Immunohistochemistry
Immunohistochemistry or IHC is a laboratory method that uses antibodies to check for certain antigens (markers) or other macromolecules in cells of a tissue sample. Immunohistochemistry involves the process of selectively identifying antigens (proteins) in cells of a tissue sample by exploiting the principle of antibodies binding specifically to antigens in biological tissues 1. The antibodies are usually linked to an enzyme or a fluorescent dye. After the antibodies bind to the antigen in the tissue sample, the enzyme or dye is activated, and the antigen can then be seen under a microscope. Immunohistochemistry is used to help diagnose diseases, such as cancer. Immunohistochemistry may also be used to help tell the difference between different types of cancer. The strength of immunohistochemistry is the intuitive visual output that reveals the existence and localization of the target-protein in the context of different cell types, biological states, and/or subcellular localization within complex tissues. Since immunohistochemistry involves specific antigen–antibody reactions, it has apparent advantage over traditionally used special enzyme staining techniques that identify only a limited number of proteins, enzymes, and tissue structures. Therefore, immunohistochemistry has become a crucial technique and is widely used in many medical research laboratories as well as clinical diagnostics 2.
Immunohistochemistry takes its name from the roots “immuno,” in reference to antibodies used in the procedure and “histo,” meaning tissue. The immunohistochemistry technique was invented during the 1940s where Coons and his colleagues 3 used Fluorescein isothiocyanate (FITC)-labeled antibodies with a fluorescent dye to localize pneumococcal antigens in infected tissues. Immunohistochemistry is routinely used as an important tool in health care and pathology for example as diagnostic purposes or to stratify patients for optimized treatment regimes 4. Immunohistochemistry or IHC is also widely used in research where molecules of interest are analyzed to study their roles in both healthy and diseased cells and tissues on the molecular, cellular or tissue level. There are many different ways to perform visualization of targets in tissues using immunohistochemistry or IHC-based methods, and numerous protocols exist for different applications and assays. Even though immunohistochemistry is generally a robust and established method, new assays often need careful optimization depending on the tissue or on the properties of the target protein, binder-molecule and/or reporter system. Many years of technical development and the hugely increased availability for specific binding-molecules have greatly improved the usefulness and areas of applications for immunohistochemistry. The progress in the field of immunohistochemistry-based techniques and reagents has enabled scientists and health care providers with more precise tools, assays and biomarkers. In addition, technical advances have enabled e.g. highly sensitive simultaneous detection of multiple proteins in the same sample, and the detection of protein-protein interactions
Immunostaining previously called immunohistochemical staining, is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. Specific molecular markers are characteristic of particular cellular events such as proliferation or cell death (apoptosis) 5. Immunohistochemistry is also widely used in basic research to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue.
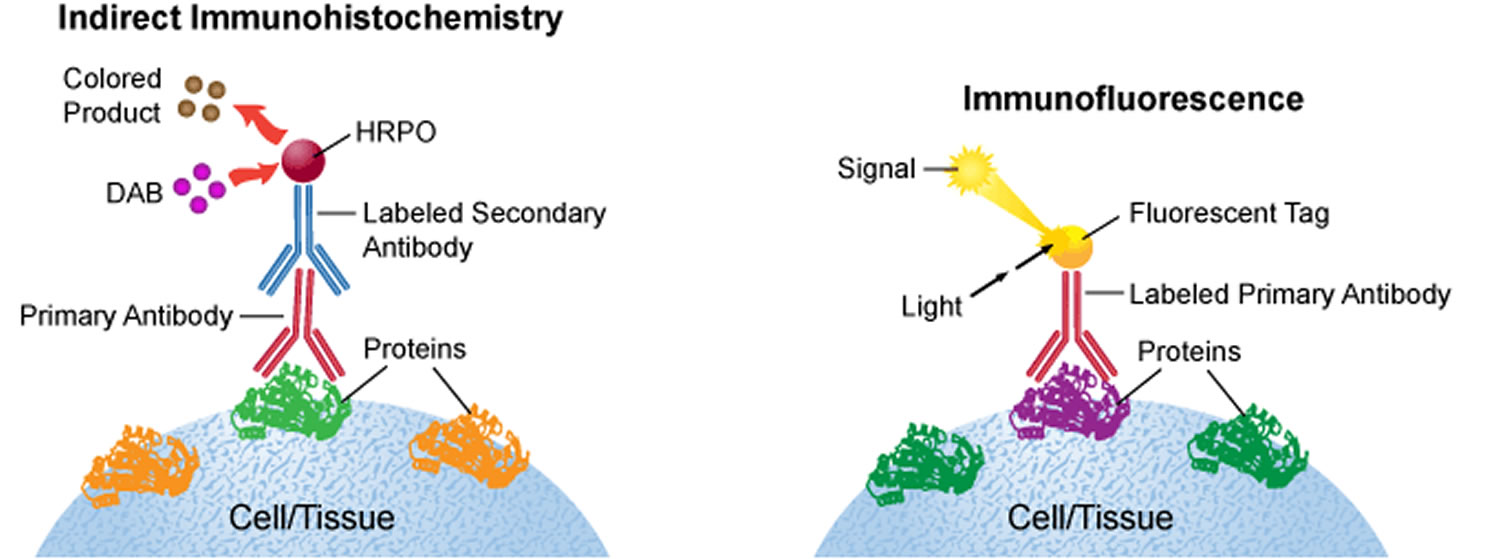
Visualizing an antibody-antigen interaction can be accomplished in a number of ways. In the most common instance, an antibody is conjugated to an enzyme, such as peroxidase, that can catalyse a color-producing reaction (immunoperoxidase staining) 6. Alternatively, the antibody can also be tagged to a fluorophore, such as fluorescein, rhodamine or Texas Red (immunofluorescence). The latter method is of great use in confocal laser scanning microscopy, which is highly sensitive and can also be used to visualize interactions between multiple proteins.
