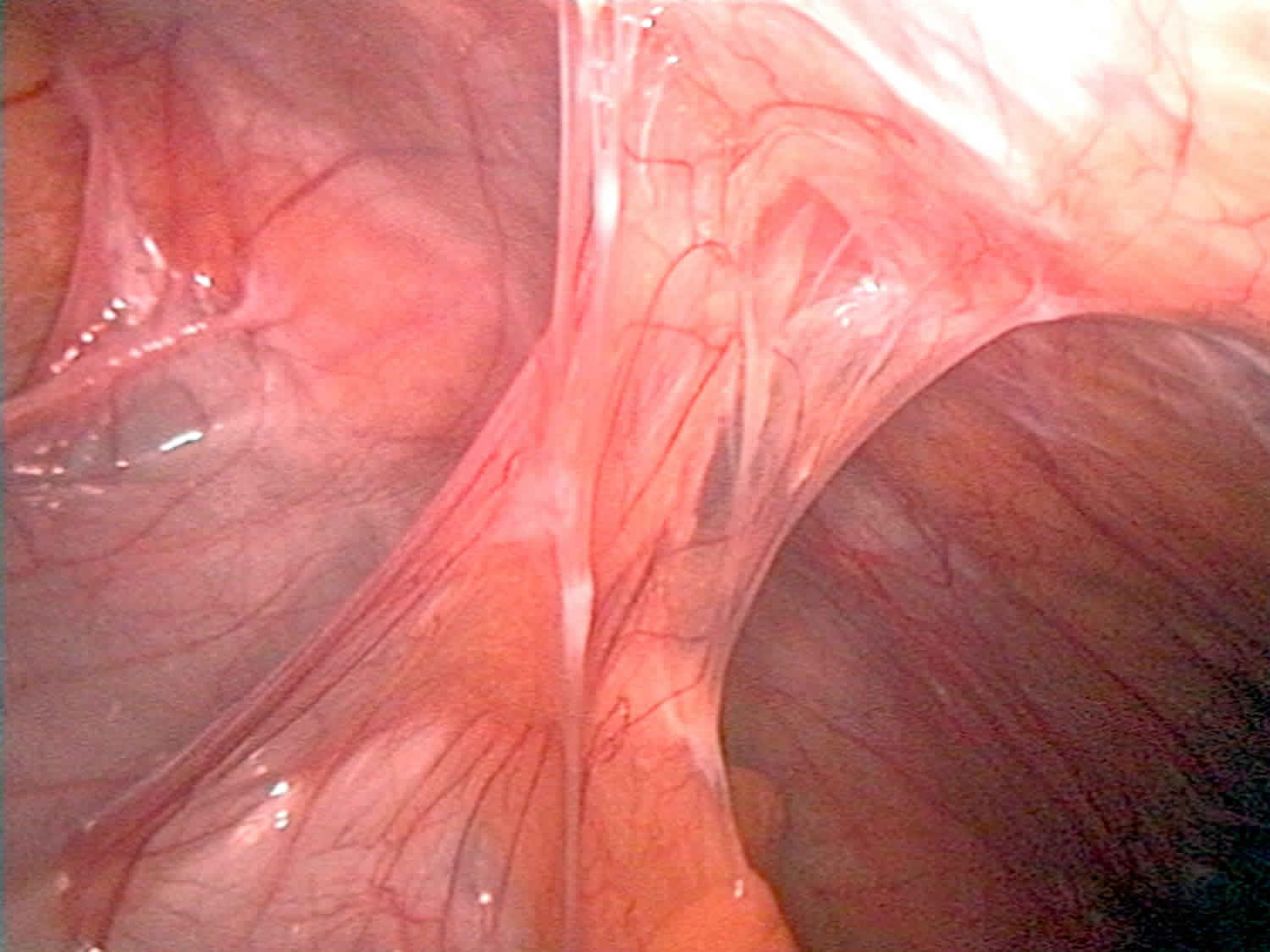
What is adhesion
Adhesions are bands of scar-like tissue. Normally, internal tissues and organs have slippery surfaces (mesothelium or serous membranes) so they can shift easily as the body moves. Adhesions cause tissues and organs to stick together. Adhesions might connect the loops of the intestines to each other, to nearby organs, or to the wall of the abdomen. Adhesions can pull sections of the intestines out of place. This may block food from passing through the intestine.
Adhesions can occur anywhere in the body. But they often form after surgery on the abdomen. Almost everyone who has surgery on the abdomen gets adhesions. Some adhesions don’t cause any problems. But when they partly or completely block the intestines, they cause symptoms such as
- Severe abdominal pain or cramping
- Vomiting
- Bloating
- An inability to pass gas
- Constipation
Adhesions can sometimes cause infertility in women by preventing fertilized eggs from reaching the uterus.
No tests are available to detect adhesions. Doctors usually find them during surgery to diagnose other problems.
Some adhesions go away by themselves. If they partly block your intestines, a diet low in fiber can allow food to move easily through the affected area. If you have a complete intestinal obstruction, it is life-threatening. You should get immediate medical attention and may need surgery.
See your healthcare provider if you have:
- Abdominal pain
- An inability to pass gas
- Nausea and vomiting that do not go away
- Pain in the belly that is severe and crampy
What causes adhesions
With movement of the body, internal organs such as the bowel or uterus are normally able to shift and to slide past each other. This is because these tissues and organs in the abdominal cavity have smooth, slippery surfaces. Inflammation (swelling), surgery, or injury can cause adhesions to form and prevent this movement.
An overview of the identified pathophysiological associations and the factors thought to be involved in the origin of adhesions is provided by Table 1 and Figure 1 below
Table 1. Overview of the principal factors affecting the fibrinolytic capacity of the mesothelium
| Factor | Fibrinolytic activity |
| Urokinase-like plasminogen activator (u-PA) | ↑ |
| Tissue plasminogen activator (t-PA) | ↑ |
| Matrix metalloproteinases (MMP) | ↑ |
| Tissue-derived inhibitors (TIMP) | ↓ |
| Plasminogen activation ‧inhibitors (PAI 1/ 2) | ↓ |
| Mechanical destruction of ‧mesothelium | ↓ |
| Mesothelial ischemia | ↓ |
| Hypoxy | ↓ |
| Radical formation | ↓ |
| Bacterial lipopolysaccharide | ↓ |
| Interleukins (e.g., IL-1, IL-6) | ↓ |
| Neurokinin-1 receptor (NK-1) | ↓ |
| Substance P (SP) | ↓ |
| Tumor necrosis factor α (TNFα) | ↓ |
| Transforming growth factor β (TGFβ) | ↓ |
| Intracellular adhesion molecule (ICAM 1) | ↓ |
| Vascular cell adhesion molecule (VCAM) | ↓ |
Figure 1. Overview of factors thought to be involved in the origin of abdominal adhesions
Footnote: Overview of pathophysiological interrelationships and factors thought to be involved in the origin of abdominal adhesions
[Source 1 ]The hypothesis that peritoneal fibrinolytic activity plays an important role in the pathophysiology of the dissociation of adhesions has been suggested 2. Tissue plasminogen activator (tPA) in mesothelial cells is a significant natural defense against the formation of adhesions following surgical operations 3. Active fibrinolysis enzymes, which emerge from inactive plasminogen via tissue plasminogen activator (tPA), turn the fibrin gel matrix into fibrin destruction products that have no effect on the formation of adhesions 2. If local fibrinolysis is sufficient, fibrinous adhesions are lysed. If it is not sufficient, connective tissue formation and adhesion development may occur 4. It was observed in many studies that closure of the parietal peritoneum increased adhesions in gynecologic operations 5, general surgical operations 6 and animal experiments 7. Based on the above data, the parietal peritoneum is routinely closed in gynecologic and obstetric operations 8. However, some other studies reported that, unlike other abdominal operations, closure of the peritoneum decreased pelvic adhesions in pregnant women 9. However, with respect to the significance of the fibrinolytic activity in the pathophysiology explained above, amnion fluid was found to show significant fibrinolytic activity after the 37th gestational week 10. Myers and Bennett 11 and Roset et al. 12 reported that the closure of the parietal peritoneum reduced adhesions in pregnant women. In another study 3, the authors found intraabdominal adhesions were not affected by whether the parietal peritoneum was closed. The healing phases of skin scars includes the inflammatory phase (including the injury and prevents infection), the proliferative phase (granulation of macrophages, proliferative degeneration, and characterized by epithelial tissue), and the remodeling phase (regulation of the extracellular matrix), which is a long process. Considering the significant points of the molecular biology behind the healing of scars, the factor that is effective at the molecular level is transforming growth factor-beta (TGF-β) 13. In adults, transforming growth factor-beta (TGF-β) and receptors are observed to be evidently active in scar tissue and involved in scar formation at the site of injury. However, transforming growth factor-beta (TGF-β) expression is temporary in the fetus and does not form scar tissue 14. Additionally, fibroblasts were observed to synthesize proteins involved in continuous transforming growth factor-beta (TGF-β) signal transduction in both hypertrophic scars and keloids 15. The number of studies on the relationship between scar tissue and intraabdominal adhesions is limited. However, Salim et al. 16 found that, among all abdominal scar characteristics, only depressive scars were associated with an increase in number and severity of evident adhesion incidence. Also, the incidence of frozen pelvis was found to increase by almost 12 times in women with depressive scars compared with those without depressive scars. Similar to many other researchers, Ferreira et al. 17 reported that hormonal, immunologic, genetic, and tissue growth factors played significant roles in scar development. Nissen et al. 18 showed that filmy intraabdominal adhesions, excessive fibrovascular structures, and depressive scars led to hypertrophic scars and might be affected by tensile strength. In another study 3, researchers found that hypopigmentation and depression of scars were associated in with intraabdominal adhesions.
