What is zeaxanthin
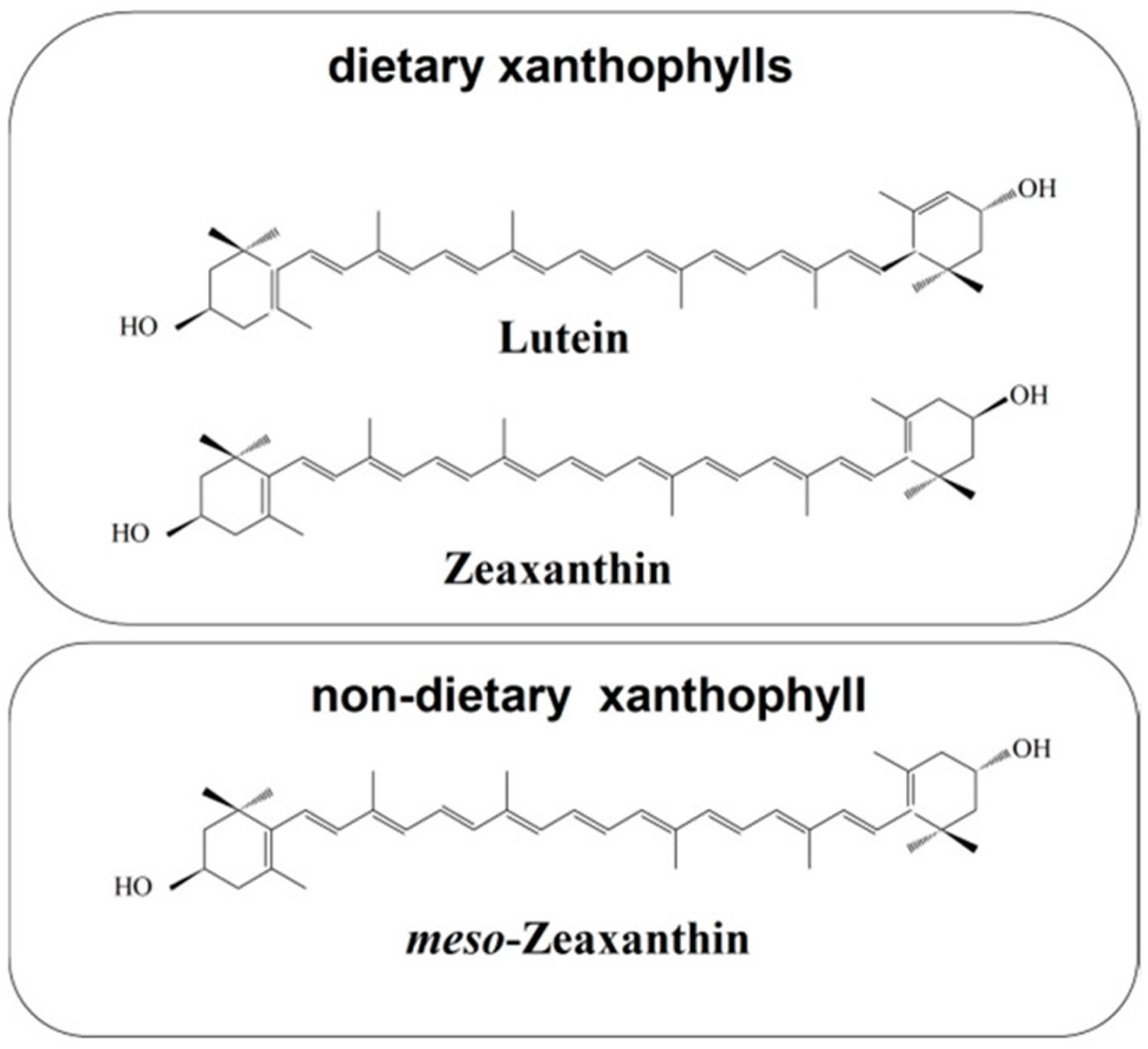
Zeaxanthin is a carotenoid alcohol (carotenol) that is involved in the xanthophyll cycle. Carotenoids are plant pigments responsible for bright red, yellow and orange hues in many fruits and vegetables. These pigments play an important role in plant health. Several plant regulatory mechanisms ensure that zeaxanthin is formed in leaves only under excess light and is removed quickly upon return to non-excessive light levels 1. Hundreds of carotenoids are present in the plant world, but the human macula contains only three: lutein, zeaxanthin (lutein structural isomer) and meso-zeaxanthin (a lutein metabolite) 2. Lutein and its close relative, zeaxanthin, are pigments called carotenoids that are related to beta-carotene and lycopene. The name lutein comes from the Latin word, lutea, meaning yellow. At normal concentrations in food, it is a yellow pigment, but can appear orange or red at high concentration. Lutein and zeaxanthin are collectively referred to as macular pigment because they are the primary carotenoids in the human macula and retina 3. Lutein and zeaxanthin play a vital role in visual health. Humans require dietary carotenoid intake because the relevant carotenoid synthesis enzymes do not exist in the human body 4. Human diet contains 5 to 12 times more lutein than zeaxanthin 5; lutein is found in higher quantities in human serum, which is consistent with the relatively high lutein content in fruits and vegetables as compared with the content of zeaxanthin 6. These proportions are reversed in the central part of the fovea (fovea centralis located in the center of the macula lutea of the retina, the region responsible for sharp, central vision) where the concentration of zeaxanthin is about two times greater than the concentration of lutein—each xanthophyll has a retina-resident active transporter 7.
In humans, dietary lutein and zeaxanthin are selectively concentrated in the visual system (eye and brain) over other carotenoids in the blood, comprising 80% to 90% of carotenoids in human eyes and the majority of carotenoids in the brain 8. Dietary lutein and zeaxanthin are selectively accumulated in the retina and constitute 100% of the total retina carotenoid content 9. Additionally, one of the stereoisomers of zeaxanthin, namely meso-zeaxanthin, is produced directly in the retina through the transformation of lutein 7. Retinal meso-zeaxanthin is a product of the conversion of lutein. The conversion of lutein to meso-zeaxanthin requires the migration of one double bond in the ε-ring of the lutein molecule and, most likely, this process takes place in the retinal pigment epithelium (RPE)/choroid 10. Meso-zeaxanthin is not commonly found in dietary sources; besides the eyes of vertebrates, meso-zeaxanthin is present in shrimp shells, turtle fat, and fish skin 4. Meso-zeaxanthin may be absorbed after oral administration and transported to the retina 11. All-trans zeaxanthin and meso-zeaxanthin are in the central part of the macula (fovea centralis located in the center of the macula lutea of the retina, the region responsible for sharp, central vision), whereas all-trans lutein dominates in the peripheral macula 12. Despite the abundance of meso-zeaxanthin in the foveal center, its specific function relative to dietary lutein and zeaxanthin remains unknown 2. Degeneration of the retina and retinal pigment epithelium (RPE) surrounding the fovea occurs in the disease state known as age-related macular degeneration (AMD).
Much of the research on zeaxanthin and lutein in humans has thus far focused on their role in photoprotection against damage, especially age-related blindness (macular degeneration) 4. Lutein and zeaxanthin are the exclusive carotenoids in the neural retina (highly concentrated in the human macula) and the lens, where they protect the eye from oxidative damage and improve visual performance 13. Zeaxanthin is highly concentrated in the fovea (located in the center of the macula lutea of the retina, the region responsible for sharp, central vision), extending from the inner to the outer limiting membranes, with especially high concentrations in the outer plexiform layer, while lutein is much more diffuse at relatively lower concentration 14. Li and colleagues 14 showed that zeaxanthin may play a more important role than lutein in human macular health and disease. There is increasing evidence that the macular pigment carotenoids, lutein and zeaxanthin, may play an important role in the prevention of age-related macular degeneration (AMD), cataract, and other blinding disorders. Lutein and zeaxanthin protect the retina against photochemical damage and oxidative stress by filtering harmful short wavelength light 15.
As a coexistent isomer of lutein, zeaxanthin is synthesized in plants and some micro-organisms. Zeaxanthin gives the distinct yellow color to many vegetables and other plants including paprika, corn, saffron and wolfberries. Zeaxanthin is one of the two primary xanthophyll (oxygen-containing) carotenoids contained within the retina of the eye and plays a predominant component in the central macula. It is available as a dietary supplement for eye health benefits and potential prevention of age-related macular degeneration (AMD). Zeaxanthin is also added as a food dye.
Lutein is a type of vitamin called a carotenoid. Lutein is related to beta-carotene and vitamin A. Foods rich in lutein include egg yolks, broccoli, spinach, kale, corn, orange pepper, kiwi fruit, grapes, orange juice, zucchini, and squash. Lutein is absorbed best when it is taken with a high-fat meal. Many people think of lutein as “the eye vitamin.” Lutein is commonly taken by mouth to prevent eye diseases such as an eye disease that leads to vision loss in older adults (age-related macular degeneration or AMD), and cataracts. There is no good scientific evidence to support the use of lutein for other conditions. Many multivitamins contain lutein. They usually provide a relatively small amount, such as 0.25 mg per tablet.
Lutein is possibly effective for:
- An eye disease that leads to vision loss in older adults such as age-related macular degeneration (AMD). People who eat higher amounts of lutein in their diet seem to have a lower risk of developing AMD. But people who already eat high amounts of lutein might not benefit from increasing their intake even more. Taking lutein supplements for up to 36 months can improve some symptoms of AMD. Greater improvement in symptoms might be seen when lutein is taken for at least 1 year at doses above 10 mg, and when it is combined with other carotenoid vitamins. Lutein does not seem to keep AMD from becoming worse over time.
- Cataracts. Eating higher amounts of lutein is linked with a lower risk of developing cataracts. Taking supplements containing lutein and zeaxanthin reduces the risk of developing cataracts that require surgical removal in people who eat low amounts of lutein and zeaxanthin as part of their diet. Also, taking lutein supplements seems to improve vision in older people who already have cataracts and do not already consume a lot of lutein and zeaxanthin.
Table 1. Major causes of vision loss in older adults
| Disease | Epidemiology 16 | Clinical presentation | Screening recommendations |
| Age-related cataracts | Cases in U.S. adults 40 years and older (2010): 24.4 million Estimated cases by 2050: 50 million By 75 years of age, 50% of whites will have cataracts in one or both eyes; 70% will have them by 80 years of age 53% of blacks and 61% of Hispanics will be affected by 80 years of age The greatest rate increase by 2050 is expected in Hispanics, with an increase from 1.76 million to 9.51 million | Vision loss Glare intolerance and halos, especially at night or while driving | No specific screening recommendations; treatment is not needed until patients experience bothersome visual symptoms |
| Age-related macular degeneration (AMD) | Cases in U.S. adults 50 years and older (2010): 2.44 million Estimated cases by 2050: 5.44 million Affects 2.5% of whites 50 years and older vs. 0.9% of blacks, Hispanics, and persons of other races Risk rises sharply for persons older than 80 years; prevalence among this age group in the United States is 11% overall and 13.8% among whites The greatest rate increase by 2050 will likely be among Hispanics, with a nearly sixfold rise | Vision loss Difficulties with dark adaptation Positive Amsler grid test result | Patients with early age-related macular degeneration or a family history of the disease should be encouraged to assess their own vision (i.e., with an Amsler grid) and to see an ophthalmologist for dilated eye examinations (age to begin screening and optimal examination interval are not specified) 17 |
| Diabetic eye disease | Cases in U.S. adults (2010): 7.7 million Estimated cases by 2050: 14.6 million Hispanics 50 years and older have the highest rates of diabetic retinopathy, with cases projected to increase from 1.2 million to 5.3 million by 2050 | Vision loss Fluctuating vision Presence of floaters Flashes of light (photopsia) Defects in the field of vision | Older adults with diabetes mellitus should be referred to an ophthalmologist at diagnosis for dilated eye examination, and reexamined at least annually17; it is reasonable to discontinue these examinations in older adults with no or mild diabetic eye disease and life-limiting conditions (e.g., end-stage renal disease) |
| Glaucoma | Cases in U.S. adults 40 years and older (2010): 2.7 million Estimated cases by 2050: 6.3 million Blacks 40 years and older are at the highest risk By 69 years of age, 6% of U.S. blacks will have glaucoma; risk increases to 12% after 80 years of age | No symptoms in the early stages Peripheral visual field loss and progressive central vision loss become apparent as the disease advances Once established, vision loss is permanent | Current evidence is insufficient to assess the balance of benefits and harms of screening for primary open-angle glaucoma in adults, according to the U.S. Preventive Services Task Force Screening of high-risk groups (e.g., blacks, Hispanics, persons with a family history of glaucoma) should be considered Complete screening includes measurement of visual acuity, measurement of intraocular pressure, and automated visual field testing 18 |
Figure 1. Chemical structures of macular xanthophylls present in the retina, including dietary xanthophylls (lutein and zeaxanthin) and non-dietary xanthophyll (meso-zeaxanthin)
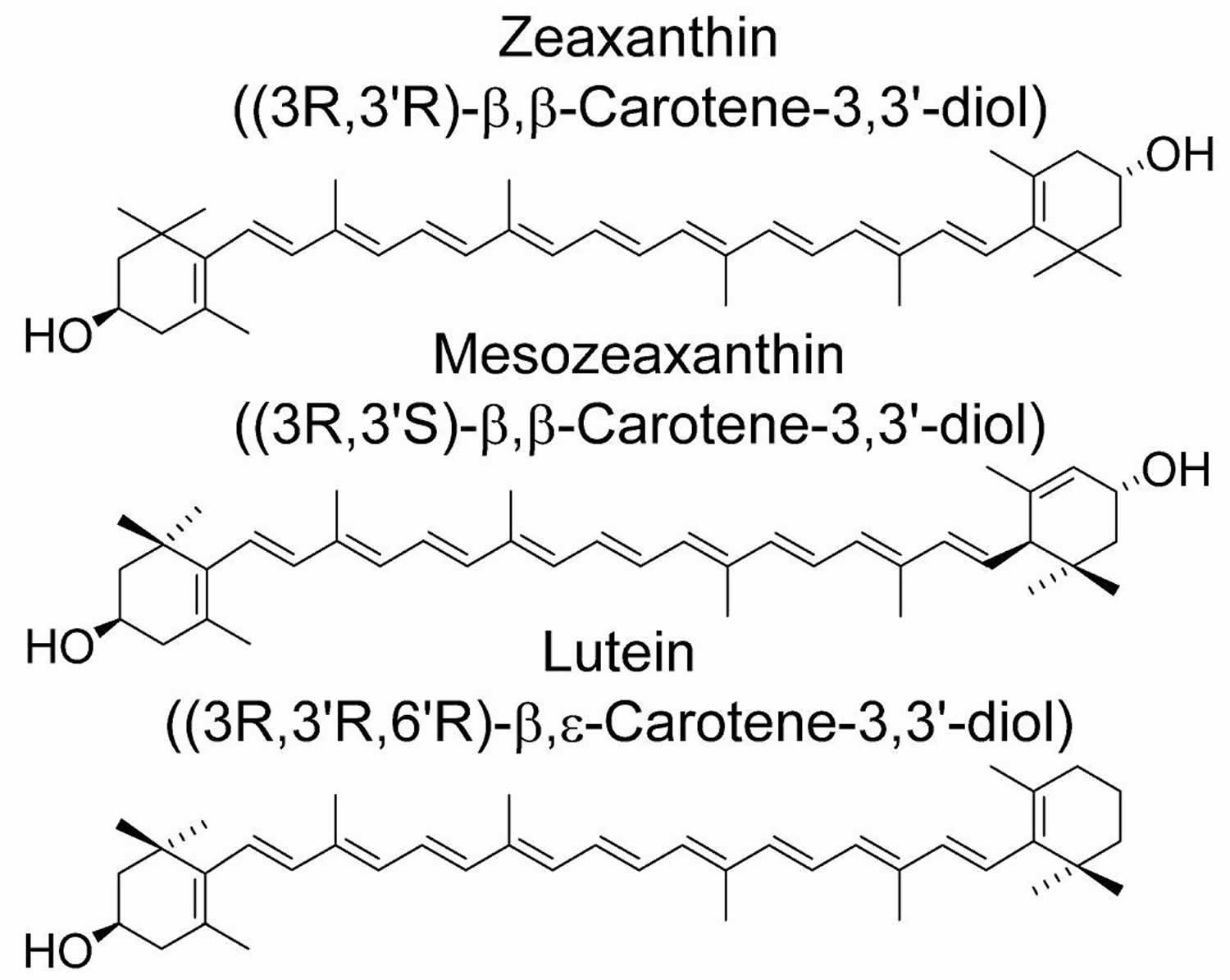
[Source 6 ]Figure 2. Lutein and zeaxanthin chemical structures
[Source 19 ]Lutein and zeaxanthin
Humans and other animals cannot synthesize carotenoids de novo, and must obtain carotenoids (or their precursors) from the diet 4. In humans, both zeaxanthin and lutein have a number of functions, including photoprotection against damage by intense light, detoxification of oxidants (reactive oxygen species [ROS] and other radicals), and the maintenance of structural and functional integrity of biological membranes 20. Despite similar qualitative roles, effects differ in degree between zeaxanthin and lutein. Zeaxanthin is the more effective antioxidant, presumably due to its longer system of conjugated double bonds 21 and has a more pronounced impact on membrane integrity
Lutein and zeaxanthin protect the retina against photochemical damage and oxidative stress by filtering harmful short wavelength light 15. It has been extensively reported that consumption of lutein-and zeaxanthin-rich green leafy vegetables and orange and yellow fruits and vegetables is associated with lower incidence of cancer, cardiovascular disease, age-related macular degeneration (AMD) and cataract formation 22.
