What is trehalose
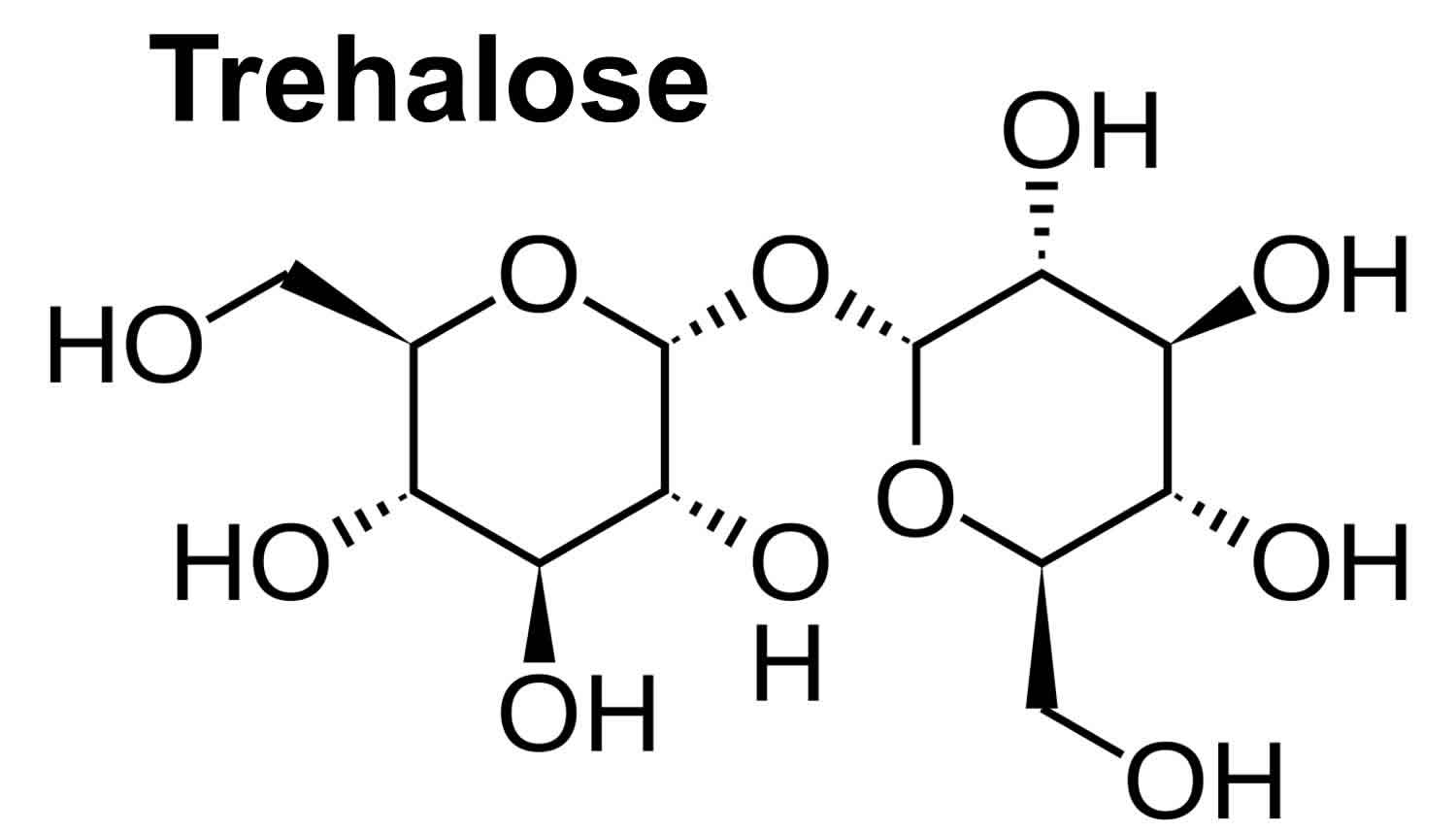
Trehalose is a nonreducing sugar containing two glucose subunits with an α,α-1,1-glycosidic linkage (Figure 1). Trehalose’s formal name is α-d-glucopyranosyl α-d-glucopyranoside (C12H22O11·2H2O [trehalose dihydrate] or C12H22O11 [anhydrous trehalose]) 1. Trehalose was granted GRAS (Generally Recognized As Safe) status by the FDA in 2000 and approved for use in food in Europe in 2001, reported expected usage ranges from concentrations of 2%-11.25% for foods including pasta, ground beef, and ice cream 2.
Trehalose is found in lower and higher plants, fungi, lichens, algae, a wide variety of bacteria, insects, and invertebrates where trehalose may serve as a source of energy and carbon, but not in mammals (outside their microbiomes) 3. The formation of trehalose in lower organisms, sometimes in amounts as high as 10% to 20% of their dry weight, is induced by stress conditions such as heat, drying, oxidative stress, and so on, and convincing evidence indicates that this accumulated cytoplasmic trehalose protects proteins and membranes from denaturation caused by these stresses 3. In addition, trehalose may act as a signaling or regulatory molecule in some cells and link trehalose metabolism to glucose transport and glycolysis. Interestingly, trehalases (trehalose-degrading enzymes) are found in the kidney and the brush border of the small intestine in mammals, including humans 4, probably to handle ingested trehalose 3. Intestinal trehalase is responsible for rapid degradation of ingested trehalose 5. Various organisms that constitute the human diet, including plants and fungi, contain trehalose. Like in lactose intolerance, having a low concentration of trehalase causes malabsorption, diarrhea, or other gastrointestinal symptoms 6. Intake of probiotic Saccharomyces boulardii by such patients had increased trehalase activity in the intestine and reduced those symptoms 7.
