What is ampullary cancer
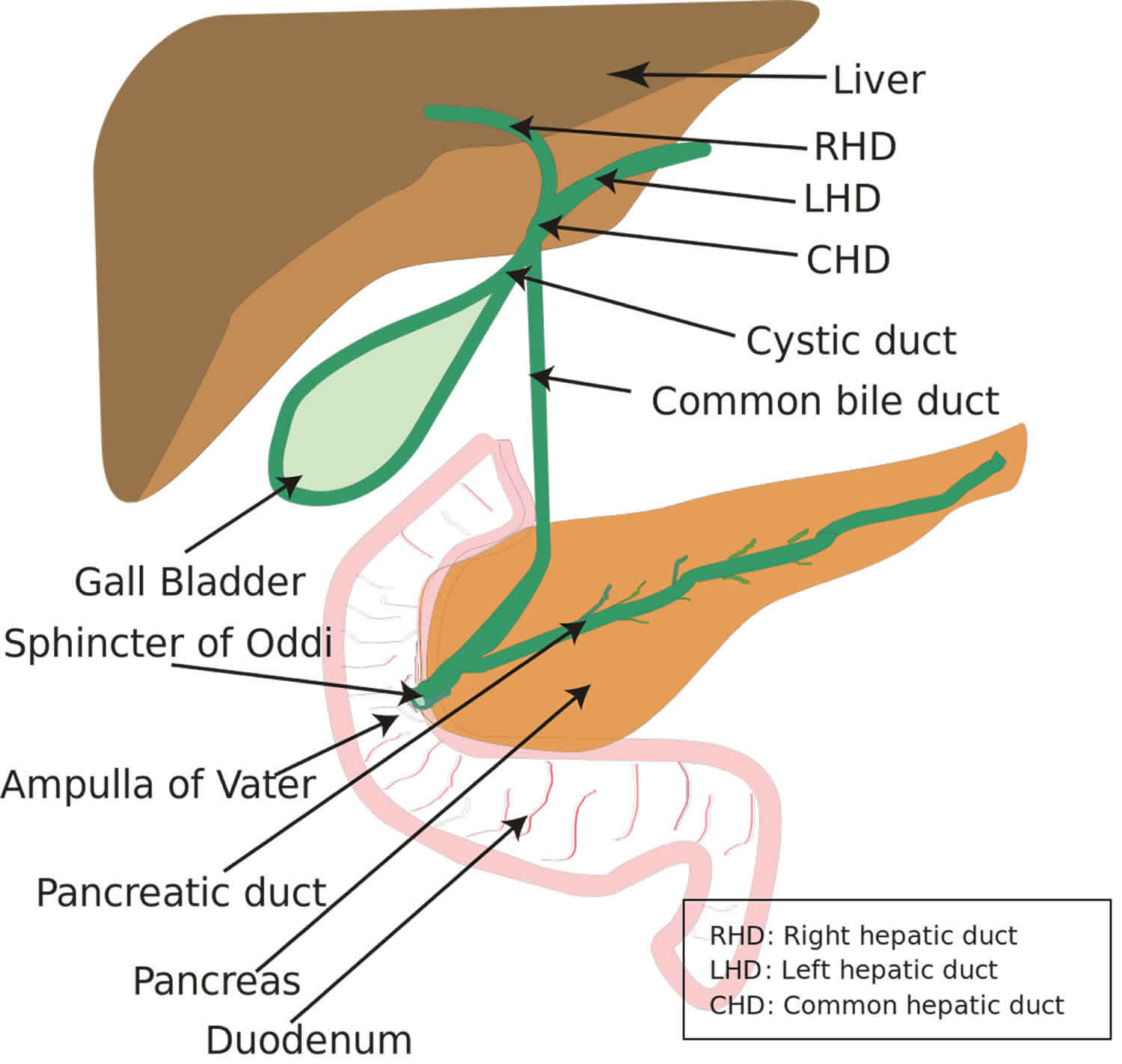
Ampullary cancer forms in the ampulla of Vater (an enlargement of the ducts from the liver and pancreas where they join and enter the small intestine), in the last centimeter of the common bile duct, where it passes through the wall of the duodenum and ampullary papilla. Ampullary cancers arise from the ampullary complex, distal to the confluence of the common bile and pancreatic duct (Figure 1). Ampullary cancers are rare, accounting for only 0.2% of gastrointestinal cancers and approximately 7% of all periampullary cancers 1. In contrast to other periampullary malignancies (tumors of the pancreas, distal bile duct and periampullary duodenum), true ampullary cancers present earlier in their disease course with symptoms that result from biliary obstruction 2. It is often difficult to distinguish primary ampullary cancers from other periampullary cancers preoperatively. In early stages, ampullary cancers are surgically treated, similar to pancreatic cancers, and typically with a pancreatico-duodenoectomy (or Whipple procedure) 2. Because of their earlier presentation, resection rates for all patients are much higher than other periampullary carcinomas. Moreover, their prognosis tends to be better than those with other periampullary- and pancreatic-originating cancers 2. In patients with true ampullary cancer, there is very limited data to guide physicians on the choice of therapy, largely because of the rarity of the disease and the paucity of related research 2.
In 90% of patient cases, this represents a primary presentation with a predominance of Caucasian males being affected over other races and gender 3. In patients with hereditary polyposis syndromes, the incidence of ampullary cancer is multifold and presents at an earlier age warranting surveillance endoscopy 4.
Surgical resection with pancreaticoduodenectomy (Whipple procedure) remains the gold standard for treatment, although local excision is an option for patients who may be unable to tolerate this 5. Several palliative options exist for patients with unresectable or metastatic disease. While certain features (eg, positive resection margins and lymph node positivity) portend poorer prognosis, patients with ampullary cancer generally have better overall survival than patients with pancreatic cancer.
Unfortunately, most patients with carcinoma of the ampulla of Vater die of recurrent disease. Treatment fails in nearly 70% of patients with poor prognostic features, and these patients ultimately die of their disease.