Age-related macular degeneration (AMD) is an eye disease that can blur the sharp, central vision you need for activities like reading and driving. “Age-related” means that it often happens in older people. “Macular” means it affects a part of your eye called the macula, the part of the eye that provides sharp, central vision needed for seeing objects clearly. AMD can cause blurriness or missing regions in central vision and is a leading cause of vision loss and blindness among older Americans.
There are 2 types of age-related macular degeneration (AMD). About 8 out of 10 people with AMD have the dry AMD. This condition is due to a breakdown or thinning of the macula. Dry AMD usually begins when tiny, yellow deposits called drusen form under the retina. Eventually, the macula may become thinner and stop working properly. Drusen alone does not cause vision loss. But when drusen grow in size or number, you are at risk for getting early or intermediate AMD. There are not always symptoms with these stages of AMD, though people with intermediate AMD might start to notice a blurred spot in their central vision. Advanced AMD develops when cells in your macula begin to break down. This is when the blurred spot in your central vision starts getting bigger and darker. That is what robs you of your central vision.
- Dry AMD: This form is quite common. About 80% (8 out of 10) of people who have AMD have the dry form. Dry AMD is when parts of the macula get thinner with age and tiny clumps of protein called drusen grow. You slowly lose central vision. There is no way to treat dry AMD yet.
- Wet AMD: This form is less common but much more serious. Wet AMD is when new, abnormal blood vessels grow under the retina. These vessels may leak blood or other fluids, causing scarring of the macula. You lose vision faster with wet AMD than with dry AMD.
Age-related macular degeneration (AMD) is a common condition — AMD is leading cause of vision loss for people age 50 and older. AMD doesn’t cause complete blindness, but losing your central vision can make it harder to see faces, drive, or do close-up work like cooking or fixing things around the house.
AMD happens very slowly in some people. Even if you have early AMD, you may not experience vision loss for a long time. For other people, AMD progresses faster and can lead to central vision loss in one eye or both eyes.
As AMD progresses, many people see a blurry area near the center of their vision. Over time, this blurry area may get bigger or you may see blank spots. Things may also seem less bright than before. Some people may also notice that straight lines start to look wavy. This can be a warning sign for late AMD. If you notice this symptom, see your eye doctor right away.
Many people don’t realize they have AMD until their vision is very blurry. This is why it is important to have regular visits to an ophthalmologist. He or she can look for early signs of AMD before you have any vision problems.
Research shows that you may be able to lower your risk of AMD (or slow its progression) by making these healthy choices:
- Quit smoking — or don’t start
- Get regular physical activity
- Maintain a healthy blood pressure and cholesterol levels
- Eat healthy foods. Eating dark leafy greens, yellow fruits and vegetables, fish, and a balanced, nutrient-rich diet have been shown beneficial for people with AMD.
There’s currently no treatment for early AMD, so your eye doctor will probably just keep track of how your eyes are doing with regular eye exams. Eating healthy, getting regular exercise, and quitting smoking can also help.
- If you are diagnosed with intermediate or late AMD, ask your eye doctor about treatment options and how the condition may affect your vision in the future.
- Right now, there is no way to treat the dry form of AMD. If you have intermediate or late AMD, people with lots of drusen or serious vision loss might benefit from taking a certain combination of dietary (vitamins and minerals) supplements may be able to stop it from getting worse (see Age-Related Eye Diseases Studies (AREDS) below). Talk with your ophthalmologist about whether nutritional supplements are recommended for you. A large study (AREDS and the later AREDS 2 study) found those people may slow their dry AMD by taking these vitamins and minerals daily:
- Vitamin C (500 mg)
- Vitamin E (400 IU)
- Lutein (10 mg)
- Zeaxanthin (2 mg)
- Zinc (80 mg)
- Copper (2 mg)
- Your ophthalmologist can tell you if vitamins and minerals are recommended for your dry AMD, as not all forms will benefit from the AREDS supplements. Beta carotene should not be used by smokers as it raised the risk of lung cancer.
- It is important to remember that nutritional supplements are not a cure for AMD, but they may help to slow the disease in some people with early- to mid-stage AMD.
For people with a type of late AMD called “wet” or neovascular AMD, there are other treatments that may be able to stop further vision loss:
- Medicines called anti-VEGF drugs (Vascular Endothelial Growth Factor Inhibitors) that the doctor injects in your eye. Anti-VEGF treatment helps reduce the number of abnormal blood vessels in your retina. It also slows any leaking from blood vessels. This medicine is delivered to your eye through a very slender needle. Injections are administered once monthly for four months or until vision stabilizes, then every three months thereafter. The safety and effectiveness of this intervention have not been evaluated past 24 months.
- Laser treatment, called photodynamic therapy (PDT). Laser surgery may also be used to treat some types of wet AMD. Your eye surgeon shines a laser light beam on the abnormal blood vessels. This reduces the number of vessels and slows their leaking.
The Age-Related Eye Disease Study (AREDS) is a randomized controlled trial that tested the effectiveness of an antioxidant vitamin supplement in 3,640 patients with AMD who were 55 to 80 years of age 23. Patients in the active treatment arm who had moderate to severe AMD at enrollment had a lower risk of disease progression compared with those receiving placebo over 6.3 years of follow-up (26.7% vs. 35.7%; number needed to treat = 11) 24. Supplements do not prevent the development of AMD 25.
Because beta-carotene supplements were subsequently associated with increased lung cancer rates in patients who smoke tobacco, a second trial (AREDS 2) 26 randomized participants to a revised supplement containing lutein plus zeaxanthin, omega-3 fatty acids, or both 27. Removal of beta-carotene had no effect on AMD progression 28. Omega-3 fatty acid supplementation offered no benefit beyond the original Age-Related Eye Disease Study (AREDS) formulation 28. Lutein plus zeaxanthin appeared to offer no additional benefit in the general study population 28. Secondary analysis of the subgroup with large drusen at enrollment, however, suggested that lutein plus zeaxanthin may provide modest additional benefit in preventing progression to advanced AMD (hazard ratio = 0.76) and development of neovascular AMD (hazard ratio = 0.65) 29. Lutein plus zeaxanthin also appears to be safe, with no increased risk of lung cancer 29.
The original AREDS and modified AREDS 2 formulations (Table 2) are sold over the counter. Current or former smokers should be counseled to avoid the original AREDS formula containing beta-carotene 28.
Table 2. Supplements to delay progression of Age-Related Macular Degeneration (AMD)
| AREDS supplements | AREDS 2 supplements |
| 500 mg vitamin C | 500 mg vitamin C |
| 400 IU vitamin E | 400 IU vitamin E |
| 15 mg beta-carotene* | 10 mg lutein plus 2 mg zeaxanthin |
| 80 mg zinc as zinc oxide | 25 mg zinc |
| 2 mg copper as cupric oxide** | 2 mg copper as cupric oxide** |
| Fish oil containing omega-3 fatty acids (docosahexaenoic acid [DHA] 350 mg + eicosapentaenoic acid [EPA] 650 mg) |
Footnote: * Associated with increased rates of lung cancer in smokers and is not recommended for current or former smokers. Consult your doctor or eye care professional about which supplement, if any, is right for you.
** Added to avoid zinc-related copper deficiency
Abbreviations: IU = international units; mg = milligrams
[Source 30 ]Dry AMD and AREDS Vitamins
Age-Related Eye Diseases Study (AREDS) researchers tested whether taking nutritional supplements could prevent or slow age-related macular degeneration (AMD) and cataract. Age-Related Eye Diseases Study (AREDS) and Age-Related Eye Diseases Study 2 (AREDS 2) formulas are nutritional supplements that reduce the risk of progressing to advanced age-related macular degeneration (AMD). There is no evidence that dietary supplements can prevent age-related macular degeneration (AMD). But certain combinations of vitamins and minerals may help delay the development of late-stage AMD. The formulations tested in the clinical trials are now sold as the AREDS and the AREDS2 formulas.
The formulation suggested by AREDS 1 is a daily dose of:
- Vitamin C (ascorbic acid) 500 mg
- Vitamin E 400 international units (IU)
- Beta carotene 15 mg
- Zinc oxide 80 mg
- Cupric oxide 2 mg (added to reduce the risk of copper-deficiency anemia).
This formulation was modified by AREDS 2. AREDS 2 (Age-Related Eye Disease Study 2) was a very large research study. It looked at taking vitamins and minerals daily for AMD. This study found that certain nutritional supplements could help some people who have a lot of drusen. These supplements may also help people who have lost a lot of vision in at least one eye from AMD. Taking the following nutritional supplements every day may help these people lower their risk of getting late-stage or wet AMD:
- Vitamin C (ascorbic acid) 500 mg
- Vitamin E 400 international units (IU)
- Lutein 10 mg
- Zeaxanthin 2 mg
- Zinc (as zinc oxide) 80 mg
- Copper (as cupric oxide) 2 mg (added to reduce the risk of copper-deficiency anemia)
- Fish oil containing omega-3 fatty acids (docosahexaenoic acid [DHA] 350 mg + eicosapentaenoic acid [EPA] 650 mg)
- Beta carotene was removed as it increased the risk of lung cancer, especially in smokers. Macular pigments lutein and zeaxanthin provided an additional reduction of risk of progression of age-related macular degeneration (AMD).
So far, only people who have a considerably higher risk of developing late-stage AMD have been shown to benefit from the AREDS supplements. People are at higher risk if they already have many deposits in their eyes, called drusen. Taking AREDS supplements regularly lowered the risk of late-stage AMD associated with loss of vision in some of them. In order to be effective, the supplements had to be taken daily for several years.
It’s important to talk with your doctor before taking these products because they aren’t suitable for everyone.
Why are the AREDS and AREDS2 formulas different?
In the AREDS trial, taking the AREDS formula reduced the risk of advanced AMD by about 25% over a five-year period. In the AREDS2 trial, adding omega-3 or lutein plus zeaxanthin to the AREDS formulation (containing beta-carotene) had no additional overall effect on the risk of advanced AMD. However, trial participants who took AREDS containing lutein and zeaxanthin and no beta-carotene had a reduction in risk of advanced AMD, compared with those who took AREDS with beta-carotene. Also, for participants with very low levels of lutein and zeaxanthin in their diet, adding these supplements to the AREDS formulation helped lower their risk of advanced AMD. Finally, former smokers who took AREDS with beta-carotene had a higher incidence of lung cancer. The investigators found no significant changes in the effectiveness of the formulation when they lowered zinc.
How do lutein and zeaxanthin compare to beta-carotene?
During the AREDS trial, two large trials funded by the National Cancer Institute found that beta-carotene may increase lung cancer risk among people who smoke. Lutein and zeaxanthin are in the same family of nutrients as beta-carotene and are believed to have important functions in the retina. Lutein and zeaxanthin have not been associated with increased cancer risk.
Some studies prior to AREDS2 found that dietary intake of lutein, zeaxanthin and omega-3 fatty acids is associated with a lower risk of developing advanced AMD. Analysis from the AREDS2 trial suggests that lutein plus zeaxanthin offers similar or better protective benefits against advanced AMD compared with beta-carotene. In the trial, participants who took an AREDS formulation containing lutein + zeaxanthin lacking beta-carotene had an 18% lower risk of progressing to advanced AMD compared with those who took AREDS containing beta-carotene (no lutein or zeaxanthin). Among participants who had the lowest dietary intake of lutein and zeaxanthin, those who took AREDS with lutein plus zeaxanthin had a 26% lower risk of progressing to advanced AMD compared to participants taking the original AREDS formula.
Are the AREDS vitamins right for me?
In clinical trials, the AREDS and AREDS2 formulas benefited people with intermediate or late AMD. There was no benefit for people with early AMD or for people who do not have AMD.
Your doctor or eye care provider is in the best position to advise you on how treat your AMD. You may wish to discuss AREDS/AREDS2 supplements with your health care providers to decide which, if any, supplements are right for you.
Will taking the AREDS or AREDS2 supplements prevent AMD?
Nutritional supplements cannot prevent AMD. However, the AREDS/AREDS2 supplements may delay progression of intermediate to advanced AMD and may help you keep your vision longer. The participants AREDS trial have now been followed for more than 10 years, and the benefits of the AREDS formulation have persisted over this time.
How effective is the AREDS formula?
The “AREDS study” (Age-Related Eye Disease Study), involving about 3,600 participants, suggest that the AREDS supplements have a positive effect. The study lasted 6 years. During this time, the progression of vision loss was slowed down a little in some people who used the AREDS formula, but most of them didn’t benefit from it. Expressed in numbers 31:
- Without treatment: Vision worsened considerably within the six years in about 43 out of 100 participants.
- With treatment: This happened in about 37 out of 100 participants who took the AREDS formula.
In other words, 6 out of 100 people benefited from taking these supplements every day for six years 31.
Can I take a daily multivitamin if I am taking one of the AREDS/AREDS2 formulas?
Yes. The AREDS and AREDS2 formulas do not substitute for multivitamins. In AREDS, two-thirds of the study participants took multivitamins along with the AREDS formulation. In AREDS2, almost nine of ten participants took multivitamins.
Can a daily multivitamin alone provide the same vision benefits as the AREDS or AREDS2 formulas?
No. The vitamins and minerals tested in AREDS and AREDS2 trials were provided in much higher doses than what is found in multivitamins. Also, it is important to remember that most of the trial participants took multivitamins. Taking an AREDS formulation clearly provided a benefit over and above multivitamins.
Can diet alone provide the same levels of antioxidants and zinc as the AREDS or AREDS2 formulas?
No. The high levels of vitamins and minerals are difficult to achieve from diet alone. However, previous studies have suggested that people who have diets rich in green, leafy vegetables—a good source of lutein and zeaxanthin—have a lower risk of developing AMD. In the AREDS2 trial, the participants who benefited most from taking lutein plus zeaxanthin were those who did not get much of these nutrients in their diet. Within this group, those who received lutein plus zeaxanthin supplements had a 26% reduced risk of developing advanced AMD compared with those who did not receive the supplements.
What is omega-3?