Urinary trehalase has been proposed to be a specific marker for kidney damages 5. In diabetes, higher trehalase activity and genetic variations in the trehalase gene were noted 8. Human trehalase (TREH) has a remarkable feature shared with yeast acid trehalase 1 (ATH1) 9. TREH rescued phenotypes of yeast ATH1 mutant but not NTH1 or NTH2. ATH1 is present in the vacuole and catalyze the hydrolysis of extracellular trehalose 10. These results suggest that human trehalase gene TREH may act as a stress-response gene involved in the utilization of exogenous trehalose 9.
In fungi, trehalose is present in spores, fruiting bodies, and vegetative cells such as hyphae. Trehalose is rapidly depleted after germination and used in central fungal metabolism and in response to specific environmental stresses 11.
Trehalose has received attention for the past few decades for its role in neuroprotection especially in animal models of various neurodegenerative diseases, such as Parkinson and Huntington diseases 12. The mechanism underlying the neuroprotective effects of trehalose remains elusive 12. The prevailing hypothesis is that trehalose protects neurons by inducing autophagy, thereby clearing protein aggregates. Some of the animal studies showed activation of autophagy and reduced protein aggregates after trehalose administration in neurodegenerative disease models, seemingly supporting the autophagy induction hypothesis. However, results from cell studies have been less certain; although many studies claim that trehalose induces autophagy and reduces protein aggregates, the studies have their weaknesses, failing to provide sufficient evidence for the autophagy induction theory. Furthermore, a recent study with a thorough examination of autophagy flux showed that trehalose interfered with the flux from autophagosome to autolysosome, raising controversy on the direct effects of trehalose on autophagy.
Trehalose Facts 12:
- Trehalose has been shown to be neuroprotective in animal models of various neurodegenerative diseases, such as Parkinson and Huntington diseases.
- Autophagy induction and aggregate clearance have been the primary hypothesis for the mechanism of neuroprotection by trehalose.
- Trehalose blocks autophagic flux from autophagosome to autolysosome in cell models.
- Trehalose may exert the neuroprotective effects through indirect mechanisms at the systemic levels, e.g., through influencing gut microbiota.
Open questions
- What is the mechanism of neuroprotection by trehalose?
- How does trehalose block the autophagic flux?
- What are the effects of trehalose on gut microbiota?
- How does the chemical chaperone activity of trehalose influence on the neuroprotective functions?
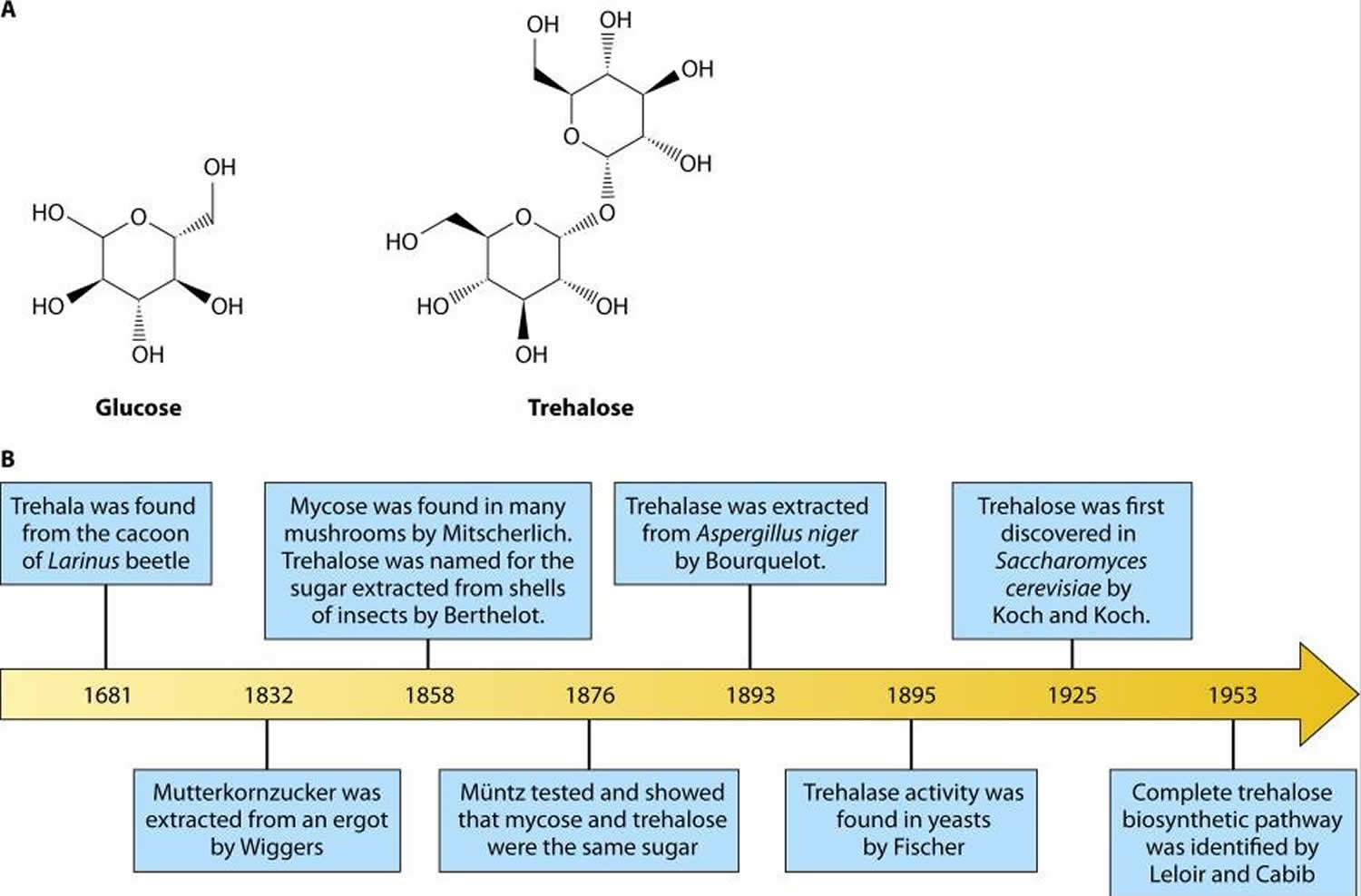
Figure 1. Trehalose and glucose chemical structures
Footnote: (A) Chemical structures of glucose and trehalose. Trehalose consists of two glucose molecules with an α,α-1,1-glycosidic linkage. (B) Timeline of trehalose and trehalose-related enzyme discovery.
[Source 1 ]Is trehalose a reducing sugar?
