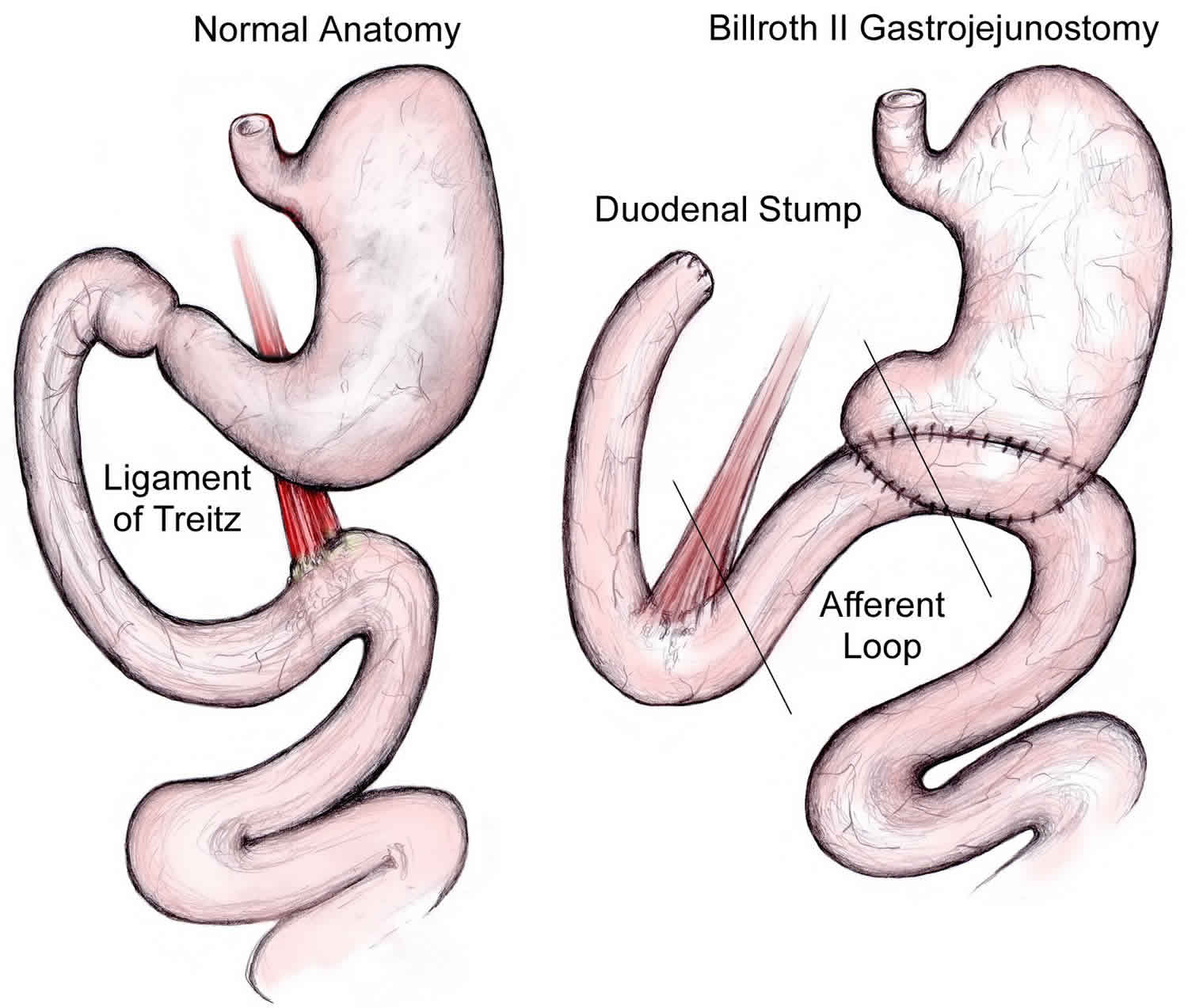
Afferent loop syndrome
Afferent loop syndrome is a rare complication of gastric surgery that results from partial or complete mechanical obstruction to the afferent jejunal loop caused by distension and accumulation of digestive juices in the afferent limb of the jejunum following creation of a gastrojejunostomy 1. Afferent loop syndrome results from mechanical obstruction at the anastomosis itself or at a point near the anastomosis 1. In general, afferent loop syndrome develops after distal gastrectomy following a Billroth 2 reconstruction 2. However, afferent loop syndrome can also occur after a Roux-en-Y reconstruction by stenosis or obstruction of “biliopancreatic limb” 3. Afferent loop syndrome has also been reported after other procedures of the foregut including pancreaticoduodenectomy. Most cases of afferent loop syndrome after pancreaticoduodenectomy, however, present with chronic symptoms 4 and although reports of acute afferent loop syndrome exist, these have occurred several years after the initial operation due to recurrence of malignancy at the anastomotic site 5.
