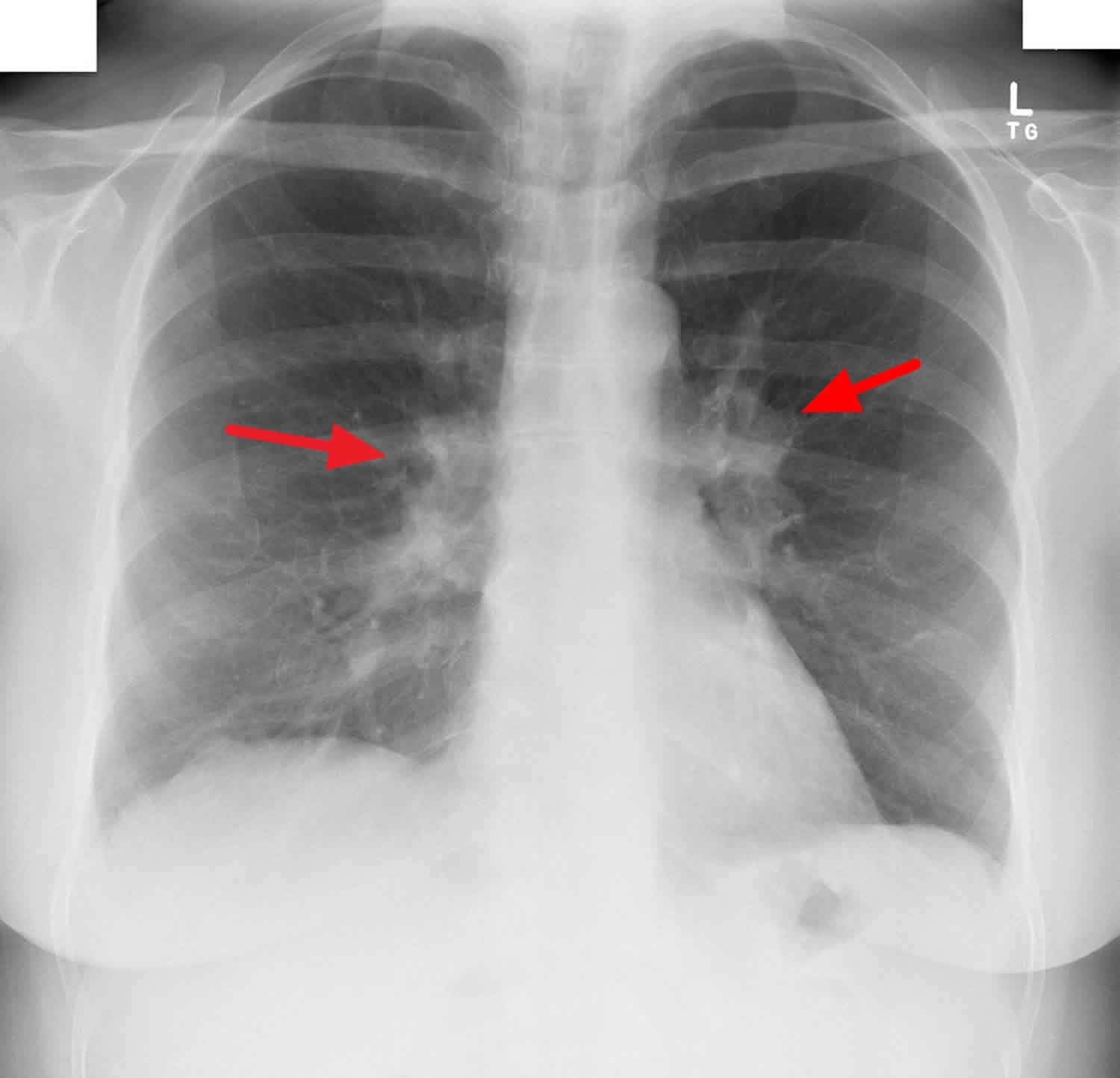
Bilateral hilar lymphadenopathy
Bilateral hilar lymphadenopathy is a bilateral enlargement of the lymph nodes of pulmonary hila 1. Bilateral hilar lymphadenopathy is a radiographic term for the enlargement of mediastinal lymph nodes and is most commonly identified by a chest x-ray.
