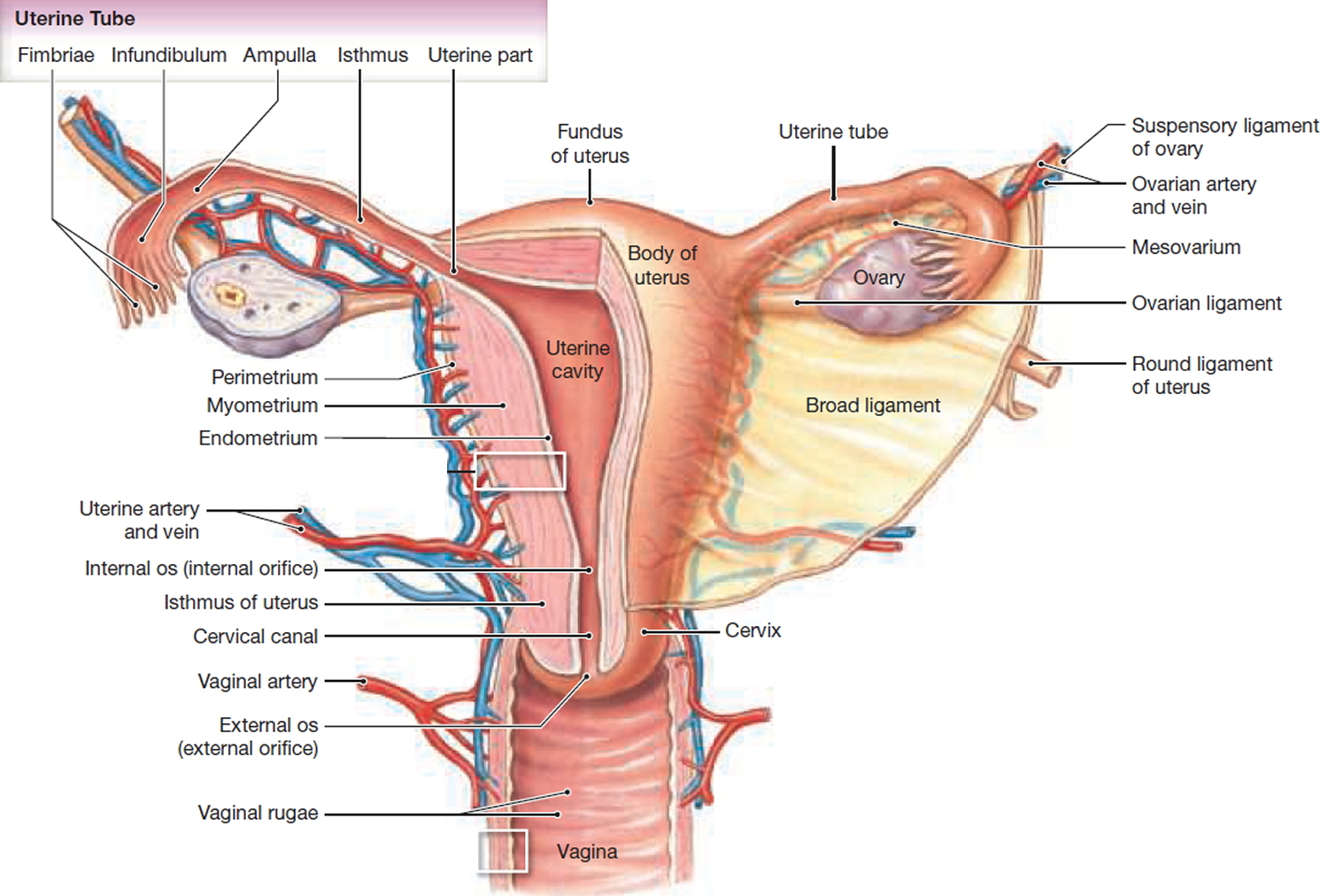
What are uterine fibroids
Uterine fibroids or leiomyomas, are the most common benign tumors in the muscular wall of the uterus, in women of reproductive age 1. These growths can be very tiny or as large as a cantaloupe. Most fibroids range from about the size of a large marble to slightly smaller than a baseball 2. Bunches or clusters of fibroids are often of different sizes. Not all fibroids grow, and some may shrink, or remain constant over time 3.
Their prevalence is age dependent; at least 20% of women 35 years of age or older have fibroids 4 and they can be detected in up to 80% of women by 50 years of age 5. Once a fibroid is formed, it tends to grow larger until menopause, after which fibroids tend to shrink, due to the effects of estrogen.
Many women have them without knowing it. Black women are at a higher risk of having fibroids than women in other racial groups. Fibroids are the leading indication for hysterectomy, accounting for 39% of all hysterectomies performed annually in the United States. Although many are detected incidentally on imaging in asymptomatic women, 20% to 50% of women are symptomatic and may wish to pursue treatment.
Symptomatic fibroids are associated with great costs to the patient and the healthcare system; it was estimated that uterine fibroids incurred a total direct cost of US$10.3 billion in the United States in one year alone 6.
Fibroids are the most common indication for a hysterectomy 7. Although highly effective, hysterectomy is associated with perioperative and postoperative morbidity and, very rarely, mortality (estimated one in 2000) 8. More conservative management techniques allowing women to preserve the uterus have become increasingly popular.
Uterine fibroids are categorize based on where in the uterine wall they grow:
- Submucosal fibroids grow just underneath the uterine lining and into the endometrial cavity.
- Intramural fibroids grow in between the muscles of the uterus.
- Subserosal fibroids grow on the outside of the uterus.
Some fibroids grow on stalks that grow out from the surface of the uterus or into the cavity of the uterus. These are called pedunculated fibroids.