Acute afferent loop syndrome results from complete obstruction, usually occurs early after surgery and runs a devastatingly lethal course unless promptly treated by reoperation. A delay in the diagnosis of afferent loop syndrome can result in serious consequences, particularly with acute afferent loop syndrome, because diagnostic delay can lead to bowel perforation or gangrene, with resultant intra-abdominal sepsis. The presentation of acute afferent loop syndrome, there is continuous upper abdominal pain, nausea and vomiting due to build up of secretions in the completely obstructed length of small bowel. In chronic afferent loop syndrome the obstruction is intermittent and produces a clinical syndrome from which a diagnostic history can usually be obtained. Patients with chronic afferent loop syndrome classically experience recurrent episodes of postprandial fullness and transient epigastric pain that is relieved by bilious vomiting as a partially obstructed afferent limb empties into the stomach 6.
Afferent loop syndrome is not an uncommon postoperative complication, especially in antecolic Billroth 2 gastrectomies and one study has estimated that it occurs in 13% of post-pancreaticoduodenectomy patients 7. Afferent limb syndromes have decreased in incidence with newer surgical techniques to decreass the size of the afferent limb.
No medical treatment is available for afferent limb syndrome and patients with no other contraindication should have revisional surgery if symptoms are clinically significant. Treatment consists of doing away with the afferent loop. In gastroenterostomy alone, takedown of the anastomosis with a Weinberg pyloroplasty is the treatment of choice. The safest and simplest treatment for patients whose original operation was Billroth 2 gastrectomy is conversion to a Roux-en-Y procedure. The Braun anastomosis has been used effectively to treat afferent loop syndrome following gastrectomy 8. This consists of an enteroenterostomy between the afferent and efferent limbs of a gastroenterostomy, and allows digestive juices in the afferent limb to flow into the efferent limb, thereby bypassing any obstruction at the gastrojejunostomy. In all cases vagotomy should be added unless previously performed. Both acute and chronic afferent loop syndromes should be completely prevented by appropriate choice of the initial operative procedure. The vagotomized stomach should be drained by pyloroplasty, not gastrojejunostomy. Vagotomy and antrectomy should be reconstructed with a Billroth 1 gastroduodenostomy.
