Vitamin E deficiency
Dietary vitamin E deficiency is common in developing countries due to malnutrition; vitamin E deficiency among adults in developed countries is uncommon and usually due to fat malabsorption, liver failure, and digestive tract diseases, which impair the absorption of dietary fats and therefore fat-soluble vitamins like vitamin E 1, 2, 3, 4. Severe vitamin E deficiency has been associated with specific genetic defects affecting the transport of alpha-tocopherol by α-tocopherol transfer protein (α-TTP) and lipoproteins 4.
Premature babies of very low birth weight (<1,500 grams) might be deficient in vitamin E. Vitamin E supplementation in these infants might reduce the risk of some complications, such as those affecting the retina, but they can also increase the risk of infections 5. In addition, a recent nested case-control study in Bangladeshi women suggested that inadequate vitamin E status during early pregnancy may be associated with an increased risk of miscarriage 6.
Because the digestive tract requires fat to absorb vitamin E, people with fat-malabsorption disorders are more likely to become deficient than people without such disorders 2, 3, 4. Vitamin E deficiency symptoms include peripheral neuropathy, ataxia, skeletal myopathy, retinopathy, and impairment of the immune response 1, 7. People with Crohn’s disease, cystic fibrosis, or an inability to secrete bile from the liver into the digestive tract, for example, often pass greasy stools or have chronic diarrhea; as a result, they sometimes require water-soluble forms of vitamin E, such as tocopheryl polyethylene glycol-1000 succinate 8.
Some people with abetalipoproteinemia, a rare inherited disorder resulting in poor absorption of dietary fat, require enormous doses of supplemental vitamin E (approximately 100 mg/kg or 5–10 g/day) 8. Vitamin E deficiency secondary to abetalipoproteinemia causes such problems as poor transmission of nerve impulses, muscle weakness, and retinal degeneration that leads to blindness 9. Ataxia and vitamin E deficiency (AVED) is another rare, inherited disorder in which the liver’s alpha-tocopherol transfer protein (α-TTP) is defective or absent 10, 11. People with AVED have such severe vitamin E deficiency that they develop nerve damage and lose the ability to walk unless they take large doses of supplemental vitamin E 9, 10, 11.
Vitamin E deficiency causes fragility of red blood cells and degeneration of neurons, particularly peripheral axons and posterior column neurons.
The main symptoms of vitamin E deficiency are hemolytic anemia and neurologic deficits. Diagnosis is based on measuring the ratio of plasma alpha-tocopherol to total plasma lipids; a low ratio suggests vitamin E deficiency. Treatment consists of oral vitamin E, given in high doses if there are neurologic deficits or if deficiency results from malabsorption.
What is Vitamin E?
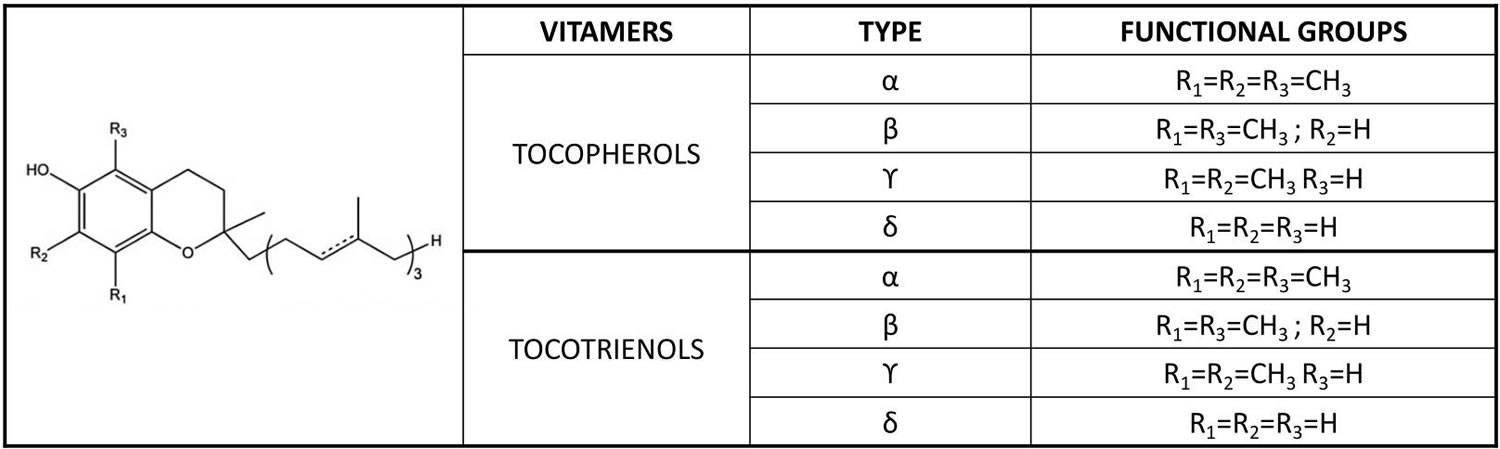
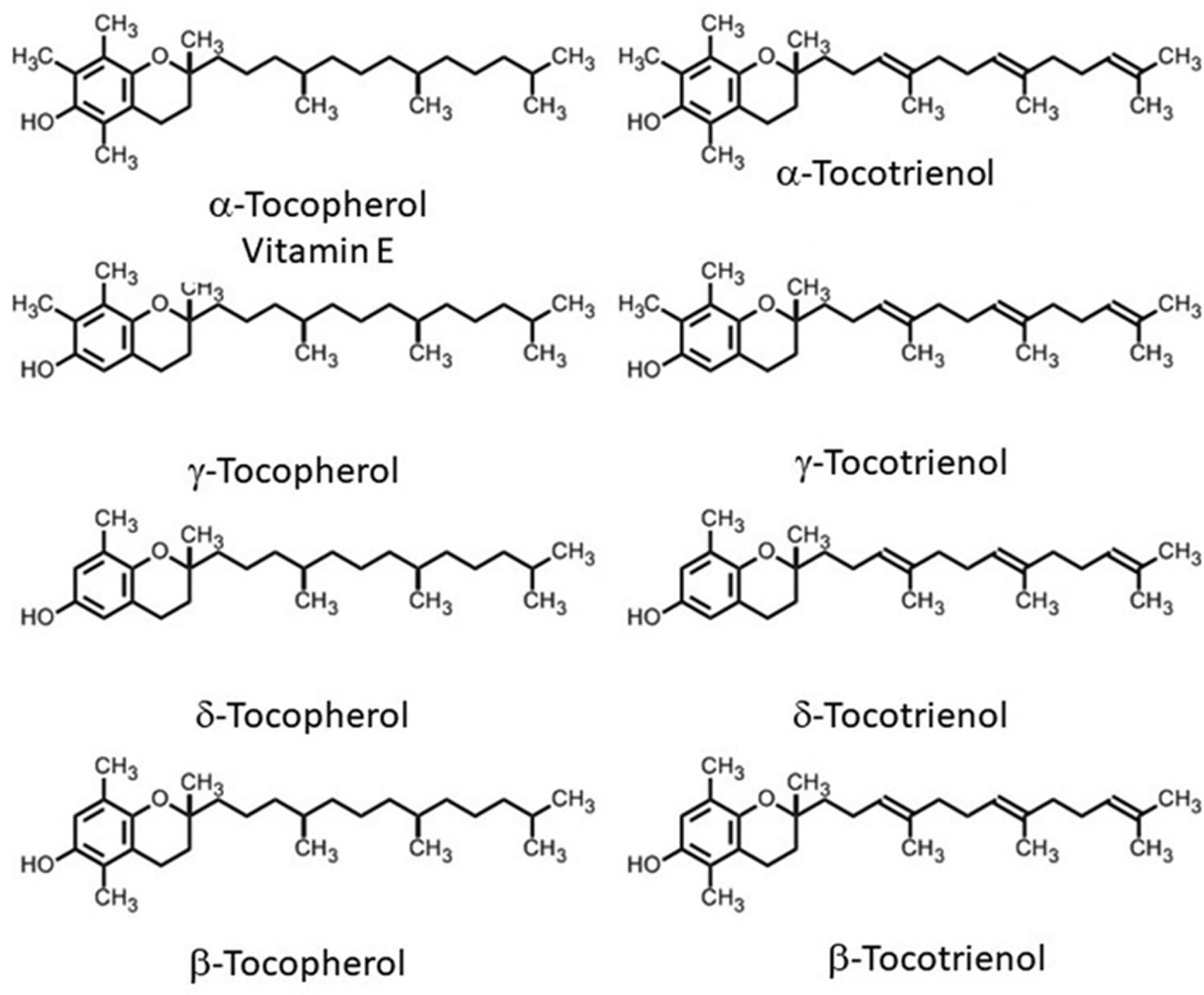
Vitamin E is a fat-soluble nutrient found in many foods, added to others, and available as a dietary supplement. “Vitamin E” is the collective name for a group of fat-soluble compounds with distinctive antioxidant activities 12. Naturally occurring vitamin E exists in eight chemical forms: four tocopherol isoforms (alpha-, beta-, gamma-, and delta-tocopherol OR α-, β-, γ-, and δ-tocopherol) and four tocotrienol isoforms (alpha-, beta-, gamma-, and delta-tocotrienol OR α-, β-, γ-, and δ-tocotrienol) that have varying levels of biological activity (see Figure 2) 12, 2. Alpha- (or α-) tocopherol is the only form that is recognized to meet human requirements and the body preferentially uses alpha-tocopherol, and only α-tocopherol supplementation can reverse vitamin E deficiency symptoms 13, 14, 15, 16, 17.
In the human liver, alpha-tocopherol (α-tocopherol) is the form of vitamin E that is preferentially bound to alpha-tocopherol transfer protein (α-TTP) and incorporated into lipoproteins that transport alpha-tocopherol (α-tocopherol) in the blood for delivery to tissues outside your liver 16. Therefore, alpha-tocopherol (α-tocopherol) is the predominant form of vitamin E found in your blood and tissues 18. In addition, alpha-tocopherol (α-tocopherol) appears to be the form of vitamin E with the greatest nutritional significance. Natural alpha-tocopherol (α-tocopherol) made by plants found in food has an RRR-configuration at the 2, 4’, and 8’-position of the alpha-tocopherol molecule wrongly referred to as d-α-tocopherol 16. Chemically synthesized all-racemic-α-tocopherol (all-rac-α-tocopherol) incorrectly labeled dl-α-tocopherol is a mixture of eight stereoisomers of alpha-tocopherol (α-tocopherol), which arose from the three chiral carbons at the 2, 4’, and 8’-positions: RRR-, RSR-, RRS-, RSS-, SRR-, SSR-, SRS-, and SSS-α-tocopherol (see Figure 2) 16. While all vitamin E stereoisomers have equal in vitro (test tube studies) antioxidant activity, only the forms in the R-conformation at position 2 (noted 2R) meet the vitamin E requirements in humans 19. Furthermore, beta-, gamma-, and delta-tocopherols, 4 tocotrienols, and several stereoisomers may also have important biologic activity 18.
In your body, vitamin E acts a fat-soluble antioxidant, helping to protect your cells from the damage caused by free radicals, which are molecules that contain an unshared electron. Unshared electrons are highly energetic and react rapidly with oxygen to form reactive oxygen species (ROS) 17. Free radicals are compounds formed when your body converts the food you eat into energy. People are also exposed to free radicals in the environment from cigarette smoke, air pollution, and ultraviolet radiation from the sun. Free radicals damage cells and might contribute to the development of cardiovascular disease and cancer 20. Vitamin E is believed to serve as a chain-breaking antioxidant that stops the oxidative degradation of fats, thus preventing free radical production and harm to the cell. Scientists are investigating whether, by limiting free-radical production and possibly through other mechanisms, vitamin E might help prevent or delay the chronic diseases associated with free radicals.
In addition to its activities as an antioxidant, vitamin E is involved in immune function and, as shown primarily by in vitro studies (test tube lab studies) of cells, cell signaling, regulation of gene expression, and other metabolic processes 18. Alpha-tocopherol inhibits the activity of protein kinase C, an enzyme involved in cell proliferation and differentiation in smooth muscle cells, platelets, and monocytes 19. Vitamin-E–packed endothelial cells lining the interior surface of blood vessels are better able to resist blood-cell components adhering to this surface. Vitamin E also increases the expression of two enzymes that suppress arachidonic acid metabolism, thereby increasing the release of prostacyclin from the endothelium, which, in turn, dilates blood vessels and inhibits platelet aggregation 19.
Your body also needs vitamin E to boost its immune system so that it can fight off invading bacteria and viruses. It helps to widen blood vessels and keep blood from clotting within them. In addition, cells use vitamin E to interact with each other and to carry out many important functions. Scientists are investigating whether, by limiting free-radical production and possibly through other mechanisms, vitamin E might help prevent or delay the chronic diseases associated with free radicals.
Aside from maintaining the integrity of cell membranes throughout the body, alpha tocopherol protects the fats in low-density lipoproteins (LDLs) from oxidation 21. Lipoproteins are particles composed of lipids and proteins that transport fats through the bloodstream. LDLs (bad cholesterol) specifically transport cholesterol from the liver to the tissues of the body. Oxidized LDLs (bad cholesterol) have been implicated in the development of cardiovascular disease 22.
Vitamin E is absorbed in the intestinal lumen, which is dependent upon various factors such as pancreatic secretions, micelle formation, and most importantly, chylomicron secretions. Chylomicron secretion is necessary for vitamin E absorption. Vitamin E is found in sunflower seeds, nuts, some oils, spinach, butternut squash, and many other food sources. Vitamin E deficiency has been linked to peripheral neuropathy in addition to spinocerebellar ataxia, skeletal myopathy and pigmented retinopathy. Interestingly, studies have reported vitamin E level in association to the development of cataracts 19.
Serum concentrations of vitamin E (alpha-tocopherol) depend on your liver, which takes up the nutrient after the various forms are absorbed from the small intestine. Yet, in the body, alpha-tocopherol (α-tocopherol) is preferentially retained in the liver by the binding to alpha-tocopherol transfer protein (α-TTP) 12, which incorporates α-tocopherol into lipoproteins for delivery to extrahepatic tissues; and other forms of vitamin E other than alpha-tocopherol (α-tocopherol) are actively broken down and excreted 23. As a result, blood and cellular concentrations of other forms of vitamin E are lower than those of alpha-tocopherol and have been the subjects of less research 24, 25. Plasma tocopherol levels vary with total plasma lipid levels. Normally, the plasma alpha-tocopherol level is 5 to 20 mcg/mL (11.6 to 46.4 mcmol/L) 26.
Vitamin E is safe for pregnancy and breastfeeding. Both vitamin K and omega-6 fatty acids requirements may increase with high doses of vitamin E.
Some food and dietary supplement labels still list vitamin E in International Units (IUs) rather than milligrams (mg). 1 IU of the natural form of vitamin E is equivalent to 0.67 mg. 1 IU of the synthetic form of vitamin E is equivalent to 0.45 mg.
International Units and Milligrams
Vitamin E is listed on the new Nutrition Facts and Supplement Facts labels in milligrams (mg) 27. The U.S. Food and Drug Administration (FDA) required manufacturers to use these new labels starting in January 2020, but companies with annual sales of less than $10 million may continue to use the old labels that list vitamin E in international units (IUs) until January 2021 28. Conversion rules are as follows:
To convert from mg to IU:
- 1 mg of alpha-tocopherol is equivalent to 1.49 IU of the natural form or 2.22 IU of the synthetic form.
To convert from IU to mg:
- 1 IU of the natural form is equivalent to 0.67 mg of alpha-tocopherol.
- 1 IU of the synthetic form is equivalent to 0.45 mg of alpha-tocopherol.
For example, 15 mg of natural alpha-tocopherol would equal 22.4 IU (15 mg x 1.49 IU/mg = 22.4 IU). The corresponding value for synthetic alpha-tocopherol would be 33.3 IU (15 mg x 2.22 IU/mg).