It was reported in a previous study 19 that there was no significant difference between women who underwent cesarean section only once and women who underwent c-section two or three times in terms of intraabdominal adhesion incidence. According to other studies 20, 21, no difference was reported between women with multiple abdominal incisions and women with a single abdominal incision in terms of intraabdominal adhesions.
Adhesions can occur almost anywhere in the body, including:
- Joints, such as the shoulder
- Eyes
- Inside the abdomen or pelvis
Adhesions can become larger or tighter over time. Problems may occur if the adhesions cause an organ or body part to:
- Twist
- Pull out of position
- Be unable to move normally
The risk of forming adhesions is high after bowel or female organ surgeries. Surgery using a laparoscope is less likely to cause adhesions than open surgery.
Other causes of adhesions in the abdomen or pelvis include:
- Appendicitis, most often when the appendix breaks open (ruptures)
- Cancer
- Endometriosis
- Infections in the abdomen and pelvis
- Radiation treatment
Adhesions around the joints may occur:
- After surgery or trauma
- With certain types of arthritis
- With overuse of a joint or tendon
Adhesions symptoms
Adhesions in joints, tendons, or ligaments make it harder to move the joint. They may also cause pain.
Adhesions in the belly (abdomen) may cause a blockage of the intestines. Symptoms include:
- Bloating or swelling of your belly
- Constipation
- Nausea and vomiting
- No longer being able to pass gas
- Pain in the belly that is severe and crampy
Adhesions in the pelvis may cause long-term (chronic) pelvic pain.
Adhesion possible complications
Adhesions can cause various disorders, depending on the tissues affected.
- In the eye, adhesion of the iris to the lens can lead to glaucoma.
- In the intestines, adhesions can cause partial or complete bowel obstruction.
- Adhesions inside the uterine cavity can cause a condition called Asherman syndrome. This can cause a woman to have irregular menstrual cycles and be unable to get pregnant.
- Pelvic adhesions that involve scarring of the fallopian tubes can lead to infertility and reproductive problems.
- Abdominal and pelvic adhesions can cause chronic pain.
Adhesions diagnosis
Most of the time, the adhesions cannot be seen using x-rays or imaging tests.
- Hysterosalpingography may help detect adhesions inside the uterus or fallopian tubes.
- X-rays of the abdomen, barium contrast studies, and CT scans may help detect a blockage of the intestines caused by adhesions.
Endoscopy (a way of looking inside the body using a flexible tube that has a small camera on the end) may help diagnose adhesions:
- Hysteroscopy looks inside the uterus
- Laparoscopy looks inside the abdomen and pelvis
Adhesions treatment
Surgery may be done to separate the adhesions. This can let the organ regain normal movement and reduce symptoms. However, the risk for more adhesions goes up with more surgeries.
Depending on the location of the adhesions, a barrier may be placed at the time of surgery to help reduce the chance of the adhesions returning.
Abdominal adhesions
Abdominal adhesions or intestinal adhesions, are bands of fibrous tissue that can form between abdominal tissues and organs. Normally, internal tissues and organs have slippery surfaces, preventing them from sticking together as the body moves. However, abdominal adhesions cause tissues and organs in the abdominal cavity to stick together. Abdominal adhesions can occur between the parietal and visceral peritoneum, and between various visceral peritonea after abdominal surgery 22. Intra-abdominal adhesions following surgery represent a major unsolved problem 23. Abdominal adhesions occur after 50% to 100% of all surgical interventions in the abdomen and can complicate the work of the surgeon considerably 24. The greater omentum is involved in 80% of cases of postoperative intra-abdominal adhesions, the bowel in only around 50% 25. Ovarian adhesions can be demonstrated in over 90% of patients after gynecological adnexal surgery 26; this is explained by the high sensitivity of the ovarian epithelium and its proximity to other peritoneal surfaces 27. Patients at high risk of already having or developing adhesions are those with previous or planned adnexal interventions, ablation of endometriosis, or bowel surgery involving large peritoneal defects, together with all those who have undergone previous abdominal surgery with pronounced formation of adhesions.
The abdominal cavity is the internal area of the body between the chest and hips that contains the lower part of the esophagus, stomach, small intestine, and large intestine. The esophagus carries food and liquids from the mouth to the stomach, which slowly pumps them into the small and large intestines. Abdominal adhesions can kink, twist, or pull the small and large intestines out of place, causing an intestinal obstruction. Intestinal obstruction, also called a bowel obstruction, results in the partial or complete blockage of movement of food or stool through the intestines.
The peritoneum primarily consists of mesothelial cells, which are associated with coagulation, fibrinolysis, and the synthesis and degradation of the extracellular matrix; they host defense capabilities as well.
Since intra-abdominal adhesions arise from aberrant peritoneal wound healing processes, any mesothelial damage by surgical trauma or bacterial inflammation can lead to their formation 28. Damage to the peritoneum is followed by capillary bleeding and increased vascular permeability with consequent exudation of fibrinogen 29. After cleavage of fibrinogen to fibrin and its bonding with fibronectin the defect is closed and a temporary wound bed forms 28. Within the ensuing 72 hours endogenous fibrinolytic activity of the mesothelial cells leads to breakdown of these fibrin deposits and thus to complete regeneration 30. Under physiological conditions, fibrinogen and fiber protease inhibitors are equally released by mesothelial cells 31. However, under pathological conditions, such as mechanical injury, tissue ischemia, extrinsic oppression and peritonitis, an increase in inflammatory cells and fibro-serous effusion/exudation leads to fibrin deposition and abdominal adhesion, thereby ultimately inducing abdominal pain and/or hiccups 32. A key role in the origin of adhesions is attributed to a pathological reduction in peritoneal fibrinolysis capacity 33. This may result from destruction of mesothelia, from their insufficient supply with blood, from increased synthesis of fibrinolysis antagonists following trauma, from hypoxia, from radical formation, or from bacterial infection 28. Of patients who undergo abdominal surgery, 93 percent develop abdominal adhesions 34. Surgery in the lower abdomen and pelvis, including bowel and gynecological operations, carries an even greater chance of abdominal adhesions. Abdominal adhesions can become larger and tighter as time passes, sometimes causing problems years after surgery.
Key facts
- Adhesions result from peritoneal trauma and aberrant wound healing processes and can therefore develop after any intra-abdominal operation.
Intra-abdominal adhesions occur in 50 to 100% of patients with previous surgery. - Abdominal adhesions are bands of fibrous tissue that can form between abdominal tissues and organs. Abdominal adhesions cause tissues and organs in the abdominal cavity to stick together.
- Abdominal surgery is the most frequent cause of abdominal adhesions. Of patients who undergo abdominal surgery, 93 percent develop abdominal adhesions.
- In most cases, abdominal adhesions do not cause symptoms. When symptoms are present, chronic abdominal pain is the most common.
- A complete intestinal obstruction is life threatening and requires immediate medical attention and often surgery.
- Abdominal adhesions cannot be detected by tests or seen through imaging techniques such as x-rays or ultrasound. However, abdominal x-rays, a lower gastrointestinal (GI) series, and computerized tomography (CT) scans can diagnose intestinal obstructions.
- Surgery is the only way to treat abdominal adhesions that cause pain, intestinal obstruction, or fertility problems.
A person with these symptoms should seek medical attention immediately.
