Angiogenesis
Angiogenesis is the formation of new blood vessels from existing blood vessels, which occurs in both physiological and pathological processes 1. Angiogenesis occurs throughout life in both health and disease, beginning in utero and continuing on through old age. No metabolically active tissue in the body is more than a few hundred micrometers from a blood capillary, which is formed by the process of angiogenesis. Capillaries grow and regress in healthy tissues according to functional demands. Exercise stimulates angiogenesis in skeletal muscle and heart. A lack of exercise leads to capillary regression. Capillaries grow in adipose tissue during weight gain and regress during weight loss. Angiogenesis process involves the migration, growth, and differentiation of endothelial cells, which line the inside wall of blood vessels.
Angiogenesis is a complex process that occurs in response to hypoxia, redox stress, and lactate concentration 2. These stimuli trigger release of growth factors, principally vascular endothelial growth factor (VEGF) from macrophages. When VEGF (vascular endothelial growth factor) and other endothelial growth factors bind to their receptors on endothelial cells, signals within these cells are initiated that promote the growth and survival of new blood vessels. Other chemical signals, called angiogenesis inhibitors, interfere with blood vessel formation.
Normally, the angiogenesis stimulating and inhibiting effects of these chemical signals are balanced so that blood vessels form only when and where they are needed, such as during growth and healing. But, for reasons that are not entirely clear, sometimes these signals can become unbalanced, causing increased blood vessel growth that can lead to abnormal conditions or disease. For example, angiogenesis is the cause of age-related wet macular degeneration.
Recognition that control of angiogenesis could have therapeutic value has stimulated great interest during the past 40 years. Stimulation of angiogenesis can be therapeutic in ischemic heart disease, peripheral arterial disease, and wound healing. Tumor angiogenesis is the growth of new blood vessels that tumors need to grow. This process is caused by the release of chemicals by the tumor and by host cells near the tumor. Decreasing or inhibiting angiogenesis can be therapeutic in cancer, ophthalmic conditions, rheumatoid arthritis, and other diseases.
Angiogenesis and cancer
Angiogenesis plays a critical role in the growth of cancer because solid tumors need a blood supply if they are to grow beyond a few millimeters in size. Tumors can actually cause this blood supply to form by giving off chemical signals that stimulate angiogenesis. Tumors can also stimulate nearby normal cells to produce angiogenesis signaling molecules.
The resulting new blood vessels “feed” growing tumors with oxygen and nutrients, allowing the tumor to enlarge and the cancer cells to invade nearby tissue, to move throughout the body, and to form new colonies of cancer cells, called metastases.
Because tumors cannot grow beyond a certain size or spread without a blood supply, scientists have developed drugs called angiogenesis inhibitors, which block tumor angiogenesis. The goal of these drugs, also called antiangiogenic agents, is to prevent or slow the growth of cancer by starving it of its needed blood supply.
Angiogenesis inhibitors are unique cancer-fighting agents because they block the growth of blood vessels that support tumor growth rather than blocking the growth of tumor cells themselves.
Angiogenesis inhibitors interfere in several ways with various steps in blood vessel growth. Some are monoclonal antibodies that specifically recognize and bind to VEGF. When VEGF is attached to these drugs, it is unable to activate the VEGF receptor. Other angiogenesis inhibitors bind to VEGF and/or its receptor as well as to other receptors on the surface of endothelial cells or to other proteins in the downstream signaling pathways, blocking their activities. Some angiogenesis inhibitors are immunomodulatory drugs—agents that stimulate or suppress the immune system—that also have antiangiogenic properties.
In some cancers, angiogenesis inhibitors appear to be most effective when combined with additional therapies. Because angiogenesis inhibitors work by slowing or stopping tumor growth without killing cancer cells, they are given over a long period.
The U.S. Food and Drug Administration (FDA) has approved a number of angiogenesis inhibitors to treat cancer. Most of these are targeted therapies that were developed specifically to target VEGF, its receptor, or other specific molecules involved in angiogenesis. Approved angiogenesis inhibitors include:
- Axitinib (Inlyta®)
- Bevacizumab (Avastin®)
- Cabozantinib (Cometriq®)
- Everolimus (Afinitor®)
- Lenalidomide (Revlimid®)
- Lenvatinib mesylate (Lenvima®)
- Pazopanib (Votrient®)
- Ramucirumab (Cyramza®)
- Regorafenib (Stivarga®)
- Sorafenib (Nexavar®)
- Sunitinib (Sutent®)
- Thalidomide (Synovir, Thalomid®)
- Vandetanib (Caprelsa®)
- Ziv-aflibercept (Zaltrap®)
Angiogenesis in wound healing
Neovascularization means the formation of any blood vessel in the adult regardless of its size or type 3. Neovascularization occurs during wound healing and encompasses both angiogenesis and vasculogenesis 2. Vasculogenesis occurs when bone-marrow-derived endothelial precursor cells migrate to damaged tissue to differentiate and grow into new vessels. It is worth noting that hyperbaric oxygen therapy (HBOT) increases the rate of vasculogenesis via its upregulation of nitric oxide, causing the bone marrow to produce greater numbers of endothelial precursor cells. Angiogenesis, in contrast to vasculogenesis, is blood vessel growth that occurs as new vessels bud off from existing blood vessels 4. VEGF (vascular endothelial growth factor) induces capillary endothelial cells to migrate into the wound, form tubules off post-capillary venules, and connect to existing blood supplies.