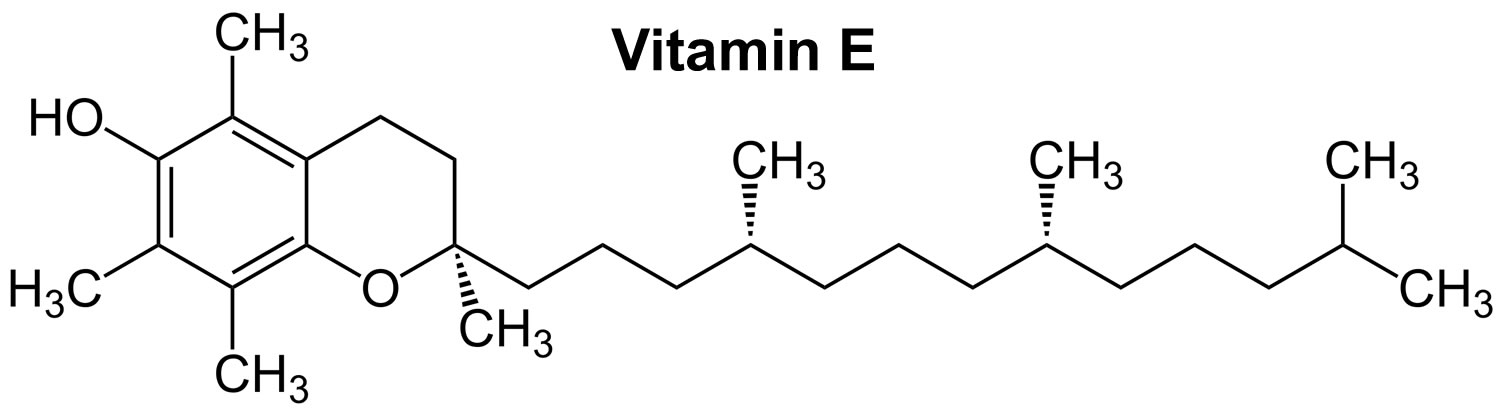
Figure 1. Vitamin E chemical structure
Figure 2. Vitamin E chemical structures
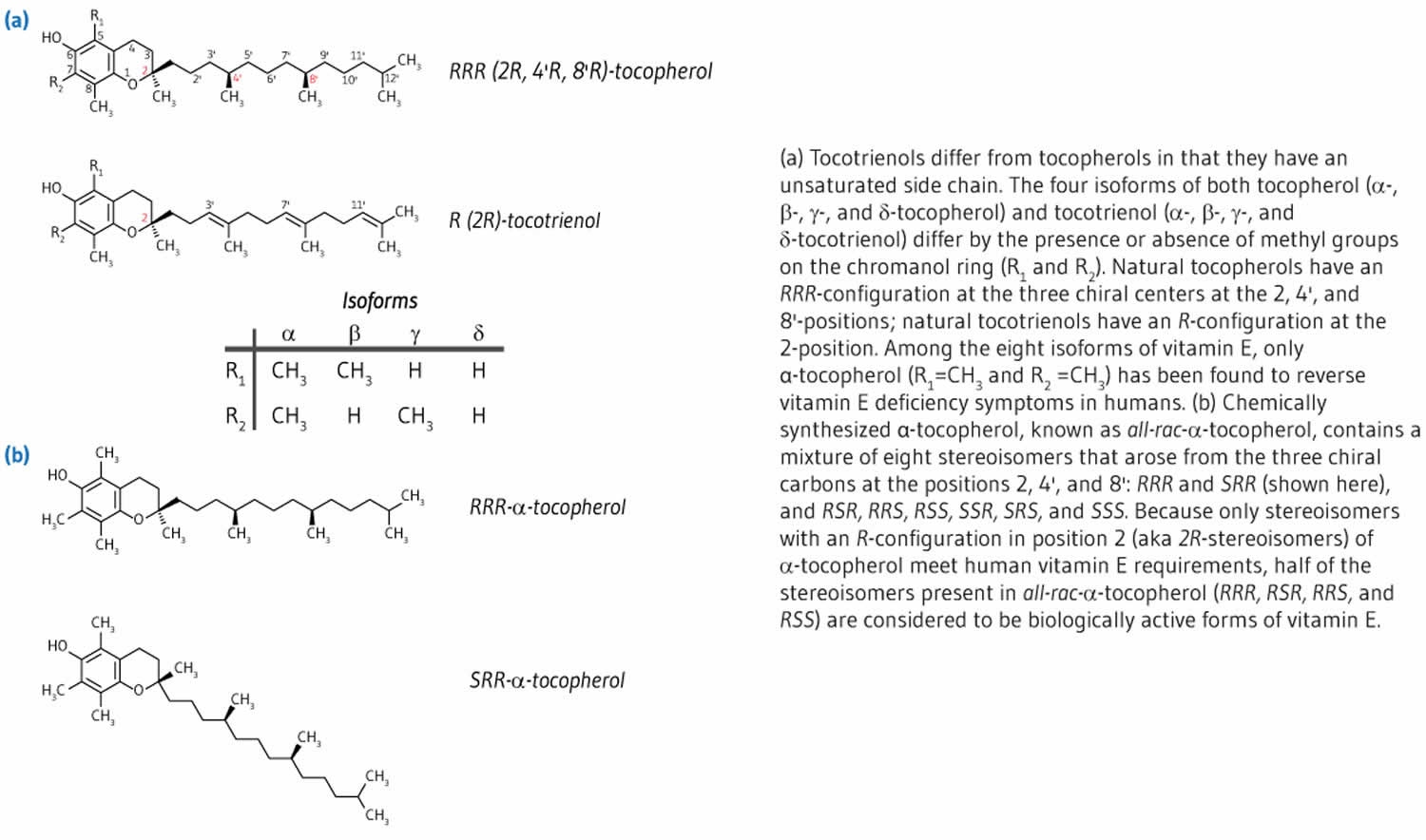 Footnote: The differences in the α, β, γ and δ forms are in the number and position of the methyl groups on the chromanol ring. Only the β- and γ- forms of tocopherols or tocotrienols can be called isomers, having the same formula but a different arrangement of atoms in the molecule. The difference between the tocopherols and the tocotrienols is in the presence of three double bonds in the side chain of the latter.
[Source 14 ]
Footnote: The differences in the α, β, γ and δ forms are in the number and position of the methyl groups on the chromanol ring. Only the β- and γ- forms of tocopherols or tocotrienols can be called isomers, having the same formula but a different arrangement of atoms in the molecule. The difference between the tocopherols and the tocotrienols is in the presence of three double bonds in the side chain of the latter.
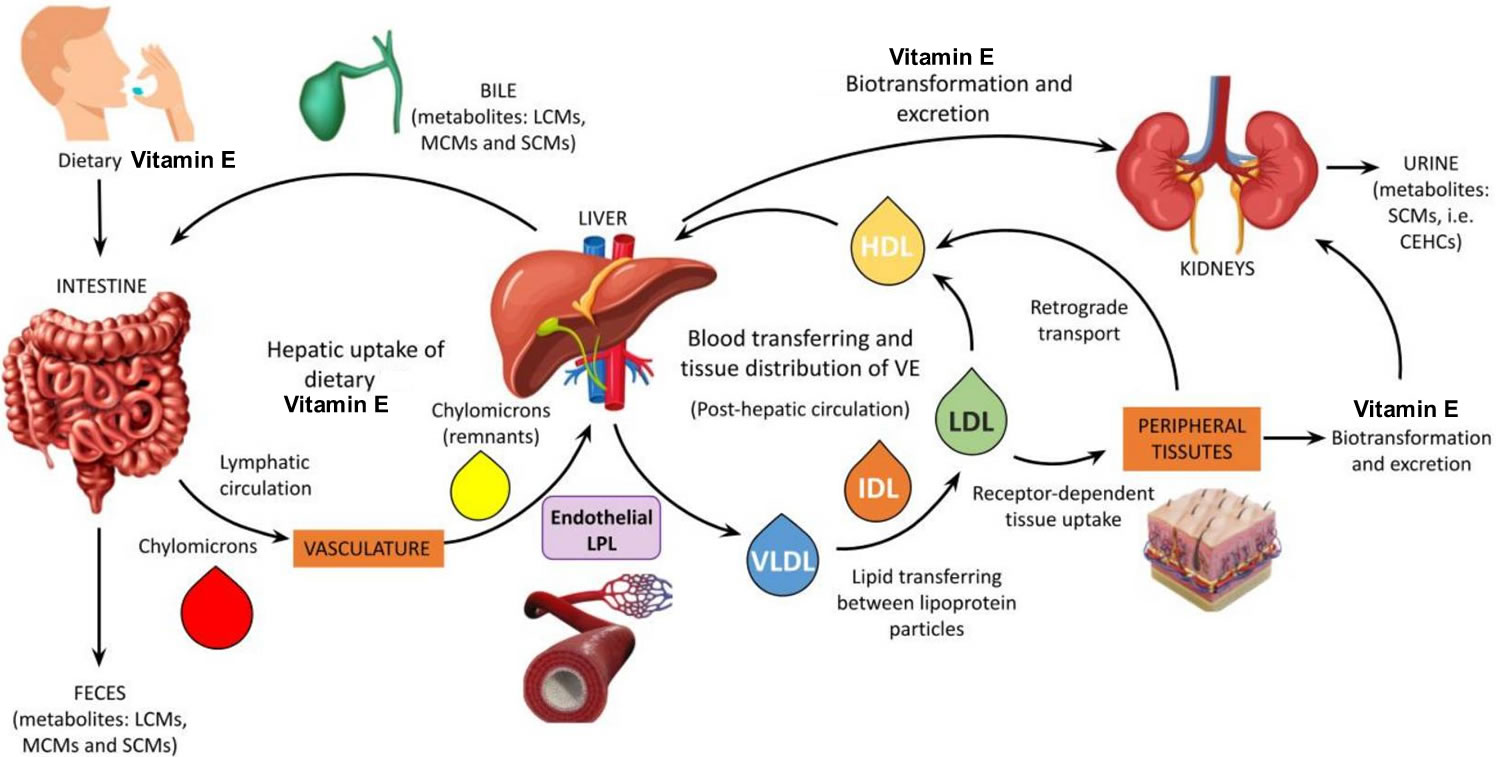
[Source 14 ]Figure 3. Vitamin E metabolism
Footnote: Schematic representation of vitamin E metabolism. The mechanism of vitamin E digestion and uptake into intestinal cells (enterocytes) is unclear but requires bile acids and pancreatic enzymes, and the packaging along with dietary fat into chylomicrons. The efficiency of vitamin E absorption increases with the amount of fat in ingested food, such that vitamin E absorption from supplements is likely to be minimal with low-fat meals 29, 30. In the circulation, all lipoproteins (i.e., VLDLs, LDLs, and HDLs) are involved in the transport and tissue distribution of alpha-tocopherol 18. Increased concentrations of fats (cholesterol and triglycerides) in the blood have been correlated to higher serum alpha-tocopherol concentrations. However, if a high blood concentration of fats is associated with a slower turnover of lipoproteins, then the distribution of alpha-tocopherol to tissues may be substantially altered 31.
Abbreviations: LCMs = long-chain-metabolites; MCMs = multi-cycling metabolites; SCMs = short-chain-metabolites; CEHCs = carboxyethyl hydroxychromans (natural vitamin E metabolites); VE = vitamin E; LPL = lipoprotein lipase.
[Source 32 ]What are the functions of vitamin E?
Vitamin E is a fat-soluble antioxidant that stops the production of reactive oxygen species (ROS) formed when fat undergoes oxidation. Scientists are investigating whether, by limiting free-radical production and possibly through other mechanisms, vitamin E might help prevent or delay the chronic diseases associated with free radicals.
Antioxidants protect cells from the damaging effects of free radicals, which are molecules that contain an unshared electron. Free radicals damage cells and might contribute to the development of cardiovascular disease and cancer 20. Unshared electrons are highly energetic and react rapidly with oxygen to form reactive oxygen species. The body forms reactive oxygen species endogenously when it converts food to energy, and antioxidants might protect cells from the damaging effects of reactive oxygen species. The body is also exposed to free radicals from environmental exposures, such as cigarette smoke, air pollution, and ultraviolet radiation from the sun. Reactive oxygen species are part of signaling mechanisms among cells.
The body also needs vitamin E to boost its immune system so that it can fight off invading bacteria and viruses. It helps to widen blood vessels and keep blood from clotting within them.
In addition to vitamin E activities as an antioxidant, vitamin E is involved in immune function and, as shown primarily by in vitro studies of cells, cell signaling, regulation of gene expression, and other metabolic processes 12, 33, 34. Alpha-tocopherol inhibits the activity of protein kinase C, an enzyme involved in cell proliferation and differentiation in smooth muscle cells, platelets, and monocytes 1. Vitamin-E–replete endothelial cells lining the interior surface of blood vessels are better able to resist blood-cell components adhering to this surface. Vitamin E also increases the expression of two enzymes that suppress arachidonic acid metabolism, thereby increasing the release of prostacyclin from the endothelium, which, in turn, dilates blood vessels and inhibits platelet aggregation 1. Moreover, vitamin E also helps improve nerve conduction 35, maintain the structural integrity of the hemoglobin membrane 36 and, along with vitamin A, plays a role in vision 37. The specific mechanism of action for most of vitamin E effects is still relatively unknown 38.
Vitamin E inhibits platelet adhesion by preventing oxidative changes to low-density lipoprotein (LDL) cholesterol also called bad cholesterol and inhibition of platelet aggregation by reducing prostaglandin E2. Another effect is inhibiting protein kinase C causing smooth-muscle proliferation.
Even though research has shown that vitamin E assists with the prevention of heart disease and atherosclerosis it has not been approved for this use by the United States Food and Drug Administration (FDA).
Antioxidant activity
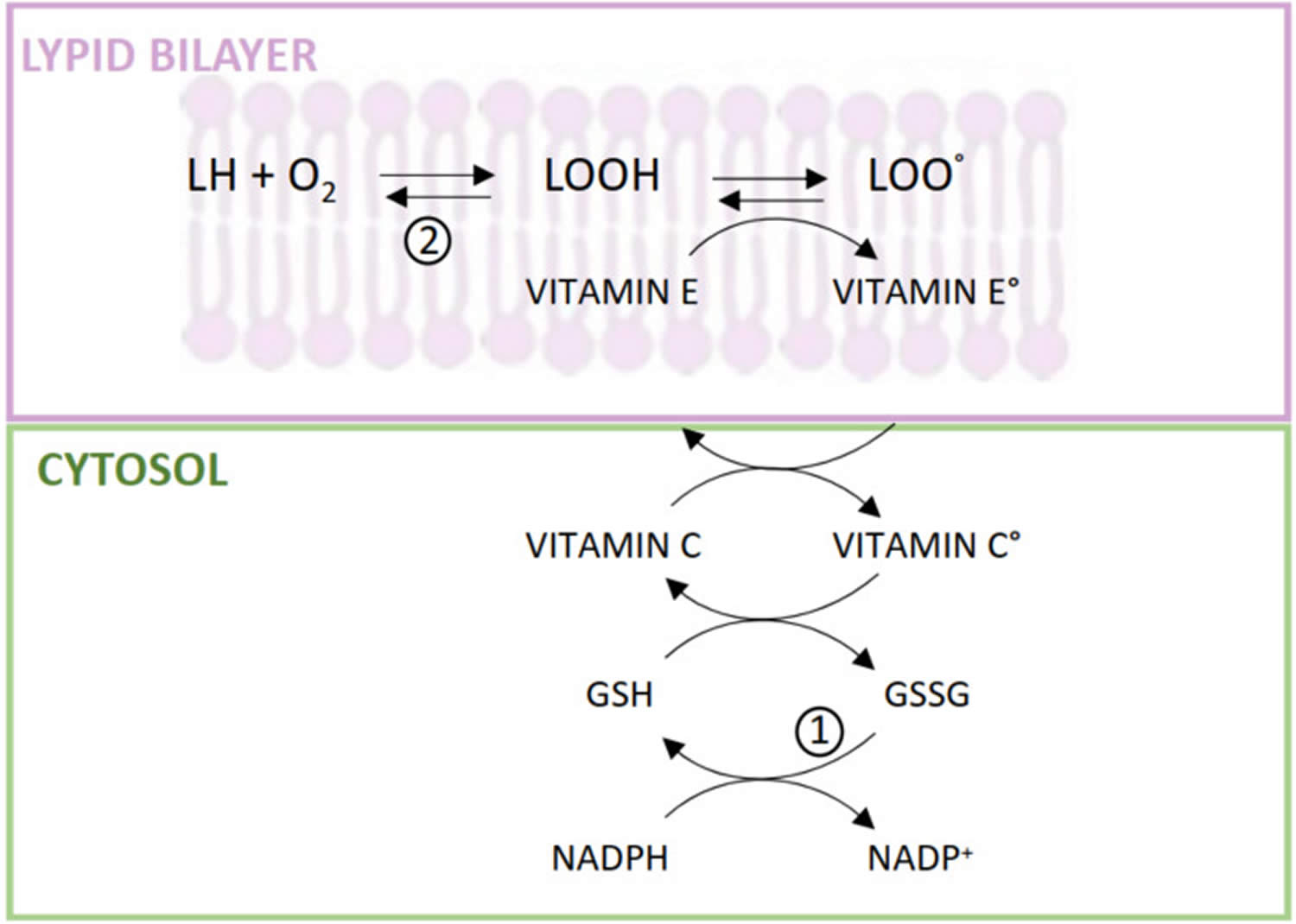
The main function of alpha-tocopherol in humans is that of a fat-soluble antioxidant. Fats, which are an integral part of all cell membranes, are vulnerable to damage through lipid peroxidation by free radicals. Alpha-tocopherol is uniquely suited to intercept peroxyl radicals and thus prevent a chain reaction of lipid oxidation (Figure 4). When a molecule of α-tocopherol neutralizes a free radical, it is oxidized and its antioxidant capacity is lost. Other antioxidants, such as vitamin C, are capable of regenerating the antioxidant capacity of α-tocopherol (Figure 4) 18.
Aside from maintaining the integrity of cell membranes throughout the body, α-tocopherol protects the fats in low-density lipoproteins (LDLs) from oxidation. Lipoproteins are particles composed of lipids and proteins that transport fats through the bloodstream. LDLs specifically transport cholesterol from the liver to the tissues of the body. Oxidized LDLs have been implicated in the development of cardiovascular disease 22.
Tocotrienols and gamma-tocopherol are thought to be better scavengers of peroxyl radicals and reactive nitrogen species, respectively, than alpha-tocopherol 39. Yet, in the body, alpha-tocopherol is preferentially retained in the liver by the binding to alpha-tocopherol transfer protein (α-TTP), which incorporates α-tocopherol into lipoproteins for delivery to extrahepatic tissues; and forms of vitamin E other than α-tocopherol are actively metabolized and excreted. Hence, while gamma-tocopherol is the most common form of vitamin E in the American diet 40, its plasma and tissue concentrations are generally significantly lower than those of alpha-tocopherol and more gamma-tocopherol is excreted in urine than alpha-tocopherol, suggesting less gamma-tocopherol is needed for use by the body 18.
Studies conducted in vitro (test tubes lab studies) and in animals have indicated that gamm-tocopherol and its major metabolite, gamma-carboxyethyl hydroxychroman (γ-CEHC), may play a role in protecting the body from free radical-induced damage in various conditions of oxidative stress and inflammation 39. Limited intervention studies highlighted in Jiang 39 have not convincingly demonstrated a potential anti-inflammatory effect of gamma-tocopherol in humans. Yet, in two recent randomized, placebo-controlled studies, the supplementation of smokers with gamma-tocopherol potentiated short-term benefits of smoking cessation (with or without nicotine replacement therapy) on vascular endothelial function 41, 42.
Numerous preclinical studies have also suggested that tocotrienols might be beneficial in the prevention of chronic diseases 43. For instance, tocotrienols especially delta-tocotrienol have shown greater anti-proliferative and pro-apoptotic effects than tocopherols in malignant cell lines 44. However, a number of factors, including dose, formulation, and type of study population, affect the bioavailability of tocotrienols and may undermine their putative efficacy in humans 45. There are currently no data available on the effectiveness of supplemental tocotrienols in humans 43.