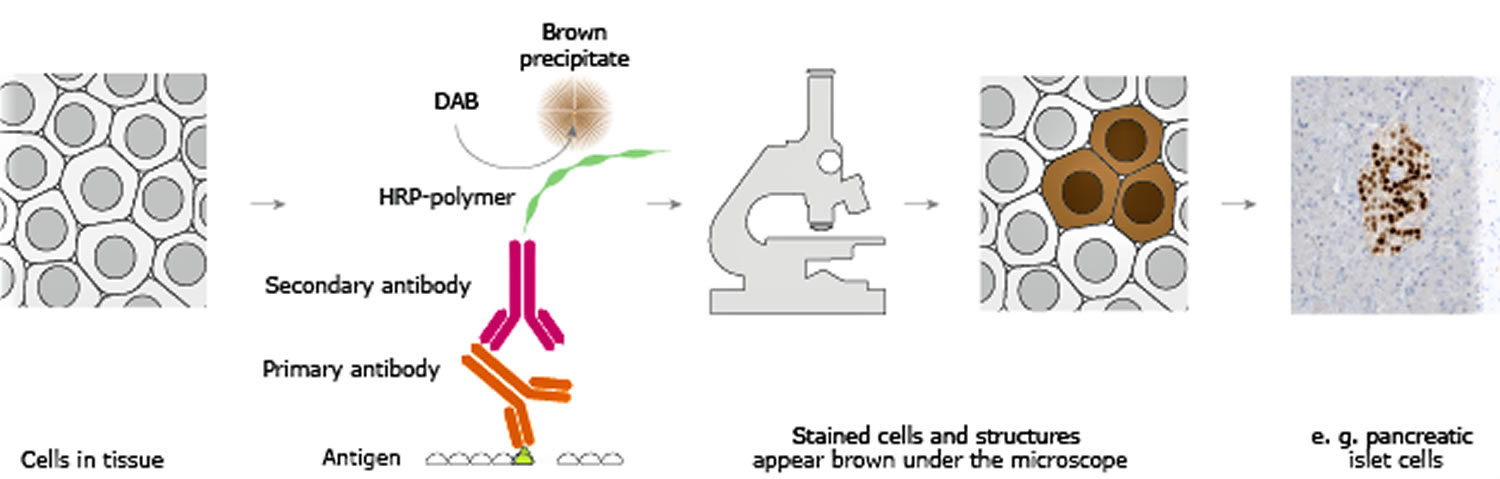
The classical immunohistochemistry assay is illustrated in Figure 1 and involves detection of epitopes expressed by a single protein-target within a tissue sample using a “primary antibody” capable of binding those epitopes with high specificity 4. After the epitope-antibody binding event, a “secondary antibody” capable of binding the primary antibody with high specificity is added. The secondary antibody is coupled to a reporter molecule and after the antibody-antibody binding event, a chemical substrate is added which reacts with the reporter molecule to produce a colored precipitate at the site of the whole epitope-antibody complex.
In the schematic illustration (Figure 1) a formalin-fixed paraffin embedded tissue section is stained using a primary antibody directed towards a specific protein target. A solution containing the primary antibody is added to the tissue section and the antibodies are allowed some time to find and bind to their target. After this step, unbound and surplus antibodies are washed away and the secondary antibody is added. The secondary antibody, which carries a linker molecule with horseradish peroxidase (HRP) enzymes, is also allowed some time to bind to the primary antibody, followed by another washing step. After this, 3,3′ Diaminobenzidine (DAB) is added. The horseradish peroxidase enzyme transforms the 3,3′ Diaminobenzidine substrate into a brownish precipitate that is deposited in the tissue at the site of the reaction, thus producing a visual representation of where the primary antibody first bound its target.
Figure 1. Immunohistochemistry basic principle
[Source 4 ]Direct and indirect immunohistochemistry
There are two strategies used for the immmunohistochemical detection of antigens in tissue, the direct method and the indirect method.In both cases, the tissue is treated to rupture the membranes, usually by using a kind of detergent called Triton X-100.