Kopelson and associates described regional nodal recurrences in 3 of 12 patients with ampullary cancers following potentially curative resection. From pooled data on 80 patients with ampullary cancer, they found that 54% developed locoregional recurrence 6.
Ampullary cancer key points
- Ampullary cancers represent a small subset of periampullary cancers and represent only 0.2% of all gastrointestinal malignancies.
- Ampullary adenocarcinomas with pancreaticobiliary histology have a much worse outcome than those with intestinal histology.
- The management of locoregional disease is primarily a surgical intervention by a pancreaticoduodenectomy (Whipple’s procedure) followed by the administration of adjuvant chemotherapy (preferably gemcitabine).
- The management of unresectable and metastatic disease is primarily through the administration of systemic therapy with an anti-metabolite (fluoropyrimidine and/or gemcitabine) combined with a platinum compound, (usually cisplatin or oxaliplatin).
Figure 1. Ampullary cancer
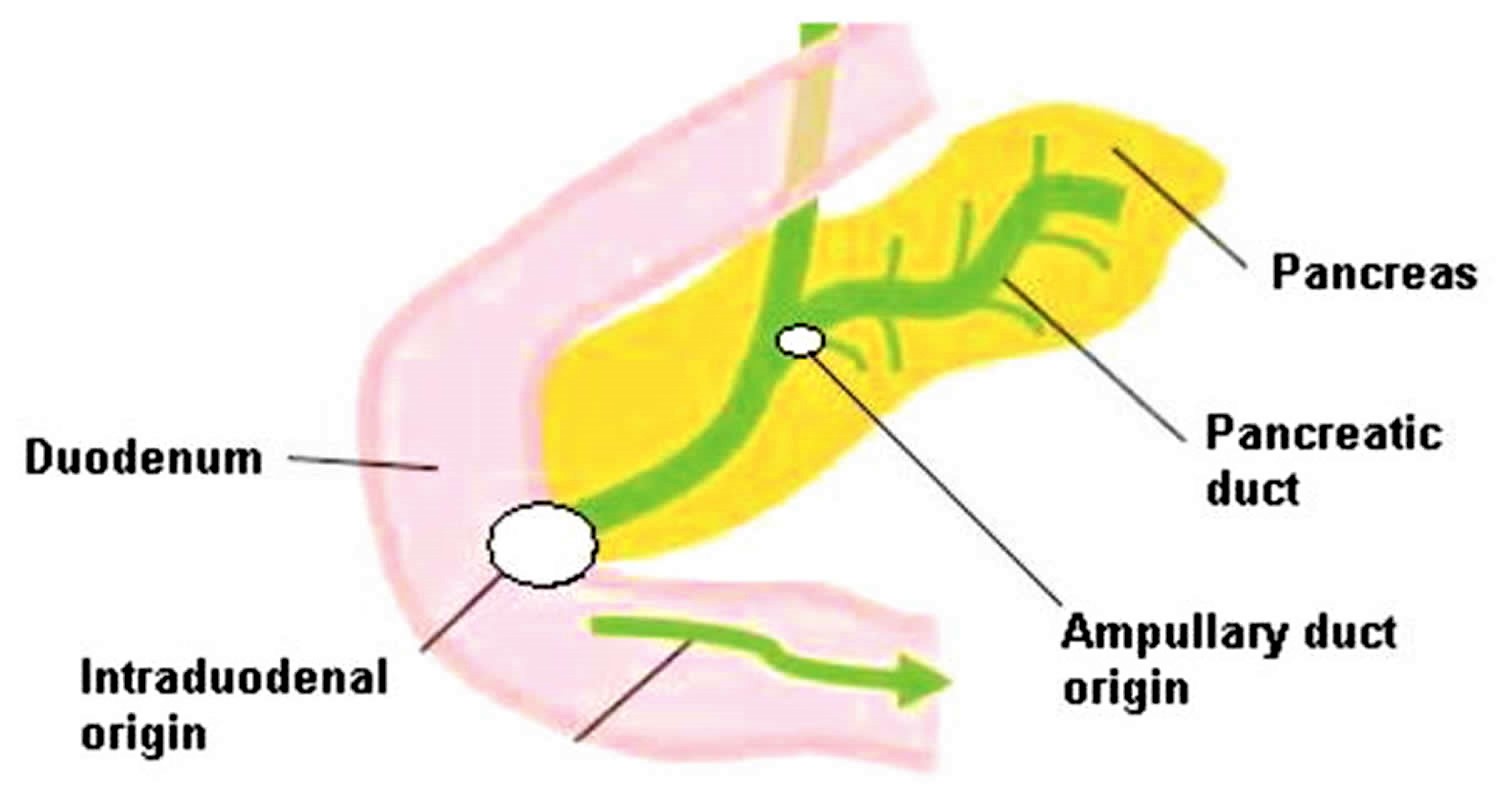
Figure 2. Carcinoma of ampulla of Vater
Footnote: Diagram of the sites of origin in ampullary cancer (Ampullary vs. Intraduodenal)
[Source 2 ]Ampullary cancer causes
Scientists don’t know what causes ampullary cancer. Most ampullary carcinomas are adenocarcinomas, but the histology varies with tumors comprising sub-types including papillary, adenosquamous, mucinous, and adenocarcinomas. Recent studies helped identify two main distinct histologic sub-types of adenocarcinoma based on their epithelium of origin: intestinal and pancreatobiliary 7. Intestinal histology originates from the intestinal epithelium overlying the ampulla, however pancreaticobiliary histology originates from the epithelium of the distal common bile duct and distal pancreatic duct.
Evidence suggests that histologic sub-types also differ in biologic behavior, bearing implications on prognosis and outcome. Differing outcomes are presumably related to the originating epithelia. Notably, ampullary adenocarcinomas with pancreaticobiliary histology have a much worse outcome than those with intestinal histology (median overall survival of 16 vs. 115.5 months) 8. When controlled for other risk factors, in resectable periampullary cancers, biologic behavior appears to be the most important prognostic indicator for patient outcome 9.