Figure 4. Alpha-tocopherol antioxidant activity
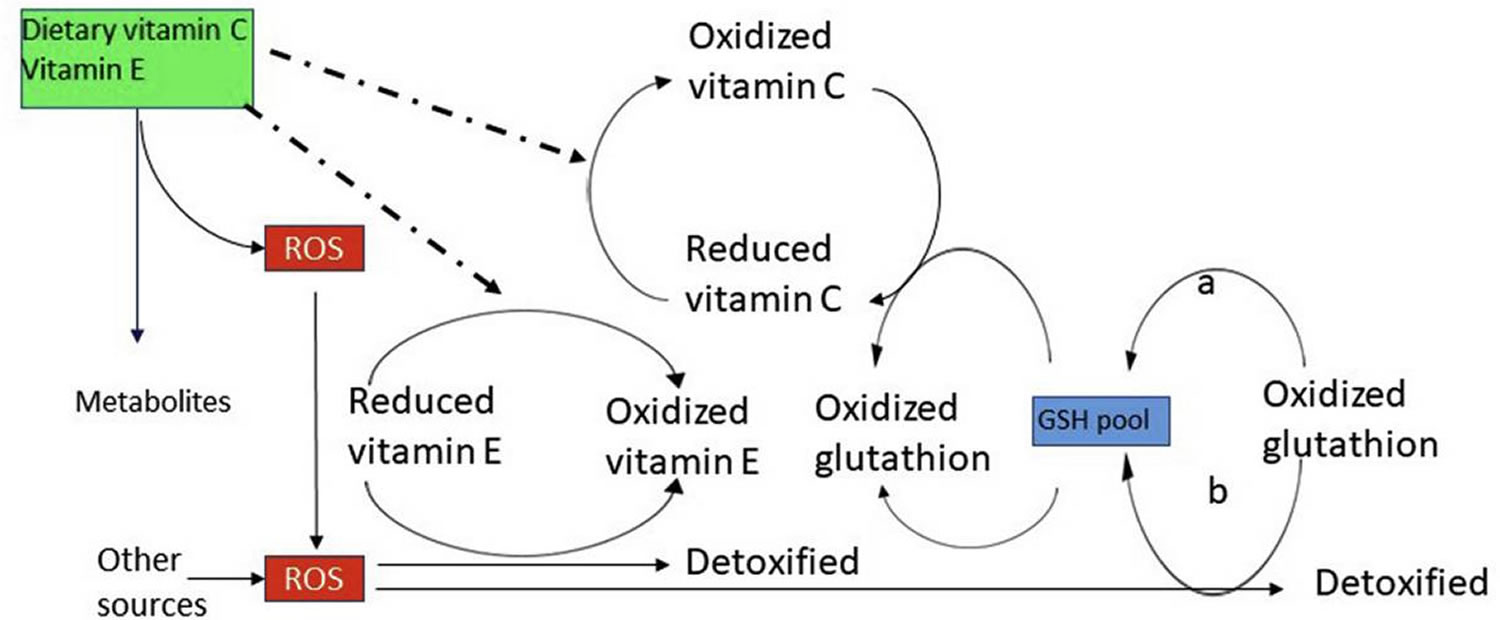
[Source 14 ]Figure 5. Vitamin E antioxidant reactions
Footnote: (1) Hexose monophosphate shunt and GSH-reductase activity; (2) Membrane GSH-peroxidase activity.
Abbreviations: L = membrane lipids; Vitamin E° = tocopheryl radical; Vitamin C°, ascorbyl radical; GSH = reduced glutathione; GSSG =oxidized glutathione; NADP+ = oxidized nicotinamide-adenine-dinucleotide phosphate; NADPH = reduced nicotinamide-adenine-dinucleotide phosphate
[Source 32 ]Immune function
Other functions of alpha-tocopherol are likely to be related to its antioxidant capacity 18. For example, alpha-tocopherol can protect the physiological properties of lipid bilayer membranes and may influence the activity of membrane proteins and enzymes 46. In cell culture studies, alpha-tocopherol was found to improve the formation of an adhesive junction known as immune synapse between naïve T lymphocytes and antigen-presenting cells (APC), which eventually prompted T cell activation and proliferation 47, 48.
The natural age-related decline of the immune function is accompanied by an increased susceptibility to infections, a poorer response to immunization, and higher risks of developing cancers and autoimmune diseases 16. Alpha-tocopherol has been shown to enhance specifically the T cell-mediated immune response that declines with advancing age 49. T cell impaired response has been partly associated with a reduced capacity of naive T cells to be activated during antigen presentation, and to produce interleukin-2 (IL-2) and proliferate as a result 48. However, very few studies have addressed the potential association between alpha-tocopherol and immune function in humans 49. In a small intervention study in older adults (mean age, 70 years), supplementation with 200 mg/day of all-rac-alpha-tocopherol (equivalent to 100 mg of RRR-α-tocopherol) for three months significantly improved natural killer (NK) cytotoxic activity, neutrophil chemotaxis, phagocytic response, and enhanced mitogen-induced lymphocyte proliferation and interleukin-2 (IL-2) production compared to baseline 50. In an earlier trial, daily supplementation of healthy older adults (≥65 years of age) with 200 mg of all-rac-alpha-tocopherol for 235 days also improved T lymphocyte-mediated immunity — as measured with the delayed-type hypersensitivity skin test and increased the production of antibodies in response to hepatitis B and tetanus vaccines 51.
Lower alpha-tocopherol doses failed to improve the delayed-type hypersensitivity response compared to a placebo in another study in healthy participants (ages, 65-80 years) 52. A randomized, placebo-controlled trial in 617 nursing home residents (≥65 years of age) reported that daily supplementation with 200 IU of synthetic alpha-tocopherol (90 mg of RRR-α-tocopherol) for one year significantly lowered the risk of contracting upper respiratory tract infections, especially the common cold, but had no effect on lower respiratory tract (lung) infections 53. More research is needed to examine whether supplemental vitamin E might enhance immune function and reduce risk of infection in older adults.
Reduction of ultraviolet (UV) radiation-induced skin damage
The primary role of vitamin E in the skin is to prevent damage induced by free radicals and reactive oxygen species (ROS); therefore, the use of vitamin E in the prevention of ultraviolet (UV) radiation-induced skin damage has been extensively studied. Ultraviolet (UV) radiation has an immunosuppressive effect on the antigen-presenting cells (APCs) within the epidermis and contributes to the likelihood of skin cancer 54. The sun is by far the strongest source of ultraviolet radiation in our environment. Solar emissions include visible light, heat and ultraviolet (UV) radiation. Just as visible light consists of different colors that become apparent in a rainbow, there are three types of ultraviolet (UV) radiation: UVC, UVB, and UVA. As sunlight passes through the atmosphere, all UVC and most UVB is absorbed by ozone, water vapor, oxygen and carbon dioxide. UVA is not filtered as significantly by the atmosphere.
The ozone layer absorbs 100% of UVC, 90% of UVB, and a minimal amount of UVA 55. For this reason, the depletion of the ozone layer increases UV transmission. UVA is associated with aging and pigmentation 55. It penetrates deep into the skin layer and produces free radical oxygen species, indirectly damaging DNA. UVA increases the number of inflammatory cells in the dermis and decreases the number of antigen-presenting cells 56. UVB causes sunburn and DNA strand breaks. UVB causes pyrimidine dimer mutations, which are associated with nonmelanoma skin cancers 57.
Although molecules in the vitamin E family can absorb light in the UVB spectrum, the “sunscreen” activity of vitamin E is considered limited since it cannot absorb UVA light or light in higher wavelengths of the UVB spectrum 58. Therefore, the primary photoprotective effect of vitamin E is attributed to its role as a lipid-soluble antioxidant.
Many studies in cell culture models (test tube lab studies) have found protective effects of vitamin E molecules on skin cells 59, 60, 61, but these models do not recreate the complex structure of skin tissues. Therefore, human studies are needed.
Studies using orally administered vitamin E have reported mixed results on its photoprotective potential. An early study of vitamin E supplementation in hairless mice found no effect of dietary α-tocopherol acetate on UV-induced carcinogenesis 62. Three other mouse studies reported inhibition of UV-induced tumors in mice fed alpha-tocopherol acetate 63, 64, 65, but one of these studies utilized vitamin E doses that were toxic to animals when combined with the UV treatment 63. Another study in mice found a reduction of UV-induced DNA damage with dietary α-tocopherol acetate, but no effects on other free radical damage were observed in the skin 66. One human study reported that subjects taking 400 IU/day of alpha-tocopherol had reduced UV-induced lipid peroxidation in the skin but concluded there was no overall photoprotective effect 67. This was supported by another human study that found that 400 IU/day of alpha-tocopherol for six months provided no meaningful protection to skin 68. Furthermore, multiple human studies have shown no effect of vitamin E on the prevention or development of skin cancers 69, 70.
In contrast to oral supplementation with alpha-tocopherol alone, multiple studies have found that the combination of vitamin C and vitamin E protects the skin against UV damage. Human subjects orally co-supplemented with vitamins C and E show increased Minimal Erythemal Dose (MED), the lowest dose of ultraviolet radiation that will produce a detectable redness 24 hours after UV exposure 71, 72. The combination of vitamin C and vitamin E was associated with lower amounts of DNA damage after UV exposure 73. Results of another study suggest a mixture of tocopherols and tocotrienols may be superior to α-tocopherol alone, as the mixture showed reduced sunburn reactions and tumor incidence after UV exposure in mice 74. However, further trials with dietary tocotrienol/tocopherol mixtures are needed in human subjects.
Topical application of vitamin E is generally effective for increasing photoprotection of the skin. In rodent models, the application of alpha-tocopherol or alpha-tocopherol acetate before UV exposure reduces UV-induced skin damage by reducing lipid peroxidation 66, 75, 76, 77, limiting DNA damage 66, 78, 79, 80, and reducing the many chemical and structural changes to skin after UV exposure 81, 82, 83, 84. Vitamin E skin applications have also been shown to reduce UV-induced tumor formation in multiple mouse studies 81, 64, 85 and to reduce the effects of photo-activated toxins in the skin 86, 87, 88, 89. Skin application of vitamin E also reduces the effects of UV radiation when applied after the initial exposure. In mice, alpha-tocopherol acetate prevents some of the redness, edema, skin swelling, and skin thickening if applied immediately after UV exposure 83, 84. A similar effect has been shown in rabbits, where applying alpha-tocopherol to skin immediately after UV increased the Minimal Erythemal Dose (the lowest dose of ultraviolet radiation that will produce a detectable redness 24 hours after UV exposure) 90. While the greatest effect was seen when vitamin E was applied immediately after UV exposure, one study showed a significant effect of application eight hours after the insult 83. In human subjects, the use of vitamin E on skin lowers peroxidation of skin surface lipids 91, decreases erythema 92, 93 and limits immune cell activation after UV exposure 94.
Like oral supplementation with vitamin C and vitamin E, skin preparations with both vitamin C and vitamin E have also been successful. Together, the application of these antioxidants to the skin of animals before UV exposure has been shown to decrease sunburned cells 95, 96, decrease DNA damage 95, 97, inhibit redness 95, 98, and decrease skin pigmentation after UV exposure 98. Similar effects have been seen in human subjects 99, 100, 101.
While a majority of studies have found benefit of topical alpha-tocopherol, there is much less evidence for the activity of esters of vitamin E in photoprotection 91. As described above, vitamin E esters require cellular metabolism to produce “free” vitamin E. Thus, skin use of vitamin E esters may provide only limited benefit or may require a delay after administration to provide significant UV protection.
Other skin functions
There is limited information concerning the effects of vitamin E supplementation on photoaging, photodamage, solar damage, or sun damage, which is commonly observed as skin wrinkling. Although vitamin E can protect mice exposed to UV from excessive skin wrinkling, this is a photoprotective effect rather than treatment of pre-existing wrinkles. Other reports using vitamin E to treat photodamage or reduce wrinkles are poorly controlled studies or unpublished observations 102, 103. An analysis of the dietary intake of Japanese women showed no correlation between vitamin E consumption and skin wrinkling 104.
Vitamin E and oils containing tocopherols or tocotrienols have been reported to have moisturizing properties, but data supporting these roles are limited. Cross-sectional studies have shown no association between vitamin E consumption and skin hydration in healthy men and women 104, 105. However, two small studies have shown topical application of vitamin E can improve skin water-binding capacity after two to four weeks of use 106, 107. Long-term studies with topical vitamin E are needed to establish if these moisturizing effects can be sustained.
Environmental pollutants like ozone can decrease vitamin E levels in the skin 108, 109, 110 and lead to free radical damage that may compound the effects of UV exposure 110. Although not well studied, topical applications of vitamin E may reduce pollution-related free radical damage 109.
Anti-inflammatory effects
Vitamin E has been considered an anti-inflammatory agent in the skin, as several studies have supported its prevention of inflammatory damage after UV exposure. As mentioned above, topical vitamin E can reduce UV-induced skin swelling, skin thickness, erythema, and edema — all signs of skin inflammation. In cultured keratinocytes, α-tocopherol and γ-tocotrienol have been shown to decrease inflammatory prostaglandin synthesis, interleukin production, and the induction of cyclooxygenase-2 (COX-2) and NADPH oxidase by UV light 111, 112, 113, as well as limit inflammatory responses to lipid hydroperoxide exposure 114. In mice, dietary gamma-tocotrienol suppresses UV-induced COX-2 expression in the skin 113. Furthermore, topical application of α-tocopherol acetate or a gamma-tocopherol derivative inhibited the induction of COX-2 and nitric oxide synthase (iNOS) following UV exposure 115. In vitro studies (test tube lab studies) have shown similar anti-inflammatory effects of alpha- and gamma-tocopherol on immune cells 116, 117, 118.
Many of these anti-inflammatory effects of vitamin E supplementation have been reported in combination with its photoprotective effects, making it difficult to distinguish an anti-inflammatory action from an antioxidant action that would prevent inflammation from initially occurring. Despite these limitations, there are many reports of vitamin E being used successfully in chronic inflammatory skin conditions, either alone 119, 120 or in combination with vitamin C 121 or vitamin D 122, therefore suggesting a true anti-inflammatory action.
Wound healing
Skin lesions have been reported in rats suffering from vitamin E deficiency, although their origin is unclear. Vitamin E levels decrease rapidly at the site of a cutaneous wound, along with other skin antioxidants, such as vitamin C or glutathione 123. Since skin antioxidants slowly increase during normal wound healing, these observations have stimulated additional studies on the effect of vitamin E on the wound healing process. However, no studies have demonstrated a positive effect of vitamin E supplementation on wound repair in normal skin. Studies have shown that α-tocopherol supplementation decreases wound closure time in diabetic mice, but no effects have been observed in normal mice 124, 125. Vitamin E increases the breaking strength of wounds pre-treated with ionizing radiation 126, but this is likely due to antioxidant functions at the wound site akin to a photoprotective effect. In contrast, intramuscular injection of α-tocopherol acetate in rats has been suggested to decrease collagen synthesis and inhibit wound repair 127.
In humans, studies with topical alpha-tocopherol have either found no effects on wound healing or appearance or have found negative effects on the appearance of scar tissue 128, 129. However, these studies are complicated by a high number of skin reactions to the vitamin E preparations, possibly due to uncontrolled formation of tocopherol radicals in the solutions used. Despite these results, vitamin E, along with zinc and vitamin C, is included in oral therapies for pressure ulcers (bed sores) and burns 130, 131.
Vitamin C interactions
A few human studies using conditions of oxidative stress have demonstrated the importance of vitamin C (ascorbic acid) in the recycling of oxidized alpha-tocopherol back to its reduced state (see Figure 4). Oxidative stress caused by cigarette smoking accelerates the depletion of plasma alpha-tocopherol in smokers compared to nonsmokers 132. In a double-blind, placebo-controlled trial in 11 smokers and 13 nonsmokers given alpha-tocopherol and gamma-tocopherol that was labeled with deuterium (hence traceable), supplementation with vitamin C reduced the rate of vitamin E loss in plasma, most probably by regenerating tocopheryl radicals back to nonoxidized forms 133.
Vitamin K interactions
One study in adults with normal blood clotting (coagulation) status found that daily supplementation with 1,000 IU (670 mg) of RRR-alpha-tocopherol for 12 weeks decreased gamma-carboxylation of prothrombin, a vitamin K-dependent factor in the coagulation cascade 134. Individuals taking anticoagulant drugs like warfarin and those who are vitamin K deficient should not take vitamin E supplements without medical supervision because of the increased risk of bleeding 135.
Vitamin E Supplements
Vitamin E supplements come in different amounts and forms. Supplements of vitamin E typically provide only alpha-tocopherol, although “mixed” products containing other tocopherols and even tocotrienols are available such as gamma-tocopherol, tocotrienols, and mixed tocopherols. Scientists do not know if any of these forms are superior to alpha-tocopherol in supplements.
Two main things to consider when choosing a vitamin E supplement are:
- The amount of vitamin E: Most once-daily multivitamin-mineral supplements provide about 13.5 mg of vitamin E, whereas vitamin E-only supplements commonly contain 67 mg or more. The doses in most vitamin E-only supplements are much higher than the recommended amounts. Some people take large doses because they believe or hope that doing so will keep them healthy or lower their risk of certain diseases.
- The form of vitamin E: Although vitamin E sounds like a single substance, it is actually the name of eight related compounds in food, including alpha-tocopherol. Each form has a different potency, or level of activity in the body.
Naturally occurring alpha-tocopherol exists in one stereoisomeric form, commonly listed as ”D-alpha-tocopherol” on food packaging and supplement labels. In contrast, synthetically produced (laboratory-made) alpha-tocopherol contains equal amounts of its eight possible stereoisomers, commonly listed as ”DL-alpha-tocopherol”; serum and tissues maintain only four of these stereoisomers 1. A given amount of synthetic alpha-tocopherol (all rac-alpha-tocopherol; commonly labeled as “DL” or “dl”) is therefore only half as active as the same amount (by weight in mg) of the natural form (RRR-alpha-tocopherol; commonly labeled as “D” or “d”). People need approximately 50% more IU of synthetic alpha tocopherol from dietary supplements and fortified foods to obtain the same amount of the nutrient as from the natural form.
- The natural vitamin E (D-alpha-tocopherol) is more potent; 1 mg vitamin E = 1 mg d-alpha-tocopherol (natural vitamin E) = 2 mg dl-alpha-tocopherol (synthetic vitamin E).
Some food and dietary supplement labels still list vitamin E in International Units (IUs) rather than mg. 1 IU of the natural form of vitamin E is equivalent to 0.67 mg. 1 IU of the synthetic form of vitamin E is equivalent to 0.45 mg.
Some vitamin E supplements provide other forms of the vitamin, such as gamma-tocopherol, tocotrienols, and mixed tocopherols. Scientists do not know if any of these forms are superior to alpha-tocopherol in supplements.
Most vitamin-E-only supplements provide ≥100 IU of the nutrient. These amounts are substantially higher than the recommended dietary allowances. The 1999–2000 National Health and Nutrition Examination Survey (NHANES) found that 11.3% of adults took vitamin E supplements containing at least 400 IU 136.
Alpha-tocopherol in dietary supplements and fortified foods is often esterified to prolong its shelf life while protecting its antioxidant properties. The body hydrolyzes and absorbs these esters (alpha-tocopheryl acetate and succinate) as efficiently as alpha-tocopherol 1.
Vitamin E interactions with medications
Vitamin E supplements have the potential to interact with several types of medications. A few examples are provided below. People taking these and other medications on a regular basis should discuss their vitamin E intakes with their healthcare providers.
Vitamin E has a few interactions with medications that are listed below:
- Anticoagulation and antiplatelet medications: due to vitamin E inhibiting platelet aggregation and disrupting vitamin K clotting factors there is a protentional increase risk of bleeding combining these two. Vitamin E can inhibit platelet aggregation and antagonize vitamin K-dependent clotting factors. As a result, taking large doses with anticoagulant or antiplatelet medications, such as warfarin (Coumadin®), can increase the risk of bleeding, especially in conjunction with low vitamin K intake. The amounts of supplemental vitamin E needed to produce clinically significant effects are unknown but probably exceed 400 IU/day 17.
- Simvastatin and niacin: Vitamin E can reduce the amount of high-density lipoprotein (HDL or “good” cholesterol) which is the opposite desired effect of taking simvastatin and/or niacin. Some people take vitamin E supplements with other antioxidants, such as vitamin C, selenium, and beta-carotene. This collection of antioxidant ingredients blunted the rise in high-density lipoprotein (HDL) cholesterol levels, especially levels of HDL, the most cardioprotective HDL component, among people treated with a combination of simvastatin (brand name Zocor®) and niacin 137.
- Chemotherapy and radiotherapy: Oncologists generally advise against the use of antioxidant supplements during cancer chemotherapy or radiotherapy because they might reduce the effectiveness of these therapies by inhibiting cellular oxidative damage in cancerous cells 138. Although a systematic review of randomized controlled trials has called this concern into question 139, further research is needed to evaluate the potential risks and benefits of concurrent antioxidant supplementation with conventional therapies for cancer.
Vitamin E deficiency causes
In developed countries, it is unlikely that vitamin E deficiency occurs due to diet intake insufficiency and the more common causes are below 140:
- In developing countries, the most common cause is inadequate intake of vitamin E.
- Premature low birth weight infants with a weight less than 1500 grams (3.3 pounds)
- Mutations in the alpha-tocopherol transfer protein (TTPA) gene causing impaired fat metabolism 141
- Disrupted fat malabsorption as the small intestine requires fat to absorb vitamin E
- Patients with cystic fibrosis fail to secrete pancreatic enzymes to absorb vitamins A, D, E, and K
- Short-bowel syndrome patients may take years to develop symptoms. Short-bowel syndrome may develop from intestinal pseudo-obstruction, surgical resection, or mesenteric vascular thrombosis. Only after 10-20 years of malabsorption do neurologic symptoms become clinically apparent.
- Chronic cholestatic hepatobiliary disease leads to a decrease in bile flow and micelle formation that is needed for vitamin E absorption. Chronic cholestatic hepatobiliary disease in infants as young as 2 years may result from this condition 142. Prior to age 1 year, neurologic function was normal in all children. Between ages 1 and 3 years, neurologic abnormalities were present in approximately 50% of the vitamin E-deficient children; after age 3 years, neurologic abnormalities were present in all vitamin E-deficient children. Areflexia was the first abnormality to develop between ages 1 and 4 years; truncal and limb ataxia, peripheral neuropathy, and ophthalmoplegia developed between ages 3 and 6 years. Neurologic dysfunction progressed to a disabling combination of findings by ages 8 to 10 years in the majority of vitamin E-deficient children. Neurologic findings are less frequent in adult patients with cholestasis secondary to cirrhosis.
- Crohn’s disease, exocrine pancreatic insufficiency, and liver disease may all not absorb fat
- Abetalipoproteinemia is a rare genetic, autosomal-recessive disease that causes an error in lipoprotein production and transportation. Infants present with steatorrhea from the time of birth. Patients have pigmented retinopathy and progressive ataxia, and they develop acanthosis of red blood cells in the first decade of life.
- Ataxia with vitamin E deficiency (AVED) also known as Familial Isolated Vitamin E Deficiency or Friedreich-like ataxia with vitamin E deficiency, is an autosomal recessive neurodegenerative disorder caused by alpha-tocopherol transfer protein (TTPA) gene mutations on chromosome 8q13 (long arm of chromosome 8) 143. Neurologic findings develop within the first decade of life, and no clinical findings distinguish deficiency from ataxia and movement disorders. Vitamin E replacement can significantly influence the outcome; therefore, screening for vitamin E deficiency is beneficial for patients with movement disorders or neuropathies that are of unknown cause.
Vitamin E deficiency often occurs secondary to disorders that impair the absorption of vitamin E from fat including liver disorders, disorders of fat metabolism and disorders of bile secretion. These disorders include choleostasis (a syndrome of various causes characterized by impaired bile secretion), cystic fibrosis (primarily a lung disorder that results in choleostasis), primary biliary cirrhosis (a liver disorder that results in choleostasis) and abetalipoproteinemia (a digestive disorder characterized by fat malabsorption). Premature infants may have low vitamin E levels due to small amounts of vitamin E cross the placenta. In rare cases vitamin E deficiency may be caused due to poor diet.
Cigarette smoking is thought to increase the utilization of alpha-tocopherol such that smokers might be at increased risk of vitamin E deficiency compared with nonsmokers 132. Also, the 19-year follow-up analysis of the Alpha-Tocopherol, Beta-Carotene cancer (ATBC) trial in older, male smokers indicated that participants in the highest versus lowest quintile of serum alpha-tocopherol concentrations (>31 μmol/L vs. <23 μmol/L) at baseline had reduced risks of total and cause-specific mortality 144.
Ataxia with Vitamin E Deficiency (AVED)
Ataxia with vitamin E deficiency (AVED), also known as Familial Isolated Vitamin E deficiency, Isolated Vitamin E deficiency or Friedreich-like ataxia with vitamin E deficiency, is a rare progressive neurodegenerative disorder of autosomal recessive cerebellar ataxia (ARCA) 145, 146, 147, 148, 149, 150. The most prominent symptoms of ataxia with vitamin E deficiency (AVED) include progressive cerebellar ataxia (difficulty coordinating movements), slurred speech (dysarthria), numbness in the hands and feet (peripheral neuropathy), absence of neurologic reflexes (areflexia), and progressive leg weakness 148. Ataxia with vitamin E deficiency (AVED) patients may also have retinitis pigmentosa (a group of rare eye diseases that affect the retina that causes severe vision impairment such as decreased vision at night or in low light and loss of side vision (tunnel vision)), a disease of the heart muscle (cardiomyopathy) and scoliosis (a sideways curvature of the spine) 151, 152. Ataxia with vitamin E deficiency (AVED) symptoms typically begin during childhood or adolescence and worsen with age, resulting in the need for a wheelchair by early adulthood 153. Vitamin E supplementation may prevent the worsening of the condition of patients with ataxia with vitamin E deficiency (AVED).
Ataxia with vitamin E deficiency (AVED) is caused by mutations in the alpha-tocopherol transfer protein (TTPA) gene, which encodes the alpha-tocopherol transfer protein (α-TTP), which in turn binds alpha-tocopherol and transports vitamin E from liver cells to circulating lipoproteins 154. More than 20 mutations in the TTPA gene have been found to cause ataxia with vitamin E deficiency 155. Some of these TTPA mutations cause no functional protein to be made, while others change a single protein building block (amino acid) in the alpha-tocopherol transfer protein (α-TTP), reducing its function 155. As a result, the body cannot retain or use dietary vitamin E, which leads to reduced levels of vitamin E in your blood and the accumulation of free radicals. Mutations in the TTPA gene that cause no functional alpha-tocopherol transfer protein (α-TTP) to be made are associated with a severe form of ataxia that begins at a young age 155. Mutations that reduce but do not eliminate the alpha-tocopherol transfer protein (α-TTP) function are associated with milder ataxia that occurs at a later age and progresses more slowly. One TTPA gene mutation that is found in the Japanese population changes the amino acid histidine to the amino acid glutamine at position 101 in the αTTP protein (written as His101Glu or H101Q). This mutation is associated with the development of an eye disorder called retinitis pigmentosa that causes vision loss in people with ataxia with vitamin E deficiency 155.
Ataxia with vitamin E deficiency (AVED) affects both males and females equally. Ataxia with vitamin E deficiency (AVED) is estimated to occur in fewer than 1 in 1,000,000 people 156. North African populations are most affected with AVED. Other cases have been reported in the Mediterranean region and Northern European countries. There have been cases in Asian countries such as Japan, China and the Philippines. The onset of AVED can occur during childhood or adulthood with cases reported ranging in children as young as 2 and adults as old as 52. Typically, the disease presents in individuals between ages 5 and 20 years. The disorder was first described in the medical literature in 1981.
Untreated ataxia with vitamin E deficiency (AVED) generally manifests between ages 5 and 15 years 147. The first signs and symptoms include progressive ataxia, clumsiness of the hands, loss of proprioception, and absence of neurologic reflexes (areflexia). Other features often observed are dysdiadochokinesia, dysarthria, positive Romberg sign, head titubation, decreased visual acuity, and positive Babinski sign 147. Although age of onset and disease course are more uniform within a given family, disease manifestations and their severity can vary even among siblings.
Ataxia with vitamin E deficiency (AVED) is caused by alpha-tocopherol transfer protein (TTPA) gene mutations on chromosome 8q13 (long arm of chromosome 8), which encodes the alpha-tocopherol transfer protein (α-TTP), which in turn binds alpha-tocopherol and transports vitamin E from liver cells to circulating lipoproteins 157, 158, 159, 160, 161, 162, 163, 164, 165. When the alpha-tocopherol transfer protein (TTPA) gene is damaged, vitamin E cannot be distributed throughout the body. Vitamin E is important because it protects the cells of the neurological system (neurons) from damaging molecules called free radicals. Ataxia with vitamin E deficiency (AVED) is inherited in an autosomal recessive manner 154.
Marriotti et al. 166 reported cerebellar atrophy in some individuals with AVED. Spinal sensory demyelination with neuronal atrophy and axonal spheroids and neuronal lipofuscin accumulation in the third cortical layer of the cerebral cortex, thalamus, lateral geniculate body, spinal horns, and posterior root ganglia are the commonest histopathology findings 167. The following criteria should be met to diagnose ataxia with vitamin E deficiency (AVED) 168:
- Friedreich ataxia‐like neurologic phenotype
- Markedly reduced plasma vitamin E
- Normal lipoprotein profile
- Exclusion of disease that cause fat malabsorption.
Individuals with AVED are treated with high doses of vitamin E supplements, but recovery is slow and incomplete 169. Early diagnosis and treatment can slow down or stop the progression of the disorder and in some people, even improve existing symptoms. Lifelong treatment with vitamin E will be needed. Genetic counseling is recommended for affected individuals and their families.
Vitamin E supplementation should be started as soon as possible, but the optimal dosage is still variable 168. The doses and the route of vitamin E supplementation depend on the causes of vitamin E deficiency. The treatment of choice for AVED is lifelong high‐dose oral vitamin E supplementation. The early use of vitamin E supplementation in the disease process helps to reverse some neurological symptoms like ataxia and intellectual deterioration 170.
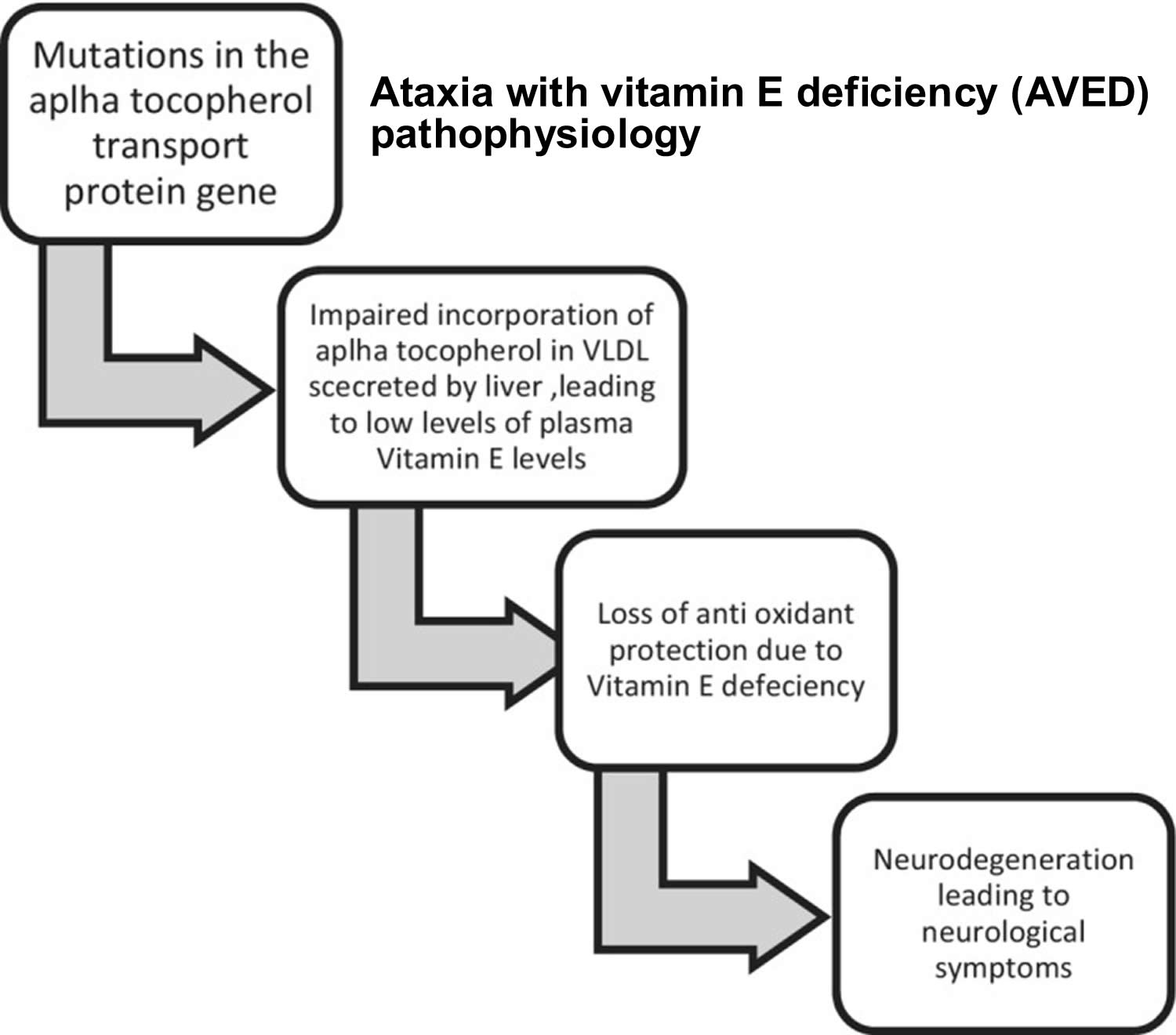
Figure 6. Ataxia with vitamin E deficiency (AVED) pathophysiology
[Source 168 ]Ataxia with Vitamin E Deficiency (AVED) causes
Ataxia with vitamin E deficiency (AVED) is caused by changes (mutations or pathogenic variants) in the TTPA gene, which provides instructions to synthesize the alpha-tocopherol transfer protein (αTTP) 143. Alpha-tocopherol transfer protein (αTTP) controls the distribution and transportation of vitamin E from the liver to other cells and tissues throughout the body, including the brain. Individuals with AVED have non-working genes for TTPA and therefore, vitamin E cannot be properly distributed throughout the body especially to the brain where it is necessary for proper function. AVED is characterized by very low levels of vitamin E in the blood and tissues in the brain. Without normal levels of vitamin E in the tissues, an individual with AVED experiences damage to the body from lack of protection from damaging free radicals.
The mechanism of neurologic dysfunction in AVED is unknown, but neuronal oxidative injury has been hypothesized 145. Brain imaging demonstrates cerebellar atrophy in up to 50% of cases 158, 171. Other brain regions are also affected. Animal models of AVED and post-mortem studies of humans with secondary vitamin E deficiency have shown evidence of degenerative changes in the substantia nigra, posterior column, nerve roots, and peripheral nervous system 172, 173, 174, 175, 176, 177, 178:915–936. doi: 10.4049/jimmunol.0903008)). Post-mortem studies of patients with AVED demonstrate loss of cerebellar Purkinje cells, dying back-type posterior column degeneration with spheroids and corpora amylacea, and lipofuscin accumulation 179, 180. Ataxia in AVED is likely caused by a combination of cerebellar and posterior column degeneration 145.
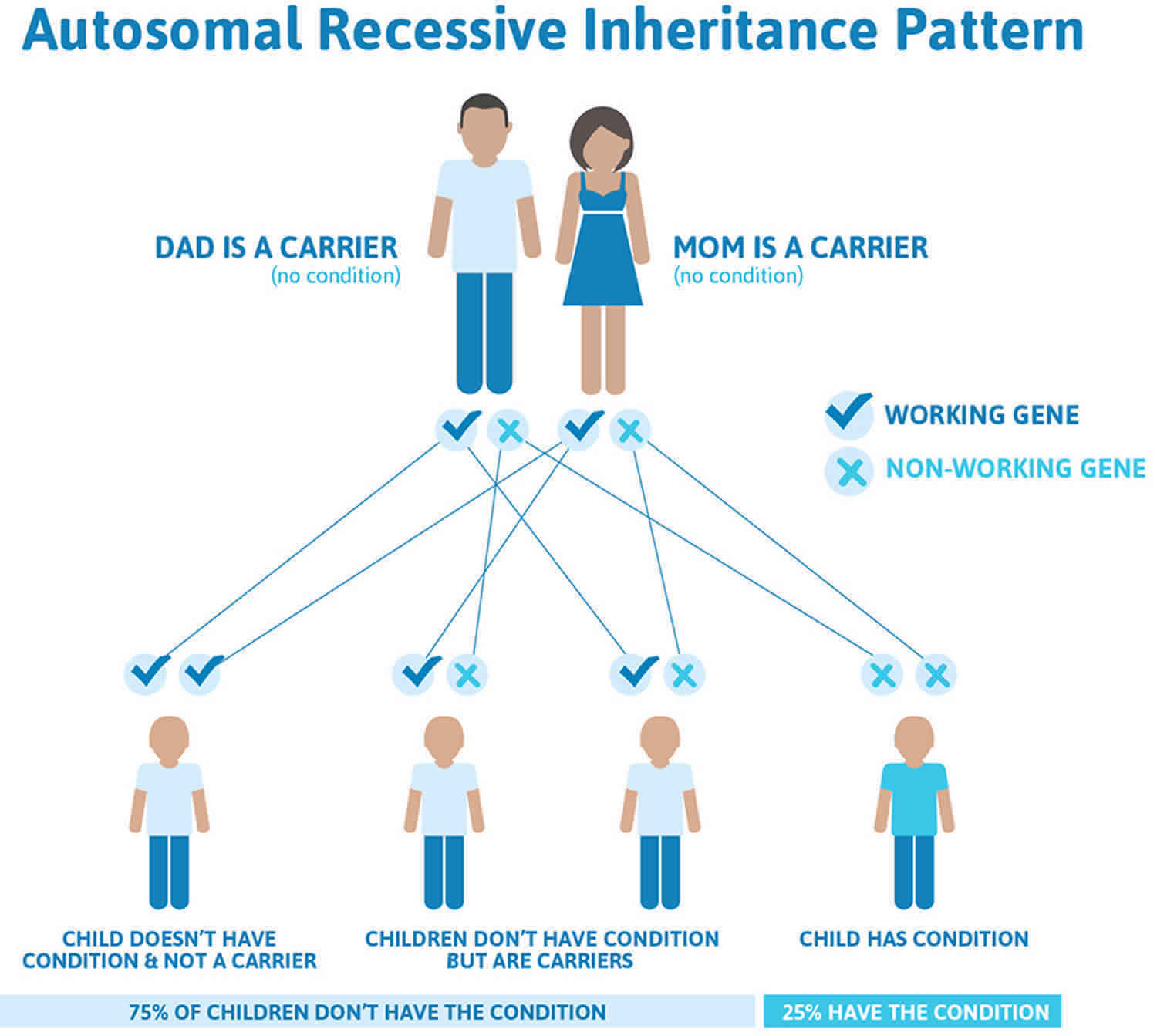
Ataxia with vitamin E deficiency (AVED) is inherited, or passed down from parent to child, in an autosomal recessive manner. Recessive genetic disorders occur when an individual inherits a non-working gene from each parent. If an individual receives one working gene and one non-working gene for the disease, the person will be a carrier for the disease, but usually will not show symptoms. The risk for two carrier parents to both pass the non-working gene and, therefore, have a child with AVED is 25% with each pregnancy. The risk of having a child who is a carrier, like the parents, is 50% with each pregnancy. The chance for a child to receive working genes from both parents is 25%. The risk is the same for males and females.