Various studies, for example, with macrophages in culture, in wound fluid, using rat models, and in human volunteers, have shown that hyperbaric oxygen therapy increases active VEGF (vascular endothelial growth factor) production. And there are various proposed mechanisms for how hyperbaric oxygen therapy does so 5.
One mechanism simply involves the correction of wound hypoxia. As part of the hemostatic process of wound healing, capillaries vasoconstrict, which increases the diffusion distance that oxygen must traverse to reach the endothelial cells. Given that VEGF requires oxygen, the greater diffusion distance decreases the amount of oxygen available to VEGF. Hyperbaric oxygen therapy becomes efficacious because it increases oxygen partial pressure gradients between healthy and hypoxic tissues.
Larger oxygen tension gradients improve oxygen diffusion and augment the rate of wound healing. Areas of the body with lower oxygen tension, such as the extremities and trunk, heal more slowly than facial wounds owing to lower oxygen tension. Moreover, it is these tissues (subcutaneous, fascial, tendon, and bone) that are at most risk of poor wound healing. Hyperbaric oxygen therapy mitigates the risk by widening the oxygen partial pressure gradient to increase diffusion distance. One study demonstrated a dramatic diffusion distance increase of 286%, from 64 μm at an oxygen partial pressure of 100 mm Hg to 247 μm with a partial pressure of 2000 mm Hg. It requires noting that the wound site must still possess some arterial inflow.
A second proposed mechanism involves oxygen’s induction of VEGF. In a study of human umbilical vein endothelial cells undergoing hyperbaric oxygen therapy, researchers found that VEGF was upregulated in those cells at both mRNA and protein levels. The study hypothesized that hyperbaric oxygen therapy induced binding of AP-1, a transcription factor, to the VEGF promoter to upregulate expression. Two pathways were identified: AP-1 via stress-activated protein kinase/c-June N-terminal kinase (SAPK/JNK) pathway and the extracellular signal-regulated kinase (ERK) pathway. To confirm, both pathways were blocked individually using specific inhibitors and luciferases, which prevented upregulation of VEGF.
A third proposed mechanism of VEGF induction involves another transcription factor: hypoxia-inducible factor-1-alpha (HIF-1alpha). During normoxic periods, HIF-1alpha is hydroxylated and then destroyed by the ubiquitin-proteasome pathway. During hypoxic periods, when reactive oxygen species’ concentration is elevated, HIF-1alpha avoids hydroxylation and thereby acts to increase the concentration of hypoxia-inducible factor (HIF). In turn, HIF induces VEGF expression and angiogenesis. hyperbaric oxygen therapy is thought to promote HIF-1alpha hydroxylation by spurring the formation of ROS via the cycling between hyperoxic and hypoxic states that occurs after hyperbaric oxygen therapy treatments.
As hyperbaric oxygen therapy increases the rate of angiogenesis, there exist myriad indications for its utilization. One such indication is the prevention of graft compromise. The very nature of grafting lends itself to hyperbaric oxygen therapy. A graft is a section of tissue completely separated from the donor tissue bed and all its vascular connections. When placed on the recipient bed, the graft requires ingrowth of new blood vessels to take. Until angiogenesis occurs, the oxygen tension in the graft is low and sustained low oxygen tension caused by ischemia is a common etiology of graft compromise. hyperbaric oxygen therapy promotes new vessel growth, and it merits underscoring that the most effective means for treating a compromised graft is preventing compromise altogether by preparing the recipient bed with hyperbaric oxygen therapy before placement.
Preparation of the wound bed is paramount for successful graft take. Initial measures include surgical debridement of necrotic tissue and infection control. Once complete, the wound should be evaluated to determine if hyperbaric oxygen therapy can raise the oxygen tension to therapeutic levels. One method is to use an oxygen challenge test with the patient breathing 100% normobaric oxygen: if transcutaneous oxygen partial pressure (tcPO2) increases at least 10mmHg, hyperbaric oxygen therapy should be efficacious. However, studies have shown that patients with a minimal increase after the oxygen challenge may still have significant increases in transcutaneous oxygen partial pressure after hyperbaric oxygen therapy. One study demonstrated an increase in transcutaneous oxygen partial pressure to greater than 200 mm Hg during hyperbaric oxygen therapy at 2.5 atmospheres absolute (ATA), which resulted in more than 80% of problem wounds healing. This was notwithstanding poor oxygen challenge results before hyperbaric oxygen therapy.