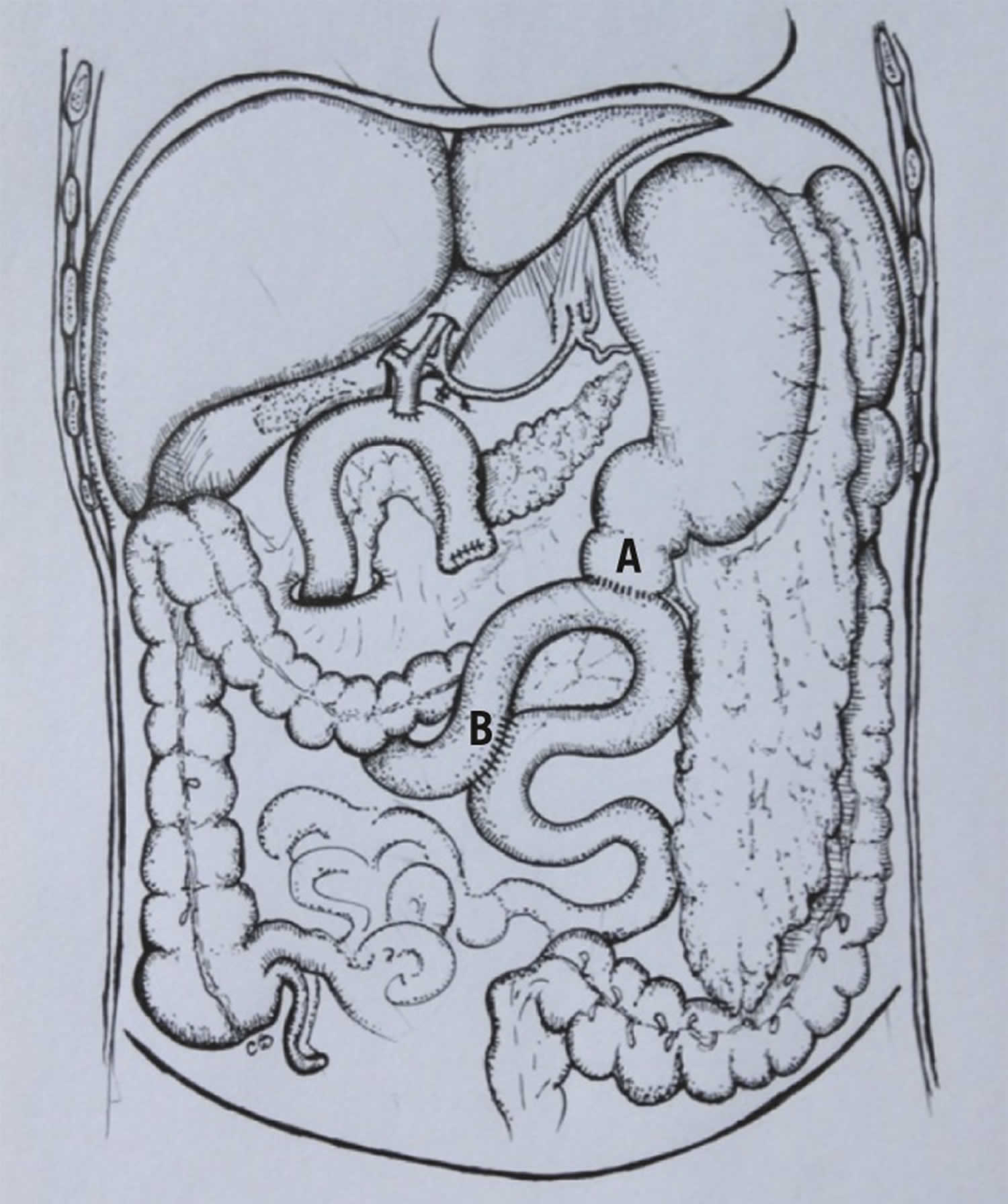
Figure 1. Braun enteroanastomosis
Footnote: Diagram of the Braun loop anastomosis during pylorus preserving pancreaticoduodenectomy: pylorojejunostomy anstomosis (A) and jejunojejunostomy (Braun) anastomosis (B).
[Source 1 ]Afferent loop syndrome causes
Afferent loop syndrome classically refers to obstruction of the upstream limb of a side-to-side gastrojejunostomy, but has also been used to refer to the biliopancreatic limb of a Roux-en-Y gastrojejunostomy. It can be seen after:
- Partial gastrectomy: Billroth II gastrojejunostomy
- Gastric bypass: Roux-en-Y gastric bypass
- Pancreaticoduodenectomy
Postoperative conditions
Each of the following postoperative conditions can cause afferent loop syndrome in a patient with a gastrojejunostomy:
- Entrapment or compression of the afferent loop by postoperative adhesions
- Internal hernia (eg, through a mesocolic defect) 9
- Volvulus of the intestinal segment
- Enteroenteral or enterogastric intussusception
- Kinking of the afferent limb at the gastrojejunostomy
- Scarring due to marginal (stomal) ulceration 10
- Recurrence of cancer at or near the anastomotic site
- Enteroliths in the afferent limb 11
- Bezoars in the afferent limb or at the anastomosis 12
- Foreign bodies in the afferent limb or at the anastomosis
Surgical technique
Patients have an increased chance of developing afferent loop syndrome if one or more of the following conditions is met:
- The jejunal portion of the afferent limb is longer than 30-40cm in length.
- The gastrojejunostomy is placed in an antecolic position instead of a retrocolic position.
- Mesocolic defects are not properly closed after construction of a retrocolic gastrojejunostomy.
Bushkin and Woodward reported an equal incidence of afferent loop syndrome in patients with short, retrocolic afferent limbs 13. However, according to Eagon and coworkers, most authors opine that longer, redundant, and antecolic afferent limbs are more prone to kinking, volvulus, and entrapment by adhesions 14.
Afferent loop syndrome symptoms
Patients with afferent loop syndrome usually present with epigastric pain, abdominal distention, nausea, and potentially bilious vomiting. It has been classified as acute (<7 days postoperative) or chronic (>7 days postoperative). Bilious vomiting is presumed to occur from regurgitation of bilious contents in the afferent limb into the stomach after release from intermittent obstruction.