A complete intestinal obstruction is life threatening and requires immediate medical attention and often surgery.
Symptoms of an intestinal obstruction include
- severe abdominal pain or cramping
- nausea
- vomiting
- bloating
- loud bowel sounds
- abdominal swelling
- the inability to have a bowel movement or pass gas
- constipation—a condition in which a person has fewer than three bowel movements a week; the bowel movements may be painful
Types of abdominal adhesions
Intra-abdominal adhesions may be congenital or acquired. Congenital adhesions arise during physiological organogenesis—like the frequently observed attachment of the sigmoid colon to the left pelvic wall—or can be traced back to abnormal embryonal development of the abdominal cavity. They are usually asymptomatic and are diagnosed incidentally 35.
Postmortem examination of patients who had not undergone surgery identified postinflammatory adhesions in 28% of cases 36. These are caused by intra-abdominal inflammation or can be attributed to endometriosis, peritonitis, radiotherapy, or long-term peritoneal dialysis 37.
Postoperative adhesions form after 50% to 100% of all abdominopelvic interventions 38. They develop as a result of wound healing and are influenced by various factors (see Table 1) 39.
Overview of factors that influence the formation of adhesions 1:
- Complexity of operation
- Extent of peritoneal trauma
- Previous illness (e.g., diabetes)
- Poor nutritional status
- Intra-abdominal placement of foreign bodies (e.g. meshes)
- Excessive coagulation with tissue necrosis
- Accompanying bacterial infection
- Laparoscopy
- Dehydration owing to high insufflation pressure and compression of capillary flow
- Laparoscopy
- Dehydration owing to dry gas
- Laparoscopy
- Mesothelial hypoxia owing to use of CO2
- Laparotomy
- Dehydration owing to light and heat
- Laparotomy
- Exposure to foreign material (e.g., glove powder)
- Laparotomy
- Mesothelial dehydration and abrasion from use of dry abdominal drapes.
Abdominal adhesions causes
Abdominal surgery is the most frequent cause of abdominal adhesions. Surgery-related causes include:
- cuts involving internal organs
- handling of internal organs
- drying out of internal organs and tissues
- contact of internal tissues with foreign materials, such as gauze, surgical gloves, and stitches
- blood or blood clots that were not rinsed away during surgery
Abdominal adhesions can also result from inflammation not related to surgery, including
- appendix rupture
- radiation treatment
- gynecological infections
- abdominal infections
Rarely, abdominal adhesions form without apparent cause.
Can abdominal adhesions be prevented?
Abdominal adhesions are difficult to prevent; however, certain surgical techniques can minimize abdominal adhesions.
Laparoscopic surgery decreases the potential for abdominal adhesions because several tiny incisions are made in the lower abdomen instead of one large incision. The surgeon inserts a laparoscope—a thin tube with a tiny video camera attached—into one of the small incisions. The camera sends a magnified image from inside the body to a video monitor. Patients will usually receive general anesthesia during this surgery.
If laparoscopic surgery is not possible and a large abdominal incision is required, at the end of surgery a special filmlike material can be inserted between organs or between the organs and the abdominal incision. The filmlike material, which looks similar to wax paper and is absorbed by the body in about a week, hydrates organs to help prevent abdominal adhesions.
Other steps taken during surgery to reduce abdominal adhesions include:
- using starch- and latex-free gloves
- handling tissues and organs gently
- shortening surgery time
- using moistened drapes and swabs
- occasionally applying saline solution
Practical tips: general strategies for reduction of adhesions 1:
- Preference for tissue-sparing and microinvasive surgical techniques
- Minimization of operating time and of heat and light
- Avoidance of peritoneal trauma by superfluous contact and coagulation
- Limited placement of intra-abdominal foreign bodies such as patches, meshes, and suture material
- Use of moistened abdominal drapes and swabs and occasional application of saline solution to minimize dehydration of mesothelial surfaces
- Irrigation of the abdominal cavity to remove residual intra-abdominal blood depots
- Reduction of infection risk by ensuring sterile working conditions and giving antibiotics as required
- Laparotomy: preferential use of latex- and powder-free gloves
- Laparoscopy: use of humidified gases at appropriately low insufflation pressure
- High-risk patients: use of barrier techniques or peritoneal instillates after appropriate explanation.
Eating, Diet, and Nutrition
Researchers have not found that eating, diet, and nutrition play a role in causing or preventing abdominal adhesions. A person with a partial intestinal obstruction may relieve symptoms with a liquid or low-fiber diet, which is more easily broken down into smaller particles by the digestive system.
Abdominal adhesions symptoms
In most cases, abdominal adhesions do not cause symptoms. When symptoms are present, chronic abdominal pain is the most common.
Abdominal adhesions complications
Abdominal adhesions can cause intestinal obstruction and female infertility—the inability to become pregnant after a year of trying.
Abdominal adhesions can lead to female infertility by preventing fertilized eggs from reaching the uterus, where fetal development takes place. Women with abdominal adhesions in or around their fallopian tubes have an increased chance of ectopic pregnancy—a fertilized egg growing outside the uterus. Abdominal adhesions inside the uterus may result in repeated miscarriages—a pregnancy failure before 20 weeks.
Abdominal adhesions diagnosis
Abdominal adhesions cannot be detected by tests or seen through imaging techniques such as x-rays or ultrasound. Most abdominal adhesions are found during surgery performed to examine the abdomen. However, abdominal x-rays, a lower gastrointestinal (GI) series, and computerized tomography (CT) scans can diagnose intestinal obstructions.
- Abdominal x-rays use a small amount of radiation to create an image that is recorded on film or a computer. An x-ray is performed at a hospital or an outpatient center by an x-ray technician, and the images are interpreted by a radiologist—a doctor who specializes in medical imaging. An x-ray does not require anesthesia. The person will lie on a table or stand during the x-ray. The x-ray machine is positioned over the abdominal area. The person will hold his or her breath as the picture is taken so that the picture will not be blurry. The person may be asked to change position for additional pictures.
- A lower GI series is an x-ray exam that is used to look at the large intestine. The test is performed at a hospital or an outpatient center by an x-ray technician, and the images are interpreted by a radiologist. Anesthesia is not needed. The health care provider may provide written bowel prep instructions to follow at home before the test. The person may be asked to follow a clear liquid diet for 1 to 3 days before the procedure. A laxative or an enema may be used before the test. A laxative is medication that loosens stool and increases bowel movements. An enema involves flushing water or laxative into the rectum using a special squirt bottle. For the test, the person will lie on a table while the radiologist inserts a flexible tube into the person’s anus. The large intestine is filled with barium, making signs of underlying problems show up more clearly on x-rays.
- CT scans use a combination of x-rays and computer technology to create images. The procedure is performed at a hospital or an outpatient center by an x-ray technician, and the images are interpreted by a radiologist. Anesthesia is not needed. A CT scan may include the injection of a special dye, called contrast medium. The person will lie on a table that slides into a tunnel-shaped device where the x-rays are taken.
Abdominal adhesions treatment
Abdominal adhesions that do not cause symptoms generally do not require treatment. Surgery is the only way to treat abdominal adhesions that cause pain, intestinal obstruction, or fertility problems. More surgery, however, carries the risk of additional abdominal adhesions. People should speak with their health care provider about the best way to treat their abdominal adhesions.
Complete intestinal obstructions usually require immediate surgery to clear the blockage. Most partial intestinal obstructions can be managed without surgery.
Pelvic adhesions
Pelvic adhesions are bands of scarlike tissue that form between two surfaces inside the body. Inflammation from infection, surgery, or trauma can cause tissues to bond to other tissues or organs. The management of pelvic adhesions in women with chronic pelvic pain is controversial 40; whilst some gynecologists will routinely perform adhesiolysis in the presence of adhesions, others do not 40. Previous studies 41, 42 have shown that adhesiolysis may only benefit a subgroup of yet uncharacterized patients.
There is some evidence to suggest that dense and vascular adhesions are more likely to result in pain, and the traditional belief that adhesions attached to pain sensitive structures such as ovaries are more likely to result in more pain 41. However, a study by Rapkin et al. 43, did not find an association between density or site of adhesions and pelvic pain. A retrospective study by Steege and Stout 44 found the presence of psychosocial compromise was associated with a lack of improvement with adhesiolysis, suggesting perhaps the ‘adhesion phenotype’ may relate, not just to the physical-mechanistic aspect of adhesions, but also to the psychosocial characteristic of the patients.