Ampullary cancer symptoms
The signs and symptoms of ampullary carcinoma are largely related to obstruction of the bile duct or pancreatic duct. They include the following 10:
- Jaundice secondary to biliary obstruction—most common clinical presentation
- Abdominal pain
- Dyspepsia
- Malaise
- Fever/chills
- Anorexia
- Pancreatitis—May be the first clinical manifestation, due to obstruction of the pancreatic duct
- Pruritus—Secondary to biliary obstruction
- Nausea
- Vomiting
- Weight loss
- Diarrhea
- Upper gastrointestinal bleeding and heme positive stools—May occur due to ulceration of ampullary mass (less common)
- Courvoisier gallbladder (ie, a distended, palpable gallbladder in a patient with jaundice)
The most common clinical manifestation of ampullary carcinoma is jaundice, which occurs due to obstruction of the biliary tract by the tumor 5. Patients may also experience scleral icterus and pruritus because of obstruction of the bile duct. Other common complaints include dyspepsia, anorexia, malaise, and weight loss.
Pancreatitis may sometimes be the initial clinical presentation due to pancreatic duct obstruction. Patients may therefore complain of symptoms of pancreatitis, such as epigastric/mid-abdominal pain, back pain, nausea, and vomiting.
Diarrhea, a common but not universal symptom, might be associated with an absence of lipase within the gut because of pancreatic duct obstruction.
Ampullary cancer diagnosis
Laboratory studies
Routine laboratory studies include the following:
- Complete blood count (CBC)
- Electrolyte panel
- Liver function studies: Prothrombin time, bilirubin (direct and indirect), transaminases, and alkaline phosphatase. A rising bilirubin level due to obstructive jaundice often is the sole presenting sign.
- CA 19-9: Serum tumor marker that is often elevated in pancreatic malignancies and may have a role in assessing response to therapy and/or predicting tumor recurrence or both.
- Carcinoembryonic antigen (CEA): A nonspecific tumor marker that is sometimes elevated in pancreatic malignancies; it may have a role in assessing response to treatment or predicting tumor recurrence. Because CEA also is elevated in patients with other gastrointestinal malignancies (in particular, colon and rectal cancer), the possibility of a second primary tumor needs to be excluded in these patients.
Ultrasonography of the abdomen
- Abdominal ultrasonography is the initial study to evaluate the common bile duct or pancreatic ducts
- Dilatation of these ducts is essentially diagnostic for extrahepatic obstruction
- Biliary or pancreatic ductal dilatation can explain abdominal pain, even in patients with localized and noninvasive disease
- 10-15% of patients with normal common bile duct findings on ultrasonography demonstrate extrahepatic biliary obstruction on a computed tomography (CT) scan
- Ultrasonography and CT scanning can help reveal metastatic disease in the liver or regional lymph nodes
CT scanning of the abdomen and/or pelvis
- Obtain a CT scan to evaluate the local region of interest and evaluate for possible metastases
- CT scanning often demonstrates a mass but is not helpful in differentiating ampullary carcinoma from tumors of the head of the pancreas or periampullary region; if the lesion is smaller than 2 cm, pancreatic or bile duct dilation may be the only abnormalities noted on the CT scan
- Such findings are highly suggestive of pancreatic malignancy and require further evaluation, usually with endoscopic retrograde cholangiopancreatography (ERCP)
- Dynamic CT scanning (ie, high-speed scans obtained during rapid intravenous administration of iodinated contrast material) can reveal tumor involvement of the vasculature
Other imaging studies
- Endoscopic retrograde cholangiopancreatography (ERCP): Obtain ERCP to evaluate the ductal architecture further
- Chest radiography: Obtain a chest radiograph to complete the workup (ie, for staging purposes)
- Positron emission tomography (PET) or PET-CT scanning: PET or PET-CT scans can detect metastases that are too small to be reliably detected on a CT scan
Ampullary cancer staging
Over the years, multiple systems for staging this tumor have been proposed. Martin proposed a 4-stage system, as follows:
- Stage 1 – Vegetating tumor limited to the epithelium with no involvement of the sphincter of Oddi
- Stage 2 – Tumor localized in the duodenal submucosa without involvement of the duodenal muscularis propria but possible involvement of the sphincter of Oddi
- Stage 3 – Tumor of the duodenal muscularis propria
- Stage 4 – Tumor of the periduodenal area or pancreas, with proximal or distal lymph node involvement
The classification system of Yamaguchi and Enjoji is similar to the Martin classification 11.
Talbot et al 12 devised a system that scored tumors according to the degree of infiltration (from 1-4 according to increasing infiltration) and according to tumor differentiation (from 1-3 for well, moderately, and poorly differentiated tumors), the sum of which separated the patients into 2 groups (scores 2-4 and scores 5-7).
The currently accepted American Joint Committee on Cancer (AJCC) staging system (7th edition) for ampullary carcinoma emphasizes the importance of pancreatic invasion and lymph node metastases (see below and see Table 1, below). Size has little impact on tumor stage. The definition of primary tumor (T), regional lymph node (N), and remote metastases (M) for classification and staging of cancer of the ampulla of Vater is provided below.
Primary tumor is defined as follows:
- TX – Primary tumor cannot be assessed
- T0 – No evidence of primary tumor
- Tis – Carcinoma in situ
- T1 – Tumor limited to ampulla of Vater or sphincter of Oddi
- T2 – Tumor invades duodenal wall
- T3 – Tumor invades pancreas
- T4 – Tumor invades peripancreatic soft tissues or other organs
Regional lymph nodes are defined as follows:
- NX – Regional lymph nodes cannot be assessed
- N0 – No regional lymph node metastases
- N1 – Regional lymph node metastases (peripancreatic lymph node metastasis, including lymph nodes along the hepatic artery and portal vein)
Distant metastases are defined as follows:
- MX – Presence of distant metastases cannot be assessed
- M0 – No distant metastases
- M1 – Distant metastases
Table 1. Staging of ampullary cancers by the American Joint Committee on Cancer (AJCC) TNM System
| Stage | T | N | M |
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2 | N0 | M0 |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T1-T3 | N1 | M0 |
| Stage III | T4 | Any N | M0 |
| Stage IV | Any T | Any N | M1 |
Ampullary cancer treatment
Early stage disease
Curative surgery is possible in approximately 50% of ampullary cancer compared to that of less than 10% in pancreatic adenocarcinoma 13. Despite the high rate of potentially curative resection, the majority of patients with ampullary carcinomas will eventually succumb to recurrent disease 14. Given the rarity of this disease, there is absence of randomized clinical trials focused on ampullary carcinomas and treatment recommendations are mainly derived from results of adjuvant clinical trials conducted in pancreaticobiliary cancers where ampullary cancers may represent a sub-group of patients. Given many of the similarities between ampullary and pancreas cancers, patients who have undergone resection are often offered adjuvant chemotherapy with or without the addition of radiotherapy.
The standard surgical approach is pancreaticoduodenal resection (Whipple procedure). The procedure involves en bloc resection of the following:
- The gastric antrum and duodenum
- A segment of the first portion of the jejunum, gallbladder, and distal common bile duct
- The head and often the neck of the pancreas
- Adjacent regional lymph nodes
In a review of 450 cases of surgical resection of ampullary adenoma or adenocarcinoma at Johns Hopkins, Winter et al 15 found that 96.7% of the patients had undergone pancreaticoduodenectomy rather than local excision. These researchers concluded that pancreaticoduodenectomy should be the preferred approach for most ampullary neoplasms that require surgical resection, given that nearly 30% of the Johns Hopkins patients with T1 disease had lymph node metastases.
Factors associated with the presence of lymph node metastasis included the following 16:
- Tumor size ≥1 cm (odds ratio [OR] 2.1)
- Poor histologic grade (OR 4.8)
- Perineural invasion (OR 3.0)
- Microscopic vessel invasion (OR 6.6)
- Depth of invasion > pT1 (OR 4.3)
- Specifically, risk of lymph node metastasis increased with T stage (T1, 28.0%; T2, 50.9%; T3, 71.7%; T4, 77.3%)
Results after radical resection of ampullary of Vater carcinoma have been improving. During recent decades, 5-year survival rates have ranged from 20-61%, averaging higher than 35%. The reported mortality rates from this operation are decreasing.
In a review of more than 1100 patients published in a surgical series, Howe et al 17 reported that the overall rate of resectability was 82%. This most likely overestimates the true resectability rate because patients in whom radiologic studies identify unresectable disease often are not included in retrospective surgical series.
A review of cases from Veterans Affairs hospitals across the United States by el-Ghazzawy et al 18 revealed that only 63% of presenting patients undergo surgery for cure. At disease presentation, 30-50% have involved lymph nodes.
A few studies have been conducted on the pattern of lymphatic spread of ampullary cancer. These studies have been difficult to interpret because of the lack of standardized nomenclature for lymph node groups, variability in the degree of superior mesenteric lymph node dissection, and the small number of patients.
Shirai and colleagues 19 meticulously reviewed 21 cases of ampullary cancer and documented the pattern of lymphatic spread. The site of greatest nodal involvement, the first echelon group, is the posterior pancreaticoduodenal nodal group. The nodal groups surrounding the inferior pancreaticoduodenal artery were the superior mesenteric lymph nodes involved most often. Finally, the para-aortic lymph node groups were involved in 3 patients with resectable disease.
Kayahara 20 reported that the inferior pancreaticoduodenal nodes (13b) and the superior mesenteric nodes (14) were the groups most often involved with metastatic carcinoma.
Local excision
Because of the mortality and morbidity associated with pancreaticoduodenectomy, surgeons have studied local excision of cancers of the ampulla of Vater to avoid major resection. Transduodenal excision of ampullary tumors has been proposed as an intermediate option between radical resection and palliative bypass for high-risk patients. However, this approach remains highly controversial.
Local resection has generally been reserved for poor operative candidates (eg, elderly patients, those with other comorbid conditions) with favorable tumors 21. Additionally, lymph node metastasis may be present even in patients with T1 tumors and local resection does not include a regional lymphadenectomy, as is performed with pancreaticoduodenectomy.
Some have argued that local resection is simpler, is better tolerated and may have acceptable survival rates. In a series of 21 patients who underwent local resection of ampullary adenomas, Posner et al 22 demonstrated overall survival of 85% and no tumor recurrence in 89% of the surviving patients (with average follow up of 38 months). However, this study was not limited to ampullary cancer; final pathology demonstrated 1 patient (5%) with invasive cancer, 2 (9%) with microinvasive cancer, 6 (28%) with high-grade dysplasia, and 1 (5%) with low-grade dysplasia.
Carcinoma in situ has been diagnosed with increasing frequency. It has been associated with polypoid growth and may be treated with endoscopic polypectomy. In these circumstances, the entire polyp should be removed and the base of the polyp should be carefully examined to ensure that no cancer is at the margin. In the case of an incomplete excision, a prompt pancreaticoduodenectomy is essential. Patients who undergo polypectomy only should be monitored endoscopically at yearly intervals to detect any recurrence.
Staging of ampullary cancer is critical to treatment. While ampullary polypectomy and ampullectomy have been performed successfully on some patients with ampullary cancer, local resection as a therapeutic approach is best reserved for patients with benign lesions, such as ampullary adenomas, or patients with carcinoma in situ or T1 tumors whose overall performance status makes the risks associated with a formal pancreaticoduodenectomy excessive. In general, for ampullary carcinoma, pancreaticoduodenectomy remains the gold standard and should be offered as long as the patient is able to tolerate the operation 10.
Adjuvant therapy
Because local and systemic failures remain problematic, physicians continue to be interested in offering adjuvant therapy. The relative rarity of this disease limits research in this area 23.
Willett and colleagues 24 summarized their experience with adjuvant radiotherapy for high-risk tumors of the ampulla of Vater (risk factors included invasion into the pancreas, poorly differentiated histology, involved lymph nodes, or positive resection margins). Twelve patients received adjuvant radiotherapy (40-50.4 Gy) to the tumor bed and some received concurrent 5-fluorouracil (5-FU) as a radiosensitizer. Comparison of these patients with 17 patients who underwent surgical resection alone showed a trend toward better locoregional control with adjuvant radiotherapy, but there was no advantage in survival. Distant metastasis to the liver, peritoneum, and pleura was the dominant failure pattern in this group of patients.
Barton and Copeland 25 reported on the M.D. Anderson Cancer Center experience of using postoperative chemotherapy for carcinoma of the ampulla of Vater. Seventeen patients received a variety of chemotherapeutic regimens (5-FU was used in combination with doxorubicin, carmustine, vincristine, methyl-lomustine, or mitomycin-C). Although no analysis was presented, the authors concluded that “no combination of drugs appeared to prolong life.”
Sikora and colleagues 26 presented their experience from a hospital in India in a retrospective review. Patients who underwent a pancreaticoduodenectomy with adjuvant chemotherapy and radiation did not do any better than the group treated with surgery alone.
Zhou et al 27 reviewed the records of 111 patients at Johns Hopkins who underwent curative surgery for ampullary adenocarcinoma, 45% of whom also received adjuvant chemotherapy and radiation. In these patients, the improvement in survival with adjuvant treatment was not statistically significant (median overall survival: 21.6 vs. 13.0 months).
In a retrospective review, Chan and colleagues 28 reported that 13 patients who received adjuvant chemotherapy (predominantly involving 5-FU, mitomycin-C, and doxorubicin) had a significantly better survival than 16 patients who underwent resection only.
Yeung and colleagues 29 used neoadjuvant chemoradiotherapy for 20 patients with presumed carcinoma of the head of the pancreas, including 4 patients with duodenal/ampullary carcinomas. Interestingly, no residual tumor was found in pancreaticoduodenectomy specimens of the 4 patients thought to have had ampullary/duodenal carcinomas.
At Stanford University, all cases of periampullary carcinoma are discussed and reviewed in detail by a multidisciplinary team that includes surgical oncologists, medical oncologists, radiation oncologists, a pathologist, a gastroenterologist, and a radiologist. All resected tumors are reviewed. Patients with tumors with poor prognostic features (eg, involved surgical margins, lymph nodes, invasion of the pancreas, perineural invasion, or poor histologic grade) are enrolled in a single-arm investigational protocol to receive adjuvant radiotherapy (45 Gy) and concurrent protracted venous infusion of 5-FU (225 mg/m²/d) during the entire treatment course.
Patients with carcinoma of the ampulla of Vater may benefit from recent advances in the treatment planning and delivery of adjuvant and definitive radiotherapy for patients with pancreatic cancer, which have produced modest gains in survival.
Pancreaticoduodenectomy is the procedure of choice for patients with resectable disease, but local recurrence plagues all surgical series, particularly when the pancreas has been invaded or lymph node metastases are discovered. In fact, whether major resection impacts survival in the setting of disease spread to the lymph nodes remains unclear. Postoperative irradiation of at least 45 Gy with 5-FU as a radiosensitizer is a reasonable treatment and reduces local recurrence in pancreatic cancer.
Early Stage Disease: Conclusions
The majority of patients with resected ampullary carcinomas will eventually die from recurrent disease 2. Multiple studies suggest a potential survival benefit from the administration of adjuvant chemotherapy, with less support for the use of radiation.
Follow-up guidelines are not well established for ampullary carcinoma. Reasonable practice includes blood studies, chest radiograph, and CT scan of the abdomen and/or pelvis every 6 months.
If treatment ultimately fails, it often does so within 5 years. Unfortunately, good salvage therapies do not yet exist. Palliative chemotherapeutic agents and effective medications for pain relief exist.
Locally advanced and metastatic disease
Systemic chemotherapy remains the mainstay in the treatment of patients with locally advanced unresectable and metastatic disease 2. Given the rarity of this disease, our reliance is on limited published data sets including a variety of trials including basket trials that allowed the inclusion of ampullary cancer in addition to small intestinal or biliary duct cancers. Agents that have been examined in this disease include anti-metabolites (fluoropyrimidine and/or gemcitabine) with or without the addition of a platinum compound (usually cisplatin or oxaliplatin). Published work suggests variability in response rates ranging from 10% to 40% and reported median overall survival up to 20 months 30. A recent phase II trial investigating the combination of capecitabine and oxaliplatin in patients with advanced small bowel or ampullary adenocarcinoma, showed that in the subset analysis of ampullary cancers, patients achieved a response rate of 33%, although the median time to progression and overall survival were reported to be 11.3 and 20.4 months, respectively 31. Another study, ABC-02, which represents the largest randomized controlled trial in biliary tract cancers, investigated the use of cisplatin with gemcitabine versus gemcitabine alone in 410 patients with advanced or metastatic biliary tract cancer. Benefits in overall survival (11.7 vs. 8.1 months) and progression-free survival (8 vs. 5 months) were observed in patients treated with the combination therapy versus gemcitabine alone 32. Subset analysis of patients with ampullary cancers, approximately 5% of the study population, suggested a similar survival benefit with the combination compared to the rest of the study population.
Local Recurrence
Recurrence patterns following resection in ampullary cancer include locoregional and distant disease. There is no known role for localized therapy approaches in cases of recurrence. One recent study suggests that palliative reoperation was associated with an unacceptable rate of postoperative mortality of 86% and a median survival of no more than 45 days 33, although another study suggested that reirradiation may have a potential benefit with improved local control 34. At this time, there is no evidence for localized approaches in patients with recurrent disease, with systemic therapy remaining the treatment of choice for those with a good performance status.
Treatment of Unresectable Disease
For patients with unresectable ampillary carcinoma, endoscopic stenting to achieve biliary decompression is an appropriate palliative procedure. Endoscopic palliation may also be performed for duodenal obstruction with expandable metal stents. Similarly, a palliative bypass may be performed for tumors found to be unresectable intraoperatively.
No established answer exists to the question of further therapy. Very little has been published on adjuvant treatment for locally advanced and advanced ampullary carcinoma. Confining the therapeutic approach to relief of symptoms is reasonable.
Given the paucity of effective standard treatment options, encourage patients to enroll in clinical trials. Radiotherapy, chemotherapy, and chemoradiotherapy have been tried, but response rates probably are low, and an effect on survival is questionable.
Ampullary cancer prognosis
Despite numerous advances in both cancer care and research, efforts in rare malignancies such as ampullary cancer remain very challenging with a clear lack of an evidence-based standard of care treatment paradigm 35. Although adding adjuvant therapies such as chemotherapy or chemoradiotherapy is likely to improve survival in high-risk disease, there is no standardized regimen for the treatment of Ampullary cancer. More research is required to elucidate whether statistically and clinically relevant differences exist that may warrant a change in the current adjuvant treatment strategies.
Ampullary cancer has a better prognosis when compared to pancreatic cancer or cholangiocarcinoma, largely in part due to the location of the tumor which is associated with an early onset of biliary obstruction-associated jaundice and thus early disease detection 36.
Curative surgery is possible in approximately 50% of ampullary cancer compared to that of less than 10% in pancreatic adenocarcinoma 37. Despite the high rate of potentially curative resection, the majority of patients with ampullary carcinomas will eventually succumb to recurrent disease 14. Given the rarity of ampullary cancer, there is absence of randomized clinical trials focused on ampullary carcinomas and treatment recommendations are mainly derived from results of adjuvant clinical trials conducted in pancreaticobiliary cancers where ampullary cancers may represent a sub-group of patients. Given many of the similarities between ampullary and pancreas cancers, patients who have undergone resection are often offered adjuvant chemotherapy with or without the addition of radiotherapy 38.
Since half of all ampullary carcinomas are anticipated to recur following the initial intervention, it is of paramount importance that features associated with recurrence risk are identified and managed accordingly 39. This risk is highlighted by the fact that up to 28% of patients with T1 disease have been reported having lymph node metastases 40. This is the primary reason why pancreaticoduodenectomy is preferred over local ampullectomy as determining benign vs. malignant tumor status is not routinely feasible using only preoperative symptoms or lesion size as predictors. Due to their earlier presentation, resection remains the only curative treatment for patients with Ampullary cancer and is feasible in approximately 50% compared to that of less than 10% in pancreatic adenocarcinoma. The mismatch between tumor size and biliary obstruction explains why, compared to pancreatic cancers, resectability of Ampullary cancer at presentation is significantly higher. As a result, the prognosis is considerably better than that for pancreatic cancer 40. However, despite such an aggressive surgical intervention, most patients will have a disease recurrence, hence justifying a possible role for adjuvant therapies.
The role of postsurgical adjuvant treatment of ampullary cancer remains to be established, because of limited data available in this rare disease. Preoperative neoadjuvant radiation, chemotherapy, or chemoradiation is the available options and has been studied with a survival benefit evident in certain subset populations: patients with multiple morbidities who need preoperative optimization which may delay surgery; patients with poor biologic behavior of neoplasm; patients with the possibility of an interruption in therapy due to postoperative surgical complications 41; or advanced disease with poor prognostic features. A significant proportion of our cohort consisted of patients with advanced disease as indicated by perineural invasion rate (26%), extension into adjacent organs (37%), and peripancreatic soft tissue (16%).
Ampullary cancer survival rate
Reviews of single-institution surgical experiences of ampullary cancer have focused on the identification of histopathologic features associated with prognosis and survival. Retrospective review, small patient numbers, and long periods of enrollment limit what can be learned from these studies. However, common themes emerge from these published clinicopathologic analyses 42.
Survival after surgical resection is related to the extent of local invasion of the primary lesion, lymph node involvement, vascular invasion, perineural invasion, cellular differentiation, and uninvolved surgical margins. Even a single lymph node with evidence of metastatic carcinoma portends a poor outcome with surgery alone. Exactly which factors are truly independent remains controversial.
El-Ghazzawy et al 18 reviewed experiences in the US Department of Veterans Affairs hospitals from 1987-1991, during which time 123 patients were diagnosed with ampullary cancer. In the group that underwent surgical resection, perineural invasion, microlymphatic invasion, vascular invasion, or tumor differentiation did not independently influence survival when the tumors were controlled for stage.
Yamaguchi et al 43 compared 18 variables among 8 long-term survivors and 12 short-term survivors with ampullary cancer and found that only perineural invasion and histologic grade were significant.
In a retrospective review 44 of 46 consecutive cases of ampullary carcinoma, multivariate analysis by Sudo et al showed perineural invasion to be a significant independent predictor of poor prognosis. On univariate analysis, other significant predictors of poor prognosis were T3 and T4 tumors (ie, pancreatic parenchymal invasion) and lymph node metastasis.
Multivariate analysis of 302 cases by Lowe et al 45 also showed that perineural invasion is associated with lower survival (hazard ratio [HR] 4.62), as was N1 disease (HR 4.50).
A retrospective study of 50 patients by Uchida et al 46 found that patients with preoperative jaundice had poorer survival than those without jaundice (5-year survival 57.2% vs. 100%, respectively).
Similarly, Carter et al 47 reported that patients with pancreaticobiliary ampullary adenocarcinomas, whose survival was worse than that of patients whose tumors had intestinal histology, were more likely to present with jaundice. This study also drew similarities in patterns of behavior based on histologic subtype, noting that intestinal ampullary adenocarcinomas behaved similarly to their duodenal counterparts, whereas pancreaticobiliary ampullary cancers were generally more aggressive and behaved like pancreatic adenocarcinomas.
In a series from Johns Hopkins, operative blood transfusions conferred a poorer 5-year survival rate on univariate analysis but not on multivariate analysis 48.
Akwari et al 49 noted that papillary histologic features portended a more favorable prognosis, with a reported 40% survival rate at 5 years, versus only 16% in those with invasive lesions. The Cleveland Clinic experience also confirmed the favorable nature of papillary histology. Table 2 summarizes the outcomes for patients with involved lymph nodes.
Table 2. Survival in patients with Localized, Node Positive Periampullary Adenocarcinoma Who Underwent Pancreaticoduodenectomy
| First Author (Year) | No. Patients | Median Survival, yrs | Estimated 5-Yr Survival, % |
|---|---|---|---|
| Monson3 (1991) | 31 | 1.4 | 16 |
| Allema4 (1995) | 35 | NA | 41 |
| Talamini5 (1997) | 40 | 2.0 | 31 |
| Harada6 (1997) | 28 | NA | 35 |
| Kayahara10 (1997) | 15 | NA | 31 |
| Howe7 (1998) | 46 | 2.0 | NA |
| Lee13 (2000) | 14 | NA | 23* |
Footnote: * 3-year survival
Abbreviation: NA, not available.
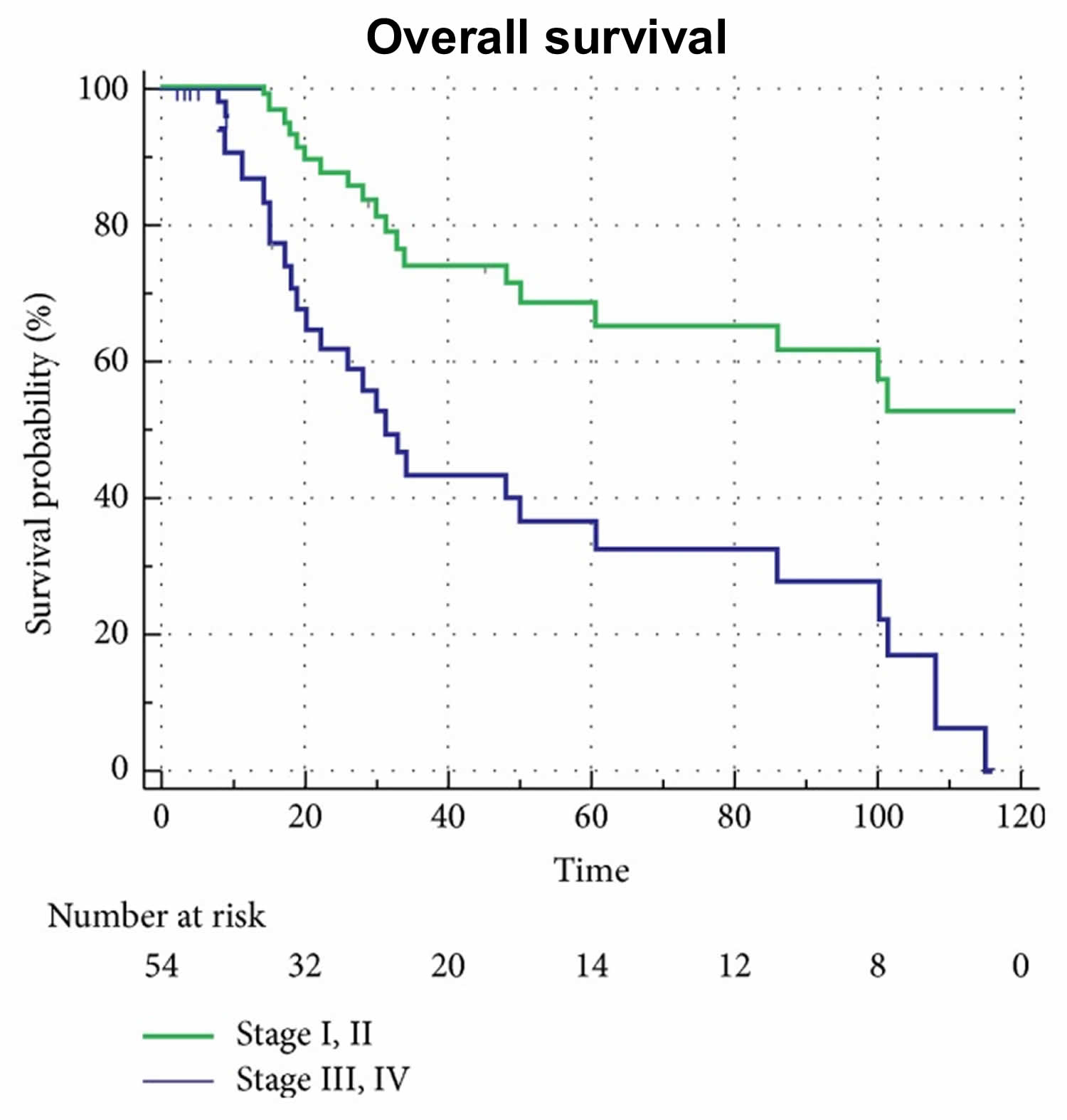
[Source 50 ]Figure 3. Overall survival probability according to ampullary cancer staging
[Source 35 ] References- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
- Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014;112–115. doi:10.14694/EdBook_AM.2014.34.112 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4966534
- Albores-Saavedra J., Schwartz A. M., Batich K., Henson D. E. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. Journal of Surgical Oncology. 2009;100(7):598–605. doi: 10.1002/jso.21374
- Herman J. M., Pawlik T. M., Merchant N. B., et al. Ampulla of vater. In: Amin M. B., editor. AJCC Cancer Staging Manual. 8th. Chicago, USA: AJCC; 2017. p. p. 327
- Ampullary Carcinoma. https://emedicine.medscape.com/article/276413-overview
- Kopelson G, Galdabini J, Warshaw AL, Gunderson LL. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1981 Mar. 7(3):413-7.
- Agoff SN, Crispin DA, Bronner MP, et al. Neoplasms of the ampulla of vater with concurrent pancreatic intraductal neoplasia: a histological and molecular study. Mod Pathol. 2001;14:139–146.
- Chang DK, Jamieson NB, Johns AL, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348–1356.
- Hatzaras I, George N, Muscarella P, et al. Predictors of survival in peri-ampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:991–997
- Jean M, Dua K. Tumors of the ampulla of Vater. Curr Gastroenterol Rep. 2003 Apr. 5 (2):171-5.
- Yamaguchi K, Enjoji M. Carcinoma of the ampulla of vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer. 1987 Feb 1. 59(3):506-15.