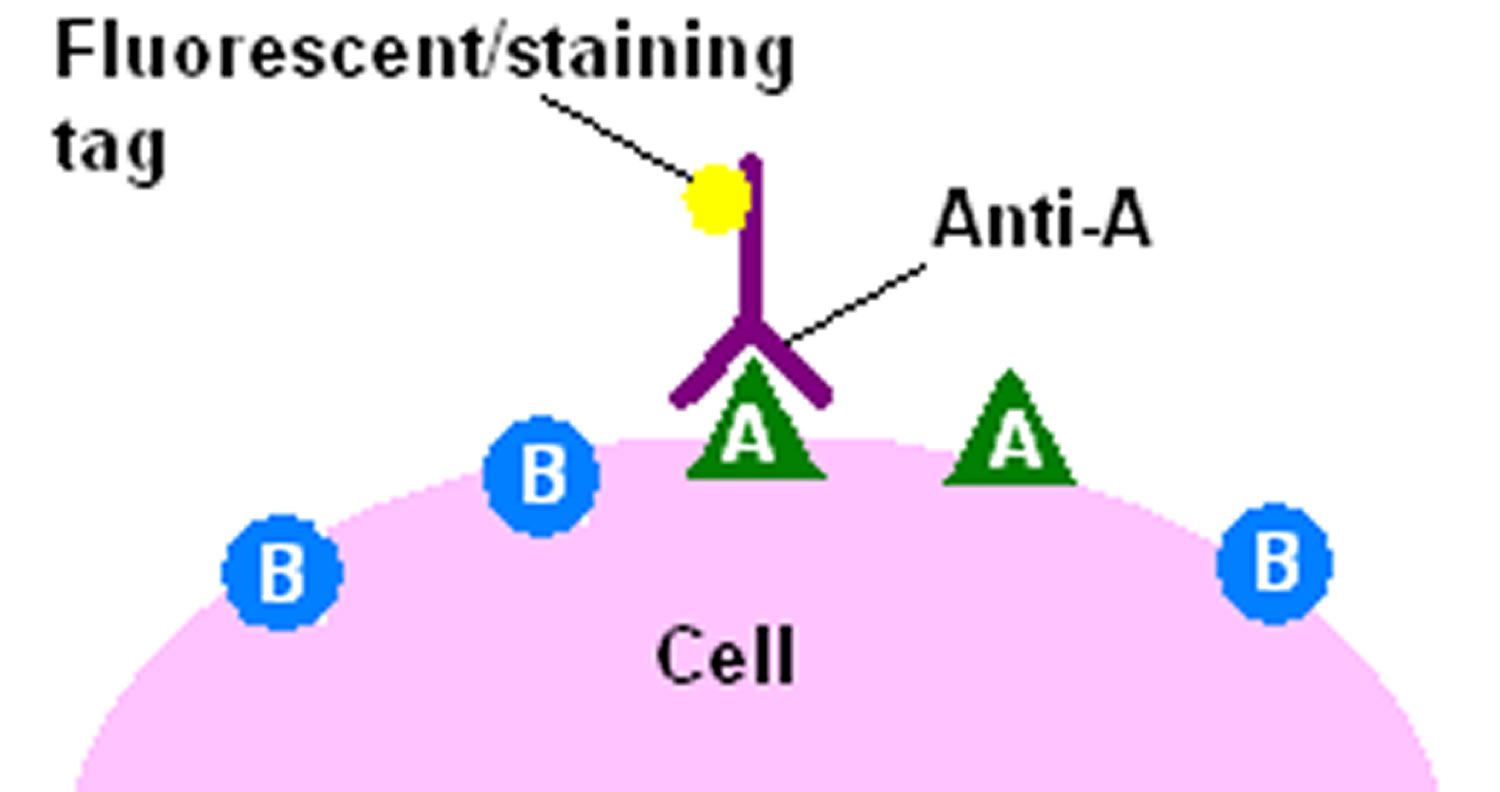
The direct immmunohistochemical detection method is a one-step staining method, and involves a labeled antibody (e.g. fluorescein isothiocyanate (FITC) conjugated antiserum) reacting directly with the antigen in tissue sections. This technique utilizes only one antibody and the procedure is therefore simple and rapid. However, it can suffer problems with sensitivity due to little signal amplification and is in less common use than indirect methods.
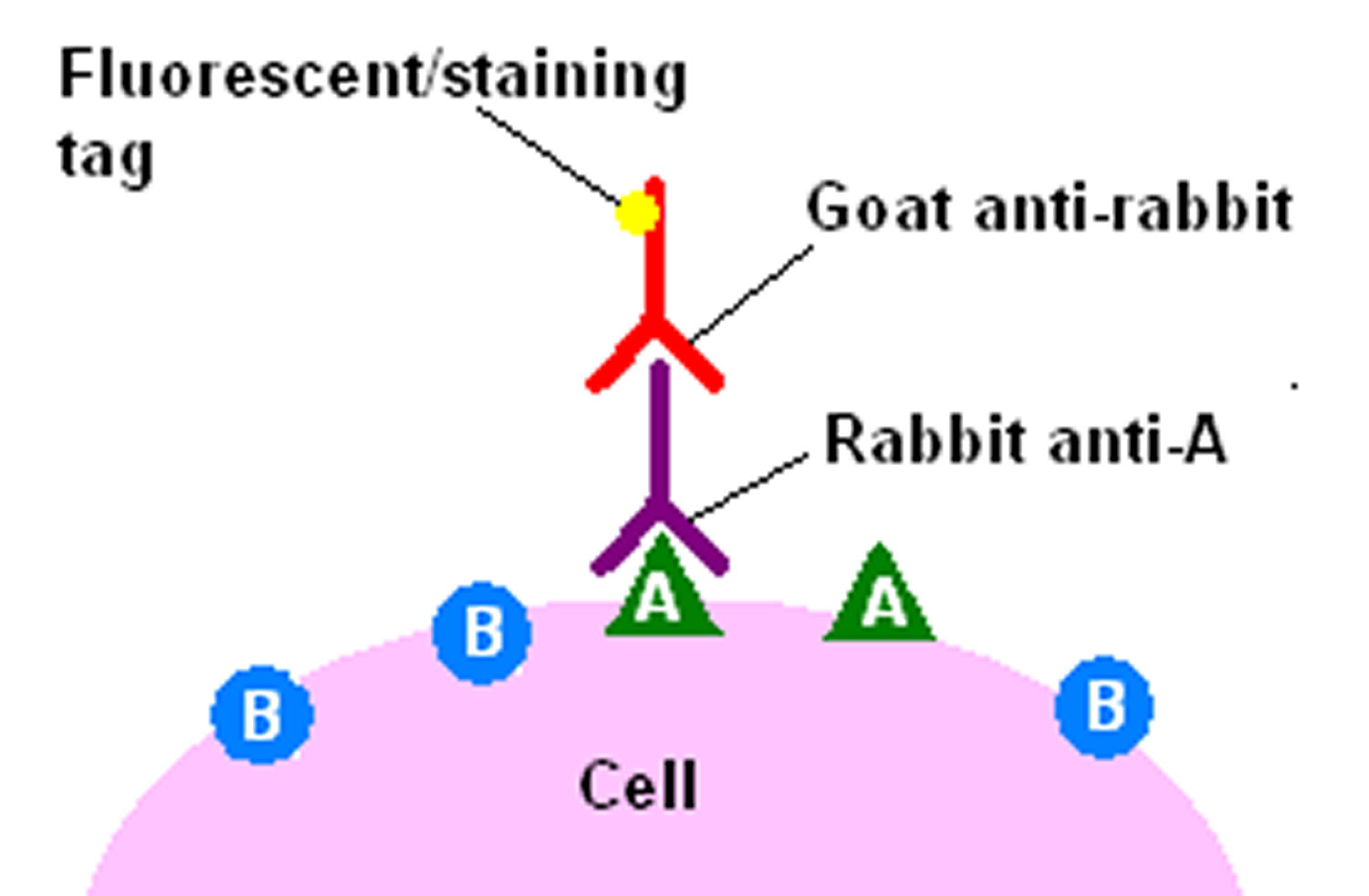
The indirect immmunohistochemical detection method involves an unlabeled primary antibody (first layer) which reacts with tissue antigen, and a labeled secondary antibody (second layer) which reacts with the primary antibody. The secondary antibody must be against the IgG of the animal species in which the primary antibody has been raised. This method is more sensitive due to signal amplification through several secondary antibody reactions with different antigenic sites on the primary antibody. The second layer antibody can be labeled with a fluorescent dye or an enzyme.
In a common procedure, a biotinylated secondary antibody is coupled with streptavidin-horseradish peroxidase. This is reacted with 3,3′-Diaminobenzidine (DAB) to produce a brown staining wherever primary and secondary antibodies are attached in a process known as DAB staining. The reaction can be enhanced using nickel, producing a deep purple/gray staining.
The indirect method, aside from its greater sensitivity, also has the advantage that only a relatively small number of standard conjugated (labeled) secondary antibodies needs to be generated. For example, a labeled secondary antibody raised against rabbit IgG, which can be purchased “off the shelf,” is useful with any primary antibody raised in rabbit. With the direct method, it would be necessary to make custom labeled antibodies against every antigen of interest.
Figure 2. Direct immmunohistochemical detection method
Footnote: The direct method of immunohistochemical staining uses one labelled antibody, which binds directly to the antigen being stained for.
Figure 3. Indirect immmunohistochemical detection method
