What is agmatine
Agmantine (1-amino-4-guanidinobutane) is a natural metabolite of the amino acid arginine that is known to cross the blood-brain barrier when given orally 1. Agmantine is formed when L-arginine is decarboxylated by the enzyme arginine decarboxylase (ADC) and is found naturally in ragweed pollen, ergot fungi, octopus muscle, herring sperm, sponges, and the mammalian brain. Agmatine is hydrolyzed to putrescine (one of the main biogenic amines associated with microbial food spoilage) and urea by agmatinase (AGM) or agmatinase-like protein (ALP) (see Figure 3A). The agmatine sulfate salt has been used as a dietary ingredient and now is available as a nutraceutical 2. Agmatine is both an experimental and investigational drug. As an investigational drug, it is being studied in a non-blinded prospective case study in the United States looking at patients who have been diagnosed with small fiber peripheral neuropathy between the ages of 18 to 75 years. Recent clinical studies have already shown that oral agmatine sulfate (daily dose of 2.67 g agmatine sulfate) given for up to 3 weeks provides a safe and, as compared with current therapeutics, more effective treatment for neuropathic pain 3. As an experimental drug, agmatine is being studied for several indications such as cardioprotection, diabetes, decreased kidney function, neuroprotection (stroke, severe central nervous system injuries, epilepsy, glaucoma, and neuropathic pain), and psychiatric conditions (depression, anxiety, schizophrenia, and cognition). The exact mechanism of action is still being investigated for all of the potential indications of agmatine.
Agmatine has been directly associated with many important cellular functions, such as the modulation of insulin release from pancreatic cells 4, renal sodium excretion 5, inhibition of nitric oxide synthase 6, neuroprotective effects 7, increased tolerance to morphine 8, modulation of ethanol anxiolysis 9 and regulation of polyamine biosynthesis 10. Agmatine is considered a neurotransmitter or neuromodulator 11 because it regulates the release of catecholamines and potentiates opioid analgesia 12. The injection of agmatine produces anticonvulsant, antineurotoxic and antidepressant-like actions in animals 13. Agmatine has also been linked to other central nervous system disorders. Specifically, preclinical studies have demonstrated the beneficial effects of agmatine administration on diseases such as depression, anxiety, hypoxic ischemia, nociception, morphine tolerance, memory, Parkinson’s disease, Alzheimer’s disease, traumatic brain injury-related disorders, and epilepsy 14. The various biological processes on which agmatine is involved suggest that it might require the fine regulation of its cellular concentrations.
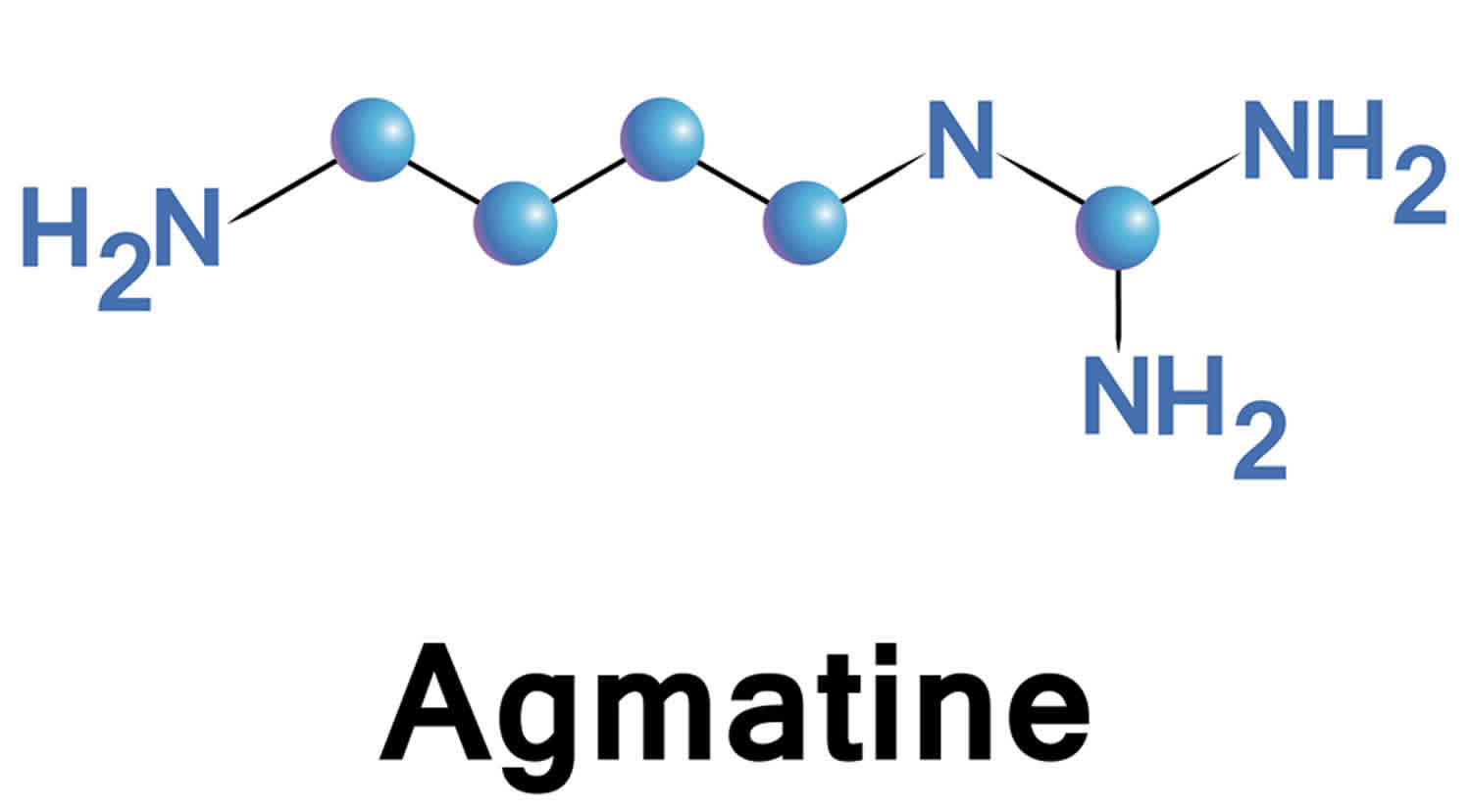
Figure 1. Agmatine chemical structure
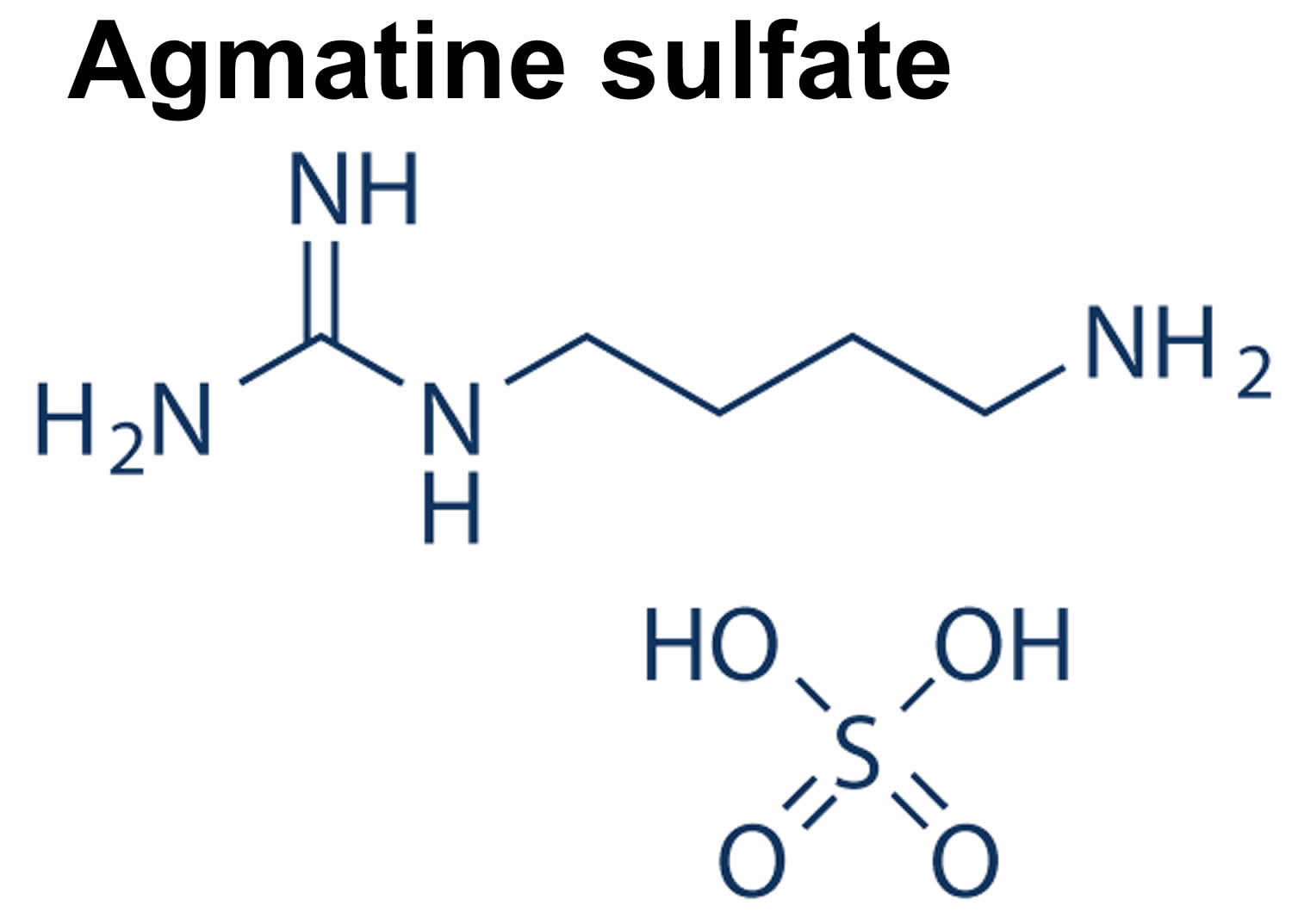
Figure 2. Agmatine sulfate chemical structure
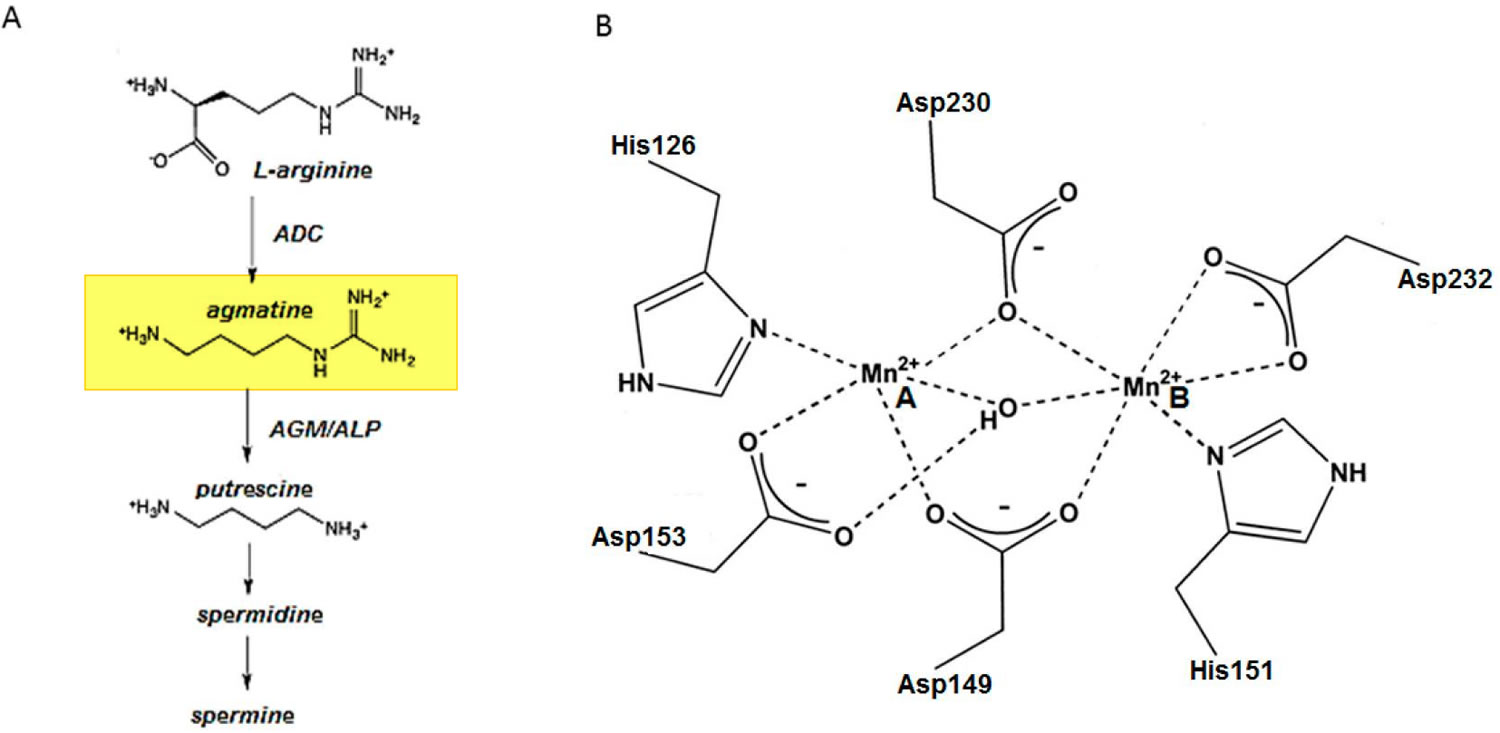
Figure 3. Agmatine biosynthesis and breakdown
Footnote: (A) Pathway of agmatine biosynthesis and breakdown. (B) Schematic illustration of the Mn2+ binding site of E. coli agmatinase (AGM). The enzyme can accommodate two closely spaced Mn2+ ions in their active sites, using highly conserved amino acid side chains 15.