Figure 1. Uterine fibroids
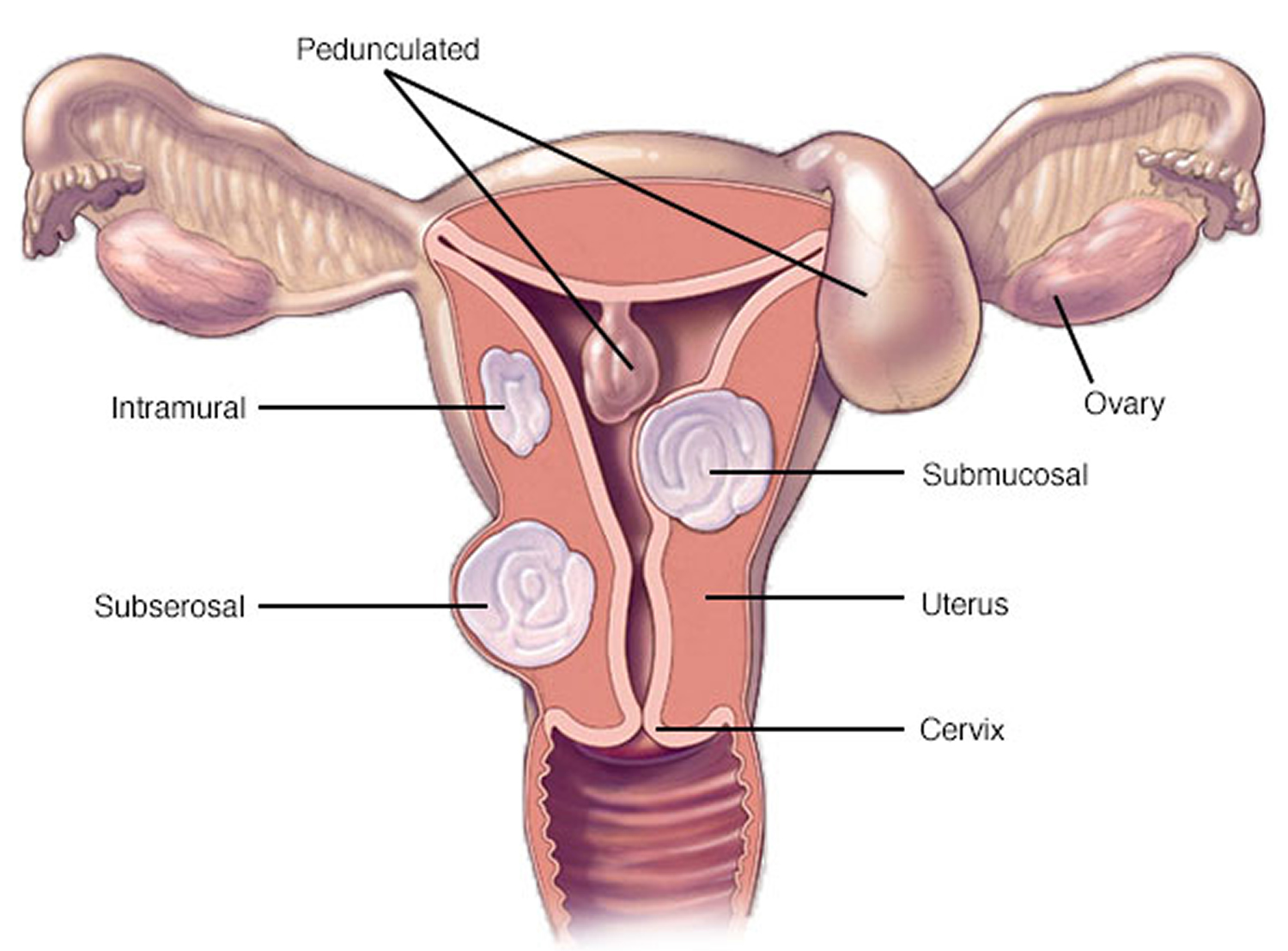
Figure 2. Uterus anatomy
Uterine Fibroids Malignancy risk
Uterine fibroids are not cancerous. Less than one in a thousand cases of fibroids develop into cancer 9.
In a very small number of patients with a condition called hereditary leiomyomatosis and renal cell cancer (HLRCC), the fibroids are linked to kidney cancer. However, this association is not seen in women who do not have HLRCC 10.
Differential diagnosis of a malignancy should be considered in women presenting with a uterine mass, particularly if they are postmenopausal 11. One to two in 1000 women with uterine masses are estimated to have a uterine malignancy 12.
Suspicion for malignancy is raised for rapidly growing fibroids, particularly in postmenopausal women who are not on hormone replacement therapy and women responding poorly to gonadotrophin releasing hormone (GnRH) agonists. A history of tamoxifen use for more than five years is associated with a threefold increase in the risk of leiomyosarcoma, and uterine sonographic surveillance is recommended in such cases, especially in patients who have had prior pelvic irradiation 13, 14.
Preoperative differentiation between benign fibroids and uterine malignancy is extremely difficult, yet increasingly important because of trends for using conservative and minimally invasive treatments. Occasionally, morcellators are used in laparoscopic fibroid resection. These instruments divide tissue into smaller sections that are otherwise too large to remove via portholes or the vaginal outlet. Shorter operating times and smaller incisions reduce postoperative morbidity.
In 2014, the US Food and Drug Administration (FDA) released a statement discouraging the use of laparoscopic power morcellation following a case of inadvertent morcellation of a leiomyosarcoma and subsequent malignant upstaging 15. Many have since considered the therapeutic challenges and risks of minimally invasive surgery. A large retrospective trial showed that the risk of unintended morcellation of a uterine leiomyosarcoma following preoperative selection of women with fibroids is one in 4791 women 16. While seemingly low, the risks of minimally invasive surgery should be carefully conveyed to patients to help them make fully informed decisions.
What causes fibroids
Doctors don’t know what causes uterine fibroids. Scientists have a number of theories, but none of these ideas explains fibroids completely.
Some factors researchers believed to be related to fibroid growth are 17, 18:
- Estrogen
- Progesterone
- Growth hormones
- Genetic changes
- Misplaced cells present in the body before birth
Other factors that may affect fibroid growth are:
- Quantity of micronutrients—nutrients such as iron that the body needs only small amounts of — in the blood 19. For instance, a deficiency of vitamin D may be associated with uterine fibroids 20.
- Major stresses 21, 22
It is likely that fibroids are caused by many factors interacting with one another. Once we know the cause or causes of fibroids, our efforts to find a cure or even prevent fibroids could move ahead more quickly.
Uterine fibroids symptoms
Many women who have fibroids don’t have symptoms. In other women, fibroids can cause heavy bleeding during the menstrual period. Periods may last much longer than usual.
Fibroids may also cause pain or a feeling of pressure or heaviness in the lower pelvic area (the area between the hip bones), the back or the legs. Some women have pain during sexual intercourse. Others have a constant feeling that they need to urinate. There may also be a feeling of pressure in the bowel. Some women have constipation or bloating.
If you do have symptoms, they may include 23:
- Longer, more frequent, or heavy menstrual periods
- Painful periods (cramps)
- Bleeding between periods
- Feeling “full” in the lower abdomen (belly)—this is sometimes called “pelvic pressure”
- Urinating often (caused by a fibroid pressing on the bladder)
- Pain during sex
- Lower back pain (often dull, heavy and aching, but may be sharp)
- Reproductive problems, such as infertility, multiple miscarriages or early onset of labor during pregnancy
- Anemia (from blood loss)
- Obstetrical problems, such as increased likelihood of cesarean section.