Another method is to consider hypoxic any wound that lacks a reconstructible vascular lesion, and that possesses a transcutaneous oxygen partial pressure of less than 40 mm Hg. The wound is then treated with hyperbaric oxygen therapy. If the transcutaneous oxygen partial pressure increases greater than 200 mm Hg, the wound has a high probability of accepting the graft. hyperbaric oxygen therapy should be continued with daily treatments of 100% oxygen at 2.0 to 2.4 ATA for 90 minutes 5 times per week. Monitor the transcutaneous oxygen partial pressure every 1 to 2 weeks at least 12 hours post-hyperbaric oxygen therapy with the patient on room air; when the transcutaneous oxygen partial pressure levels crest 40 mm Hg, hyperbaric oxygen therapy can be discontinued and the graft placed.
Hyperbaric oxygen therapy is an effective means of promoting angiogenesis. Via its ability to increase the oxygen partial pressure gradient and its induction of VEGF, hyperbaric oxygen therapy is an invaluable tool in a physician’s armamentarium when confronted with compromised skin grafts and problem wounds. In conclusion, consider hyperbaric oxygen therapy for osteoradionecrosis and soft-tissue radionecrosis as well. Both of these conditions are produced by radiation-induced small vessel loss and would benefit from angiogenesis aided by hyperbaric oxygen therapy 6.
Angiogenesis process
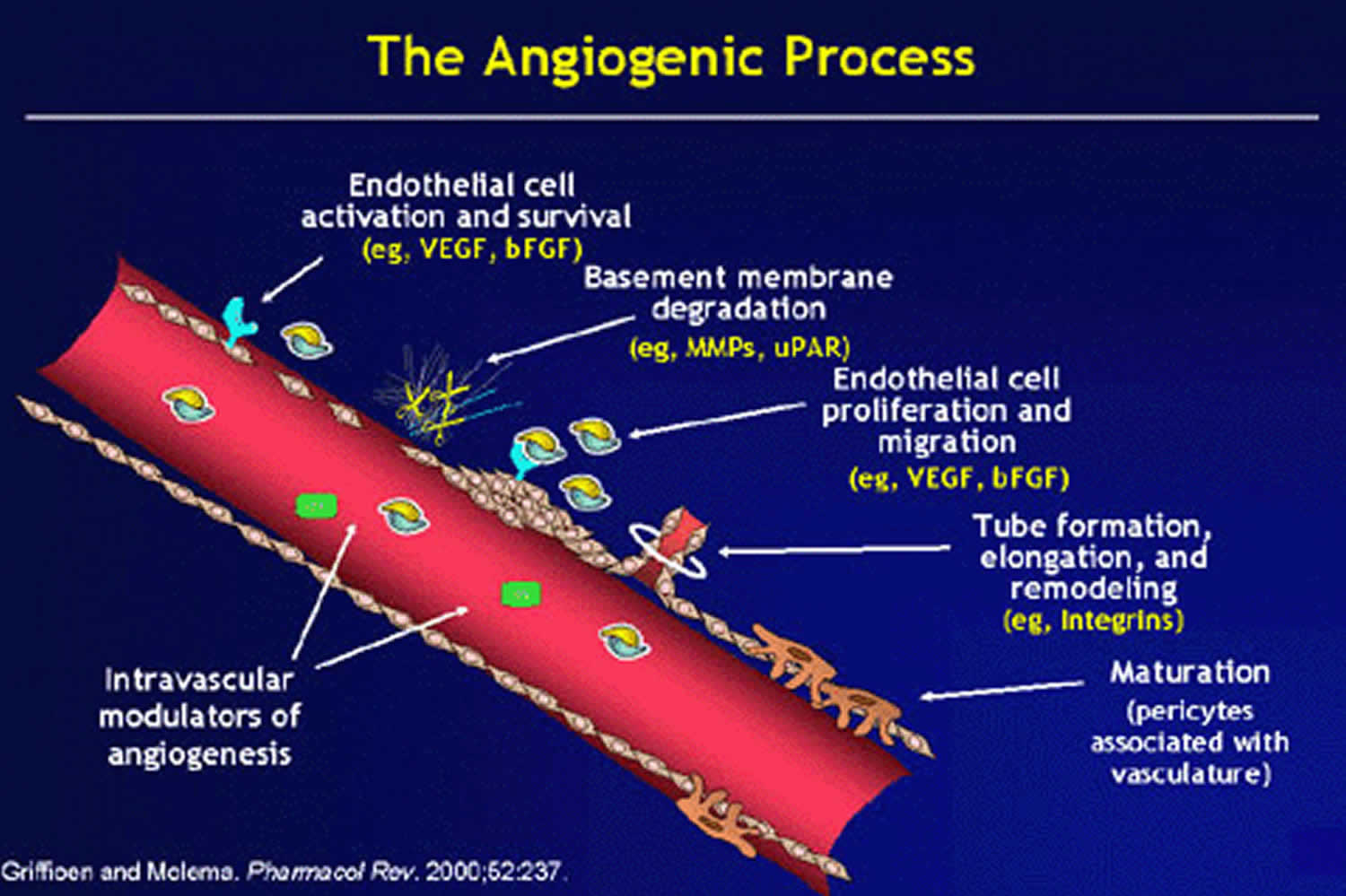
Angiogenesis is driven by the coordinated action of endothelial cells, involves remodeling of the extracellular matrix and recruitment of mural cells, such as pericytes, fibroblasts and smooth muscle cells 7. There are two significant forms of angiogenesis:
- Intussusceptive angiogenesis, where new vessels are formed through pillars that result from the fusing of the plasma membrane of pre-existing vessels
- Sprouting angiogenesis, which is driven by coordinated movements of endothelial cells in response to cytokine gradients and tissue hypoxia (Figure 2).