Abbreviations: ADC = arginine decarboxylase; ALP = agmatinase-like protein; AGM = agmatinase; Mn2+ = manganese ion.
[Source 16 ]Agmatine function
Moinard et al. 17 have reported that agmatine concentrations in the brain have been found to be comparable to that of typical neurotransmitters, thus suggesting that agmatine might be a neurotransmitter.
A variety of effects have been demonstrated in response to agmatine in vivo and in vitro, but the biological significance of these effects is still under debate, because the concentrations at which the effects were induced were much higher than those present in blood and tissues 18. However, agmatine is known to have secretagogue properties, such as the modulation of insulin release and glucose metabolism, the stimulation of adrenaline, and noradrenaline secretion. agmatine antagonizes some hyperalgesic states and enhances the dose-related analgesic effect of morphine 19.
Agmatine appears to influence appetite regulation. The administration of a high dose of agmatine to satiated rats triggered an increase in their caloric intake and their carbohydrate preferences, whereas agmatine does not modulate caloric intake in hungry rats 20. agmatine also plays an important role in polyamines homeostasis: by inhibiting ornithine decarboxylase, agmatine inhibits smooth muscle proliferation 17.
Agmatine is recognized as an inhibitor of nitric oxide synthase, an enzyme that, starting from arginine, catalyzes the production of nitric oxide (NO), an intercellular messenger implicated in the regulation of various physiological functions in central neurotransmission. It has been suggested that NO (nitric oxide) may be involved in the mechanism of anxiety, therefore NO (nitric oxide) inhibitors, such as agmatine, may be a potential tool for the treatment of anxiety 21, even though Krass et al. 22 failed to demonstrate the reported antidepressant and anxiolytic-like activity of agmatine.
However, it is highly probable that agmatine will play a role in the therapeutic treatment of a number of different diseases. In the treatment of cancer, it has been established that agmatine has a antiproliferative effect due to its suppression of polyamine synthesis and cellular polyamine uptake through the induction of antizyme 23.
It has also been suggested that agmatine could play an important role in the control of ureagenesis, representing a significant contribution to the therapeutic removal of waste toxic nitrogen from the body. Moreover, agmatine could be useful in the treatment of sepsis, because its administration to endotoxemic rats has been shown to prevent the decrease in blood pressure and renal function usually associated with sepsis 17.
Agmatine is also able to protect brain mitochondria against the drop in energy capacity by the Ca2+-dependant induction of permeability transition in rat brain mitochondria 24. Furthermore, agmatine may also be considered as a regulator of mitochondria cell death. In addition, by increasing the expression of antizyme, a protein that inhibits polyamine biosynthesis and transport, agmatine exhibits a regulatory effect on cell proliferation 23.
The high content of agmatine in the lumen of the gastrointestinal tract may also originate from the bacteria of the physiological gut microflora, as well as from pathogens such as Helicobacter pylori.
After oral administration of radiolabeled agmatine to rats in vivo, radioactivity was retrieved in all organs investigated, as well as in blood and urine indicating that agmatine is absorbed from the stomach and the gut by means of an energy-dependent transport mechanism and distributed to the organs via the blood stream. Moreover, the accumulation of radioactivity in organs and distal gut luminal content decreases with the administration of increasing doses of putrescine 18.
Because of the low arginine decarboxylase activity in mammalians, only a fraction of agmatine in the tissues of the organism can be due to endogenous de novo synthesis by arginine decarboxylase, while a substantial portion can be of dietary origin 18.
Agmatine foods
Several food items contain only small amounts of polyamines, such as agmatine, while higher concentrations can be found in fermented foods. Polyamines can also be considered as indicators of freshness in fish and meat products, as they are produced during food storage, thus confirming the main role of microorganisms in their synthesis. In particular, agmatine is present at high levels in alcoholic beverages, such as wine, beer, and especially in sake (114 mg/L) 25, while low levels have been found in fermented non-alcoholic beverages, such as Turkish shalgam 26. This fact could confirm the role of yeasts in agmatine production.
Table 1. Agmatine content in different foodstuffs (ppm)
| Foodstuffs | Mean | Range | N (A) |
| WINE | |||
| Red wines | n.s. | n.d.–22 | 286 |
| White wines | n.s. | n.d.–6.5 | 103 |
| BEER | 12 | 0.5–42 | 211 |
| SAKE | 114 | n.s. | n.s. |
| COFFEE | |||
| Green coffee | n.d. | n.d. | n.s. |
| Roasted | n.s. | n.d.–1.2 | n.s. |
| Instant coffee | 0.4 | 0.4–5.3 | 68 |
| CEREALS AND VEGETABLES | |||
| Flour | n.d. | n.d. | 5 |
| Bread | 3.3 | n.d.–4.7 | 9 |
| Soy sauce | n.d. | n.d. | 4 |
| Soybean paste (Doenjang) | 473 | n.d.–5508 | 23 |
| Miso | 6.1 | n.d.–30 | 5 |
| Sauerkraut brine | 4.3 | 2.2–6.7 | 6 |
| Fermented cabbage juice | n.d. | n.d. | 5 |
| FISH AND DERIVED PRODUCTS | |||
| Fresh fish | 92 | n.d.–401 | 13 |
| Fish paste | n.d. | n.d. | 3 |
| Cooked fish paste | 29 | n.d.–161 | 28 |
| MEAT AND MEAT PRODUCTS | |||
| Fresh meat | 0.8 | n.d.–3.1 | 22 |
| Fermented meat | 6.2 | n.d.–43 | 23 |
| Cooked meat | 5.4 | n.d.–27 | 20 |
| DAIRY PRODUCTS | |||
| Fermented milk (Kefir) | n.d. | n.d. | 10 |
| Ripened cheese | 1.1 | n.d–18 | 69 |
| Fresh cheese | 0.1 | n.d.–1.3 | 13 |
| Grated cheese | 1.2 | n.d.–14 | 12 |
Footnotes: (A) Number of samples examined; n.d. = Not detected; n.s. = Not specified.
[Source 27 ]Wine
The concentration of biogenic amines in wine has been reported to range from a few mg/L to about 50 mg/L. The type and concentration of amines in wines depends on several factors, such as winemaking processes, time and storage conditions, raw material quality, and microbial contamination during winery operations. Moreover, some amines are normal constituents of grapes, and their level may vary with grape variety and degree of ripening, as well as with soil type and composition. Therefore, geographical characterization based on the biogenic amine content has also been proposed as a criterion to discriminate several types of wines from different countries or regions 28. Biogenic amines usually investigated in wines are cadaverine (CAD), histamine (HIS), 2-phenylethylamine (2-PHE), putrescine (PUT) and tyramine (TYR); agmatine and ethanolamine can be abundant in wines, but they are rarely investigated 27. Low amounts of biogenic amine, as normal constituents of the raw materials, can be released in must from grapes during the winemaking process, and the biogenic amine concentration may increase as a consequence of alcoholic fermentation, yeast autolysis, malolactic fermentation, and wine aging, red wines usually having a richer amine content than white wines. Generally putrescine and agmatine can contribute significantly to the total amine content in alcoholic beverages, while cadaverine, spermine, and spermidine are rare 27. In particular, putrescine is the most abundant biogenic amine in wine, while agmatine is the most prevalent in beer 29.
For agmatine, the concentration has been reported to vary from not detected to 22 mg/L in red wines and from not detected to 6.5 mg/L in white wines, while putrescine has been found in red wines at concentrations ranging from 0.6 to 21 mg/L and from not detected to 9 mg/L in white wines 30. Spermidine and spermine originate from putrescine, which derives from agmatine; the latter is generally recognized as an intermediate in the putrescine formation pathway, starting from arginine, the most abundant amino acid in grape. Putrescine can also derive from decarboxylation of ornithine. However, putrescine levels increase during fermentation from must to alcoholic, to malolactic fermentation, thus supporting the hypothesis that the principal biosynthetic pathway for putrescine formation is via arginine-agmatine rather than via ornithine 30. Moreover, arginine, from which agmatine derives, is present in must at concentrations higher than those found in the finished wine 30. In addition, wines with a low putrescine content have been shown to possess a high level of agmatine and total polyphenols and vice versa 28. The phenolic compounds seem to be a natural way of reducing putrescine formation in wine, because these moieties can protect the cells against oxidative stress 31.
Beer
Polyamines are natural beer constituents and are present in malt and yeast at higher concentrations then in hops 32, while tyramine, histamine, and cadaverine are considered to be indicators of microbial contamination during brewing 33. In several beers agmatine is the most abundant amine with concentrations ranging from 0.5 to 42 mg/L 34. Barley variety, malting technology, and fermentation conditions can influence the biogenic amine content in beer both qualitatively and quantitatively. In particular, during wort processing, significant increases of agmatine, and putrescine have been observed, together with the decrease of spermidine and spermine 35.
Coffee
The profile and levels of biogenic amines in green and roasted coffee are different; in particular, the degree of roasting significantly influences the amine profile and levels. Agmatine has been detected in coffee roasted at a high temperature for a long time. In fact, extensive roasting (12 min at 300°C) contributes to agmatine formation (1.2 mg/kg) by decarboxylation of arginine, which is present in green coffee at levels ranging from 2 to 5 g/100 g. Conversely, agmatine was not found in American coffee, which was roasted for 6 min at 300°C. Furthermore, putrescine is the most abundant amine in green coffee (10 mg/kg), while it is not present in roasted coffee 36. Also the processing of instant coffee can influence both the type and level of biogenic amines; instant coffee contains low levels of agmatine (0.4 mg/kg), while the most abundant biogenic amine is serotonin, followed by cadaverine, tyramine and spermidine 37.