- Constipation, rectal pain, or difficult bowel movements.
Fibroids also may cause no symptoms at all. Fibroids may be found during a routine pelvic exam or during tests for other problems.
What complications can occur with fibroids ?
Fibroids that are attached to the uterus by a stem may twist and can cause pain, nausea, or fever. Fibroids that grow rapidly, or those that start breaking down, also may cause pain. Rarely, they can be associated with cancer. A very large fibroid may cause swelling of the abdomen. This swelling can make it hard to do a thorough pelvic exam.
Fibroids also may cause infertility, although other causes are more common. Other factors should be explored before fibroids are considered the cause of a couple’s infertility. When fibroids are thought to be a cause, many women are able to become pregnant after they are treated.
How is uterine fibroids diagnosed ?
Clinical History
Following a detailed medical and gynaecological history, consider if the patient has had the following 12:
- abnormal uterine bleeding: menorrhagia, dysmenorrhoea, breakthrough bleeding
- symptoms of anaemia and iron deficiency from long-term menorrhagia
- pressure symptoms, such as urinary frequency, retention, tenesmus or evidence of hydronephrosis
- a history of subfertility: distortion of the uterine cavity may be associated with implantation failure and even later stage pregnancy losses
- acute pelvic pain: this can occur in the setting of fibroid degeneration as its vascular supply is outgrown.
Clinical Examination
Abdominal and pelvic examination may reveal a firm palpable uterine mass. Fibroids may palpate as smooth and be similar to a gravid uterus, or irregular and nodular if there are multiple fibroids. Larger fibroids may distend the abdomen.
Differential diagnoses are:
Uterine
- Pregnancy
- Haematoma
- Leiomyosarcoma
Extra-uterine
- Ovarian cyst
- Ovarian malignancy
- Ectopic pregnancy
- Pyosalpinx
- Hydrosalpinx
- Primary fallopian tube neoplasm
- Pelvic abscess
- Colorectal carcinoma
- Bladder carcinoma
Investigations
Biochemical
There are no specific blood tests to diagnose fibroids. Depending on symptoms, tests that may help in the patient workup include a full blood count, iron studies, thyroid function tests and measurement of follicular stimulating hormone, luteinising hormone, oestrogen and ß human chorionic gonadotropin levels. The usefulness of assessing levels of Ca-125 and other tumour markers is debatable in a routine workup. Elevation of Ca-125 levels with benign large fibroids has been observed and is likely to be due to peritoneal irritation or concurrent adenomyosis 24. Tumour markers may have a more accurate role in follow-up after treatment 24.
Ultrasonography
Ultrasonography is a non-invasive, highly tolerable diagnostic technique and is usually the modality of choice for detailed evaluation of the endometrium and myometrium. It provides information about the number, size and position of fibroids, and the uterine vasculature. Serial assessments can improve accuracy and positive predictive value in distinguishing benign from malignant uterine masses 25.
Sonohysterography is a test in which fluid is put into the uterus through the cervix. Ultrasonography is then used to show the inside of the uterus. The fluid provides a clear picture of the uterine lining.
Magnetic resonance imaging (MRI)
MRI has a prominent evolving role in assessing uteropelvic masses. MRI provides more accurate morphological soft-tissue detail when compared with computed tomography (CT), and has a useful pre-operative role in some cases, particularly in monitoring fibroid degeneration and identifying sarcomatous changes 26.
Computed tomography
CT is not believed to be as specific as other imaging modalities in differentiating between fibroids and malignancy 27 and is generally reserved for postoperative follow-up to assess the extent of metastatic disease 28.
Hysterosalpingography is a special X-ray test. It may detect abnormal changes in the size and shape of the uterus and fallopian tubes.
Hysteroscopy
Hysteroscopy uses a slender device (the hysteroscope) to see the inside of the uterus. It is inserted through the vagina and cervix (opening of the uterus). This permits the doctor to see fibroids inside the uterine cavity.
Laparoscopy
Laparoscopy uses a slender device (the laparoscope) to help the doctor see the inside of the abdomen. It is inserted through a small cut just below or through the navel. The doctor can see fibroids on the outside of the uterus with the laparoscope.
Uterine fibroids treatment
There are many treatment options for women who have fibroids. Fibroids that don’t cause any symptoms may not need treatment. For fibroids that do cause symptoms, treatment options include medicine, noninvasive or minimally invasive procedures, or traditional surgery. Your doctor will help you figure out the best treatment option for your fibroid.
Conservative
Asymptomatic women, or those with small or slow-growing fibroids, usually benefit from expectant management. Women with larger fibroids who decline medical treatment and have no significant complications may only require annual serial ultrasounds to monitor growth 29.
Medicines
Medicines don’t get rid of fibroids, but they can help control symptoms and make fibroids smaller.
Medical therapy to reduce heavy menstrual bleeding includes hormonal contraceptives, tranexamic acid, and nonsteroidal anti-inflammatory drugs. Gonadotropin-releasing hormone agonists or selective progesterone receptor modulators are an option for patients who need symptom relief preoperatively or who are approaching menopause.
Birth control pills and other types of hormonal birth control methods—These drugs often are used to control heavy bleeding and painful periods.
Tranexamic acid and non-steroidal anti-inflammatory drugs used alone are marginally effective in managing fibroid related menorrhagia 30.
Progestin–releasing intrauterine device— can help with abnormal uterine bleeding. It reduces heavy and painful bleeding but does not treat the fibroids themselves. Depending on fibroid size and position, associated cavity distortion may create difficulty with insertion and retention of the device.
GnRH agonists reduce oestrogen production and may reduce fibroid size and decrease vascularity. These effects are transitory and fibroids usually grow back to the pre-treatment size several months after treatment cessation. Additionally, they carry significant side effects, such as hot flushes, sleep disturbances, vaginal dryness and headaches. Long-term use (>6 months) can predispose to osteoporosis 29. GnRH analogues are currently recommended for temporary symptomatic relief and pre-operative fibroid size reduction.
Radiological
Uterine artery embolisation (UAE)
During uterine fibroid embolization, the doctor injects tiny particles into the arteries that supply blood to the fibroids. These particles stop the blood flow to the fibroid. Over time, the fibroid will shrink. The procedure works even if you have several fibroids. Uterine artery embolisation (UAE) is considered to be safe and minimally invasive, with demonstrable improvement in menstrual bleeding, pressure and urinary symptoms, as well as dysmenorrhea for most patients 31.