Figure 7. Ataxia with vitamin E deficiency autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Ataxia with Vitamin E Deficiency (AVED) signs and symptoms
Individuals usually show symptoms between 5 and 20 years of age. Symptoms and the severity of ataxia with vitamin E deficiency (AVED) may be different from person to person. Without treatment, symptoms may get worse as the person grows older.
Untreated individuals with Ataxia with Vitamin E Deficiency (AVED)
| Signs and symptoms | % of Persons with signs and symptoms | ||
|---|---|---|---|
| Cerebellar involvement | Gait impairment | 93.4% | |
| Dysarthria Dysarthria occurs when the muscles you use for speech are weak or you have difficulty controlling them. Dysarthria often causes slurred or slow speech that can be difficult to understand. | 61.8% | ||
| Head titubation (involuntary tremor or uncontrollable rhythmic shaking that occurs in the head) | 33% | ||
| Nystagmus | 5.3% | ||
| Lower motor neuron involvement | Areflexia | 94.7% | |
| Deep sensory disturbances | 67.1% | ||
| Upper motor neuron involvement | Babinski sign | 85.5% | |
| Urinary urgency | 22.4% | ||
| Pigmentary retinopathy | 2.3% | ||
| Cardiomyopathy | 1.5% | ||
Footnote: Frequency of signs and symptoms associated with untreated individuals with ataxia with vitamin E deficiency (AVED) based on findings in 132 individuals of North African heritage.
[Source 149 ]AVED affects the brain and spinal cord (central nervous system or CNS) as well as the motor and sensory nerves that connect the central nervous system (CNS) to the rest of the body (peripheral nervous system) 156. This results in ataxia, which is difficulty controlling body movements and numbness of the hands and feet (peripheral neuropathy). Individuals with AVED develop increasing weakness of the legs, which may appear as an unsteady or staggering way of walking (gait) or trembling when standing still 156. If symptoms become very severe, an individual with AVED may require a wheelchair if they cannot walk 156. Clinical symptoms like progressive ataxia with decreased reflex remain the prominent symptoms of AVED. According to the study done in North Africa, most of the individuals with AVED had mild neuropathy and were mostly combined types of neuropathies (both sensory and motor) 181.
Additional symptoms related to the central nervous system (CNS) include loss of proprioception, which is an awareness of joint position in relation to other parts of the body 156. With time, reflexes in the legs may slow down or be absent (areflexia). Involvement of the throat muscles may lead to impaired swallowing or choking and may cause difficulty in eating. Slurred speech or difficulty speaking (dysarthria) may also be present. Some affected individuals may develop a tremor or shaking of the head (titubation). Intellect and emotions are rarely affected.
In some people with AVED, psychotic episodes and intellectual decline have been described 147. In rare individuals, school performance declines secondary to loss of intellectual capacities 147.
In addition to neurological symptoms, individuals with AVED may develop symptoms affecting other systems of the body including the eyes 156. Retinitis pigmentosa (RP) is a large group of rare eye diseases that cause progressive degeneration of the membrane lining the eyes (retina). This results in visual impairment or decreased vision. A high percentage of affected individuals (e.g., 8/11 individuals in one series) experience decreased visual acuity 152. Some affected individuals may have yellow “fatty” deposits (xanthelasma) in the retina.
Affected individuals may also develop sideways curvature of the spine (scoliosis), degenerative changes of the heart muscle (cardiomyopathy) or “fatty” deposits (xanthomas) affecting the Achilles tendon around the ankle 156.
Some individuals with ataxia with vitamin E deficiency (AVED) may experience a form of dystonia 182, 145. Dystonia is the name for a group of movement disorders generally characterized by involuntary muscle contractions that force the body into abnormal, sometimes painful, movements and positions (postures). These contractions result in twisting and repetitive movements. Dystonia can affect just one muscle, a group of muscles or all of your muscles. Symptoms can include tremors, voice problems or a dragging foot. Symptoms often start in childhood. They can also start in the late teens or early adulthood. Some cases worsen over time. Others are mild.
Ataxia with Vitamin E Deficiency (AVED) diagnosis
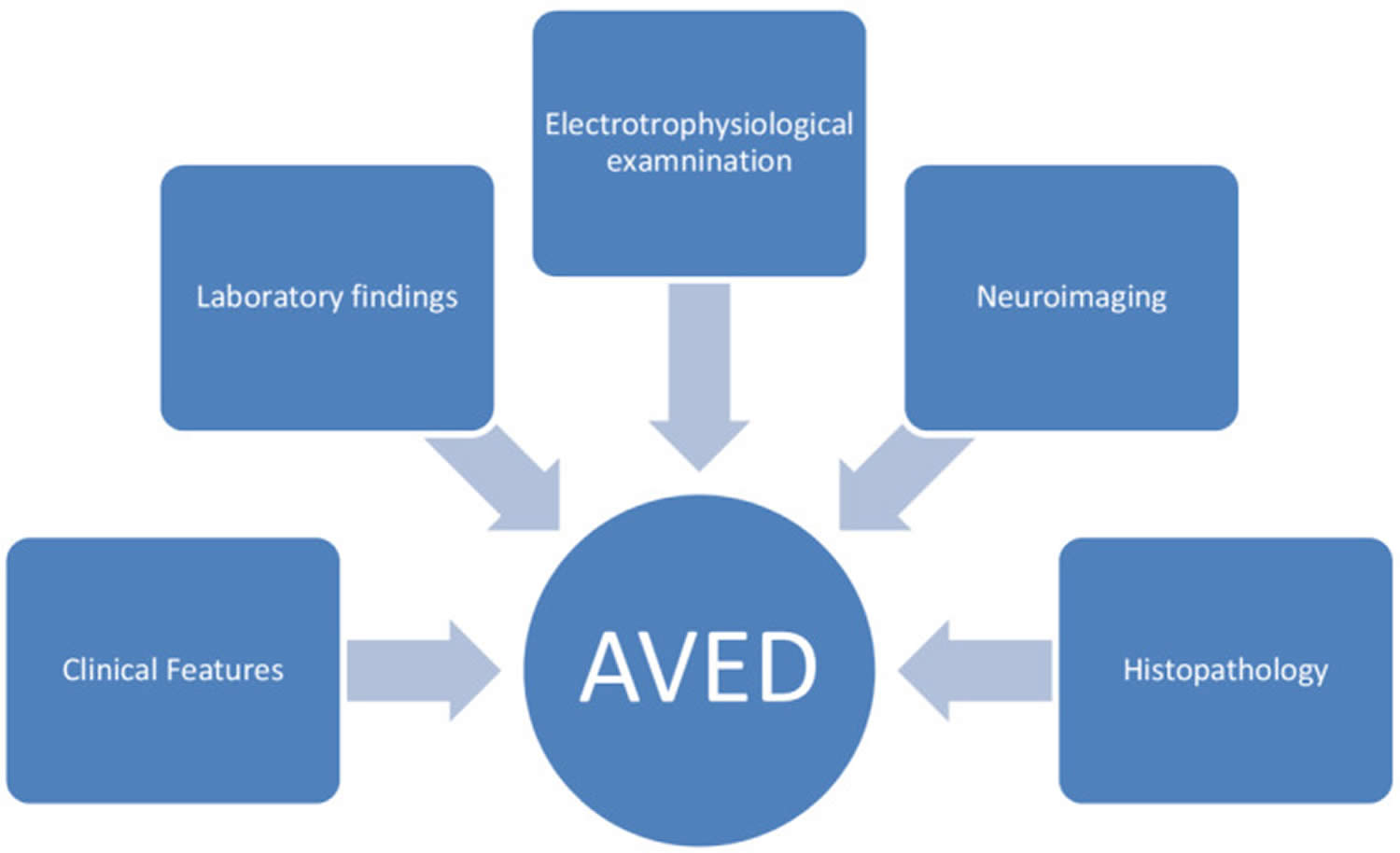
A diagnosis of ataxia with vitamin E deficiency (AVED) is made upon a thorough clinical evaluation, a detailed patient history, and a variety of tests and characteristic findings (e.g., low levels of vitamin E with normal levels of lipoproteins and lipids with no evidence of fat malabsorption and abnormalities in the TTPA gene).
AVED should be suspected in an individual with the following clinical and laboratory findings and family history 147:
- Family history
- Family history is consistent with autosomal recessive inheritance (e.g., affected sibs and/or parental consanguinity). Absence of a known family history does not preclude the diagnosis.
- Clinical features
- Onset between ages 5 and 15 years
- Progressive cerebellar findings including the following:
- Gait ataxia
- Clumsiness of the hands
- Loss of proprioception (especially distal joint position and vibration sense)
- Dysdiadochokinesia
- Positive Romberg sign
- Head titubation
- Lower motor neuron involvement. Areflexia
- Upper motor neuron involvement. Positive Babinski sign
- Ophthalmologic involvement. Decreased visual acuity due to macular degeneration, pigmentary retinopathy
- Supportive laboratory findings
- Normal lipid and lipoprotein profile
- Very low plasma vitamin E (alpha-tocopherol, or α-tocopherol) concentration
- Note: There is no universal normal range of plasma vitamin E concentration, as it depends on the test method and varies among laboratories.
- In Finckh et al 183, the plasma vitamin E (alpha-tocopherol, or α-tocopherol) concentration normal range lies between 9.0 and 29.8 µmol/L. In El Euch-Fayache et al 149, the normal range is given as 16.3-34.9 µmol/L, while individuals with AVED had vitamin E levels between 0.00 and 3.76 µmol/L (mean 0.95 µmol/L). In individuals with AVED, the plasma vitamin E concentration is generally lower than 4.0 µmol/L (<1.7 mg/L) 160, 158.
- Because oxidation of alpha-tocopherol (α-tocopherol) by air may invalidate test results, the following precautions with a blood sample should be taken:
- Centrifugation of the EDTA blood soon after venipuncture
- Quick separation of plasma from blood cells after centrifugation and subsequent flash freezing of the plasma in liquid nitrogen
- Filling the space above the plasma with an inert gas (e.g., argon or nitrogen)
- Protecting the sample from light by wrapping the container in aluminum foil, or using a black or light-shielded Eppendorf tube
- Shipment of the sample to the test laboratory in dry ice
- Electrophysiologic findings
- No electrophysiologic findings (motor nerve conduction velocities, compound muscle action potentials, or nerve sensory action potentials) are specific to or diagnostic of AVED; even the presence of a severe neuropathy does not exclude the diagnosis of AVED.
- Somatosensory evoked potentials show increased central conduction time between the segment C1 (N13b) and the sensorimotor cortex (N20) and increased latencies of the N20 (median nerve) and P40 (tibial nerve) waves. The P40 wave may be missing completely 170.
- Neuroimaging
The diagnosis of ataxia with vitamin E deficiency (AVED) is established by molecular genetic testing with the identification of abnormalities in the TTPA gene 147.
Figure 8. Ataxia with vitamin E deficiency (AVED) diagnosis
[Source 168 ]Ataxia with Vitamin E Deficiency (AVED) treatment
Individuals with ataxia with vitamin E deficiency (AVED) are treated with high doses of vitamin E supplements. Early diagnosis and treatment can slow down or stop the progression of the disorder and in some people, even improve existing symptoms. The treatment of choice for ataxia with vitamin E deficiency (AVED) is lifelong high-dose oral vitamin E supplementation. With treatment, plasma vitamin E concentrations can become normal. Genetic counseling is recommended for affected individuals and their families.
One of the following vitamin E preparations is used 147:
- The chemically manufactured racemic form, all-rac-alpha-tocopherol acetate
- The naturally occurring form, RRR-alpha-tocopherol
- It is currently unknown whether affected individuals should be treated with all-rac-alpha-tocopherol acetate or with RRR-alpha-tocopherol. It is known that alpha-tocopherol transfer protein (α-TTP) stereoselectively binds and transports 2R-alpha-tocopherols 185, 186, 187. For some TTPA pathogenic variants, this stereoselective binding capacity is lost and affected individuals cannot discriminate between RRR- and SRR-alpha-tocopherol 162, 160. In this instance, affected individuals would also be able to incorporate non-2R-alpha-tocopherol stereoisomers into their bodies if they were supplemented with all-rac-alpha-tocopherol. Since potential side effects of the synthetic stereoisomers have not been studied in detail, it seems appropriate to treat with RRR-alpha-tocopherol, despite the higher cost.
When vitamin E treatment is initiated in presymptomatic individuals (e.g., younger age), manifestations of ataxia with vitamin E deficiency (AVED) do not develop 188, 149.
No large-scale therapeutic studies have been performed to determine optimal vitamin E dosage and to evaluate outcomes 147. The reported vitamin E dose ranges from 800 mg to 1,500 mg (or 40 mg/kg body weight in children) 189, 190, 188, 160, 170, 191, 171, 158. The ideal dose of vitamin E ranges from 800 mg to 1500 mg (40 mg/kg/day) in children 166, 169.
Periodic follow‐up is required during vitamin E therapy. The plasma concentration of vitamin E should be measured in regular intervals, usually, every 6 months to maintain the level of vitamin E in the high normal range especially in children. Ideally the plasma vitamin E concentration should be maintained in the high-normal range 147.
Individuals with AVED should avoid smoking and occupations requiring quick responses or good balance. Smoking reduces the total radical trapping antioxidant parameter of plasma (TRAP), which is regarded as the best prognostic marker during the supplementation of vitamin E, leading to the reduction in plasma vitamin level 192.
Symptomatic individuals
Some clinical signs and symptoms (e.g., ataxia and intellectual deterioration) can be reversed in symptomatic individuals if treatment is initiated early in the disease process 170.
In older individuals, disease progression can be stopped, but deficits in proprioception and gait unsteadiness generally remain 171, 158, 149.
Supportive Care
The goals of supportive care in those with clinical signs and symptoms of AVED are to maximize function and reduce complications. Depending on the clinical signs and symptoms, it is recommended that each individual be managed by a multidisciplinary team of relevant specialists such as neurologists, occupational therapists, physical therapists, physiatrists, orthopedists, nutritionists, speech-language pathologists, pulmonologists, and mental health specialists 147.
Pregnancy Management
Because reduced vitamin E levels are associated with low fertility and embryo resorption in mice 193 and α-tocopherol transfer protein is highly expressed in the human placenta 194, it is advisable for women with AVED to maintain vitamin E levels in the high-normal range during pregnancy 147.
Ataxia with Vitamin E Deficiency (AVED) prognosis
Ataxia with Vitamin E Deficiency (AVED) prognosis despite the supplementation of vitamin E depends on the timing of the supplementation and the age of the onset of vitamin E deficiency. Vitamin E treatment in pre‐symptomatic individuals with a history of family cases of AVED can help to prevent the individual from primary manifestations. The symptoms do not develop in the individuals with the initiation of vitamin E earlier in pre‐symptomatic individuals who are at risk 195, 158, 171. Older individuals usually remain deficient in proprioception and gait unsteadiness although the progression of the disease can be stopped with vitamin E supplementation 181.
Without treatment, most patients with ataxia with vitamin E deficiency (AVED) disease onset during childhood (age <18 years) become wheelchair-bound within 5–20 years of disease onset 145.
The specific response of dystonia in AVED to vitamin E treatment is variable 145. Dystonia has been reported to improve in two patients 158, 182 and most patients with dystonia as a feature of their disease had stabilization or improvement of their other symptoms on high dose vitamin E 145. One patient, however, experienced progressive dystonia despite escalating doses of vitamin E supplementation 196. There are anecdotal reports of dystonia improvement in response to symptomatic treatment with botulinum toxin, trihexyphenidyl and clonazepam 196, 182.
Vitamin E deficiency prevention
The amount of vitamin E you need each day depends on your age. Average daily recommended amounts are listed below in milligrams (mg) and in International Units (IU). Package labels list the amount of vitamin E in foods and dietary supplements in International Units (IU). Intake recommendations for vitamin E and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of The National Academies 1. The Food and Nutrition Board’s vitamin E recommendations are for alpha-tocopherol alone, the only form maintained in plasma. The Food and Nutrition Board (FNB) based these recommendations primarily on serum levels of the nutrient that provide adequate protection in a test measuring the survival of red blood cells when exposed to hydrogen peroxide, a free radical. Acknowledging “great uncertainties” in these data, the Food and Nutrition Board (FNB) has called for research to identify other biomarkers for assessing vitamin E requirements.
Recommended Dietary Allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy people. The RDAs for vitamin E are provided in milligrams (mg) and are listed in Table 1. Because insufficient data are available to develop RDAs for infants, Adequate Intake (AI) were developed based on the amount of vitamin E consumed by healthy breastfed babies.
At present, the vitamin E content of foods and dietary supplements is listed on labels in international units (IUs), a measure of biological activity rather than quantity. Naturally sourced vitamin E is called RRR-alpha-tocopherol (commonly labeled as d-alpha-tocopherol); the synthetically produced form is all rac-alpha-tocopherol (commonly labeled as dl-alpha-tocopherol). Conversion rules are as follows:
To convert from mg to IU:
- 1 mg of alpha-tocopherol is equivalent to 1.49 IU of the natural form or 2.22 IU of the synthetic form.
To convert from IU to mg:
- 1 IU of the natural form is equivalent to 0.67 mg of alpha-tocopherol.
- 1 IU of the synthetic form is equivalent to 0.45 mg of alpha-tocopherol.
Table 1 lists the RDAs for alpha-tocopherol in both mg and IU of the natural form; for example, 15 mg x 1.49 IU/mg = 22.4 IU. The corresponding value for synthetic alpha-tocopherol would be 33.3 IU (15 mg x 2.22 IU/mg).
Table 1. Recommended Dietary Allowances (RDAs) for Vitamin E (Alpha-Tocopherol)
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 4 mg (6 IU) |
| Infants 7–12 months* | 5 mg (7.5 IU) |
| Children 1–3 years | 6 mg (9 IU) |
| Children 4–8 years | 7 mg (10.4 IU) |
| Children 9–13 years | 11 mg (16.4 IU) |
| Teens 14–18 years | 15 mg (22.4 IU) |
| Adults | 15 mg (22.4 IU) |
| Pregnant teens and women | 15 mg (22.4 IU) |
| Breastfeeding teens and women | 19 mg (28.4 IU) |
Footnote: *Adequate Intake (established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy)
What foods provide vitamin E?