For the diagnosis of pathologically enlarged nodes, information about normal node size is required. Commonly used metrics for lymph node measurement include the maximum and minimum axial diameters, and the ratio of these two values 2. The shortest axial diameter appears to be a more useful parameter than the greatest axial diameter, although a different threshold should be used for each nodal station 3.
The generally accepted size criterion for mediastinal lymph node enlargement (>10 mm along the short axis) has been applied to all patients when staging lymphoma or bronchogenic carcinoma 4. Other authors use the following standard maximum normal short-axial diameters for nodal regions: region 7, 12 mm; regions 4 and 10R, 10 mm; and other regions, 8 mm. Maximum greatest axial diameters show wider variation, ranging from 10 to 25 mm 5.
Bilateral hilar lymphadenopathy causes
Bilateral hilar lymphadenopathy or bilateral hilar lymph node enlargement can arise from many causes, which include 1:
- Sarcoidosis: Sarcoidosis is a systemic granulomatous disease that affects primarily the lung and lymphatic system. Five radiological stages of intrathoracic changes have been defined: stage 0, no visible intrathoracic finding; stage 1, lymphadenopathy only; stage 2, lymphadenopathy with parenchymal infiltration; stage 3, parenchymal disease only; and stage 4, pulmonary fibrosis 6. Lymphadenopathy is seen in stages 1 and 2. The most common pattern is bilateral hilar and right paratracheal lymphadenopathy, also known as Garland’s triad. The left paratracheal nodes and the aortopulmonary window nodes are also commonly enlarged 7. In general, when multiple lymph node groups in the hila and mediastinum are symmetrically enlarged in a young patient, sarcoidosis is the most likely diagnosis. Amorphous, punctate, or eggshell-like calcifications may also be seen and are suggestive of a chronic condition, occurring in 3% of patients after 5 years and in 20% after 10 years 8.
- Infection
- Tuberculosis
- Nycoplasma
- Histoplasmosis
- Pulmonary coccidioidomycosis
- Malignancy
- Lymphoma: more common in Hodgkin lymphoma than non-Hodgkin lymphoma.
- Carcinoma
- Inorganic dust disease
- Silicosis: Silicosis is caused by the inhalation of free silica particles during occupational exposure –such as in mining, drilling, and sandblasting –which leads to a fibrous tissue reaction in the lungs 9. The classical features of patients with silicosis are diffuse interstitial shadowing with bilateral enlargement of hilar lymph nodes, which may or may not be calcified 10. Antao et al. 11 also found that 74% of silicotic patients had enlarged mediastinal lymph nodes and 66% of exposed workers had evidence of lymph node calcification. Eggshell calcification is the most common pattern described in silicosis 12, although some authors have reported that the puncate pattern is most prevalent 11.
- Berylliosis: Berylliosis is a lung disease caused by the inhalation of beryllium (Be) compounds. Berylliosis can lead to acute chemical pneumonitis – due to intense exposure to beryllium within a short time – or chronic interstitial lung disease, after prolonged exposure to lower beryllium concentrations. The chronic form is more common and presents as intraalveolar accumulation of lymphocytes and macrophages, alveolitis, and noncaseating granulomas 13. CT appearances of chronic berylliosis are similar to those of sarcoidosis, although mediastinal and hilar lymphadenopathy are less common, occurring in about 25% of patients. Berylliosis should be included in the differential diagnosis for all patients with imaging appearances suggestive of sarcoidosis 14.
- Heart failure: Chronic left heart failure may cause mediastinal lymphadenopathy. Several mediastinal lymphatic chains are involved, but the subcarinal, paratracheal, and hilar nodes are more frequently affected. The mechanisms underlying the pathogenesis of lymphadenopathy in cardiogenic pulmonary edema are not completely clear, but it has been suggested to be the expression of diffuse intrathoracic edema affecting the pulmonary parenchyma and neighboring structures, including the mediastinum and associated lymph nodes 15. Lymphadenopathy has been suspected based on features observed on radiographs taken to investigate dyspnea and heart failure. The identification of suspicious features is theoretically easier on follow-up radiographic examinations, which provide better visibility of hilar and mediastinal areas, after treatment to reduce pulmonary edema 15.