In comparison to myomectomy, uterine artery embolisation is associated with shorter procedural times and hospital stays, and faster resumption of usual activities 32. Patient satisfaction with fibroid symptom relief after uterine artery embolisation or hysterectomy are closely comparable 33. However, uterine artery embolisation is associated with higher rates of minor postoperative complications and increased likelihood of surgical re-intervention within two years 32. The overall failure rate is estimated to be around 32% within the first two years, compared with 7% of patients after hysterectomy or myomectomy 33. The higher re-intervention rate may balance out an initial cost advantage, for which patients should be carefully counselled 33.
Effects of uterine artery embolisation on premature ovarian failure, overall fertility and pregnancy outcomes are not well established 34. Women undergoing this treatment are believed to receive a radiation dose equivalent to approximately 10 times that of a pelvic CT scan 35. Patients should be aware of the risk that, although rare, complications of UAE may ultimately necessitate a life-saving hysterectomy 34.
MRI-guided focused ultrasound
This is a relatively new technique that has been trialled with promising effects. Ultrasonic energy is focused to generate heat at focal points in the fibroid to denature proteins and cause cell death, thus reducing fibroid size. MRI is used for precise tissue targeting and temperature monitoring 36. The procedure is associated with low morbidity and quick recovery. Published results have shown reductions in fibroid volume of up to 33% at six months after the procedure 37.
The disadvantages of MRI are similar to those of uterine artery embolisation (UAE), with a risk of requiring further surgical treatment. At this stage there is insufficient evidence for pregnancy outcomes after MRI-guided focused ultrasound, and the procedure should be recommended with caution for women who are planning to become pregnant 29.
Surgical
Myomectomy
During this procedure, your doctor surgically removes the fibroids from your uterus. The surgical tools are inserted in the body either through very small cuts in the abdomen, or through the vagina and cervix.
Myomectomy is a uterine-sparing procedure involving surgical removal of fibroids from the uterine wall. Some women may require this to improve their reproductive chances if there is a suggestion that the fibroid is causing recurrent miscarriages, fallopian tube compression or significant distortion of the uterine cavity.
Myomectomy may be performed via laparotomy, laparoscopy or hysteroscopy in the case of submucosal fibroids. The laparoscopic approach is associated with decreased peri-operative and postoperative morbidity and shorter hospital stays when performed by a skilled laparoscopic surgeon, particularly when compared with open myomectomy 38. However, this approach may have a higher recurrence rate of fibroids, compared with open myomectomy 39.
Submucosal fibroids can be removed hysteroscopically with a resectoscope or morcellator. This is usually a day procedure and is minimally invasive with reduced surgical trauma and positive outcomes. Most women avoid further surgery and experience improved heavy menstrual bleeding symptoms, sometimes without combined endometrial ablation 40. A recent Cochrane review suggested that hysteroscopic myomectomy may improve reproductive chances, but the evidence is not conclusive 41.
Hysterectomy
During a hysterectomy, your doctor removes the entire uterus from your body. A hysterectomy can be done through an abdominal incision (cut) or through a vaginal incision. Women with symptomatic fibroids who do not desire future fertility may be candidates for a hysterectomy. Complete removal of the uterus has the best outcome for symptom reduction, recurrence of fibroids and requirements for further surgery.
There are three main surgical approaches to a hysterectomy: vaginal, abdominal (laparotomy) and laparoscopic with or without the use of a surgical robot. A vaginal hysterectomy is the preferred option when possible. In the cases of large fibroids, this may not be technically achievable.
A laparoscopic hysterectomy has similar benefits to the vaginal approach in terms of reduced post-operative pain, cosmetic results, shorter hospitalisation and speedier return to work. It uses expensive equipment, but most costs are offset by savings in hospital stas and post-operative care when compared with an abdominal hysterectomy. Further evolution of technology may allow cheaper and more accessible options to become available.
Compared with total laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy, vaginal hysterectomy is associated with shorter operative time, less blood loss, shorter paralytic ileus time, and shorter hospitalization.
An estimated 15% to 33% of fibroids recur after myomectomy, and approximately 10% of women undergoing myomectomy will undergo a hysterectomy within five to 10 years.
Do uterine fibroids ever go away without treatment ?
In most cases, fibroids stop growing or they shrink without treatment. Once a woman goes through menopause, but this is not true for all women. Interestingly, each of a woman’s fibroids may grow or shrink at different times.
Some studies have suggested a link between uterine fibroids and the hormone replacement therapy (HRT) used to reduce the symptoms of menopause, but the nature of this relationship is still unclear 42. More research is needed on this topic.
Fibroids and pregnancy
If you have fibroids, you may still be able to get pregnant. Many women who have fibroids get pregnant naturally. Advances in treatments for fibroids and for infertility have greatly improved the chances for a woman to conceive. If you have fibroids and wish to become pregnant, it is wise to consult with a knowledgeable provider about the location of the fibroids and possible related problems with pregnancy or growth of a baby in the uterus.
However, some women with fibroids do have trouble getting pregnant. Current research suggests that submucosal and intramural fibroids—fibroids that change the shape and size of the uterine cavity—seem to affect a woman’s ability to get pregnant, even with in vitro fertilization 43. These fibroids may reduce fertility by as much as 70% 44. However, if the fibroid is treated, fertility may be restored.
Fibroids can also cause pregnancy complications, such as miscarriage, preterm delivery, abnormal position of the fetus, and the need for cesarean (C-section or surgical) delivery. Fibroids can also increase the risk of heavy bleeding after delivery 45.
Breast Fibroids
Breast fibroids or fibroadenomas are common benign lesions of the breast that usually present as a single breast mass in young women 46. They are assumed to be aberrations of normal breast development or the product of hyperplastic processes, rather than true cancer.
There are no clear-cut data on the incidence of breast fibroids in the general population. In one study, the rate of occurrence of breast fibroids in women who were examined in breast clinics was 7% to 13% 47, while it was 9% in another study of autopsies 48. Fibroadenomas comprise about 50% of all breast biopsies, and this rate rises to 75% for biopsies in women under the age of 20 years 49, 50.
Breast fibroids usually form during menarche (15 –25 years of age), a time at which lobular structures are added to the ductal system of the breast. Hyperplastic lobules are common at that time, and may be regarded as a normal phase of breast development 51. Hyperplastic lobules were shown to be histologically identical with fibroadenomas 52. Analyses of the cellular components of fibroadenomas by means of polymerase chain reaction demonstrated that both the stromal and the epithelial cells are polyclonal 53, supporting the theory that fibroadenomas are hyperplastic lesions associated with aberration of the normal maturation of the breast, rather than true neoplasms 54.
