What is BPC-157
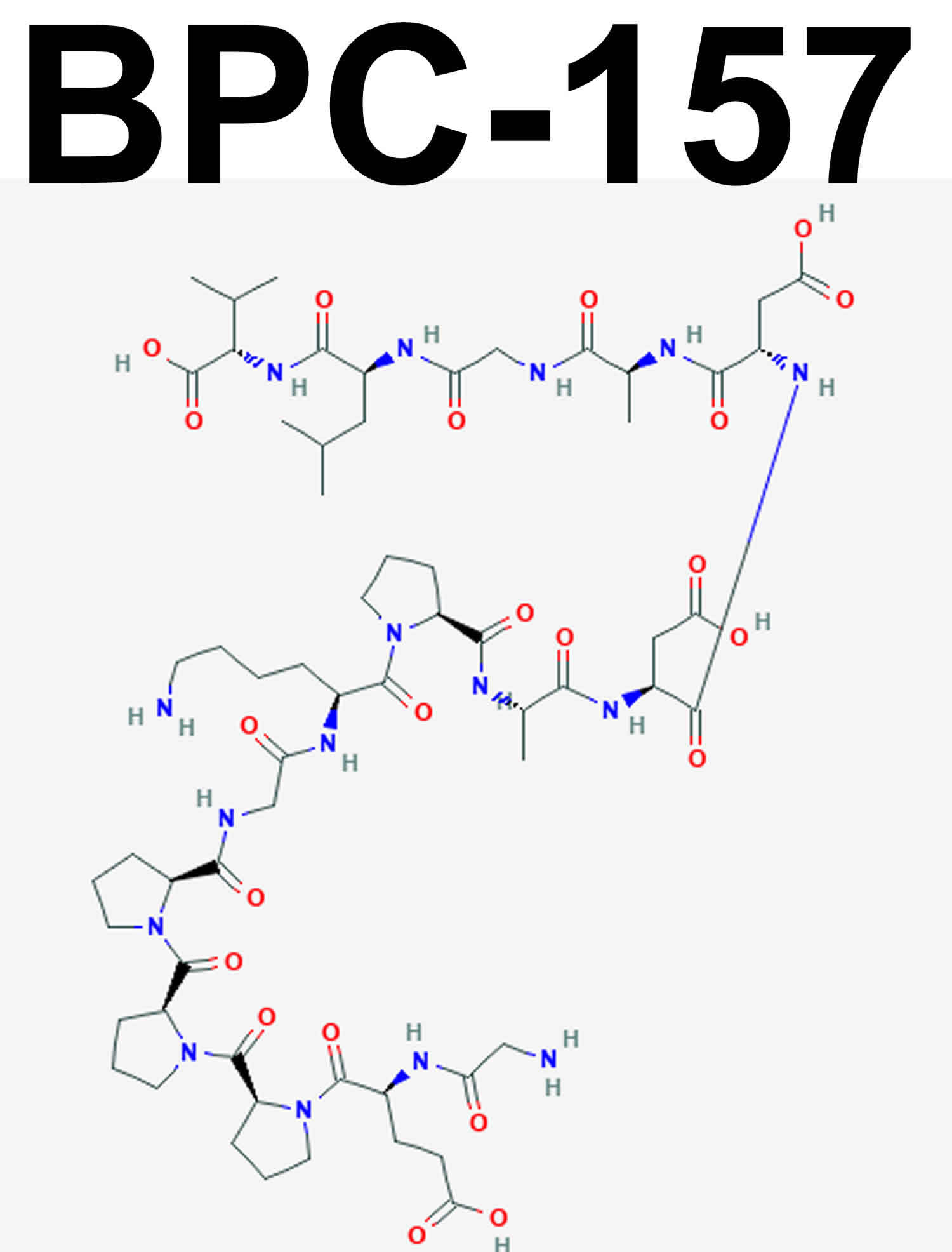
BPC-157 is short for Body Protection Compound 15 also known as Bepecin, is a stable gastric pentadecapeptide composed of 15 amino acids derived from human gastric juice 1, that has been demonstrated in many animal studies (lab rats) to have anti-inflammatory and therapeutic effects to promote the healing of different tissues, including skin, muscle, bone, ligament, tendon 2, somatosensory neurons and the rescue of in the sciatic nerve after transection, upon brain injury after concussive trauma, and in severe encephalopathies 3. BPC 157 is comprised of a peptide chain (a string of amino acids in a particular order) made up of 15 amino acids fragment (Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val) that is often termed as ‘synthetic’; although the compounds are ‘natural’, they are not known to exist in nature and are derived from another protein 4. BPC-157 got its name from its ability to quickly heal body injuries, ward-off fatigue of the central nervous system as a result of time-intensive exercise and retain an anabolic state post-workout. BPC 157 has been shown in clinical trials to be both safe in inflammatory bowel disease (PL-10, PLD-116, PL 14736) and wound healing, stable in human gastric juice, with no toxicity being reported 5. However, the underlying mechanism has not been fully clarified.
BPC 157 is native and stable in human gastric juice and maintains gastrointestinal mucosal integrity 6 and it represents a prototype of a more effective class of gastrointestinal tract cytoprotective agents with both prophylactic and therapeutic effects for pre-existing lesions in individuals with the most complex disturbances, such as internal and external fistulas, or anastomosis complicated with severe colitis 7, unlike standard cytoprotective agents that exhibit only prophylactic effectiveness (shared limitation of activity) 8. BPC 157 had been used in ulcerative colitis and now in multiple sclerosis trials also counteracts colitis (in various models of colitis) 9 and its complications, such as fistulas 10, failed healing of anastomoses 11 and other gastrointestinal lesions 12, given parenterally or orally. Furthermore, BPC 157 is known to counteract thrombosis, not only after abdominal aorta anastomosis 13 or inferior caval vein ligation 14, but also after bleeding prolongation upon amputation and the administration of anticoagulants and aspirin 15 or inferior caval vein ligation 14. This effect is related to its endothelial maintenance and its interaction with the nitric oxide (NO) system, which provides the counteraction of both the opposite effects of L-NAME (prothrombotic) and L-arginine (antithrombotic) application 15. Otherwise, bleeding from the perforated lesion and perforated lesion outcome might be complicated with these innate L-NAME and NOS substrate L-arginine effects 15.
In a study conducted by Hsieh et al. 16, the authors aimed to explore the proangiogenic and therapeutic effect of BPC 157. Demonstrated by chick chorioallantoic membrane (CAM) and endothelial tube formation assays, both in vivo and in vitro, BPC 157 was shown to increase both vessel density and accelerate the recovery of blood flow in ischemic muscle, indicating a promotional effect on angiogenesis. Histological analysis was shown to confirm enhanced expression of vascular endothelial growth factor receptor 2 (VEGFR2) in rats treated with BPC 157. This was further confirmed in vitro using human vascular endothelial cells. BPC 157 was also shown to time-dependently activate the VEGFR2-Akt-eNOS signalling pathway. The study therefore demonstrates the pro-engiogenic effects of BPC 157 associated with VEGFR2 and VEGFR2-Akt-eNOS signalling.
In addition, there is a particular effect on endothelial integrity 17. Together, these may result in the particular activation of blood vessels during injury, vessel occlusion, or organ perforation, the recruitment of the vessel to organize an adequate shunting and bypass occlusion 18. Furthermore, the effect of BPC 157 is due to its interaction with and modulation of the nitric oxide (NO) system, and its interaction with prostaglandin, dopamine, and serotonin systems has also been documented 19. BPC 157 also acts as a free radical scavenger, counteracts free radical-induced lesions, and normalizes nitric oxide (NO) and malondialdehyde levels in tissues and during ischemia and reperfusion 20. Pleiotropic effects involving distinctive receptors, including vascular endothelial growth factor 2 (VEGFR2) and growth hormone receptors, distinctive pathways, including VEGFR2-AKT-eNOS, ERK ½, FAK-paxillin, FoxO3a, p-AKT, p-mTOR and p-GSK-3β, and distinctive loops, including stimulation of the egr-1 gene and its corepressor gene naB2, and counteraction of increases in pro-inflammatory and procachectic cytokines 21, likely minimize the inherent lack of full understanding of the mechanisms that may be involved. However, more important is the practical evidence from a considerable number of the studies, particularly in gastrointestinal research, that intragastric administration or per os administration in drinking water, is equally effective as injections of the supplement administered to rodents, which has been performed in the majority of studies on BPC 157 7. In reality, in particular along with its safety profile, Lethal Dose 1 (LD1) is not achieved, and there are no reported adverse effects in clinical trials 22. LD1 (Lethal Dose 1) is the amount of a material, given all at once, which causes the death of 1% (one percent) of a group of test animals. The LD1 is one way to measure the short-term poisoning potential (acute toxicity) of a material.
Is BPC 157 a steroid?
BPC-157 is not a anabolic steroid. BPC-157 is a peptide (protein) or chain of 15 amino acids. BPC-157 does not have the anabolic or androgenic effects of anabolic-androgenic steroids. Instead, it may assist athletes by assisting the repair and regeneration of muscles and connective tissues among other benefits.
Anabolic steroids are synthetic hormones that help with the growth and repair of muscle tissue. They imitate the male sex hormone, testosterone. Doctors prescribe anabolic-androgenic steroids to treat hormonal problems (such as delayed puberty in males or loss of muscle caused by diseases like cancer or HIV). Anabolic steroids are also misused. People who illegally use anabolic steroids often do so to increase lean muscle mass, reduce fat and speed up recovery from injury.
Does BPC 157 build muscle?
BPC-157 does not directly build muscle, however in bodybuilders looking to optimize recovery between sessions and stay injury-free, it may increase how frequently you can train to a high intensity without having to take a step back.
BPC 157 health benefits
Therapeutically, the synthetically produced peptide BPC-157 is not currently approved for use as a human drug. BPC-157 is an experimental compound that has been investigated for inflammatory bowel disease and soft tissue healing, although there is a concerning lack of published clinical trial data because studies appear to have been cancelled or stopped without any published conclusions. There are also no U.S. Food and Drug Administration (FDA) approved medications that contain BPC-157 and it is not clinically indicated for any medical condition.
Even though there are no studies or clinical trials that show BPC-157 is safe or effective in humans, some websites related to performance-enhancing drugs advertise that it can be injected or taken orally for bone and joint healing, stomach ulcers, organ damage, and a number of other purposes, including athletic performance enhancement. It is important to realize that these are unproven claims, and that the use of BPC-157 for these or any other reasons is not supported by medical literature or by any medical associations.
Research on BPC-157, which began in 1993, has continued without any reported side effects 23. BPC-157 has been used in research studies for the repair of bones, intestines, muscles, teeth, tendons to name a few. The BPC-157 studies have been performed in-vivo on human subjects and lab rats as well as in laboratory in-vitro. Moreover, test subjects were injected both subcutaneously and intramuscularly.
BPC-157 interacts with peptidergic sensory afferent neurons and may rescue adult and newborn capsaicin rats 24, improves the healing of damaged enteric nerves and increases the survival of cultured enteric neurons and the proliferation of cultured enteric glial cells 25. Also, BPC-157 attenuated morphine analgesia and counteracted the haloperidol-induced enhancement of the antinociceptive action of morphine 26. Also, BPC-157 counteracts the adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs), both cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2) inhibitors 27. BPC-157 markedly improved rat sciatic nerve healing following nerve transection and/or anastomosis 28. After an induced traumatic brain injury, there is a marked attenuation of damage with an improved early outcome and minimal postponed mortality throughout a 24 hour postinjury period with less intense subarachnoid and intraventricular haemorrhage and brain laceration and subsequent brain edema considerably improved 29. Also, BPC-157 counteracts encephalopathies after NSAID treatment 30, insulin overdose 31 and a multiple sclerosis rat model induced by neurotoxin cuprizone application 32. BPC-157 has beneficial effects on inflammation, hemorrhage, and edema after traumatic brain injury 33, various severe encephalopathies (which follow gastrointestinal and/or liver lesions), NSAID overdose 34 or insulin overdose seizures 35 and on severe muscle weakness after exposure to the specific neurotoxin cuprizone in a rat multiple sclerosis model 36 or magnesium overdose 37. In other studies, it was shown that BPC-157 counteracts increased levels of proinflammatory and procachectic cytokines such as IL-6 and TNF-α 38. BPC-157 improves sciatic nerve healing when applied intraperitoneally, intragastrically, or locally at the site of anastomosis shortly after injury or directly into the tube after non-anastomosed nerve tubing (7-mm nerve segment resection) 39. Finally, BPC-157 counteracts various induced seizures in rats and mice 27.
Health benefits of BPC-157 that were discovered during several studies concluded that BPC-157 was:
- Responsible for improved tendon outgrowth, cell survival, and migration, thereby promoting ligament and tendon recovery when administered via drinking water for test rats with damaged collateral medial ligaments 40.
- Effective in the direct healing of tendon to bone, that may perhaps effectively replace current reconstructive surgical procedures 41.
- Highly effective in neutralizing the damage if the gut lining, caused by nonsteroidal anti-inflammatory drugs (NSAIDs) 5.
- Able to reverse damage caused by inflammatory bowel disease within several days of administration in lab rats with IBS 42.
- Successful in curing periodontal disease in rats when regularly administered.
- Effective in reversing systemic corticosteroid damaged muscle recovery in rat subjects. As a result of a set dose, administered once per day for 14 days to rats with a compressed gastrocnemius muscle.
- Responsible for promoting the healing of bone in rabbits inflicted with an experimental segmental bone defect.
Additionally, BPC-157, is known to be a stable form of gastric pentadecapeptide as a result of its stability in the gastric juice of humans, its anabolic healing properties for both the lower and upper gastrointestinal tract, a reversing effect on ulcers as well as healing properties for inflammatory bowel disease without any side effects.
Furthermore, research studies have shown that BPC-157 increases the rate of wound healing, and through its reaction with the nitric oxide system, it produces a protective effect of endothelial tissue.
It is worth noting that there does appear to be a trial administering BPC 157 (rectally administered) in human participants for the treatment and healing of acute to mild ulcerative colitis 43; however, details on the studies are limited and are not overly informative. Similarly, a pilot study in 2015, a clinical trial (randomized) on 42 healthy human participants, receiving oral (tablet form) dosages of BPC 157 was carried out: 0.25, 0.5, 1 and 2 μg/kg 44. The details and results for this trial are still pending.
Tendon and ligament healing
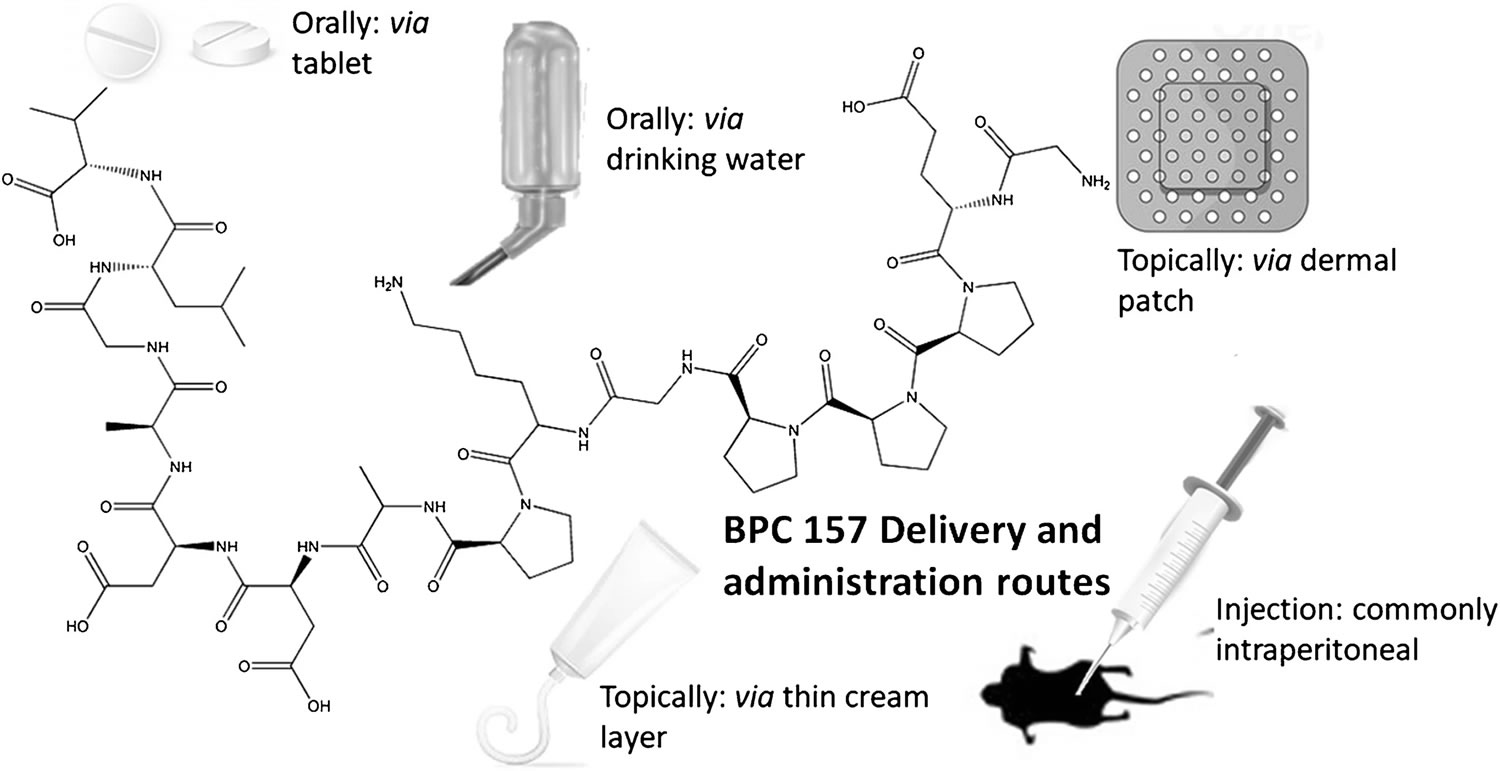
Due to a limited blood supply, the spontaneous healing of both tendons and ligaments is inherently poor. Thus, these soft tissues are highly prevalent in BPC-157 research. One of the earlier studies in literature investigated the applicability and therapeutic efficacy of BPC-157 via intraperitoneal administration, specifically the effect of BPC 157 on healing following the transection of the Achilles tendon in rats 45. It was revealed that, in comparison to the severely compromised healing observed in sham and control rats, the systemic delivery of BPC-157 significantly improved recovery measures 45. This was evidenced biochemically, micro- and macroscopically. Biomechanically, the healed tendons (over 14 days) showed an increased load to tendon failure and significantly higher functionality (Achilles functional Index—AFI). Microscopic analysis revealed a greater mononuclear count, less granulocytes, an increase in fibroblasts and superior formation of the reticulin and collagen fibers. Macroscopically, the defects treated with BPC 157 were smaller in size and depth and subsequently full tendon integrity was re-established 45. Similar results have also been shown in rat models investigating the healing of the medial collateral ligament (MCL) following surgical transection 40. Treatment of BPC 157 was administered orally in drinking water, topically via a thin cream and via intraperitoneal administration over a 90-day period. This suggests that BPC-157 peptide has a therapeutic benefit via a wide range of delivery mechanisms (see Figure 1).
A further study investigating tendon-to-bone healing following the transection and detachment of the Achilles tendon in rats went on to reveal that BPC 157 is also capable of promoting tendon-to-bone healing despite the presence of corticosteroids 41. This is of significance since although corticosteroid use has long been and remains controversial for healing of tissues 46, it still remains a prevalent treatment choice clinically for soft tissue damage and inflammation. Thus, the fact that BPC 157 has the apparent ability to counteract corticosteroid aggravation 41 lends itself to being applied alongside conventional treatments to improve both understanding and biomechanical outcomes.
Chang et al. 47 attempted to elucidate the potential mechanism by which BPC 157 stimulates the outgrowth and proliferation of tendon fibroblasts. Results showed that BPC 157 significantly accelerated the outgrowth of tendon explants; furthermore, the in vitro migration and rate of spreading of tendon fibroblasts increased in a dose-dependent manner, which was attributed to the activation of the FAK-paxillin pathway. Although BPC 157 had no direct effect on the proliferation of cultured tendon fibroblasts (Achilles), cell survival following H2O2 stress was significantly increased. The apparent lack of a direct effect was noted due to the in vitro environment not mimicking the inherent environment of a tendon in vivo.
Figure 1. BPC-157 route of administration
Footnote: Examples of successful administration mechanisms for the delivery of BPC 157; all routes, local and systemic, have been reported to have positive healing outcomes.
[Source 48 ]Skeletal muscle healing
In addition to tendon and ligament healing, the positive therapeutic effect of BPC-157 has also been extended and applied to muscle injury models, both traumatic and systemic 48. Following the compete transection of the quadriceps muscle in rats, a traumatic definitive defect under normal healing conditions would not be compensated 49; it was reported that the systemic delivery of BPC-157 promoted healing. More importantly, the study demonstrated that this healing continued for a sustained period (72 days) whilst maintaining the functional restoration 49.
Novinscak et al. 50 went on to compare the effectiveness of both systemic (intraperitoneal) and local treatment (as a thin cream layer) over a period of 14 days in crushed muscle (gastrocnemius muscle complex) in rats. BPC-157 significantly improved healing outcomes in both sites of treatment: macroscopically, microscopically and functionally, in addition to improving enzymatic activity (a decrease in muscle proteolysis) 51. The authors concluded that BPC-157 accelerated post-injury skeletal muscle healing in addition to restoring full muscle function that is similar to the finding in tendons reported by Staresinic et al. 49.
Pevec et al. 52, akin to the tendon-to-bone healing study of Krivic et al. 41, proposed BPC-157 as an effective treatment that could improve muscle healing, when administered despite corticosteroid treatment, 6a-methylprednisolone, following gastrocnemius muscular injury. With a similar administrative procedure as previously outlined (topical and intraperitoneal), corticosteroid administration was shown to aggravate healing across all parameters. BPC-157, in contrast, induced faster rates of healing and induced full functional restoration, which is also in agreement with the aforementioned studies on tendons 41. When administered with corticosteroid, the restorative effects of BPC-157 were not significantly affected in functional, macroscopic or histological measures.
In addition to muscle injuries caused by direct trauma, there have been a number of studies that have indicated that BPC-157 may have the ability to recover systemic muscular disturbances in response to induced nervous, electrolyte disturbances and/or skeletal muscle wasting 38. Since systemic muscle pain is attributed to infection, autoimmune conditions, illness or side effects from medication, they are considered to be more serious that stress or exercise-related muscle injuries.
BPC 157 dosage
Although BPC-157 is not currently prescribed for human use, it is important for athletes to proceed with caution when looking at potential agents to prevent and treat injury. While BPC-157 not currently on the WADA list of banned substances (https://www.usada.org/spirit-of-sport/education/bpc-157-peptide-risk), due to what some individuals would term as the synthetic nature of BPC 157, there may be issues associated with the use of this peptide, as seen by some sport organisations.
The majority of the BPC-157 research suggests a daily dose in the range between 1-10 mcg per kilogram of body weight to be most effective. For the average man, the dose runs between 200 mcg and 800 mcg. Some users of BPC-157 have claimed greater success by splitting the dose into twice per day.
BPC-157 performs systematically, meaning that regardless of whether you inject it or spray it in your mouth, the benefit will be the same. In the event that you choose to inject BPC-157 subcutaneously under the skin, aim as close as possible to the area of pain. This also applies if you choose to inject it intramuscularly. With oral administration, the key is to keep it in your mouth for 1.5 to 2 minutes before swallowing it.
BPC-157 side effects
Because BPC-157 has not been extensively studied in humans, no one knows if there is a safe dose, or if there is any way to use this compound safely to treat specific medical conditions. As an original antiulcer peptide, BPC-157 has virtually no known toxicity of its own, a Lethal Dose 1 (LD1) value has not yet been reported, and there have been no side effects in clinical trials, such as ulcerative colitis and multiple sclerosis 22. LD1 (Lethal Dose 1) is the amount of a material, given all at once, which causes the death of 1% (one percent) of a group of test animals. The LD1 is one way to measure the short-term poisoning potential (acute toxicity) of a material.
References- Cox HD, Miller GD, Eichner D. Detection and in vitro metabolism of the confiscated peptides BPC 157 and MGF R23H. Drug Test Anal. 2017 Oct;9(10):1490-1498. doi: 10.1002/dta.2152
- Chang C-H, Tsai W-C, Hsu Y-H, Pang J-HS. Pentadecapeptide BPC 157 Enhances the Growth Hormone Receptor Expression in Tendon Fibroblasts. Molecules. 2014; 19(11):19066-19077. https://doi.org/10.3390/molecules191119066
- Perovic, D., Kolenc, D., Bilic, V. et al. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J Orthop Surg Res 14, 199 (2019). https://doi.org/10.1186/s13018-019-1242-6
- Bódis B, Karádi O, Németh P, Dohoczky C, Kolega M, Mózsik G (1997) Evidence for direct cellular protective effect of PL-10 substances (synthesized parts of body protection compound, BPC) and their specificity to gastric mucosal cells. Life Sci 61:PL243–PL248
- Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Aralica G, Safic H, Suran J, Rak D, Dzidic S, Vrcic H, Sebecic B. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19(1):76-83. doi: 10.2174/13816128130111
- Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, Sever M, Zoricic I, Zoricic Z, Batelja L, Ziger T, Luetic K, Vlainic J, Rasic Z, Bencic ML. Stress in Gastrointestinal Tract and Stable Gastric Pentadecapeptide BPC 157. Finally, do we have a Solution? Curr Pharm Des. 2017;23(27):4012-4028. doi: 10.2174/1381612823666170220163219
- Sikiric, P., Hahm, K. B., Blagaic, A. B., Tvrdeic, A., Pavlov, K. H., Petrovic, A., Kokot, A., Gojkovic, S., Krezic, I., Drmic, D., Rucman, R., & Seiwerth, S. (2020). Stable Gastric Pentadecapeptide BPC 157, Robert’s Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye’s Stress Coping Response: Progress, Achievements, and the Future. Gut and liver, 14(2), 153–167. https://doi.org/10.5009/gnl18490
- Sikiric P, Seiwerth S, Grabarevic Z, Petek M, Rucman R, Turkovic B, Rotkvic I, Jagic V, Duvnjak M, Mise S, et al. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994;54(5):PL63-8. doi: 10.1016/0024-3205(94)00796-9
- Duzel, A., Vlainic, J., Antunovic, M., Malekinusic, D., Vrdoljak, B., Samara, M., Gojkovic, S., Krezic, I., Vidovic, T., Bilic, Z., Knezevic, M., Sever, M., Lojo, N., Kokot, A., Kolovrat, M., Drmic, D., Vukojevic, J., Kralj, T., Kasnik, K., Siroglavic, M., … Sikiric, P. (2017). Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World journal of gastroenterology, 23(48), 8465–8488. https://doi.org/10.3748/wjg.v23.i48.8465
- Baric M, Sever AZ, Vuletic LB, Rasic Z, Sever M, Drmic D, Pavelic-Turudic T, Sucic M, Vrcic H, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci. 2016 Mar 1;148:63-70. doi: 10.1016/j.lfs.2016.02.029
- Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, Zoricic I, Sever M, Seiwerth S, Sikiric P. Effects of Diclofenac, L-NAME, L-Arginine, and Pentadecapeptide BPC 157 on Gastrointestinal, Liver, and Brain Lesions, Failed Anastomosis, and Intestinal Adaptation Deterioration in 24 Hour-Short-Bowel Rats. PLoS One. 2016 Sep 14;11(9):e0162590. doi: 10.1371/journal.pone.0162590
- Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Aralica G, Stupnisek M, Suran J, Barisic I, Dzidic S, Vrcic H, Sebecic B. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20(7):1126-35. doi: 10.2174/13816128113190990411
- Hrelec M, Klicek R, Brcic L, Brcic I, Cvjetko I, Seiwerth S, Sikiric P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J Physiol Pharmacol. 2009 Dec;60 Suppl 7:161-5.
- Vukojević J, Siroglavić M, Kašnik K, Kralj T, Stanćić D, Kokot A, Kolarić D, Drmić D, Sever AZ, Barišić I, Šuran J, Bojić D, Patrlj MH, Sjekavica I, Pavlov KH, Vidović T, Vlainić J, Stupnišek M, Seiwerth S, Sikirić P. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol. 2018 Jul;106:54-66. doi: 10.1016/j.vph.2018.02.010
- Stupnisek M, Kokot A, Drmic D, Hrelec Patrlj M, Zenko Sever A, Kolenc D, Radic B, Suran J, Bojic D, Vcev A, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 Reduces Bleeding and Thrombocytopenia after Amputation in Rats Treated with Heparin, Warfarin, L-NAME and L-Arginine. PLoS One. 2015 Apr 21;10(4):e0123454. doi: 10.1371/journal.pone.0123454
- Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JH (2017) Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med 95(3):323–333.
- Seiwerth S, Rucman R, Turkovic B, et al. BPC 157 and standard angiogenic growth factors: gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr Pharm Des. 2018;24:1972–1989. doi: 10.2174/1381612824666180712110447
- Drmic, D., Samara, M., Vidovic, T., Malekinusic, D., Antunovic, M., Vrdoljak, B., Ruzman, J., Milkovic Perisa, M., Horvat Pavlov, K., Jeyakumar, J., Seiwerth, S., & Sikiric, P. (2018). Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World journal of gastroenterology, 24(48), 5462–5476. https://doi.org/10.3748/wjg.v24.i48.5462
- Kang EA, Han YM, An JM, et al. BPC157 as potential agent rescuing from cancer cachexia. Curr Pharm Des. 2018;24:1947–1956. doi: 10.2174/1381612824666180614082950
- Belosic Halle Z, Vlainic J, Drmic D, et al. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology. 2017;25:511–522. doi: 10.1007/s10787-017-0358-8
- Hsieh MJ, Liu HT, Wang CN, et al. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl) 2017;95:323–333. doi: 10.1007/s00109-016-1488-y
- Marin Lozic, Vasilije Stambolija, Ivan Krezic, Aleksandra Dugandzic, Gordana Zivanovic-Posilovic, Slaven Gojkovic, Josip Kovacevic, Luka Vrdoljak, Ivan Mirkovic, Antonio Kokot, Andreja Petrovic, Katarina Horvat Pavlov, Domagoj Drmic, Jelena Suran, Alenka Boban Blagaic, Sven Seiwerth, Predrag Sikiric, “In relation to NO-System, Stable Pentadecapeptide BPC 157 Counteracts Lidocaine-Induced Adverse Effects in Rats and Depolarisation In Vitro”, Emergency Medicine International, vol. 2020, Article ID 6805354, 20 pages, 2020. https://doi.org/10.1155/2020/6805354
- Zivanovic-Posilovic G, Balenovic D, Barisic I, Strinic D, Stambolija V, Udovicic M, Uzun S, Drmic D, Vlainic J, Bencic ML, Sindic A, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur J Pharmacol. 2016 Dec 15;793:56-65. doi: 10.1016/j.ejphar.2016.10.035
- L. Kalogjera, M. Ries, T. Baudoin, Z. Ferencic, R. Trotic, and B. Pegan, “Dose-dependent protective effect of BPC 157 on capsaicin-induced rhinitis in rats,” European Archives of Oto-Rhino-Laryngology, vol. 254, no. S1, pp. S9–S11, 1997.
- X.-Y. Wang, M. Qu, R. Duan et al., “Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells,” Neuroscience Bulletin, vol. 35, no. 1, pp. 167–170, 2019.
- A. Boban Blagaic, P. Turcic, V. Blagaic et al., “Gastric pentadecapeptide BPC 157 counteracts morphine-induced analgesia in mice,” J Physiol Pharmacol, vol. 60, no. 7, pp. 177–181, 2009.
- S. Ilic, D. Drmic, K. Zarkovic et al., “High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736),” Journal of Physiology and Pharmacology:An Official Journal of the Polish Physiological Society, vol. 61, no. 61, pp. 241–250, 2010.
- M. Gjurasin, P. Miklic, B. Zupancic et al., “Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury,” Regulatory Peptides, vol. 160, no. 1–3, pp. 33–41, 2010.
- M. Tudor, I. Jandric, A. Marovic et al., “Traumatic brain injury in mice and pentadecapeptide BPC 157 effect,” Regulatory Peptides, vol. 160, no. 1–3, pp. 26–32, 2010.
- D. Drmic, D. Kolenc, S. Ilic et al., “Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-Name,” World Journal of Gastroenterology, vol. 23, no. 29, pp. 5304–5312, 2017.
- S. Ilic, I. Brcic, M. Mester et al., “Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats,” Journal of Physiology and Pharmacology, vol. 60, no. 7, pp. 107–114, 2009.
- R. Klicek, D. Kolenc, J. Suran et al., “Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability,” Journal of Physiology and Pharmacology:An Official Journal of the Polish Physiological Society, vol. 64, no. 64, pp. 597–612, 2013.
- Tudor M, Jandric I, Marovic A, Gjurasin M, Perovic D, Radic B, et al. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regul Pept. 2010;160(1–3):26–32.
- Drmic D, Kolenc D, Ilic S, Bauk L, Sever M, Zenko Sever A, et al. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol. 2017;23(29):5304–12.
- Ilic S, Brcic I, Mester M, Filipovic M, Sever M, Klicek R, et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J Physiol Pharmacol. 2009;60(Suppl 7):107–14.
- Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64(5):597–612.
- Medvidovic-Grubisic M, Stambolija V, Kolenc D, Katancic J, Murselovic T, Plestina-Borjan I, et al. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology. 2017;25(4):439–49.
- Kang EA, Han YM, An JM, Park YJ, Sikiric P, