Ventricular septal defect
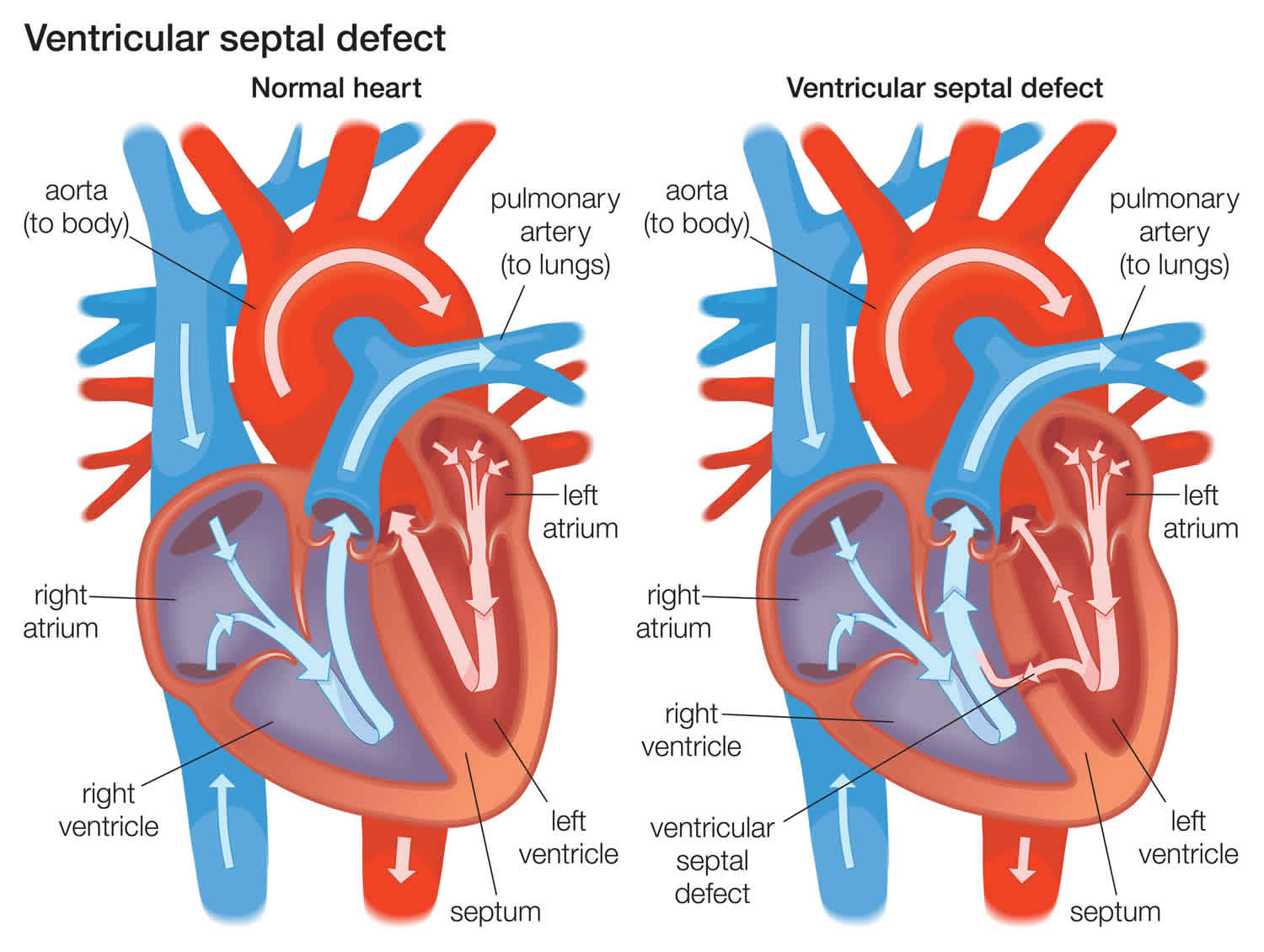
Ventricular septal defect or VSD, is a hole in the muscle wall that separates the right and left ventricles of the heart. Ventricular septal defect is one of the most common congenital (present from birth) heart defects in children and is the second most common congenital abnormality in adults, second only to a bicuspid aortic valve 1. Ventricular septal defect occurs in nearly half of all children with congenital heart disease. It may occur by itself or with other congenital diseases.