Sprouting angiogenesis and intussusceptive angiogenesis both occur in utero and in adults 3. Sprouting angiogenesis is better understood having been discovered nearly 200 years ago: intussusceptive angiogenesis was discovered by Burri 8 in 1990. Figure 1 shows the basic morphological events for angiogenesis. As implied by its name, sprouting angiogenesis is characterized by sprouts composed of endothelial cells, which usually grow toward an angiogenic stimulus such as VEGF-A. Sprouting angiogenesis can therefore add blood vessels to portions of tissues previously devoid of blood vessels. On the other hand, intussusceptive angiogenesis involves formation of blood vessels by a splitting process in which elements of interstitial tissues invade existing vessels, forming transvascular tissue pillars that expand. Both types of angiogenesis are thought to occur in virtually all tissues and organs.
Figure 1. Angiogenesis process
Sprouting angiogenesis
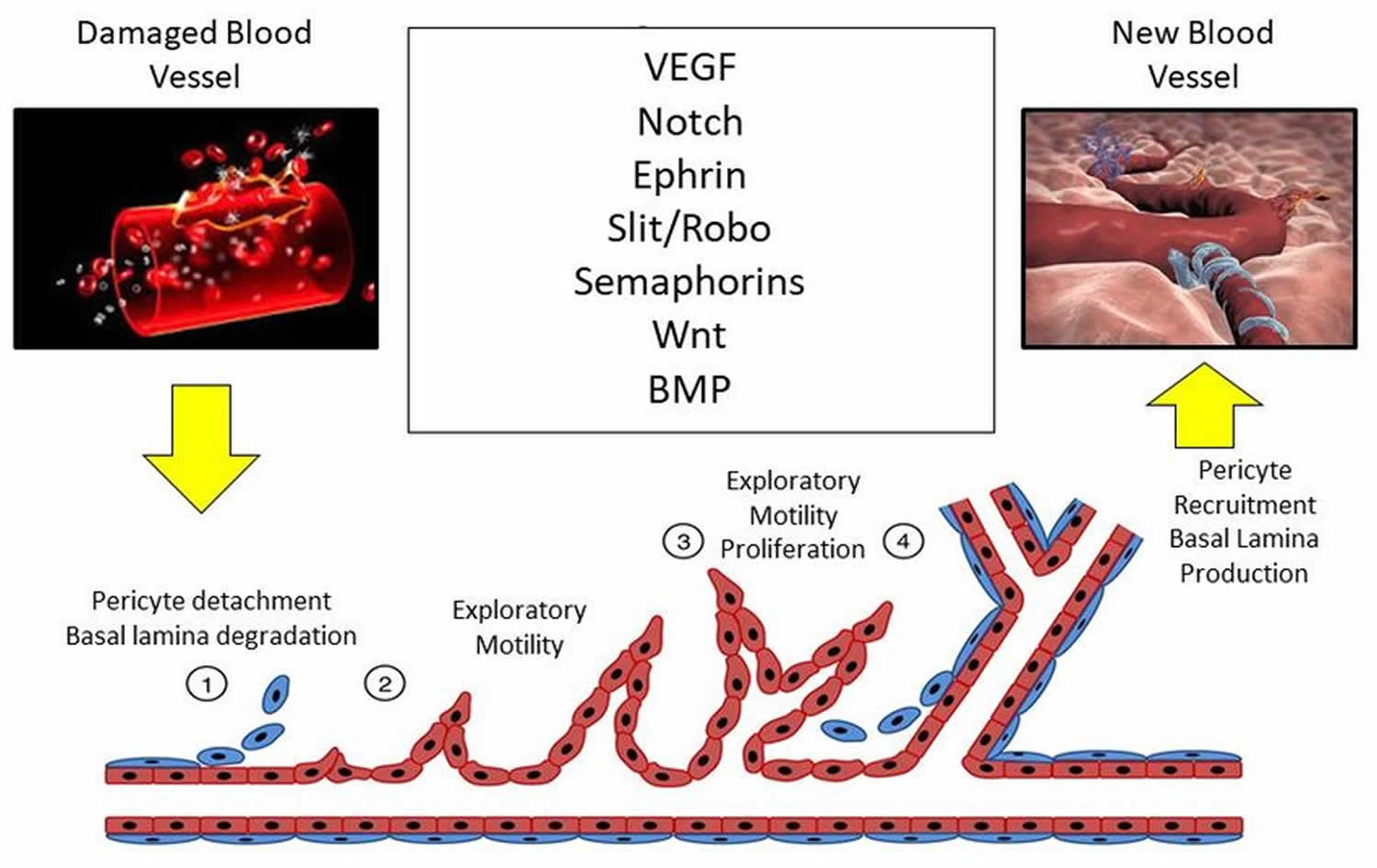
Sprouting angiogenesis is a complex process in which a vascular sprout arises from a pre-existing vessel and subsequently forms a new blood vessel. The basic steps of sprouting angiogenesis include enzymatic degradation of capillary basement membrane, endothelial cell proliferation, directed migration of endothelial cells, tubulogenesis (endothelial cell tube formation), vessel fusion, vessel pruning, and pericyte stabilization. Sprouting angiogenesis is initiated in poorly perfused tissues when oxygen sensing mechanisms detect a level of hypoxia that demands the formation of new blood vessels to satisfy the metabolic requirements of parenchymal cells (Figure 2). Most types of parenchymal cells (myocytes, hepatocytes, neurons, astrocytes, etc.) respond to a hypoxic environment by secreting a key proangiogenic growth factor called vascular endothelial growth factor (VEGF-A). There does not appear to be redundant growth factor mechanisms that can replace the role of VEGF-A in hypoxia-induced angiogenesis.
