Balantidiasis
Balantidiasis also known as Balantidium coli infection or balantidiosis, is a rare infectious disease in the US that is caused by single celled intestinal protozoan parasite called Balantidium coli, a ciliated protozoan that is usually associated with intestinal infection in areas associated with pig rearing. Balantidium coli parasites can be transmitted through the fecal-oral route by contaminated food and water. Balantidium coli infects humans occasionally, mostly immunocompromised patients. Some infected people may have no symptoms or only mild diarrhea and abdominal discomfort but others may experience more severe symptoms reminiscent of an acute inflammation of the intestines. People with other serious illnesses can experience persistent diarrhea, abdominal pain, and sometimes a perforated colon. Symptoms of Balantidiasis may be similar to those of other infections that cause intestinal inflammation, for example, amoebic dysentery. On very rare occasions Balantidium coli may invade extra-intestinal organs, that include liver, lungs and genitourinary tract 1. Genitourinary sites of infection, including uterine infection, vaginitis and cystitis are thought to occur via direct spread from the anal area or secondary to rectovaginal fistula created from infection with Balantidium coli 2. The three medications often used to treat Balantidium coli are tetracycline, metronidazole, and iodoquinol. See your health care provider for treatment.
Is balantidiasis contagious?
Yes. Balantidium coli is contagious by the fecal-oral route.
Balantidiasis causes
Risk factors for balantidiasis include contact with pigs, handling fertilizer contaminated with pig excrement, and living in areas where the water supply may be contaminated by the excrement of infected animals. Poor nutrition, achlorhydria, alcoholism, and immunosuppression may also be contributing factors.
Balantidiasis life cycle
Balantidium coli, a large ciliated protozoan, is the only ciliated protozoan known to be capable of infecting humans 3. Balantidium coli is often associated with pigs, the primary reservoir host. Recent molecular analyses have suggested the need for taxonomic revision, and it is now sometimes referred to as Neobalantidium coli or Balantioides coli, although this nomenclature has neither been resolved nor widely adopted in the medical community.