Numerous foods provide vitamin E. Nuts, seeds, and vegetable oils are among the best sources of alpha-tocopherol, and significant amounts are available in green leafy vegetables and fortified cereals (see Table 2 for a more detailed list) 197. Most vitamin E in American diets is in the form of gamma-tocopherol from soybean, canola, corn, and other vegetable oils and food products 25.
Vitamin E is found naturally in foods and is added to some fortified foods. You can get recommended amounts of vitamin E by eating a variety of foods including the following:
- Vegetable oils like wheat germ, sunflower, and safflower oils are among the best sources of vitamin E. Corn and soybean oils also provide some vitamin E.
- Nuts (such as peanuts, hazelnuts, and, especially, almonds) and seeds (like sunflower seeds) are also among the best sources of vitamin E.
- Green vegetables, such as spinach and broccoli, provide some vitamin E.
- Food companies add vitamin E to some breakfast cereals, fruit juices, margarines and spreads, and other foods. To find out which ones have vitamin E, check the product labels.
Vitamin E from natural (food) sources is commonly listed as “d-alpha-tocopherol” on food packaging and supplement labels. Synthetic (laboratory-made) vitamin E is commonly listed as “dl-alpha-tocopherol”. The natural form is more potent. For example, 100 IU of natural vitamin E is equal to about 150 IU of the synthetic form.
Some vitamin E supplements provide other forms of the vitamin, such as gamma-tocopherol, tocotrienols, and mixed tocopherols. Scientists do not know if any of these forms are superior to alpha-tocopherol in supplements.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods, including, in some cases, the amounts of alpha-, beta-, gamma-, and delta-tocopherol arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminE-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminE-Food.pdf).
Table 2. Food Sources of Vitamin E (Alpha-Tocopherol)
| Food | Milligrams (mg) per serving | Percent DV* |
| Wheat germ oil, 1 tablespoon | 20.3 | 100 |
| Sunflower seeds, dry roasted, 1 ounce | 7.4 | 37 |
| Almonds, dry roasted, 1 ounce | 6.8 | 34 |
| Sunflower oil, 1 tablespoon | 5.6 | 28 |
| Safflower oil, 1 tablespoon | 4.6 | 25 |
| Hazelnuts, dry roasted, 1 ounce | 4.3 | 22 |
| Peanut butter, 2 tablespoons | 2.9 | 15 |
| Peanuts, dry roasted, 1 ounce | 2.2 | 11 |
| Corn oil, 1 tablespoon | 1.9 | 10 |
| Spinach, boiled, ½ cup | 1.9 | 10 |
| Broccoli, chopped, boiled, ½ cup | 1.2 | 6 |
| Soybean oil, 1 tablespoon | 1.1 | 6 |
| Kiwifruit, 1 medium | 1.1 | 6 |
| Mango, sliced, ½ cup | 0.7 | 4 |
| Tomato, raw, 1 medium | 0.7 | 4 |
| Spinach, raw, 1 cup | 0.6 | 3 |
Footnote: *DV = Daily Value. DVs were developed by the FDA to help consumers compare the nutrient content of different foods within the context of a total diet. The DV for vitamin E is 30 IU (approximately 20 mg of natural alpha-tocopherol) for adults and children age 4 and older. However, the FDA does not require food labels to list vitamin E content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 198 ]Vitamin E deficiency signs and symptoms
Severe vitamin E deficiency results mainly in neurologic symptoms, including impaired balance and coordination (spinocerebellar ataxia), injury to the sensory nerves (peripheral neuropathy), muscle weakness (myopathy), and damage to the retina of the eye (retinopathy) 16. For this reason, people who develop peripheral neuropathy, ataxia, or retinitis pigmentosa of unknown causes should be screened for vitamin E deficiency 4. Severe vitamin E deficiency individuals may present with profound muscle weakness and visual-field constriction 199. Patients with severe, prolonged vitamin E deficiency may develop complete blindness, cardiac arrhythmia, and dementia 199.
The results of one randomized controlled trial in 601 patients with common forms of retinitis pigmentosa indicated that daily supplementation with 400 IU of all-rac-alpha-tocopherol (180 mg of RRR-α-tocopherol) modestly but significantly increased the loss of retinal function 200. In contrast, daily supplementation with 15,000 IU of vitamin A (4,500 μg RAE) significantly slowed the loss of retinal function over a period of four to six years, suggesting that patients with common forms of retinitis pigmentosa may benefit from long-term vitamin A supplementation but should avoid high-dose supplemental vitamin E 200.
Inherited defects in alpha-tocopherol transfer protein (α-TTP) are associated with a characteristic syndrome called Ataxia with Vitamin E Deficiency (AVED). A recent case study reported that visual impairment in a middle-age patient with AVED was caused by both retinitis pigmentosa and early-onset macular degeneration 201. Supplementation with high-dose vitamin E (800-1,200 mg/day) is used to prevent neurologic deterioration in AVED subjects 4.
The developing nervous system appears to be especially vulnerable to vitamin E deficiency. For instance, children with severe vitamin E deficiency at birth rapidly experience irreversible neurologic symptoms if not treated with vitamin E. In contrast, individuals who develop gastrointestinal disorders affecting vitamin E absorption in adulthood may not develop neurologic symptoms for 10-20 years 4. It should also be noted that neurologic symptoms caused by vitamin E deficiency have not been reported in healthy individuals who consume diets low in vitamin E 16. In addition, a recent nested case-control study in Bangladeshi women suggested that inadequate vitamin E status during early pregnancy may be associated with an increased risk of miscarriage 6.
If vitamin E deficiency is expected, a full neurological exam is recommended as well as a standard physical exam. Patients presenting early may show hyporeflexia, decreased night vision, loss/decreased vibratory sense, however, have normal cognition. A more moderate stage of this deficiency may show limb and truncal ataxia, profuse muscle weakness, and limited upward gaze. Late presentations may show cardiac arrhythmias and possible blindness with reduced cognition. Ataxia is the most common exam finding.
Patients that have abetalipoproteinemia have eye problems often including pigmented retinopathy and visual field issues. However, patients suffering from cholestatic liver disease often have personality and behavioral disorders.
Vitamin E deficiency diagnosis
A low alpha-tocopherol level or low ratio serum alpha-tocopherol to serum lipids measurement is the mainstay of diagnosis. In adults, alpha-tocopherol levels should be less than 5 mcg/mL. In an adult with hyperlipidemia, the abnormal lipids may affect the vitamin E levels and a serum alpha-tocopherol to lipids level, needing to be less than 0.8 mg/g) is more accurate. A pediatric patient with abetalipoproteinemia will have serum alpha-tocopherol levels that are not detectable.
Vitamin E deficiency treatment
Treatment addresses the underlying cause of the deficiency (fat malabsorption, fat metabolism disorders, among others) and then provide oral vitamin E supplementation 140. Also, a modification in diet can assist in vitamin E supplementation, by increasing intake of leafy vegetables, whole grains, nuts, seeds, vegetable oils and fortified cereals is highly recommended. Though normally presented in our diets, adults need 15 mg of vitamin E per day. A supplement of 15 to 25 mg/kg once per day or mixed tocopherols 200 IU can both be used. If a patient has issues with the small intestine and/or oral ingestion intramuscular vitamin E injection is necessary 202, 203, 204, 205. The recommended daily allowance of alpha-tocopherol is as follows 140, 206:
- Age 0 to 6 months: 3 mg
- Age 6 to 12 months: 4 mg
- Age 1 to 3 years: 6 mg
- Age 4 to 10 years: 7 mg
- Adults and elderly patients: 8 to 10 mg
Replacement recommendations vary by the disease and are as follows 207, 206:
- Abetalipoproteinemia: 100 to 200 IU/kg per day
- Chronic cholestasis: 15 to 25 IU/kg per day
- Cystic fibrosis: 5 to 10 IU/kg per day
- Short-bowel syndrome: 200 to 3600 IU per day
- Isolated vitamin E deficiency: 800 to 3600 IU per day
Larger doses are required in short-bowel syndrome and isolated vitamin E deficiency state.
The recommended doses of vitamin E for age group and associated causes are shown in Table 3.
Table 3. Recommended doses of vitamin E according to age group and associated causes
| Age group | Associated symptoms | Dose and route of supplementation |
|---|---|---|
| Infants and children | Cholestasis |
|
| Adults | Fat malabsorption |
|
| Any age group | Severe cholestasis/genetic disorders related to transport of vitamin E |
|
Vitamin E deficiency prognosis
If left untreated, vitamin E deficiency symptoms may worsen. However, once diagnosed, the outcome of vitamin E deficiency is very good as most symptoms will resolve quickly. However, as vitamin E deficiency becomes more pronounced, the therapy will be more restricted. Patients who are at risk for vitamin E deficiency should undergo a thorough neurologic examination, as well as periodic testing of serum vitamin E levels 9. The results of a systematic review by Sherf-Dagan et al suggest that patients who undergo weight loss surgery (bariatric surgery), particularly malabsorptive procedures, are at greater risk for the development of vitamin E deficiency 210.
References- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids
- Galli F., Azzi A., Birringer M., Cook-Mills J.M., Eggersdorfer M., Frank J., Cruciani G., Lorkowski S., Özer N.K. Vitamin E: Emerging Aspects and New Directions. Free Radic. Biol. Med. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017
- Ulatowski L.M., Manor D. Vitamin E and neurodegeneration. Neurobiol. Dis. 2015;84:78–83. doi: 10.1016/j.nbd.2015.04.002
- Traber MG. Vitamin E. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2014:293-304.
- Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665. doi: 10.1002/14651858.CD003665
- Shamim AA, Schulze K, Merrill RD, Kabir A, Christian P, Shaikh S, Wu L, Ali H, Labrique AB, Mehra S, Klemm RD, Rashid M, Sungpuag P, Udomkesmalee E, West KP Jr. First-trimester plasma tocopherols are associated with risk of miscarriage in rural Bangladesh. Am J Clin Nutr. 2015 Feb;101(2):294-301. doi: 10.3945/ajcn.114.094920
- Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992 Jun;102(6):2139-42. doi: 10.1016/0016-5085(92)90344-x
- Traber MG. Vitamin E. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006;396-411
- Tanyel MC, Mancano LD. Neurologic findings in vitamin E deficiency. Am Fam Physician. 1997 Jan;55(1):197-201.
- Martinello F., Fardin P., Ottina M., Ricchieri G.L., Koenig M., Cavalier L., Trevisan C.P. Supplemental Therapy in Isolated Vitamin E Deficiency Improves the Peripheral Neuropathy and Prevents the Progression of Ataxia. J. Neurol. Sci. 1998;156:177–179. doi: 10.1016/S0022-510X(98)00038-0
- Kohlschutter A., Finckh B., Nickel M., Bley A., Hübner C. First Recognized Patient with Genetic Vitamin e Deficiency Stable after 36 Years of Controlled Supplement Therapy. Neurodegener. Dis. 2020;20:583–587. doi: 10.1159/000508080
- Traber MG. Vitamin E. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006;396-411.
- Jiang Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035
- Azzi A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019 Sep;26:101259. doi: 10.1016/j.redox.2019.101259
- Suskind DL. Nutritional deficiencies during normal growth. Pediatr Clin North Am. 2009 Oct;56(5):1035-53. doi: 10.1016/j.pcl.2009.07.004
- Vitamin E. https://lpi.oregonstate.edu/mic/vitamins/vitamin-E
- Vitamin E. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional
- Traber MG. Vitamin E. In: Erdman JWJ, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, D.C.: Wiley-Blackwell; 2012:214-229.
- National Academies of Sciences, Engineering, and Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press. Chapter: 6 Vitamin E. https://doi.org/10.17226/9810
- Verhagen H, Buijsse B, Jansen E, Bueno-de-Mesquita B. The state of antioxidant affairs. Nutr Today 2006;41:244-50.
- Kagan VE, Serbinova EA, Forte T, Scita G, Packer L. Recycling of vitamin E in human low density lipoproteins. J Lipid Res. 1992 Mar;33(3):385-97. https://www.jlr.org/article/S0022-2275(20)41529-9/pdf
- Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52(2):70-85. doi: 10.3109/10408363.2014.992063
- Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347-62. doi: 10.1146/annurev.nutr.27.061406.093819
- Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006 Mar 27;78(18):2088-98. doi: 10.1016/j.lfs.2005.12.001
- Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does gamma-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J Am Coll Nutr. 2006 Aug;25(4):292-9. doi: 10.1080/07315724.2006.10719538
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin E (Tocopherol). https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-e
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that
- Bruno RS, Leonard SW, Park SI, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled alpha-tocopheryl acetate. Am J Clin Nutr. 2006 Feb;83(2):299-304. doi: 10.1093/ajcn/83.2.299
- Leonard SW, Good CK, Gugger ET, Traber MG. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am J Clin Nutr. 2004 Jan;79(1):86-92. doi: 10.1093/ajcn/79.1.86
- Traber MG, Leonard SW, Bobe G, Fu X, Saltzman E, Grusak MA, Booth SL. α-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am J Clin Nutr. 2015 Apr;101(4):752-9. doi: 10.3945/ajcn.114.100966. Epub 2015 Mar 4. Erratum in: Am J Clin Nutr. 2017 Feb;105(2):543.
- Galli F, Bonomini M, Bartolini D, Zatini L, Reboldi G, Marcantonini G, Gentile G, Sirolli V, Di Pietro N. Vitamin E (Alpha-Tocopherol) Metabolism and Nutrition in Chronic Kidney Disease. Antioxidants (Basel). 2022 May 18;11(5):989. doi: 10.3390/antiox11050989
- Azzi A. Molecular mechanism of alpha-tocopherol action. Free Radic Biol Med. 2007 Jul 1;43(1):16-21. doi: 10.1016/j.freeradbiomed.2007.03.013
- Zingg JM, Libinaki R, Lai CQ, Meydani M, Gianello R, Ogru E, Azzi A. Modulation of gene expression by α-tocopherol and α-tocopheryl phosphate in THP-1 monocytes. Free Radic Biol Med. 2010 Dec 15;49(12):1989-2000. doi: 10.1016/j.freeradbiomed.2010.09.034
- Cynamon HA, Milov DE, Valenstein E, Wagner M. Effect of vitamin E deficiency on neurologic function in patients with cystic fibrosis. J Pediatr. 1988 Oct;113(4):637-40. doi: 10.1016/s0022-3476(88)80371-8
- Swann IL, Kendra JR. Anaemia, vitamin E deficiency and failure to thrive in an infant. Clin Lab Haematol. 1998 Feb;20(1):61-3. doi: 10.1046/j.1365-2257.1998.00099.x
- Bines JE, Truby HD, Armstrong DS, Carzino R, Grimwood K. Vitamin A and E deficiency and lung disease in infants with cystic fibrosis. J Paediatr Child Health. 2005 Dec;41(12):663-8. doi: 10.1111/j.1440-1754.2005.00755.x
- Brigelius-Flohé R. Vitamin E: the shrew waiting to be tamed. Free Radic Biol Med. 2009 Mar 1;46(5):543-54. doi: 10.1016/j.freeradbiomed.2008.12.007
- Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014 Jul;72:76-90. doi: 10.1016/j.freeradbiomed.2014.03.035
- Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001 Dec;74(6):714-22. doi: 10.1093/ajcn/74.6.714
- Mah E, Pei R, Guo Y, Ballard KD, Barker T, Rogers VE, Parker BA, Taylor AW, Traber MG, Volek JS, Bruno RS. γ-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic Biol Med. 2013 Dec;65:1291-1299. doi: 10.1016/j.freeradbiomed.2013.09.016
- Mah E, Pei R, Guo Y, Masterjohn C, Ballard KD, Taylor BA, Taylor AW, Traber MG, Volek JS, Bruno RS. Greater γ-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F2α. Exp Biol Med (Maywood). 2015 Apr;240(4):527-33. doi: 10.1177/1535370214556948
- Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond). 2014 Nov 12;11(1):52. doi: 10.1186/1743-7075-11-52
- Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008 Aug 15;123(4):739-52. doi: 10.1002/ijc.23689
- Fu JY, Che HL, Tan DM, Teng KT. Bioavailability of tocotrienols: evidence in human studies. Nutr Metab (Lond). 2014 Jan 13;11(1):5. doi: 10.1186/1743-7075-11-5
- Davis S, Davis BM, Richens JL, Vere KA, Petrov PG, Winlove CP, O’Shea P. α-Tocopherols modify the membrane dipole potential leading to modulation of ligand binding by P-glycoprotein. J Lipid Res. 2015 Aug;56(8):1543-50. doi: 10.1194/jlr.M059519
- Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J Immunol. 2007 Feb 1;178(3):1443-9. doi: 10.4049/jimmunol.178.3.1443
- Molano A, Meydani SN. Vitamin E, signalosomes and gene expression in T cells. Mol Aspects Med. 2012 Feb;33(1):55-62. doi: 10.1016/j.mam.2011.11.002
- Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283-9. doi: 10.2174/1871530314666140922143950
- De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res. 2008 Mar;42(3):272-80. doi: 10.1080/10715760801898838
- Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997 May 7;277(17):1380-6. doi: 10.1001/jama.1997.03540410058031
- Pallast EG, Schouten EG, de Waart FG, Fonk HC, Doekes G, von Blomberg BM, Kok FJ. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr. 1999 Jun;69(6):1273-81. doi: 10.1093/ajcn/69.6.1273
- Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004 Aug 18;292(7):828-36. doi: 10.1001/jama.292.7.828. Erratum in: JAMA. 2004 Sep 15;292(11):1305. Erratum in: JAMA. 2007 May 2;297(17):1882
- Latha MS, Martis J, Shobha V, Sham Shinde R, Bangera S, Krishnankutty B, Bellary S, Varughese S, Rao P, Naveen Kumar BR. Sunscreening agents: a review. J Clin Aesthet Dermatol. 2013 Jan;6(1):16-26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543289
- Gabros S, Nessel TA, Zito PM. Sunscreens and Photoprotection. [Updated 2023 Mar 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537164
- Lavker RM, Gerberick GF, Veres D, Irwin CJ, Kaidbey KH. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol. 1995 Jan;32(1):53-62. doi: 10.1016/0190-9622(95)90184-1
- Rhodes LE. Topical and systemic approaches for protection against solar radiation-induced skin damage. Clin Dermatol. 1998 Jan-Feb;16(1):75-82. doi: 10.1016/s0738-081x(97)00171-5
- Kagan V, Witt E, Goldman R, Scita G, Packer L. Ultraviolet light-induced generation of vitamin E radicals and their recycling. A possible photosensitizing effect of vitamin E in skin. Free Radic Res Commun. 1992;16(1):51-64. doi: 10.3109/10715769209049159
- Kondo S, Mamada A, Yamaguchi J, Fukuro S. Protective effect of dl-alpha-tocopherol on the cytotoxicity of ultraviolet B against human skin fibroblasts in vitro. Photodermatol Photoimmunol Photomed. 1990 Aug;7(4):173-7.
