width, fundus diameter, uterine volume, and ovarian circumference. The authors suggest that increased uterine and ovarian measurements may be an early and sensitive sign of precocious puberty (Table 1) and that pelvic ultrasound may give the clinician a complementary indication to the GnRH test in distinguishing isolated premature thelarche from early-stage precocious puberty in girls with early breast budding.
Table 1. Cut-off values to predict precocious puberty by ultrasound and their sensitivity and specificity
| Value | Sensitivity % | Specificity % | |
| Uterine volume (cc) | >2.0 | 88.8 | 89.4 |
| Uterine length (cm) | >3.4 | 80.2 | 57.8 |
| Uterine transverse diameter (cm) | >1.5 | 67.9 | 100 |
| Fundus (cm) | >0.8 | 82.5 | 76.4 |
| Presence of endometrial echo | 57.3 | 100 | |
| Ovarian circumference (cm) | >4.5 | 66.6 | 85.5 |
The measurement of baseline estradiol blood concentrations may also be helpful in distinguishing premature thelarche from precocious puberty, although the differential diagnosis is based on the results of the classic gonadotropin releasing-hormone (GnRH) stimulation test (100 mg LH-RH as i.v. bolus). Baseline LH and FSH plasma levels are often higher, and peak LH levels are significantly and constantly elevated in precocious puberty than in premature thelarche patients, whereas peak FSH levels may not be significantly different in the two groups. A stimulated LH/FSH ratio greater than 1, is suggestive of precocious puberty (185). In recent years, the stimulation with an LH-RH analogue such as leuprolide acetate, was proved to be particularly useful in the differential diagnosis of pubertal disorders. Peak LH was shown to be significantly higher and consistently >8 IU/l in pubertal in contrast to pre-pubertal subjects 24. Moreover, the gonadal response, which is maximal 24h post-stimulation, was also discriminating between pre-pubertal and pubertal conditions (estradiol in females >150 pmol/L, and testosterone >3.15 nmol/L in males) 24. Other Authors reported a 100% sensitivity and 84% specificity for a peak LH/FSH ratio >0.24 25. Breast ultrasound alone has limited ability to distinguish precocious puberty from premature thelarche 26.
New markers for the differential diagnosis of premature thelarche and precocious puberty have been investigated. Particularly, serum kisspeptin, leptin, and neurokinin B were reported to be higher in patients with central precocious puberty and premature thelarche compared to controls 27. However, these markers are not able to differentiate patients with central precocious puberty from premature thelarche 28.
References- Remien K, Pillarisetty LS. Female Development. [Updated 2019 Mar 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539695
- Breehl L, Caban O. Physiology, Puberty. [Updated 2018 Nov 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534827
- Blondell RD, Foster MB, Dave KC. Disorders of puberty. Am Fam Physician. 1999 Jul;60(1):209-18, 223-4.
- Beccuti G, Ghizzoni L. Normal and Abnormal Puberty. [Updated 2015 Aug 8]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279024
- Dumic M, Tajic M, Mardesic D, Kalafatic Z. Premature thelarche: a possible adrenal disorder. Arch Dis Child 1982; 57:200-203
- Carantoni M, Abbasi F, Azhar S, Schaaf P, Reaven GM. Can changes in plasma insulin concentration explain the variability in leptin response to weight loss in obese women with normal glucose tolerance? J Clin Endocrinol Metab 1999; 84:869-872
- Pasquino AM, Piccolo F, Scalamandre A, Malvaso M, Ortolani R, Boscherini B. Hypothalamic-pituitary-gonadotropic function in girls with premature thelarche. Arch Dis Child 1980; 55:941-944
- Freedman SM, Kreitzer PM, Elkowitz SS, Soberman N, Leonidas JC. Ovarian microcysts in girls with isolated premature thelarche. J Pediatr 1993; 122:246-249
- Roman R, Johnson MC, Codner E, Boric MA, aVila A, Cassorla F. Activating GNAS1 gene mutations in patients with premature thelarche. J Pediatr 2004; 145:218-222