The pattern of stromal growth in a fibroadenoma depends on its epithelial component: stromal mitotic activity was found to be higher near this component 55. Fibroadenomas are stimulated by estrogen and progesterone, and by lactation during pregnancy, and they undergo atrophic changes in menopause 51. Some fibroadenomas have receptors and respond to growth hormone and epidermal growth factor 56.
Risk Factors for Breast Fibroids
Fibroadenomas are more frequent among women in higher socioeconomic classes 5–7 and in dark-skinned populations 57. The age of menarche, the age of menopause, and hormonal therapy, including oral contraceptives, were shown not to alter the risk of these lesions 58. Conversely, body mass index and the number of full-term pregnancies were found to have a negative correlation with the risk of fibroadenomas 59. Moreover, consumption of large quantities of vitamin C and cigarette smoking were found to be associated with reduced risk of a fibroadenoma 60.
No genetics factors are known to alter the risk of fibroadenoma. However, a family history of breast cancer in first-degree relatives was reported by some investigators to be related with increased risk of developing these tumors 61.
Symptoms and Signs of Breast Fibroids
A fibroadenoma is most often detected incidentally during a medical examination or during self examination, usually as a discrete solitary breast mass of 1 to 2 cm 61. Although they can be located anywhere in the breast, the majority are situated in the upper outer quadrant 62. A fibroadenoma is usually smooth, mobile, nontender, and rubbery in consistency. Several other breast lesions have similar characteristics, and physical examinations provided an accurate diagnosis in only one half to two thirds of cases studied. 63 However, most of the masses that are erroneously diagnosed by palpation as fibroadenomas are found on histologic examination to be another benign form of breast disease, such as cystic fibrosis 64.
Multiple Fibroadenomas
From 10% to 16% of patients with multiple fibroadenomas have two to four in a single breast, which may present initially or be discovered over several years 61. Unlike women with a single fibroadenoma, most of the patients with multiple fibroadenomas have a strong family history of these tumors 65. A possible connection between multiple fibroadenomas and oral contraceptives was proposed but has not yet been substantiated 66.
Giant and Juvenile Fibroadenomas
Fibroadenomas larger than 5 cm (about 4% of the total) are commonly defined as being giant fibroadenomas 56; however, this terminology is not universally accepted 61. Giant fibroadenomas are usually encountered in pregnant or lactating women. When found in an adolescent girl, the term juvenile fibroadenoma is more appropriate. These lesions in young women constitute 0.5% to 2% of all fibroadenomas, and are rapidly growing masses that cause asymmetry of the breast, distortion of the overlying skin, and stretching of the nipple. Histologically, they appear to be more cellular and have less lobular components than do simple fibroadenomas. However, giant fibroadenomas are benign lesions that do not undergo transformation into malignancy 67.
How is Breast Fibroids Diagnosed ?
Ultrasonography
Breast sonography is often used for the diagnosis of fibroadenomas. The sonographic criteria that support the diagnosis of a fibroadenoma are a round or oval solid mass with a smooth contour and weak internal echoes in a uniform distribution and intermediate acoustic attenuation 68. This imaging technique is very useful for differentiating between solid and cystic lesions. However, attempts to correlate between the sonographic features of solid masses compatible with fibroadenomas and pathologic findings were disappointing 69. There is some overlap in the sonographic criteria for fibroadenomas and for breast cancer 70 and approximately 25% of fibroadenomas appear with irregular margins, which may imply that the lesions are malignant 68. Also, only 82% of biopsy-proven fibroadenomas were visualized by sonography in one study 68.
Mammography
The yield of mammography in young women is low, and its role in the diagnosis of fibroadenomas is limited. However, it may disclose features of infiltrative lesions in older women. In the mammographic image, fibroadenomas appear as soft, homogenous, and well-circumscribed nodules, and inner coarse calcifications are often observed.
Fine needle aspiration cytology
Fine needle aspiration (FNA) has become a popular method in the evaluation of breast masses. The characteristic cytologic features of fibroadenomas are: clusters of spindle cells without inflammatory or fat cells, found in 96% of all fibroadenomas; aggregates of cells with a papillary configuration resembling elk antler (antler horn clusters), found in 93% of all cases; and uniform cells with well-defined cytoplasm lying in rows and columns (honeycomb sheets), found in 95% of all fibroadenomas 71. Taken together with the clinical diagnosis of fibroadenoma, FNA can improve the sensitivity of the diagnosis to 86% with a specificity of 76% 69 while for breast cancer FNA is 96% sensitive and 98% specific. Thus, while aspiration cytology may confuse fibroadenomas with other benign breast lesions, incorrect diagnosis of a malignant process is rare.
The overall diagnostic efficacy of these three modalities—namely, manual breast examination, imaging and cytology is approximately 70% to 80%, but they provide a 95% accurate differentiation between a benign and a malignant lesion. A follow-up period of 1 to 3 years after fibroadenoma is diagnosed and breast cancer is excluded using the three modalities can enhance the accuracy of the diagnosis 72.
Breast Fibroids and Breast Cancer
Any analysis of the associations of fibroadenomas with breast cancer must address two main questions: whether or not a fibroadenoma is a marker for increased risk of breast cancer, and whether or not breast cancer can evolve from the epithelial component of a fibroadenoma. The first issue was originally assessed in several retrospective studies, which demonstrated a 1.3 to 2.1 increased risk of breast cancer in women with fibroadenomas compared with the general population 73. The elevated risk was persistent, and did not decrease with time. A more recent study designed to delineate the possible correlation between the histologic features of the fibroadenomas and the risk for subsequent breast cancer used the term “complex fibroadenoma” 61. This term applies to fibroadenomas having the histologic characteristic of being more than 3 mm in diameter, or with elements of sclerosing adenosis, epithelial calcifications, or papillary apocrine metaplasia, which were associated with a 3.1 elevated risk of breast cancer. Proliferative changes in the parenchyma adjacent to the fibroadenoma were related to a further increase of the risk to 3.88. The relative risk for women with a familial history of breast cancer and complex fibroadenoma was 3.72, compared with control women with a family history of breast cancer without fibroadenoma. In these studies, women with noncomplex fibroadenomas and no family history of breast cancer were not at a greater risk of breast cancer.
Malignant transformations in the epithelial components of fibroadenomas are generally considered rare. The incidence of a carcinoma evolving within a fibroadenoma was reported to be 0.002% to 0.0125% 74. About 50% of these tumors were lobular carcinoma in situ (LCIS), 20% were infiltrating lobular carcinoma, 20% were ductal carcinoma in situ (DCIS), and the remaining 10% were infiltrating ductal carcinoma. The clinical, sonographic and mammographic findings are usually similar to those of benign fibroadenomas 75 and the malignant changes are often noted only when the fibroadenoma is excised.