Acute afferent loop syndrome symptoms
Acute afferent loop syndrome is caused by complete obstruction of the afferent loop. However, it is rare and may either occur within a few days postoperatively or present unexpectedly several years after a Billroth 2 gastrectomy as described by Ballas et al 15 and Valdivielso Cortázar et al 16. In both circumstances, this condition is caused by an acute obstruction of the afferent limb due to herniation or volvulus of the afferent loop posterior to the efferent limb. Patients with acute afferent loop syndrome typically present with a sudden onset of epigastric and/or right or left upper quadrant abdominal pain, with associated nausea and vomiting.
With acute afferent loop syndrome, the vomitus is not bilious because the biliary and pancreatic secretions remain trapped in the obstructed bowel loop. If the afferent loop is not decompressed, the patient becomes acutely ill and can subsequently develop peritonitis and shock if intestinal perforation or infarction ensues.
Chronic afferent loop syndrome symptoms
Chronic afferent loop syndrome is caused by partial obstruction of the afferent loop and may be more difficult to diagnose than acute afferent loop syndrome. Approximately 10-20 minutes to an hour postprandially, the patient experiences abdominal fullness and epigastric pain. These symptoms usually last from several minutes to an hour, although they occasionally may last as long as several days.
Projectile bilious vomiting is a classic manifestation of afferent loop syndrome with partial obstruction. The distended afferent loop decompresses forcefully, providing rapid relief of symptoms. Note that the vomitus usually contains no food which passes through the unobstructed efferent limb. Vomiting may occur after each meal or only occasionally 17. Also, symptoms in the immediate postprandial period may be minimized if the patient assumes a recumbent position.
Prolonged chronic afferent loop syndrome with stasis and bacterial overgrowth can be further complicated by steatorrhea, diarrhea, and vitamin B-12 deficiency anemia. These effects are primarily due to bacterial deconjugation of bile salts. The aforementioned factors, in addition to bypassing the duodenum and proximal jejunum, can result in iron deficiency anemia.
Afferent loop syndrome diagnosis
Physical examination can reveal one or more of the following findings:
- An ill-defined mass in the right upper abdominal quadrant may be present in one-third of patients with acute afferent loop syndrome.
- Localized midepigastric or right upper abdominal quadrant tenderness
- Peritonitis and/or a rigid abdomen if necrosis or perforation of the bowel wall has occurred
- Jaundice
- Signs of pancreatitis (eg, upper abdominal pain radiating to the flank or back)
Laboratory studies
Complete blood count
Blood should be drawn for a complete blood count (CBC). Areas of interest include the hemoglobin and hematocrit values, white blood cell (WBC) count, and red blood cell (RBC) characteristics (eg, mean corpuscular volume, cell size, iron content).
These studies aid in confirming the diagnosis of anemia (hemoglobin and hematocrit), the possibility of infection or acute illness (WBC count), and a possible cause for anemia related to afferent loop syndrome (eg, vitamin B-12 deficiency anemia, iron deficiency anemia).
Liver function tests and pancreatic enzymes
Elevated levels of serum bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, amylase, and lipase may be detected when biliary and/or pancreatic duct obstruction is prominent.
Occasionally, abnormalities of these hepatic and pancreatic products are early clues to the diagnosis in the proper patient scenario (eg, those with a Billroth II gastrojejunostomy) 18.
Electrolyte panel
Serum electrolytes should be examined, especially in patients with prolonged vomiting and possible dehydration. These conditions can lead to hyponatremia or hypernatremia, hypokalemia, and hypochloremia. Metabolic alkalosis may be present.
Albumin
Serum albumin levels should be measured, especially because afferent loop syndrome requires surgical correction.
In patients with chronic afferent loop syndrome and significant malnutrition, a period of preoperative specialized nutritional support might be appropriate.
Carbon 14 xylose breath test
When bacterial overgrowth is present, a carbon 14 xylose breath test reveals an increased concentration of hydrogen in exhaled gas following a glucose-containing meal. This test is mainly helpful in diagnostic dilemmas and has no role in acute afferent loop syndrome.
Radiography
Imaging studies may be of greater utility in patients with chronic afferent loop syndrome in which the diagnosis is often elusive. Although imaging tests are often performed in patients with acute afferent loop syndrome, the vast majority of such patients require urgent surgery regardless of the results of the imaging tests.
Upper gastrointestinal series
Findings from an upper gastrointestinal series may suggest the diagnosis when orally administered contrast agents fail to provide adequate opacification of the afferent loop. However, test results are not specific because nonopacification of the afferent loop is not unusual in normally functioning Billroth II anastomoses.