Omega-3 fatty acids are made by marine algae and enriched in fish oils. They are believed to be responsible for the health benefits associated with regularly eating fish, including lower rates of cardiovascular disease. The AREDS2 study focused on the omega-3 fatty acids docosahexanoic acid (DHA) and its precursor eicosapentanoic acid (EPA). DHA is needed for the integrity of retinal cells and has been shown to promote retinal development and repair in prior studies.
What is the function of copper in the AREDS and AREDS2 supplements?
In AREDS/AREDS2 trials, copper (as cupric oxide) was added to supplement formulas containing zinc. The goal was to reduce the risk of copper deficiency anemia, a condition associated with high levels of zinc intake. The studies showed clear benefits for patients who took an AREDS formula with zinc, with no evidence of anemia. There was no evidence that 2 mg copper was harmful, nor reason to suspect that it would be. So, in the AREDS investigators’ hands, the use of copper was safe and may have helped balance the effects of zinc.
What is the basis for the concentration of zinc in the AREDS supplements?
In the AREDS trial, the 80 mg zinc dose (alone or in combination with antioxidant vitamins) was found to be effective compared to a placebo. Although zinc was found to be an essential component of the AREDS formulation, some nutritional experts recommended a lower dose. In the AREDS2 trial, there was no placebo control. Instead, participants were given the option to take the original formula or to be randomly assigned to receive a modified version, such as a formula containing 25 mg zinc. The investigators did not find a difference in the effects of 80 mg vs. 25 mg zinc. Because AREDS2 did not include a placebo control, results from AREDS, placebo-controlled trial, are still considered the gold standard.
Zinc is found in vegetables, grains, and meat. Vegetables and grains contain other molecules that can prevent zinc absorption and thus reduce its bioavailability. Supplements contain purified zinc, without these competing molecules. Although the chemical form of zinc affects its rate of absorption in the stomach, it is not clear how this affects bioavailability (i.e., the amount of zinc that reaches the retina).
Lutein and zeaxanthin foods
Carotenoids are plant pigments responsible for bright red, yellow and orange hues in many fruits and vegetables. Carotenoids cannot be synthesized in your body and they must be obtained from dietary consumption. Major sources of lutein are leafy greens, corn, and green vegetables such as broccoli, kale, spinach, brussel sprouts, green beans, peas, and zucchini 32. Major sources of zeaxanthin are egg yolks, corn, corn meal, Japanese persim-mons, and leafy greens 33. The carotenoid compositions of foods vary qualitatively and quantitatively 34. Factors such as species, cultivation, part of the plant, degree of maturity at harvest, and post-harvest handling practices affect carotenoid levels 34. The differences in lutein and zeaxanthin among green leafy vegetables studied are often attributed to species variations 35. Lutein concentration during maturation differs depending on the vegetable; in some cases, an increase in lutein concentration has been reported, whereas in other cases, a decrease has also been reported 36.