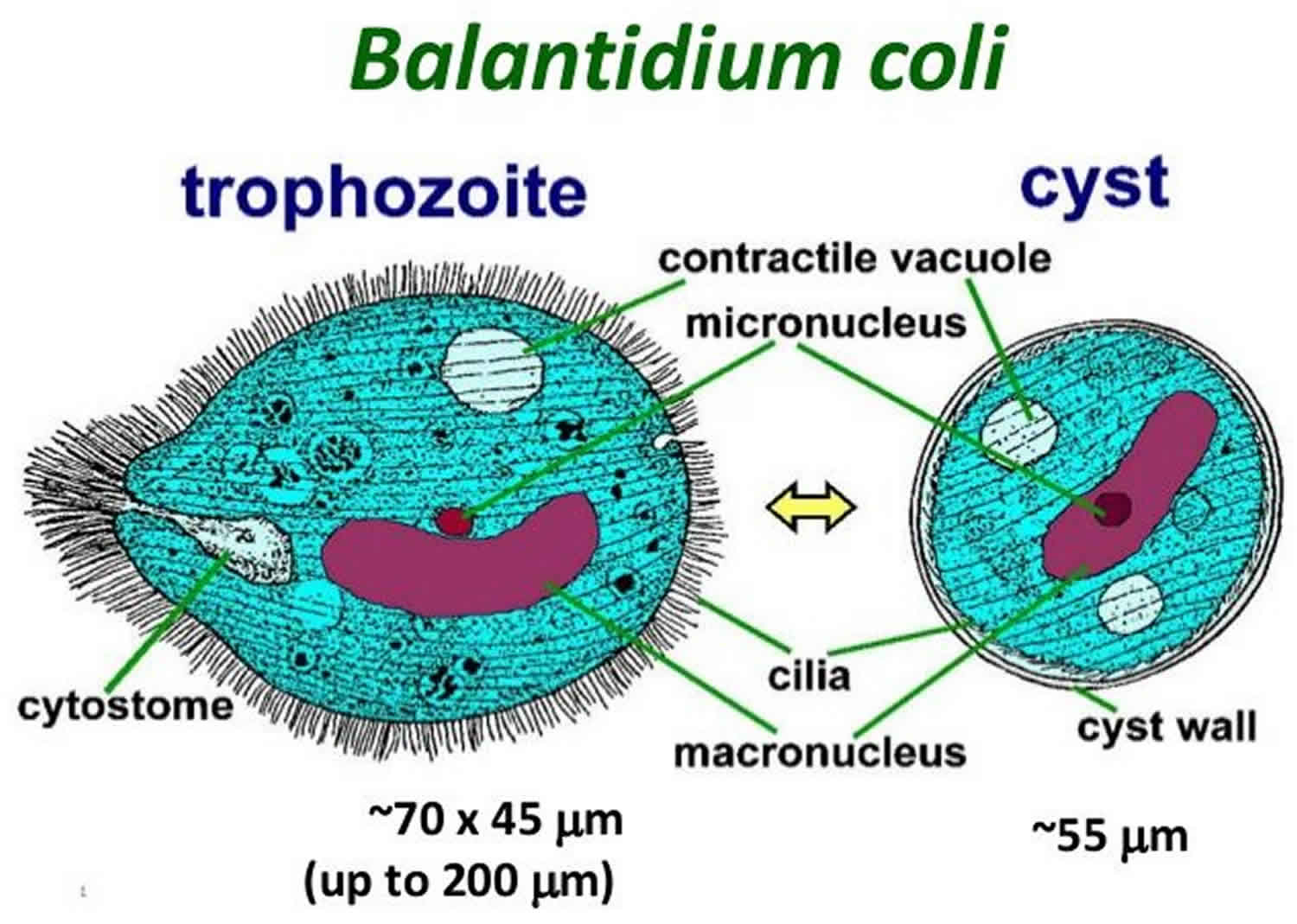
Balantidium coli inhabits the large intestine of humans, pigs and monkeys. The parasite exists in two stages; trophozoite stage found in dysenteric stool and encysted stage found in chronic cases and carriers. B. coli passes its life cycle in two stages, but in one host only. Pig is the natural host and man is an accidental host. Cyst is the infective stage of the parasite and the route of transmission is feco-oral. The pig serves as the usual source of infection. Transmission occurs through the ingestion of food or water contaminated by cysts obtained from the feces of a pig or man. The infection is found world-wide. Most human cases have been reported from South and Central America, China, Iran, Indonesia, Philippines, New Guinea and Pacific Islands 4.
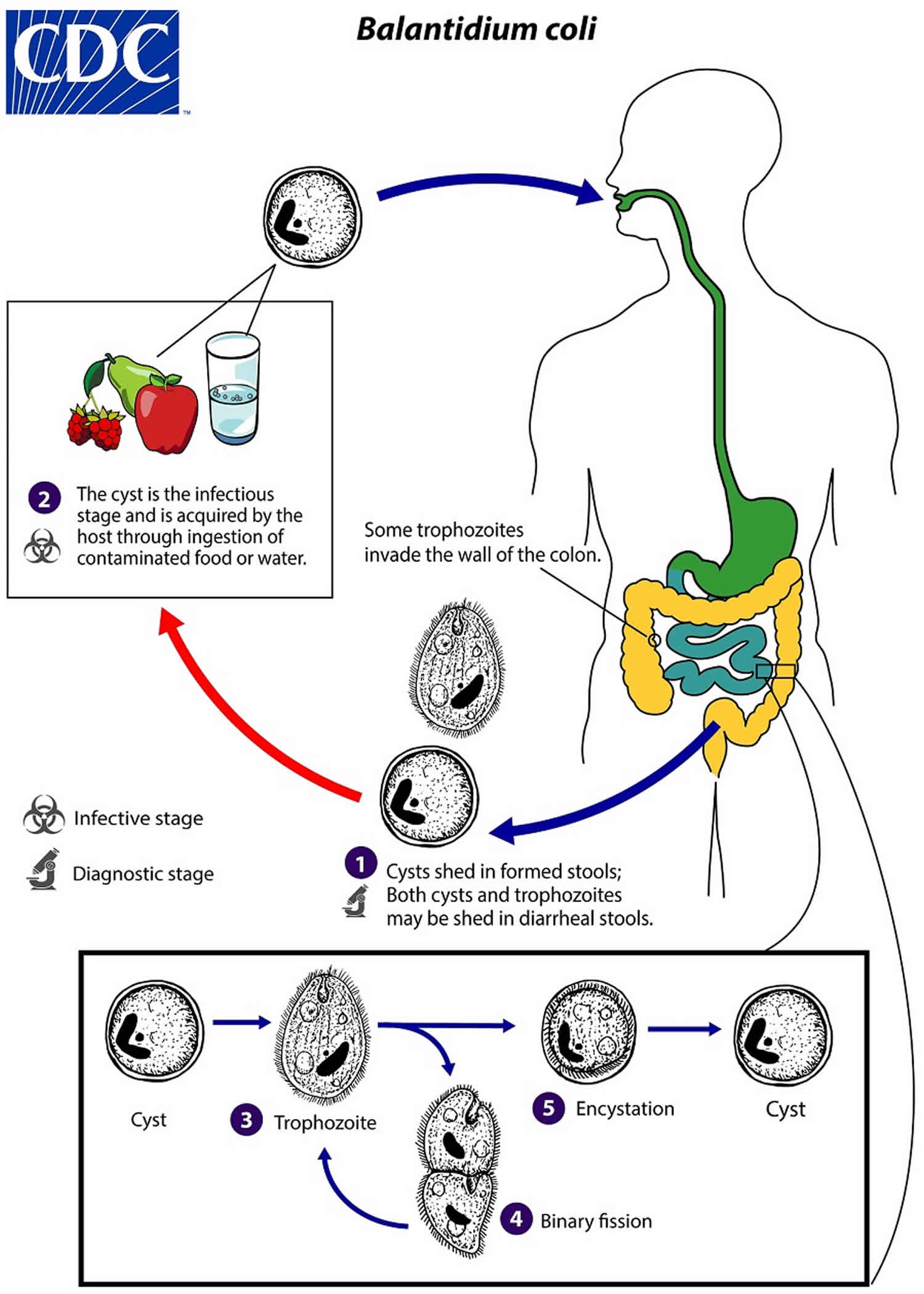
Cysts are the stage responsible for transmission of balantidiasis (number 1). The host most often acquires the cyst through ingestion of contaminated food or water (number 2). Following ingestion, excystation occurs in the small intestine, and the trophozoites colonize the large intestine (number 3). The trophozoites reside in the lumen of the large intestine and appendix of humans and animals, where they replicate by binary fission, during which conjugation may occur (number 4). Trophozoites undergo encystation to produce infective cysts (number 5). Some trophozoites invade the wall of the colon and multiply, causing ulcerative pathology in the colon wall. Some return to the lumen and disintegrate. Mature cysts are passed with feces.
Figure 1. Balantidium coli
[Source 3 ]Figure 2. Balantidiasis life cycle
 [Source 5 ]
[Source 5 ]
How is balantidiasis transmitted?
Balantidium coli is transmitted through the fecal-oral route. Humans can become infected by eating and drinking contaminated food and water that has come into contact with infective animal or human fecal matter. Infection can occur in several ways, including the following examples:
- eating meat, fruits, and vegetables that have been contaminated by an infected person or contaminated by fecal matter from an infected animal,
- drinking and washing food with contaminated water, or
- having poor hygiene habits.
Balantidiasis prevention
Balantidium coli is found throughout the world, but it is most prevalent in tropical and subtropical regions and developing countries. Because pigs are an animal reservoir, human infections occur more frequently in areas where pigs are raised, especially if good hygiene is not practiced. When traveling to endemic tropical countries, Balantidium coli infection can be prevented by following good hygiene practices. Wash your hands with soap and warm water after using the toilet, changing diapers, and before handling food. Teach children the importance of washing hands to prevent infection. Wash all fruits and vegetables with clean water when preparing or eating them, even if they have a removable skin.
Balantidiasis symptoms
Balantidium coli is the largest ciliated protozoa infecting humans by the feco-oral transmission from pigs. Large gut is the most common site of involvement.
Most people infected with Balantidium coli experience no symptoms (asymptomatic carrier). Balantidium coli infects the large intestine in humans and produces infective microscopic cysts that are passed in the feces, potentially leading to re-infection or infection of others. People who are immune-compromised are the most likely to experience more severe signs and symptoms 6. These include persistent diarrhea, dysentery, abdominal pain, weight loss, nausea, and vomiting. If left untreated, perforation of the colon can occur.
Potential symptoms of balantidiasis include the following:
- Diarrhea (watery, bloody, mucoid)
- Nausea
- Vomiting
- Abdominal pain
- Anorexia
- Weight loss
- Headache
- Mild colitis
- Fever
- Severe and marked fluid loss (resembling amebic dysentery) 7
Patients with balantidiasis may present with abdominal tenderness, fever, and prolonged diarrhea, which may result in signs of dehydration.
Extra-intestinal infections can occur in liver, lung and urogenital tract. There are very few case reports of urinary balantidiasis.
Balantidiasis diagnosis
Stool samples can be examined by a laboratory. Microscopic examination can detect Balantidium coli in the stool. An experienced parasitologist should review such cases. Rapid spiraling motility and cytosmears stained with Giemsa and H&E satins are very useful in morphological identification.