- Cryptogenic organizing pneumonia or bronchiolitis obliterans-organizing pneumonia 16
- Amyloidosis: Amyloidosis is characterized by the accumulation of inappropriately folded protein deposits. Amyloidosis may be localized, but it is much more commonly diffuse, affecting organ function by replacing the normal cell structure. Based on the structure of the fibrillar protein –one of the deposit’s components–amyloidosis is classified into several types, most commonly primary and secondary 17. Hilar and mediastinal lymph node enlargement is not described in secondary amyloidosis, and is uncommon in localized amyloidosis. However, it is quite common in primary amyloidosis. Thoracic lymphadenopathy, alone or with interstitial disease, is the most common CT finding in primary amyloidosis. Mediastinal and hilar lymph nodes may be involved, bilaterally and often massively, resulting in a pattern that may resemble sarcoidosis. Calcification is usually described as coarse or nonspecific, and eggshell calcification may be seen. Associations with nephrotic syndrome, congestive heart failure, and neuropathy can help identify the disease 18.
- Castleman’s disease: Castleman’s disease is an uncommon, mainly benign, lymphoproliferative disorder of unknown cause. The overall prevalence of the disease is estimated to be less than 1/100,000 19. Castleman’s disease may be localized or multifocal. The former presents few or no symptoms, so its diagnosis is made typically through incidental radiological findings. On chest radiographs, it may appear as an incidental, rounded, solitary mediastinal or hilar mass with a differential diagnosis that includes thymoma, lymphoma, neurogenic tumor, and bronchial adenoma 20. On CT, it usually manifests as a homogeneous, noninvasive, large, solitary mass with soft-tissue attenuation, most commonly in the mediastinum or hila 21.
- Idiopathic pulmonary fibrosis: Idiopathic pulmonary fibrosis is a specific form of chronic fibrosing interstitial pneumonia limited to the lung 22. The reported prevalence of mediastinal lymphadenopathy in idiopathic pulmonary fibrosis is 66% (90/136 patients) 23 or, similarly, 70% 24. The presence of enlarged mediastinal nodes may indicate a more chronic and advanced disease process 25. Jung et al. 26 showed that the total number of enlarged lymph nodes increased with the severity score of pulmonary fibrosis. Idiopathic pulmonary fibrosis was reported to be associated with an increased risk of lung cancer, due to the occurrence of atypical or dysplastic epithelial changes in fibrosis, which progress to invasive malignancy 27. Consensus on this association is lacking, but epidemiological reports have described an increased incidence of lung cancer during idiopathic pulmonary fibrosis follow-up. In addition, lung cancer was found simultaneously with idiopathic pulmonary fibrosis in some autopsy studies 28. The relation between the idiopathic pulmonary fibrosis severity score of – reflected in the number of enlarged lymph nodes – and the emergence of lung cancer also remains uncertain and offers a potential area for further investigation 27.
- Chronic obstructive pulmonary disease (COPD): Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction, inflammation in the airways, and systemic effects or comorbidities 29. Approximately 50% of patients with chronic obstructive pulmonary disease present enlarged hilar and mediastinal lymph nodes. These lymph nodes are located predominantly in the lower paratracheal space, in the aortopulmonary window, and below the carina. Lymph node enlargement is identified more often in patients with signs of severe bronchitis, which seems to be logical because the inflammatory processes seen in chronic bronchitis may result in airway remodeling and narrowing and reactive nodal enlargement. All enlarged lymph nodes in patients with chronic obstructive pulmonary disease have well-defined contours, and most are oval. Neither calcification nor central low attenuation of enlarged lymph nodes is seen in the majority of patients 30.