Table 3. Lutein and zeaxanthin content of vegetables (1 cup, cooked)
| Lutein + Zeaxanthin Content of Vegetables (1 cup, cooked) | |
| Kale | 23 mg |
| Spinach | 20 mg |
| Turnip greens | 12 mg |
| Green peas | 2 mg |
| Corn | 2 mg |
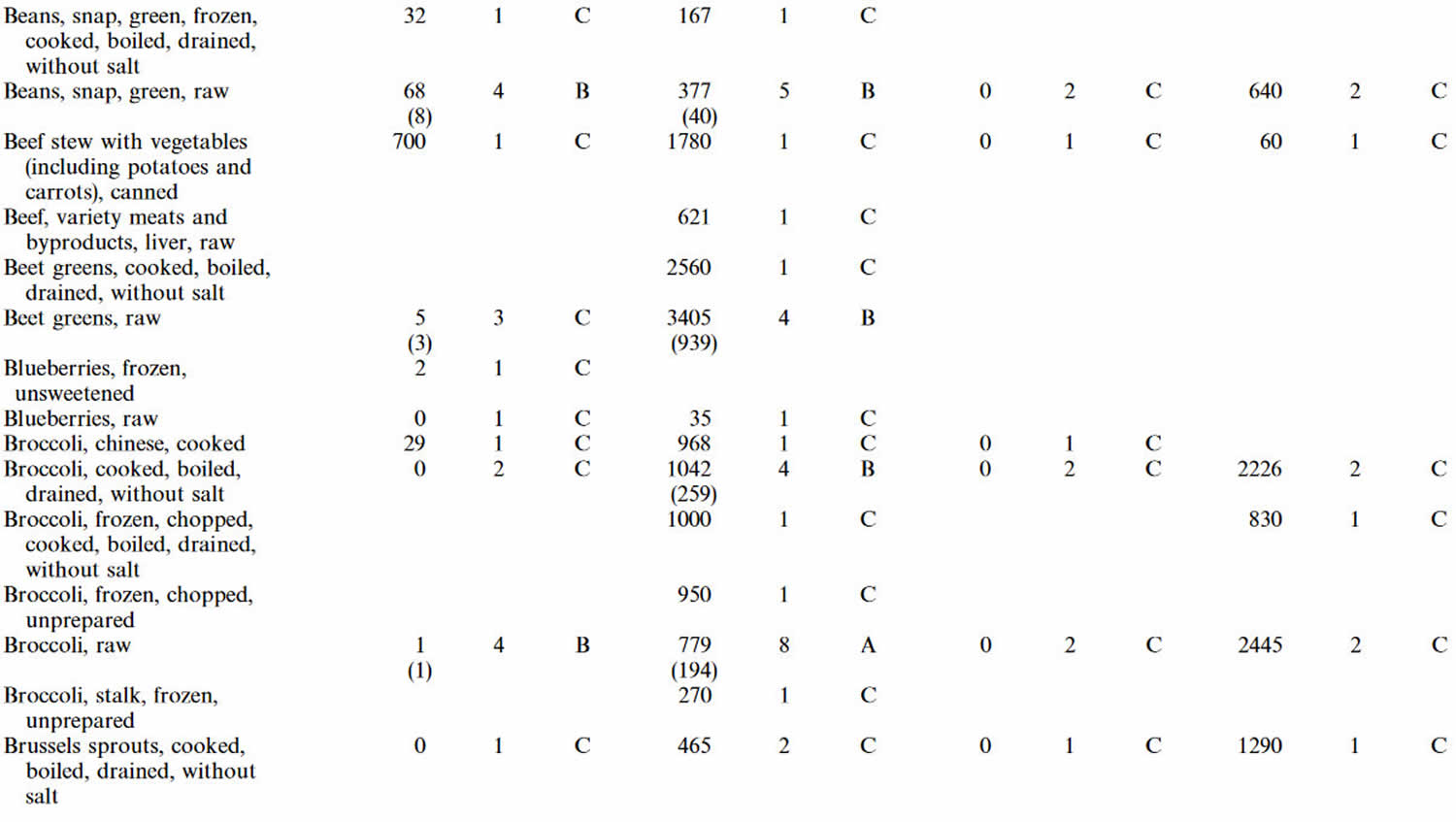
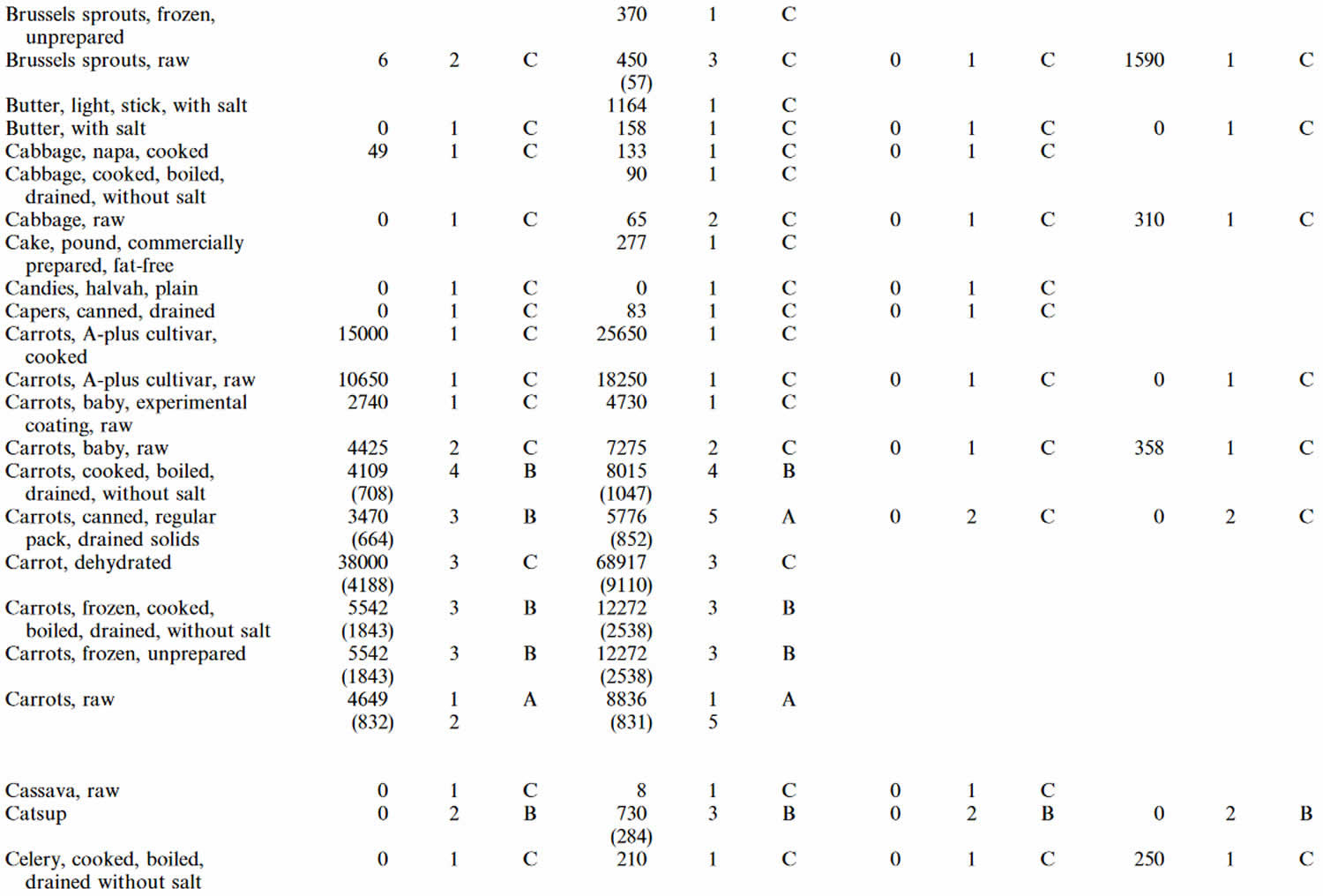
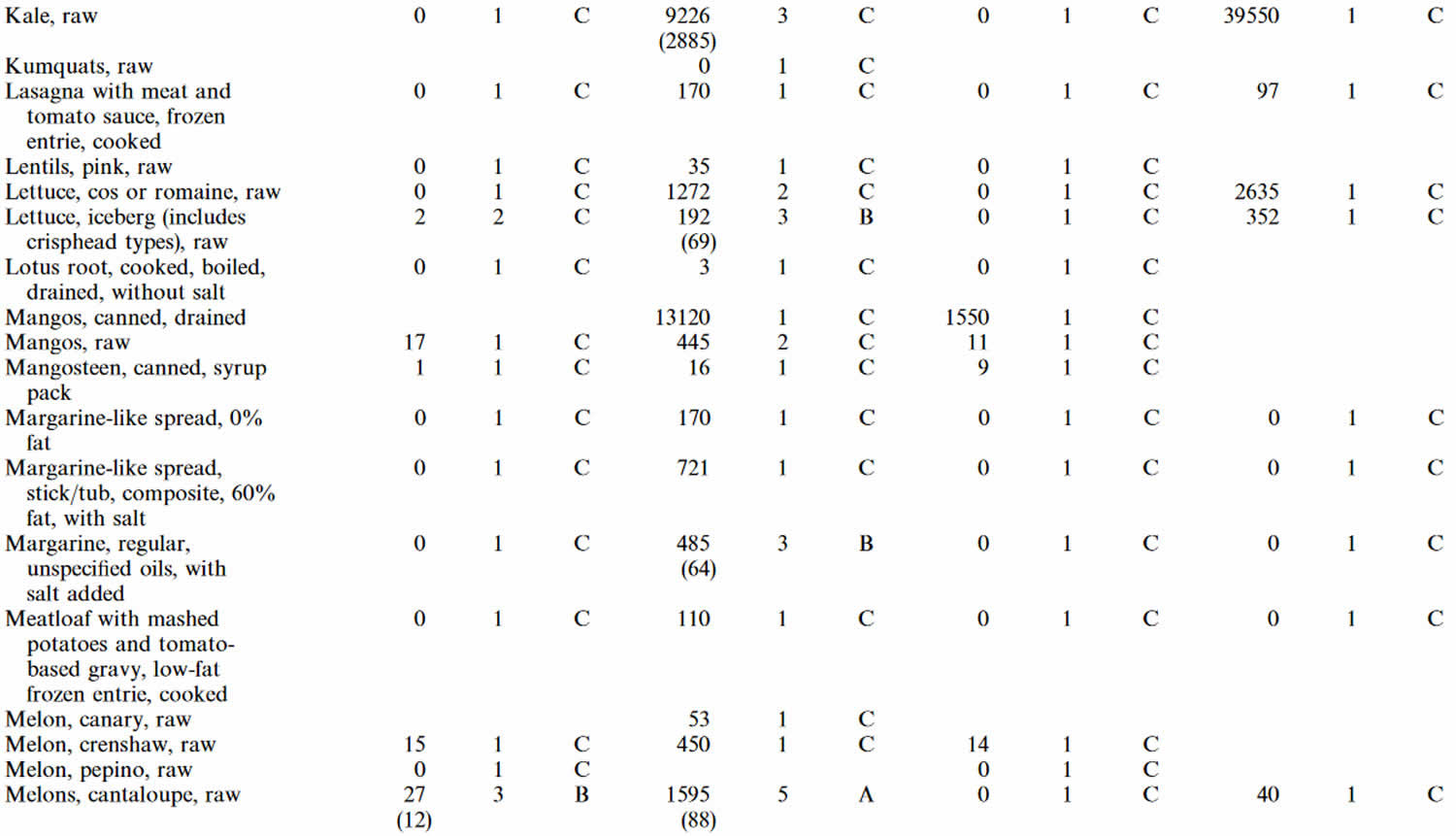
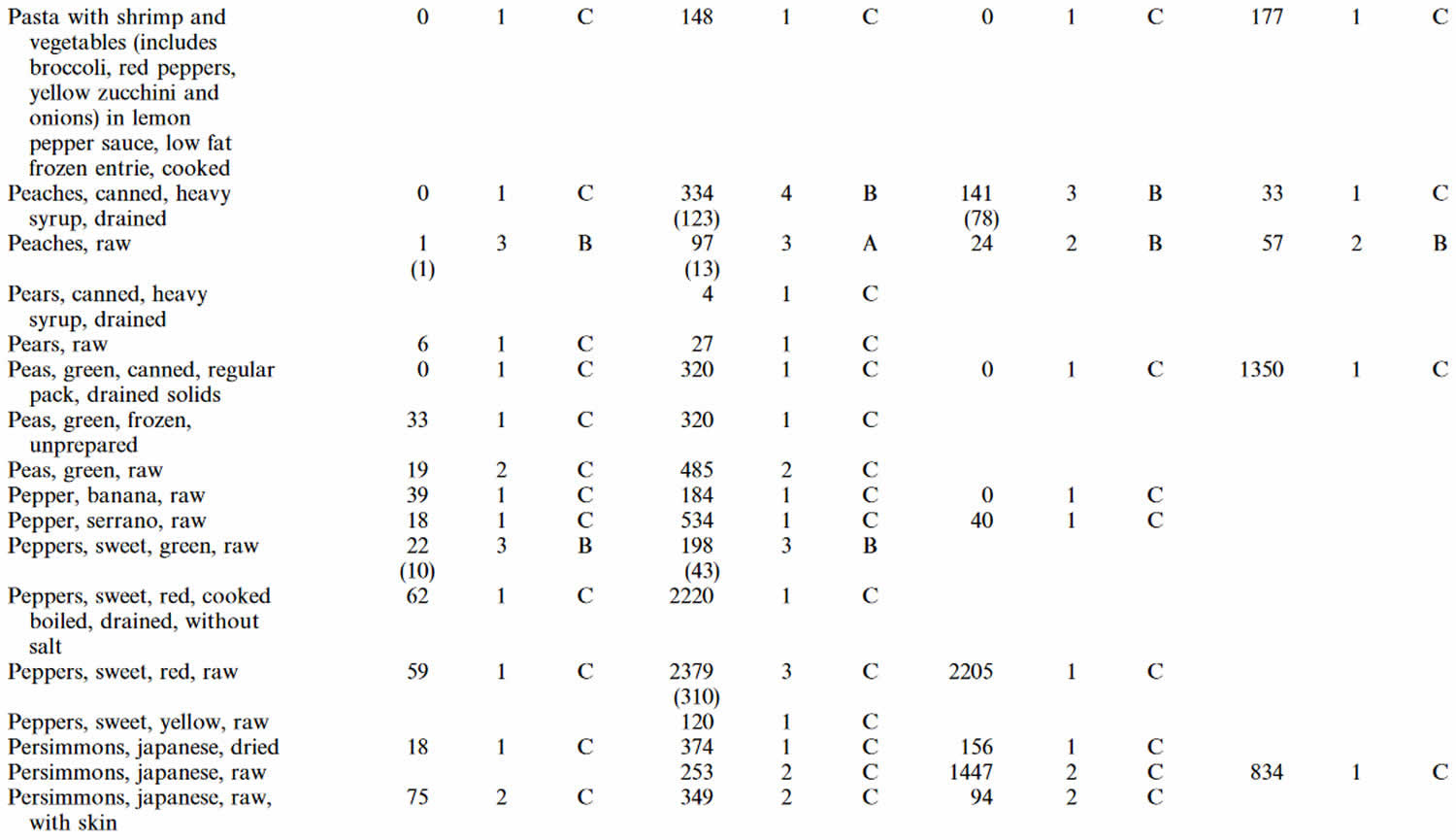
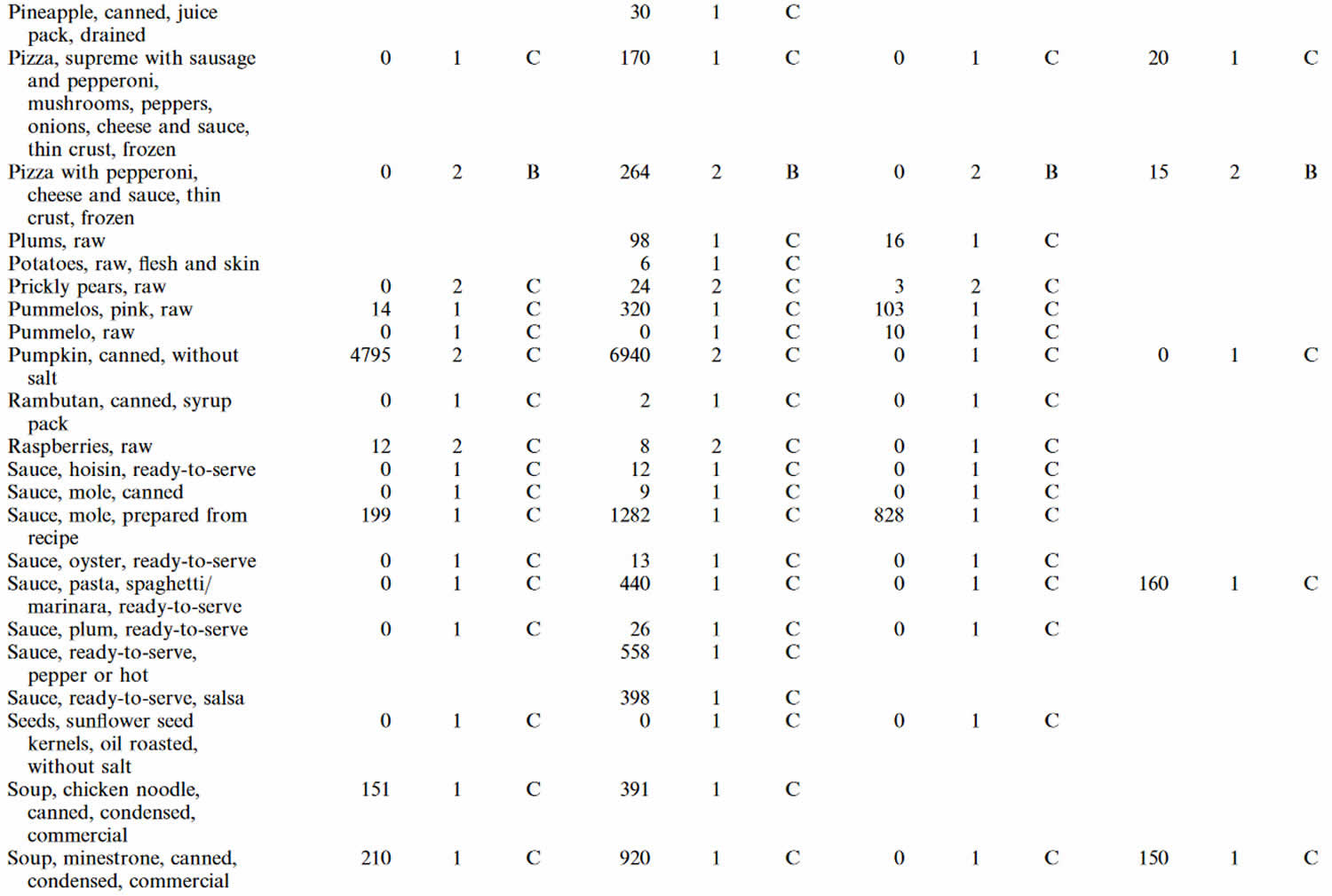
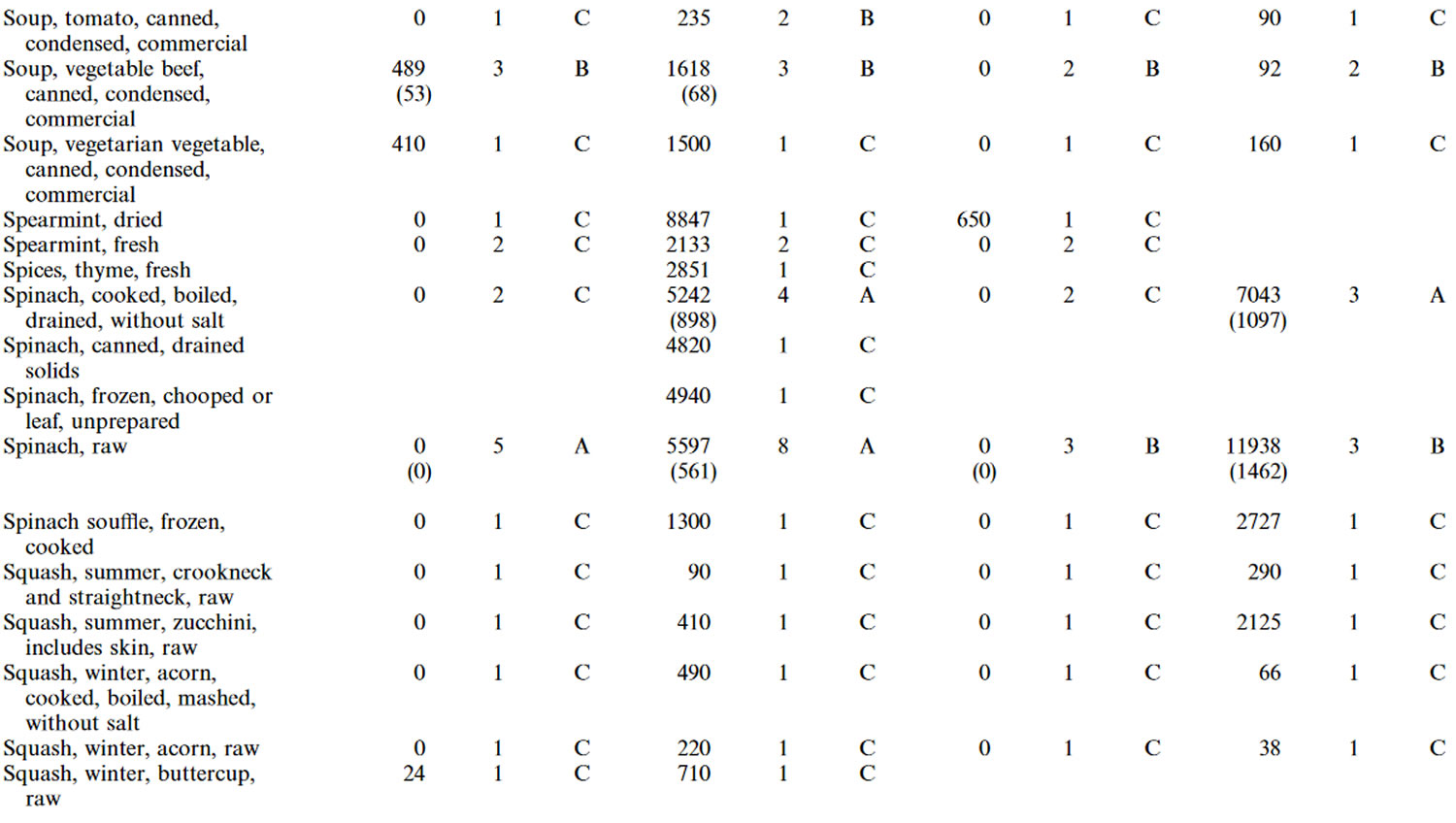
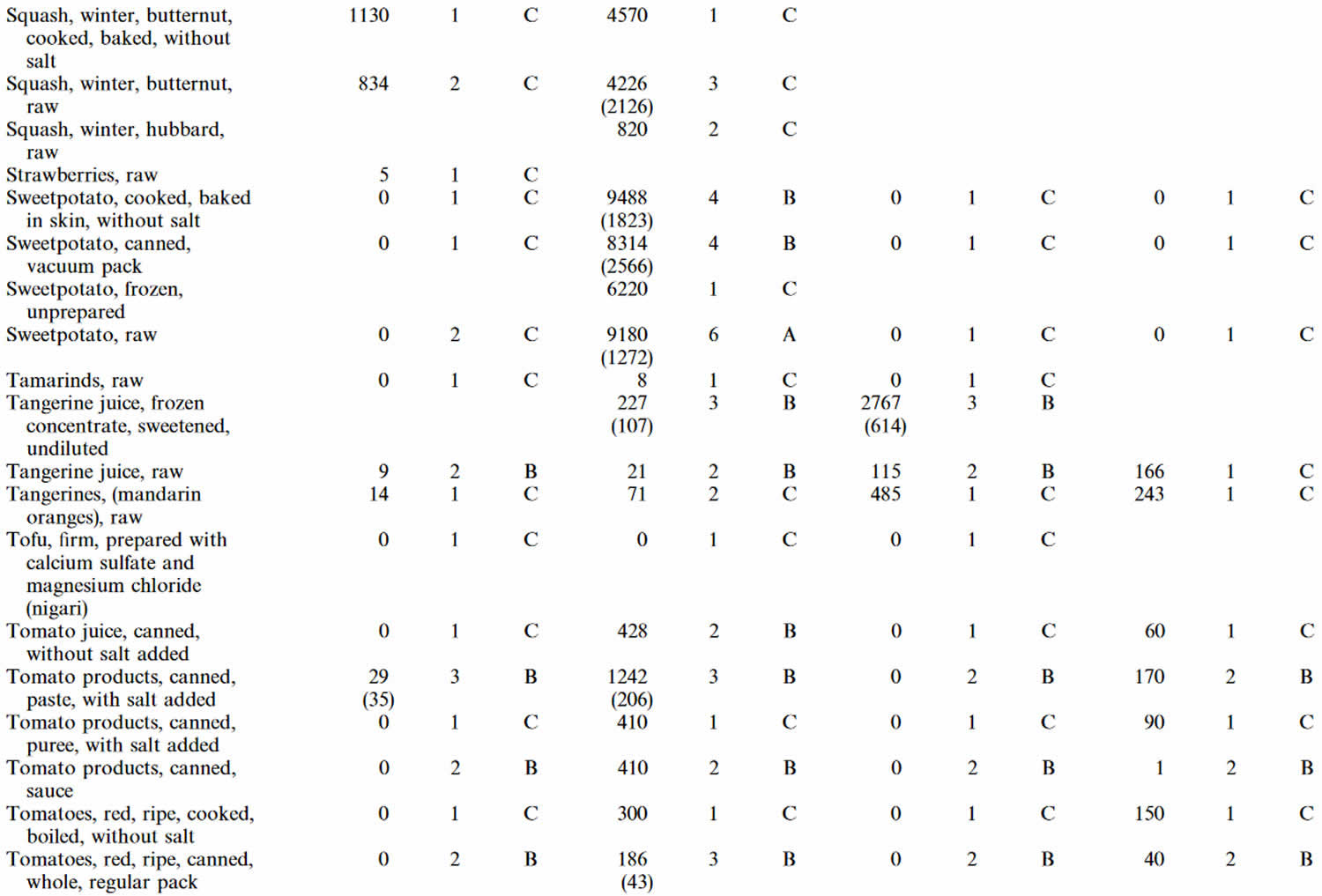
Table 4. Lutein and zeaxanthin content of U.S. foods (μg (microgram) per 100 g edible portion) (plus alpha-carotene, beta-carotene, beta-cryptoxanthin)
Footnotes: 1 = Weighted mean; 2 = Number of samples; 3 = Confidence code; 4 = Weighted standard error of the mean.
[Source 32 ]Bio-accessibility of carotenoids from green leafy vegetables is low, and various dietary factors affect their oral bioavailability 37. Given their hydrophobic nature, there is evidence that consuming carotenoid-rich foods in the presence of oils or cholesterol may increase their uptake 38. In addition to vegetables, which are less bio-available, egg yolk 39 and fortified milk 40, are also good dietary and bioavailable sources of lutein and zeaxanthin. This may explain why some study results suggest a higher bioavailability of lutein from lutein-enriched eggs versus leafy greens such as spinach or other forms of supplementation 41 The dietary intake of carotenoids varies widely between individuals, and epidemiological studies have consistently shown that all age groups and ethnicities, as well as both sexes, have overall greater lutein than zeaxanthin consumption 42.
Meso-zeaxanthin is rarely found in the human diet, but it has been detected in shrimp carapace (hard upper shell), fish skin, and turtle fat, where all three isomers of zeaxanthin were found 43 and Nolan et al 44 has recently confirmed its presence in fish skin using more modern methods. A significant amount of meso-zeaxanthin has been detected in commercially produced chicken eggs in Mexico where it is commonly added to the feed to achieve desirable coloration 45.
Lutein and zeaxanthin supplements
Recent evidence for the beneficial effects of the macular carotenoids has increased consumer demand for these products. Commercially available lutein preparations such as Lutemax 2020 (OmniActive Health Technologies, Ltd., Mumbai, India), Hi-Fil (Industrial Organica, Topo Chico, Mexico), FloraGlo (Kemin Industries, Inc., Des Moines, USA), and others are made from marigold oleoresin 4. Lutein produced for human nutritional supplements typically contains 6-16% of waxes, and their zeaxanthin composition varies from one to another. Lutemax 2020 has approximately 13% of zeaxanthin (a mix of (3R, 3′R)-zeaxanthin and (3R, 3′S)-zeaxanthin), while FloraGlo has 2- 9% of zeaxanthin with minimal meso-zeaxanthin 4.
Carotenoids such as lutein and zeaxanthin are generally recognized as safe (GRAS) for human consumption, which allows food manufacturers to use them as additives. Recently, the European Food Safety Authority (EFSA) Panel on Food Additives and Nutrient Sources added to Food established an acceptable daily intake of 1 mg per kg bodyweight per day for lutein preparations derived from Mexican marigold or Aztec marigold (Tagetes erecta) containing at least 80% carotenoids 46. Based on the available data, the European Food Safety Authority Panel on Food Additives and Nutrient Sources concluded that an intake of 0.75 mg per kg bodyweight per day of synthetic zeaxanthin does not raise any safety concerns 47. These values correspond to a daily intake of 53 mg of zeaxanthin and 70 mg of lutein for a person weighing 70 kg. These numbers are much higher than the earlier claims that 20 mg/day/person was safe in dietary supplements 48. Mutagenic studies have revealed that lutein and zeaxanthin are safe for human consumption 49. The no observed-adverse-effect-level (NOAEL) for lutein and zeaxanthin concentrate was determined to be 400 mg per kg bodyweight per day, the highest dose tested in rats 50. The safety of supplemental meso-zeaxanthin was recently reviewed 51 and the no observed-adverse-effect-level (NOAEL) of meso-zeaxanthin in rats is 300 mg per kg bodyweight per day when administered orally for 13 consecutive weeks 52.
Lutein and zeaxanthin side effects
The safety data for lutein have been evaluated by the European Food Safety Authority (EFSA). Due to the close similarity of lutein and zeaxanthin, it is probable that the toxicology for the pure substances is very similar, although it has to be remembered that within the eye a highly specific biological stereoisomeric differentiation may occur. Many of the analytical methods used in the past did not differentiate zeaxanthin and lutein such that the information on the differential occurrence of lutein and zeaxanthin in fruits and vegetables for many years was incomplete. The Joint FAO (Food and Agriculture Organization of the United Nations)/WHO (World Health Organization) Expert Committee on Food Additives (JECFA) in 2006 53 in their safety evaluation of lutein and zeaxanthin defined a “group” ADI (Acceptable Daily Intake) for lutein and zeaxanthin of 0–2 mg per kg body weight per day, covering both substances.
Lutein as a human dietary supplement is often obtained as an extract from marigold (Tagetes) and the extract always contains some zeaxanthin. Zeaxanthin itself, on the other hand, tends to be produced from both biological sources and in a highly pure form synthetically. The predominant zeaxanthin stereoisomer in nature and consequently in the diet is the 3R, 3R′-stereoisomer, which is also the predominant stereoisomer of synthetic zeaxanthin (Figures 1 and 2).
Subchronic OECD guideline studies with mice and rats receiving beadlet formulations of high purity synthetic zeaxanthin in the diet at dosages up to 1000 mg/kg body weight per day, and in dogs at over 400 mg/kg body weight per day, produced no adverse effects or histopathological changes. In developmental toxicity studies, there was no evidence of fetal toxicity or teratogenicity in rats or rabbits at dosages up to 1000 or 400 mg/kg body weight per day, respectively. Formulated zeaxanthin was not mutagenic or clastogenic in a series of in vitro and in vivo tests for genotoxicity. A 52-week chronic oral study in Cynomolgus monkeys at doses of 0.2 and 20 mg/kg body weight per day, mainly designed to assess accumulation and effects in primate eyes, showed no adverse effects. In a rat two-generation study, the no-observed-adverse-effect level (NOAEL) was 150 mg/kg body weight per day. In 2012, this dosage was used by European Food Safety Authority Panel, in association with a 200-fold safety factor, to propose an Acceptable Daily Intake (ADI) equivalent to 53 mg/day for a 70 kg adult. The requested use level of 2 mg/day was ratified by the EU Commission.
Based on the lack of reported side effects in the studies that have been done, up to 20 mg per day of a lutein supplement should be safe for adults. There is no evidence available to determine a safe lutein supplement dose in children. As with many other medications and supplements, there is no information about safety in pregnant or breastfeeding women.
Very large doses of carotenoids such as lutein and zeaxanthin can cause carotenodermia – a yellow-orange skin discoloration. It can look like jaundice, but the abnormal skin color can be removed with an alcohol swab.
Zeaxanthin benefits
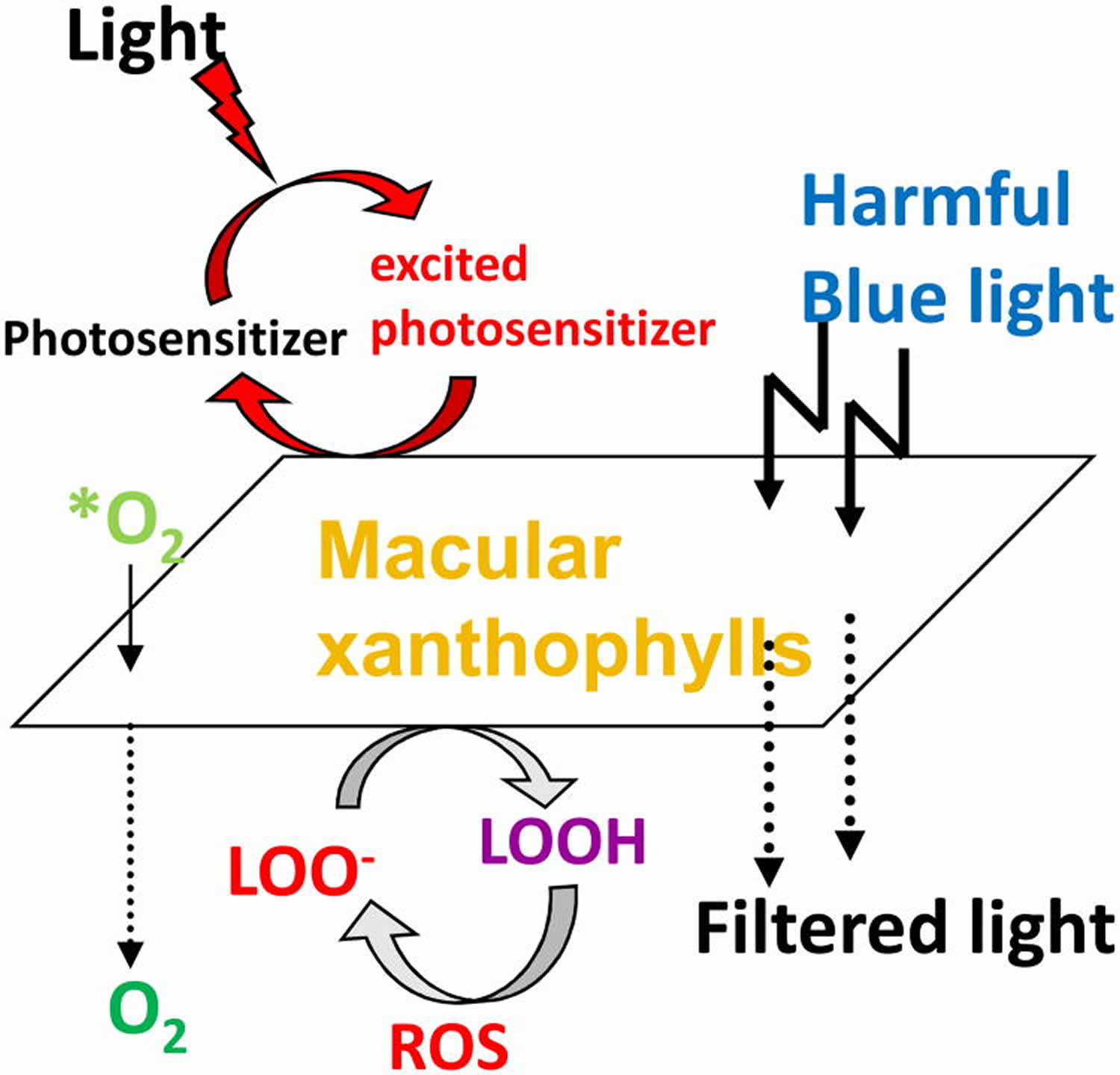
Over two decades of research has shown that macular pigment carotenoids is a short-wavelength (blue) light filter 54 and a powerful antioxidant 55 and because of these properties it is believed to protect against AMD 56. Carotenoids are excellent quenchers of singlet oxygen that react at the limits of diffusion without being consumed in the process 57. Reactive oxygen species (ROS) are either radicals such as hydroxyl radical or peroxyl radical, or they are reactive non-radical compounds such as singlet oxygen, peroxynitrite, or hydrogen peroxide 58. Singlet-state molecules rapidly form and can create triplet-state molecules via intersystem crossing. These long-lived molecules can then react with oxygen to produce ROS (reactive oxygen species), including superoxide anions, hydroxyl radicals, hydrogen peroxide, and singlet oxygen. These, in turn, can cause lipid peroxidation by attacking polyunsaturated fatty acids, resulting in DNA damage, protein and transmembrane glycoprotein oxidation, and other forms of cellular vandalism 59. Among radicals, hydroxyl radical is the most reactive species 60. Due to its high reactivity, this radical immediately reacts with surrounding target molecules at the site where it is generated. In general, carotenoids react with ROS in three possible mechanisms oxidation, electron transfer or hydrogen abstraction 61. Macular carotenoids may neutralize the ROS generated due to various free radical reactions in the eye and other tissues. Lutein and zeaxanthin are very efficient at absorbing and transmitting excited energy when needed, and they can harmlessly release the energy as heat without chemical degradation 62. The steps involved in the formation of ROS in the human retina and absorption of ROS by macular pigment carotenoids are outlined in Figure 3.
Both low macular pigment optical density (MPOD) and low serum xanthophylls have been previously associated with an increased susceptibility to age-related macular degeneration (AMD) 63. Although contradictory results have been reported in various studies concerning the efficiency of dietary supplementation with zeaxanthin and lutein for the prevention of AMD, valuable findings were provided by the AREDS2 study. The addition of lutein (10 mg), zeaxanthin (2 mg) and omega-3 fatty acids (1 g) to the original AREDS formula determined a reduction of 26% in risk of the progression of advanced AMD compared to the aforementioned formula (for the quintile with the lowest intake) 64. Furthermore, lutein and zeaxanthin supplementation could also be associated with a lower risk of progression to cataract surgery 65.
Based on their preferential accumulation in the human brain and the acknowledged correlation between macular pigment optical density and brain carotenoids, lutein and combinations of lutein and zeaxanthin have been investigated for their contribution in cognitive function. Zeaxanthin concentration in the brain tissue of centenarians decedents was significantly correlated with premortem memory retention, verbal fluency and dementia 66. In addition to a significant increase in macular pigment optical density, several cognitive parameters such as complex attention and cognitive flexibility were improved in both older women and men (mean age 72.51 years) after twelve months supplementation with 10 mg of lutein and 2 mg of zeaxanthin, with the composite memory being improved only in men 67.
Lutein and zeaxanthin were among the major carotenoids found in the infant brain and the detection of higher concentrations of lutein and zeaxanthin in almost all the brains of term infants as opposed to preterm infants may indicate an important role in cognition 68.
Similarly, better cognitive performance and neural efficiency were observed in children with higher macular pigment optical density (MPOD) 69. Lutein and zeaxanthin seem to play an important role in pre-natal and post-natal development, as suggested by their presence in cord blood and incipient macula in human fetuses 70. Henriksen et al. 71 found a correlation between zeaxanthin concentration in serum and macular pigment optical density in healthy term infants, as well as a correlation between the mother’s zeaxanthin concentration in serum and infant macular pigment optical density. These results indicated that maternal zeaxanthin has a more relevant role in macular pigment deposition in utero than lutein. As breast milk is the only source of lutein and zeaxanthin for young infants, the prospect of maternal supplementation and the development of macular pigment-fortified milk formulas are of great interest 70.
The blue light-filtering ability and the antioxidant properties of zeaxanthin have also been proven to have an important role in skin protection. Several studies on cell cultures and animal models displayed the protective effect of lutein and zeaxanthin through cell viability improvement, inhibition of matrix metalloproteinases and of inflammation and immunosuppression associated with UV-induced oxidative damage 72. After twelve weeks of supplementation with 5 mg of lutein and 0.3 mg of zeaxanthin (in capsule form, twice per day), a significant reduction in lipid peroxidation associated with an improvement in skin hydration, skin lipid content, elasticity and photoprotection has been observed in women exhibiting signs of premature aging 73.
Recently, Christensen et al. 74 used the data reported by the 2003–2014 National Health and Nutrition Examination Survey (NHANES) to investigate cross-sectional associations between dietary and serum levels of carotenoids in relation to non-alcoholic fatty liver disease (NAFLD). The carotenoid intake was estimated by a 24-hour recall and for some groups the serum carotenoids were measured by HPLC. A lower intake of carotenoids (including zeaxanthin) was observed for NAFLD subjects. Moreover, a higher level of serum carotenoids has been associated with a reduced risk of developing NAFLD. Although rodents do not accumulate xanthophylls due to the high activity of Beta-Carotene Oxygenase 2 75, a protective effect of zeaxanthin (free or esterified) against ethanol induced hepatic damage in animal models (rats, mice) and in nonalcoholic steatohepatitis (gerbils) was indicated in several studies reviewed by Murillo et al. 76.
The antioxidant activity of zeaxanthin has a major importance in limiting the oxidation of both HDL cholesterol (high-density lipoprotein or “good” cholesterol) and LDL cholesterol (low-density lipoprotein or “bad” cholesterol), thus contributing to the prevention of atherosclerosis and other associated cardiovascular diseases. A study carried out over the course of 18 months on 573 middle-aged healthy subjects at baseline revealed that the change in carotid intima-media thickness was significantly inversely correlated with the serum concentration of lutein, zeaxanthin, β-cryptoxanthin and α-carotene 77. Although zeaxanthin and beta-carotene were negatively correlated with right common carotid artery stiffness, elastic modulus and pulse wave velocity in subjects with early atherosclerosis, no statistical differences were observed as regards zeaxanthin serum concentration of the cases and the controls 78.
Considering that eggs constitute rich sources of highly bioavailable lutein and zeaxanthin, a supplementation study with 1 soft boiled egg per day for 4 weeks was conducted in moderately hypercholesterolemic Japanese males in order to investigate its effect on LDL cholesterol (low-density lipoprotein or “bad” cholesterol) oxidation. Despite the higher cholesterol intake, the total cholesterol level was not affected by the egg supplementation and an increase in both lutein and zeaxanthin serum concentration was observed. In addition, a decrease in malondialdehyde modified low-density lipoprotein concentrations and a prolonged LDL cholesterol (low-density lipoprotein or “bad” cholesterol) oxidation lag were recorded, emphasizing the antioxidant protection of these xanthophylls 79.
Figure 3. Protective roles of lutein and zeaxanthin, as an absorber of harmful light and as an antioxidant reacting with reactive oxygen species (ROS).
Footnote: *O2 = singlet oxygen; LOO- = lipid peroxyl radicals;LOOH = lipid peroxides.
Protection of the eye against intense visible light
The photoprotective role of zeaxanthin and lutein in the eye involves several mechanisms, including attenuation of blue light in a sunscreen-like function, as well as the removal of ROS by de-excitation 80, as well as the reduction of other reactive radicals 4. The role of zeaxanthin and lutein in photoprotection of the eye is supported by correlative evidence, as well as experimental or trial-based manipulation.
- The central portion (macula) of the retina that receives the most intense light features the highest concentration of zeaxanthin and lutein.
- Experimental induction of eye damage (photoreceptor death) in a bird model, the Japanese quail Coturnix japonica, established that photoreceptor death by exposure to intense light, can be prevented by dietary zeaxanthin 81 or dietary zeaxanthin and lutein 82.