The presence, site and nature of adhesions observed intra-operatively is often unpredictable pre-operatively and whilst one expects patients with previous laparotomy to have more abdominal or pelvic adhesions than those who had previous laparoscopic surgery, laparotomy and laparoscopy are associated with comparable risks of adhesion related operative and non-operative morbidity 45. There is only moderate correlation between skin scar characteristics and intra-abdominal adhesions 46. Moreover, significant number of patients in the aforementioned study did not have chronic pelvic pain and many with chronic pelvic pain may not have had previous surgery.
Whilst clinical phenotyping has been shown to be helpful for the management of patients with chronic pain syndromes 47, a clinical phenotype for adhesive disorders in terms of pain, physical, emotional and functional characteristics of women presenting with adhesions and non-adhesions related chronic pelvic pain has not yet been defined. Identifiable clinical characteristics may help facilitate better surgical decisions making and serve as better tools for pre-operative counseling.
In this study 40, researchers found that there is little or no correlation between women with chronic pelvic pain, physical, emotional and functional characteristics scores with the presence or absence of pelvic adhesions/intra-abdominal found during investigative laparoscopy. Most women who had adhesions had the lowest reported current pain scores. It is, therefore important clinicians place due attention to the fact that history and evaluation of patients with chronic pelvic pain must include cognitive, emotional behavior and other physical assessments prior to embarking on surgical management.
Uterine adhesions
Intrauterine adhesions also known as Asherman syndrome, is the formation of scar tissue inside the uterine cavity and/or cervix, leading to infertility or changes to your menstruation (e.g., amenorrhea, dysmenorrhea and oligomenorrhea) 48. In many cases the front and back walls of the uterus stick to one another. In other cases, adhesions only occur in a small portion of the uterus. The extent of the adhesions defines whether the case is mild, moderate, or severe. The adhesions can be thin or thick, spotty in location, or confluent. They are usually not vascular, which is an important attribute that helps in treatment.
Women with Asherman syndrome often experience reduced menstrual flow, increased cramping and abdominal pain, eventual cessation of menstrual cycles (amenorrhea), and, in many instances, infertility. Most often these symptoms are the result of severe inflammation of the lining of the uterus (endometriosis) that is caused by the development of bands of scar tissue that join parts of the walls of the uterus to one another, thus reducing the volume of the uterine cavity (intrauterine adhesions and synechiae). Endometrial scarring and intrauterine adhesions may occur as a result of surgical scraping or cleaning of tissue from the uterine wall (dilatation and curettage [D and C]), infections of the endometrium (e.g., tuberculosis), or other factors. Such changes may have an effect on implantation and may lead to infertility since the hypotrophic endometrium becomes unreceptive to an embryo resulting in recurrent pregnancy loss 49. Obstetrical complications and recurrent miscarriages have been reported with Asherman syndrome 50.
Asherman syndrome is also known as uterine atresia, amenorrhea traumatica, endometrial sclerosis, and intrauterine adhesions or synechiae 51. In most cases, Asherman syndrome occurs in women who have had several dilatation and curettage (D&C) procedures. A severe pelvic infection unrelated to surgery may also lead to Asherman syndrome. Intrauterine adhesions can also form after infection with tuberculosis or schistosomiasis 52. These infections are rare in the United States. Uterine complications related to these infections are even less common. The true incidence of Asherman syndrome remains unknown since the majority of the patients are asymptomatic 53.
Asherman syndrome is thought to be under-diagnosed because it is usually undetectable by routine diagnostic procedures such as an ultrasound scan. The condition is estimated to affect 1.5% of women undergoing a hysterosalpingogram (HSG) 54, between 5 and 39% of women with recurrent miscarriage 55, and up to 40% of patients who have undergone D&C for retained products of conception following childbirth or incomplete abortion 56.
Intrauterine adhesions can lead to partial or complete dysfunction of the endometrium with impairment of fertility and menstrual pattern (amenorrhea and hypomenorrhea) 57. When the adhesions are exclusively located in the lower uterine tract and functioning endometrium persists, Asherman syndrome can also cause severe pelvic pain and retrograde menstruation.
Pregnant or early pregnant uterus seems to be more susceptible to develop uterine scarring after curettage 57. Nonetheless any uterine insult or trauma following even less invasive surgical procedure can lead to intrauterine adhesions development.
The impact of the Asherman syndrome on pregnancy is well documented with a high rate of infertility, miscarriage, poor implantation following in vitro fertilization and abnormal placentation 58.
Although the first case of intrauterine adhesion was published in 1894 by Heinrich Fritsch 59, it was only after 54 years that a full description of Asherman syndrome was carried out by Israeli gynaecologist Joseph Asherman 57. Specifically, he identified this pathology in 29 women who showed amenorrhea with stenosis of internal cervical ostium 60. The author speculated that such a manifestation could be a consequence of endometrium trauma. Two years later, he published another case series of intrauterine adhesions, this time involving the uterine cavity and characterized by evident filling defects during hysterography 61.
The cause of Asherman syndrome is not clear 53; however, an event that causes damage to the endometrium can lead to the development of adhesions. Asherman syndrome most frequently occur after repeated dilatation and curettage (D&C), postpartum hemorrhage (PPH), and elective abortion 53. Additionally, Asherman syndrome may occur after a simple operation on the uterus like a cesarean section and myomectomy 62. Presently, Asherman syndrome is known to be Asherman syndromesociated with nontraumatic factors, for example, puerperal sepsis 63, infections such Asherman syndrome tuberculous endometritis, and even after a normal delivery 64.
Direct visualization of the uterus via hysteroscopy is the most reliable method for diagnosis. Hysteroscopic adhesiolysis is the treatment of choice for the management of intrauterine adhesions 65. Hysteroscopic adhesiolysis can be an effective treatment of Asherman syndrome, with an overall pregnancy rate of 40% following surgery 66.
Grades of uterine adhesions severity
Society for Hysteroscopy, 1989 67:
- I – Thin or filmy adhesions easily ruptured by hysteroscope sheath alone, cornual areas normal;
- II – Singular firm adhesions connecting separate parts of the uterine cavity, visualization of both tubal ostia possible, cannot be ruptured by hysteroscope sheath alone;
- IIa – Occluding adhesions only in the region of the internal cervical OS. Upper uterine cavity normal;
- III – Multiple firm adhesions connecting separate parts of the uterine cavity, unilateral obliteration of ostial areas of the tubes;
- IIIa – Extensive scarring of the uterine cavity wall with amenorrhea or hypomenorrhea;
- IIIb – Combination of III and IIIa;
- IV – Extensive firm adhesions with agglutination of the uterine walls. Both tubal ostial areas occluded
Valle and Sciarra’s 1988 classification
- Mild- Filmy adhesions composed of basal endometrium producing partial or complete uterine cavity occlusion;
- Moderate – Fibromuscular adhesions that are characteristically thick, still covered by endometrium that may bleed on division, partially or totally occluding the uterine cavity;
- Severe – Composed of connective tissue with no endometrial lining and likely to bleed upon division, partially or totally occluding the uterine cavity.
Donnez and Nisolle 1994 classification
- I – Central adhesions
- a) thin filmy adhesions (endometrial adhesions)
- b) myofibrous (connective adhesions)
- II – Marginal adhesions (always myofibrous or connective)
- a) wedge like projection
- b) obliteration of one horn
- III – Uterine cavity absent on hysterosalpingogram
- a) occlusion of the internal os (upper cavity normal)
- b) extensive agglutination of uterine walls (absence of uterine cavity – true Asherman’s)
Uterine adhesions symptoms
Most patients with intrauterine adhesions present with scanty or absent menstrual blood flows (amenorrhea). In a few instances, the menstrual cycle may be normal. In some instances, the affected individual may experience an interrupted menstrual blood flow with substantial pain. Some patients have no periods but feel pain at the time that their period would normally arrive each month. This pain may indicate that menstruation is occurring but the blood cannot exit the uterus because the cervix is blocked by adhesions. Recurrent miscarriage and infertility could also be symptoms 68.
This may occur as a result of blockage of the cervix (the neck of the uterus) by adhesions. Recurrent miscarriages and/or infertility may also be signs of intrauterine adhesions.
Menstrual cycle of a woman with intrauterine adhesions
The hormonal influences remain the same; however, scar tissue inside the uterus will generally prevent the full scope of uterine changes. One theory suggests that the lack of endometrial growth is due to a complex biochemical feedback loop in which the uterus restricts the proliferation of endometrium. Others include significant damage to the basal layer of the endometrium (critical for the growth of the functional layer) or reduced blood flow to the endometrium either from fibrosis of the muscular layer of the uterus or because the scars which seal the uterus closed (partially or completely) reduce blood flow to the endometrium.