Plain abdominal radiography
Plain abdominal radiographs can be helpful by demonstrating abnormal bowel gas patterns or air-fluid levels, but these findings are not specific to the diagnosis of afferent loop syndrome.
Computed tomography scanning
Abdominal computed tomography (CT) scanning helps in the visualization of the obstructed segment directly and yields detailed information regarding the biliary tree, pancreas, and other structures 19. Yilmaz et al 20 reported that CT scanning should be the radiographic study of choice in the diagnosis of afferent loop syndrome.
Zissin and coworkers 21 reported salient CT scan features of afferent loop syndrome. They described the typical appearance as that of a U-shaped, fluid-filled tubular structure crossing the midline of the abdomen between the abdominal aorta and the superior mesenteric artery.
Kim and coauthors 22 demonstrated the accuracy of CT scanning not only in detecting afferent loop syndrome but also in predicting the underlying pathology causing the condition. They performed helical CT scans on 18 patients presenting with afferent loop syndrome. CT scanning helped correctly predict locally recurrent gastric cancer or carcinomatosis as the cause in 16 patients and adhesion formation and internal herniation as the cause in the other 2 patients.
Gayer and colleagues 23 described CT scan findings in 5 patients with afferent loop syndrome. The afferent limb appeared as a dilated (average 5.3 cm diameter), fluid-filled tubular mass. Valvulae conniventes were observed in all cases, and intraluminal air was detected in 80%. The dilated loop was confined to the subhepatic area in 60%, but it crossed the midline in the other patients. Biliary dilation was identified in all patients, and radiographic evidence of pancreatitis was discovered in one. Notably, orally administered contrast opacified the afferent limb in just one patient.
Gale and coworkers 24 stated that the afferent limb can appear as multiple, uniformly sized, peripancreatic cystic masses on CT scans. This description was confirmed by Swayne and Love 25, who added that the cysts featured attenuation numbers consistent with water density.
The initial description of afferent loop syndrome on CT scans was offered by Kuwabara and associates in 1980 26. They characterized an obstructed afferent limb as a U-shaped cystic mass in continuity with the biliary system, appearing posterior to the superior mesenteric artery.
Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP)
Chevallier and associates 27 described a case of a 77-year-old man presenting with obstructive jaundice. Magnetic resonance imaging (MRI) revealed biliary and pancreatic ductal dilation and a dilated afferent limb. A mass was visualized between the afferent loop and gastric remnant. Endoscopy with biopsy proved this mass to be an adenocarcinoma that was completely obstructing the afferent limb.
A variety of investigators have used magnetic resonance cholangiopancreatography (MRCP) to diagnose afferent loop syndrome 28. For example, McKee and coworkers 29 described the use of magnetic resonance cholangiopancreatography (MRCP) in afferent loop syndrome manifesting with cholangitis.
Abdominal ultrasonography
Ultrasound may demonstrate a peripancreatic cystic mass or a fluid-filled tubular structure in the right upper abdominal quadrant that may cross the midline.
Kitamura and associates 30 performed ultrasound as an adjunct to percutaneous small bowel drainage in patients with afferent loop syndrome. They used ultrasound to identify a segment of the afferent limb in apposition to the abdominal wall in 3 patients with afferent loop syndrome. In each case, the small bowel segment was successfully cannulated and decompressed.
Derchi and colleagues 31 reported their experience with ultrasound in 4 patients with afferent loop syndrome caused by tumor recurrence at or near a Billroth II gastrojejunostomy. In each case, ultrasound was able to define the distended afferent limb as a fluid-filled structure with a multilayered wall and effacement of the mucosal folds. The investigators described being able to trace the obstructed afferent loop from the hepatic hilum to the anastomosis with the stomach.
Lee and coworkers 32 reviewed the sonographic findings of afferent loop syndrome in 7 patients. In their group of patients, the etiology of afferent loop syndrome was internal herniation in 3, recurrent cancer in 2, marginal ulceration in 1, and a new primary cancer at the anastomosis in 1. The obstructed afferent limb appeared as a dilated, fluid-filled structure in the upper abdominal quadrants, which crossed the midline. These investigators also described the ability to trace this distended bowel loop to the area of the gastrojejunostomy.
Matsusue and colleagues 33 published their experience with ultrasound in 3 patients with afferent loop syndrome. In their report, the salient features of afferent loop syndrome on ultrasound images included a dilated intestinal loop without accompanying gas echoes in the upper abdomen and associated echolucent, edematous swelling of the pancreas.