No. Trehalose is a non-reducing stable sugar containing two glucose subunits with an α,α-1,1-glycosidic linkage, which is not readily hydrolyzed by acid or α-glucosidase 5. Trehalose inert characteristic ensures that it does not readily interact with proteins or other biomolecules 13. As a nonreducing sugar with a low free energy of activation of the glycosidic linkage, trehalose is more resistant to hydrolysis than other disaccharides 1. Under mildly acidic conditions, other disaccharides go through a Maillard (browning) reaction that forms many compounds, e.g., furans, imidazoles, and N-nitroso derivatives, which may negatively affect dried food nutrition. However, trehalose is relatively stable in this context and does not hydrolyze 14. O’Brien observed in 1996 15 that under suboptimal conditions, trehalose undergoes ∼2,000-fold fewer Maillard reactions than those observed for sucrose. Consequently, although there is still some controversy about the mechanisms of trehalose’s protective properties, it is well known and widely used in food preservative processes and in mammalian cell and plant preservations 15. For example, trehalose is used as a cryogenic preservative for spermatozoa and ovarian tissue and also in the formulation of commercial products such as Herceptin and Avastin 1. Trehalose unique properties that preserve protein structures allow its use in everyday products 16. Further experiments to unravel the biological properties of trehalose are necessary to fully reveal its biological mechanisms and its potential for preservation of fragile cells and molecules. This is particularly true for the role of trehalose itself in the pathogenesis mechanisms of human-pathogenic fungi.
What is trehalose used for?
Trehalose is an ingredient, along with hyaluronic acid, in an artificial tears product (Thealoz Duo®) used to treat dry eye 17. Trehalose has been shown not only to stabilize both bilipid membranes and labile proteins against desiccation, but also to have a protective effect against desiccation and oxidative insult in the mammalian eye 18.
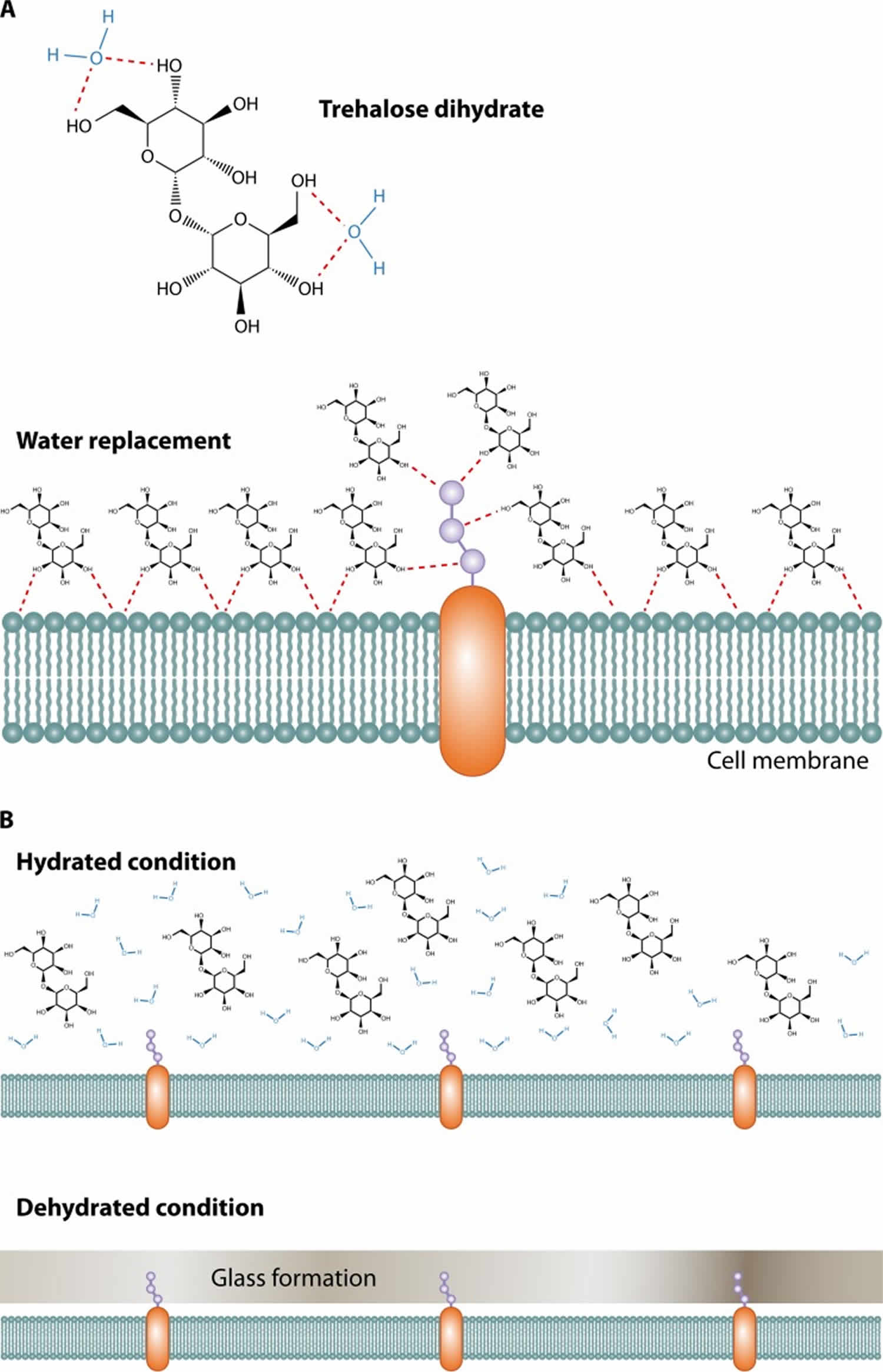
There are three suggested mechanisms by which trehalose stabilizes proteins: water replacement, glass transition, and chemical stability 19. Trehalose inhibits protein denaturation by the exclusion of water molecules from the surface of proteins when cells are in the dehydrated condition 20. In the dry state, it maintains proteins in the folded state by replacing water molecules and forming hydrogen bonds directly with proteins 21. The unique property of trehalose to create a stable non-hygroscopic glass at high temperatures in the dry state also allows maintenance of the protein structure 22. The amount of trehalose accumulation in different yeast species is related to the ability to survive heat and dehydration 23. In nematodes, trehalose accumulates at the onset of dehydration 24. Trehalose is rapidly broken down once the stress is relieved, bringing it down to the normal level.
Trehalose, therefore, acts as a natural stabilizer of life processes, withstanding extreme temperatures, nutrient deprivation, osmotic pressures, and dehydration in many species of invertebrates 25. Trehalose serves as an excellent desiccant for many organisms. Even human primary fibroblasts, artificially producing trehalose, could be maintained in the dry state for up to 5 days 26.
