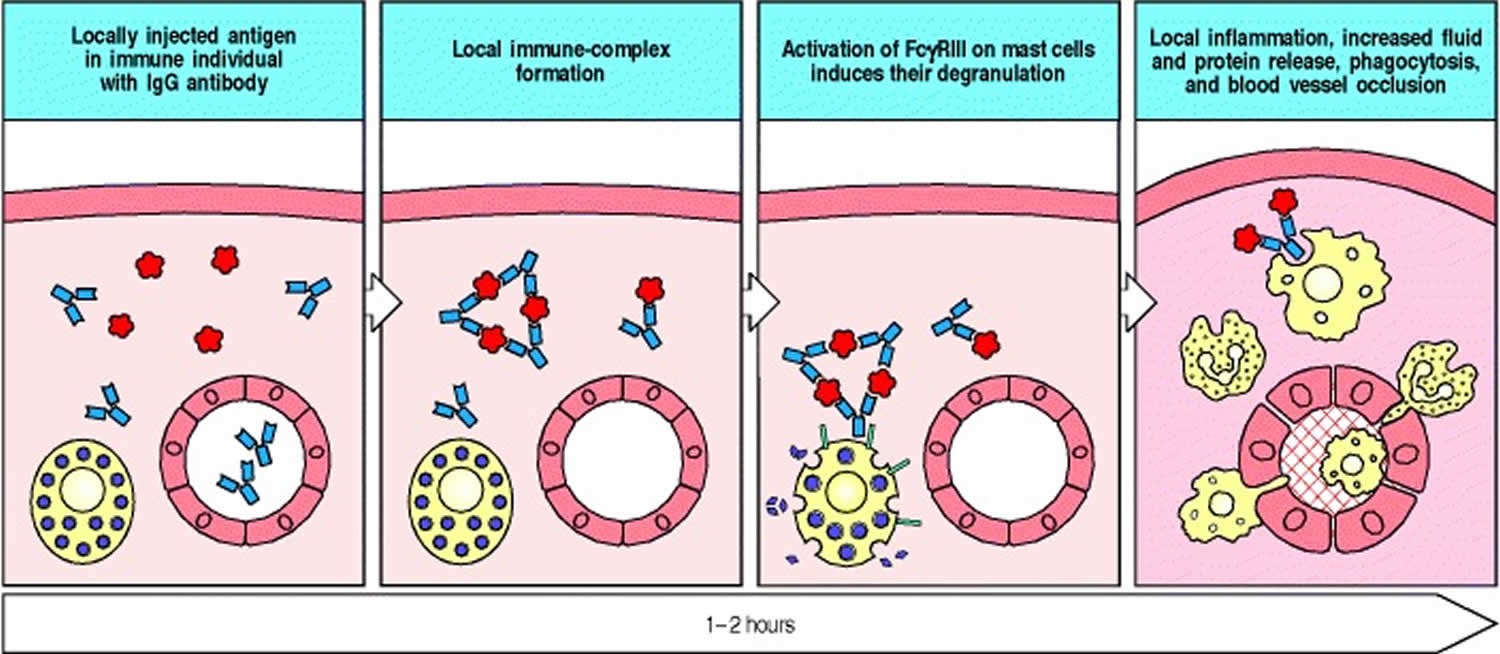
Arthus reaction
Arthus reaction is a rare adverse reaction that usually occurs after vaccination with large and more severe local reactions, belonging to type 3 hypersensitivity reaction 1. Arthus reaction is a type III hypersensitivity reaction involving IgG antibodies bound to foreign antigens in the blood. These antibody–antigen complexes can precipitate and get stuck in certain locations, such as blood vessels in the skin, kidneys and joints, where they activate the complement cascade to cause local damage 2. Arthus reaction is characterized by pain, swelling, induration (tissue that becomes firm) and edema, even accompanied by severe necrosis or ulceration at the injection sites. However, most of mild cases generally can be cured without treatment, and only severe cases need to be treated with anti-allergy. Therefore, this adverse reaction is often ignored by people.
Arthus reaction generally develops over 6 to 12 hours if antibody levels are already high, or it can develop over several days (e.g., in serum sickness) as antibody levels increase and antigen persists. In Arthus reaction, immune complexes in the walls of blood vessels initiate an inflammatory reaction involving complement and leukocytes, particularly neutrophils. Tissue sections show acute inflammation, and profound tissue destruction can occur.
Localized Arthus reactions have been reported to be common at the site of injection of some vaccines and occur when reimmunization is performed in the presence of high levels of circulating IgG antibody 3. They are characterized by pain, swelling, induration, and edema beginning several hours after immunization and usually reaching a peak 12 to 36 hours after immunization. They are self-limited, resolving over the course of a few days. Their frequency and severity can be lessened by spacing immunizations more widely, as has been recommended for tetanus-diphtheria toxoid booster injections.
Generalized Arthus reactions of a serum sickness-like character have also been invoked following vaccine administration. Such generalized serum sickness-like reactions were common in the era when horse serum was used to treat or prevent many infectious diseases and when very large quantities of immunogenic foreign protein were infused (sometimes repeatedly). These reactions require both IgG antibody and circulating excess antigen. Considering the small quantity of protein in present-day vaccines that is injected, it is not clear that such reactions could occur as a result of immunization. In animal models, symptoms and pathology tend to localize in the kidney, skin, joints, lung, and brain 4. The manifestations after vaccination most commonly ascribed to serum sickness-like mechanisms are arthritis and fever.
Figure 1. Arthus reaction
What is serum sickness?