Hysteroscopy with lysis of the scar tissue can in many cases help restore normal uterine function in terms of endometrial response to hormonal stimulation. Unfortunately sometimes even after the scar tissue is resected the prior damage to the endometrium will lead to a chronically thin endometrium (i.e., it will undergo the correct proliferative and secretory changes, however full endometrial growth potential will not be recognized). This may be due to a complex chemical feedback mechanism not yet fully understood by medical science.
Thin endometrium is counteracted by a short course of sequential exogenous estrogen to encourage endometrial re-epithilialization (regrowth) and progesterone. In many cases once the uterus is mostly free of scar tissue this course of action can help restore more normal uterine function, and decrease the chance of rescarring.
In a few cases however the endometrial basal layer has been so badly denuded (scarred) that estrogen therapy will not help. This result is more common after fibroid removal surgery.
What causes intrauterine adhesions
Intrauterine adhesions occurs when trauma to the endometrial lining triggers the normal wound-healing process, which causes the damaged areas to fuse together. Most commonly, intrauterine adhesions occur after a dilation and curettage (D&C) that was performed because of a missed or incomplete miscarriage, retained placenta with or without hemorrhage after a delivery, or elective abortion. Pregnancy-related D&Cs have been shown to account for 90% of Asherman’s Syndrome cases 69. Sometimes adhesions also occur following other pelvic surgeries such as Cesarean section, surgery to remove fibroids or polyps, or in the developing world, as a result of infections such as genital tuberculosis 70 and schistosomiasis 71.
There is a 25% risk of developing intrauterine adhesions from a D&C that is performed 2 to 4 weeks after delivery 72. Dilation and Curettages (D&C) may also lead to intrauterine adhesions in 30.9% of procedures for missed miscarriages 73 and 6.4% of procedures for incomplete miscarriages 69. The risk of Asherman syndrome increases with the number of D&Cs performed; after a single termination the risk is 16%, however, after 3 or more D&Cs, the risk increases to 32% 74. Each case of intrauterine adhesions is different, and the cause must be determined on a case-by-case basis. In some cases, intrauterine adhesions may have been caused by an “overly aggressive” D&C. However, this is not often considered to be the case. The placenta may have attached very deeply in the endometrium or fibrotic activity of retained products of conception could have occurred, both of which make it difficult to remove retained tissue.
There is a variant of intrauterine adhesions called “Unstuck Asherman” or endometrial sclerosis that is more difficult to treat. In this condition, which may coexist with the presence of adhesions, the uterine walls are not stuck together. Instead, the endometrium has been denuded. Although curettage can cause this condition, it is more likely after uterine surgery, such as myomectomy. In these cases the endometrium, or at least its basal layer, has been removed or destroyed.
Sporadic inflammation of the mucous membrane lining the uterus (endometriosis), endometritis or endometriosis caused by a tuberculosis infection or certain other infectious diseases may also be causes of Asherman’s syndrome.
Intrauterine adhesions are also seen as a consequence of congenital uterine abnormalities, the use of IUD (intrauterine device) birth control devices and genetic predisposition 75. It is well-known that the endometrium is more susceptible to trauma in a pregnant uterus and the incidence of intrauterine adhesions following curettage for retained placental tissue is reported up to 40% 3 months after curettage 76. In patients who develop Asherman syndrome, curettage of a pregnant uterus has been shown as the cause in 64% of the cases.
Uterine adhesions prevention
Incidence of intrauterine adhesions might be lower following medical evacuation (e.g. misoprostol) of the uterus, thus avoiding any intrauterine instrumentation.
Also, the use of systematic antibiotics after D&C to avoid sub-clinical infections, likely a cofactor in the genesis of scarring, might be beneficial.
There is no evidence to suggest suction D&C is less likely to result in adhesions than sharp D&C. Cases of Asherman syndrome have been reported even following manual evacuation, and the rate of Asherman syndrome has not dropped since the introduction of suction D&C.
Uterine adhesions diagnosis
Unless the physician is careful, the diagnosis of intrauterine adhesions may be needlessly overlooked. A simple X-ray of the uterus with a small tube placed in the cervix is usually diagnostic. However, many physicians will, in order to save time, use a small balloon catheter placed in the uterus. The latter technique will overlook a number of cases of intrauterine adhesions. The gold standard for diagnosis uses a hysteroscope that pictures the interior of the uterus directly.
- Blood tests: Estrogen, luteinizing hormone (LH), progesterone, follicle-stimulating hormone (FSH), thyroid, prolactin to rule out hormonal problems affecting ovulation. Some women may not want to jump into hysteroscopy or hysterosalpingogram (HSG), which are not without some risks.
- Gold standard for diagnosis: diagnostic hysteroscopy
- Imaging tests:
- Ultrasound to measure endometrial thickness at ovulation and to check normal follicle development
- Sonohysterography (SHG) or hysterosalpingogram (HSG) these procedures are not as accurate as hysteroscopy, but will confirm patency of tubes if infertility is a problem
Uterine adhesions chances of pregnancy
Pregnancy success following intrauterine adhesions treatment is around 90% for stage I, 60% for stage II and 30% for stage III with pregnancy after stage IV uncommon 77.
Pregnancy risks after treatment of intrauterine adhesions include: small for dates, slightly higher incidence of miscarriage and preterm delivery, and 5% risk of major haemorrhagic complication at delivery due to placenta accreta.
Pregnancy with untreated uterine adhesions and the risks
For women who become pregnant BEFORE having had the chance to correct their intrauterine adhesions:
- If a woman gets pregnant with adhesions still in her uterus, the risks are different. Getting pregnant with adhesions remaining in the uterus is not recommended because the chances of miscarriage, problems with fetal growth and/or other serious complications of pregnancy are high.
- If you become pregnant before your intrauterine adhesion has been treated, it is imperative that you speak to your doctor right away and that you be closely monitored. Even then, your risk of complications is significantly higher than if you wait and become pregnant after you are cleared by your doctor.
RECOMMENDED MONITORING FOR PREGNANCIES WITH ADHESIONS PRESENT IN UTERUS:
- Weeks 4 to 7: Regular human chorionic gonadotropin (hCG) and progesterone blood tests
- Weeks 6 to 10: Regular ultrasounds, the first at approximately around 6 weeks. This ultrasound will verify that the baby is in the right place (not in a fallopian tube) and is growing normally. Subsequent ultrasounds could be performed weekly in order to identify a normal heartbeat and to verify good interval growth of the baby and that all is going well. If a miscarriage happens, the doctor can get onto it straight away. There should be no delay as this can cause further damage to your uterus.
- Weeks 18 to 20: Regular bi-weekly scans in order to evaluate for an incompetent cervix which is often a risk for those with post-Asherman syndrome pregnancies because of multiple prior cervical dilatations. It involves performing ultrasounds in order to measure cervical length and to detect “funneling” of the fetal membranes. These ultrasound examinations need to be performed by someone with the skill needed to detect these changes which are often subtle. If placental abnormalities or inadequate fetal growth are not detected, the patient can be monitored by a routine schedule.
- Third trimester: An evaluation, often by MRI, in order to detect placenta accreta, increta or percreta. Continued assessment to identify IUGR (intrauterine growth restriction of the fetus) as well as placenta previa. If any of these complications are identified, you should be monitored more closely.
Pregnancy AFTER uterine adhesions diagnosis
In some cases pregnancy may occur spontaneously and without complication depending on the severity of uterine adhesions. In some cases where the endometrium has been badly denuded down to the basal layer a condition called “Unstuck Asherman’s” can occur and the endometrium will fail to respond to hormonal influence, even in the presence of exogenous estrogen. In this case surrogacy is the only viable option for a biological child.
In some cases pregnancy can occur with the aid of fertility drugs and/or treatments such as IVF (in vitro fertilization). Pregnancy after treatment for Asherman syndrome will greatly increase the chance of placental implantation issues such as placenta previa (low lying placenta growing over the cervix which mandates a cesarean section and which can be caused by previous scarring of the fundus of the uterus), placenta accreta (placenta attaches too deeply causing problems with placental removal and hemorrhage at the time of delivery), cervical incompetence secondary to multiple dilations of the cervix from multiple curettages and/or multiple hysteroscopies to treat the Asherman’s and abnormal fetal lie (the fetus lies is and grows in an abnormal position presumably due to abnormal and restricted uterine shape). These conditions can be detected during routine ultrasound.