Balantidiasis treatment
Three medications are used most often to treat Balantidium coli: tetracycline, metronidazole, and iodoquinol.
Tetracycline*: adults, 500 mg orally four times daily for 10 days; children ≥ 8 years old, 40 mg/kg/day (max. 2 grams) orally in four doses for 10 days. Note: Tetracyclines are contraindicated in pregnancy and in children < 8 years old. Tetracycline should be taken 1 hour before or 2 hours after meals or ingestion of dairy products.
- Tetracycline is in pregnancy category D. Tetracycline should not be used in pregnant women due to positive evidence of maternal and fetal risk. Use during pregnancy should be limited to instances when there are contraindications to the use of other appropriate antibiotics and the potential benefit justifies the known risk.
- Tetracycline is excreted in breast milk. The American Academy of Pediatrics and the World Health Organization (WHO) both classify tetracycline as compatible with breast-feeding, although data on the use of tetracycline during lactation is limited. Tetracycline should be used during lactation only if the potential benefit of therapy to the mother justifies the known risk to the infant.
- Tetracycline is contraindicated in children age 8 and younger as it may cause permanent discoloration of the teeth. The safety of intravenous tetracycline has not been established. Use of tetracycline in children age 8 and younger should be limited to instances when there are contraindications to the use of other appropriate antibiotics and the potential benefit justifies the known risk.
Alternatives:
- Metronidazole*: adults, 500-750 mg orally three times daily for 5 days; children, 35-50 mg/kg/day orally in three doses for 5 days.
- Metronidazole is in pregnancy category B. Data on the use of metronidazole in pregnant women are conflicting. The available evidence suggests use during pregnancy has a low risk of congenital anomalies. Metronidazole may be used during pregnancy in those patients who will clearly benefit from the drug, although its use should be weighed against any potential risks.
- Metronidazole is excreted in breast milk. The American Academy of Pediatrics classifies metronidazole as a drug for which the effect on nursing infants is unknown but may be of concern. The World Health Organization (WHO) advises to avoid metronidazole treatment in lactating women. Metronidazole should be used during lactation only if the potential benefit of therapy to the mother justifies the potential risk to the infant.
- The safety of metronidazole in children has not been established. Metronidazole is listed as an antiamebic and antigiardiasis medicine on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
OR
- Iodoquinol*: adults, 650 mg orally three times daily for 20 days; children, 30-40 mg/kg/day (max 2 g) orally in three doses for 20 days. Note: iodoquinol should be taken after meals.
- Oral iodoquinol has not been assigned a pregnancy category by the Food and Drug Administration. Data on the use of iodoquinol in pregnant women are limited, and risk to the embryo-fetus is unknown. Iodoquinol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- It is not known whether iodoquinol is excreted in breast milk. Iodoquinol should be used with caution in breastfeeding women.
- The safety of iodoquinol in children has not been established.
Nitazoxanide*: has been tried in small studies, which suggest some therapeutic benefit (adults, 500 mg orally twice daily for 3 days; children age 4-11 years old 200 mg orally twice daily for 3 days; children 1-3 years old 100 mg orally twice daily for 3 days).
*Not FDA-approved for this indication
* Tetracycline is available for human use in the United States.
* Metronidazole is available for human use in the United States.
* Iodoquinol is available for human use in the United States.
References- Koopowitz A, Smith P, van Rensburg N, Rudman A. Balantidium coli-induced pulmonary haemorrhage with iron deficiency. S Afr Med J. 2010;100:534–6.
- Umesh S. Balantidium coli on urine microscopy. Natl Med J India. 2007;20:270
- Karuna T, Khadanga S. A rare case of urinary balantidiasis in an elderly renal failure patient. Trop Parasitol. 2014;4(1):47–49. doi:10.4103/2229-5070.129165 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3992804
- Parija SC. Textbook of Medical Parasitology. 3rd ed. New Delhi: All India Publishers and Distributors; 2011.
- Parasites – Balantidiasis Biology. https://www.cdc.gov/parasites/balantidium/biology.html
- Schuster FL, Ramirez-Avila L. Current world status of Balantidium coli. Clin Microbiol Rev. 2008;21:626–38.
- Dysenteric syndrome. Bellanger AP, Scherer E, Cazorla A, Grenouillet F. Dysenteric syndrome due to Balantidium coli: a case report. New Microbiol. 2013 Apr. 36(2):203-5.