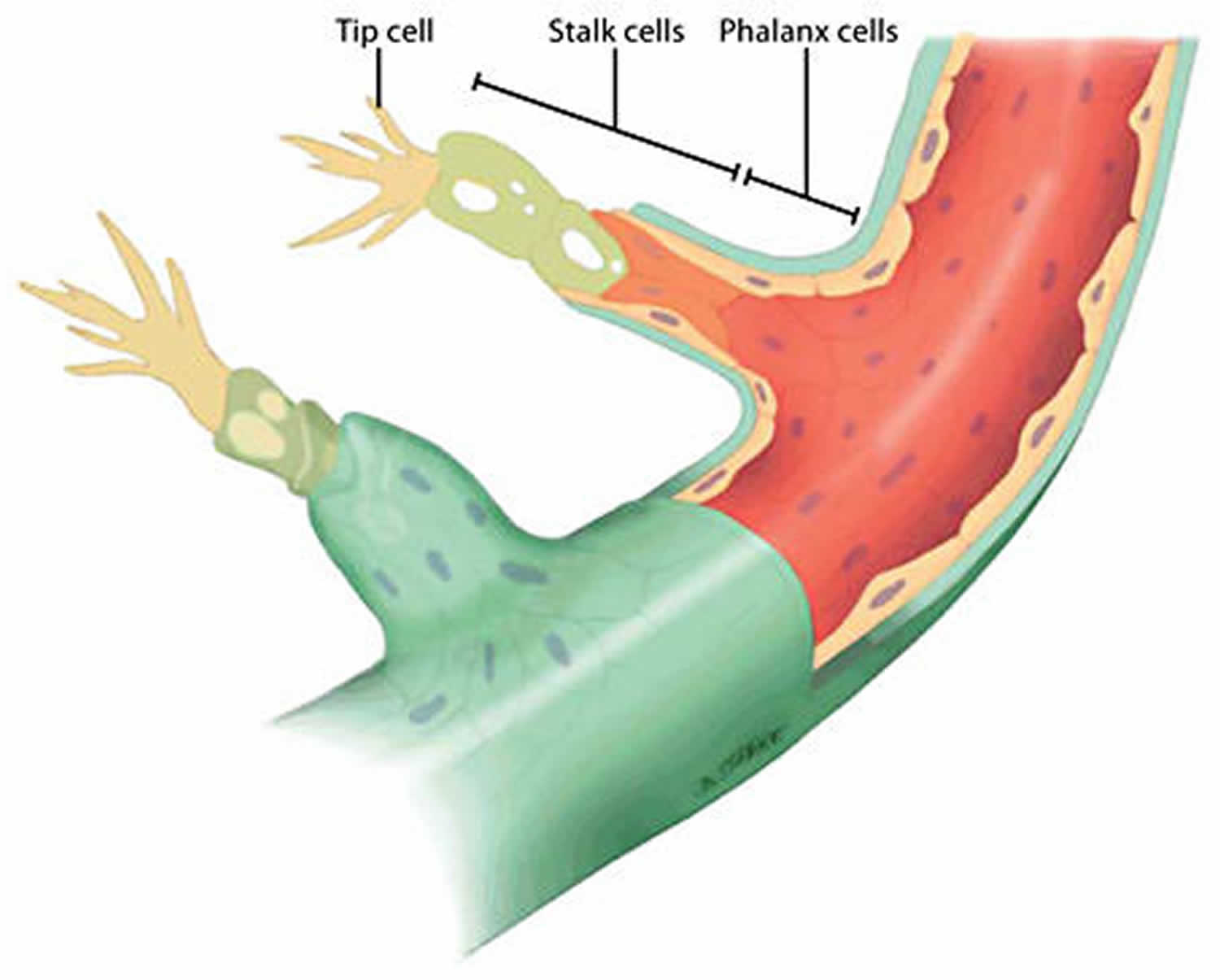
In sprouting angiogenesis, a highly motile, filopodia-enriched “endothelial tip cell” acts as the “pathfinder” that creates a path for its adjacent neighbors (the highly proliferative “stalk” cells) through the extracellular matrix (ECM) toward an angiogenic stimulus such as VEGF-A 9. Long, thin cellular processes on tip cells called filopodia secrete large amounts of proteolytic enzymes, which digest a pathway through the extracellular matrix for the developing sprout 10. The filopodia of tip cells are heavily endowed with VEGF-A receptors (VEGFR2), allowing them to “sense” differences in VEGF-A concentrations and causing them to align with the VEGF-A gradient. When a sufficient number of filopodia on a given tip cell have anchored to the substratum, contraction of actin filaments within the filopodia literally pull the tip cell along toward the VEGF-A stimulus. Meanwhile, endothelial stalk cells proliferate as they follow behind a tip cell causing the capillary sprout to elongate. Vacuoles develop and coalesce, forming a lumen within a series of stalk cells. These stalk cells become the trunk of the newly formed capillary. When the tip cells of two or more capillary sprouts converge at the source of VEGF-A secretion, the tip cells fuse together creating a continuous lumen through which oxygenated blood can flow. When the local tissues receive adequate amounts of oxygen, VEGF-A levels return to near normal. Maturation and stabilization of the capillary requires recruitment of pericytes and deposition of extracellular matrix along with shear stress and other mechanical signals 11.
As a result of the coordinated action of tip and stalk cells, a new vessel branch arises that reaches through zones of hypoxia to merge with neighboring vessels for the reestablishment of blood flow and oxygen delivery to these areas. Through a stochastic process, any endothelial cell is intrinsically capable of becoming a tip or stalk cell, or to remain as a quiescent endothelial cell, or phalanx cell, in the stable vessel 12. Interestingly, individual endothelial cells actively rotate between each phenotype by the rapid induction and repression of a complex genetic program driven by the integration of extracellular pro- and anti-angiogenic chemotactic cues from surrounding tissues and neighboring vascular endothelial cells 13. Furthermore, the ratio of tip cells to stalk cells is crucial for the proper formation of new vessels and is tightly regulated by crosstalk among several signaling pathways, such as ephrin, notch, bone morphogenetic protein (BMP), and Wnt signaling (Figure 2) 14.