In a clinicopathologic study of 105 women with carcinoma developing within fibroadenomas, the mean age was higher than in patients with benign fibroadenomas (44 vs 23 years) 76. However, in that study, DCIS and LCIS in equal frequencies comprised 95% of the cases, and carcinoma in situ was also present in the adjacent breast tissue in about 20% of these women. No axillary metastases were found in any of the study patients.
Breast Fibroids Natural History
There are inherent obstacles in studying the natural course of breast fibroadenomas, and the data are not unequivocal. Some investigators believe that most fibroadenomas grow over a 12-month period to gain a size of 2 to 3 cm, after which they remain unchanged for several years 61. As definite diagnosis can be obtained only from histologic sections, solitary solid masses usually have been excised, and long term follow-up surveys are limited in number. These studies followed young women for up to 29 years, and regression or complete resolution of the fibroadenomas were noted in 16% to 59% of all cases 77. It was extrapolated that the probability that a fibroadenoma would resolve after 5 years is approximately 50%, and the “lifetime” of a fibroadenoma is about 15 years 78 Among the 50% of fibroadenomas that did not regress spontaneously, about half did not change, and the remaining 25% enlarged in size during the follow-up 56.
From their incidence in mastectomy specimens, it has been assumed that fibroadenomas tend to regress and loss their cellularity with age. The rare finding of fibroadenomas in the older age groups also supports the hypothesis of regression of fibroadenomas 79. The mechanisms offered to explain the regression of fibroadenomas are infarction, calcification, and hyalinization 80.
How are Fibroids of Breast Treated ?
As fibroadenomas are benign breast lesions, it could be argued that they should not be excised and can be expected to regress spontaneously. Moreover, 30% of breast tumors that are diagnosed as fibroadenomas are found postsurgically to be other types of benign lesions. In Cant et al.’s follow-up studies on clinically diagnosed fibroadenomas, persistent lesions were excised after 3 years: fibroadenomas were found in the histologic examinations of 97% of these cases 72. These findings suggest that the other benign lesions had resolved spontaneously during 1 to 3 years, that the remaining masses were true fibroadenomas, and that conservative management is warranted. Not all women can be candidates for conservative treatment: the patient’s age, a family history of malignancy, and any data on proliferative changes in the breasts from previous biopsies must be taken into consideration.
The risk of missing breast cancer in women under 25 years of age who have fibroadenomas as diagnosed by physical examination, sonography, and FNA is 1 in 229 to 1 in 700 81. This risk remains very low in women under the age of 35 years. Therefore, it has been recommended that young patients should be observed with frequent clinical evaluations, and the lesions excised in women over the age of 35 years 69. Other investigators suggested that the cutoff age should be 25 years 82.
The preferred management of multiple fibroadenomas is complete excision. However, this approach can lead to undesirable scarring or to extensive ductal damage if all the fibroadenomas are excised through one incision 83. Giant fibroadenomas tend to shrink after cessation of lactation, so their removal should be delayed until the patient’s hormonal status returns to normal, and a smaller excision can be performed 56. It may be very disfiguring to excise juvenile fibroadenomas because of their large sizes; nevertheless, no recurrences were reported after complete excision, and normal and symmetrical development of the breasts can be anticipated 84.
References
- Uterine Fibroids: Diagnosis and Treatment. Am Fam Physician. 2017 Jan 15;95(2):100-107. http://www.aafp.org/afp/2017/0115/p100.html
- Agency for Healthcare Research and Quality (AHRQ). (2007). Management of uterine fibroids: https://archive.ahrq.gov/downloads/pub/evidence/pdf/uterupdate/uterup.pdf
- Peddada, S. D., Laughlin, S. K., Miner, K., Guyon, J.-P., Haneke, K., Vahdat, H. L., et al. (2008). Growth of uterine leiomyomata among premenopausal black and white women. Proceedings of the National Academy of Sciences of the United States of America, 105, 19887–19892. http://www.pnas.org/content/105/50/19887.long
- Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 2007;87(4):725–36.
- Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: A systematic review. J Minim Invasive Gynecol 2015;22(1):26–33.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206(3):211.e1–9.
- Soliman AM, Yang H, Du EX, Kelkar SS, Winkel C. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol 2015;213(2):141–60.
- Visvanathan D, Bown S, Cutner A. Review of the conservative surgical treatment of uterine fibroids. Rev Gynaecol Pract 2004;4(1):20–26. http://discovery.ucl.ac.uk/62598/
- Levy, B., Mukherjee, T., & Hirschhorn, K. (2000). Molecular cytogenetic analysis of uterine leiomyoma and leiomyosarcoma by comparative genomic hybridization. Cancer Genetics and Cytogenetics 121(1), 18.
- Berger, L. (2008, October 23). A Decade of Developments in Fibroid Research. New York Times. http://www.nytimes.com/ref/health/healthguide/esn-fibroids-expert.html
- D ’Angelo E, Prat J. Uterine sarcomas: A review. Gynecol Oncol 2010;116(1):131–39.
- Pérez López FR, Ornat L, Ceausu I, et al. EMAS position statement: Management of uterine fibroids. Maturitas 2014;79(1):106–16.
- del Carmen MG. Uterine Leiomyosarcoma. In: Uncommon gynecologic cancers. Chichester, UK: John Wiley & Sons, 2014; p. 167–77. doi:10.1002/9781118655344.ch15.
- Samuel A, Fennessy FM, Tempany CMC, Stewart EA. Avoiding treatment of leiomyosarcomas: The role of magnetic resonance in focused ultrasound surgery. Fertil Steril 2008;90(3):850.e9–12.
- Wallis L. FDA warns against power morcellation for hysterectomy and fibroids. Am J Nurs 2014;114(7):16.
- Lieng M, Berner E, Busund B. Risk of morcellation of uterine leiomyosarcomas in laparoscopic supracervical hysterectomy and laparoscopic myomectomy: A retrospective trial including 4791 women. J Minim Invasive Gynecol 2015;22(3):410–14.