Footnote: The indirect method of immunohistochemical staining uses one antibody against the antigen being probed for, and a second, labelled, antibody against the first.
Immunohistochemistry technology
Immunohistochemistry requires the availability of biopsies; these are processed into sections with a microtome and then the sections are incubated with an appropriate antibody. The site of antibody binding is visualized under an ordinary or fluorescent microscope by a marker such as fluorescent dye, enzyme, radioactive element, or colloidal gold, which is directly linked to the primary antibody or to an appropriate secondary antibody.
Tissue preparation
The tissue plays a central role in the experiment and it is important that it is processed so that epitopes and proper morphology is preserved. The most common processing for immunohistochemistry is to prepare formalin-fixed paraffin-embedded tissue blocks. The purpose of formalin fixation is to produce chemical cross-linking of proteins within the tissue. This terminates all cellular processes and freezes the cellular components at the place and in the conformation they were in at the time of fixation and also prevent degradation. After adequate fixation, the tissue is further processed and ultimately embedded in paraffin blocks, which are then sectioned into thin slices (usually 4-10µm) using a microtome. The sections are transferred to glass slides and allowed to adhere prior to further processing.
Other methods for fixation besides formalin are sometimes used. These include other types of aldehydes or using different alcohol solutions. The best choice of fixative is very much dependent on the assay. A common alternative to formalin-fixed paraffin-embedded is to prepare frozen tissue samples. In this case, the tissue is embedded in a cryoprotective medium and frozen, and fixation is performed post-sectioning. Frozen tissues are sectioned in cryostats and have the advantage of short processing times and of better preservation of sensitive epitopes, but can often be inferior to formalin-fixed paraffin-embedded tissues in terms of preserving histological morphology.