- In human clinical trials, individuals receiving zeaxanthin/lutein supplements experienced less vision loss over time than controls 4
There is, furthermore, evidence for a greater photoprotective capacity of zeaxanthin compared to lutein 20. The ratio of zeaxanthin (and meso-zeaxanthin) to lutein is highest in the macula where the strongest light is received, and lowest in the peripheral, low-light-vision regions of the eye 83; the ratio of zeaxanthin to lutein increases from the diet to the retina; and a portion of dietary lutein is converted to meso-zeaxanthin, a stereoisomer of zeaxanthin (Figure 1). This preference for zeaxanthin has been suggested to be due to a greater antioxidant capacity and membrane-stabilizing function of zeaxanthin (and meso-zeaxanthin) compared to lutein 84. More research is needed to explore whether the employment of lutein alongside zeaxanthin and meso-zeaxanthin is related to the dramatically limited dietary availability of zeaxanthin compared to lutein or whether lutein has any roles that zeaxanthin and meso-zeaxanthin cannot fulfill. Attenuation of blue light is presumably accomplished by all three isomers, since xanthophylls absorb strongly between 350 and 500 nm 85.
Enhancement of eye and brain function
It is noteworthy that the macular xanthophylls also enhance the function of the healthy eye—via improved contrast sensitivity 86 and reduced glare, which may be related to the filtering of blue light 4. In addition, zeaxanthin and lutein enhance the processing of visual signals in the brain 87 as well as the processing of auditory signals 88. Recent evidence, furthermore, indicates that zeaxanthin and lutein function broadly in a number of brain regions that are associated with visual perception, cognition, decision-making, and motor coordination 89. A correlative study 90 reported lower Alzheimer’s mortality in individuals, with higher levels of zeaxanthin, lutein, and lycopene, but not of other carotenoids (beta-carotene, alpha-carotene, or the intermediate beta-cryptoxanthin in the synthetic pathway from beta-carotene to zeaxanthin). A recent nutritional intervention with Alzheimer’s patients, consisting of supplementation with either (1) zeaxanthin, meso-zeaxanthin, and lutein, or with (2) the three latter xanthophylls plus two omega-3 fatty acids (docosahexaenoic acid and eicosapentaenoic acid) resulted in a greater increase in blood xanthophyll concentrations, and greater caregiver-reported improvements in memory, sight, and mood of patients 91. More research is needed into the possible effects of lutein and zeaxanthin and their interaction with omega-3 fatty acids, with respect to the solubility and bioavailability of the largely hydrophobic xanthophylls and their function in the brain.
Anti-inflammatory agents
Age-related macular degeneration (AMD) and Alzheimer’s disease are both pro-inflammatory diseases involving immune system dysfunction and uncontrolled inflammation. The realization that a state of chronic low-grade inflammation plays a key role in a host of additional chronic diseases (e.g., cardiovascular disease, diabetes, certain cancers, autoimmune diseases) and disorders (e.g., anxiety, depression, bipolar disease, schizophrenia, post-traumatic stress disorder) has been called “one of the most important scientific discoveries in health research in recent years” 92. Strikingly, memory, attention, learning, and overall cognitive performance is also impaired by systemic inflammation—even in adults considered healthy and with no diagnosis of a disease or disorder 93. One can hypothesize that carotenoids with antioxidant and anti-inflammatory functions may ameliorate some or all of these conditions. There is a substantial body of evidence for correlations between higher carotenoid levels and a lower risk for various pro-inflammatory diseases 94.
Like intense visible light, ionizing radiation (that increases in the upper atmosphere and especially in outer space) produces multiple ROS that can trigger system-wide uncontrolled inflammation 95. There is correlative evidence for a possible role of zeaxanthin in protection against ionizing radiation; pilots who reported consuming more antioxidants, including zeaxanthin and lutein, exhibited less inflammation 96. Sufficient dietary zeaxanthin and lutein may be of particular concern for future human spaceflight and the associated radiation exposure, and a reliable food source that can be grown in limited space with minimal resources and delivers high levels of zeaxanthin and lutein may be critical 97.
While more research is needed to assess the question of causality between zeaxanthin and lutein and a lessening of systemic inflammation, evidence for a causal relationship is beginning to emerge from manipulative studies in animal models and humans. For example, Zhou et al. 98 reported that long-term zeaxanthin supplementation lowered the levels of pro-inflammatory hormones and lessened diabetic symptoms, anxiety, and depression in diabetic rats. Stringham et al. 99 extended these findings to humans and, in particular, healthy young subjects that received a supplement with zeaxanthin, meso-zeaxanthin, and lutein for six months. Outcomes of this trial included significantly lower levels of pro-inflammatory hormones and enhanced cognitive performance on a variety of complex tasks, including processing speed, as well as various aspects of memory and attention. Similar improvements of cognitive function in young healthy adults as a result of supplementation with zeaxanthin and lutein were reported by Renzi-Hammond et al. 100.
Zeaxanthin foods
The overall occurrence of zeaxanthin in natural food products is low (see Tables 4 and 5) 101. The broad majority of xanthophyll-rich foods contain more lutein than zeaxanthin. Moreover, many of the zeaxanthin-containing products listed in Table 4 are not commercially available around the world. Consequently, the most predominant sources of zeaxanthin present in the human diet are corn-based foods along with pepper and egg yolk 101.
Corn and egg yolks contain approximately the same content of both macular carotenoids; the molar ratio of zeaxanthin-to-lutein is about 1 in both foods 6. Few foods contain more zeaxanthin than lutein. One food with a higher zeaxanthin-to-lutein molar ratio is the orange pepper, with a zeaxanthin-to-lutein molar ratio of approximately 10. Another such food is the fruit of the Lycium barbarum, commonly known as goji berries or wolfberries. Goji berries are the richest source of zeaxanthin (Table 5) and are widely used in traditional Chinese medicine for eye health. Daily supplementation with goji berries for 90 days increases serum zeaxanthin and in early AMD patients; goji berries can be used to prevent the progression of AMD 102. Another rich source of zeaxanthin is the fruit of Physalis alkekengi or the Chinese lantern 103, wherein zeaxanthin comprises more than one-half of the total carotenoid content. The berries of the sea buckthorn also have zeaxanthin ester as major compound, with zeaxanthin dipalmitate comprising up to 38% of the total carotenoid content and a zeaxanthin-to-lutein molar ratio of about 10 104. Despite the fact that eggs have a low zeaxanthin content compared with zeaxanthin-rich berries, the egg yolk provides an excellent dietary source of zeaxanthin because the oral bioavailability from the yolk matrix is much higher than from the leaves of green vegetables. The high oral bioavailability of a fat-soluble nutrient such as zeaxanthin from the egg is due to the rich lipid matrix of the yolk. Egg yolk is a good dietary source of both zeaxanthin and lutein, particularly as part of a typical western diet, which is poor in vegetables and fruits. It was reported that egg supplementation may increase plasma zeaxanthin by 142% 105. Furthermore, it should be noted that a high intake of lutein can also increase the macular content of meso-zeaxanthin because the lutein can convert to meso-zeaxanthin in the central retina.
In vegetables, zeaxanthin is present in its free form, while in ripped fruits it usually occurs in a more stable and less soluble form, i.e., esterified with various fatty acids 106. After the ingestion of these zeaxanthin-rich fruits, the mono- or di-esters need to be enzymatically hydrolyzed into their free form in the gastrointestinal tract before absorption by the intestinal cells 107. Some fruits with distinguished zeaxanthin content such as goji (Lycium barbarum L.) berries and sea buckthorn (Hippophae rhamnoides L.) berries have been studied in terms of zeaxanthin content and bioaccessibility 108 but a large number of exotic fruits with a high content of zeaxanthin still remain uninvestigated.
Animal-based food sources of zeaxanthin are limited and fully dependent on the animal’s diet. For instance, by supplementing the feed of laying hens, the content of both lutein and zeaxanthin in egg yolk can be enhanced 109. Due to the high-lipid matrix, xanthophylls from egg yolk, present in a lipid-dissolved form, are more bioavailable than from plant-based sources 110.
Apart from plant and animal food sources, the dried edible biomass of microalgae constitutes a potential rich source of zeaxanthin. Several microalgae such as Dunaliella sp. and Chlorella sp. can accumulate impressive amounts of zeaxanthin (Table 4). Considering the steady increase in the human population and Earth’s limited resources, microalgae could be regarded as reliable sources of zeaxanthin and other beneficial byproducts in the near future. In addition to their less labor-intensive production and faster growing rate, the carotenoid content of microalgae is clearly superior to that of higher plants. Furthermore, micro algae can be amended through genetic engineering with the aim of improving the accumulation of high-value compounds such as carotenoids 111.
Food processing leads in most cases to a decrease in the content of zeaxanthin in varying degrees 112. However, this slight disadvantage appears to be somewhat counterbalanced by a higher zeaxanthin bioaccessibility from the processed food than from its raw state 113. In the case of microalgae, processing represents a critical step as it facilitates the disruption of the cellulose-rich wall of some microalgal strains, which further translates into an enhanced bioaccessibility of valuable bioactive compounds 114.
As previously mentioned, the intake of zeaxanthin is significantly lower as opposed to lutein and since frequently consumed fruits and vegetables such as apples, oranges, tomatoes and potatoes have a naturally low content of zeaxanthin, unexplored or novel foods are of utmost importance.
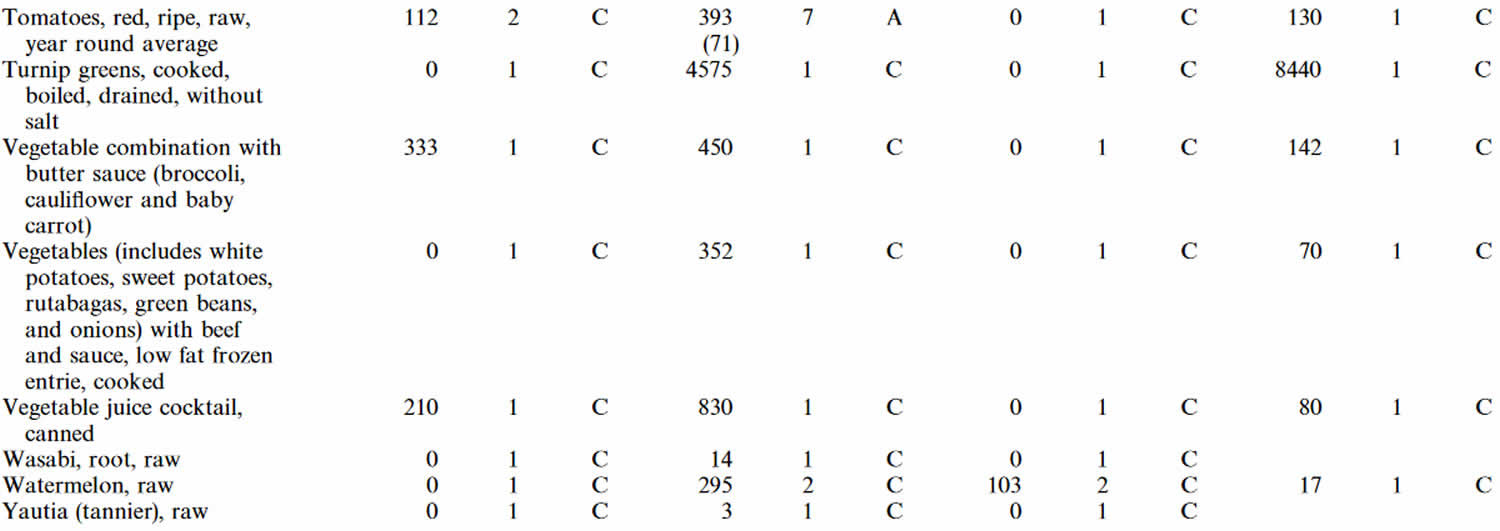
Table 4. Zeaxanthin content of foods (μg (microgram) per gram (g) dry weight a or μg/g fresh weight b)
| Plant Sources | Zeaxanthin |
| Einkorn wheat (Triticum monococcum) | 0.94 a |
| Khorasan wheat (Triticum turgidum subsp. turanicum) | 0.71 a |
| Durum wheat (Triticum turgidum subsp. durum) | 0.49 a |
| Corn (Zea mays L.) | 10.31 a |
| Corn flakes | 1.02–2.97 a |
| Corn chips | 1.05 a |
| Corn tortilla | 0.93 a |
| Corn masa | 1.13 a |
| Corn flour | 9.4 a |
| Boiled corn | 3.7 a |
| Potato (Solanum tuberosum L.) | 7.7 a |
| Sweet potato (Ipomoea batatas) | 0.3 a |
| Squash (Cucurbita maxima) | 1.9 a |
| Kidney been (Phaseolus vulgaris L.) | 0.1 a |
| Okra (Abelmoschus esculentus) | 0.1 a |
| Beet (Beta vulgaris L.) | 0.7 a |
| Tomato (Solanum lycopersicum L.) | 1.3 a |
| Hot chili peppers (Capsicum frutescens L.) | 1230 a* |
| Pepper (Capsicum annuum L.) | |
| red | 55.0–97.0 a |
| green | 1.7–5.7 a |
| orange | 62.0 a |
| yellow | 4.4 a |
| India mustard (Brassica juncea) | 0.8 a |
| Watercress (Nasturtoum officinale) | 0.4 a |
| Endive (Cichorium endivia L.) | 0.5 a |
| Romaine lettuce (Lactuca sativa L. var. longifolia) | 0.7 a |
| Lettuce (Lactuca sativa L.) | 0.1 a |
| Cabbage (Brassica oleracea L.) | 0.1 a |
| Spinach (Spinacia oleracea L.) | 0.7 a |
| Kale (Brassica oleracea L. var. sabellica) | 163–2460 a |
| Zucchini blossoms (Cucurbita pepo L.) | 32.7 b* |
| Artichoke heart (Cynara cardunculus L. var. scolymus) | 0.18 b |
| Avocado (Persea americana) | 0.08–0.18 b |
| Apple (Malus domestica) | |
| flesh | nd– 0.04 a |
| peel | nd–0.52 a |
| Apricot (Prunus armeniaca L.) | nd–0.39 b |
| European plum (Prunus domestica L.) | 0.1 a |
| Nectarine (Prunus persica) | 0.2 a |
| Orange ‡ (Citrus sinensis) | 0.3 a |
| Orange juice ‡ (Citrus sinensis) | 0.1 a |
| Grafted orange ‡ (Citrus sinensis) | 1.1 a |
| Grafted orange (juice) ‡ | 0.6 a |
| Mandarin ‡ (Citrus reticulata) | 2.1 a |
| Mandarin juice ‡ (Citrus reticulata) | 1.7 a |
| Red grapefruit ‡ (Citrus paradisi) | 0.2 a |
| Peruvian groundcherry (Physalis peruviana L.) | 0.4 a |
| Strawberry tree (Arbutus unedo L.) fruits | 0.7–2.0 a |
| Raspberry (Rubus idaeus L.) | 0.14–0.49 a |
| Rose hip (Rosa spp.) | 23–107 a* |
| Wolfberry (goji berry) (Lycium barbarum L.) | 1231.1 a* |
| Red Chinese lantern fruit (Physalis alkekengi L.) | 847–1035 a* |
| Sea buckthorn (Hippophae rhamnoides L.) | |
| berries | 193–424 a* |
| oil (cold-pressed) | 2312.2 b* |
| Murici fruit (Byrsonima crassifolia) | 5.4 a* |
| Arazá fruit (Eugenia stipitata) | |
| peel | 1.14 b |
| pulp | 0.17 b |
| Astringent persimmon (Diospyros kaki Thunb. var. Rojo brillante) | 10.2 b* |
| Cashew apples (Anacardium occidentale L.) | |
| peel | 0.51–2.69 b* |
| pulp | 0.04–0.58 b* |
| Corozo ‡ (Aiphanes aculeata) | 79.2 a |
| South American sapote ‡ (Quararibea cordata) | 46.2 a |
| Passion fruit ‡ (Passiflora edulis) | 0.2 a |
| Mango ‡ (Mangifera indica) | 0.5 a |
| Red papaya ‡ (Carica papaya) | 0.6 a |
| Yellow guava ‡ (Psidium guajava L.) | 0.2 a |
| Pineapple ‡ (Ananas comosus) | 0.1 a |
| Melon ‡ (Cucumis melo L.) | 0.1 a |
| Tahitian apple ‡ (Spondias dulcis) | 0.1 a |
| Cassabanana ‡ (Sicana odorífera) | 0.4 a |
| Tree tomato ‡ (Cyphomandra betacea) | 1.7 a |
| Red tree tomato ‡ (Cyphomandra betacea) | 2.4 a |
| Roselle ‡ (Hibiscus sabdariffa L.) | 0.8 a |
| Membrillo # (Gustavia superba) | 37.6 a |
| Canistel # (Pouteria campechiana) | 19.7 a |
| Chinese passion fruit # (Cionosicyos macranthus) | 2.8 a |
| Sastra # (Garcinia intermedia) | 84.7 a |
| Yellow mombin # (Spondias mombin L.) | 1.2 a |
| Guanabana toreta # (Annona purpurea) | 6.8 a |
| Purple mombin # (Spondias purpurea L.) | 0.8 a |
| Chinese rose # (Pereskia bleo) | 0.8 a |
| Nance # (Byrsonima crassiflora) | 0.2 a |
| Lucuma fruit (Pouteria lucuma) | |
| Molina variety | 3.44–5.76 b* |
| Beltran variety | 5.74 –6.66 b* |
| Sarsaparilla (Smilax aspera L.) berries | 8.56 b* |
| Animal sources | |
| Butter | nd – 0.02 b |
| Marine crab (Charybdis cruciata) | |
| meat | 0.02 b |
| Freshwater crab (Potamon potamon) | |
| meat | 1.72 b |
| Eggs | |
| raw | 1.5 a |
| boiled | 1.3 a |
| poached | 1.3 a |
| omelette | 1.14 a |
| Microalgal sources | |
| Nannochloropsis sp. | |
| suspension | 420 a |
| oil | 1930 b |
| Chlorella ellipsoidea | 1999 a |
| Dunaliella salina | 11270 a |
| Phaeodactylum tricornutum | 679.2 a |
| Scenedesmus almeriensis | 370 a |
Footnotes: nd = not detected; * = zeaxanthin + zeaxanthin mono- and diesters; # = Panamanian wild fruit; ‡ = fruit cultivated in Panama.