- Atay Z, Turan S, Guran T, Furman A, Bereket A. The prevalence and risk factors of premature thelarche and pubarche in 4- to 8-year-old girls. Acta Paediatr 2012; 101:e71-75
- Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics 2009; 123:84-88
- Teilmann G, Juul A, Skakkebaek NE, Toppari J. Putative effects of endocrine disrupters on pubertal development in the human. Best Pract Res Clin Endocrinol Metab 2002; 16:105-121
- Pasquino AM, Pucarelli I, Passeri F, Segni M, Mancini MA, Municchi G. Progression of premature thelarche to central precocious puberty. J Pediatr 1995; 126:11-14
- Ucar A, Saka N, Bas F, Bundak R, Gunoz H, Darendeliler F. Is premature thelarche in the first two years of life transient? J Clin Res Pediatr Endocrinol 2012; 4:140-145
- de Vries L, Guz-Mark A, Lazar L, Reches A, Phillip M. Premature thelarche: age at presentation affects clinical course but not clinical characteristics or risk to progress to precocious puberty. J Pediatr 2010; 156:466-471
- Volta C, Bernasconi S, Cisternino M, Buzi F, Ferzetti A, Street ME, Da Milano AM. Isolated premature thelarche and thelarche variant: clinical and auxological follow-up of 119 girls. J Endocrinol Invest 1998; 21:180-183
- Salardi S, Cacciari E, Mainetti B, Mazzanti L, Pirazzoli P. Outcome of premature thelarche: relation to puberty and final height. Arch Dis Child 1998; 79:173-174
- Sales DS, Moreira AC, Camacho-Hubner C, Ricco RG, Daneluzzi JC, Campos AD, Martinelli CE, Jr. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 in girls with premature thelarche. J Pediatr Endocrinol Metab 2003; 16:827-833
- Takakuwa S. Premature thelarche in later childhood demonstrates a pubertal response to GnRH stimulation test at one year after breast development. Clin Pediatr Endocrinol 2011; 20:81-87
- Bizzarri C, Spadoni GL, Bottaro G, Montanari G, Giannone G, Cappa M, Cianfarani S. The response to gonadotropin releasing hormone (GnRH) stimulation test does not predict the progression to true precocious puberty in girls with onset of premature thelarche in the first three years of life. J Clin Endocrinol Metab 2014; 99:433-439
- Haber HP, Wollmann HA, Ranke MB. Pelvic ultrasonography: early differentiation between isolated premature thelarche and central precocious puberty. Eur J Pediatr 1995; 154:182-186
- Herter LD, Golendziner E, Flores JA, Moretto M, Di Domenico K, Becker E, Jr., Spritzer PM. Ovarian and uterine findings in pelvic sonography: comparison between prepubertal girls, girls with isolated thelarche, and girls with central precocious puberty. J Ultrasound Med 2002; 21:1237-1246; quiz 1247-1238
- de Vries L, Horev G, Schwartz M, Phillip M. Ultrasonographic and clinical parameters for early differentiation between precocious puberty and premature thelarche. Eur J Endocrinol 2006; 154:891-898
- Ibanez L, Potau N, Zampolli M, Virdis R, Gussinye M, Carrascosa A, Saenger P, Vicens-Calvet E. Use of leuprolide acetate response patterns in the early diagnosis of pubertal disorders: comparison with the gonadotropin-releasing hormone test. J Clin Endocrinol Metab 1994; 78:30-35
- Catli G, Erdem P, Anik A, Abaci A, Bober E. Clinical and laboratory findings in the differential diagnosis of central precocious puberty and premature thelarche. Turk Pediatri Ars 2015; 50:20-26
- Youn I, Park SH, Lim IS, Kim SJ. Ultrasound assessment of breast development: distinction between premature thelarche and precocious puberty. AJR Am J Roentgenol 2015; 204:620-624
- Abaci A, Catli G, Anik A, Kume T, Calan OG, Dundar BN, Bober E. Significance of serum neurokinin B and kisspeptin levels in the differential diagnosis of premature thelarche and idiopathic central precocious puberty. Peptides 2015; 64:29-33
- Akinci A, Cetin D, Ilhan N. Plasma kisspeptin levels in girls with premature thelarche. J Clin Res Pediatr Endocrinol 2012; 4:61-65