In a study in Atlanta, the Centers for Disease Control and Prevention (CDC) estimated that 42 of every 10,000 babies born had a ventricular septal defect 2. This means about 16,800 babies are born each year in the United States with a ventricular septal defect. In other words, about 1 in every 240 babies born in the United States each year are born with a ventricular septal defect.
Normally, the left ventricle pumps oxygen-rich blood into the aorta, which carries blood away from the heart and lungs to the rest of the body. But with ventricular septal defect, some of the blood gets pushed through the hole into the right ventricle instead of flowing normally to the rest of the body. A ventricular septal defect allows oxygenated blood to mix with deoxygenated blood, causing the heart to work harder to provide enough oxygen to the body’s tissues. Furthermore, from the right ventricle, the blood goes through the pulmonary artery to the lungs. The blood vessels in the lungs may become damaged by the high volume of blood being pumped into them by the right ventricle.
The left and right ventricles can also become overworked. In an attempt to supply the body with enough blood, the left ventricle may pump harder and faster than normal. This extra work can cause the heart to become enlarged.
Ventricular septal defects may be various sizes, and they can be present in several locations in the wall between the ventricles. There may be one or more ventricular septal defect.
Many children with ventricular septal defect do not need surgery. The hole may close naturally during the first 7 years of life, or it may be too small to cause problems.
But if the hole is large, surgery may be needed. Pulmonary artery banding is one type of surgery used to temporarily fix ventricular septal defects. It uses a band to narrow the pulmonary artery, which reduces blood flow and pressure to the lungs. Later, when the child is older, the band can be removed and the defect can be fixed with open heart surgery.
If the hole needs to be repaired, the edges of the hole may be stitched together, or it may be covered with a patch.
See your doctor if your baby or child:
- Tires easily when eating or playing
- Is not gaining weight
- Becomes breathless when eating or crying
- Breathes rapidly or is short of breath
See your doctor if you develop:
- Shortness of breath when you exert yourself or when you lie down
- Rapid or irregular heartbeat
- Fatigue or weakness
VSD types
Different anatomic locations and histologic variation of ventricular septal defects has led to several classifications and nomenclature systems. The interventricular septum is an asymmetric curved structure due to the pressure difference in ventricular chambers. It is composed of five parts: the membranous, muscular (frequently referred to as trabecular), infundibular, atrioventricular and the inlet 3. Complexities in describing ventricular septal defects and multiple synonyms have been improved after a new unified classification was established and categorized ventricular septal defects into four major groups:
- Type 1 Infundibular ventricular septal defect or conoventricular ventricular septal defect: This ventricular septal defect is located below the semilunar valves (aortic and pulmonary) in the outlet septum of the right ventricle above the crista supraventricularis, that is why sometimes also referred to as supracristal. It is the most uncommon type representing only 6% of all ventricular septal defects with the exception being in the Asian population where it accounts for approximately 30%. Aortic valve prolapse and regurgitation are common because of loss of support of the right and/or the noncoronary cusps of the aortic valve. It is unusual for these defects to close spontaneously.
- Type 2 Membranous ventricular septal defect: This ventricular septal defect is, by far the most common type, accounting for 80% of all defects. It is located in the membranous septum inferior to the crista supraventricularis. It often involves the muscular septum when it is commonly known as perimembranous. The septal leaflet of the tricuspid valve sometimes forms a “pouch” that reduces the shunt and can result in spontaneous closure.
- Type 3 Inlet ventricular septal defect or atrioventricular septal defect: This ventricular septal defect is located just inferior to the inlet valves (tricuspid and mitral) within the inlet part of the right ventricular septum. It only represents 8% of all defects. It is seen in patients with Down syndrome.
- Type 4 Muscular ventricular septal defect: This ventricular septal defect is located in the muscular septum, bordered by muscle usually in the apical, central and outlet parts of the interventricular septum. They can be multiple, assuming a “Swiss cheese” appearance. They represent up to 20% of ventricular septal defects in infants. However, the incidence is lower in adults due to the tendency of spontaneous closure.
While ventricular septal defects are classified according to location, they can also be classified into size. The size is described in comparison to the diameter of the aortic annulus. They are considered small if they measure less or equal to 25% of the aortic annulus diameter, medium if they measure more than 25% but less than 75%, and large if they are greater than 75% of the aortic annulus diameter.
Ventricular septal defect causes
The causes of ventricular septal defect among most babies are unknown. Ventricular septal defect develops when there is a developmental abnormality or an interruption of the interventricular septum formation during the complex embryologic heart morphogenesis. Ventricular septal defects are frequently isolated; however, they can occur in association with other congenital heart defects such as atrial septal defects (ASD), patent ductus arteriosus (PDA), right aortic arch and pulmonic stenosis. Ventricular septal defects are also found in cases of aortic coarctation and sub-aortic stenosis, and they are a frequent component of complex congenital heart disease such as Tetralogy of Fallot and transposition of great arteries. Several genetic factors have been identified to cause ventricular septal defect including chromosomal, a single gene and polygenic inheritance. A TBX5 mutation was recently discovered to cause septal defects in patients with Holt-Oram Syndrome. Non-inherited risk factors have been implicated in the development of ventricular septal defects; these include maternal infection (rubella, influenza, and febrile illness), maternal diabetes mellitus and phenylketonuria. Exposure to toxins like alcohol, marijuana, cocaine and certain medications such as metronidazole and ibuprofen is also linked to ventricular septal defects 4.
Ventricular septal defects may run in families and sometimes may occur with other genetic problems, such as Down syndrome. If you already have a child with a heart defect, a genetic counselor can discuss the risk of your next child having one.
It’s also possible to acquire a ventricular septal defect later in life, usually after a heart attack or as a complication following certain heart procedures.
Isolated ventricular septal defect accounts for 37% of all congenital heart disease in children. The incidence of isolated ventricular septal defect is about 0.3% of newborns. Because as many as 90% may eventually close spontaneously; the incidence is significantly lower in adults. Ventricular septal defects have no gender predilection.
Ventricular septal defect prevention
In most cases, you can’t do anything to prevent having a baby with a ventricular septal defect. However, it’s important to do everything possible to have a healthy pregnancy. Here are the basics:
- Get early prenatal care, even before you’re pregnant. Talk to your doctor before you get pregnant about your health and discuss any lifestyle changes that your doctor may recommend for a healthy pregnancy. Also, be sure you talk to your doctor about any medications you’re taking.
- Eat a balanced diet. Include a vitamin supplement that contains folic acid. Also, limit caffeine.
- Exercise regularly. Work with your doctor to develop an exercise plan that’s right for you.
- Avoid risks. These include harmful substances such as alcohol, tobacco and illegal drugs.
- Avoid infections. Be sure you’re up to date on all of your vaccinations before becoming pregnant. Certain types of infections can be harmful to a developing fetus.
- Keep diabetes under control. If you have diabetes, work with your doctor to be sure it’s well-controlled before getting pregnant.
If you have a family history of heart defects or other genetic disorders, consider talking with a genetic counselor before getting pregnant.
Ventricular septal defect symptoms
Symptoms of ventricular septal defect may not appear until days or weeks after birth. You and your doctor may not notice signs of a ventricular septal defect at birth. If the defect is small, symptoms may not appear until later in childhood — if at all. Signs and symptoms vary depending on the size of the hole and other associated heart defects. However, if the hole is large, the baby often has symptoms related to heart failure.
The most common symptoms of ventricular septal defect include:
- Shortness of breath
- Pale skin
- Fast breathing
- Rapid heart rate (pulse)
- Hard breathing
- Failure to gain weight
- Fast heart rate
- Sweating while feeding
- Frequent respiratory infections
- Slow growth
Your doctor may first suspect a heart defect during a regular checkup if he or she hears a murmur while listening to your baby’s heart with a stethoscope. Sometimes ventricular septal defects can be detected by ultrasound before the baby is born.
Sometimes a ventricular septal defect isn’t detected until a person reaches adulthood. Symptoms and signs can include shortness of breath or a heart murmur your doctor hears when listening to your heart with a stethoscope.
Ventricular septal defect complications
A small ventricular septal defect may never cause any problems. Medium or large defects can cause a range of disabilities — from mild to life-threatening. Treatment can prevent many complications.
- Heart failure. In a heart with a medium or large VSD, the heart needs to work harder to pump enough blood to the body. Because of this, heart failure can develop if medium to large ventricular septal defects aren’t treated.
- Pulmonary hypertension. Increased blood flow to the lungs due to the ventricular septal defect causes high blood pressure in the lung arteries (pulmonary hypertension), which can permanently damage them. This complication can cause reversal of blood flow through the hole (Eisenmenger syndrome).
- Endocarditis. This heart infection is an uncommon complication.
- Other heart problems. These include abnormal heart rhythms and valve problems.
Ventricular septal defect diagnosis
Ventricular septal defects often cause a heart murmur that your doctor can hear using a stethoscope. If your doctor hears a heart murmur or finds other signs or symptoms of a heart defect, he or she may order several tests including:
- Echocardiogram. In this test, sound waves produce a video image of the heart. Doctors may use this test to diagnose a ventricular septal defect and determine its size, location and severity. It may also be used to see if there are any other heart problems. Echocardiography can be used on a fetus (fetal echocardiography).
- Colored Doppler transthoracic echocardiography is the most valuable tool for diagnosis due to its high sensitivity. Colored Doppler transthoracic echocardiography can detect up to 95% of VSDs, especially non-apical lesions larger than 5 mm; it provides morphologic information such as size, location, and the number of the defects as well as hemodynamic information such as jet size, severity, and estimation of pulmonary artery pressure. transthoracic echocardiography is useful in detecting any associated aortic insufficiency and other associated congenital heart defects. Lastly, transthoracic echocardiography is also helpful in evaluating the right and left ventricular chamber size and function. Limitations include operator dependent and poor acoustic window. When conventional transthoracic echocardiography is equivocal, a trans-esophageal echo is recommended 5.
- Electrocardiogram (ECG). This test records the electrical activity of the heart through electrodes attached to the skin and helps diagnose heart defects or rhythm problems. Electrocardiography is entirely normal in half of the patients with VSD. When the ECG is abnormal, it may detect left ventricle hypertrophy in those with large shunts. In patients with pulmonary arterial hypertension, the ECG may show right bundle branch block, right axis deviation, and right ventricle hypertrophy and strain.
- Chest X-ray (CXR). An X-ray image helps the doctor view the heart and lungs. This can help doctors see if the heart is enlarged and if the lungs have extra fluid. Chest radiography is often normal in those with small defects. Enlarged cardiac silhouette can be observed in those with larger defects and increased left ventricle size. Right ventricle enlargement and increased pulmonary diameter can be observed in those with pulmonary arterial hypertension.
- Cardiac catheterization. In this test, a thin, flexible tube (catheter) is inserted into a blood vessel at the groin or arm and guided through the blood vessels into the heart. Through cardiac catheterization, doctors can diagnose congenital heart defects and determine the function of the heart valves and chambers. Cardiac catheterization gives accurate hemodynamic information regarding the pulmonary vascular resistance and response to vasodilators; this is particularly useful in those who are being evaluated for surgical closure. It provides more details on coexisting aortic regurgitation, in multiple VSDs and when coronary artery disease is suspected.
- Pulse oximetry. A small clip on the fingertip measures the amount of oxygen in the blood.
- Cardiac Magnetic Resonance Imaging (MRI) and computed tomography (CT) are useful in cases where anatomy is complex such as VSD accompanied with other congenital heart anomalies and in defects in unusual locations that are hard to visualize by conventional transthoracic echocardiography.
Ventricular septal defect treatment
Approximately 85% to 90% of small isolated ventricular septal defects (VSDs) close spontaneously during the first year of life. Many babies born with a small ventricular septal defect (VSD) won’t need surgery to close the hole. After birth, your doctor may want to observe your baby and treat symptoms while waiting to see if the defect closes on its own.
Patients with small, asymptomatic VSDs with the absence of pulmonary arterial hypertension have an excellent prognosis without any intervention. Otherwise, the management approach includes endocarditis prophylaxis and VSD closure.
Babies who need surgical repair often have the procedure in their first year. Children and adults who have a medium or large ventricular septal defect or one that’s causing significant symptoms may need surgery to close the defect.
Some smaller ventricular septal defects are closed surgically to prevent complications related to their locations, such as damage to heart valves. Many people with small VSDs have productive lives with few related problems.
Babies who have large VSDs or who tire easily during feeding may need extra nutrition to help them grow. To make sure babies have a healthy weight gain, a special high-calorie formula might be prescribed. Some babies become extremely tired while feeding and might need to be fed through a feeding tube.
Patients with Eisenmenger syndrome are usually managed in advanced centers due to the complexity of managing of such cases. Historically, surgical repair of VSDs was the only option; however, recent advances in interventional techniques make percutaneous VSD closure possible. Endocarditis prophylaxis is mainly indicated in cyanotic congenital heart disease, prior episodes of endocarditis and in those who have prosthetic heart valves or had repair with prosthetic material. In general, VSD closure is indicated in medium to large defects with a significant hemodynamic compromise such as those who are symptomatic and have left ventricle dysfunction. An intervention should be also considered in cases of progressive aortic insufficiency or after an episode of endocarditis. The indications of a surgical closure according to the American College of Cardiology and the American Heart Association 2008 guidelines are summarized in the following:
- Those who suffered an episode of endocarditis.
- When the ratio of the pulmonary blood flow to the systemic blood flow (Qp/Qs) is equal to or more than 2 plus clinical evidence of left ventricular fluid overload.
- In milder shunts such as those with ratio of the pulmonary blood flow to the systemic blood flow (Qp/Qs) above 1.5, it is reasonable to intervene when there is evidence of left ventricle systolic or diastolic dysfunction, or when the pulmonary artery pressure and pulmonary vascular resistance are less than two-thirds of systemic pressure and systemic vascular resistance, respectively.
Surgical repair reduces the risk for endocarditis, might improve pulmonary arterial hypertension and overall it increase survival. Without pulmonary arterial hypertension, the operative mortality rate is approximately 1%. Complications include residual or recurrent VSD, valvular incompetence such as tricuspid regurgitation and aortic insufficiency, arrhythmias, left ventricular dysfunction and progression of pulmonary arterial hypertension. The arrhythmias which accompany VSD repair include atrial fibrillation, complete heart block and uncommonly, ventricular tachycardia. The main contraindication for surgical VSD closure is the presence of irreversible pulmonary arterial hypertension; this is due to the high surgical perioperative mortality and pulmonary complications.
Percutaneous device VSD closure is reserved for those whom surgery is very risky due to severe pulmonary arterial hypertension, multiple comorbidities, and those who had prior cardiothoracic surgery such as residual or recurrent VSD. Muscular VSDs are the main type amenable to this procedure, the proximity of other defects to the inlet valves makes performing this technique challenging in such cases. Despite the fact that it is still unpopular in the United States, current data shows excellent outcomes with complete closure and low mortality. The most frequent complication is complete atrioventricular block mostly related to perimembranous defects.
In conclusion, VSD is the most common congenital anomaly at birth. Small defects are expected to close spontaneously in the first year of life; however, larger defects can result in severe complications. Surgical VSD closure and device closure are the main intervention for large defects 6.
VSD medications
Medications for ventricular septal defect may include those to:
- Decrease the amount of fluid in circulation and in the lungs. Doing so reduces the volume of blood that must be pumped. These medications, called diuretics, include furosemide (Lasix).
- Keep the heartbeat regular. Examples include beta blockers, such as metoprolol (Lopressor), propranolol (Inderal LA) and others, and digoxin (Lanoxin, Lanoxin Pediatric).
VSD surgery
Surgical treatment for ventricular septal defects involves plugging or patching the abnormal opening between the ventricles. If you or your child is having surgery to repair a ventricular defect, consider having surgery performed by surgeons and cardiologists with training and expertise in conducting these procedures. Procedures may include:
- Surgical repair. This procedure of choice in most cases usually involves open-heart surgery under general anesthesia. The surgery requires a heart-lung machine and an incision in the chest. The doctor uses a patch or stitches to close the hole.
- Catheter procedure. Closing a ventricular septal defect during catheterization doesn’t require opening the chest. Rather, the doctor inserts a thin tube (catheter) into a blood vessel in the groin and guides it to the heart. The doctor then uses a specially sized mesh device to close the hole.
- Hybrid procedure. A hybrid procedure uses surgical and catheter-based techniques. Access to the heart is usually through a small incision, and the procedure may be performed without stopping the heart and using the heart-lung machine. A device closes the ventricular septal defect via a catheter placed through the incision.
After repair, your doctor will schedule regular medical follow-up to ensure that the ventricular septal defect remains closed and to look for signs of complications. Depending on the size of the defect and the presence of other problems, your doctor will tell you how frequently you or your child will need to be seen.
After your ventricular septal defect (VSD) is repaired, you or your child will need follow-up care throughout life for doctors to monitor your condition and check for any signs of complications.
Your doctor may suggest that you or your child have regular follow-up appointments with a doctor who specializes in congenital heart disease. In follow-up appointments, your doctor may evaluate you or your child and order imaging tests to monitor your or your child’s condition.
Here are a few tips for managing your or your child’s condition:
- Consider pregnancy carefully. Before becoming pregnant, talk to a doctor trained in heart conditions (cardiologist) to determine if you can undergo pregnancy safely. This is especially important if you’re taking medications. It’s also important to see both an obstetrician and a cardiologist during pregnancy. Having a repaired VSD without complications or having a small defect doesn’t pose an additional pregnancy risk. However, having an unrepaired, larger defect; heart failure; pulmonary hypertension; abnormal heart rhythms; or other heart defects poses a high risk to both mother and fetus. Doctors strongly advise women with Eisenmenger syndrome not to become pregnant because of the high risk of complications.
- Prevent endocarditis. You or your child usually won’t need to take antibiotics before certain dental procedures to prevent an infection of the heart’s inner lining (endocarditis). However, your doctor may recommend antibiotics if you’ve had prior endocarditis, a heart valve replacement, if you have had a recent VSD repair with artificial material, if you still have leaks through the VSD, if the repaired VSD is next to a defect that’s been repaired with artificial material, or if you have a large ventricular septal defect that’s causing low oxygen levels. For most people with a ventricular septal defect, good oral hygiene and regular dental checkups can prevent endocarditis.