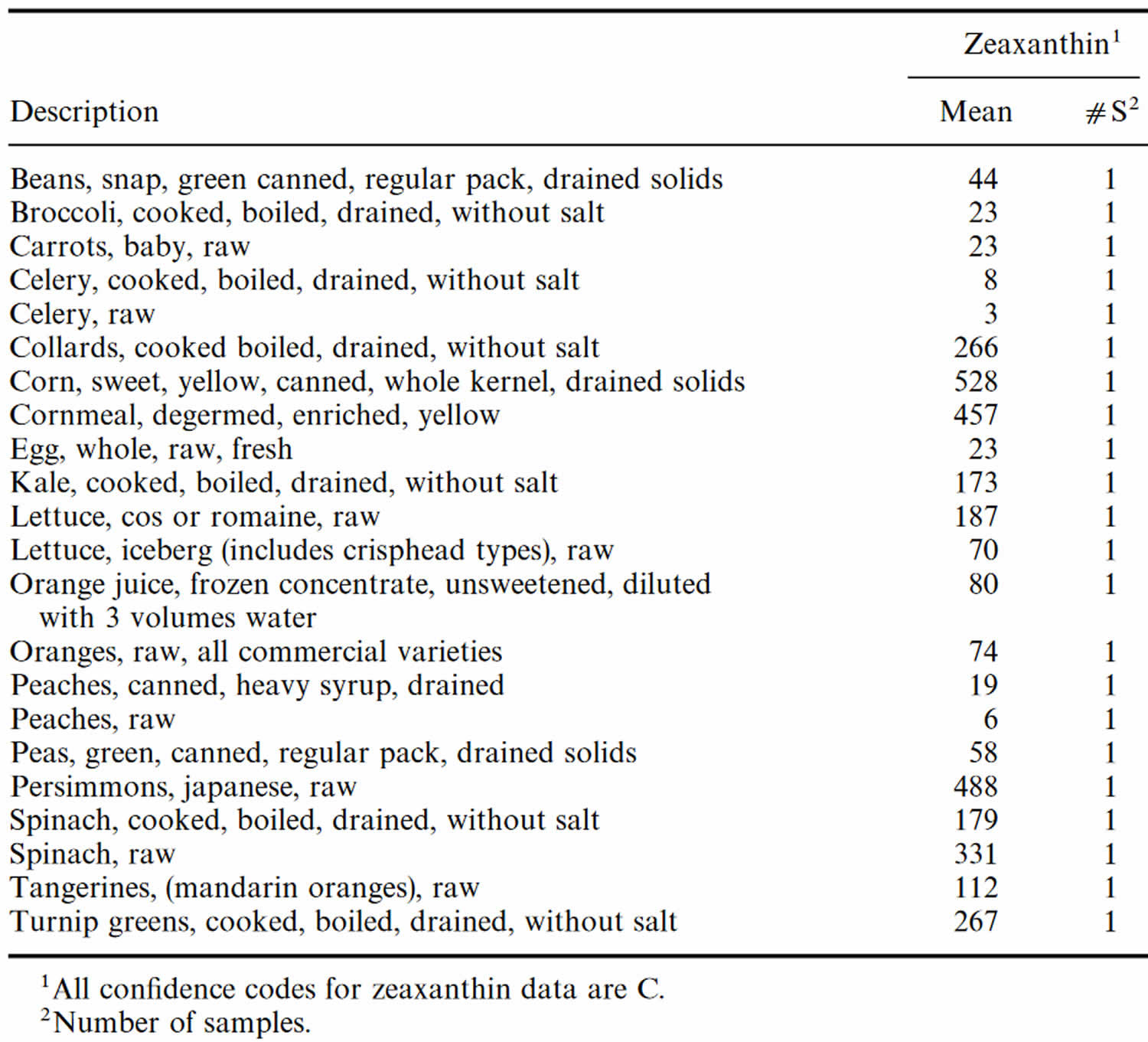
[Source 115 ]Table 5. Zeaxanthin content of foods
| Food | Zeaxanthin Content (μg (microgram) per 100 g) |
| Goji berry | 280000 |
| Red Chinese lantern fruit | 84700 |
| Orange pepper | 5580 |
| Sea buckthorn | 1930 |
| Egg yolk (raw) | 762 |
| Corn | 105 |
| Orange juice | 26 |
| Peach | 39 |
| Spinach | 75 |
| Kale | 62 |
| Papaya | 6 |
Table 6. Zeaxanthin content of U.S. foods (μg (microgram) per 100 g edible portion)
[Source 32 ]Zeaxanthin dosage
Zeaxanthin intake of up to 0.75 mg/kg body weight and lutein intake of up to 1 mg/kg body weight (20 to 40 mg/day) are considered safe 116. However, one report 117 noted that overdose lutein resulted in yellow deposits in the retina; hence, caution is needed to avoid excessive intake of lutein.
Zeaxanthin side effects
Although neither animal nor human data on the lung cancer risk of zeaxanthin are available, based on the current data available the European Food Safety Authority Panel considers it unlikely that supplemental intake of zeaxanthin would increase the risk of lung cancer in heavy smokers 48. The European Food Safety Authority Panel identifies a no observed-adverse-effect-level (NOAEL) of 150 mg per kg body weight per day in the two-generation reproduction toxicity study with synthetic zeaxanthin in rats and has no concerns with regard to genotoxicity. Given the absence of a chronic toxicity and carcinogenicity study, the European Food Safety Authority Panel applies an uncertainty factor of 200 on the NOAEL in the two-generation study. This results in 0.75 mg per kg body weight per day for synthetic zeaxanthin corresponding to a daily intake of 53 mg for a person with a body weight of 70 kg 48.
The European Food Safety Authority Panel concludes that based on the available data, intakes of 0.75 mg per kg body weight per day for synthetic zeaxanthin, corresponding to a daily intake of 53 mg for a person with a body weight of 70 kg, do not raise safety concerns 48.
References- Demmig-Adams, B., Gilmore, A., and Adams, W. W. III (1996b). Carotenoids 3: in vivo functions of carotenoids in higher plants. FASEB J. 10, 403–412. doi: 10.1096/fasebj.10.4.8647339
- Shyam, R., Gorusupudi, A., Nelson, K., Horvath, M. P., & Bernstein, P. S. (2017). RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proceedings of the National Academy of Sciences of the United States of America, 114(41), 10882–10887. https://doi.org/10.1073/pnas.1706332114
- Mozaffarieh M, Sacu S, Wedrich A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: A review based on controversial evidence. Nutr J. 2003; 2: 20.
- Bernstein, P. S., Li, B., Vachali, P. P., Gorusupudi, A., Shyam, R., Henriksen, B. S., & Nolan, J. M. (2016). Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Progress in retinal and eye research, 50, 34–66. https://doi.org/10.1016/j.preteyeres.2015.10.003
- Olmedilla-Alonso B, Beltrán-de-Miguel B, Estévez-Santiago R, Cuadrado-Vives C. Markers of lutein and zeaxanthin status in two age groups of men and women: dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr J. 2014 Jun 3;13:52. doi: 10.1186/1475-2891-13-52
- Widomska, J., SanGiovanni, J. P., & Subczynski, W. K. (2020). Why is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea?. Nutrients, 12(5), 1333. https://doi.org/10.3390/nu12051333
- Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993 May;34(6):2033-40.
- Vishwanathan R, Schalch W, Johnson EJ. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr Neurosci. 2016;19(3):95-101. doi: 10.1179/1476830514Y.0000000141
- Whitehead AJ, Mares JA, Danis RP. Macular Pigment: A Review of Current Knowledge. Arch Ophthalmol. 2006;124(7):1038–1045. doi:10.1001/archopht.124.7.1038
- Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):10882-10887. doi: 10.1073/pnas.1706332114
- Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond). 2007 May 11;4:12. doi: 10.1186/1743-7075-4-12
- Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997 Feb;64(2):211-8. doi: 10.1006/exer.1996.0210
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001 Mar;72(3):215-23. doi: 10.1006/exer.2000.0954
- Li, B., George, E. W., Rognon, G. T., Gorusupudi, A., Ranganathan, A., Chang, F. Y., Shi, L., Frederick, J. M., & Bernstein, P. S. (2020). Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proceedings of the National Academy of Sciences of the United States of America, 117(22), 12352–12358. https://doi.org/10.1073/pnas.1922793117
- Waugh N, Loveman E, Colquitt J, et al. Treatments for dry age-related macular degeneration and Stargardt disease: a systematic review. Southampton (UK): NIHR Journals Library; 2018 May. (Health Technology Assessment, No. 22.27.) Chapter 6, Nutritional interventions in dry age-related macular degeneration. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500492
- Vision Problems in the U.S. http://www.visionproblemsus.org
- What Is Macular Degeneration? https://www.aao.org/eye-health/diseases/amd-macular-degeneration
- Glaucoma: Screening. https://uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/glaucoma-screening
- Black, H. S., Boehm, F., Edge, R., & Truscott, T. G. (2020). The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants (Basel, Switzerland), 9(3), 264. https://doi.org/10.3390/antiox9030264
- Demmig-Adams, B., López-Pozo, M., Stewart, J. J., & Adams, W. W., 3rd (2020). Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules (Basel, Switzerland), 25(16), 3607. https://doi.org/10.3390/molecules25163607
- Havaux M, Dall’osto L, Bassi R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007 Dec;145(4):1506-20. doi: 10.1104/pp.107.108480
- Paula R Trumbo, Kathleen C Ellwood, Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration’s evidence-based review system for health claims, The American Journal of Clinical Nutrition, Volume 84, Issue 5, November 2006, Pages 971–974, https://doi.org/10.1093/ajcn/84.5.971
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436.
- Gordon JE, Schooff M. Can high-dose supplementation with vitamins C and E, beta carotene, and zinc slow the progression of macular degeneration? J Fam Pract. 2002;51(2):105.
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. 2012;(6):CD000253.
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. Erratum in: JAMA. 2013 Jul 10;310(2):208.
- Aronow ME, Chew EY. Age-related Eye Disease Study 2: perspectives, recommendations, and unanswered questions. Curr Opin Ophthalmol. 2014;25(3):186–190.
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxan-thin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial [published correction appears in JAMA. 2013;310(2):208]. JAMA. 2013;309(19):2005–2015.
- Chew EY, Clemons TE, Sangiovanni JP, et al.; Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014;132(2):142–149.
- Age-Related Eye Disease Studies (AREDS/AREDS2). https://www.nei.nih.gov/research/clinical-trials/age-related-eye-disease-studies-aredsareds2
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Age-related macular degeneration (AMD): Do dietary supplements prevent AMD? 2015 Jul 29 [Updated 2018 May 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK315802
- Holden JM, Eldridge AL, Beecher G, Buzzard IM, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S. Carotenoid Content of U.S. Foods: An Update of the Database. Journal of Food Compositon and Analysis. 1999;12:169–196. https://doi.org/10.1006/jfca.1999.0827
- Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis. 2009;22:9–15.
- Rodriguez-Amaya DB. Food carotenoids: analysis, composition and alterations during storage and processing of foods. Forum Nutr. 2003;56:35-7.
- Azevedo-Meleiro CH, Rodriguez-Amaya DB. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J Agric Food Chem. 2007 May 16;55(10):4027-33. doi: 10.1021/jf063413d
- Calvo MM. Lutein: a valuable ingredient of fruit and vegetables. Crit Rev Food Sci Nutr. 2005;45(7-8):671-96. doi: 10.1080/10408690590957034
- van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000 Mar;130(3):503-6. doi: 10.1093/jn/130.3.503
- Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004 Aug;80(2):396-403. doi: 10.1093/ajcn/80.2.396
- Goodrow EF, Wilson TA, Houde SC, Vishwanathan R, Scollin PA, Handelman G, Nicolosi RJ. Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. J Nutr. 2006 Oct;136(10):2519-24. doi: 10.1093/jn/136.10.2519
- Granado-Lorencio F, Herrero-Barbudo C, Olmedilla-Alonso B, Blanco-Navarro I, Pérez-Sacristán B. Lutein bioavailability from lutein ester-fortified fermented milk: in vivo and in vitro study. J Nutr Biochem. 2010 Feb;21(2):133-9. doi: 10.1016/j.jnutbio.2008.12.002
- Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr. 2004 Aug;134(8):1887-93. doi: 10.1093/jn/134.8.1887
- Johnson EJ, Maras JE, Rasmussen HM, Tucker KL. Intake of lutein and zeaxanthin differ with age, sex, and ethnicity. J Am Diet Assoc. 2010 Sep;110(9):1357-62. doi: 10.1016/j.jada.2010.06.009
- Maoka T, Arai A, Shimizu M, Matsuno T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp Biochem Physiol B. 1986;83(1):121-4. doi: 10.1016/0305-0491(86)90341-x
- Nolan JM, Beatty S, Meagher KA, Howard AN, Kelly D, Thurnham DI. Verification of Meso-Zeaxanthin in Fish. J Food Process Technol. 2014 Jun 1;5(6):335. doi: 10.4172/2157-7110.1000335
- Wang Y, Connor SL, Wang W, Johnson EJ, Connor WE. The selective retention of lutein, meso-zeaxanthin and zeaxanthin in the retina of chicks fed a xanthophyll-free diet. Exp Eye Res. 2007 Mar;84(3):591-8. doi: 10.1016/j.exer.2006.11.013
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of lutein preparations other than lutein with high concentrations of total saponified carotenoids at levels of at least 80% EFSA Journal. 2011;9:2144.