- Pulmonary embolism. Lymphadenopathy is common in patients with pulmonary arterial hypertension caused by proven chronic pulmonary thromboembolism, and may not necessarily implicate additional pulmonary disease. Enlarged nodes are found on CT in more than one-third of patients with chronic pulmonary thromboembolism with no clinical or radiological evidence of comorbid conditions. The frequent association between enlarged hilar lymph nodes and pleural and pericardial effusions raises the possibility of a common pathophysiological mechanism related to the slowing of lymphatic flow in the mediastinum, caused by increased pressure in the systemic venous system 31.
- Drug-induced lymphadenopathy. Hypersensitivity reactions to drugs can cause mediastinal or hilar lymph node enlargement. Anticonvulsants, particularly phenytoin, can cause a pseudolymphoma syndrome with generalized lymphadenopathy in addition to fever, skin rash, eosinophilia, and hepatosplenomegaly. Methotrexate, sulfonamides, penicillin, allopurinol, aspirin, and erythromycin are other examples of drugs with this effect. These reactions tend to follow several months of drug therapy and decrease after discontinuation of the drug 18.
Bilateral hilar lymphadenopathy treatment
Bilateral hilar lymphadenopathy treatment involves treating the underlying cause.
References- Thoracic lymphadenopathy in benign diseases: A state of the art review. Respiratory Medicine Volume 112, March 2016, Pages 10-17 https://doi.org/10.1016/j.rmed.2016.01.021
- L.H. Schwartza, J. Bogaertsb, R. Fordc, L. Shankard, P. Therassee, S. Gwytherf, et al. Evaluation of lymph nodes with RECIST 1.1. Eur. J. Cancer, 45 (2009), pp. 261-267
- S. Ganeshalingam, D.M. Koh. Nodal staging. Cancer Imaging, 9 (2009), pp. 104-111
- T. Suwatanapongched, D.S. Gierada. CT of thoracic lymph nodes. Part I: anatomy and drainage. Br. J. Radiol., 70 (2006), pp. 922-928
- K. Kiyono, S. Sone, F. Sakai, Y. Imai, T. Watanabe, I. Izuno, et al. The number and size of normal mediastinal lymph nodes: a postmortem study. AJR Am. J. Roentgenol., 150 (1988), pp. 771-776
- The American Thoracic Society (ATS), The European Respiratory Society (ERS), The World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG). Statement on sarcoidosis. Am. J. Respir. Crit. Care. Med., 160 (1999), pp. 736-755
- H. Al-Jahdali, P. Rajiah, S.S. Koteyar, C. Allen, A.N. Khan. Atypical radiological manifestations of thoracic sarcoidosis: a review and pictorial essay. Ann. Thorac. Med., 8 (2013), pp. 186-196
- B.H. Miller, M.L. Rosado-de-Christenson, H.P. McAdams, N.F. Fishback. Thoracic sarcoidosis: radiologic-pathologic correlation. RadioGraphics, 15 (1995), pp. 421-437
- B. Satija, S. Kumar, U.C. Ojha, D. Gothi. Spectrum of high-resolution computed tomography imaging in occupational lung disease. Indian. J. Radiol. Imaging, 23 (2013), pp. 287-296
- D.R. Baldwin, L. Lambert, C.F. Pantin, K. Prowse, R.B. Cole. Silicosis presenting as bilateral hilar lymphadenopathy. Thorax, 51 (1996), pp. 1165-1167
- V.C.S. Antao, G.A. Pinheiro, M. Terra-Filho, J. Kavakama, N. Müller. High-resolution CT in silicosis: correlation with radiographic findings and functional impairment. J. Comput. Assist. Tomogr., 29 (2005), pp. 350-356