- Jin GH, Liu Y, Jin SZ, Liu XD, Liu SZ. UVB induced oxidative stress in human keratinocytes and protective effect of antioxidant agents. Radiat Environ Biophys. 2007 Mar;46(1):61-8. doi: 10.1007/s00411-007-0096-1
- Stewart MS, Cameron GS, Pence BC. Antioxidant nutrients protect against UVB-induced oxidative damage to DNA of mouse keratinocytes in culture. J Invest Dermatol. 1996 May;106(5):1086-9. doi: 10.1111/1523-1747.ep12339344
- Pauling L, Willoughby R, Reynolds R, Blaisdell BE, Lawson S. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19(2):125-33. doi: 10.1080/01635589309514243
- Burke KE, Clive J, Combs GF Jr, Nakamura RM. Effects of topical L-selenomethionine with topical and oral vitamin E on pigmentation and skin cancer induced by ultraviolet irradiation in Skh:2 hairless mice. J Am Acad Dermatol. 2003 Sep;49(3):458-72. doi: 10.1067/s0190-9622(03)00900-9
- Burke KE, Clive J, Combs GF Jr, Commisso J, Keen CL, Nakamura RM. Effects of topical and oral vitamin E on pigmentation and skin cancer induced by ultraviolet irradiation in Skh:2 hairless mice. Nutr Cancer. 2000;38(1):87-97. doi: 10.1207/S15327914NC381_13
- Record IR, Dreosti IE, Konstantinopoulos M, Buckley RA. The influence of topical and systemic vitamin E on ultraviolet light-induced skin damage in hairless mice. Nutr Cancer. 1991;16(3-4):219-25. doi: 10.1080/01635589109514160
- McArdle F, Rhodes LE, Parslew RA, Close GL, Jack CI, Friedmann PS, Jackson MJ. Effects of oral vitamin E and beta-carotene supplementation on ultraviolet radiation-induced oxidative stress in human skin. Am J Clin Nutr. 2004 Nov;80(5):1270-5. doi: 10.1093/ajcn/80.5.1270
- Werninghaus K, Meydani M, Bhawan J, Margolis R, Blumberg JB, Gilchrest BA. Evaluation of the Photoprotective Effect of Oral Vitamin E Supplementation. Arch Dermatol. 1994;130(10):1257–1261. doi:10.1001/archderm.1994.01690100041005
- van der Pols JC, Heinen MM, Hughes MC, Ibiebele TI, Marks GC, Green AC. Serum antioxidants and skin cancer risk: an 8-year community-based follow-up study. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1167-73. doi: 10.1158/1055-9965.EPI-08-1211
- McNaughton SA, Marks GC, Green AC. Role of dietary factors in the development of basal cell cancer and squamous cell cancer of the skin. Cancer Epidemiol Biomarkers Prev. 2005 Jul;14(7):1596-607. doi: 10.1158/1055-9965.EPI-05-0026
- Eberlein-König B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998 Jan;38(1):45-8. doi: 10.1016/s0190-9622(98)70537-7
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998 Dec;25(9):1006-12. doi: 10.1016/s0891-5849(98)00132-4
- Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol. 2005 Feb;124(2):304-7. doi: 10.1111/j.0022-202X.2004.23560.x
- Yamada Y, Obayashi M, Ishikawa T, Kiso Y, Ono Y, Yamashita K. Dietary tocotrienol reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J Nutr Sci Vitaminol (Tokyo). 2008 Apr;54(2):117-23. doi: 10.3177/jnsv.54.117
- Saral Y, Uyar B, Ayar A, Naziroglu M. Protective effects of topical alpha-tocopherol acetate on UVB irradiation in guinea pigs: importance of free radicals. Physiol Res. 2002;51(3):285-90.
- Yuen KS, Halliday GM. alpha-Tocopherol, an inhibitor of epidermal lipid peroxidation, prevents ultraviolet radiation from suppressing the skin immune system. Photochem Photobiol. 1997 Mar;65(3):587-92. doi: 10.1111/j.1751-1097.1997.tb08610.x
- Lopez-Torres M, Thiele JJ, Shindo Y, Han D, Packer L. Topical application of alpha-tocopherol modulates the antioxidant network and diminishes ultraviolet-induced oxidative damage in murine skin. Br J Dermatol. 1998 Feb;138(2):207-15. doi: 10.1046/j.1365-2133.1998.02062.x
- Chen W, Barthelman M, Martinez J, Alberts D, Gensler HL. Inhibition of cyclobutane pyrimidine dimer formation in epidermal p53 gene of UV-irradiated mice by alpha-tocopherol. Nutr Cancer. 1997;29(3):205-11. doi: 10.1080/01635589709514625
- McVean M, Liebler DC. Inhibition of UVB induced DNA photodamage in mouse epidermis by topically applied alpha-tocopherol. Carcinogenesis. 1997 Aug;18(8):1617-22. doi: 10.1093/carcin/18.8.161
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999 Mar;24(3):169-76. doi: 10.1002/(sici)1098-2744(199903)24:3<169::aid-mc3>3.0.co;2-a
- Bissett DL, Chatterjee R, Hannon DP. Photoprotective effect of superoxide-scavenging antioxidants against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Photodermatol Photoimmunol Photomed. 1990 Apr;7(2):56-62.
- Ritter EF, Axelrod M, Minn KW, Eades E, Rudner AM, Serafin D, Klitzman B. Modulation of ultraviolet light-induced epidermal damage: beneficial effects of tocopherol. Plast Reconstr Surg. 1997 Sep;100(4):973-80. doi: 10.1097/00006534-199709001-00021
- Trevithick JR, Shum DT, Redae S, Mitton KP, Norley C, Karlik SJ, Groom AC, Schmidt EE. Reduction of sunburn damage to skin by topical application of vitamin E acetate following exposure to ultraviolet B radiation: effect of delaying application or of reducing concentration of vitamin E acetate applied. Scanning Microsc. 1993 Dec;7(4):1269-81.
- Trevithick JR, Xiong H, Lee S, Shum DT, Sanford SE, Karlik SJ, Norley C, Dilworth GR. Topical tocopherol acetate reduces post-UVB, sunburn-associated erythema, edema, and skin sensitivity in hairless mice. Arch Biochem Biophys. 1992 Aug 1;296(2):575-82. doi: 10.1016/0003-9861(92)90613-2
- Gensler HL, Aickin M, Peng YM, Xu M. Importance of the form of topical vitamin E for prevention of photocarcinogenesis. Nutr Cancer. 1996;26(2):183-91. doi: 10.1080/01635589609514474
- Beijersbergen van Henegouwen GM, Junginger HE, de Vries H. Hydrolysis of RRR-alpha-tocopheryl acetate (vitamin E acetate) in the skin and its UV protecting activity (an in vivo study with the rat). J Photochem Photobiol B. 1995 Jul;29(1):45-51. doi: 10.1016/1011-1344(95)90251-1
- Schoonderwoerd SA, Beijersbergen van Henegouwen GM, Persons KC. Effect of alpha-tocopherol and di-butyl-hydroxytoluene (BHT) on UV-A-induced photobinding of 8-methoxypsoralen to Wistar rat epidermal biomacromolecules in vivo. Arch Toxicol. 1991;65(6):490-4. doi: 10.1007/BF01977362
- Slaga TJ, Bracken WM. The effects of antioxidants on skin tumor initiation and aryl hydrocarbon hydroxylase. Cancer Res. 1977 Jun;37(6):1631-5.
- Potapenko AYa, Abijev GA, Pistsov MYu, Roshchupkin DI, Vladimirov YuA, Pliquett F, Ermolayev AV, Sarycheva IK, Evstigneeva RP. PUVA-induced erythema and changes in mechanoelectrical properties of skin. Inhibition by tocopherols. Arch Dermatol Res. 1984;276(1):12-6. doi: 10.1007/BF00412556
- Roshchupkin DI, Pistsov MY, Potapenko AY. Inhibition of ultraviolet light-induced erythema by antioxidants. Arch Dermatol Res. 1979 Aug;266(1):91-4. doi: 10.1007/BF00412867
- Thiele JJ, Hsieh SN, Ekanayake-Mudiyanselage S. Vitamin E: critical review of its current use in cosmetic and clinical dermatology. Dermatol Surg. 2005 Jul;31(7 Pt 2):805-13; discussion 813. doi: 10.1111/j.1524-4725.2005.31724
- Zhai H, Behnam S, Villarama CD, Arens-Corell M, Choi MJ, Maibach HI. Evaluation of the antioxidant capacity and preventive effects of a topical emulsion and its vehicle control on the skin response to UV exposure. Skin Pharmacol Physiol. 2005 Nov-Dec;18(6):288-93. doi: 10.1159/000088014
- Montenegro L, Bonina F, Rigano L, Giogilli S, Sirigu S. Protective effect evaluation of free radical scavengers on UVB induced human cutaneous erythema by skin reflectance spectrophotometry. Int J Cosmet Sci. 1995 Jun;17(3):91-103. doi: 10.1111/j.1467-2494.1995.tb00113.x
- Chung JH, Seo JY, Lee MK, Eun HC, Lee JH, Kang S, Fisher GJ, Voorhees JJ. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J Invest Dermatol. 2002 Aug;119(2):507-12. doi: 10.1046/j.1523-1747.2002.01844.x
- Lin JY, Selim MA, Shea CR, Grichnik JM, Omar MM, Monteiro-Riviere NA, Pinnell SR. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003 Jun;48(6):866-74. doi: 10.1067/mjd.2003.425
- Darr D, Dunston S, Faust H, Pinnell S. Effectiveness of antioxidants (vitamin C and E) with and without sunscreens as topical photoprotectants. Acta Derm Venereol. 1996 Jul;76(4):264-8. doi: 10.2340/0001555576264268
- Lin FH, Lin JY, Gupta RD, Tournas JA, Burch JA, Selim MA, Monteiro-Riviere NA, Grichnik JM, Zielinski J, Pinnell SR. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005 Oct;125(4):826-32. doi: 10.1111/j.0022-202X.2005.23768.x
- Quevedo WC Jr, Holstein TJ, Dyckman J, McDonald CJ, Isaacson EL. Inhibition of UVR-induced tanning and immunosuppression by topical applications of vitamins C and E to the skin of hairless (hr/hr) mice. Pigment Cell Res. 2000 Apr;13(2):89-98. doi: 10.1034/j.1600-0749.2000.130207.x
- Dreher F, Gabard B, Schwindt DA, Maibach HI. Topical melatonin in combination with vitamins E and C protects skin from ultraviolet-induced erythema: a human study in vivo. Br J Dermatol. 1998 Aug;139(2):332-9. doi: 10.1046/j.1365-2133.1998.02447.x
- Quevedo WC Jr, Holstein TJ, Dyckman J, McDonald CJ. The responses of the human epidermal melanocyte system to chronic erythemal doses of UVR in skin protected by topical applications of a combination of vitamins C and E. Pigment Cell Res. 2000 Jun;13(3):190-2. doi: 10.1034/j.1600-0749.2000.130312.x
- Murray JC, Burch JA, Streilein RD, Iannacchione MA, Hall RP, Pinnell SR. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol. 2008 Sep;59(3):418-25. doi: 10.1016/j.jaad.2008.05.004
- Passi S, De Pità O, Grandinetti M, Simotti C, Littarru GP. The combined use of oral and topical lipophilic antioxidants increases their levels both in sebum and stratum corneum. Biofactors. 2003;18(1-4):289-97. doi: 10.1002/biof.5520180233
- Burke KE. Photodamage of the skin: protection and reversal with topical antioxidants. J Cosmet Dermatol. 2004 Jul;3(3):149-55. doi: 10.1111/j.1473-2130.2004.00067.x
- Nagata C, Nakamura K, Wada K, Oba S, Hayashi M, Takeda N, Yasuda K. Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br J Nutr. 2010 May;103(10):1493-8. doi: 10.1017/S0007114509993461
- Boelsma E, van de Vijver LP, Goldbohm RA, Klöpping-Ketelaars IA, Hendriks HF, Roza L. Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003 Feb;77(2):348-55. doi: 10.1093/ajcn/77.2.348
- Gehring W, Fluhr J, Gloor M. Influence of vitamin E acetate on stratum corneum hydration. Arzneimittelforschung. 1998 Jul;48(7):772-5.
- Gönüllü U, Sensoy D, Uner M, Yener G, Altinkurt T. Comparing the moisturizing effects of ascorbic acid and calcium ascorbate against that of tocopherol in emulsions. J Cosmet Sci. 2006 Nov-Dec;57(6):465-73.
- Weber SU, Thiele JJ, Cross CE, Packer L. Vitamin C, uric acid, and glutathione gradients in murine stratum corneum and their susceptibility to ozone exposure. J Invest Dermatol. 1999 Dec;113(6):1128-32. doi: 10.1046/j.1523-1747.1999.00789.x
- Thiele JJ, Traber MG, Podda M, Tsang K, Cross CE, Packer L. Ozone depletes tocopherols and tocotrienols topically applied to murine skin. FEBS Lett. 1997 Jan 20;401(2-3):167-70. doi: 10.1016/s0014-5793(96)01463-9
- Valacchi G, Weber SU, Luu C, Cross CE, Packer L. Ozone potentiates vitamin E depletion by ultraviolet radiation in the murine stratum corneum. FEBS Lett. 2000 Jan 21;466(1):165-8. doi: 10.1016/s0014-5793(99)01787-1
- Wu S, Gao J, Dinh QT, Chen C, Fimmel S. IL-8 production and AP-1 transactivation induced by UVA in human keratinocytes: roles of D-alpha-tocopherol. Mol Immunol. 2008 Apr;45(8):2288-96. doi: 10.1016/j.molimm.2007.11.019
- Kato E, Sasaki Y, Takahashi N. Sodium dl-α-tocopheryl-6-O-phosphate inhibits PGE₂ production in keratinocytes induced by UVB, IL-1β and peroxidants. Bioorg Med Chem. 2011 Nov 1;19(21):6348-55. doi: 10.1016/j.bmc.2011.08.067
- Shibata A, Nakagawa K, Kawakami Y, Tsuzuki T, Miyazawa T. Suppression of gamma-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J Agric Food Chem. 2010 Jun 9;58(11):7013-20. doi: 10.1021/jf100691g
- Nakagawa K, Shibata A, Maruko T, Sookwong P, Tsuduki T, Kawakami K, Nishida H, Miyazawa T. gamma-Tocotrienol reduces squalene hydroperoxide-induced inflammatory responses in HaCaT keratinocytes. Lipids. 2010 Sep;45(9):833-41. doi: 10.1007/s11745-010-3458-4
- Yoshida E, Watanabe T, Takata J, Yamazaki A, Karube Y, Kobayashi S. Topical application of a novel, hydrophilic gamma-tocopherol derivative reduces photo-inflammation in mice skin. J Invest Dermatol. 2006 Jul;126(7):1633-40. doi: 10.1038/sj.jid.5700236
- Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990 Sep;52(3):557-63. doi: 10.1093/ajcn/52.3.557
- Han SN, Meydani SN. Impact of vitamin E on immune function and its clinical implications. Expert Rev Clin Immunol. 2006 Jul;2(4):561-7. doi: 10.1586/1744666X.2.4.561
- Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003 May;17(8):816-22. doi: 10.1096/fj.02-0877com
- Tsoureli-Nikita E, Hercogova J, Lotti T, Menchini G. Evaluation of dietary intake of vitamin E in the treatment of atopic dermatitis: a study of the clinical course and evaluation of the immunoglobulin E serum levels. Int J Dermatol. 2002 Mar;41(3):146-50. doi: 10.1046/j.1365-4362.2002.01423.x
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998 Oct;39(4 Pt 1):611-25. doi: 10.1016/s0190-9622(98)70011-8
- Hayakawa R, Ueda H, Nozaki T, Izawa Y, Yokotake J, Yazaki K, Azumi T, Okada Y, Kobayashi M, Usuda T, Ishida J, Kondo T, Adachi A, Kawase A, Matsunaga K. Effects of combination treatment with vitamins E and C on chloasma and pigmented contact dermatitis. A double blind controlled clinical trial. Acta Vitaminol Enzymol. 1981;3(1):31-8.
- Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, Seirafi H, Ehsani AH, Chamari M, Mirshafiey A. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011 Jun;22(3):144-50. doi: 10.3109/09546630903578566
- Shukla A, Rasik AM, Patnaik GK. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defence enzymes in a healing cutaneous wound. Free Radic Res. 1997 Feb;26(2):93-101. doi: 10.3109/10715769709097788
- Musalmah M, Nizrana MY, Fairuz AH, NoorAini AH, Azian AL, Gapor MT, Wan Ngah WZ. Comparative effects of palm vitamin E and alpha-tocopherol on healing and wound tissue antioxidant enzyme levels in diabetic rats. Lipids. 2005 Jun;40(6):575-80. doi: 10.1007/s11745-005-1418-9
- Musalmah M, Fairuz AH, Gapor MT, Ngah WZ. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac J Clin Nutr. 2002;11 Suppl 7:S448-51. doi: 10.1046/j.1440-6047.11.s.7.6.x
- Taren DL, Chvapil M, Weber CW. Increasing the breaking strength of wounds exposed to preoperative irradiation using vitamin E supplementation. Int J Vitam Nutr Res. 1987;57(2):133-7.