- Agostoni C, Bresson J, Fairweather-Tait S, Flynn A, Golly I, Korhonen H, Lagiou P, Løvik M, Marchelli R, Martin A, Moseley B, Neuhäuser-Berthold M, Przyrembel H, Salminen S, Sanz Y, Strain S, Strobel S, Tetens I, Tome D, van Loveren H, Verhagen H. Scientific Opinion on the substantiation of health claims related to lutein and maintenance of normal vision (ID 1603, 1604, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2012;10
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Statement on the safety of synthetic zeaxanthin as an ingredient in food supplements. EFSA Journal 2012;10(10):2891. [14 pp.] doi:10.2903/j.efsa.2012.2891
- Ma L, Lin XM. Effects of lutein and zeaxanthin on aspects of eye health. J Sci Food Agric. 2010 Jan 15;90(1):2-12. doi: 10.1002/jsfa.3785
- Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpande J, Shankaranarayanan J, Shankaranarayana ML, Soni MG. Safety assessment of lutein and zeaxanthin (Lutemax 2020): subchronic toxicity and mutagenicity studies. Food Chem Toxicol. 2011 Nov;49(11):2841-8. doi: 10.1016/j.fct.2011.08.011
- Nolan JM, Meagher K, Kashani S, Beatty S. What is meso-zeaxanthin, and where does it come from? Eye (Lond). 2013 Aug;27(8):899-905. doi: 10.1038/eye.2013.98
- Xu X, Zhang L, Shao B, Sun X, Ho CT, Li S. Safety evaluation of meso-zeaxanthin. Food Control. 2013;32:678–686.
- JECFA. Safety evaluation of certain food additives. Proceedings of the 63rd Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA ’06); 2006; World Health Organization
- Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Res. 1992 Jan;32(1):105-10. doi: 10.1016/0042-6989(92)90118-3
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997 Aug;38(9):1802-11.
- Sabour-Pickett S, Nolan JM, Loughman J, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res. 2012 Feb;56(2):270-86. doi: 10.1002/mnfr.201100219
- Foote CS, Chang YC, Denny RW. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J Am Chem Soc. 1970 Aug 26;92(17):5216-8. doi: 10.1021/ja00720a036
- Stahl W, Sies H. Carotenoids and protection against solar UV radiation. Skin Pharmacol Appl Skin Physiol. 2002 Sep-Oct;15(5):291-6. doi: 10.1159/000064532
- Winkler, B. S., Boulton, M. E., Gottsch, J. D., & Sternberg, P. (1999). Oxidative damage and age-related macular degeneration. Molecular vision, 5, 32.
- Woodall AA, Lee SW, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biochim Biophys Acta. 1997 Jul 19;1336(1):33-42. doi: 10.1016/s0304-4165(97)00006-8
- Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995 Dec;9(15):1551-8.
- Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7(6):617-35. doi: 10.1016/0891-5849(89)90143-3
- Nolan J.M., Kenny R., O’Regan C., Cronin H., Loughman J., Connolly E.E., Kearney P., Loane E., Beatty S. Macular Pigment Optical Density in an Ageing Irish Population: The Irish Longitudinal Study on Ageing. Ophthalmic. Res. 2010;44:131–139. doi: 10.1159/000315531
- Sadda S. Lutein/Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration. The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. Ophthalmologica. 2013;230:10–11.
- Chew E.Y., SanGiovanni J.P., Ferris F.L., Wong W.T., Agron E., Clemons T.E., Sperduto R., Danis R., Chandra S.R., Blodi B.A., et al. Lutein/Zeaxanthin for the Treatment of Age-Related Cataract AREDS2 Randomized Trial Report No. 4. JAMA Ophthalmol. 2013;131:843–850. doi: 10.1001/jamaophthalmol.2013.4412
- Johnson E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012;96:1161S–1165S. doi: 10.3945/ajcn.112.034611
- Hammond B.R., Miller L.S., Bello M.O., Lindbergh C.A., Mewborn C., Renzi-Hammond L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017;9:254. doi: 10.3389/fnagi.2017.00254
- Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr. 2014 Nov;59(5):659-65. doi: 10.1097/MPG.0000000000000389
- Walk A.M., Khan N.A., Barnett S.M., Raine L.R., Kramer A.F., Cohen N.J., Moulton C.J., Renzi-Hammond L.M., Hammond B.R., Hillman C.H. From neuro-pigments to neural efficiency: The relationship between retinal carotenoids and behavioral and neuroelectric indices of cognitive control in childhood. Int. J. Psychophysiol. 2017;118:1–8. doi: 10.1016/j.ijpsycho.2017.05.005
- Giordano E., Quadro L. Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Arch. Biochem. Biophys. 2018;647:33–40. doi: 10.1016/j.abb.2018.04.008
- Henriksen B.S., Chan G., Hoffman R.O., Sharifzadeh M., Ermakov I.V., Gellermann W., Bernstein P.S. Interrelationships between Maternal Carotenoid Status and Newborn Infant Macular Pigment Optical Density (MPOD) and Carotenoid Status. Invest. Ophthalmol. Vis. Sci. 2013;54:5568–5578. doi: 10.1167/iovs.13-12331
- Roberts R.L. Lutein, Zeaxanthin, and Skin Health. Am. J. Lifestyle Med. 2013;7:182–185. doi: 10.1177/1559827613477827
- Palombo P., Fabrizi G., Ruocco V., Ruocco E., Fluhr J., Roberts R., Morganti P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Double-Blind, Placebo-Controlled Study. Skin Pharmacol. Phys. 2007;20:199–210. doi: 10.1159/000101807
- Christensen K., Lawler T., Mares J. Dietary Carotenoids and Non-Alcoholic Fatty Liver Disease among US Adults, NHANES 2003–2014. Nutrients. 2019;11:1101. doi: 10.3390/nu11051101
- Lintig J., Moon J., Lee J., Srinivasagan R. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids. 2019;1865:158580. doi: 10.1016/j.bbalip.2019.158580
- Murillo A.G., Hu S., Fernandez M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants. 2019;8:390. doi: 10.3390/antiox8090390
- Dwyer J.H., Paul-Labrador M.J., Fan J., Shircore A.M., Merz C.N.B., Dwyer K.M. Progression of Carotid Intima-Media Thickness and Plasma Antioxidants: The Los Angeles Atherosclerosis Study. Arterioscler. Thromb. Vasc. Biol. 2004;24:313–319. doi: 10.1161/01.ATV.0000109955.80818.8a
- Zou Z., Xu X., Huang Y., Xiao X., Ma L., Sun T., Dong P., Wang X., Lin X. High serum level of lutein may be protective against early atherosclerosis: The Beijing Atherosclerosis Study. Atherosclerosis. 2011;219:789–793. doi: 10.1016/j.atherosclerosis.2011.08.006
- Kishimoto Y., Taguchi C., Saita E., Suzuki-Sugihara N., Nishiyama H., Wang W., Masuda Y., Kondo K. Additional consumption of one egg per day increases serum lutein plus zeaxanthin concentration and lowers oxidized low-density lipoprotein in moderately hypercholesterolemic males. Food Res. Int. 2017;99:944–949. doi: 10.1016/j.foodres.2017.03.003
- Stahl W., Nicolai S., Briviba K., Hanusch M., Broszeit G., Peters M., Martin H.D., Sies H. Biological activities of natural and synthetic carotenoids: Induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis. 1997;18:89–92. doi: 10.1093/carcin/18.1.89
- Thomson L.R., Toyoda Y., Langner A., Delori F.C., Garnett K.M., Craft N., Nichols C.R., Cheng K.M., Dorey C.K. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Investig. Ophthalmol. Vis. Sci. 2002;43:3538–3549.
- Toyoda Y., Thomson L.R., Langner A., Craft N.E., Garnett K.M., Nichols C.R., Cheng K.M., Dorey C.K. Effect of dietary zeaxanthin on tissue distribution of zeaxanthin and lutein in quail. Investig. Ophthalmol. Vis. Sci. 2002;43:1210–1221.
- Bone R.A., Landrum J.T., Hime G.W., Cains A., Zamor J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993;34:2033–2040.
- Chung H.Y., Rasmussen H.M., Johnson E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004;134:1887–1893. doi: 10.1093/jn/134.8.1887
- Niedzwiedzki D.M., Enriquez M.M., LaFountain A.M., Frank H.A. Ultrafast time-resolved absorption spectroscopy of geometric isomers of xanthophylls. Chem. Phys. 2010;373:80–89. doi: 10.1016/j.chemphys.2010.01.019
- Hammond B.R., Wooten B.R., Engles M., Wong J.C. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vis. Res. 2012;63:58–62. doi: 10.1016/j.visres.2012.04.019
- Ceravolo S.A., Hammond B.R., Oliver W., Clementz B., Miller L.S., Renzi-Hammond L.M. Dietary carotenoids lutein and zeaxanthin change brain activation in older adult participants: A randomized, double-masked, placebo-controlled trial. Mol. Nutr. Food Res. 2019;63:1801051. doi: 10.1002/mnfr.201801051
- Wong J.C., Kaplan H.S., Hammond B.R. Lutein and zeaxanthin status and auditory thresholds in a sample of young healthy adults. Nutr. Neurosci. 2017;20:1–7. doi: 10.1179/1476830514Y.0000000138
- Mewborn C.M., Terry D.P., Renzi-Hammond L.M., Hammond B.R., Miller L.S. Relation of retinal and serum lutein and zeaxanthin to white matter integrity in older adults: A diffusion tensor imaging study. Arch. Clin. Neuropsychol. 2018;33:861–874. doi: 10.1093/acn/acx109
- Min J.Y., Min K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014;37:246–256. doi: 10.1159/000356486
- Nolan J.M., Mulcahy R., Power R., Moran R., Howard A.N. Nutritional intervention to prevent Alzheimer’s disease: Potential benefits of xanthophyll carotenoids and omega-3 fatty acids combined. J. Alzheimers Dis. 2018:367–378. doi: 10.3233/JAD-180160
- Slavich G.M. Understanding inflammation, its regulation, and relevance for health: A top scientific and public priority. Brain Behav. Immun. 2015;45:13–14. doi: 10.1016/j.bbi.2014.10.012
- Ashraf-ganjouei A., Moradi K., Bagheri S., Aarabi M.H. The association between systemic inflammation and cognitive performance in healthy adults. J. Neuroimmunol. 2020;345:577272. doi: 10.1016/j.jneuroim.2020.577272
- Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466
- Baselet B., Sonveaux P., Baatout S., Aerts A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019;76:699–728. doi: 10.1007/s00018-018-2956-z
- Yong L.C., Petersen M.R., Sigurdson A.J., Sampson L.A., Ward E.M. High dietary antioxidant intakes are associated with decreased chromosome translocation frequency in airline pilots. Am. J. Clin. Nutr. 2009;90:1402–1410. doi: 10.3945/ajcn.2009.28207
- Stewart J.J., Adams W.W., III, Escobar C.M., López-Pozo M., Demmig-Adams B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020;11:480. doi: 10.3389/fpls.2020.00480
- Zhou X., Wang S., Ding X., Qin L., Mao Y., Chen L., Li W., Ying C. Zeaxanthin improves diabetes-induced cognitive deficit in rats through activiting PI3K/AKT signaling pathway. Brain Res. Bull. 2017;132:190–198. doi: 10.1016/j.brainresbull.2017.06.001
- Stringham J.M., Johnson E.J., Hammond B.R. Lutein across the lifespan: From childhood cognitive performance to the aging eye and brain. Curr. Dev. Nutr. 2019;3:nzz066. doi: 10.1093/cdn/nzz066
- Renzi-Hammond L.M., Bovier E.R., Fletcher L.M., Miller L.S., Mewborn C.M., Lindbergh C.A., Baxter J.H., Hammond B.R. Effects of a lutein and zeaxanthin intervention on cognitive function: A randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients. 2017;9:1246. doi: 10.3390/nu9111246
- Perry A., Rasmussen H., Johnson E.J. Xantophyll (lutein, zeaxanthin) conten in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009;22:9–15. doi: 10.1016/j.jfca.2008.07.006
- Li S., Liu N., Lin L., Sun E.-D., Li J.-D., Li P.-K. Macular pigment and serum zeaxanthin levels with Goji berry supplement in early age-related macular degeneration. Int. J. Ophthalmol. 2018;11:970–975. doi: 10.18240/ijo.2018.06.12
- Wen X., Hempel J., Schweiggert R.M., Ni Y., Carle R. Carotenoids and carotenoid esters of red and yellow physalis (physalis alkekengi L. and P. pubescens L.) fruits and calyces. J. Agric. Food Chem. 2017;65:6140–6151. doi: 10.1021/acs.jafc.7b02514
- Tudor C., Bohn T., Iddir M., Dulf F.V., Focşan M., Rugină D.O., Pintea A. Sea buckthorn oil as a valuable source of bioaccessible xanthophylls. Nutrients. 2020;12:76. doi: 10.3390/nu12010076
- Handelman G.J., Nightingale Z.D., Lichtenstein A.H., Schaefer E.J., Blumberg J.B. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am. J. Clin. Nutr. 1999;70:247–251. doi: 10.1093/ajcn.70.2.247
- Mariutti L.R.B., Mercadante A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018;648:36–43. doi: 10.1016/j.abb.2018.04.005
- Xavier A.A.O., Mercadante A.Z. The bioaccessibility of carotenoids impacts the design of functional foods. Curr. Opin. Food Sci. 2019;26:1–8. doi: 10.1016/j.cofs.2019.02.015
- Tudor C., Bohn T., Iddir M., Dulf F.V., Focsan M., Rugină D.O., Pintea A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients. 2020;12:76. doi: 10.3390/nu12010076
- Shin H.S., Kim J.W., Kim J.H., Lee D.G., Lee S., Kil D.Y. Effect of feeding duration of diets containing corn distillers dried grains with solubles on productive performance, egg quality, and lutein and zeaxanthin concentrations of egg yolk in laying hens. Poult. Sci. 2016;95:2366–2371. doi: 10.3382/ps/pew127
- Chung H.Y., Rasmussen H.M., Johnson E.J. Lutein Bioavailability Is Higher from Lutein-Enriched Eggs than from Supplements and Spinach in Men. J. Nutr. 2004;134:1887–1893. doi: 10.1093/jn/134.8.1887
- Huang W., Lin Y., He M., Gong Y., Huang J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018;66:891–897. doi: 10.1021/acs.jafc.7b05400
- Nimalaratne C., Lopes-Lutz D., Schieber A., Wu J. Effect of Domestic Cooking Methods on Egg Yolk Xanthophylls. J. Agric. Food Chem. 2012;60:12547–12552. doi: 10.1021/jf303828n
- Zhang S., Ji J., Zhang S., Guan C., Wang G. Effects of three cooking methods on content changes and absorption efficiencies of carotenoids in maize. Food Funct. 2020;11:944–954. doi: 10.1039/C9FO02622C
- Cha K.H., Koo S.Y., Song D.G., Pan C.H. Effect of Microfluidization on Bioaccessibility of Carotenoids from Chlorella ellipsoidea during Simulated Digestion. J. Agric. Food Chem. 2012;60:9437–9442. doi: 10.1021/jf303207x
- Tudor, C., & Pintea, A. (2020). A Brief Overview of Dietary Zeaxanthin Occurrence and Bioaccessibility. Molecules (Basel, Switzerland), 25(18), 4067. https://doi.org/10.3390/molecules25184067
- Alves-Rodrigues A, Shao A. The science behind lutein. Toxicol Lett. 2004 Apr 15;150(1):57-83. doi: 10.1016/j.toxlet.2003.10.031
- Choi RY, Chortkoff SC, Gorusupudi A, Bernstein PS. Crystalline Maculopathy Associated With High-Dose Lutein Supplementation. JAMA Ophthalmol. 2016 Dec 1;134(12):1445-1448. doi: 10.1001/jamaophthalmol.2016.4117