Trehalose is also widely used in food preservative processes and in mammalian cell and plant preservations 15. For example, trehalose is used as a cryogenic preservative for spermatozoa and ovarian tissue and also in the formulation of commercial products such as Herceptin and Avastin 1. Trehalose unique properties that preserve protein structures allow its use in everyday products 16. Further experiments to unravel the biological properties of trehalose are necessary to fully reveal its biological mechanisms and its potential for preservation of fragile cells and molecules. This is particularly true for the role of trehalose itself in the pathogenesis mechanisms of human-pathogenic fungi.
With regard to glass transformation, sugars may solidify in a glass state that helps biomolecules to stabilize and protect small hydrophobic volatile esters from evaporation in cold and desiccating environments. The glass state of trehalose is different from other sugars because it does not retain water molecules and thus does not form crystals as other sugars do (Figure 2B). Due to this unique property, the trehalose glass state is stable at high temperatures and under desiccation conditions 27. What role this property of trehalose has, if any, on fungal biology is unclear.
Figure 2. Trehalose uses
Footnote: (A) Trehalose forms hydrogen bonds with two water molecules and functions as a replacement for water by interacting with the phospholipids or other macromolecules on the cell membrane to protect their structures under stress conditions. (B) Trehalose forms a glass state without any water retention or crystallization, thereby protecting the cell membrane under dehydrated conditions.
[Source 1 ]Trehalose sources
Trehalose is a naturally occurring disaccharide that is widespread throughout the biological world. Trehalose in lower orders of the plant kingdom has been known for many years, with the first tentative report being in 1832 in ergot of rye 28. Trehalose is quite common in yeast and fungi where it occurs in spores, fruiting bodies, and vegetative cells 29. For example, the spores and macrocysts of Dictyostelium mucoroides have been reported to contain as much as 7% trehalose on a dry-weight basis 30, and the ascospores of Neurospora tetrasperma have as much as 10% trehalose 31. When these spores germinate, the trehalose rapidly disappears suggesting that this sugar is stored as a source of carbon, and/or energy. Trehalose is also present in high concentrations in baker’s yeast 32 and brewer’s yeast 33; in these organisms, the levels of trehalose depend on the age of the cells, as well as on their stage of growth and their nutritional state. Many species of lichens 34 and algae 35 also contain this disaccharide, although in considerably lower concentrations than those found in yeast. Trehalose is probably also present in many higher plants since it has been isolated from the resurrection plant (Selaginella lepidophylla) 36 and also from Arabidopsis thaliana 37. It is apparently also a component of the wound exudates of Fraxsinus aras 38.