- A.S. Ferreira, V.B. Moreira, H.M. Ricardo, R. Coutinho, J.M. Gabetto, E. Marchiori. Progressive massive fibrosis in silica-exposed workers, high-resolution computed tomography findings. J. Bras. Pneumol., 32 (2006), pp. 523-528
- E.M. Capitani, A.M.A. Altemani, J.I. Kawakama. Pulmonary beriliosis: literature review and case report. J. Pneumol., 21 (1995), pp. 135-142
- C.W. Cox, C.S. Rose, D.A. Lynch. State of the Art: Imaging of Occupational Lung Disease. Radiology, 270 (2014), pp. 681-696
- A. Ngom, P. Dumont, P. Diot, E. Lemarié. Benign mediastinal lymphadenopathy in congestive heart failure. Chest, 119 (2001), pp. 653-656
- Varma S, Gupta S, Elsoueidi R, Dhar M, Talwar J, Mobarakai N. Bilateral hilar lymphadenopathy in a young female: a case report. J Med Case Rep. 2007;1:60. Published 2007 Aug 3. doi:10.1186/1752-1947-1-60 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1995203
- C.S. Georgiades, E.G. Neyman, M.A. Barish. Amyloidosis: review and CT manifestations. Radiographics, 24 (2004), pp. 405-416
- J.R. Brown, A.T. Skarin. Clinical mimics of lymphoma. Oncologist, 9 (2004), pp. 406-416
- T. Dégot, A. Métivier, S. Casnedi, M. Chenard, R. Kessler. Thoracic manifestations of Castleman’s disease. Rev. Pneumol. Clin., 65 (2009), pp. 101-107
- H.P. McAdams, M. Rosado-de-Christenson, N.F. Fishback, P.A. Templeton. Castleman disease of the thorax: radiologic features with clinical and histopathologic correlation. Radiology, 209 (1998), pp. 221-228
- H. Racil, S. Cheikh Rouhou, O. Ismail, S. Hantous-Zannad, N. Chaouch, M. Zarrouk, et al. Castleman’s disease: an intrapulmonary form with intrafissural development. Sci. World J., 14 (2009), pp. 940-945
- J. Park, D.S. Kim, T.S. Shim, C.M. Lim, Y. Koh, S.D. Lee, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur. Respir. J., 17 (2001), pp. 1216-1219
- C.A. Souza, N.L. Muller, K.S. Lee, T. Johkoh, H. Mitsuhiro, S. Chong. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am. J. Roentgenol., 168 (2006), pp. 995-999
- H. Niimi, E.Y. Kang, J.S. Kwong, S. Carignan, N.L. Müller. CT of chronic infiltrative lung disease: prevalence of mediastinal lymphadenopathy. J. Comput. Assist. Tomogr., 20 (1996), pp. 305-308
- M.K. Lim, J.G. Im, J.M. Ahn, J.H. Kim, S.K. Lee, K.M. Yeon, et al. Idiopathic pulmonary fibrosis vs. pulmonary involvement of collagen vascular disease: HRCT findings. J. Korean Med. Sci., 12 (1997), pp. 492-498
- J.I. Jung, H.H. Kim, Y.J. Jung, S.H. Park, J.M. Lee, S.T. Hahn. Mediastinal lymphadenopathy in pulmonary fibrosis: correlation with disease severity. J. Comput. Assist. Tomogr., 24 (2000), pp. 706-710
- R. Haddad, D. Massaro. Idiopathic diffuse interstitial pulmonary fibrosis (fibrosing alveolitis), a typical epithelial proliferation and lung cancer. Am. J. Med., 45 (1968), pp. 211-219
- A. Nagai, A. Chiyotani, T. Nakadate, K. Konno. Lung Cancer in Patients with idiopathic pulmonary fibrosis. Tohoku J. Exp. Med., 167 (1992), pp. 231-237
- M. Decramer, W. Janssens, M. Miravitlles. Chronic obstructive pulmonary disease. Lancet, 379 (2012), pp. 1341-1351
- J. Kirchner, E.M. Kirchner, J.P. Goltz, A. Obermann, R. Kickuth. Enlarged hilar and mediastinal lymph nodes in chronic obstructive pulmonary disease. J. Med. Imaging Radiat. Oncol., 54 (2010), pp. 333-338
- C.J. Bergin, K.J. Park. Lymph node enlargement in pulmonary arterial hypertension due to chronic thromboembolism. J. Med. Imaging Radiat. Oncol., 52 (2008), pp. 18-23