Figure 4. Immunohistochemistry technique
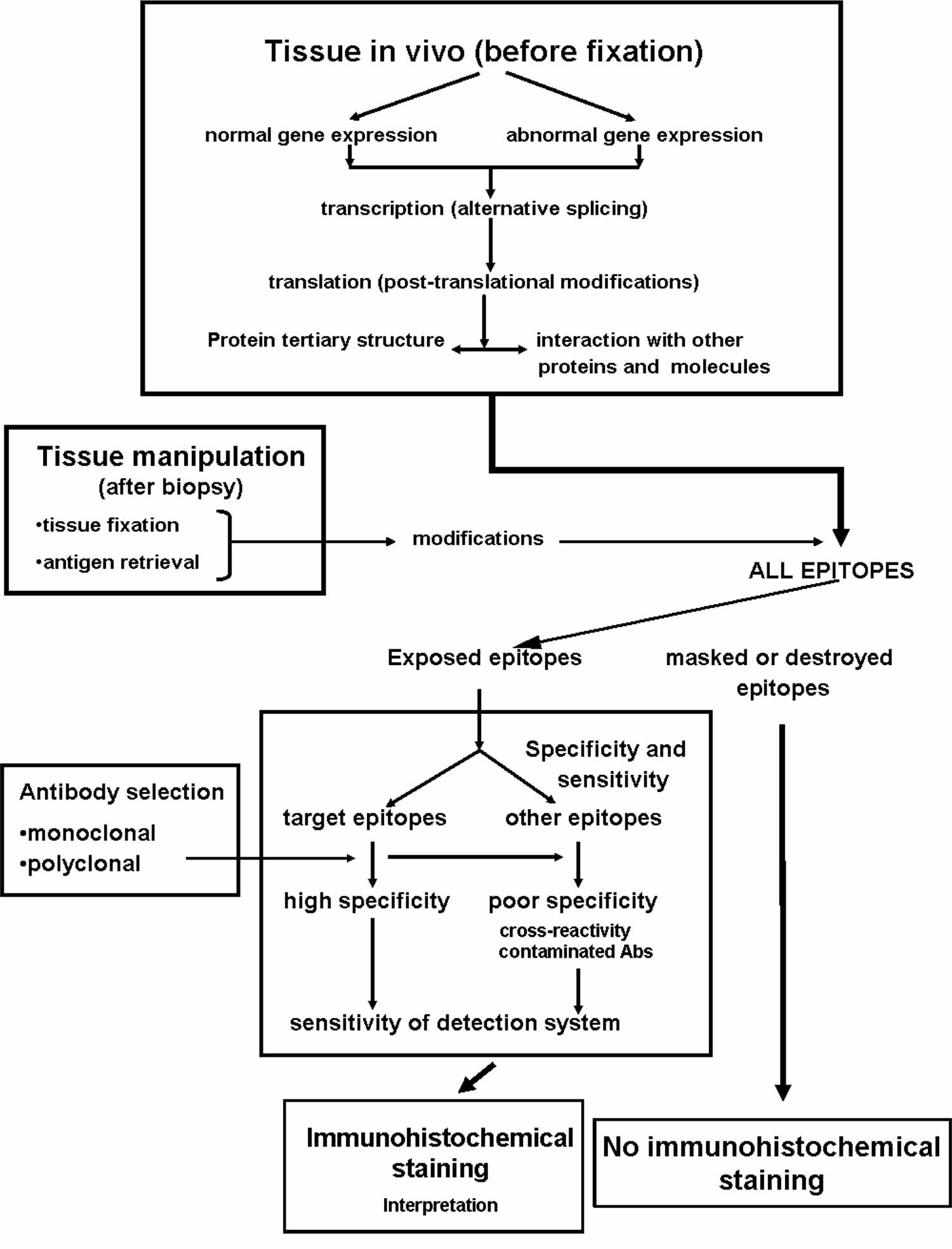
Footnote: Principal factors affecting the outcome of immunohistochemical studies
[Source 7 ]Antigen (epitope) retrieval
A concern associated with cross-linking fixatives like formalin, or too long time spent in fixative medium is the masking of epitopes, which can obstruct the primary antibody from binding to its target. Especially with formalin-fixed paraffin-embedded samples, there is often a need to revert some of the chemical crosslinking and “retrieve” the epitopes before proceeding to the actual immunohistochemistry. There are several antigen retrieval protocols available and the main strategies include treating the tissue slide with heat, digestive enzymes, detergents, or combinations thereof. The most common method for antigen retrieval in formalin-fixed paraffin-embedded samples is to pressure-boil the tissue slides in an acidic citrate buffer for around 15-20 minutes.
Antibody binding
The quality and specificity of the binding molecule is crucial for any immunohistochemistry based technique, and the choice of binder can directly affect the outcome, reliability, and possibly also the interpretation of the assay. Antibodies are by far the most common type of binding-molecule used for immunohistochemistry, and although most antibodies are able to adequately detect the correct molecule of interest, they may also vary greatly in their specificity for their intended target. Antibodies with high specificity are therefore more reliable when interpreting “on-target” binding, since they produce little or no “off-target” binding or “background”. Antibodies that are less specific can produce more off-target binding, and the resulting background will possibly interfere with the correct interpretation of the true on-target signals. There are two main types of antibodies; polyclonal antibodies which is a heterogeneous mix of antibodies that bind different epitopes on the target and monoclonal antibodies that all bind the same epitope. Polyclonal antibodies are often very potent due to their ability to detect and bind multiple epitopes on the same target. However, the epitopes they bind are often poorly defined and with multiple and varying epitope-specificities comes the increased likelihood of off-target binding events and background noise. However, the potency of polyclonal antibodies can be advantageous since the concentration of binding events around the on-target molecule usually outweighs potential background noise. A drawback is that polyclonal antibodies are usually limited resources since they are derived from animal sera. Monoclonal antibodies, by contrast, have more continuity since they can be produced in hybridoma cell lines. Monoclonal antibodies are also often well defined in terms of epitope binding, but can still generate results that are hard to interpret if the specificity is low or if the target epitope is present in low abundance.
Careful optimization and titration of antibody concentration for each assay is needed, since the result is dependent not only on the antibody’s specificity and affinity for the target, but also on the concentration and availability of on-target and potential off-target epitopes present in the sample. Adding too much antibodies to the sample will increase the number of possible low-affinity off-target binding events once the on-target epitope(s) are saturated with binders. By lowering the antibody concentration, off-target binding events become rarer as they usually have lower affinity than on-target binding events. The risk when attempting to reduce background while using a low-affinity antibody is that the on-target signals are concomitantly weakened to the point of providing a false negative result.
Other types of binder molecules sometimes used in immunohistochemistry-based techniques include affibodies, peptides, antibody fragments or other small molecules.
Detection systems
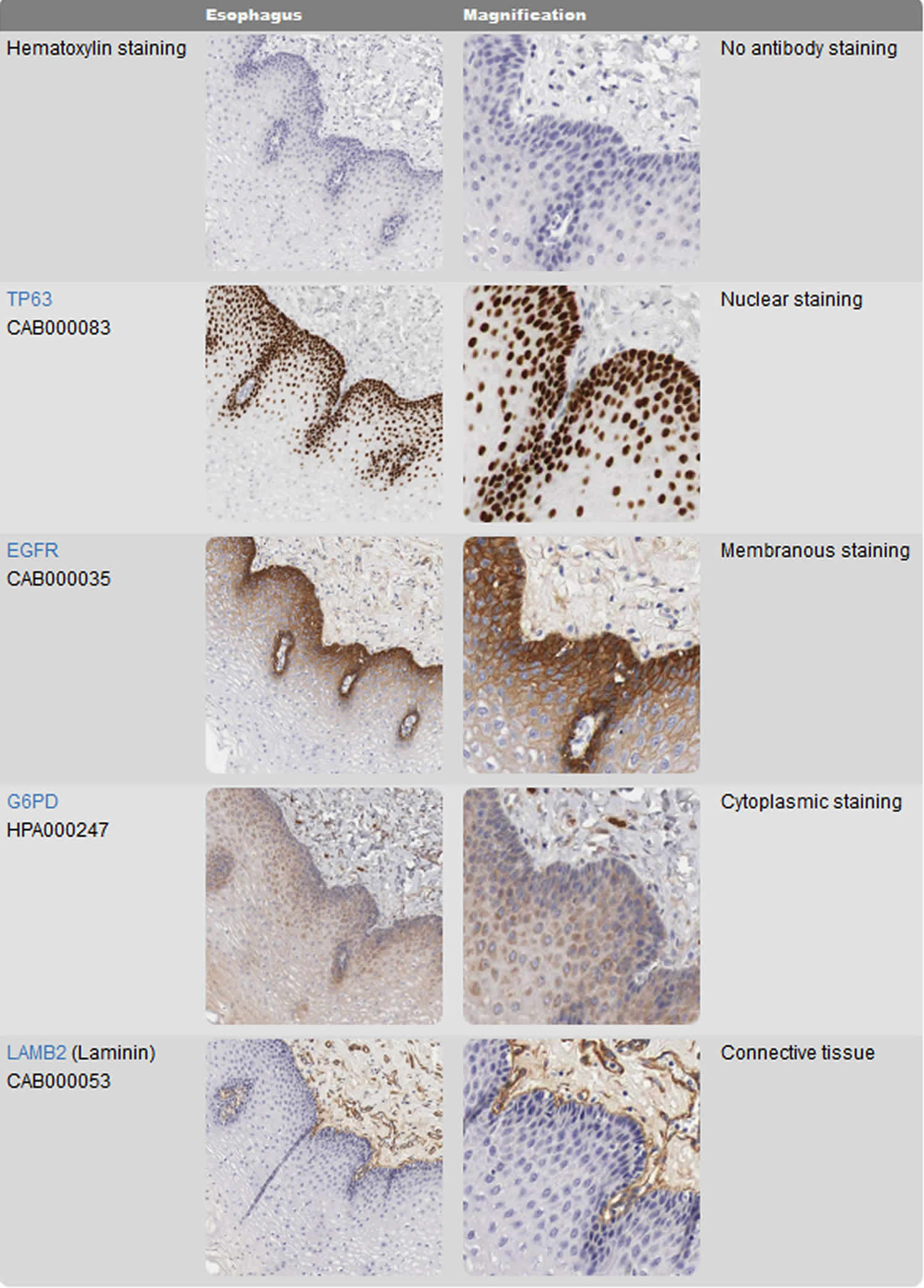
The whole purpose of performing immunohistochemistry is to obtain a visual representation of where the target can be found within the experimental tissue, and preferably also gain information about the target’s expression pattern among heterogeneous cell populations and/or subcellular localizations. This is exemplified in Figure 5, which illustrates how different antibodies are used to visualize different cellular or tissue compartments within a complex tissue. To visualize the target-antibody interaction, some kind of detection system that produces an observable stain or signal is needed. The most common method for introducing a detection system to the experiment is to use a secondary antibody that carries a pre-bound reporter molecule, i.e. enzyme or fluorophore. Secondary antibodies are usually targeted specifically towards antibody molecules from a different animal species. For example, if the primary antibody is raised in a rabbit, then the secondary antibody must be raised in another animal and targeted specifically towards rabbit antibodies.