- Follow exercise recommendations. Your doctor can advise you about which activities are safe for you or your child. If some activities pose special dangers, encourage your child to engage in other, safer activities. Keep in mind that many children with VSDs can lead healthy, fully active, productive lives. Children with small defects or a repaired hole in the heart will usually have few or no restrictions on activity or exercise. Children whose hearts don’t pump as normally will need to follow some limits. A child with irreversible pulmonary hypertension (Eisenmenger syndrome) has the greatest number of restrictions.
Ventricular septal defect prognosis
Children with small VSDs are asymptomatic and have excellent long-term prognoses. The prognosis is good for patients who have undergone VSD repair. However, they have a higher risk of arrhythmia, endocarditis and congestive heart failure in the long run in comparison to the general population 7.
The outcome of medical therapy for children with moderate or large VSDs varies, as follows.
Many infants improve, showing evidence of a gradual decrease in the magnitude of the left-to-right shunt between the ages of 6 and 24 months. It is important to assess the cause of the decrease in left-to-right flow, which can reflect an increase in pulmonary vascular resistance, a decrease in the relative size of the defect, or the development of right ventricle outflow tract hypertrophy, resulting in functional or anatomic obstruction.
Most children with VSDs remain in stable condition or improve after infancy. Heart failure rarely occurs after infancy. Anemia, respiratory infection, endocarditis, or the development of an associated lesion (eg, aortic insufficiency) can trigger a recurrence of symptoms.
A few patients who develop severe pulmonary vascular obstructive disease with predominant right-to-left shunts (Eisenmenger syndrome) at the time of referral require symptomatic therapy. Cyanosis progressively increases, and exercise capacity decreases. Select patients with large VSDs may be candidates for lung or heart-lung transplantation.
Red blood cell (RBC) reduction by means of partial-exchange transfusion may relieve symptoms associated with extreme polycythemia (eg, headache, extreme fatigue).
The surgical mortality is less than 1% for isolated VSDs.
References- Dakkak W, Oliver TI. Ventricular Septal Defect. [Updated 2019 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470330
- Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of Congenital Heart Defects in Metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153:807-13.
- Lopez L, Houyel L, Colan SD, Anderson RH, Béland MJ, Aiello VD, Bailliard F, Cohen MS, Jacobs JP, Kurosawa H, Sanders SP, Walters HL, Weinberg PM, Boris JR, Cook AC, Crucean A, Everett AD, Gaynor JW, Giroud J, Guleserian KJ, Hughes ML, Juraszek AL, Krogmann ON, Maruszewski BJ, St Louis JD, Seslar SP, Spicer DE, Srivastava S, Stellin G, Tchervenkov CI, Wang L, Franklin RCG. Classification of Ventricular Septal Defects for the Eleventh Iteration of the International Classification of Diseases-Striving for Consensus: A Report From the International Society for Nomenclature of Paediatric and Congenital Heart Disease. Ann. Thorac. Surg. 2018 Nov;106(5):1578-1589.
- Muthialu N, Balakrishnan S, Sundar R. Single patch closure of multiple VSDs through right atrial approach. Indian Heart J. 2018 Jul – Aug;70(4):578-579.
- Maagaard M, Heiberg J, Eckerström F, Asschenfeldt B, Rex CE, Ringgaard S, Hjortdal VE. Biventricular morphology in adults born with a ventricular septal defect. Cardiol Young. 2018 Dec;28(12):1379-1385.
- Garg N, Nayyar M, Khouzam RN, Salem SA, Ardeshna D. Peri-procedural antibiotic prophylaxis in ventricular septal defect: a case study to re-visit guidelines. Ann Transl Med. 2018 Jan;6(1):18.
- Goldberg JF. Long-term Follow-up of “Simple” Lesions–Atrial Septal Defect, Ventricular Septal Defect, and Coarctation of the Aorta. Congenit Heart Dis. 2015 Sep-Oct;10(5):466-74.