- Talbot IC, Neoptolemos JP, Shaw DE, Carr-Locke D. The histopathology and staging of carcinoma of the ampulla of Vater. Histopathology. 1988 Feb. 12(2):155-65.
- Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772
- O’Connell JB, Maggard MA, Manunga J, Jr, et al. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820–1827
- Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, et al. Clinicopathologic Analysis of Ampullary Neoplasms in 450 Patients: Implications for Surgical Strategy and Long-Term Prognosis. J Gastrointest Surg. 2009 Nov 13
- Sakata J, Shirai Y, Wakai T, Ajioka Y, Akazawa K, Hatakeyama K. Assessment of the nodal status in ampullary carcinoma: the number of positive lymph nodes versus the lymph node ratio. World J Surg. 2011 Sep. 35(9):2118-24
- Howe JR, Klimstra DS, Moccia RD, et al. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998 Jul. 228(1):87-94.
- el-Ghazzawy AG, Wade TP, Virgo KS, Johnson FE. Recent experience with cancer of the ampulla of Vater in a national hospital group. Am Surg. 1995 Jul. 61(7):607-11.
- Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Patterns of lymphatic spread of carcinoma of the ampulla of Vater. Br J Surg. 1997 Jul. 84(7):1012-6.
- Kayahara M, Nagakawa T, Ohta T, et al. Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery. 1997 Jun. 121(6):611-7.
- Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov. 2004 Dec. 11 (4):255-63.
- Posner S, Colletti L, Knol J, Mulholland M, Eckhauser F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery. 2000 Oct. 128 (4):694-701.
- Palta M, Patel P, Broadwater G, Willett C, Pepek J, Tyler D, et al. Carcinoma of the Ampulla of Vater: Patterns of Failure Following Resection and Benefit of Chemoradiotherapy. Ann Surg Oncol. 2011 Nov 2.
- Willett CG, Warshaw AL, Convery K, et al. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet. 1993 Jan. 176(1):33-8.
- Barton RM, Copeland EM 3d. Carcinoma of the ampulla of Vater. Surg Gynecol Obstet. 1983 Mar. 156(3):297-301.
- Sikora SS, Balachandran P, Dimri K, et al. Adjuvant chemo-radiotherapy in ampullary cancers. Eur J Surg Oncol. 2005 Mar. 31(2):158-63.
- Zhou J, Hsu CC, Winter JM, Pawlik TM, Laheru D, Hughes MA, et al. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of Vater. Radiother Oncol. 2009 Aug. 92(2):244-8.
- Chan C, Herrera MF, de la Garza L, et al. Clinical behavior and prognostic factors of periampullary adenocarcinoma. Ann Surg. 1995 Nov. 222(5):632-7.
- Yeung RS, Weese JL, Hoffman JP. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer. 1993 Oct 1. 72(7):2124-33.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281.
- Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598–2603.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281
- Plastaras JP, Berman A, Apisarnthanarax S, et al. Proton reirradiation of locally recurrent pancreatic and ampullary adenocarcinomas. J Clin Oncol. 2012;30(suppl 34) abstr 317
- Boone BA, Moser AJ, Mock BK, et al. Palliative reoperation for recurrent perampullary adenocarcinoma: Primum non nocer? J Clin Oncol. 2012;30(suppl 34) abstr 257
- Al-Jumayli M, Batool A, Middiniti A, et al. Clinical Outcome of Ampullary Carcinoma: Single Cancer Center Experience. J Oncol. 2019;2019:3293509. Published 2019 May 2. doi:10.1155/2019/3293509 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6521487
- Panzeri F., Crippa S., Castelli P., et al. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World Journal of Gastroenterology. 2015;21(26):7970–7987. doi: 10.3748/wjg.v21.i26.7970.
- Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772.
- National Comprehensive Cancer Network. https://www.nccn.org/patients/guidelines/pancreatic/files/assets/basic-html/page-1.html
- Talamini M. A., Moesinger R. C., Pitt H. A., et al. Adenocarcinoma of the ampulla of vater. A 28-year experience. Annals of Surgery. 1997;225(5):590–599
- Winter J. M., Cameron J. L., Olino K., et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: Implications for surgical strategy and long-term prognosis. Journal of Gastrointestinal Surgery. 2010;14(2):379–387. doi: 10.1007/s11605-009-1080-7
- Cloyd J. M., Wang H., Overman M., et al. Influence of preoperative therapy on short- and long-term outcomes of patients with adenocarcinoma of the ampulla of vater. Annals of Surgical Oncology. 2017;24(7):2031–2039. doi: 10.1245/s10434-017-5777-7
- Lazaryan A, Kalmadi S, Almhanna K, Pelley R, Kim R. Predictors of clinical outcomes of resected ampullary adenocarcinoma: a single-institution experience. Eur J Surg Oncol. 2011 Sep. 37(9):791-7.
- Yamaguchi K, Nishihara K. Long- and short-term survivors after pancreatoduodenectomy for ampullary carcinoma. J Surg Oncol. 1992 Jul. 50(3):195-200.
- Sudo T, Murakami Y, Uemura K, Hayashidani Y, Hashimoto Y, Ohge H, et al. Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig Dis Sci. 2008 Aug. 53(8):2281-6.
- Lowe MC, Coban I, Adsay NV, Sarmiento JM, Chu CK, Staley CA, et al. Important prognostic factors in adenocarcinoma of the ampulla of Vater. Am Surg. 2009 Sep. 75(9):754-60; discussion 761.
- Uchida H, Shibata K, Iwaki K, Kai S, Ohta M, Kitano S. Ampullary cancer and preoperative jaundice: possible indication of the minimal surgery. Hepatogastroenterology. 2009 Jul-Aug. 56(93):1194-8.
- Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008 Aug. 207(2):210-8.
- Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg. 1997 May. 225(5):590-9; discussion 599-600.
- Akwari OE, van Heerden JA, Adson MA, Baggenstoss AH. Radical pancreatoduodenectomy for cancer of the papilla of Vater. Arch Surg. 1977 Apr. 112(4):451-6.
- Yen TWF, Wolff RA, Evans DB. Neoplasms of the Ampulla of Vater. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Chapter 103b. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13495