In the immunohistochemistry image (Figure 5), consecutive sections of human esophagus stained using four different antibodies allows for direct comparison of different protein expression patterns within the tissue and within subcellular compartments. The top images are only counterstained for hematoxylin for comparison. The p63 antibody stains cell nuclei in a population of cells that reside in the basal part of the esophageal epithelium. The EGFR (Epidermal growth factor receptor) antibody appears to stain the same cell population as p63, but stains cellular membranes instead of nuclei. The G6PD (Glucose-6-phosphate dehydrogenase) antibody stains the cytoplasm of a wider repertoire of esophageal epithelial cells and also cells residing in the connective tissue. The Laminin (LAMB2) antibody stains only cells and structures in the connective tissue underlying the esophagus.
For formalin-fixed paraffin-embedded tissue samples the most common detection method is to use enzymatic reactions to generate a colored precipitate at the site of antibody binding. The secondary antibodies then carry an enzyme, e.g. horseradish peroxidase (HRP) or alkaline phosphatase (AP), that are capable of converting chromogens like 3,3′ Diaminobenzidine (DAB) or 5-bromo-4-chloro-3-indolyl phosphate/ p-nitroblue tetrazolium chloride (BCIP/NBT) into brown or bluish precipitates that are deposited in the tissue at the site of the reaction. Chromogenic stains are observable in light-microscopy and are usually very stable over long periods of time, which is beneficial if the experiment needs to be archived or reviewed at a later time point.
For frozen tissue sections it is more common to use fluorophore-linked secondary antibodies that emit a specific color (usually green, red, or blue) when excited by the correct wavelengths of light. Moreover, fluorophores are usually not stable for long periods of time. However, the benefit of using fluorophores is that they provide an easy method for performing double-labeling experiments where several antibodies towards multiple targets are assayed in the same sample. The secondary antibodies need to be targeted towards different primary antibodies and also to be coupled to different fluorophores. The different secondary antibodies are then observed separately by exciting them sequentially with different wavelengths of light. These different excitation results are saved as separate images (or color channels) and may later be overlaid to infer protein co-localizations etc.
Using reporter-carrying secondary antibodies for detection is in itself an amplification step since several secondary antibodies are able to bind a single primary antibody, but sometimes further amplification steps are desired to increase the signal and sensitivity of the experiment. In such cases, the secondary antibody may instead carry “linker molecules”, for instance biotin polymers, which are able to recruit a larger number of reporter molecules in subsequent steps. This strategy for amplifying signals is useful for both enzymatic and fluorescent detection methods.
Figure 5. Immunohistochemistry image
Footnote: Visualizing different protein targets in complex tissues. The right column shows a magnification of the corresponding images in the left column.
[Source 4 ]Counterstaining
Immunohistochemical staining using chromogens often benefits from having a counterstain applied that enhances the contrast and facilitates the observation of histological features. The most common type of counterstain used for formalin-fixed paraffin-embedded samples is hematoxylin that stains cellular cytoplasm with a pale bluish color, and stain cell nuclei in a darker bluish nuance. Fluorescent stainings are usually not counterstained with hematoxylin, since the detection method is not based on light microscopy. Instead, the most common way to obtain counterstaining for fluorescence is to label cell nuclei by adding fluorescent dyes that bind nucleic acids. After the actual immunohistochemical reaction, the only remaining steps are to coverslip and seal the sample for protection and longterm storage. The most common way is to “glue” the coverslip to the sample using commercially available purpose-made resins.
Immunohistochemistry clinical applications
Immunohistochemistry (IHC) is an important application of monoclonal as well as polyclonal antibodies to determine the tissue distribution of an antigen of interest in health and disease. Immunohistochemistry is an excellent detection technique and has the tremendous advantage of being able to show exactly where a given protein is located within the tissue examined. This has made it a widely-used technique in the neurosciences, enabling researchers to examine protein expression within specific brain structures. Its major disadvantage is that, unlike immunoblotting techniques where staining is checked against a molecular weight ladder, it is impossible to show in Immunohistochemistry that the staining corresponds with the protein of interest. For this reason, primary antibodies must be well-validated in a Western Blot or similar procedure. The technique is even more widely used in diagnostic surgical pathology for typing tumors (e.g. carcinoma vs melanoma).
- Carcinoembryonic antigen (CEA): used for identification of Adenocarcinomas. Not specific for site.
- CD15 and CD30 : used for Hodgkin’s disease
- Alpha fetoprotein: for yolk sac tumors and hepatocellular carcinoma
- CD117: for gastrointestinal stromal tumors (GIST)
- Prostate specific antigen (PSA): for prostate cancer
- Estrogens and progesterone staining for tumour identification
- Identification of B-cell lymphomas using CD20
Prognostic markers in cancer
To predict the prognosis of tumors by identification of enzymes, tumor-specific antigens, oncogenes, tumor suppressor genes, and tumor cell proliferation markers. Analysis of tumors by these methods is a significant improvement over the conventional prognostic considerations by clinical staging and histologic grading. Immunohistochemistry is used for disease diagnosis, drug development, and biological research. Using specific tumor markers, physicians use Immunohistochemistry to diagnose a cancer as benign or malignant, determine the stage and grade of a tumor, and identify the cell type and origin of a metastasis to find the site of the primary tumor. Immunohistochemistry is also used in drug development to test drug efficacy by detecting either the activity or the up- or down-regulation of disease targets 8.