Upper endoscopy
Esophagogastroduodenoscopy offers the advantage of direct visualization of the gastrojejunostomy and portions of its afferent and efferent limbs. The anastomosis can be inspected for kinking or marginal ulceration. A twist near the anastomosis suggests volvulus or internal herniation. Worrisome masses in the region of the anastomosis can be identified, and biopsy samples can be taken. In addition, enteroliths may also be present, which may mimic mitotic disease on imaging studies and can only be diagnosed with upper endoscopy as described by Yavuz et al 34.
According to Eagon et al 14, esophagogastroduodenoscopy is helpful in discriminating between afferent loop syndrome and alkaline reflux gastritis, which is an important entity in the differential diagnosis.
Afferent loop syndrome treatment
The cornerstone of treatment in afferent loop syndrome remains corrective surgery. Occasionally, patients are too debilitated to withstand operative therapy or recurrent cancer may preclude a successful reoperation.
In patients with acute afferent loop syndrome, a favorable outcome is correlated with an expedient diagnosis and corrective surgery. Medical therapy has no role, although nasogastric tube drainage may temporarily provide relief of symptoms while patients are resuscitated before surgery. Kim et al 35 reported a case of a 67-year-old patient with afferent loop syndrome and coexisting acute pancreatitis who was not considered to be an ideal surgical candidate. As a result, the patient was treated with an endoscopically placed nasogastric/enteric tube, with excellent relief of symptoms.
Patients with chronic afferent loop syndrome can be severely malnourished and anemic. These patients may benefit from preoperative specialized nutritional support or transfusion before undergoing corrective surgery. However, surgery should not be delayed if symptoms and signs consistent with complete obstruction develop.
Afferent loop syndrome surgical therapy
The treatment of afferent loop syndrome is surgical. Conservative measures can be temporarily used to resuscitate the patient, but the definitive treatment is corrective surgery. When afferent loop syndrome is caused by recurrent or unresectable malignancies, successful palliation is frequently accomplished using interventional radiologic techniques. Several references are provided in the preceding section.
Surgical correction is accomplished by deconstructing the Billroth 2 gastrojejunostomy and restoring gastrointestinal continuity with an alternate method. Several procedures have been described, but the two predominant operations are Billroth 1 gastroduodenostomy and Roux-en-Y gastrojejunostomy.
Interestingly, based on their retrospective study of 19 patients with postgastrectomy syndromes of whom 3 had afferent loop syndrome, Borrelli et al 36 reported that a significant proportion of patients required minor surgical intervention. The authors raised the question that in selected patients, laparoscopic surgery may be considered.
Vettoretto and associates 37 reported a case of afferent loop obstruction caused by an adhesive band following distal gastrectomy and reconstruction for gastric cancer. The authors performed diagnostic laparoscopy and laparoscopic lysis of adhesions, resulting in resolution of the afferent loop syndrome.
Aimoto and colleagues 38 described two cases of malignant afferent loop syndrome in patients who had undergone pancreaticoduodenectomy. In both patients, recurrence of the pancreatic cancer was found at laparotomy. Bypass procedures were performed in each case to achieve palliation.
Korean investigators 39 have suggested that placement of partially covered self-expandable dual stents may be effective in patients who develop afferent loop syndrome following different types of surgical procedures. The investigators retrospectively evaluated data from 13 consecutive patients who underwent placement of dual-stents (15 dual stents, 1 fully covered esophageal stent) via either the percutaneous transhepatic biliary drainage tract (n = 9) or the perioral route (n = 4). The stent placements were technically successful in all 13 patients, with 12 of 13 achieving postprocedure normalization of their blood tests and bowel decompression. The single patient that showed no change following stent placement subsequently underwent surgical jejunojejunostomy 39.
Afferent loop syndrome surgery complications
Patients undergoing surgery for afferent loop syndrome are at risk of developing any of the following complications:
- Wound infection
- Wound dehiscence with or without evisceration
- Urinary tract infection
- Atelectasis
- Pneumonia
- Anastomotic disruption
- Anastomotic stricture
- Marginal ulceration
- Intra-abdominal abscess formation
- Cholangitis
- Delayed gastric emptying/gastroparesis
- Internal or enterocutaneous fistulae
- Small bowel obstruction
- Dumping syndrome
- Alkaline reflux gastritis
- Roux stasis syndrome
- Cardiac arrhythmias
- Deep venous thrombosis
- Pulmonary embolism
Afferent loop syndrome prognosis
After a proper corrective procedure, the prognosis is usually very good, except in cases of advanced or recurrent malignancy.