- Ehrlich HP, Tarver H, Hunt TK. Inhibitory effects of vitamin E on collagen synthesis and wound repair. Ann Surg. 1972 Feb;175(2):235-40. doi: 10.1097/00000658-197202000-00013
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999 Apr;25(4):311-5. doi: 10.1046/j.1524-4725.1999.08223.x
- Jenkins M, Alexander JW, MacMillan BG, Waymack JP, Kopcha R. Failure of topical steroids and vitamin E to reduce postoperative scar formation following reconstructive surgery. J Burn Care Rehabil. 1986 Jul-Aug;7(4):309-12. doi: 10.1097/00004630-198607000-00002
- Barbosa E, Faintuch J, Machado Moreira EA, Gonçalves da Silva VR, Lopes Pereima MJ, Martins Fagundes RL, Filho DW. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: a randomized, double-blind, placebo-controlled pilot study. J Burn Care Res. 2009 Sep-Oct;30(5):859-66. doi: 10.1097/BCR.0b013e3181b487a8
- Ellinger S, Stehle P. Efficacy of vitamin supplementation in situations with wound healing disorders: results from clinical intervention studies. Curr Opin Clin Nutr Metab Care. 2009 Nov;12(6):588-95. doi: 10.1097/MCO.0b013e328331a5b5
- Leonard SW, Bruno RS, Ramakrishnan R, Bray T, Traber MG. Cigarette smoking increases human vitamin E requirements as estimated by plasma deuterium-labeled CEHC. Ann N Y Acad Sci. 2004 Dec;1031:357-60. doi: 10.1196/annals.1331.044
- Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006 Feb 15;40(4):689-97. doi: 10.1016/j.freeradbiomed.2005.10.051
- Booth SL, Golly I, Sacheck JM, Roubenoff R, Dallal GE, Hamada K, Blumberg JB. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am J Clin Nutr. 2004 Jul;80(1):143-8. doi: 10.1093/ajcn/80.1.143
- Pastori D, Carnevale R, Cangemi R, Saliola M, Nocella C, Bartimoccia S, Vicario T, Farcomeni A, Violi F, Pignatelli P. Vitamin E serum levels and bleeding risk in patients receiving oral anticoagulant therapy: a retrospective cohort study. J Am Heart Assoc. 2013 Oct 28;2(6):e000364. doi: 10.1161/JAHA.113.000364
- Ford ES, Ajani UA, Mokdad AH. Brief communication: the prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann Intern Med 2005;143:116-20. https://www.ncbi.nlm.nih.gov/pubmed/16027453?dopt=Abstract
- Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001 Nov 29;345(22):1583-92. doi: 10.1056/NEJMoa011090
- Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008 Jun 4;100(11):773-83. doi: 10.1093/jnci/djn148
- Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007 Aug;33(5):407-18. doi: 10.1016/j.ctrv.2007.01.005
- Kemnic TR, Coleman M. Vitamin E Deficiency. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519051
- Kono N, Ohto U, Hiramatsu T, Urabe M, Uchida Y, Satow Y, Arai H. Impaired α-TTP-PIPs interaction underlies familial vitamin E deficiency. Science. 2013 May 31;340(6136):1106-10. doi: 10.1126/science.1233508
- Sokol RJ, Guggenheim MA, Heubi JE, Iannaccone ST, Butler-Simon N, Jackson V, Miller C, Cheney M, Balistreri WF, Silverman A. Frequency and clinical progression of the vitamin E deficiency neurologic disorder in children with prolonged neonatal cholestasis. Am J Dis Child. 1985 Dec;139(12):1211-5. doi: 10.1001/archpedi.1985.02140140045024
- Di Donato I, Bianchi S, Federico A. Ataxia with vitamin E deficiency: update of molecular diagnosis. Neurol Sci. 2010 Aug;31(4):511-5. doi: 10.1007/s10072-010-0261-1
- Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2006 Nov;84(5):1200-7. doi: 10.1093/ajcn/84.5.1200
- Becker AE, Vargas W, Pearson TS. Ataxia with Vitamin E Deficiency May Present with Cervical Dystonia. Tremor Other Hyperkinet Mov (N Y). 2016 May 17;6:374. doi: 10.7916/D8B85820
- Elkamil A, Johansen KK, Aasly J. Ataxia with vitamin e deficiency in norway. J Mov Disord. 2015 Jan;8(1):33-6. doi: 10.14802/jmd.14030
- Schuelke M. Ataxia with Vitamin E Deficiency. 2005 May 20 [Updated 2023 Mar 16]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1241
- Zhang LW, Liu B, Peng DT. Clinical and genetic study of ataxia with vitamin E deficiency: A case report. World J Clin Cases. 2022 Aug 16;10(23):8271-8276. doi: 10.12998/wjcc.v10.i23.8271
- El Euch-Fayache G, Bouhlal Y, Amouri R, Feki M, Hentati F. Molecular, clinical and peripheral neuropathy study of Tunisian patients with ataxia with vitamin E deficiency. Brain. 2014 Feb;137(Pt 2):402-10. doi: 10.1093/brain/awt339
- Hamza W, Ali Pacha L, Hamadouche T, Muller J, Drouot N, Ferrat F, Makri S, Chaouch M, Tazir M, Koenig M, Benhassine T. Molecular and clinical study of a cohort of 110 Algerian patients with autosomal recessive ataxia. BMC Med Genet. 2015 Jun 12;16:36. doi: 10.1186/s12881-015-0180-3
- Esmer C, Salazar MA, Rentería PE, Bravo OA. Clinical and molecular findings in a patient with ataxia with vitamin E deficiency, homozygous for the c.205-1G>C mutation in the TTPA gene. Bol Med Hos Infant Mex. 2013;70:313–317.
- Benomar A, Yahyaoui M, Meggouh F, Bouhouche A, Boutchich M, Bouslam N, Zaim A, Schmitt M, Belaidi H, Ouazzani R, Chkili T, Koenig M. Clinical comparison between AVED patients with 744 del A mutation and Friedreich ataxia with GAA expansion in 15 Moroccan families. J Neurol Sci. 2002 Jun 15;198(1-2):25-9. doi: 10.1016/s0022-510x(02)00057-6
- Ataxia with vitamin E deficiency. https://rarediseases.info.nih.gov/diseases/8595/ataxia-with-vitamin-e-deficiency
- Chung S, Ghelfi M, Atkinson J, Parker R, Qian J, Carlin C, Manor D. Vitamin E and Phosphoinositides Regulate the Intracellular Localization of the Hepatic α-Tocopherol Transfer Protein. J Biol Chem. 2016 Aug 12;291(33):17028-39. doi: 10.1074/jbc.M116.734210
- TTPA gene. https://medlineplus.gov/genetics/gene/ttpa
- Ataxia with Vitamin E Deficiency. https://rarediseases.org/rare-diseases/ataxia-with-vitamin-e-deficiency
- Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet. 1995 Feb;9(2):141-5. doi: 10.1038/ng0295-141
- Mariotti C, Gellera C, Rimoldi M, Mineri R, Uziel G, Zorzi G, Pareyson D, Piccolo G, Gambi D, Piacentini S, Squitieri F, Capra R, Castellotti B, Di Donato S. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow-up and novel mutations in TTPA gene in Italian families. Neurol Sci. 2004 Jul;25(3):130-7. doi: 10.1007/s10072-004-0246-z
- Gotoda T, Arita M, Arai H, Inoue K, Yokota T, Fukuo Y, Yazaki Y, Yamada N. Adult-onset spinocerebellar dysfunction caused by a mutation in the gene for the alpha-tocopherol-transfer protein. N Engl J Med. 1995 Nov 16;333(20):1313-8. doi: 10.1056/NEJM199511163332003
- Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations and phenotypic variability in a large number of families. Am J Hum Genet. 1998 Feb;62(2):301-10. doi: 10.1086/301699
- Sokol RJ. Vitamin E deficiency and neurologic disease. Annu Rev Nutr. 1988;8:351-73. doi: 10.1146/annurev.nu.08.070188.002031
- Traber MG, Sokol RJ, Kohlschütter A, Yokota T, Muller DP, Dufour R, Kayden HJ. Impaired discrimination between stereoisomers of alpha-tocopherol in patients with familial isolated vitamin E deficiency. J Lipid Res. 1993 Feb;34(2):201-10.
- Yokota T, Shiojiri T, Gotoda T, Arai H. Retinitis pigmentosa and ataxia caused by a mutation in the gene for the alpha-tocopherol-transfer protein. N Engl J Med. 1996 Dec 5;335(23):1770-1. doi: 10.1056/NEJM199612053352315
- Yokota T, Shiojiri T, Gotoda T, Arita M, Arai H, Ohga T, Kanda T, Suzuki J, Imai T, Matsumoto H, Harino S, Kiyosawa M, Mizusawa H, Inoue K. Friedreich-like ataxia with retinitis pigmentosa caused by the His101Gln mutation of the alpha-tocopherol transfer protein gene. Ann Neurol. 1997 Jun;41(6):826-32. doi: 10.1002/ana.410410621
- Yokota T, Uchihara T, Kumagai J, Shiojiri T, Pang JJ, Arita M, Arai H, Hayashi M, Kiyosawa M, Okeda R, Mizusawa H. Postmortem study of ataxia with retinitis pigmentosa by mutation of the alpha-tocopherol transfer protein gene. J Neurol Neurosurg Psychiatry. 2000 Apr;68(4):521-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1736898/pdf/v068p00521.pdf
- Mariotti C, Gellera C, Rimoldi M, et al. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow‐up and novel mutations in TTPA gene in Italian families. Neurol Sci. 2004;25(3):130‐137. doi: 10.1007/s10072-004-0246-z
- Ulatowski L, Parker R, Warrier G, Sultana R, Butterfield DA, Manor D. Vitamin E is essential for Purkinje neuron integrity. Neuroscience. 2014;260:120‐129. doi: 10.1016/j.neuroscience.2013.12.001
- Thapa S, Shah S, Chand S, Sah SK, Gyawali P, Paudel S, Khanal P. Ataxia due to vitamin E deficiency: A case report and updated review. Clin Case Rep. 2022 Sep 6;10(9):e6303. doi: 10.1002/ccr3.6303
- Gabsi S, Gouider‐Khouja N, Belal S, et al. Effect of vitamin E supplementation in patients with ataxia with vitamin E deficiency. Eur J Neurol. 2001;8(5):477‐481. doi: 10.1046/j.1468-1331.2001.00273.x
- Schuelke M, Mayatepek E, Inter M, Becker M, Pfeiffer E, Speer A, Hübner C, Finckh B. Treatment of ataxia in isolated vitamin E deficiency caused by alpha-tocopherol transfer protein deficiency. J Pediatr. 1999 Feb;134(2):240-4. doi: 10.1016/s0022-3476(99)70424-5
- Gabsi S, Gouider-Khouja N, Belal S, Fki M, Kefi M, Turki I, Ben Hamida M, Kayden H, Mebazaa R, Hentati F. Effect of vitamin E supplementation in patients with ataxia with vitamin E deficiency. Eur J Neurol. 2001 Sep;8(5):477-81. doi: 10.1046/j.1468-1331.2001.00273.x
- Dexter DT, Nanayakkara I, Goss-Sampson MA, et al. Nigral dopaminergic cell loss in vitamin E deficient rats. Neuroreport. 1994;5:1773–1776. doi: 10.1097/00001756-199409080-00022
- Geller A, Gilles F, Shwachman H. Degeneration of fasciculus gracilis in cystic fibrosis. Neurology. 1977;27:185–187. doi: 10.1212/WNL.27.2.185
- Nelson JS, Fitch CD, Fischer VW, Broun GO, Chou AC. Progressive neuropathologic lesions in vitamin E-deficient rhesus monkeys. J Neuropathol Exp Neurol. 1981;40:166–186. doi: 10.1097/00005072-198103000-00008
- Rosenblum JL, Keating JP, Prensky AL, Nelson JS. A progressive neurologic syndrome in children with chronic liver disease. N Engl J Med. 1981;304:503–508. doi: 10.1056/NEJM198102263040902
- Sung JH, Park SH, Mastri AR, Warwick WJ. Axonal dystrophy in the gracile nucleus in congenital biliary atresia and cystic fibrosis (mucoviscidosis): beneficial effect of vitamin E therapy. J Neuropathol Exp Neurol. 1980;39:584–597. doi: 10.1097/00005072-198009000-00007
- Weder B, Meienberg O, Wildi E, Meier C. Neurologic disorder of vitamin E deficiency in acquired intestinal malabsorption. Neurology. 1984;34:1561–1565. doi: 10.1212/WNL.34.12.1561
- Southam E, Thomas PK, King RHM, Goss-Sampson MA, Muller DPR. Experimental vitamin E deficiency in rats. Morphological and functional evidence of abnormal axonal transport secondary to free radical damage. Brain. 1991;114((Pt 2
- Larnaout A, Belal S, Zouari M, et al. Friedreich’s ataxia with isolated vitamin E deficiency: a neuropathological study of a Tunisian patient. Acta Neuropathol. 1997;93:633–637. doi: 10.1007/s004010050662
- Yokota T, Uchihara T, Kumagai J, et al. Postmortem study of ataxia with retinitis pigmentosa by mutation of the alpha-tocopherol transfer protein gene. J Neurol Neurosurg Psychiatry. 2000;68:521–525. doi: 10.1136/jnnp.68.4.521
- El Euch‐Fayache G, Bouhlal Y, Amouri R, Feki M, Hentati F. Molecular, clinical and peripheral neuropathy study of Tunisian patients with ataxia with vitamin E deficiency. Brain. 2014;137(Pt 2):402‐410. doi: 10.1093/brain/awt339
- Angelini L, Erba A, Mariotti C, Gellera C, Ciano C, Nardocci N. Myoclonic dystonia as unique presentation of isolated vitamin E deficiency in a young patient. Mov Disord. 2002 May;17(3):612-4. doi: 10.1002/mds.10026
- Finckh B, Kontush A, Commentz J, Hübner C, Burdelski M, Kohlschütter A. Monitoring of ubiquinol-10, ubiquinone-10, carotenoids, and tocopherols in neonatal plasma microsamples using high-performance liquid chromatography with coulometric electrochemical detection. Anal Biochem. 1995 Dec 10;232(2):210-6. doi: 10.1006/abio.1995.0009
- Usuki F, Maruyama K. Ataxia caused by mutations in the alpha-tocopherol transfer protein gene. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):254-6. doi: 10.1136/jnnp.69.2.254
- Weiser H, Riss G, Kormann AW. Biodiscrimination of the eight alpha-tocopherol stereoisomers results in preferential accumulation of the four 2R forms in tissues and plasma of rats. J Nutr. 1996 Oct;126(10):2539-49. doi: 10.1093/jn/126.10.2539
- Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997 Jun 2;409(1):105-8. doi: 10.1016/s0014-5793(97)00499-7
- Leonard SW, Terasawa Y, Farese RV Jr, Traber MG. Incorporation of deuterated RRR- or all-rac-alpha-tocopherol in plasma and tissues of alpha-tocopherol transfer protein–null mice. Am J Clin Nutr. 2002 Mar;75(3):555-60. doi: 10.1093/ajcn/75.3.555
- Amiel J, Maziere JC, Beucler I, Koenig M, Reutenauer L, Loux N, Bonnefont D, Féo C, Landrieu P. Familial isolated vitamin E deficiency. Extensive study of a large family with a 5-year therapeutic follow-up. J Inherit Metab Dis. 1995;18(3):333-40. doi: 10.1007/BF00710425
- Burck U, Goebel HH, Kuhlendahl HD, Meier C, Goebel KM. Neuromyopathy and vitamin E deficiency in man. Neuropediatrics. 1981 Aug;12(3):267-78. doi: 10.1055/s-2008-1059657
- Harding AE, Matthews S, Jones S, Ellis CJ, Booth IW, Muller DP. Spinocerebellar degeneration associated with a selective defect of vitamin E absorption. N Engl J Med. 1985 Jul 4;313(1):32-5. doi: 10.1056/NEJM198507043130107
- Schuelke M, Finckh B, Sistermans EA, Ausems MG, Hübner C, von Moers A. Ataxia with vitamin E deficiency: biochemical effects of malcompliance with vitamin E therapy. Neurology. 2000 Nov 28;55(10):1584-6. doi: 10.1212/wnl.55.10.1584
- Bruno RS, Traber MG. Vitamin E biokinetics, oxidative stress and cigarette smoking. Pathophysiology. 2006;13(3):143‐149. doi: 10.1016/j.pathophys.2006.05.003
- Traber MG, Manor D. Vitamin E. Adv Nutr. 2012 May 1;3(3):330-1. doi: 10.3945/an.112.002139
- Müller-Schmehl K, Beninde J, Finckh B, Florian S, Dudenhausen JW, Brigelius-Flohé R, Schuelke M. Localization of alpha-tocopherol transfer protein in trophoblast, fetal capillaries’ endothelium and amnion epithelium of human term placenta. Free Radic Res. 2004 Apr;38(4):413-20. doi: 10.1080/10715760310001659611
- Amiel J, Maziere JC, Beucler I, et al. Familial isolated vitamin E deficiency. Extensive study of a large family with a 5‐year therapeutic follow‐up. J Inherit Metab Dis. 1995;18(3):333‐340. doi: 10.1007/BF00710425
- Roubertie A, Biolsi B, Rivier F, Humbertclaude V, Cheminal R, Echenne B. Ataxia with vitamin E deficiency and severe dystonia: report of a case. Brain Dev. 2003 Sep;25(6):442-5. doi: 10.1016/s0387-7604(03)00054-8
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/
- Vitamin E Deficiency Clinical Presentation. https://emedicine.medscape.com/article/126187-clinical
- Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993 Jun;111(6):761-72. doi: 10.1001/archopht.1993.01090060049022
- Iwasa K, Shima K, Komai K, Nishida Y, Yokota T, Yamada M. Retinitis pigmentosa and macular degeneration in a patient with ataxia with isolated vitamin E deficiency with a novel c.717 del C mutation in the TTPA gene. J Neurol Sci. 2014 Oct 15;345(1-2):228-30. doi: 10.1016/j.jns.2014.07.001
- Boltshauser E, Weber KP. Laboratory investigations. Handb Clin Neurol. 2018;154:287-298. doi: 10.1016/B978-0-444-63956-1.00017-5
- Tokgöz Y, Terlemez S, Karul A. Fat soluble vitamin levels in children with newly diagnosed celiac disease, a case control study. BMC Pediatr. 2018 Apr 9;18(1):130. doi: 10.1186/s12887-018-1107-x
- Teriaky A, Mosli M, Chandok N, Al-Judaibi B, Marotta P, Qumosani K. Prevalence of fat-soluble vitamin (A, D, and E) and zinc deficiency in patients with cirrhosis being assessed for liver transplantation. Acta Gastroenterol Belg. 2017 Apr-Jun;80(2):237-241.
- Gomez-Pomar E, Hatfield E, Garlitz K, Westgate PM, Bada HS. Vitamin E in the Preterm Infant: A Forgotten Cause of Hemolytic Anemia. Am J Perinatol. 2018 Feb;35(3):305-310. doi: 10.1055/s-0037-1607283
- Vitamin E Deficiency Medication. https://emedicine.medscape.com/article/126187-medication
- Kemnic TR, Coleman M. Vitamin E Deficiency. [Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519051
- Thébaut A, Nemeth A, Le Mouhaër J, et al. Oral Tocofersolan corrects or prevents vitamin E deficiency in children with chronic cholestasis. J Pediatr Gastroenterol Nutr. 2016;63(6):610‐615. doi: 10.1097/MPG.0000000000001331
- Perlmutter DH, Gross P, Jones HR, Fulton A, Grand RJ. Intramuscular vitamin E repletion in children with chronic cholestasis. Am J Dis Child. 1987;141(2):170‐174. doi: 10.1001/archpedi.1987.04460020060027
- Sherf-Dagan S, Buch A, Ben-Porat T, Sakran N, Sinai T. Vitamin E status among bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2021 Apr;17(4):816-830. doi: 10.1016/j.soard.2020.10.029