Serum sickness is a self-limited allergic reaction following exposure to foreign proteins. Serum sickness is sometimes called a type 3 hypersensitivity reaction. The resulting immune complex of the patient’s antibodies bound to the foreign protein is deposited in small blood vessels and stimulates the complement cascade and hence an inflammatory reaction. It presents with a classic triad:
- Fever
- Skin rash
- Joint symptoms.
Serum sickness typically followed exposure to foreign, non-human proteins, especially antivenoms and antitoxins made in horse, for example, rattlesnake antivenom used in the USA. More recently, reactions have been reported with the increasing use of thymoglobulin and chimeric monoclonal antibody therapy (biological response agents).
Thymoglobulin (anti-thymocyte globulin) is made in rabbits. It is used particularly in the perioperative period following solid organ transplants to reduce the post-operative doses of immunosuppressive drugs. The incidence of serum sickness due to thymoglobulin in renal transplant recipients has been estimated to be between 7 and 27%. There is an increased risk of developing serum sickness to thymoglobulin if there has been significant past exposure to rabbits or horses.
Chimeric monoclonal antibodies are a group of biologic agents that are being used with increasing frequency for many immunological disorders such as rheumatoid arthritis, psoriasis and in cancer therapies.
What are the clinical features of serum sickness?
The typical triad of serum sickness comprises fever, rash and joint pain/swelling (arthritis or arthralgia). There is also often facial swelling and lymph node enlargement. The kidneys are commonly affected.
The classical rash is similar to hives (urticaria), but following thymoglobulin this may be absent or a brief erythematous morbilliform rash may be visible at the onset.
How is serum sickness diagnosed?
In classic serum sickness, immune complexes deposit in small blood vessels, activating the complement cascade and resulting in inflammation. This is recognised by typical clinical features. Blood tests may detect these immune complexes or the antibodies against the foreign protein and may reveal that complement levels (C3, C4) are reduced.
Skin biopsy of the rash will show a leukocytoclastic vasculitis (inflammation of blood vessels) with immune complex deposits composed of a combination of complement, IgG, IgM and IgA.
Serum sickness due to thymoglobulin can be confirmed by a blood test for antiheterologous antibodies against rabbit immunoglobulin G by enzyme-linked immunosorbent assay (ELISA).
Proposed criteria for making the diagnosis of thymoglobulin-induced serum sickness:
Major criteria
- More than seven days since starting thymoglobulin therapy
- Persistent high fevers
- Persistent joint pain or swelling
- Presence of antibodies
Minor criteria
- +/- Acute kidney failure
- +/- Skin rash
- +/- Restricted mouth opening
- +/- Low blood complement levels
What is the treatment of serum sickness?
If possible the triggering therapy should be ceased.
High dose IV corticosteroids should be given for three days then reducing doses of oral steroids following clinical response.
If there has been no response, then plasma exchange can be used to remove the immune complexes, antibodies and protein.
Serum sickness usually resolves without long-term complications.
Arthus reaction symptoms
Arthus reaction generally develops over 6 to 12 hours if antibody levels are already high, or it can develop over several days (e.g., in serum sickness) as antibody levels increase and antigen persists. In Arthus reaction, immune complexes in the walls of blood vessels initiate an inflammatory reaction involving complement and leukocytes, particularly neutrophils. Tissue sections show acute inflammation, and profound tissue destruction can occur.
Localized Arthus reactions have been reported to be common at the site of injection of some vaccines and occur when reimmunization is performed in the presence of high levels of circulating IgG antibody 3. They are characterized by pain, swelling, induration, and edema beginning several hours after immunization and usually reaching a peak 12 to 36 hours after immunization. They are self-limited, resolving over the course of a few days. Their frequency and severity can be lessened by spacing immunizations more widely, as has been recommended for tetanus-diphtheria toxoid booster injections.
Generalized Arthus reactions of a serum sickness-like character have also been invoked following vaccine administration. Such generalized serum sickness-like reactions were common in the era when horse serum was used to treat or prevent many infectious diseases and when very large quantities of immunogenic foreign protein were infused (sometimes repeatedly). These reactions require both IgG antibody and circulating excess antigen. Considering the small quantity of protein in present-day vaccines that is injected, it is not clear that such reactions could occur as a result of immunization. In animal models, symptoms and pathology tend to localize in the kidney, skin, joints, lung, and brain 4. The manifestations after vaccination most commonly ascribed to serum sickness-like mechanisms are arthritis and fever.
Arthus reaction treatment
The treatment for type 3 hypersensitivity reactions is aimed at controlling the underlying disease. It often involves immunosuppression with systemic glucocorticoids and disease-modifying drugs, such as methotrexate, ciclosporin and cyclophosphamide.
References- Peng B, Wei M, Zhu FC, Li JX. The vaccines-associated Arthus reaction. Hum Vaccin Immunother. 2019;15(11):2769–2777. doi:10.1080/21645515.2019.1602435
- Institute of Medicine (US) Vaccine Safety Committee; Stratton KR, Howe CJ, Johnston RB Jr., editors. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. Washington (DC): National Academies Press (US); 1994. 4, Immunologic Reactions. Available from: https://www.ncbi.nlm.nih.gov/books/NBK236294
- Facktor MA, Bernstein RA, Fireman P. Hypersensitivity to tetanus toxoid. Journal of Allergy and Clinical Immunology 1973; 52:1-12.
- Henson PM. Antibody and immune-complex-mediated allergic and inflammatory reactions. In: Lachmann PJ, editor; , Peters DK, editor. , eds. Clinical Aspects of Immunology, 4th edition. Oxford: Blackwell: 1982.