A recent study by Roy et al. 66 reported pregnancy rates of 58% in mild Asherman, 30% in moderate Asherman, and 33.3% in severe cases of Asherman syndrome following hysteroscopic adhesiolysis. Furthermore, the live birth rate reported was 86.1%, the miscarriage rate 11.1% and the cumulative pregnancy rate showed that 97.2% of the patients conceived within 24 months postoperatively 66.
Studies evaluating obstetric complications in pregnant women with previous intrauterine adhesions are sparse. A number of cases have been reported but large observational studies are lacking. In 1982, Schenker and Margalioth 78 found an incidence of placenta accreta in 13–14% of patients with previous Asherman syndrome. Roy et al. 66 reported an incidence of postpartum hemorrhage due to adherent placenta in 12.5% of the 89 women who had undergone hysteroscopic adhesiolysis due to intrauterine adhesions. These numbers present a remarkably low incidence of abnormal placentation in a group of women, who all had known trauma of the endometrium (i.e., 87.5% did not have an adherent placenta and subsequent postpartum hemorrhage).
Conventional 2 dimensional ultrasonography is currently the best screening tool for detecting placenta accreta with a sensitivity of 77–90.7%, a specificity of 96–98%, a positive predictive value of 65–93%, and a negative predictive value of 98% 79. Another diagnostic tool for detection of abnormal placentation is Magnetic Resonance Imaging (MRI). MRI has a sensitivity of 80–85% and a specificity of 65–100% 80. MRI can be used in conjunction with conventional ultrasonography 81, and can be helpful in some cases, especially if the placenta is located on the posterior uterine wall. Though it is important to notice, that for cases of placenta accreta, MRI is not a good prognostic tool for changing of surgical management 82. Retrospective studies have shown that women with an antenatal diagnosis of placenta accreta have less blood loss and requirement for blood transfusion than women in whom the abnormal placentation was diagnosed during cesarean section 83.
Any patient with a previous history of intrauterine surgery or intrauterine adhesions should be thoroughly examined by a skilled sonographer for possible abnormal placentation. In case of any suspicion of abnormal placentation, the patient should be scheduled for planned cesarean section with a set-up of skilled clinicians due to risk of severe postpartum hemorrhage.
In conclusion, it is important to be aware of the risk of a placenta accreta in patients with a previous history of Asherman syndrome and uterine scarring. Antenatal diagnosis is important to counsel the pregnant woman and to plan mode of delivery.
Pregnancy Risks in a Post-intrauterine adhesions pregnancy
You should ask your doctor about the risks involved in a post-Asherman syndrome pregnancy. Also, note that the information provided here applies only to women who have had their adhesions removed. If a woman gets pregnant with adhesions still in her uterus, the risks are different. Getting pregnant with adhesions remaining in the uterus is usually not recommended.
What are the risks involved in a post-intrauterine adhesions pregnancy?
The risks involved in a post-Asherman syndrome pregnancy vary, depending on how severe the Asherman’s was. If the Asherman syndrome was very mild, then after the adhesions are removed, pregnancy is statistically no riskier than for a woman who never had Asherman syndrome. Obviously this can vary in individual cases, but the statistics show no increased risk.
If the Asherman syndrome was moderate or severe, there is some increased risk in subsequent pregnancies.
The risks are:
- First-trimester miscarriage. This is a significant risk in any pregnancy, but it’s higher after Asherman syndrome .
- Placenta accreta. One study of 137 post-Asherman syndrome pregnancies found a 9% risk of placenta accreta. Placenta accreta means the placenta grows into the wall of the uterus. This isn’t harmful to the baby, but after the baby is born, when it’s time for the placenta to come out, if you have placenta accreta the placenta will be “stuck” and not come out. The doctor might have to do a D&C to remove it. In the worst cases of placenta accreta, in which they cannot get the placenta out without causing the mother to hemorrhage uncontrollably, they sometimes have to do a hysterectomy. Several members of the Asherman syndrome message board have had placenta accreta but most just needed a D&C, not a hysterectomy. Note that when they do the hysterectomy, they can usually just remove the uterus but leave the ovaries in place, which means you’ll continue to ovulate each month, and you won’t go through menopause until you reach the normal age for menopause. (Of course you won’t get a period, but that’s not the same as menopause.) Besides placenta accreta, placenta increta and placenta percreta are also risks, although they are extremely rare. means the placenta grows even more deeply and firmly into the uterine wall than placenta accreta. means the placenta grows all the way through the wall of the uterus and sometimes extends to nearby organs.
- Placenta previa. This means low-lying placenta. Normally the placenta should implant high up in your uterus but if the uterine walls aren’t in good condition (for example due to you having had Asherman syndrome ), sometimes the placenta implants lower, sometimes even covering the cervix. Placenta previa can increase your risk for bleeding during pregnancy, increase the risk of preterm delivery, and increase the risk of harm to mother and baby due to blood loss during delivery. However, if discovered early, more than 99% of mothers with placenta previa are just fine, and most of the babies come out of it just fine too. You could end up on bed rest for a substantial part of the pregnancy though. Fortunately the risk of placenta previa seems to be much lower than the risk of placenta accreta. Some of the A-list doctors think placenta previa is not a significant risk at all, if you’ve had all your adhesions removed. We have seen it occasionally on the Asherman’s message board though.
- Incompetent cervix (this is where the cervix dilates way too early in the pregnancy, resulting in loss of the baby or resulting in preterm delivery, depending on what week of pregnancy you’re in). Asherman’s doesn’t increase your risk of incompetent cervix, but having multiple D&Cs does increase the risk. Most doctors think having a lot of hysteroscopies doesn’t increase the risk, but a few doctors think it does. So it’s wise to be monitored for incompetent cervix if you get pregnant, just in case.
- Some perinatologists say that Asherman syndrome theoretically increases the risk of intrauterine growth retardation (IUGR), which is where the fetus doesn’t grow as well as it should, possibly due to placental insufficiency, but we have never seen a case of post- Asherman’s IUGR on the Asherman’s message board.
- Vasa Previa. This is a rare (1:3000), heartbreaking condition which occurs when one or more of the baby’s placental or umbilical blood vessels cross the entrance to the birth canal beneath the baby. When the cervix dilates or the membranes rupture, the unprotected vessels can tear, causing rapid fetal hemorrhage. When the baby drops in to the pelvis, the vessels can be compressed, compromising the baby’s blood supply and causing oxygen deprivation.
Non Reproductive Consequences of Uterine adhesions
The reproductive consequences of Asherman syndrome, including infertility, recurrent miscarriage, intrauterine growth restriction, placenta accreta and others, are well known. However, for all women with intrauterine scarring and amenorrhea, including those who may have completed childbearing, there are other concerns. Although the lack of menstrual periods could be secondary to hormonal abnormalities, it is more likely caused by either complete destruction of the uterine lining or by obstruction of the cervix or lower portion of the uterus; thus, menses are either retained in the uterus (leading to pelvic pain and a condition called hematometra) or flow into the abdominal cavity leading to endometriosis. Women with Asherman’s syndrome may develop uterine cancer, either before or after menopause. This risk is NOT increased and may be lower than in the general population. However, the usual warning sign of uterine cancer is excessive uterine bleeding: those with obstructed menstrual flow cannot have that symptom even if they harbor a uterine growth. Therefore, pelvic ultrasound should be a routine part of their annual gynecologic visit.
Uterine adhesions treatment
First-line treatment of uterine adhesions is to remove the scar tissue and promote the growth of the endometrium to reduce the formation of new scar tissue. Hysteroscopic lysis of adhesions is the main method of treatment.
Hormone support therapy is used in almost all cases, except in stage I. Use of a stent to keep the uterine walls separated during the healing phase, is recommended in stages III or IV. Repeat intervention is common in stage III and IV. Increasingly early reintervention either with standard hysteroscopy, or most recently with flexible hysteroscopy, has been advocated.
Many physicians argue against the use of lasers or energy sources inside the uterus (this means removing scars with scissors rather than with energy-generating instruments such as resectoscopes or lasers, although not all surgeons agree with this) to remove the adhesions. These doctors claim that the use of small cutting devices is less likely to irritate the lining of the uterus or to cause infection. Adhesions have a tendency to reform, especially in more severe cases. There are different methods to prevent re-scarring after surgery for Asherman syndrome. Many surgeons prescribe estrogen supplementation to stimulate uterine healing and place a splint or balloon to prevent apposition of the walls during the immediate post-operative healing phase. Other surgeons recommend weekly in-office hysteroscopy after the main surgery to cut away any newly formed adhesions. As of yet, studies have not confirmed the method of treatment that is most likely to have a successful outcome, which would be one where the uterus/cervix remains scar-free and fertility is restored.