Delta-Notch signaling is a key component of sprout formation 3. It is a cell–cell signaling system in which the ligand, Delta-like-4 (Dll4) mates with its notch receptor on neighboring cells. Both the receptor and ligand is cell bound and thus act only through cell–cell contact. VEGF-A induces Dll4 production by tip cells, which leads to activation of notch receptors in stalk cells. Notch receptor activation suppresses VEGFR2 production in stalk cells, which dampens migratory behavior compared with that of tip cells. Hence, endothelial cells exposed to the highest VEGF-A concentration are most likely to become tip cells 15. Although tip cells are exposed to the highest VEGF-A concentration, their rate of proliferation is far less compared with that of stalk cells.
Not all aspects of the Delta-Notch signaling pathway are fully understood, but it is clear that production of a normal vasculature is heavily dependent upon the concentration of VEGF-A in the tissues. A 50% reduction of VEGF-A expression is lethal embryonically because of vascular defects 16 and excess VEGF-A in tumors induces overproduction of tip cells leading to a disorganized vasculature 17. This critical dependence on physiological concentrations of VEGF-A for construction of viable blood vessels might help explain why attempts to induce angiogenesis in poorly perfused tissues with VEGF-A administration and gene therapy have not been highly successful.
Figure 2. Sprouting angiogenesis
[Source 7 ]Intussusceptive angiogenesis
Intussusceptive angiogenesis is also also called splitting angiogenesis or nonsprouting angiogenesis, because the vessel wall extends into the lumen causing a single vessel to split in two 3. Intussusceptive angiogenesis is thought to be fast and efficient compared with sprouting angiogenesis because, initially, it only requires reorganization of existing endothelial cells and does not rely on immediate endothelial proliferation or migration 3. Intussusceptive angiogenesis occurs throughout life but plays a prominent role in vascular development in embryos where growth is fast and resources are limited 18. However, intussusception mainly causes new capillaries to develop where capillaries already exist.
Evidence for the occurrence of intussusceptive angiogenesis is based upon the presence of transcapillary tissue pillars 3. Identification of tissue pillars requires scanning electron micrographs of vascular casts or three-dimensional reconstruction of serial micrographs. This type of angiogenesis was discovered in postnatal lungs of rats and humans 8, but it also occurs in many other tissues and organs, especially in capillary networks that abut an epithelial surface, e.g., choroid of the eye, vascular baskets around glands, intestinal mucosa, kidney, ovary, and uterus 19. It also occurs in skeletal muscle, heart, and brain. In addition to forming new capillary structures, intussusceptive growth plays a major role in the formation of artery and vein bifurcations as well as pruning of larger microvessels.
The control of intussusceptive angiogenesis is poorly understood compared with sprouting angiogenesis. This difference is only partly due to its recent discovery in 1986 20. A rate-limiting step in intussusceptive growth research can be pinned to the laborious methods required to prove its presence, which, again, involve determining the frequency of tissue pillars from scanning electron micrographs of vascular casts. However, it is known that intussusceptive angiogenesis can be stimulated in the chick chorioallantoic membrane with application of VEGF-A and there is little doubt that many growth factors and signaling systems are involved 19. Mechanical stresses related to increases in blood flow can initiate intussusceptive growth in some high flow regions of the circulation 19.
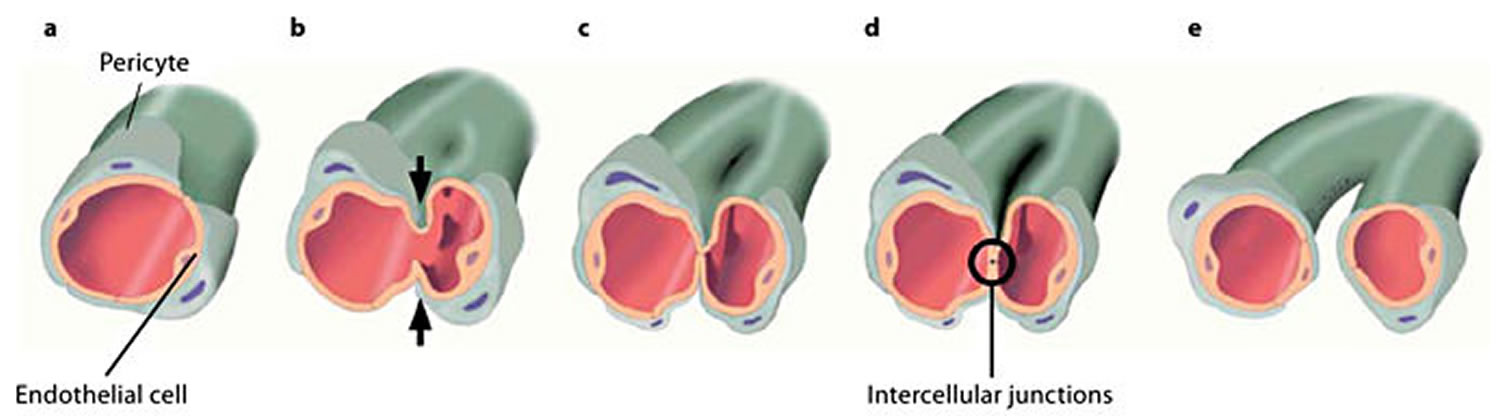
A typical characteristic of intussusceptive angiogenesis is the formation of so-called intraluminal tissue pillars that are formed by an invagination of the capillary walls into the vascular lumen 21. The formation of intraluminal pillars proceeds through a multistep process 22. It starts when the endothelial walls of the opposite sides of a vessel migrate to each other (Figure 3a, b), forming an intraluminal pillar (Figure 3c). The interendothelial junctions are reorganized (Figure 3c), and a central perforation is formed in the core of the pillar. Subsequently, this pillar is invaded by pericytes and myofibroblasts that deposit extracellular matrix into the pillar (Figure 3d). Finally, several pillars increase in size and fuse with each other, splitting up the initial capillary into two new capillaries (Figure 3e) 22.
Figure 3. Intussusceptive angiogenesis
Footnote: Schematic illustration of a small capillary surrounded by pericytes (a) undergoing intussusceptive angiogenesis. The opposite walls of this capillary start to migrate to each other (b), an intraluminal pillar is formed (c), and the cellular junctions of the opposing endothelial cells are rearranged (d). Subsequently, further growth of the pillar leads to spitting of the blood vessel into two new vessels (e).