Trehalose is also found in a number of different bacteria, including Streptomyces hygroscopicus and other species of Streptomyces 39, various mycobacteria, including Mycobacterium smegmatis and tuberculosis 40 and corynebacteria 41. In mycobacteria and corynebacteria, this disaccharide plays a structural role as a cell wall component, but it may also serve other functions in these organisms. It is also present in Escherichia coli 42 and a number of other bacteria, such as Rhizobium sp. 43, Sulfdolobus acidocaldarius 44, Pimelobacter sp. R48 45, Arthrobacter sp. Q36 45, and so on. In many of these organisms, the function of trehalose is still not clear. Several of the organisms listed appear to have rather unusual biosynthetic pathways for synthesizing trehalose.
In the animal kingdom, trehalose was first reported in insects, where it is present in hemolymph 46 and also in larvae or pupae 47. In the adult insect, the levels of trehalose fall rapidly during certain energy-requiring activities, such as flight 48, indicating a role for this disaccharide as a source of glucose for energy. In addition to insects, trehalose has also been identified in the eggs of the roundworm Ascaris lumbricoides, in which it may be present at levels as high as 8% of the dry weight 49, and in adult roundworms and Porrocaecum larvae where its levels are as high as 6% of the dry weight 50. This sugar also occurs in a number of invertebrates 51. This profile of the occurrence of α,α-trehalose among such diverse living organisms demonstrates its widespread distribution and suggests that it plays an important role in the biological world.
A series of trehalose oligosaccharides was isolated from the cytoplasm of Mycobacterium smegmatis and characterized by nuclear magnetic resonance, mass spectrometry, methylation analysis, and enzymatic digestions. These oligosaccharides all had trehalose as the base with one or two other sugars attached to it. Two trisaccharides were identified as Glcα1-4Trehalose and Glcβ1-6Trehalose, whereas three tetrasaccharides were characterized as Glcβ1-6Glcβ1-6Trehalose, Galα1-6Galα1-6Trehalose, and a trehalose with an α1-6Gal on one of its glucose residues and an α1-4Glc on the other glucose 52. The function of these trehalose oligosaccharides is not known at this time, but they may be involved in stabilizing cellular structures. For example, sucrose, which is the major nonreducing disaccharide in plants, may be somewhat analogous to trehalose in terms of some of its functions. Thus, many plants produce higher homologs of sucrose such as raffinose (Galα1-6Sucrose) and stachyose (Galα1-6Galα1-6Sucrose); these higher sucrose oligosaccharides have been proposed to play a role in stabilizing or protecting cells against stress 53.
Chemical synthesis of trehalose
α,α-Trehalose has been synthesized chemically using the ethylene oxide addition reaction between 2,3,4,5-tetra- O-acetyl-D-glucose and 3,4,6-tri-O-acetyl-1,2-anhydro-D- glucose 54. This same series of reactions also gives rise to one of the other trehalose anomers, specifically, α,β-trehalose, also referred to as neotrehalose. Neotrehalose has also been synthesized using the Koenigs-Knorr reaction 55. On the other hand, this anomer has not been isolated from any living organisms, although it was identified in koji extract 56. The other anomer of trehalose, β,β-trehalose, or isotrehalose, also has not been isolated from any living organisms, but it was found in starch hydrolysates 57; it also has been synthesized chemically using the Koenigs-Knorr reaction, as well as by a dehydration reaction 58. Trehalose also can be produced chemically by an acid reversion of glucose 59. On the other hand, α,α-trehalose is the only anomer of trehalose that has been shown to be biosynthesized in many different types of organisms.
Trehalose in food
Trehalose is commonly used in prepared frozen foods, like ice cream, because it lowers the freezing point of foods 60. Trehalose is rapidly broken down into glucose by the enzyme trehalase, which is present in the brush border of the intestinal mucosa of humans. Trehalose causes less of a spike in blood sugar than glucose 60. Trehalose has about 45% the sweetness of sucrose at concentrations above 22%, but when the concentration is reduced, its sweetness decreases more quickly than that of sucrose, so that a 2.3% solution tastes 6.5 times less sweet as the equivalent sugar solution 61.
Trehalose side effects
A new study in the journal Nature 2 indicates that trehalose-laden food may have helped fuel the recent epidemic spread of Clostridium difficile, which is a microbe that can cause life-threatening gastrointestinal distress, especially in older patients getting antibiotics and antacid medicines 62. In laboratory experiments, a team found that the two strains of Clostridium difficile most likely to make people sick possess an unusual ability to thrive on trehalose, even at very low levels. And that’s not all: a diet containing trehalose significantly increased the severity of symptoms in a mouse model of Clostridium difficile infection.
Clostridium difficile is a common bacterium that many people already have in their gut. In most cases, the bacterium doesn’t make people sick. The trouble often comes when taking antibiotics, especially in a hospital or nursing home. These drugs can upset the normal balance of healthy gut microbes, and because Clostridium difficile is naturally resistant to many common antibiotics, this opportunistic microbe can multiply and produce toxins that cause inflammation and diarrhea.