Cereals and vegetables
In cereal foods, in particular in flour and bread samples, putrescine is reported to be the most abundant biogenic amine, at even higher levels than 40 mg/kg, while agmatine has been found only in cereal derivates, in particular in different types of bread samples, at concentrations ranging from 3 to 5 mg/kg of dry matter 38. Agmatine is also a natural constituent of the seedlings of the winged bean, while is not found in the common bean nor in soybean 39. Since several varieties of molds, yeasts, and lactic acid bacteria are involved in the fermentation processes occurring in the production of several soybean products, and as the substrate is very rich in proteins, the formation of various amines might be expected during fermentation. Several studies have highlighted that biogenic amines in fermented soybean products are most probably formed by the lactic microflora that remains active during fermentation 40. Among many fermented foods that have been consumed for thousands of years in Asian countries, soy sauce, and miso are the most important, and they are obtained by the fermentation of soybean with or without the addition of rice and wheat (in the case of soy sauce) or rice and barley (in the case of miso), using a mix of molds, yeasts, and lactic acid bacteria. In these products agmatine has rarely been detected, whereas putrescine is one of the most abundant amines that can be found 40. Conversely, in Doenjang, a traditional Korean soybean paste produced by the fermentation of naturally occurring bacteria and fungi, the presence of agmatine was reported, in concentrations ranging from not detected to over 5500 mg/kg 41.
Recently Özdestan et al. 42 have investigated the biogenic amine content in Kumru, a traditional Turkish fermented cereal food produced from chickpeas. In this case not detectable amounts of agmatine were found in the product, while other biogenic amines, such as putrescine, cadaverine, spermidine, spermine, and histamine were present in all samples.
Fermentation temperature, salt concentration, and starter selection have a significant effect on biogenic amine production in sauerkraut (fermented cabbage). In particular, the agmatine level seems to be dependent on the inoculum concentration of Lactobacillus curvatus. In sauerkraut brine, agmatine was found to be the prevalent amine, reaching a maximum value of 12 mg/L 43. Conversely, other authors have reported that lactic fermented cabbage juices do not contain agmatine, while putrescine, estimated at levels of up to 360 mg/L, is the most abundant amine, with assessed levels up to 360 mg/L; 40.
Fish and fish derived products
Spermine and spermidine are usually the main polyamines present in fresh tissues, at concentrations of less than 10 mg/kg. Depending on the fish species, the free amino acids present in the tissues, and the exposure conditions to spoilage bacteria, other amines can originate during storage or processing, reaching harmful levels, as reported for histamine in the case of animals belonging to the mackerel and herring families 44. The existence of a synergic relationship between spermine, spermidine, and histamine with potentially negative consequences on health, has been hypothesized 45. Therefore, various indexes have been proposed for fish derived products, such as the “biogenic amine index,” corresponding to the sum of the histamine, putrescine, cadaverine, and tyramine content 46 or the “quality index,” directly related to the histamine, putrescine, and cadaverine content and inversely related to the amounts of spermine and spermidine 47. Moreover, several authors have indicated that agmatine may be used as a freshness index, because immediately after catch agmatine can not be detected or its level is very low, but then in various fish species the agmatine content increases progressively during chilling storage, reaching concentrations of over 300 mg/kg after 7 days of storage 48. The formation of biogenic amines, commonly related to fish spoilage (histamine, tyramine, cadaverine and agmatine), was found to be significantly higher in the first stage of ripening in ungutted anchovies than in gutted ones. Conversely, no differences have been observed regarding the content of spermine and spermidine 49. In Rihaakuru, a cooked fish paste, the agmatine concentration is extremely variable, ranging from not detected to 161 mg/kg 50, whereas in other fermented and non-fermented fish paste, agmatine is not detectable 40. Fish storage conditions can help to reduce the processes favoring the formation of biogenic amines. In particular, refrigeration with flaked ice, traditionally applied to fish, significantly reduces agmatine and the formation of other biogenic amines compared with refrigeration at the same temperature, but without ice 48. Protective atmosphere packaging is frequently used to prolong the shelf-life of foodstuffs, and effectively reduces biogenic amine concentrations in different fish. In particular, Ruiz-Capillas and Moral 51 have shown that for tuna a gas mixture containing 60% CO2 is more effective in reducing these moieties than a gas mixture containing 40% CO2. This was particularly true in the case of white muscle of tuna, where after 25 days of storage an atmosphere containing 60% CO2 caused a 10-fold reduction of the agmatine level compared with the control stored in air.
Meat and meat products
Biogenic amines can be found in processed meat products as a consequence of microbial activity related to fermentation occurring during processing, or to microbial contamination of poor quality raw meat. Therefore, in cooked meat products (non-fermented) biogenic amines may represent a useful indicator for assessing the hygienic quality of the raw meat utilized for preparing these foodstuffs 52. Furthermore, the nitrosable secondary amines (agmatine, spermidine, and spermine) can form nitrosamines by reaction with nitrites, chemical agents considered to possess major carcinogenic properties. This is particularly important in some meat products with high polyamine levels and whose production process requires the use of nitrates and nitrites 53. As with fish, also fresh meat normally contains polyamines such as spermine and spermidine, the levels of which may vary slightly in fermented and ripened meat products. In fresh meat agmatine can be found at levels ranging from not detectable to about 3 mg/kg, while in fermented and ripened meat products the agmatine content may range more widely from not detectable to about 43 mg/kg. However, it has been reported that in these products a low formation of putrescine can be related to the presence of high levels of agmatine, the latter being an intermediate metabolite in the putrescine production pathway from arginine. Generally cooked meat products show lower polyamine concentrations than fresh meat 52.
Dry-cured “lacón” is a traditional cured meat product made in Spain, following manufacturing processes very similar to those utilized in the production of dry-cured ham. During the manufacture of dry-cured lacón the content of biogenic amine may vary greatly, as is also the case with ham. In addition, higher levels of tryptamine have been found in fresh lacón pieces than in fresh meat, whereas agmatine has not been detected. However, agmatine was generated during the drying-ripening stage, both with and without additives and the values reached in the final product (around 8 mg/kg) were higher than those found in ham 54.
The technology applied in meat processing may influence biogenic amine production. For example, sugar omission in the production of slightly fermented sausages is not recommended, because it may cause an increase in agmatine and the accumulation of other biogenic amines during manufacture and storage 52. Furthermore, different concentrations of biogenic amines have been found in fresh, fermented, and cooked meat products, either treated with high pressure processing or under protective atmosphere packaging, which were closely related to the type of product and the processing conditions. In general, protective atmosphere packaging has a positive effect in reducing biogenic amine formation in these products. In commercial meat products treated with high pressure processing, higher biogenic amine concentrations, particularly agmatine, have been reported compared with non-treated samples. This negative effect of high pressure processing has also been observed in frankfurters, butifarra, cooked ham, and chorizo; for the latter product, it has been observed how commercially available non-treated samples may contain agmatine concentrations of about 2 mg/kg, while chorizo treated by high pressure processing may show a 20-fold increase in agmatine content 55.
Milk and dairy products
In milk, as in other fresh foods derived from animals, spermine and spermidine are the prevalent polyamines, whereas it must be reported that agmatine was not detectable 56. Also in industrial kefir, a milk product belonging to the category of mixed lactic acid and ethanol fermented beverages, agmatine was not detectable. The estimated biogenic amine content in kefir, ranging from 2 to 35 mg/L, is generally lower than that found in other fermented foods. Short fermentation time, the use of starter culture, controlled production conditions such as heating at 90–95°C before fermentation and a low microbial count in the raw material are the main reasons for the low biogenic amine content. Higher biogenic amine levels can be expected in artisanal or home-made kefir compared with industrial samples, because of worse hygienic conditions 57.
Cheeses represent an ideal environment for the production of amines. The factors influencing the formation, accumulation, and type of amines are: the availability of amino acids, the presence of bacteria capable of decarboxylating amino acids, pH, salt concentration, water availability, temperature and duration of ripening and storage, bacterial density, the presence of cofactors, and amine catabolism. The pH of cheese is appropriate for biogenic amine production, generally between 5.0 and 6.5, depending on the age and type of the products. Cheeses are also rich in pyridoxal phosphate, which is required for amino acid decarboxylase activity. Studies have shown that in cheese the concentration of biogenic amines decreases with increased fat content. This phenomenon has been attributed to changes in the water activity, which inhibits the growth of proteolytic bacteria, causing a reduction of free amino acid concentration in the medium 53. Numerous bacteria, both intentional or adventitious and isolated from cheese, have been reported as biogenic amine producers 56. Several outbreaks of histamine poisoning have occurred following the consumption of cheese; also cases of hypertensive crisis and migraine headache have been observed after the ingestion of cheeses with high levels of tryptamine and 2-phenylethylamine. The use of bacteriocin-producing starters can prevent the formation of histamine. Moreover, the addition of microbial isolates that degrade biogenic amines in order to prevent the presence of hazardous levels of amines in the final products has been proposed. Although the type and levels of amines in each kind of cheese may vary considerably, agmatine is a polyamine which is rarely present in cheeses 58.
Agmatine benefits
In 1995, Gilad, et al. 59 firstly reported the neuroprotective action of agmatine. Henceforward, more and more studies showed neuroprotection of agmatine in stroke, traumatic brain injuries, neurodegenerative disorders, neuropathic pain, epilepsy, and even in mental disorders. Anti-oxidation, anti-apoptosis, anti-inflammation, brain blood barrier (BBB) protection and brain edema prevention are the common mechanisms involved in the neuroprotective effects.
Agmatine has been shown in preclinical experiments to interact with multiple molecular targets important for nervous system function. These include: 1) modulation of several neurotransmitter receptors and receptor ionophores (e.g., nicotine, NMDA, imidazoline, and alpha 2-adrenoceptors) 60; 2) blockade of key ionic channels (e.g., ATP-sensitive K+ channels and voltage-gated Ca++ channels) 61; 3) inhibition of nitric oxide (NO) synthase and thus, modulation of NO production 62; 4) inhibition of protein ADP-ribosylation 63 and thus, interference with cell signalling; 5) inhibition of matrix metalloproteases 64, enzymes implicated in nerve cell death and neuropathic pain 65; and 6) inhibition of advanced glycation end (AGE)-product formation, a process involved in the pathology of diabetes and neurodegenerative diseases 66.
Agmatine in ischemic stroke
Stroke has been the second most common disease to cause death and disability in adults around the world 67. Ischemic stroke, which accounts for about 87% of cases, is the most common subtype of stroke 68. Ischemic stroke is the result of insufficient blood and oxygen supply to the brain. The cell in central portion of the ischemic tissue, known as infarct core, is afflicted with irreversible damage and the area around the infarct core, called penumbra, is at risk of infarction and can be reverted 69.
Previous studies have proved that the occurrence of stroke promoted the expression of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), which could destroy the structure of blood brain barrier, leading to brain edema 70. In addition, the disruption of neuro-inflammatory or oxidative stress balance could produce excessive inflammatory cytokines, reactive oxygen species (ROS) and free radicals, which initiate cell death ultimately 71.
Numerous experimental researches, most of which focused on the salvation of the tissue in the penumbra, had been carried out to explore the optimal neuroprotective drugs for cerebral ischemia 72. But the severe side-effects of these drugs posed great limitation on further clinical trials. In contrast, agmatine displayed its safety in both experimental and clinical trials. The sulfate salt of agmatine had been used as a dietary ingredient many years ago and now is available as a nutraceutical 27. Besides, many studies confirmed the neuroprotective role of agmatine in strokes 73.