[Source 23 ]Morphological characteristics of intussusceptive angiogenesis
Three forms of intussusceptive angiogenesis are recognized, depending on the outcome or phenotype of these forms, i.e. intussusceptive microvascular growth (intussusceptive microvascular growth), intussusceptive arborization (intussusceptive arborization), and intussusceptive branching remodeling (intussusceptive branching remodeling) 22. Intussusceptive microvascular growth leads to the rapid expansion of an already existing vascular network through the continuous new formation and expansion of pillars into the network. This results in a simple network of similarly sized capillaries. Intussusceptive microvascular growth is characterized by a diffuse appearance of numerous pillars. In a mechanism that is most likely driven by blood flow, these pillars fuse and split the vessels, expanding the capillary network and forming the organ-specific architecture 24. In contrast, intussusceptive arborization contributes to the remodeling of a previously unhierarchical capillary network into a hierarchical vascular tree in which major arterioles, venules, and capillaries can be discerned. intussusceptive arborization can be recognized in a dense capillary network by the occurrence of a series of pillars, delineating the prospective supply vessel from the neighboring capillaries. The third form of intussusceptive angiogenesis, intussusceptive branching remodeling, can be defined as the mechanism that optimizes the number of vessels to efficiently supply a tissue with blood by either changing the branching pattern of blood vessels or pruning the vascular network from superfluous vessels 25. intussusceptive branching remodeling is commonly recognized by the occurrence of a tissue pillar close to the bifurcation of two blood vessels. These pillars enlarge and eventually merge with the perivascular connective tissue. As such, the bifurcation is further narrowed and the bifurcation point is relocated more proximally. In the case of pruning, pillars also appear close to a bifurcation, but they are situated more eccentrically and asymmetrically 26. The elongation and fusion of these pillars cuts off the blood flow from the targeted blood vessel, which subsequently regresses.
References- Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Griffioen AW, Molema G. Pharmacol Rev. 2000 Jun; 52(2):237-68.
- Buckley CJ, Cooper JS. Hyperbaric, Angiogenesis. [Updated 2019 May 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482485
- Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Overview of Angiogenesis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53238
- Hatibie MJ, Islam AA, Hatta M, Moenadjat Y, Susilo RH, Rendy L. Hyperbaric Oxygen Therapy for Second-Degree Burn Healing: An Experimental Study in Rabbits. Adv Skin Wound Care. 2019 Mar;32(3):1-4.
- Hadanny A, Lang E, Copel L, Meir O, Bechor Y, Fishlev G, Bergan J, Friedman M, Zisman A, Efrati S. Hyperbaric oxygen can induce angiogenesis and recover erectile function. Int. J. Impot. Res. 2018 Nov;30(6):292-299.
- Buboltz JB, Cooper JS. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Feb 16, 2019. Hyperbaric Soft Tissue Radionecrosis.
- Ranchoux, Benoît & Harvey, Lloyd & Ayon, Ramon & Babicheva, Aleksandra & Bonnet, Sébastien & Chan, Stephen & Yuan, Jason & Perez, Vinicio. (2017). EXPRESS: Endothelial Dysfunction in Pulmonary Arterial Hypertension: An Evolving Landscape (2017 Grover Conference Series). Pulmonary Circulation. 8. 204589321775291. 10.1177/2045893217752912.
- Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec 228: pp. 35–45, 1990.10.1002/ar.1092280107
- Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4: pp. 241–6, 2008.10.4161/org.4.4.7414.
- van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc Res 78: pp. 203–12, 2008.
- Chien S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am J Physiol Heart Circ Physiol 292: pp. H1209–24, 2007.
- De Bock K, Georgiadou M, Carmeliet P. Role of Endothelial Cell Metabolism in Vessel Sprouting. Cell Metab 2013; 18: 634–647.
- Beets K, Huylebroeck D, Moya IM, et al. Robustness in angiogenesis: notch and BMP shaping waves. Trends Genet TIG 2013; 29: 140–149.
- Aspalter IM, Gordon E, Dubrac A, et al. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun 2015; 6: 7264.
- Carmeliet P, De Smet F, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: Tip cells lead the way. Nat Rev Clin Oncol 6: pp. 315–26, 2009.
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: pp. 439–42, 1996.10.1038/380439a0
- Jain RK. Normalization of tumor vasculature: An emerging concept in anti-antiangiogenic therapy. Science 307: pp. 58–62, 2005.
- Kurz H, Burri PH, Djonov VG. Angiogenesis and vascular remodeling by intussusception: From form to function. News Physiol Sci 18: pp. 65–70, 2003.
- Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev Dyn 231: pp. 474–88, 2004.
- Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec 216: pp. 154–64, 1986.10.1002/ar.1092160207.
- Djonov V, Baum O, Burri P: Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res 2003;314:107–117.
- Burri PH, Djonov V: Intussusceptive angiogenesis: the alternative to capillary sprouting. Mol Aspects Med 2002;23:S1–S27.
- De Spiegelaere W, Casteleyn C, Van den Broeck W, Plendl J, Bahramsoltani M, Simoens P, Djonov V, Cornillie P: Intussusceptive Angiogenesis: A Biologically Relevant Form of Angiogenesis. J Vasc Res 2012;49:390-404. doi: 10.1159/000338278
- Makanya AN, Hlushchuk R, Djonov VG: Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis 2009;12:113–123.
- Djonov VG, Kurz H, Burri PH: Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev Dyn 2002;224:391–402.
- Hlushchuk R, Ehrbar M, Reichmuth P, Heinimann N, Styp-Rekowska B, Escher R, Baum O, Lienemann P, Makanya A, Keshet E, Djonov V: Decrease in VEGF expression induces intussusceptive vascular pruning. Arterioscler Thromb Vasc Biol 2011;31:2836–2844.