Tumors of uncertain histogenesis
Immunohistochemistry methods have brought about a revolution in approach to diagnosis of tumors of uncertain origin, primary as well as metastatic from unknown primary tumor. A panel of antibodies is chosen to resolve such diagnostic problem cases. The selection of antibodies being made is based on clinical history, morphological features, and results of other relevant investigations. Immunohistochemical stains for intermediate filaments are expressed by tumor cells (keratin, desmin, vimentin, neurofilaments, and glial fibrillary acidic proteins) 8.
Prediction of response to therapy
Immunohistochemistry is widely used to predict therapeutic response in two important tumors, i.e. carcinoma of breast and prostate. Both these tumors are under the growth regulation of the hormones estrogen and androgen, respectively. The specific receptors for these growth regulating hormones are located on respective tumor cells. Tumors expressing high level of receptor positivity would respond favorably to removal of the endogenous source of such hormones or hormonal therapy is administered to lower their levels – estrogen therapy in prostate cancer and androgen therapy in breast cancer 8.
Infections
Immunohistochemical methods are also being applied to confirm infectious agent in tissues by use of specific antibodies against microbial DNA or RNA, e.g. in Cytomegalo virus, Hepatitis B virus, Hepatitis C virus, etc. The application is used routinely in validation of disease targets as it allows visualizing expression of the target in the affected tissue during the disease process. The concept was introduced as early as the 1940s when fluorescein dye (visible under ultraviolet light) was tagged to antibodies directed against pneumococci for identification of this organism with specific anti-serum 9. This method, often abbreviated IFA for “immunofluorescence assay”, has been widely used for the detection of specific pathogens, viral as well as bacterial and protozoal, in “fresh”/unfixed tissues in both human and veterinary medicine.
Another important advantage of immunohistochemistry is that it can also be used to detect organisms in cytological preparations such as fluids, sputum samples, and material obtained from fine needle aspiration procedures. This can be very helpful in certain situations such as detection of pneumocystis from the sputum of an immunocompromised patient who needs rapid and precise confirmation of infection in order to begin immediate and appropriate therapy.
In Genetics
Immunohistochemistry can also be used to determine the function of specific gene products in fundamental biological processes such as development and apoptosis. Using a custom made monoclonal antibody against p53 homologue of the pro-apoptotic pathways of p53 was identified.
Neurodegenerative disorders
Degenerative disorders of the nervous system include a wide range of diseases characterized by the dysfunction and death of specific, selectively vulnerable populations of nerve cells. It has played an increasingly important role in the subclassification of neurodegenerative disorders and the development of consensus criteria for their diagnosis.
Brain trauma
In 1994, immunohistochemical staining for beta amyloid precursor protein has been validated as a method to detect axonal injury within as little as 2–3 hour of head injury 10. Immunohistochemical detection of axonal injury can be useful in establishing timing of a traumatic insult in medico-legal settings.
Immunohistochemistry in muscle diseases
Specific diagnosis of muscular dystrophy is important because of the genetic counseling implications of inherited disease and accurate prognostication. In recent years, abnormalities in several muscle proteins have been identified in muscular dystrophies. Such abnormalities involve proteins located in the sarcolemma, extracellular matrix, cytosol, nucleus, and other sites within muscle fibers 11. Skeletal muscle biopsy can play a main role in differentiating vascular dystrophy from non-dystrophic disorders and immunohistochemistry can assist in establishing a specific diagnosis of the dystrophies for which specific protein abnormalities are known.
Research application
Much of the current research into the causes of neurodegenerative diseases is directed at identifying the factors that result in the formation of paired helical filaments, the deposition of beta amyloid, cytoplasmic accumulations of alpha synuclein, etc. Consequently, studies to localize and quantify the abnormal proteins that constitute reasons of neurodegenerative diseases are of central importance. Immunohistochemistry using antibodies to beta amyloid, alpha synuclein, ubiquitin, huntingtin, polyglutamine, and others has become a routine tool for a sensitive detection and quantification of these abnormal proteins in both human tissues and in experimental animals that are used to model some of the features of these diseases. Immunohistochemistry is an important tool in diagnostic and research laboratories.
References- Immunohistochemistry: Basics and Methods – 2010. ISBN 9783642046094 DOI:10.1007/978-3-642-04609-4 https://link.springer.com/book/10.1007%2F978-3-642-04609-4
- Rajendran . Shafer’s textbook of oral pathology. 6th edition. India: Elsevier; 2009. p. 932.
- Coons AH, Creech HJ, Jones RN: Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med 1941; 47: 200-202.
- Immunohistochemistry. https://www.proteinatlas.org/learn/method/immunohistochemistry
- Whiteside, G; Munglani, R (1998). “TUNEL, Hoechst and immunohistochemistry triple-labelling: an improved method for detection of apoptosis in tissue sections—an update”. Brain Research Protocols. 3 (1): 52–53. doi:10.1016/s1385-299x(98)00020-8
- Ramos-Vara, J. A. (2005). “Technical Aspects of Immunohistochemistry”. Veterinary Pathology. 42 (4): 405–426. doi:10.1354/vp.42-4-405
- Ramos-Vara, J. A. (2005). Technical Aspects of Immunohistochemistry. Veterinary Pathology, 42(4), 405–426. https://doi.org/10.1354/vp.42-4-405
- Harsh Mohan. Essential pathology for dental students. 3rd edition. New Delhi: Jaypee brother’s medical publishers; 2005. p. 14.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol. 1941;47:200–2.
- Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunohistochemistry for beta-amyloid precursor protein. Actaneuropathologica. 1994;87:55–62.
- Vainzof M, Zata M. Protein defects in neuromuscular diseases. Braz J Med Biol Res. 2003;36:543–55.