- Lethaby, A., & Vollenhoven, B. (2007). Fibroids (uterine myomatosis, leiomyomas). Clinical Evidence 5. http://www.clinicalevidence.com/x/systematic-review/0814/overview.html
- American Congress of Obstetricians and Gynecologists. (2009). Uterine fibroids. https://www.acog.org/~/media/For%20Patients/faq074.pdf?dmc=1&ts=20120430T1057019423
- Martin, C.L., Huber, L.R., Thompson, M.E., & Racine, E.F. (2011). Serum micronutrient concentrations and risk of uterine fibroids. Journal of Women’s Health, 20, 915-922. http://online.liebertpub.com/doi/abs/10.1089/jwh.2009.1782
- Eunice Kennedy Shriver National Institute of Child Health and Human Development. (2012). Vitamin D shrinks fibroid tumors in rats. https://www.nichd.nih.gov/news/releases/Pages/030112-vitaminD-fibroids.aspx
- Baird, D., & Wise, L. (2011). Childhood abuse and fibroids. Epidemiology, 22, 15-17. http://journals.lww.com/epidem/Citation/2011/01000/Childhood_Abuse_and_Fibroids.3.aspx
- Vines, A.I., Ta, M., & Esserman, D.A. (2010). The association between self-reported major life events and the presence of uterine fibroids. Women’s Health Issues, 20, 294-298. http://www.whijournal.com/article/S1049-3867(10)00041-1/fulltext
- Uterine Fibroids. Medline Plus. https://medlineplus.gov/uterinefibroids.html
- Babacan A, Kizilaslan C, Gun I, Muhcu M, Mungen E, Atay V. CA 125 and other tumor markers in uterine leiomyomas and their association with lesion characteristics. International Journal of Clinical and Experimental Medicine. 2014;7(4):1078-1083. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4057864/
- Kurjak A, Kupesic S, Shalan H, Jukic S, Kosuta D, Ilijas M. Uterine sarcoma: A report of 10 cases studied by transvaginal color and pulsed Doppler sonography. Gynecol Oncol 1995;59(3):342–46.
- Green D, Johnson IR. Magnetic resonance imaging for gynaecological masses. Rev Gynaecol Pract 2004;4(2):133–40.
- Rha SE, Byun JY, Jung SE, et al. CT and MRI of uterine sarcomas and their mimickers. Am J Roentgenol 2003;181(5):1369–74.
- Bansal N, Herzog TJ, Brunner Brown A, et al. The utility and cost effectiveness of preoperative computed tomography for patients with uterine malignancies. Gynecol Oncol 2008;111(2):208−12.
- Parker WH. Uterine myomas: Management. Fertil Steril 2007;88(2):255–71.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma induced menorrhagia. Obstet Gynecol 1986;68(1):10–12.
- Worthington Kirsch RL, Popky GL, Hutchins FLJ. Uterine arterial embolization for the management of leiomyomas: Quality-of-life assessment and clinical response. Radiology 1998;208(3):625–29.
- Dutton S, Hirst A, McPherson K, Nicholson T, Maresh M. A UK multicentre retrospective cohort study comparing hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids (HOPEFUL study): Main results on medium-term safety and efficacy. BJOG 2007;114(11):1340–51.
- Gupta J, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2012;(5):CD005073. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005073.pub2/pdf/standard
- van der Kooij SM, Hehenkamp WJK, Volkers NA, Birnie E, Ankum WM, Reekers JA. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 5 year outcome from the randomized EMMY trial. Am J Obstet Gynecol 2010;203(2):105.e1–13.
- Visvanathan D, Bown S, Cutner A. Review of the conservative surgical treatment of uterine fibroids. Rev Gynaecol Pract 2004;4(1):20–26.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Radiol 2013;36(1):5–13.
- Morita Y, Ito N, Hikida H, Takeuchi S, Nakamura K, Ohashi H. Non-invasive magnetic resonance imaging guided focused ultrasound treatment for uterine fibroids – Early experience. Eur J Obstet Gynecol Reprod Biol 2008;139(2):199−203.
- Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev 2014;10:CD004638.
- Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomectomies with 49 open myomectomies. J Am Assoc Gynecol Laparosc 1997;4(4):457–64.
- Emanuel MH. Hysteroscopy and the treatment of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2015;29(7):920–29.
- Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev 2001;2:CD000547
- Berger, L. (2008, October 23). A Decade of Developments in Fibroid Research. New York Times. http://www.nytimes.com/ref/health/healthguide/esn-fibroids-expert.html
- Evans, P., & Brunsell, S. (2007). Uterine fibroid tumors: Diagnosis and treatment. American Family Physician, 75(10), 1503-1508.
- Berger, L. (2008, October 23). A Decade of Developments in Fibroid Research. New York Times.
- Uterine fibroids. Medline Plus. https://medlineplus.gov/ency/article/000914.htm
- Greenberg R, Skornick Y, Kaplan O. Management of Breast Fibroadenomas. Journal of General Internal Medicine. 1998;13(9):640-645. doi:10.1046/j.1525-1497.1998.cr188.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1497021/
- Dent DM, Hacking EA, Wilkie W. Benign breast disease clinical classification and disease distribution. Br J Clin Pract. 1988;42(suppl 56):69–71.
- Franyz VK, Pickern JW, Melcher GW, Auchincoloss JR. Incidence of chronic cystic disease in so-called normal breast: a study based on 225 post mortem examinations. Cancer. 1951;4:762–7. https://www.ncbi.nlm.nih.gov/pubmed/14859197
- Schuerch C, Rosen PP, Hirota T, Itabashi M. A pathologic study of benign breast disease in Tokyo and New York. Cancer. 1982;50:1899–902. https://www.ncbi.nlm.nih.gov/pubmed/7116314
- Onuigb WIB. Adolescent mass in Nigerian igbos. Am J Surg. 1979;137:367–71. https://www.ncbi.nlm.nih.gov/pubmed/434332
- Hughes LE, Mansel RE, Webster DJT. Aberration of normal development and involution: a new perspective on pathogenesis and nomenclature of benign breast disorders. Lancet. 1987;11:1316–9. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(87)91204-9/abstract
- Parks AG. The Micro-Anatomy of the Breast: Hunterian Lecture delivered at the Royal College of Surgeons of England on 12th March 1959. Annals of The Royal College of Surgeons of England. 1959;25(4):235-251. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2413909/pdf/annrcse00354-0029.pdf
- Noquchi S, Motomura K, Inaji H, Imorka S. Clonal analysis of fibroadenoma and phylloides tumor of the breast. Cancer Res. 1993;53(17):4071–4. http://cancerres.aacrjournals.org/content/53/17/4071.long
- Sawhney N, Garrahan N, Douglas Jones AG, Williams ED. Epithelial stromal interaction in tumors: a morphologic study of fibroepithelial tumors of the breast. Cancer. 1992;70(8):2115–20. https://www.ncbi.nlm.nih.gov/pubmed/1327488
- Van Agthoven T, Timmermans M, Foekens JA, Dorssers LC, Henzen-Logmans SC. Differential expression of estrogen, progesterone, and epidermal growth factor receptors in normal, benign, and malignant human breast tissues using dual staining immunohistochemistry. The American Journal of Pathology. 1994;144(6):1238-1246. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1887470/
- Dent DM, Cant PJ. Fibroadenoma. World J Surg. 1989;13:706–10. https://www.ncbi.nlm.nih.gov/pubmed/2696223
- Yu H, Rohan TE, Cook MG, Howe GR. Risk factor for fibroadenoma: a case control study in Australia. Am J Epidemiol. 1992;135:247–58. https://www.ncbi.nlm.nih.gov/pubmed/1546700
- Ravnihar B, Segel DG, Lindther J. An epidemiologic study of breast cancer and benign breast neoplasm in relation to the oral contraceptive and estrogen use. Eur J Cancer. 1979;15:395–405. https://www.ncbi.nlm.nih.gov/pubmed/436904
- Canny PF, Berkowitz GS, Kelsey JL. Fibroadenoma and the use of exogenous hormones: a case control study. Am J Epidemiol. 1988;127:454–61. https://www.ncbi.nlm.nih.gov/pubmed/3341352
- Parazzini F, La Vecchia C, Franceshi S. Risk factors for pathologically confirmed benign breast disease. Am J Epidemiol. 1984;120:115–22. https://www.ncbi.nlm.nih.gov/pubmed/6741913
- Haagensen CD. Disease of the breast. 3rd ed. Philadelphia, Pa: W.B. Saunders; 1996. 267–83.