Kim et al. 74 showed the neuroprotective effects of agmatine both in vivo and in vitro through the mechanism of reducing the production of nitric oxide (NO) by competitively inhibiting neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS). Meanwhile, agmatine can also activate endothelial nitric oxide synthase (eNOS) in endothelial cells, and thus increase the production of NO, which acts as a vasodilator to increase the blood flow in the ischemic areas 75. Feng et al. 76 reported that both endogenous and exogenous agmatine can exert their functions on the NOS (nitric oxide synthase) and reduce hypoxic-ischemic brain injury in neonatal rats. Besides, many other studies verified the effects of agmatine on the three kinds of NOS mentioned above in the setting of cerebral ischemia 77.
Brain blood barrier (BBB) is extremely important in maintaining homeostasis and microvascular integrity 78. However, the cerebral ischemic attack could induce upregulation of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), which could destroy the integrity of blood brain barrier. Hyun et al. 79 and Yang et al. 80 both demonstrated that exogenous agmatine could inhibit the expression of MMP-2 and MMP-9 by induction of eNOS in vitro. Moreover, Hyun and his colleagues 79 utilized retrovirus to induce the endogenous agmatine, and their findings suggested that endogenous agmatine could reduce the MMP-2 and MMP-9 expression by regulation of eNOS, NO and activation of transcription factor 3(ATF3). In addition to detecting MMP-2 and MMP-9, Ahn and his colleagues 81 took advantage of dynamic contrast-enhanced MR imaging to evaluate the beneficial effects of agmatine on blood brain barrier stabilization.
Brain edema frequently was observed to be involved in the worsening process of cerebral ischemia and contributed to increasing mortality after attack of stroke. Jae and his colleagues 82 explored the mechanism of brain edema and the effects of agmatine. Their findings suggested that the blood brain barrier disruption and the upregulation of aquaporins-1 and -9 (AQPs) were well correlated with brain edema, and these disadvantages can be significantly attenuated by agmatine treatment. Besides, some other studies revealed the effects of agmatine on reducing the expression of AQP-4 and thus attenuated the brain edema as well 83.
Additionally, studies also showed that agmatine contributed to reducing the apoptosis of neuron and cerebral astrogliosis after cerebral ischemia in a rat model 84. Jeong et al. 83 found anti-inflammatory character of agmatine in diabetic middle cerebral artery obstruction (MCAO) rats by decreasing the expression of high-mobility group box 1, RAGE, toll-like receptor (TLR)-2,4.
Overall, the agmatine exerted its neuroprotective effect on ischemic stroke both in in vivo and in vitro experimental models 85.
Agmatine in traumatic brain injury
Traumatic brain injury (TBI) is a condition of emergency and ranks second to conventional stroke in causing death and disability, which place tremendous burden on the family and society 86. It can be divided into primary injury and secondary injury 87. A series of pathophysiological changes are involved in the progress of traumatic brain injury, which include brain edema, cellular apoptosis, blood brain barrier disruption, inflammation and so on 88. However, there were no ideal therapeutic drugs for the patients suffering from traumatic brain injury so far. Recently, many studies verified the beneficial effects of agmatine in traumatic brain injury. In 1996, Gad and his colleagues 89 explored the neuroprotective effects of agmatine in rodent brain injury. Previous researches reported that excessive accumulation of glutamate or NO could lead to cellular ischemia and neurotoxicity 90. In a rat model of fluid percussion brain injury, Jinn et al. 91 found that agmatine could reduce excessive glutamate and NO, and attenuated traumatic brain injury resultantly. Furthermore, they also demonstrated that traumatic brain injury-induced intracranial hypertension, cerebral hypotension, cerebral infarction, motor and proprioception deficits and body weight loss could all be alleviated by agmatine 91. In 2010, Jinn and his colleagues 92 conducted a series of experiments on the neuroprotective effects of agmatine, and found that agmatine could improve the outcome of traumatic brain injury in rats by attenuating neuronal and glial apoptosis, inhibiting gliosis, promoting angiogenesis and neurogenesis. In addition, Jae and his colleagues 93 found that agmatine could reduce brain edema by suppressing the expression of AQP1, 4 and 9. Besides, they also found that agmatine could inhibit cellular apoptosis by inhibiting the phosphorylation of MAPKs and increasing the nuclear translocation of NF-κB after traumatic brain injury. In addition, agmatine had also been proved to play neuroprotective role in rat spinal cord model. It could significantly restore the locomotor function and reduce tissue damage by blocking the N-methyl-D-aspartate (NMDA) receptor and NOS 94.
Agmatine in Parkinson’s disease
Parkinson’s disease commonly presented as motor dysfunction, is one of the most common neurodegenerative diseases in the aged 95. It was reported that the degeneration of dopaminergic neurons in the substantia nigra pars, which leads to motor dysfunction, is the on-off step in the progression of Parkinson’s disease. Recently, many studies showed that glutamatergic neurotransmission also contributed greatly to the pathogenesis of Parkinson’s disease 96.
Excitatory amino acids were assumed to have neurotoxicity and aggravate the condition of patients with Parkinson’s disease 74. N-methyl-D-aspartate (NMDA) is the receptor of excitatory amino acid, and its antagonists like memantine, amantadine and agmatine were extensively proved to ameliorate motor function of Parkinson’s disease patients 97.
In the experimental model of Parkinson’s disease induced by rotenone, agmatine could significantly decrease the level of oxidative stress in SH-SY5Y cells. Besides, agmatine could also reduce the cellular apoptosis induced by rotenone 98. In addition, a recent in vitro study demonstrated that agmatine could prevent redox reaction and cellular injury 99.
Gilad and his colleagues 78 developed a new mouse Parkinson’s disease model by intranasal administration of 1-methyl-4-phenyl-4-1,2,3,6-tetrahydropyridine (MPTP), and found that agmatine could provide a partial (31%) protection of Parkinson’s disease induced by MPTP. Matheus and his colleagues delivered agmatine to a group of aged mice before the treatment with MPTP. Their results demonstrated that agmatine could prevent the occurrence of motor, cognitive and neurological impairments induced by MPTP 100.
Agmatine in Alzheimer’s disease
Alzheimer’s disease commonly accompanied with progressive cognitive dysfunction, is also one of the most common neurodegenerative diseases in the aged. Its pathological hallmarks are extracellular deposition of amyloid-beta peptide, and presence of neurofibrillary tangles in the neuron 101 which are mainly distributed in the hippocampus, cerebral cortex and basal ganglia 102. Currently, the accumulation of amyloid-beta peptide, abnormal phosphorylation of Tau peptide, oxidative stress and radical injury has been recognized as the main pathophysiologic mechanism of Alzheimer’s disease 103. Agmatine showed its neuroprotective role in oxidative stress, radical injury and other pathological process. For example, Baranov et al. 104 revealed that agmatine could exert its anti-oxidative effect by activating many kinds of antioxidants including glutathione. Besides, agmatine could decrease the level of free radical and inhibit the accumulation of amyloid-beta peptide 105.
N-methyl-D-aspartate (NMDA) is the receptor of the excitatory amino acid and its dysfunction can lead to the apoptosis of neuron 74. It was reported that the level of endogenous agmatine rose sharply on the setting of brain injury 91 and exerted its neuroprotective effect by competitively inhibiting the NMDA receptor and activity of excitatory amino acid 71. In the Alzheimer’s disease patients, accumulation of amyloid-beta peptide in neuron increased the level of excitatory amino acid, which then produced neurotoxicity. Agmatine was reported to postpone apoptosis of neuron by inhibiting the neurotoxicity of excitatory amino acids 106. Except for its effect on neurotoxicity, amyloid-beta peptide could also down-regulate the insulin receptor which widely exists in the central nervous system. However, retaining the insulin receptor and its ligand is quite important in the treatment of Alzheimer’s disease 107. Somang and his colleagues demonstrated that agmatine could promote the secretion of insulin and protect the insulin receptor via binding with the imidazoline receptors 108. This process could then reduce the accumulation of amyloid-beta peptide and inhibit the abnormal phosphorylation of Tau peptide, which ultimately ameliorate the cognition and memory injury of Alzheimer’s disease patients 109.
Agmatine in epilepsy
Epilepsy is a chronic process characterized by recurrent paroxysmal seizures 110. Epileptogenesis resulted from synchronization and propagation of excessive excitability, which spread from hyper-excitable neurons and glial cells to the normal non-epileptogenic tissue 111. The glutamatergic receptors and NMDA receptor were reported to be involved in the initiation of some kinds of epilepsies 112. Besides, epileptic seizures could increase the production of NO, and pre-treatment with NOS inhibitors could give precaution against some types of seizure 113. As aforementioned, the agmatine could act as an antagonist of NAMD receptor, thus several studies had been carried out to test the neuroprotective effects of agmatine in experiment animals with epilepsies. Their results demonstrated that agmatine could reduce both the incidence and intensity of epilepsy and also exert anticonvulsant effect 114. Additionally, agmatine could inhibit the activation of NOS and reduce the production of NO. It was reported that both the NOS inhibitor and exogenous agmatine had neuroprotective effects on epilepsy 115. Except for the anticonvulsant effect, agmatine could also strengthen the anticonvulsant effect of some other drugs, such as phenobarbital, valproate and lithium chloride 116. Overall, agmatine proved to have neuroprotective effects in epileptic patients.
In the meanwhile, several other studies 117, 118 contradicted this inference, Abe et al. 117 reported that agmatine (200-800μM) could induce the release of glutamate, which could lead to neuronal death. Besides, Luszczki and his colleagues 118 also suggested that the synergistic effect of agmatine in anticonvulsant is also uncertain, which meant that further researches of agmatine in epilepsy are needed before it can be extensively clinically used.
Agmatine in mental disorders
Mental disorder is a group of systemic diseases characterized by a variety of physical and mental discomfort, such as depression, anxiety, addiction, schizophrenia and so on 119. Agmatine has been long-term studied for its neuroprotective effects in mentor diseases. Many studies reported that agmatine exerts its antidepressant-like action by inhibiting the NMDA receptors or interacting with 5-HT1A/1B and 5-HT2 receptors 120. Besides, agmatine could suppress the expression of NOS, NMDA receptors or imidazoline receptors, and alleviate drug addiction induced by opioid, morphine, ethanol or psychostimulants 121. Additionally, agmatine may have beneficial effects on anxiety, schizophrenia and some other mentor diseases in experimental model 122.
Anti-apoptotic effects of agmatine
Apoptosis is one type of cell death characterized by energy dependence and programmed cell death 123. Apoptosis is of vital importance to normal physiological metabolism, growth and development, keeping hemostasis by scavenging the aging or damaged cells, shaping of organs or regulating immune system by removal of defective and excessive cells 124. However, uncontrolled apoptosis may result in various pathological processes of different diseases, like cancers, Alzheimer’s disease and stroke 125. El-Sherbeeny et al. 126 demonstrated that agmatine could protect rat liver from nicotine-induced damage by inhibiting the production of proapoptotic protein Bax. In vitro, Mary and his colleagues 127 identified the anti-apoptotic effects of agmatine to the Ha-Ras-transformed murine NIH-3T3 cell line by reducing the expression of Bax and caspase-3. In addition, agmatine was also observed to be involved in the modulation of programmed cell death in rats and inhibit the proliferation of human mast cell leukemia cells (HMC-1) and HL-60 cells 128. Many researches have demonstrated the anti-apoptotic effect of agmatine in various neurological disorders. For example, Kim and his colleagues 95 demonstrated that agmatine could reduce cellular apoptosis in traumatic brain injury of rats by inhibiting phosphorylation of MAPKs and increasing nuclear translocation of NF-kappaB. In addition, Zarifkar 129 reported that inhibition of caspase-3 expression by agmatine could also prevent hippocampal apoptosis and spatial memory impairment induced by lipopolysaccharide (LPS). Besides, in rat model of Alzheimer’s disease, agmatine reduced cellular apoptosis through inhibiting the expression of caspase-3 and Bax, and improved the level of Bcl2, PI3K, Nrf2, and gamma-glutamyl cysteine 109.
Several in vitro studies reported the protection of agmatine on the human-derived dopaminergic neuroblastoma cell line (SH-SY5Y). Agmatine exerted its anti-apoptotic effects by increasing the amount of phosphorylated Akt /Akt, inhibiting the GSK-3β activity and decreasing the expression of apoptotic markers, such as caspase 3, Bax and cytochrome c.
Overall, the anti-apoptotic effect of agmatine had been well demonstrated in neurological diseases.
Agmatine anti-inflammatory effects
Inflammation is a complex immune response of organisms to the injury. Under normal conditions, inflammation could help to scavenge the necrotic cells or tissues, and initiate the tissue repair process 130. However, excessive activation of immune responses is harmful to the organisms and can cause injury 131. Agmatine exerted its anti-inflammatory effect in many ways. For example, in vivo, Taksande 132 demonstrated that agmatine could attenuate the symptom of arthritis by reducing the level of inflammatory cytokines, like tumor necrosis factor (TNF)-α and interleukins (IL)-6. Meanwhile, an in vitro study showed that agmatine could inhibit the production of pro-inflammatory cytokines, such as IL-6, TNF-α and CCL2, and reduce the cell death 133.
Inflammatory neurodegeneration also plays a key role in the pathogenesis of neurological diseases, including acute diseases (stroke or traumatic injury) and chronic neurodegenerative diseases (Alzheimer’s disease or Parkinson’s disease) 131. Sahin 134 showed that agmatine could attenuate sub-chronic stress by down-regulating the gene expression of nod-like receptor protein 3 (NLRP3) inflammasome components (NLRP3, NF-kappaB, PYCARD, caspase-1, IL-1β and IL-18) and reducing the level of pro-inflammatory cytokines. Besides, agmatine could also revert the change of anti-inflammatory cytokines, such as IL-4 and IL-10. In addition, the anti-inflammatory effect of agmatine was also proved in many other neurological diseases, such as transient brain ischemia, depression, traumatic brain injury and micro-opioid receptor tolerance, by regulating the expression of inflammatory cytokines 135.
Agmatine anti-oxidant effects
Oxidative stress, which is mainly caused by overbalance of pro-oxidant/anti-oxidant system in cells, takes part in the pathogenesis of many diseases 136. Various agents including agmatine had been demonstrated to decrease oxidative stress and protect organisms from injury. Iizuka 137 reported that agmatine could protect the retinal ganglion cells from H2O2-induced injury through the alpha 2-adrenergic receptor signaling pathway. Bratislav 138 found that agmatine could exert its anti-oxidative effect by protecting antioxidant defense system and restoring the antioxidant capacity in liver tissue. Besides, several studies had demonstrated the anti-oxidative effect of agmatine in the setting of neurological diseases. Gawali and his colleagues 139 reported that agmatine could ameliorate depressive-like behavior by reducing the oxidative/nitrosative stress evoked by LPS in hippocampus and prefrontal cortex, and this effect may be achieved via preventing lipid peroxidation and regulating the activity of superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase 140. In addition, agmatine has also been proved to exert anti-oxidative stress in stroke and traumatic brain injury by reducing the generation of reactive oxygen species (ROS) and free radicals 77.
Blood brain barrier protection and brain edema prevention
Blood brain barrier is a continuous, non-fenestrated system which regulates the movement of many particles and cells, such as ion, toxicant and inflammatory cells. Any factors disrupting the blood brain barrier would deteriorate the condition of neurological diseases, including stoke, traumatic brain injury and neurodegenerative diseases 141. The most common complication of blood brain barrier disturbance was the vasogenic brain edema, which was reported to be related to the early expression of matrix metalloproteinases-2,9 after brain injury 142. Agmatine was reported to have significant effect in inhibiting the expression of matrix metalloproteinases 79. Therefore, regulation of agmatine on the expression of matrix metalloproteinases may be the potential mechanism of blood brain barrier protection and vasogenic brain edema attenuation.
In addition, another type of brain edema that commonly occurs during brain injury is cytotoxic brain edema. One of the most frequent and elaborated mechanisms of cytotoxic edema is the dysfunction of aquaporin (AQP) in the brain. The aquaporin are a group of water channel proteins which regulate the movement of water molecule between plasma membrane. AQP1, 4 and 9 were clearly identified in the brain tissue. AQP1, 4 were reported to be involved in cerebrospinal fluid formation and their dysfunction could also contribute to brain edema 143. Several studies demonstrated that the mechanism of agmatine in preventing cytotoxic edema is to suppress the expression of aquaporin (AQP)-1, 4, and 9. The changes had been demonstrated in many types of neurological diseases, such as traumatic brain injury, stroke or degenerative diseases 144.
Although the effect of agmatine in reducing the brain edema had been well demonstrated, its therapeutic effect was doubted by some researchers and more studies should be launched 145.
Agmatine dosage
At this time there is not enough scientific information to determine an appropriate range of doses for agmatine.
Agmatine side effects
No adverse effect of 5 years daily agmatine intake of 2.67 g agmatine sulfate/day for humans 3 and 21 days of 3.560 g agmatine sulfate/day for humans 2. Gilad and his colleagues 3 assessed long-term safety of oral agmatine treatment by consuming a 2.67 g agmatine sulfate daily dosage of oral agmatine over a period of 4-5 years. All measurements remained within normal values and no discomfort was observed during the follow-up period 3.
In a randomized, double-blind, placebo-controlled trial of agmatine sulfate (1.335 g/day agmatine sulfate for 10 days, 2.670 g/day for 10 days, 3.560 g/day for 10 days, and 3.560 g/day for 21 days) in patients with herniated lumbar disc-associated radiculopathy 146, three participants reported discomfort as a result of mild-to-moderate diarrhea and nausea that appeared 2–3 days after taking the high dose (3.560 g/day) of agmatine sulfate. These symptoms disappeared within 1–2 days after treatment cessation. Only one participant, however, chose to discontinue for that reason. None of the affected participants required any treatment related to the side-effects. In summary, only 2 of the 34 participants failed to complete the study, one as a result of free will discontinuation and the other as a result of mild-to-moderate adverse effects. These mild side-effects were initially predicted for two reasons: One, sulfate salts (e.g., magnesium sulfate [Epsom salt] and sodium sulfate [Glauber’s salt]) are long known to stimulate peristaltic action and are used therapeutically as purgative or cathartic agents; and two, guanidine group-containing dietary ingredients, arginine and creatine, are also known to cause mild diarrhea and nausea at high doses 146. Additionally, these mild side effects were expected based on previous experience with a group of nine people, who elected, on their own cognizance, to take agmatine sulfate on a continuous basis for a year. Three individuals developed transient mild diarrhea and gas which began 1–3 days after treatment initiation and subsided within several days thereafter 146.
References- Mishra, Shwetakshi & Mishra, Ravi & Rahim, Iqra & Singh, Archana & Jhariya, Dalchand & Manzoor, Nadiya & Manjari, K. & Rafeeq, Jauhar & Tekam, Manish & Mullasseri, Sileesh & Mehraj, Ufaid & Arjumand, Tahera & Hans, Aradhana. (2021). Neuroinflammatory Diseases: Agmatine for Memory Defects. Current Science. 120. 610-613.
- Keynan O, Mirovsky Y, Dekel S, Gilad VH, Gilad GM. Safety and Efficacy of Dietary Agmatine Sulfate in Lumbar Disc-associated Radiculopathy. An Open-label, Dose-escalating Study Followed by a Randomized, Double-blind, Placebo-controlled Trial. Pain Med. 2010 Mar;11(3):356-68. doi: 10.1111/j.1526-4637.2010.00808.x
- Gilad GM, Gilad VH. Long-term (5 years), high daily dosage of dietary agmatine–evidence of safety: a case report. J Med Food. 2014 Nov;17(11):1256-9. doi: 10.1089/jmf.2014.0026
- Lee J.-P., Chen W., Wu H.-T., Lin K.-C., Cheng J.-T. Metformin can activate imidazoline I-2 Receptors to lower plasma glucose in type 1-like diabetic rats. Horm. Metab. Res. 2011;43:26–30. doi: 10.1055/s-0030-1267169
- Satriano J., Cunard R., Peterson O.W., Dousa T., Gabbai F.B., Blantz R.C. Effects on kidney filtration rate by agmatine requires activation of ryanodine channels for nitric oxide generation. Am. J. Physiol. Ren. Physiol. 2008;294:F795–F800. doi: 10.1152/ajprenal.00392.2007
- Mun C.H., Lee W.T., Park K.A., Lee J.E. Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain. Anat. Cell Biol. 2010;43:230–240. doi: 10.5115/acb.2010.43.3.230
- Lee W.T., Hong S., Yoon S.H., Kim J.H., Park K.A., Seong G.J., Lee J.E. Neuroprotective effects of agmatine on oxygen-glucose deprived primary-cultured astrocytes and nuclear translocation of nuclear factor-kappa B. Brain Res. 2009;1281:64–70. doi: 10.1016/j.brainres.2009.05.046
- Li F., Wu N., Su R., Chen Y., Lu X., Liu Y., Li J. Imidazoline receptor antisera-selected/Nischarin regulates the effect of agmatine on the development of morphine dependence. Addict. Biol. 2012;17:392–408. doi: 10.1111/j.1369-1600.2011.00373.x
- Taksande B.G., Kotagale N.R., Patel M.R., Shelkar G.P., Ugale R.R., Chopde C.T. Agmatine, an endogenous imidazoline receptor ligand modulates ethanol anxiolysis and withdrawal anxiety in rats. Eur. J. Pharmacol. 2010;637:89–101. doi: 10.1016/j.ejphar.2010.03.058
- Vargiu C., Cabella C., Belliardo S., Cravanzola C., Grillo M.A., Colombatto S. Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur. J. Biochem. 1999;259:933–938. doi: 10.1046/j.1432-1327.1999.00126.x
- Piletz J.E., Aricioglu F., Cheng J.-T., Fairbanks C.A., Gilad V.H., Haenisch B., Halaris A., Hong S., Lee J.E., Li J., et al. Agmatine: Clinical applications after 100 years in translation. Drug Discov. Today. 2013;18:880–893. doi: 10.1016/j.drudis.2013.05.017
- Wu N., Su R.-B., Li J. Agmatine and imidazoline receptors: Their role in opioid analgesia, tolerance and dependence. Cell. Mol. Neurobiol. 2008;28:629–641. doi: 10.1007/s10571-007-9164-y
- Dixit M.P., Thakre P.P., Pannase A.S., Aglawe M.M., Taksande B.G., Kotagale N.R. Imidazoline binding sites mediates anticompulsive-like effect of agmatine in marble-burying behavior in mice. Eur. J. Pharmacol. 2014;732:26–31. doi: 10.1016/j.ejphar.2014.02.045
- Barua S., Kim J.Y., Kim J.Y., Kim J.H., Lee J.E. Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors. Neurochem. Res. 2019;44:735–750. doi: 10.1007/s11064-018-02712-1
- Uribe E, Reyes MB, Martínez I, Mella K, Salas M, Tarifeño-Saldivia E, López V, García-Robles M, Martínez-Oyanedel J, Figueroa M, Carvajal N, Schenk G. Functional analysis of the Mn2+ requirement in the catalysis of ureohydrolases arginase and agmatinase – a historical perspective. J Inorg Biochem. 2020 Jan;202:110812. doi: 10.1016/j.jinorgbio.2019.110812
- Reyes, M. B., Martínez-Oyanedel, J., Navarrete, C., Mardones, E., Martínez, I., Salas, M., López, V., García-Robles, M., Tarifeño-Saldivia, E., Figueroa, M., García, D., & Uribe, E. (2020). Insights into the Mn2+ Binding Site in the Agmatinase-Like Protein (ALP): A Critical Enzyme for the Regulation of Agmatine Levels in Mammals. International journal of molecular sciences, 21(11), 4132. https://doi.org/10.3390/ijms21114132
- Moinard C, Cynober L, de Bandt JP. Polyamines: metabolism and implications in human diseases. Clin Nutr. 2005 Apr;24(2):184-97. doi: 10.1016/j.clnu.2004.11.001
- Molderings GJ, Heinen A, Menzel S, Göthert M. Exposure of rat isolated stomach and rats in vivo to [(14)C]agmatine: accumulation in the stomach wall and distribution in various tissues. Fundam Clin Pharmacol. 2002 Jun;16(3):219-25. doi: 10.1046/j.1472-8206.2002.00073.x
- Wade CL, Schuster DJ, Domingo KM, Kitto KF, Fairbanks CA. Supraspinally-administered agmatine attenuates the development of oral fentanyl self-administration. Eur J Pharmacol. 2008 Jun 10;587(1-3):135-40. doi: 10.1016/j.ejphar.2008.04.007
- Prasad A, Prasad C. Agmatine enhances caloric intake and dietary carbohydrate preference in satiated rats. Physiol Behav. 1996 Oct;60(4):1187-9. doi: 10.1016/0031-9384(96)00151-5
- Uzbay IT, Lal H. Effects of NG-nitro-L-arginine methyl ester, 7-nitro indazole, and agmatine on pentylenetetrazol-induced discriminative stimulus in Long-Evans rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002 Apr;26(3):567-73. doi: 10.1016/s0278-5846(01)00309-8
- Krass M, Wegener G, Vasar E, Volke V. Antidepressant-like effect of agmatine is not mediated by serotonin. Behav Brain Res. 2008 Apr 9;188(2):324-8. doi: 10.1016/j.bbr.2007.11.013
- Agostinelli E, Marques MP, Calheiros R, Gil FP, Tempera G, Viceconte N, Battaglia V, Grancara S, Toninello A. Polyamines: fundamental characters in chemistry and biology. Amino Acids. 2010 Feb;38(2):393-403. doi: 10.1007/s00726-009-0396-7
- Battaglia V, Grancara S, Satriano J, Saccoccio S, Agostinelli E, Toninello A. Agmatine prevents the Ca(2+)-dependent induction of permeability transition in rat brain mitochondria. Amino Acids. 2010 Feb;38(2):431-7. doi: 10.1007/s00726-009-0402-0
- Okamoto A, Sugi E, Koizumi Y, Yanagida F, Udaka S. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci Biotechnol Biochem. 1997 Sep;61(9):1582-4. doi: 10.1271/bbb.61.1582
- Ozdestan O, Uren A. Biogenic amine content of shalgam (salgam): a traditional lactic acid fermented Turkish beverage. J Agric Food Chem. 2010 Feb 24;58(4):2602-8. doi: 10.1021/jf903775z
- Galgano, F., Caruso, M., Condelli, N., & Favati, F. (2012). Focused review: agmatine in fermented foods. Frontiers in microbiology, 3, 199. https://doi.org/10.3389/fmicb.2012.00199
- Galgano F., Caruso M., Perretti G., Favati F. (2011). Authentication of Italian red wines on the basis of the polyphenols and biogenic amines. Eur. Food Res. Technol. 232, 889–89710.1007/s00217-011-1457-1
- Alberto M. R., Arena M. E., Manca De Nadra M. C. (2007). Putrescine production from agmatine by Lactobacillus hilgardii: effect of phenolic compounds. Food Control 18, 898–90310.1016/j.foodcont.2006.05.006
- Galgano F., Caruso M., Favati F. (2009). “Biogenic amines in wines: a review,” in Red Wine and Health, ed. O’Byrne P. (New York: Nova Science Publishers Inc.), 173–203.
- Rodríguez H, Curiel JA, Landete JM, de las Rivas B, López de Felipe F, Gómez-Cordovés C, Mancheño JM, Muñoz R. Food phenolics and lactic acid bacteria. Int J Food Microbiol. 2009 Jun 30;132(2-3):79-90. doi: 10.1016/j.ijfoodmicro.2009.03.025
- Sohrabvandi S., Mortazavian A. M., Rezaei K. (2012). Health-related aspects of beer: a review. Int. J. Food Properties 15, 350–37310.1080/10942912.2010.487627
- Izquierdo-Pulido M. I., Jover T. H., Font A. M., Vidal-Carou M. C. (1996). Biogenic amines in European beers. J. Agric. Food Chem. 44, 3159–316310.1021/jf960155j
- De Borba B. M., Rohrer J. S. (2007). Determination of biogenic amines in alcoholic beverages by ion chromatography with suppressed conductivity detection and integrated pulsed amperometric detection. J. Chromatogr. 1155, 22–3010.1016/j.chroma.2007.01.114
- Halász A., Baráth Á., Holzapfel W. H. (1999a). The biogenic amine content of beer; the effect of barley, malting and brewing on amine concentration. Eur. Food Res. Technol. 208, 418–426
- Cirilo M. P. G., Coelho A. F., Araúio C. M., Gonçalves F. R. B., Nogueira F. D., Glória M. B. A. (2003). Profile and levels of bioactive amines in green and roasted coffee. Food Chem. 82, 397–40210.1016/S0308-8146(02)00560-5
- Leite da Silveira T. M. L., Tavares E., Glória M. B. A. (2007). Profile and levels of bioactive amines in instant coffee. J. Food Compost. Anal. 20, 451–45710.1016/j.jfca.2007.02.003
- Farkas S., Hajós G. (1998). Monitoring of biologically amines in cereals and cereals based food products by HPLC. Chromatographia 48, 37–4210.1007/BF02467513
- Morris B. (2003). Bio-functional legumes with nutraceutical, pharmaceutical, and industrial uses. Econ. Bot. 57, 254–26110.1663/0013-0001(2003)057[0254:BLWNPA]2.0.CO;2
- Kirschbaum J., Rebscher K., Brückner H. (2000). Liquid chromatographic determination of biogenic amines in fermented foods after derivatization with 3,5-dinitrobenzoyl chloride. J. Chromatogr. A 881, 517–53010.1016/S0021-9673(00)00257-0
- Shukla S., Park H. E., Kim J. K., Kim M. (2010). Determination of biogenic amines in Korean traditional fermented soybean paste (Doenjang). Food Chem. Toxicol. 48, 1191–119510.1016/j.fct.2010.01.034
- Özdestan Ö., Alpozen E., Guven G., Üren A. (2011). Monitoring of biogenic amines in Kumru: a traditional fermented cereal food. Int. J. Food Properties 10.1080/10942912.2010.511754
- Halász A., Baráth Á., Holzapfel W. H. (1999b). The influence of starter culture selection on sauerkraut fermentation. Eur. Food Res. Technol. 208, 434–438.
- Önal A. (2007). A review: current analytical methods for the determination of biogenic amines in foods. Food Chem. 103, 1475–147610.1016/j.foodchem.2006.08.028
- Silla Santos MH. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996 Apr;29(2-3):213-31. doi: 10.1016/0168-1605(95)00032-1
- Veciana-Nogues M. T., Marine-Font A., Vidal-Carou M. C. (1997). Biogenic amines as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines, and organoleptic changes. J. Agric. Food Chem. 45, 2036–204110.1021/jf9605714
- Mietz J. L., Karmas E. (1997). Chemical quality index of canned tuna as determined by high-performance liquid chromatography. J. Food Sci. 42, 155–15810.1111/j.1365-2621.1977.tb01240.x
- Chotimarkorn C. (2011). Quality changes of anchovy (Stolephorus heterolobus) under refrigerated storage of different practical industrial methods in Thailand. J. Food Sci. Technol. 10.1007/s13197-011-0505-y
- Pons-Sanchez-Cascado S., Veciana-Nogues M. Y., Vidal-Carou M. C. (2003). Effect of delayed gutting on biogenic amine contents during ripening of European anchovies. Eur. Food Res. Technol. 216, 489–483.
- Naila A., Flint S., Fletcher G. C., Bremer P. J., Meerdink G. (2011). Biogenic amines and potential histamine-forming bacteria in Rihaakuru (a cooked fish paste). Food Chem. 128, 479–48410.1016/j.foodchem.2011.03.057
- Ruiz-Capillas C., Moral A. (2004). Free amino acids and biogenic amines in red and white muscle of tuna stored in controlled atmosphere. Amino Acids 26, 125–13210.1007/s00726-003-0054-4
- Bover-Cid S., Izquierdo-Pulido M., Vidal Carou M. C. (2001). Changes in biogenic amine and polyamine contents in slightly fermented sausages manufactured with and without sugar. Meat Sci. 57, 215–22110.1016/S0309-1740(00)00096-6
- Ruiz-Capillas C, Jiménez-Colmenero F. Biogenic amines in meat and meat products. Crit Rev Food Sci Nutr. 2004;44(7-8):489-99. doi: 10.1080/10408690490489341
- Lorenzo J. M., Martínez S., Franco I., Carballo J. (2007). Biogenic amines content during the manufacture of dry-cured lacón, a Spanish traditional meat product: effect of some additives. Meat Sci. 77, 287–29310.1016/j.meatsci.2007.03.020
- Ruiz-Capillas C., Carballo J., Jiménez-Colmenero F. (2007). Consequences of high-pressure processing of vacuum-packaged frankfurters on the formation of polyamines: effect of chilled storage. Food Chem. 104, 202–20810.1016/j.foodchem.2006.11.024
- Novella-Rodríguez S., Veciana-Nogués M. T., Roig-Sagués A. X., Trujillo-Mesa A. J., Vidal-Carou M. C. (2002). Influence of starter and nonstarter on the formation of biogenic amine in goat cheese during ripening. J. Dairy Sci. 85, 2471–247810.3168/jds.S0022-0302(02)74329-4
- Özdestan Ö., Üren A. (2010b). Biogenic amine content of kefir: a fermented dairy product. Eur. Food Res. Technol. 231, 101–10710.1007/s00217-010-1258-y
- Custódio F. B., Tavares E., Glória M. B. A. (2007). Extraction of bioactive amines from grated Parmesan cheese using acid, alkaline and organic solvent. J. Food Compost. Anal. 20, 280–28810.1016/j.jfca.2006.06.009
- Gilad G. Agmatine metabolism and neuroprotection.; 1995.
- Yang XC Reis DJ. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Therap 1999;288:544–9.
- Xie-Chuan W Xiao-Dan G Jian-Quan Z Jin L. Agmatine blocked voltage-gated calcium channel in cultured rat hippocampal neurons. Acta Pharmacol Sin 2003;24:746–50.
- Galea E Regunathan S Eliopoulus V Feinstein DL Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J 1996;316:247–9.
- Murayama T Tsai S-C Adamik R Moss J Vaughan M. Effects of temperature on ADP-ribosylation factor stimulation of cholera toxin activity. Biochem 1993;32:561–7.
- Yang MZ Mun CH Choi YJ et al. Agmatine inhibits matrix metalloproteinase-9 via endothelial nitric oxide synthase in cerebral endothelial cells. Neurol Res 2007;29:749–54.
- Kawasaki Y Xu ZZ Wang X et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 2008;14(3):331–6.
- Marx M Trittenwein G Aufricht C Hoeger H Lubec B. Agmatine and spermidine reduce collagen accumulation in kidneys of diabetic db/db mice. Nephron 1995;69:155–8.
- Imam Y.Z., D’Souza A., Malik R.A., Shuaib A. Secondary stroke prevention: Improving diagnosis and management with newer technologies. Transl. Stroke Res. 2016;7(6):458–477. http://dx.doi.org/10.1007/s12975-016-0494-2
- Cai W., Liu H., Zhao J., Chen L.Y., Chen J., Lu Z., Hu X. Pericytes in brain injury and repair after ischemic stroke. Transl. Stroke Res. 2017;8(2):107–121. http://dx.doi.org/10.1007/s12975-016-0504-4
- Jiang X., Pu H., Hu X., Wei Z., Hong D., Zhang W., Gao Y., Chen J., Shi Y. A Post-stroke therapeutic regimen with omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia. Transl. Stroke Res. 2016;7(6):548–561. http://dx.doi.org/10.1007/ s12975-016-0502-6
- Ji B., Zhou F., Han L., Yang J., Fan H., Li S., Li J., Zhang X., Wang X., Chen X., Xu Y. Sodium Tanshinone IIA sulfonate enhances effectiveness Rt-PA treatment in acute ischemic stroke patients associated with ameliorating blood-brain barrier damage. Transl. Stroke Res. 2017;8(4):334–340. http://dx.doi.org/10. 1007/s12975-017-0526-6
- Cunha A.S., Matheus F.C., Moretti M., Sampaio T.B., Poli A., Santos D.B., Colle D., Cunha M.P., Blum-Silva C.H., Sandjo L.P., Reginatto F.H., Rodrigues A.L., Farina M., Prediger R.D. Agmatine attenuates reserpine-induced oral dyskinesia in mice: Role of oxidative stress, nitric oxide and glutamate NMDA receptors. Behav. Brain Res. 2016;312:64–76. http://dx.doi.org/10. 1016/j.bbr.2016.06.014
- Huang Y.C., Tzeng W.S., Wang C.C., Cheng B.C., Chang Y.K., Chen H.H., Lin P.C., Huang T.Y., Chuang T.J., Lin J.W., Chang C.P. Neuroprotective effect of agmatine in rats with transient cerebral ischemia using MR imaging and histopathologic evaluation. Magn. Reson. Imaging. 2013;31(7):1174–1181. http:// dx.doi.org/10.1016/j.mri.2013.03.026
- Kim DJ, Kim DI, Lee SK, Suh SH, Lee YJ, Kim J, Chung TS, Lee JE. Protective effect of agmatine on a reperfusion model after transient cerebral ischemia: Temporal evolution on perfusion MR imaging and histopathologic findings. AJNR Am J Neuroradiol. 2006 Apr;27(4):780-5.
- Kim J.H., Yenari M.A., Giffard R.G., Cho S.W., Park K.A., Lee J.E. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp. Neurol. 2004;189(1):122–130. http:// dx.doi.org/10.1016/j.expneurol.2004.05.029
- Morrissey JJ, Klahr S. Agmatine activation of nitric oxide synthase in endothelial cells. Proc Assoc Am Physicians. 1997 Jan;109(1):51-7.
- Feng Y., Piletz J.E., Leblanc M.H. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr. Res. 2002;52(4):606–611. http://dx.doi. org/10.1203/00006450-200210000-00023
- John E. Piletz; Feyza, A.; Juei-Tang, C.; Carolyn, A. Fairbanks, V. H.; Gilad, B. H. Agmatine: clinical applications after 100 years in translation. Drug Discov. Today. 2010;18(17-18):880–893.
- Gilad G.M., Gilad V.H., Finberg J.P., Rabey J.M.
- Jung H.J., Yang M.Z., Kwon K.H., Yenari M.A., Choi Y.J., Lee W.T., Park K.A., Lee J.E. Endogenous agmatine inhibits cerebral vascular matrix metalloproteinases expression by regulating activating transcription factor 3 and endothelial nitric oxide synthesis. Curr. Neurovasc. Res. 2010;7(3):201–212. http://dx. doi.org/10.2174/156720210792231804
- Yang M.Z., Mun C.H., Choi Y.J., Baik J.H., Park K.A., Lee W.T., Lee J.E. Agmatine inhibits matrix metalloproteinase-9 via endothelial nitric oxide synthase in cerebral endothelial cells. Neurol. Res. 2007;29(7):749–754. http://dx.doi.org/10.1179/ 016164107X208103
- Ahn S.S., Kim S.H., Lee J.E., Ahn K.J., Kim D.J., Choi H.S., Kim J., Shin N.Y., Lee S.K. Effects of agmatine on blood-brain barrier stabilization assessed by permeability MRI in a rat model of transient cerebral ischemia. AJNR Am. J. Neuroradiol. 2015;36(2):283–288. http://dx.doi.org/10.3174/ajnr.A4113
- Kim J.H., Lee Y.W., Park K.A., Lee W.T., Lee J.E. Agmatine attenuates brain edema through reducing the expression of aquaporin-1 after cerebral ischemia. J. Cereb. Blood Flow Metab. 2010;30(5):943–949. http://dx.doi.org/10.1038/jcbfm.2009.260
- Kim J.M., Lee J.E., Cheon S.Y., Lee J.H., Kim S.Y., Kam E.H., Koo B.N. The anti-inflammatory effects of agmatine on transient focal cerebral ischemia in diabetic rats. J. Neurosurg. Anesthesiol. 2016;28(3):203–213.
- Wang C.C., Chio C.C., Chang C.H., Kuo J.R., Chang C.P. Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 2010;10:11. [http://dx.doi.org/10.1186/1471-2210-10-11
- Xu, W., Gao, L., Li, T., Shao, A., & Zhang, J. (2018). Neuroprotective Role of Agmatine in Neurological Diseases. Current neuropharmacology, 16(9), 1296–1305. https://doi.org/10.2174/1570159X15666170808120633
- Sahuquillo J., Poca M.A., Amoros S. Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr. Pharm. Des. 2001;7(15):1475–1503. http://dx.doi.org/10.2174/ 1381612013397311
- Reilly P.L. Brain injury: the pathophysiology of the first hours.‘Talk and Die revisited’. J. Clin. Neurosci. 2001;8(5):398–403. http://dx.doi.org/10.1054/jocn.2001.0916
- Liang D., Bhatta S., Gerzanich V., Simard J.M. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg. Focus. 2007;22(5):E2. http://dx.doi.org/10.3171/foc.2007.22.5.3
- Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58(2):PL 41-6. doi: 10.1016/0024-3205(95)02274-0
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20(3):132–139. http://dx.doi.org/ 10.1016/S0166-2236(96)10074-6
- Kuo J.R., Lo C.J., Chio C.C., Chang C.P., Lin M.T. Resuscitation from experimental traumatic brain injury by agmatine therapy. Resuscitation. 2007;75(3):506–514. http://dx.doi.org/10.1016/ j.resuscitation.2007.05.011
- Chong-Jeh L., Ching-Ping C., Kao-Chang L., Mao-Tsun L., Chung-Ching C. Agmatine-promoted angiogenesis, neurogenesis, and Inhibition of ghliosis-reduced traumatic brain injury in rats. J. Trauma Inj. Infect. Crit. Care. 2011;71(4):E87–E93. http://dx.doi. org/10.1097/TA.0b013e31820932e
- Kim J.Y., Lee Y.W., Kim J.H., Lee W.T., Park K.A., Lee J.E. Agmatine attenuates brain edema and apoptotic cell death after traumatic braini. J. Korean Med. Sci. 2015;30(7):943–952. http://dx.doi.org/10.3346/jkms.2015.30.7.943
- Yu C.G., Marcillo A.E., Fairbanks C.A., Wilcox G.L., Yezierski R.P. Agmatine improves locomotor function and reduces tissue damage following spinal cord injury. Neuroreport. 2000;11(14):3203–3207. http://dx.doi.org/10.1097/00001756-200009280-00031
- Hegarty S.V., Sullivan A.M., O’Keeffe G.W. The Epigenome as a therapeutic target for Parkinson’s disease. Neural Regen. Res. 2016;11(11):1735–1738. http://dx.doi.org/10.4103/1673-5374. 194803
- Rodriguez M.C., Obeso J.A., Olanow C.W. Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann. Neurol. 1998;44(3) Suppl. 1:S175–S188. http:// dx.doi.org/10.1002/ana.410440726
- Sawada H., Oeda T., Kuno S., Nomoto M., Yamamoto K., Yamamoto M., Hisanaga K., Kawamura T. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS One. 2010;5(12):e15298. http://dx.doi.org/10.1371/journal. pone.0015298
- Condello S., Currò M., Ferlazzo N., Caccamo D., Satriano J., Ientile R. Agmatine effects on mitochondrial membrane potential and NF-κB activation protect against rotenone-induced cell damage in human neuronal-like SH-SY5Y cells. J. Neurochem. 2011;116(1):67–75. http://dx.doi.org/10.1111/j.1471-4159.2010.07085. x
- Condello S., Calabrò E., Caccamo D., Currò M., Ferlazzo N., Satriano J., Magazù S., Ientile R. Protective effects of agmatine in rotenone-induced damage of human SH-SY5Y neuroblastoma cells: fourier transform infrared spectroscopy analysis in a model of Parkinson’s disease. Amino Acids. 2012;42(2-3):775–781. http:// dx.doi.org/10.1007/s00726-011-0994-z
- Matheus F.C., Aguiar A.S., Jr, Castro A.A., Villarinho J.G., Ferreira J., Figueiredo C.P., Walz R., Santos A.R., Tasca C.I., Prediger R.D. Neuroprotective effects of agmatine in mice infused with a single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Behav. Brain Res. 2012;235(2):263–272. http://dx.doi.org/10.1016/j.bbr.2012.08.017
- Drummond E., Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133(2):155–175. http://dx.doi.org/10.1007/s00401-016-1662-x
- Yang Y., Bu S. The current status of the Low temperature on the pathogenesis of Alzheimer’s disease. Shiyong Laonian Yixue. 2014;28(8):681–684.
- Uchoa M.F., Moser V.A., Pike C.J. Interactions between inflammation, sex steroids, and Alzheimer’s disease risk factors. Front. Neuroendocrinol. 2016;43:60–82. http://dx.doi.org/10.1016/j. yfrne.2016.09.001
- Baranov D., Bickler P.E., Crosby G.J., Culley D.J., Eckenhoff M.F., Eckenhoff R.G., Hogan K.J., Jevtovic-Todorovic V., Palotás A., Perouansky M., Planel E., Silverstein J.H., Wei H., Whittington R.A., Xie Z., Zuo Z. Consensus statement: First international workshop on anesthetics and Alzheimer’s disease. Anesth. Analg. 2009;108(5):1627–1630. http://dx.doi.org/10. 1213/ane.0b013e318199dc72
- Song J., Hur B.E., Bokara K.K., Yang W., Cho H.J., Park K.A., Lee W.T., Lee K.M., Lee J.E. Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced Alzheimer rat model. Yonsei Med. J. 2014;55(3):689–699. http://dx.doi.org/10.3349/ymj.2014.55.3.689
- Zhu M.Y., Piletz J.E., Halaris A., Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell. Mol. Neurobiol. 2003;23(4-5):865–872. [http://dx.doi.org/10.1023/A:1025069407173
- Zhao W.Q., Alkon D.L. Role of insulin and insulin receptor in learning and memory. Mol. Cell. Endocrinol. 2001;177(1-2):125–134. http://dx.doi.org/10.1016/S0303-7207(01)00455-5
- Kang S, Kim CH, Jung H, Kim E, Song HT. 2017.
- Li X., Can G. The research progress of relationship between the Alzheimer’s disease and outside the synapses, and synaptic NMDA receptor. Prog. Biochem. Biophys. 2014;41(9):823–829.
- Murray C.J., Lopez A.D., Jamison D.T. The global burden of disease in 1990: summary results, sensitivity analysis and future directions. Bull. World Health Organ. 1994;72(3):495–509.
- Alarcón G., Martinez J., Kerai S.V., Lacruz M.E., Quiroga R.Q., Selway R.P., Richardson M.P., García Seoane J.J., Valentín A. In vivo neuronal firing patterns during human epileptiform discharges replicated by electrical stimulation. Clin. Neurophysiol. 2012;123(9):1736–1744. http://dx.doi.org/10.1016/j.clinph.2012. 02.062
- Bence A.K., Worthen D.R., Stables J.P., Crooks P.A. An in vivo evaluation of the antiseizure activity and acute neurotoxicity of agmatine. Pharmacol. Biochem. Behav. 2003;74(3):771–775. http://dx.doi.org/10.1016/S0091-3057(02)01079-1
- Arhan E., Serdaroglu A., Ozturk B., Ozturk H.S., Ozcelik A., Kurt N., Kutsal E., Sevinc N. Effects of epilepsy and antiepileptic drugs on nitric oxide, lipid peroxidation and xanthine oxidase system in children with idiopathic epilepsy. Seizure. 2011;20(2):138–142. http://dx.doi.org/10.1016/j.seizure.2010.11.003
- Luszczki J.J., Czernecki R., Wojtal K., Borowicz K.K., Czuczwar S.J. Agmatine enhances the anticonvulsant action of phenobarbital and valproate in the mouse maximal electroshock seizure model. J. Neural Transm. (Vienna) 2008;115(11):1485–1494. http://dx. doi.org/10.1007/s00702-008-0046-3
- Feng Y., LeBlanc M.H., Regunathan S. Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: a potential mechanism for the anticonvulsive effects. Neurosci. Lett. 2005;390(3):129–133. http://dx.doi.org/10.1016/ j.neulet.2005.08.008
- Bahremand A., Ziai P., Khodadad T.K., Payandemehr B., Rahimian R., Ghasemi A., Ghasemi M., Hedayat T., Dehpour A.R. Agmatine enhances the anticonvulsant effect of lithium chloride on pentylenetetrazole-induced seizures in mice: Involvement of L-arginine/nitric oxide pathway. Epilepsy Behav. 2010;18(3):186–192. http://dx.doi.org/10.1016/j.yebeh.2010.04.014
- Abe K., Abe Y., Saito H. Agmatine induces glutamate release and cell death in cultured rat cerebellar granule neurons. Brain Res. 2003;990(1-2):165–171. http://dx.doi.org/10.1016/S0006-8993(03)03454-1
- Luszczki J.J., Czernecki R., Dudra-Jastrzebska M., Borowicz K.K., Czuczwar S.J. Influence of agmatine on the protective action of numerous antiepileptic drugs against pentetrazole-induced seizures in mice. Pharmacol. Rep. 2009;61(2):252–260. http://dx. doi.org/10.1016/S1734-1140(09)70029-5
- Shen X., Zhao Z., Luo X., Wang H., Hu B., Guo Z. Systems pharmacology based study of the molecular mechanism of SiNiSan formula for application in nervous and mental diseases. Evid. Based Complement. Alternat. Med. 2016;2016:9146378. http://dx.doi.org/10.1155/2016/9146378
- Taksande B.G., Kotagale N.R., Tripathi S.J., Ugale R.R., Chopde C.T. Antidepressant like effect of selective serotonin reuptake inhibitors involve modulation of imidazoline receptors by agmatine. Neuropharmacology. 2009;57(4):415–424. http://dx. doi.org/10.1016/j.neuropharm.2009.06.035
- Zaniewska M., McCreary A.C., Sezer G., Przegaliński E., Filip M. Effects of agmatine on nicotine-evoked behavioral responses in rats. Pharmacol. Rep. 2008;60(5):645–654.
- Pålsson E., Fejgin K., Wass C., Klamer D. Agmatine attenuates the disruptive effects of phencyclidine on prepulse inhibition. Eur. J. Pharmacol. 2008;590(1-3):212–216. http://dx.doi.org/10.1016/ j.ejphar.2008.06.022
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. http://dx.doi.org/10.1080/ 01926230701320337
- Rathmell J.C., Thompson C.B. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl.):S97–S107. [http://dx.doi.org/10.1016/S0092-8674(02) 00704-3
- Shao A, Wang Z, Wu H, Dong X, Li Y, Tu S, Tang J, Zhao M, Zhang J, Hong Y. Enhancement of Autophagy by Histone Deacetylase Inhibitor Trichostatin A Ameliorates Neuronal Apoptosis After Subarachnoid Hemorrhage in Rats. Mol Neurobiol. 2016 Jan;53(1):18-27. doi: 10.1007/s12035-014-8986-0
- El-Sherbeeny N.A., Nader M.A., Attia G.M., Ateyya H. Agmatine protects rat liver from nicotine-induced hepatic damage via antioxidative, antiapoptotic, and antifibrotic pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2016;389(12):1341–1351. http://dx.doi.org/10.1007/s00210-016-1284-9
- Arndt M.A., Battaglia V., Parisi E., Lortie M.J., Isome M., Baskerville C., Pizzo D.P., Ientile R., Colombatto S., Toninello A., Satriano J. The arginine metabolite agmatine protects mitochondrial function and confers resistance to cellular apoptosis. Am. J. Physiol. Cell Physiol. 2009;296(6):C1411–C1419. [http://dx. doi.org/10.1152/ajpcell.00529.2008
- Haenisch B., Bönisch H., Cichon S., Allam J.P., Novak N., Molderings G.J. Effects of exogenous agmatine in human leukemia HMC-1 and HL-60 cells on proliferation, polyamine metabolism and cell cycle. Leuk. Res. 2011;35(9):1248–1253. http://dx. doi.org/10.1016/j.leukres.2010.12.023
- Zarifkar A., Choopani S., Ghasemi R., Naghdi N., Maghsoudi A.H., Maghsoudi N., Rastegar K., Moosavi M. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur. J. Pharmacol. 2010;634(1-3):84–88. http://dx. doi.org/10.1016/j.ejphar.2010.02.029
- Turrin N.P., Rivest S. Molecular and cellular immune mediators of neuroprotection. Mol. Neurobiol. 2006;34(3):221–242. http://dx.doi.org/10.1385/MN:34:3:221
- Brown G.C., Neher J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010;41(2-3):242–247. http://dx.doi.org/10.1007/s12035-010-8105-9
- Taksande B.G., Gawande D.Y., Chopde C.T., Umekar M.J., Kotagale N.R. Agmatine ameliorates adjuvant induced arthritis and inflammatory cachexia in rats. Biomed. Pharmacother. 2017;86:271–278. http://dx.doi.org/10.1016/j.biopha.2016.12.039
- Song J., Lee B., Kang S., Oh Y., Kim E., Kim C.H., Song H.T., Lee J.E. Agmatine ameliorates high glucose-induced neuronal cell senescence by regulating the p21 and p53 signaling. Exp. Neurobiol. 2016;25(1):24–32. [http://dx.doi.org/10.5607/ en.2016.25.1.24
- Sahin C., Albayrak O., Akdeniz T.F., Akbulut Z., Yanikkaya Demirel G., Aricioglu F. Agmatine reverses sub-chronic stress induced nod-like receptor protein 3 (NLRP3) activation and cytokine response in rats. Basic Clin. Pharmacol. Toxicol. 2016;119(4):367–375. http://dx.doi.org/10.1111/bcpt.12604
- Wade C.L., Eskridge L.L., Nguyen H.O., Kitto K.F., Stone L.S., Wilcox G., Fairbanks C.A. Immunoneutralization of agmatine sensitizes mice to micro-opioid receptor tolerance. J. Pharmacol. Exp. Ther. 2009;331(2):539–546. http://dx.doi.org/10.1124/ jpet.109.155424
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. http://dx.doi.org/10.1016/j.biocel.2006.07.001
- Iizuka Y., Hong S., Kim C.Y., Yang W.I., Lee J.E., Seong G.J. Protective mechanism of agmatine pretreatment on RGC-5 cells injured by oxidative stress. Braz. J. Med. Biol. Res. 2010;43(4):356–358. http://dx.doi.org/10.1590/S0100-879X2010007500018
- Bratislav D., Irena L., Milica N., Ivana S., Ana D., Sanda D., Ivana S. Effects of agmatine on chlorpromazine toxicity in the liver of Wistar rats: the possible role of oxidant/antioxidant imbalance. Exp. Anim. 2017;66(1):17–27. http://dx.doi.org/10.1538/ expanim.16-0010
- Gawali N.B., Bulani V.D., Chowdhury A.A., Deshpande P.S., Nagmoti D.M., Juvekar A.R. Agmatine ameliorates lipopolysaccharide induced depressive-like behaviour in mice by targeting the underlying inflammatory and oxido-nitrosative mediators. Pharmacol. Biochem. Behav. 2016;149:1–8. http://dx.doi.org/10.1016/ j.pbb.2016.07.004
- Freitas A.E., Bettio L.E., Neis V.B., Santos D.B., Ribeiro C.M., Rosa P.B., Farina M., Rodrigues A.L. Agmatine abolishes restraint stress-induced depressive-like behavior and hippocampal antioxidant imbalance in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;50:143–150. http://dx.doi.org/10.1016/j.pnpbp. 2013.12.012
- Liu Y-C., Lee Y-D., Wang H-L., Liao K.H., Chen K.B., Poon K.S., Pan Y.L., Lai T.W. Anesthesia-induced hypothermia attenuates early-phase blood-brain barrier disruption but not infarct volume following cerebral ischemia. PLoS One. 2017;12(1):e0170682. http://dx.doi.org/10.1371/journal.pone.0170682
- Fujimura M., Gasche Y., Morita-Fujimura Y., Massengale J., Kawase M., Chan P.H. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842(1):92–100. http://dx.doi.org/10.1016/S0006-8993(99)01843-0
- Agre P., King L.S., Yasui M., Guggino W.B., Ottersen O.P., Fujiyoshi Y., Engel A., Nielsen S. Aquaporin water channels–from atomic structure to clinical medicine. J. Physiol. 2002;542(Pt 1):3–16. [http://dx.doi.org/10.1113/jphysiol.2002.020818
- Hegarty SV, Sullivan AM, O’Keeffe GW. The Epigenome as a therapeutic target for Parkinson’s disease. Neural Regen Res. 2016 Nov;11(11):1735-1738. doi: 10.4103/1673-5374.194803
- Wang C.C., Chio C.C., Chang C.H., Kuo J.R., Chang C.P. Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 2010;10:11. http://dx.doi.org/10.1186/1471-2210-10-11
- Ory Keynan, MD, Yigal Mirovsky, MD, Samuel Dekel, MD, Varda H. Gilad, CRA, Gad M. Gilad, PhD, Safety and Efficacy of Dietary Agmatine Sulfate in Lumbar Disc-associated Radiculopathy. An Open-label, Dose-escalating Study Followed by a Randomized, Double-blind, Placebo-controlled Trial, Pain Medicine, Volume 11, Issue 3, March 2010, Pages 356–368, https://doi.org/10.1111/j.1526-4637.2010.00808.x