The recent rise in Clostridium difficile infections has been driven in large part by a particular group of bacterial strains, known collectively as RT027. Their spread may be explained in part by a mutation that lent the bugs resistance to certain antibiotics. But still unanswered was how this strain and another one called RT078 emerged so rapidly and became so prevalent.
An earlier study led by Robert Britton at Baylor College of Medicine, Houston, provided an important lead. He and his colleagues showed that the RT027 strains outcompeted many other Clostridium difficile strains in cell culture and mouse studies 63.
To learn why in the new study, Britton and colleagues tested the strains’ ability to use various sugars that might be present in the gut to fuel their growth and give them a competitive advantage over other bacteria. Those studies suggested that RT027 strains have a special ability to grow on trehalose. In fact, further study of 21 different strains showed that the RT027 and RT078 strains, and only those strains, grow unusually well on a diet of trehalose.
Britton’s team found in the new study that RT027’s ability to grow on trehalose traces to a new mutation in the bacterial DNA. The change allows the bacterium to sense the sugar and produce an enzyme to metabolize it for food, even at extremely low concentrations. In contrast, RT078 is able to grow exceptionally well on low levels of trehalose, thanks to a cluster of four genes involved in metabolizing the sugar that were apparently acquired from another microbe. This shows the two Clostridium difficile strains have adapted to feed on trehalose in two completely different ways!
In mouse studies, Britton and colleagues found that a diet including trehalose makes infections with the RT027 strain more severe and sometimes deadly. The researchers also found that when trehalose is present, the epidemic Clostridium difficile strains are not only more abundant, they produce more toxins for reasons that aren’t yet entirely clear. The team also has preliminary evidence from mice and people to suggest that enough dietary trehalose may make its way to the intestine to fuel the growth of those infectious strains.
Britton says the ability of these Clostridium difficile strains to grow on trehalose isn’t new. Strains of RT027 that efficiently metabolized trehalose were first isolated in the 1980s. Back then, people got most of their trehalose in small amounts from foods that naturally have it, such as mushrooms and shellfish.
What has changed is the recent addition of man-made trehalose into the food supply, often in large quantities. This shift was prompted by a new method to manufacture trehalose from cornstarch, which made the sugar much less costly. In 2000, FDA approved the sugar as a safe food additive. Trehalose quickly found its way into processed foods in the U.S. and around the world for its mild, flavor-enhancing sweetness and protection of frozen foods. In some store-bought ice creams, it’s found at concentrations of up to 11 percent.
What Britton and colleagues noticed is the more widespread use of manufactured trehalose coincided with early reports of Clostridium difficile outbreaks. Those outbreaks popped up not only in the U.S., but also in countries all around the world where trehalose consumption increased. While more study is surely needed to nail down the possible connection, the circumstantial and experimental evidence has led Britton’s team to propose that widespread use of trehalose inadvertently played a key role in the recent emergence of Clostridium difficile infections.
This doesn’t mean that everyone needs to start worrying about trehalose. In fact, Britton says the sugar does have some advantages. For instance, because it’s harder to break down, trehalose doesn’t cause blood glucose to spike in the way some other sugars do.
Britton’s team continues to study the interaction between these Clostridium difficile strains and trehalose. They are especially curious to learn more about how and why trehalose causes some Clostridium difficile strains to produce more toxins in the gut. The findings certainly offer food for thought about the complex interplay between our diets, gut microbes, and health—and the unintended consequences that dietary changes might bring.
References- Thammahong A, Puttikamonkul S, Perfect JR, Brennan RG, Cramer RA. Central Role of the Trehalose Biosynthesis Pathway in the Pathogenesis of Human Fungal Infections: Opportunities and Challenges for Therapeutic Development. Microbiol Mol Biol Rev. 2017;81(2):e00053-16. Published 2017 Mar 15. doi:10.1128/MMBR.00053-16 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5485801
- Collins J, Robinson C, Danhof H, et al. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature. 2018;553(7688):291-294. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5984069/
- Alan D. Elbein, Y.T. Pan, Irena Pastuszak, David Carroll; New insights on trehalose: a multifunctional molecule, Glycobiology, Volume 13, Issue 4, 1 April 2003, Pages 17R–27R, https://doi.org/10.1093/glycob/cwg047
- Elbein AD. 1974. The metabolism of alpha,alpha-trehalose. Adv Carbohydr Chem Biochem 30:227–256. doi:10.1016/S0065-2318(08)60266-8
- Lee HJ, Yoon YS, Lee SJ. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis. 2018;9(7):712. Published 2018 Jun 15. doi:10.1038/s41419-018-0749-9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6003909
- Richards AB, et al. Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002;40:871–898. doi: 10.1016/S0278-6915(02)00011-X
- Buts JP, Stilmant C, Bernasconi P, Neirinck C, De Keyser N. Characterization of alpha,alpha-trehalase released in the intestinal lumen by the probiotic Saccharomyces boulardii. Scand. J. Gastroenterol. 2008;43:1489–1496. doi: 10.1080/00365520802308862
- Muller YL, et al. Identification of genetic variation that determines human trehalase activity and its association with type 2 diabetes. Hum. Genet. 2013;132:697–707. doi: 10.1007/s00439-013-1278-3
- Ouyang Y, Xu Q, Mitsui K, Motizuki M, Xu Z. Human trehalase is a stress responsive protein in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2009;379:621–625. doi: 10.1016/j.bbrc.2008.12.134
- Huang J, Reggiori F, Klionsky DJ. The transmembrane domain of acid trehalase mediates ubiquitin-independent multivesicular body pathway sorting. Mol. Biol. Cell. 2007;18:2511–2524. doi: 10.1091/mbc.e06-11-0995
- Elbein AD, Pan YT, Pastuszak I, Carroll D. 2003. New insights on trehalose: a multifunctional molecule. Glycobiology 13:17R–27R. doi:10.1093/glycob/cwg047
- Lee HJ, Yoon YS, Lee SJ. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis. 2018;9(7):712. Published 2018 Jun 15. doi:10.1038/s41419-018-0749-9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6003909/
- Amorphous stability and trehalose. Colaco C, Kampinga J, Roser B. Science. 1995 May 12; 268(5212):788.