Mortality and morbidity
Mortality rates of up to 57% have been reported for acute afferent loop syndrome. Mortality is most frequently associated with a delay in the diagnosis that leads to bowel infarction or rupture and peritonitis. Patients in whom a timely diagnosis is made or who present with chronic manifestations of the disease can undergo corrective surgery with acceptably low morbidity and mortality rates.
References- Nageswaran H, Belgaumkar A, Kumar R, et al. Acute afferent loop syndrome in the early postoperative period following pancreaticoduodenectomy. Ann R Coll Surg Engl. 2015;97(5):349–353. doi:10.1308/003588414X14055925061036 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5096581
- Katagiri H, Tahara K, Yoshikawa K, Lefor AK, Kubota T, Mizokami K. Afferent Loop Syndrome after Roux-en-Y Total Gastrectomy Caused by Volvulus of the Roux-Limb. Case Rep Surg. 2016;2016:4930354. doi:10.1155/2016/4930354 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939196
- Aoki M., Saka M., Morita S., Fukagawa T., Katai H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World Journal of Surgery. 2010;34(10):2389–2392. doi: 10.1007/s00268-010-0602-5
- Kim JK, Park CH, Huh JH et al. Endoscopic management of afferent loop syndrome after a pylorus preserving pancreatoduodenctomy presenting with obstructive jaundice and ascending cholangitis. Clin Endosc 2011; : 59–64.
- Aimoto T, Uchida E, Nakamura Y et al. Malignant afferent loop obstruction following pancreaticoduodenectomy: report of two cases. J Nippon Med Sch 2006; : 226–230.
- Pannala R, Brandabur JJ, Gan SI et al. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc 2011; : 295–302.
- Pannala R, Brandabur JJ, Gan SI et-al. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest. Endosc. 2011;74 (2): 295-302. doi:10.1016/j.gie.2011.04.029
- Kim DJ, Lee JH, Kim W. Afferent loop obstruction following laparoscopic distal gastrectomy with Billroth-II gastrojejunostomy. J Korean Surg Soc 2013; : 281–286.
- Ogata M, Ishikawa T. Acute afferent loop obstruction caused by retroanastomotic hernia. J Ultrasound Med. 1993 Nov. 12(11):697-9.
- Tsutsui S, Kitamura M, Shirabe K, Baba H, Sugimachi K. Afferent loop syndrome due to scarring of a stomal ulcer following a Billroth II gastrectomy. Endoscopy. 1995 Jun. 27(5):410.
- Alves AR, Almeida N, Ferreira M, Tome L. Endoscopic management of afferent loop syndrome caused by enteroliths and anastomotic stricture. A case report. Rev Esp Enferm Dig. 2017 Jun. 109(6):457.
- Hui MS, Perng HL, Choi WM, Chem LK, Yang KC, Chen TJ. Afferent loop syndrome complicated by a duodenal phytobezoar after Billroth-II subtotal gastrectomy. Am J Gastroenterol. 1997 Sep. 92(9):1550-2.
- Bushkin FL, Woodward ER. The afferent loop syndrome. Major Probl Clin Surg. 1976. 20:34-48.
- Eagon JC, Miedema BW, Kelly KA. Postgastrectomy syndromes. Surg Clin North Am. 1992 Apr. 72(2):445-65.
- Ballas KD, Rafailidis SE, Konstantinidis HD, et al. Acute afferent loop syndrome: a true emergency. A case report. Acta Chir Belg. 2009 Jan-Feb. 109(1):101-3.
- Valdivielso Cortazar E, Redondo Martinez J, Romay Cousido G, Alonso-Aguirre P. Cholangitis secondary to afferent loop syndrome from a gastric stump adenocarcinoma. Rev Esp Enferm Dig. 2018 Apr. 110(4):253.
- Golioto M. A woman with abdominal pain and bilious vomiting. A very late aftermath of Billroth II gastrectomy. N C Med J. 2000 Nov-Dec. 61(6):338-40.
- Yue P, Meng W, Luo Z, Bai B, Li X. Double pigtail stents healed acute pancreatitis resulting from afferent loop obstruction. Turk J Gastroenterol. 2018 Nov. 29 (6):705-7.
- Juan YH, Yu CY, Hsu HH, et al. Using multidetector-row CT for the diagnosis of afferent loop syndrome following gastroenterostomy reconstruction. Yonsei Med J. 2011 Jul. 52(4):574-80.