- You will need to have Hysteroscopic and possibly Laparascopic surgery to remove your adhesions. This is the most important phase of your treatment. Only a highly skilled surgeon with experience in AS should do this. Protecting your uterine lining is very important.
- After your surgery you will most likely have a balloon catheter inserted into your uterus, this is used to keep your uterine walls from adhering together during the healing process. Your doctor may want this to stay inside for 5-14 days. You will also take an antibiotic to prevent infection. Note, not all Dr.’s use a balloon.
- Once the balloon is removed you will be prescribed a regimen of estrogen and progesterone. The dose and length of this regimen will vary depending on your doctor.
- 2-3 months after your surgery you should have an HSG, SHG or diagnostic hysteroscopy to view the inside of your uterus and your fallopian tubes for remaining scar tissue.
- Subsequent surgery may be necessary.
Hormonal therapy is also used to encourage menstruation.
Treatment with estrogen has shown good results in preventing reformation of adhesions following hysteroscopic surgery for Asherman syndrome 84. Use of peroral estrogen gives better fertility and menstrual outcome when given in combination with ancillary treatment (IUD, balloon or hyaluronic acid) 79.
If you have healed from your surgery and your uterus is free of scar tissue your doctor may give you the “green light” to try and conceive. It is very important that this not be rushed and that your uterus is at least 90% free of scar tissue before getting pregnant. Some of the risks that you now face with carrying a child are: Placenta Previa, Placenta Accreta, Premature rupture of membranes and possibly incompetent cervix.
During your continued treatment your doctor may want to track your ovulation and measure your endometrial lining and follicles during ovulation. Your doctor may also suggest that you purchase a fertility monitor to pinpoint your ovulation day and schedule intercourse appropriately. Your doctor may also consider fertility medication. This is usually prescribed when you have a blocked tube or when blood tests indicate a hormonal imbalance. Fertility medication is not necessary for every woman with Asherman syndrome. If your doctor prescribes this for you, ask why and which type would be the best for you.
References- Brüggmann D, Tchartchian G, Wallwiener M, Münstedt K, Tinneberg HR, Hackethal A. Intra-abdominal adhesions: definition, origin, significance in surgical practice, and treatment options. Dtsch Arztebl Int. 2010;107(44):769-75. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992017/
- Holmdahl L, Eriksson E, al-Jabreen M, Risberg B. Fibrinolysis in human peritoneum during operation. Surgery. 1996;119:701–5.
- Çim N, Elçi E, Güneş Elçi G, Almalı N, Yıldızhan R. Are the skin scar characteristics and closure of the parietal peritoneum associated with pelvic adhesions?. Turk J Obstet Gynecol. 2018;15(1):28-32. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5894533/
- Holmdahl L, Eriksson E, Eriksson BI, Risberg B. Depression of peritoneal fibrinolysis during operation is a local response to trauma. Surgery. 1998;123:539–44.
- Tulandi T, Hum HS, Gelfand MM. Closure of laparotomy incisions with or without peritoneal suturing and second-look laparoscopy. Am J Obstet Gynecol. 1988;158:536–7.
- O’Leary DP, Coakley JB. The influence of suturing and sepsis on the development of post-operative peritoneal adhesions. Ann R Coll Surg Engl. 1992;74:134–7.
- Swanwick RA, Stockdale PHG, Milne FJ. Healing of parietal peritoneum in the horse. Br Vet J. 1973;129:29–35.
- Bamigboye AA, Hofmeyr GJ. Closure versus non-closure of the peritoneum at cesarean section. Cochrane Database Syst Rev. 2003;CD000163
- Lyell DJ, Caughey AB, Hu E, Daniels K. Peritoneal closure at primary cesarean delivery and adhesions. Obstet Gynecol. 2005;106:275–80.
- Pschera H, Kjaeldgaard A, Larsson B. Fibrinolytic activity in amniotic fluid during late pregnancy. Acta Obstet Gynecol Scand. 1986;65:417–20.
- Myers SA, Bennett TL. Incidence of significant adhesions at repeat cesarean section and the relationship to method of prior peritoneal closure. J Reprod Med. 2005;50:659–62.
- Roset E, Boulvain M, Irion O. Nonclosure of the peritoneum during caesarean section: long-term follow-up of a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. 2003;108:40–4.
- Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics: Part II. Strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol. 2012;66:13–24.
- Martin P, Dickson MC, Millan FA, Akhurst RJ. Rapid induction and clearance of TGF beta 1 is an early response to wounding in the mouse embryo. Dev Genet. 1993;14:225–38
- Lee TY, Chin GS, Kim WJ, Chau D, Gittes GK, Longaker MT. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–84.
- Salim R, Kadan Y, Nachum Z, Edelstein S, Shalev E. Abdominal scar characteristics as a predictor of intra-abdominal adhesions at repeat cesarean delivery. Fertil Steril. 2008;90:2324–7.
- Ferreira LM, Gragnani A, Furtado F, Hochman B. Control of the skin scarring response. An Acad Bras Cienc. 2009;81:623–9.
- Nissen FB, Spauwen PH, Robinson PH, Fidler V, Kon M. The use of silicone occlusive sheeting (Sil-K) and silicone occlusive gel (Epiderm) in the prevention of hypertrophic scar formation. Plast Reconstr Surg. 1998;102:1962–72.
- Salim R, Kadan Y, Nachum Z, Edelstein S, Shalev E. Abdominal scar characteristics as a predictor of intra-abdominal adhesions at repeat cesarean delivery. Fertil Steril. 2008;90:2324–7
- Levrant SG, Bieber E, Barnes R. Risk of anterior abdominal wall adhesions increases with number and type of previous laparotomy. J Am Assoc Gynecol Laparosc. 1994;1:S19
- Ashrafinia M, Vazirichimeh Z, Dastjerdi MV, Moiini A. Adhesion formation in patients with previous laparotomies. J Am Assoc Gynecol Laparosc. 1996;3:S2.
- Benefits and harms of adhesion barriers for abdominal surgery: a systematic review and meta-analysis. Ten Broek RPG, Stommel MWJ, Strik C, van Laarhoven CJHM, Keus F, van Goor H. Lancet. 2014 Jan 4; 383(9911):48-59.
- Regulation of expression of tissue plasminogen activator and plasminogen activator inhibitor-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Diamond MP, El-Hammady E, Wang R, Kruger M, Saed G. Am J Obstet Gynecol. 2004 Apr; 190(4):926-34.
- Contemporary adhesion prevention. diZerega GS. Fertil Steril. 1994 Feb; 61(2):219-35. https://www.ncbi.nlm.nih.gov/pubmed/8299773/
- Menzies D, Ellis H. Intestinal obstruction from adhesions: How big is the problem? Ann R Coll Surg Engl. 1990;72:60–63
- Pittaway DE, Daniell JF, Maxson WS. Ovarian surgery in an infertility patient as an indication for a short-interval second-look laparoscopy: a preliminary study. Fertil Steril. 1985;44:611–614.
- Diamond MP, Pellicer A, Boyers SP, DeCherney AH. The effect of periovarian adhesions on follicular development in patients undergoing ovarian stimulation for in vitro fertilization-embryo transfer. Fertil Steril. 1988;49:100–103
- Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. Reed KL, Fruin AB, Bishop-Bartolomei KK, Gower AC, Nicolaou M, Stucchi AF, Leeman SE, Becker JM. J Surg Res. 2002 Nov; 108(1):165-72.
- Peritoneal healing and adhesion formation/reformation. Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Hum Reprod Update. 2001 Nov-Dec; 7(6):556-66.
- Role of plasminogen activators in peritoneal adhesion formation. Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Biochem Soc Trans. 2002 Apr; 30(2):126-31.
- Yung S, Chan TM. Mesothelial cells. Perit Dial Int 2007;27:110–5.
- van den Beukel BA, de Ree R, van Leuven S, et al. Surgical treatment of adhesion-related chronic abdominal and pelvic pain after gynaecological and general surgery: a systematic review and meta-analysis. Hum Reprod Update 2017;23:276–88.
- Fibrinolysis in human peritoneum during operation. Holmdahl L, Eriksson E, al-Jabreen M, Risberg B. Surgery. 1996 Jun; 119(6):701-5.
- Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. Journal of Surgical Research. 2011;165(1):91–111.
- Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Dig Surg. 2001;18:260–273.