- Foster ME, Garrahan N, Williams S. Fibroadenoma of the breast: a clinical and pathological study. J R Coll Surg Edinb. 1988;33:13–6. https://www.ncbi.nlm.nih.gov/pubmed/3418570
- Wilkinson S, Anderson TJ, Rifkind E, Chetty U. Fibroadenoma of the breast: a follow up of conservative management. Br J Surg. 1989;76:390–1. https://www.ncbi.nlm.nih.gov/pubmed/2720350
- Carty NJ, Carter C, Rubin C, Ravichandran D, Royle GT, Taylor I. Management of fibroadenoma of the breast. Annals of The Royal College of Surgeons of England. 1995;77(2):127-130. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2502143/pdf/annrcse01594-0057.pdf
- Williamson ME, Lyons K, Hughes LE. Multiple fibroadenomas of the breast: a problem of uncertain incidence and management. Annals of The Royal College of Surgeons of England. 1993;75(3):161-163. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2497873/pdf/annrcse01581-0027.pdf
- Gregg WI. Gallactorrhea after contraceptive hormones. N Engl J Med. 1966;274:1432–4. http://www.nejm.org/doi/full/10.1056/NEJM196606232742510
- Pike AM, Oberman HA. Juvenile cellular adenofibromas. Am J Surg Pathol. 1985;9:730–5. https://www.ncbi.nlm.nih.gov/pubmed/2998214
- Cale Beuglet C, Soriano RZ, Kurtz AB, Goldberg BB. Fibroadenoma of the breast sonomammography correlated with pathology in 122 patients. AJR. 1983;140:369–75. https://www.ncbi.nlm.nih.gov/pubmed/6600356
- Wilkinson S, Forrest APN. Fibroadenoma of the breast. Br J Surg. 1985;72:835–8.
- Jackson VP, Rothschild PA, Kreipke DL, Mal TJ. The spectrum of sonographic finding of fibroadenoma of the breast. Invest Radiol. 1986;21:34–9. https://www.ncbi.nlm.nih.gov/pubmed/3511000
- Bottels K, Chan JS, Holly EA. Cytologic criteria for fibroadenoma: a step wise logistic regression analysis. Am J Clin Pathol. 1988;89:707–13. https://www.ncbi.nlm.nih.gov/pubmed/2835896
- Cant PJ, Madden MV, Coleman MG, Dent DM. Nonoperative management of breast mass diagnosed as fibroadenoma. Br J Surg. 1995;82:792–4. https://www.ncbi.nlm.nih.gov/pubmed/7627513
- Krieger N, Hiatt RA. Risk of breast cancer after benign breast diseases: variation by histologic type, degree of atypia, age at biopsy, and length of follow up. Am J Epidemiol. 1992;135:619–31. https://www.ncbi.nlm.nih.gov/pubmed/1580238
- Deschenes L, Jacob S, Fobia J, Christen A. Beware of breast fibroadenoma in middle aged women. Can J Surg. 1985;28:372–3. https://www.ncbi.nlm.nih.gov/pubmed/2990650
- Oyyello L, Gump PE. The management of patients with carcinomas in fibroadenomatous tumors of the breast. Surg Gynecol Obstet. 1985;160:99–104. https://www.ncbi.nlm.nih.gov/pubmed/2982218
- Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenoma of the breast: a clinicopathological study of 105 patients. Am J Clin Pathol. 1991;95:614–22. https://www.ncbi.nlm.nih.gov/pubmed/1850948
- Smallwood JA, Roberts A, Guyer DP, Taylor I. The natural history of fibroadenoma. Br J Clin Pract. 1988;56(suppl):86–7.
- Hutchinson WB, Thomas DB, Hamlin WB, Roth GJ. Risk of breast cancer in women with benign breast disease. J Natl Cancer Inst. 1980;65:13–20. https://www.ncbi.nlm.nih.gov/pubmed/6930509
- Sainsbury JRL, Nicholson S, Needham GK, Wadehra V. Natural history of benign breast lumps. Br J Surg. 1988;75:1080–2. https://www.ncbi.nlm.nih.gov/pubmed/3208039
- Kerm WH, Clark RW. Retrogression of fibroadenoma of the breast. Am J Surg. 1973;126:59–62. https://www.ncbi.nlm.nih.gov/pubmed/4123521
- Cant PJ, Learmonth GM, Dent DM. When can fibroadenoma be managed conservatively. Br J Clin Pract. 1988;42(suppl 56):62–6.
- Cant PJ, Madden MV, Close PM, Learmonth GM. Case for conservative management of selected fibroadenoma of the breast. Br J Surg. 1987;74:857–9. https://www.ncbi.nlm.nih.gov/pubmed/3664257
- Naraynsingh V, Raju GC. Familial bilateral fibroadenoma of the breast. Postgrad Med J. 1985;64:430–40.
- Mies C, Rosen PP. Juvenile fibroadenoma with atypical epithelium. Am J Surg Pathol. 1987;11:184–7. https://www.ncbi.nlm.nih.gov/pubmed/3826478