- Yilmaz S, Yekeler E, Dural C, et al. Afferent loop syndrome secondary to Billroth II gastrojejunostomy obstruction: Multidetector computed tomography findings. Surgery. 2007 Apr. 141(4):538-9.
- Zissin R, Hertz M, Paran H, Osadchy A, Gayer G. Computed tomographic features of afferent loop syndrome: pictorial essay. Can Assoc Radiol J. 2005 Apr. 56(2):72-8.
- Kim HC, Han JK, Kim KW, et al. Afferent loop obstruction after gastric cancer surgery: helical CT findings. Abdom Imaging. 2003 Sep-Oct. 28(5):624-30.
- Gayer G, Barsuk D, Hertz M, Apter S, Zissin R. CT diagnosis of afferent loop syndrome. Clin Radiol. 2002 Sep. 57(9):835-9.
- Gale ME, Gerzof SG, Kiser LC, et al. CT appearance of afferent loop obstruction. AJR Am J Roentgenol. 1982 Jun. 138(6):1085-8.
- Swayne LC, Love MB. Computed tomography of chronic afferent loop obstruction: a case report and review. Gastrointest Radiol. 1985. 10(1):39-41.
- Kuwabara Y, Nishitani H, Numaguchi Y, Kamoi I, Matsuura K, Saito S. Afferent loop syndrome. J Comput Assist Tomogr. 1980 Oct. 4(5):687-9.
- Chevallier P, Gueyffier C, Souci J, Oddo F, Diaine B, Padovani B. [MRI of an afferent loop syndrome presenting as obstructive icterus]. J Radiol. 2001 Feb. 82(2):177-9.
- Benallal DC, Hoibian S, Caillol F, et al. EUS-guided gastroenterostomy for afferent loop syndrome treatment stent. Endosc Ultrasound. 2018 Nov-Dec. 7(6):418-9.
- McKee JD, Raju GP, Edelman RR, Levine H, Steer M, Chuttani R. MR cholangiopancreatography (MRCP) in diagnosis of afferent loop syndrome presenting as cholangitis. Dig Dis Sci. 1997 Oct. 42(10):2082-6.
- Kitamura H, Miwa S, Nakata T, et al. Sonographic detection of visceral adhesion in percutaneous drainage of afferent-loop small-intestine obstruction. J Clin Ultrasound. 2000 Mar. 28(3):133-6.
- Derchi LE, Bazzocchi M, Brovero PL. Sonographic diagnosis of obstructed afferent loop. Gastrointest Radiol. 1992. 17(2):105-7.
- Lee DH, Lim JH, Ko YT. Afferent loop syndrome: sonographic findings in seven cases. AJR Am J Roentgenol. 1991 Jul. 157(1):41-3.
- Matsusue S, Kashihara S, Takeda H, Koizumi S. Three cases of afferent loop obstruction–the role of ultrasonography in the diagnosis. Jpn J Surg. 1988 Nov. 18(6):709-13.
- Yavuz N, Erguney S, Ogut G, Alver O. Enteroliths developed in a chronically obstructed afferent loop coexisting with gastric remnant carcinoma: Case report and review of the literature. J Gastroenterol Hepatol. 2006 Mar. 21(3):495-8.
- Kim HJ, Kim JW, Kim KH, et al. [A case of afferent loop syndrome treated by endoscopic drainage procedure using nasogastric tube] [Korean]. Korean J Gastroenterol. 2007 Mar. 49(3):173-6.
- Borrelli D, Borrelli A, Presenti L, Bergamini C, Basili G. [Surgical approach of the functional post-partial gastrectomy syndromes] [Italian]. Ann Ital Chir. 2007 Jan-Feb. 78(1):3-10.
- Vettoretto N, Pettinato G, Romessis M, Bravo AF, Barozzi G, Giovanetti M. Laparoscopy in afferent loop obstruction presenting as acute pancreatitis. JSLS. 2006 Apr-Jun. 10(2):270-4.
- Aimoto T, Uchida E, Nakamura Y, et al. Malignant afferent loop obstruction following pancreaticoduodenectomy: report of two cases. J Nippon Med Sch. 2006 Aug. 73(4):226-30.
- Han K, Song HY, Kim JH, et al. Afferent loop syndrome: treatment by means of the placement of dual stents. AJR Am J Roentgenol. 2012 Dec. 199(6):W761-6.