- Weibel MA, Mayno G. Peritoneal adhesions and their relationship to abdominal surgery. A postmortem study. Am J Surg. 1973;126:345–353.
- Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Human Reprod Update. 2001;7:556–566.
- DiZerega GS. Contemporary adhesion prevention. Fertil Steril. 1994;61:219–235.
- Monk BJ, Berman ML, Montz FJ. Adhesions after extensive gynecologic surgery: clinical significance, etiology and prevention. Am J Obstet Gynecol. 1994;170:1396–1403.
- Cheong Y, Saran M, Hounslow JW, Reading IC. Are pelvic adhesions associated with pain, physical, emotional and functional characteristics of women presenting with chronic pelvic pain? A cluster analysis. BMC Womens Health. 2018;18(1):11. Published 2018 Jan 8. doi:10.1186/s12905-017-0509-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759355/
- Peters AA, Trimbos-Kemper GC, Admiraal C, Trimbos JB, Hermans J. A randomized clinical trial on the benefit of adhesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. Br J Obstet Gynaecol. 1992;99(1):59–62. doi: 10.1111/j.1471-0528.1992.tb14394.x
- Cheong YC, Reading I, Bailey S, Sadek K, Ledger W, Li TC. Should women with chronic pelvic pain have adhesiolysis? BMC Womens Health. 2014;14(1):36. doi: 10.1186/1472-6874-14-36
- Rapkin AJ. Adhesions and pelvic pain: a retrospective study. Obstet Gynecol. 1986;68(1):13–15.
- Steege JF, Stout AL. Resolution of chronic pelvic pain after laparoscopic lysis of adhesions. Am J Obstet Gynecol. 1991;165(2):278–281. doi: 10.1016/0002-9378(91)90079-7
- Scholin J, Buunen M, Hop W, Bonjer J, Anderberg B, Cuesta M, et al. Bowel obstruction after laparoscopic and open colon resection for cancer: results of 5 years of follow-up in a randomized trial. Surg Endosc. 2011;25(12):3755–3760. doi: 10.1007/s00464-011-1782-2
- Stocker LJ, Glazebrook JE, Cheong YC. Are skin scar characteristics associated with the degree of pelvic adhesions at laparoscopy? Fertil Steril. 2014;101(2):501–505. doi: 10.1016/j.fertnstert.2013.10.026.
- Guan X, Zhao C, Ou ZY, Wang L, Zeng F, Qi L, et al. Use of the UPOINT phenotype system in treating Chinese patients with chronic prostatitis/chronic pelvic pain syndrome: a prospective study. Asian J Androl. 2015;17(1):120–123. doi: 10.4103/1008-682X.138189
- Intrauterine adhesions. March CM. Obstet Gynecol Clin North Am. 1995 Sep; 22(3):491-505.
- March CM. Asherman’s syndrome. Semin. Reprod. Med. 2011;29:83–94.
- Posttraumatic intrauterine synechiae and pregnancy. Forssman L. Obstet Gynecol. 1965 Nov; 26(5):710-3.
- Management of Asherman’s syndrome. March CM. Reprod Biomed Online. 2011 Jul; 23(1):63-76. https://www.ncbi.nlm.nih.gov/pubmed/21549641/
- Asherman syndrome. https://medlineplus.gov/ency/article/001483.htm
- Baradwan S, Baradwan A, Al-Jaroudi D. The association between menstrual cycle pattern and hysteroscopic march classification with endometrial thickness among infertile women with Asherman syndrome. Chatzistamatiou. K, ed. Medicine. 2018;97(27):e11314. doi:10.1097/MD.0000000000011314. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6076072/
- Dmowski WP, Greenblatt RB. Asherman?s syndrome and risk of placenta accreta. Obstet Gynecol 1969; 34:288-299.
- Ventolini G, Zhang M, Gruber J. Hysteroscopy in the evaluation of patients with recurrent pregnancy loss: a cohort study in a primary care population. Surg Endosc 2004; 18:1782-1784.
- Westendorp ICD, Ankum WM, Mol BWJ, Vonk J. Prevalence of Asherman?s syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum Reprod 1998; 13:3347-3350.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reproductive Biology and Endocrinology : RB&E. 2013;11:118. doi:10.1186/1477-7827-11-118. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3880005/
- Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;11:555–569. doi: 10.1016/j.jmig.2010.04.016
- Amenorrhoea traumatica (atretica). ASHERMAN JG. J Obstet Gynaecol Br Emp. 1948 Feb; 55(1):23-30.
- Asherman JG. Amenorrhoea traumatica (atretica) J Obstet Gynaecol Br Emp. 1948;11:23–30. doi: 10.1111/j.1471-0528.1948.tb07045.x
- Asherman JG. Traumatic intrauterine adhesions. J Obstet Gynaecol Br Emp. 1950;11:892–896. doi: 10.1111/j.1471-0528.1950.tb06053.x.
- Orhue AA, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet 2003;82:49–56.
- Polishuk WZ, Anteby SO, Weinstein D. Puerperal endometritis and intrauterine adhesions. Int Surg 1975;60:418–20.
- Zondek R, Rozin S. Filling defect in the hysterogram simulating intrauterine synechae which disappear after denervation. Am J Obstet Gynecol 1964;88:123–7.
- March C, Israel R. Intrauterine adhesions secondary to elective abortion. Hysteroscopic diagnosis and management. Obstet Gynecol 1976;48:422–4.
- Roy KK, Baruah J, Sharma JB, Kumar S, Kachawa G. Singh N. Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman’s syndrome. Arch. Gynecol. Obstet. 2010;281:355–361.
- Grades of Ashermans Syndrome Severity. http://www.ashermans.org/information/ashermans-grades
- Valle RF, and Sciarra JJ. Intrauterine adhesions: Hystreoscopic diagnosis, classification, treatment and reproductive outcome. Am J Obstet 1988; 158:1459-1470.
- Schenker JG, Margalioth EJ. Intra-uterine adhesions: an updated appraisal. Fertility Sterility 1982; 37:593-610.
- Netter AP, Musset R, Lambert A et al. Traumatic intrauterine synechiae: a common cause of menstrual insufficiency, sterility and abortion. Am J Obstet Gynecol 1956; 71:368.
- Krolikowski A, Janowski K, Larsen JV. Asherman syndrome caused by schistosomiasis.Obstet Gynecol. 1995; 85(5 Pt 2):898-9.
- Fedele L, Bianchi S, Frontino G. Septums and synechiae: approaches to surgical correction. Clin Obstet Gynecol 2006; 49:767-788.
- Adoni, A, Palti, Z, Milwidsky, A, and Dolberg, M. The incidence of intrauterine adhesions following spontaneous abortion. Int J Fertil 1982;27(2):117-8.
- Friedler S, Margalioth EJ, Karfka I, Yaffe H. Incidence of post-abortion intrauterine adhesions evaluated by hysteroscopy-a prospective study. Hum Reprod 1993; 8:442-4.
- March CM. Management of Asherman’s syndrome. Reprod. Biomed. Online. 2011;23:63–76.
- Westendorp IC, Ankum WM, Mol BW. Vonk J. Prevalence of Asherman’s syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum. Reprod. 1998;13:3347–3350.
- Asherman’s syndrome: A rare infertility disorder – Medical Observer 12 April 2013. https://jeanhailes.org.au/contents/documents/Resources/Medical__health_articles/Medical_Observer/2013/Ashermans_12_April_2013.pdf
- Schenker JG. Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertil. Steril. 1982;37:593–610.
- D’Antonio F, Iacovella C. Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013;42:509–517.
- Gielchinsky Y, Mankuta D, Rojansky N, Laufer N, Gielchinsky I. Ezra Y. Perinatal outcome of pregnancies complicated by placenta accreta. Obstet. Gynecol. 2004;104:527–530.
- Levine D, Hulka CA, Ludmir J, Li W. Edelman RR. Placenta accreta: evaluation with color Doppler US, power Doppler US, and MR imaging. Radiology. 1997;205:773–776.
- McLean LA, Heilbrun ME, Eller AG, Kennedy AM. Woodward PJ. Assessing the role of magnetic resonance imaging in the management of gravid patients at risk for placenta accreta. Acad. Radiol. 2011;18:1175–1180.
- Tikkanen M, Paavonen J, Loukovaara M. Stefanovic V. Antenatal diagnosis of placenta accreta leads to reduced blood loss. Acta Obstet. Gynecol. Scand. 2011;90:1140–6.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clinical Case Reports. 2015;3(3):175-178. doi:10.1002/ccr3.194. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4377250/