- Colaço CALS, Roser B. 1995. Trehalose—a multifunctional additive for food preservation, p 123–140. In Mathlouthi M, editor. (ed), Food packaging and preservation. Blackie Professional, London, United Kingdom.
- O’Brien J. 1996. Stability of trehalose, sucrose and glucose to nonenzymatic browning in model systems. J Food Sci 61:679–682. doi:10.1111/j.1365-2621.1996.tb12180.x
- Ohtake S, Wang YJ. 2011. Trehalose: current use and future applications. J Pharm Sci 100:2020–2053. doi:10.1002/jps.22458.
- Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729. Published 2016 Feb 23. doi:10.1002/14651858.CD009729.pub2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5045033/
- Trehalose: an intriguing disaccharide with potential for medical application in ophthalmology. Luyckx J, Baudouin C. Clin Ophthalmol. 2011; 5():577-81.
- Colaco C, Kampinga J, Roser B. Amorphous stability and trehalose. Science. 1995;268:788. doi: 10.1126/science.7754360
- Arakawa T, Carpenter JF, Kita YA, Crowe JH. The basis for toxicity of certain cryoprotectants: A hypothesis. Cryobiology. 1990;27:401–415. doi: 10.1016/0011-2240(90)90017-X
- Allison SD, Chang B, Randolph TW, Carpenter JF. Hydrogen Bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem. Biophys. 1999;365:289–298. doi: 10.1006/abbi.1999.1175
- Slade L, Levine H. A food polymer science approach to structure-property relationships in aqueous food systems: non-equilibrium behavior of carbohydrate-water systems. Adv. Exp. Med. Biol. 1991;302:29–101. doi: 10.1007/978-1-4899-0664-9_3
- Van Dijck P, Colavizza D, Smet P, Thevelein JM. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 1995;61:109–115
- Womersley C, Smith L. Anhydrobiosis in nematodes i. The role of glycerol myo-inositol and trehalose during desiccation. Comp. Biochem. Physiol. 1981;70B:579–586.
- McBride MJ, Ensign JC. Effects of intracellular trehalose content on Streptomyces griseus spores. J. Bacteriol. 1987;169:4995–5001. doi: 10.1128/jb.169.11.4995-5001.1987
- Guo N, Puhlev I, Brown DR, Mansbridge J, Levine F. Trehalose expression confers desiccation tolerance on human cells. Nat. Biotechnol. 2000;18:168–171. doi: 10.1038/72616.
- Crowe JH, Crowe LM. 2000. Preservation of mammalian cells—learning nature’s tricks. Nat Biotechnol 18:145–146. doi:10.1038/72580
- Elbein, A.D. (1974) The metabolism of α,α-trehalose. Adv. Carbohyd. Chem. Biochem. , 30, 227–256.
- Nwaka, S. and Holzer, H. (1998) Molecular biology of trehalose and trehalases in the yeast, Saccharomyces cerevcesiae. Prog. Nucleic Acid Res. Mol. Biol. , 58, 197–237.
- Clegg, J.S. and Filosa, M.F. (1961) Trehalose in the cellular slime mold, Dictyostelium mucoroides. Nature , 192, 1077–1078.
- Sussman, A.S. and Lingappa, B.T. (1959) Role of trehalose in ascospores of Neurospora tetrasperma. Science , 130, 1343–1344.
- Elander, E. and Myrback, K. (1949) Isolation of crystalline trealose after fermentation of glucose by maceration juice. Arch. Biochem. Biophys. , 21, 249–255.
- Stewart, L.C., Richtmyer, N.K., and Hudson, C.S. (1950) Preparation of trehalose from yeast. J. Am. Chem. Soc. , 72, 2059–2061.
- Lindberg, B. (1955) Studies on the chemistry of lichens. Investigation of a Dermatocarpon and some Roccella species. Acta Chem. Scand. , 9, 917–919.
- Augier, J. (1954) The biochemistry of a North American algae, Tuomeyafluviatilis. Compt. Rend. , 239, 87–89.
- Adams, R.P., Kendall, E., and Kartha, K.K. (1990) Comparison of free sugars in growing and desiccated plants of Selaginella lepidophylla. Biochem. Syst. Ecol. , 18, 107–110.
- Muller, J., Aeschbacher, R.A., Wingler, A., Boller, T., and Wiemken, A. (2001) Trehalose and trehalase in Arabidopsis. Plant Physiol. , 125, 1086–1093.
- Sabry, Z.I. and Atallah, N.A. (1961) Identification of sugars in the manna of northern Iraq. Nature , 190, 915–916.
- Martin, M.C., Diaz, L.A., Manzanal, M.B., and Hardisson, C. (1986) Role of trehalose in the spores of Streptomyces. FEMS Micro. Lett. , 35, 49–54.
- Elbein, A.D. and Mitchell, M. (1973) Levels of glycogen and trehalose in Mycobacterium smegmatis, and the purification and properties of the glycogen synthase. J. Bacteriol. , 113, 863–873.
- Shimakata, T. and Minatagawa, Y. (2000) Essential role of trehalose in the synthesis and subsequent metabolism of corynomycolic acid in Corynebacterium matruchotii. Arch. Biochem. Biophys. , 380, 331–338.
- Kaasen, I., McDougall, J., and Strom, A.R. (1994) Analysis of the otsBA operon for osmoregulatory trehalose synthesis in Escherichia coli. Gene , 145, 9–15.
- Maruta, K., Hattori, K., Nakada, T., Kubota, M., Sugimoto, T., and Kurimoto, M. (1996) Cloning and sequencing of trehalose biosynthesis genes from Rhizobium sp. M-11. Biosci. Biotechnol. Biochem. , 60, 717–720.
- Maruta, K., Mitsuzumi, H., Nakada, T., Kubota, M., Chaen, H., Fukuda, S., Sugimoto, T., and Kurimoto, M. (1996) Cloning and sequencing of a cluster of genes encoding novel enzymes of trehalose biosynthesis from thermophilic archaebacterium Sulfolobus acidocaldarius. Biochim. Biophys. Acta , 1291, 177–181.
- Nishimoto, T., Nakano, M., Bnakada, T., Chaen, H., Fukuda, S., Sugimoto, T., Kurimoto, M., and Tsujisaka, Y. (1995) Purification and properties of a novel enzyme, trehalose synthase, from Pimelobacter sp. R48. Biosci. Biotechnol. Biochem. , 60, 640–644.
- Wyatt, G.R. and Kalf, G.F. (1957) The chemistry of in,sect hemolymph. Trehalose and other carbohydrates. J. Gen. Physiol. , 40, 833–846.
- Fairbairn, D. (1958) Glucose, trehalose and glycogen in Porrocaecum decipiens larvae. Nature , 181, 1593–1594.
- Evans, D.R. and Dethier, V.G. (1957) The regulation of taste thresholds for sugars in the blowfly. J. Insect Physiol. , 1, 3–17.
- Fairbairn, D. and Passey, R.F. (1957) Occurrence and distribution of trehalose and glycogen in the eggs and tissues of Ascaris lumbricoides. Exp. Parasitol. , 6, 566–574.
- Kalf, G.F. and Rieder, S.V. (1958) The purification and properties of trehalase. J. Biol. Chem. , 230, 691–698.
- Friedman, S. (1960) The purification and properties of trehalase isolated from Phormia regina, MEIG. Arch. Biochem. Biophys. , 87, 252–258.
- Ohta, M., Pan, Y.T., Laine, R.A., and Elbein, A.D. (2002) Trehalose-based oligosaccharides isolated from the cytoplasm of Mycobacterium smegmatis. Eur. J. Biochem. , 269, 1–8.
- Peterbauer, T., Mucha, J., Mach, L., and Richter, A. (2002) Chain elongation of raffinose in pea seeds. J. Biol. Chem. , 277, 194–200.
- Lemieux, R.U. and Bauer, H.F. (1954) A chemical synthesis of D-trehalose. Can. J. Chem. , 32, 340–344.
- Helferich, B. and Weis, K. (1956) The synthesis of glucosides and of nonreducing disaccharides. Chem. Ber. , 89, 314–321.
- Matsuda, K. (1956) Studies on the unfermentable disaccharides in koji extract. IV. Isolation of α,α- and α,β-trehalose. Tohoku J. Agric. Res. , 6, 271–277.
- Sato, A. and Aso, K. (1957) Kojibiose, 2-O-α-D-glucopyranosyl-D-glucose. Nature , 180, 984–985.
- Bredereck, H., Hoschele, G., and Ruck, K. (1953) Synthesis of non-reducing disaccharides. Chem. Ber. , 86, 1277–128.
- Thompson, A., Anno, K., Wolfrom, M.L., and Inatome, M. (1954) Acid reversion products from D-glucose. J. Am. Chem. Soc. , 76, 1309–1311.
- Has an Alternative to Table Sugar Contributed to the C. Diff. Epidemic? https://directorsblog.nih.gov/2018/01/09/has-a-sucrose-alternative-contributed-to-the-c-diff-epidemic/
- O’Brien-Nabors, Lyn, ed. (2012). Alternative sweeteners (4th ed.). Boca Raton: CRC Press. ISBN 978-1-4398-4614-8.
- Evaluating the risk factors for hospital-onset Clostridium difficile infections in a large healthcare system. Watson T, Hickok J, Fraker S, Korwek K, Poland RE, Septimus E. Clin Infect Dis. 2017 Dec 20.
- Epidemic Clostridium difficile strains demonstrate increased competitive fitness compared to nonepidemic isolates. Robinson CD, Auchtung JM, Collins J, Britton RA. Infect Immun. 2014 Jul;82(7):2815-25.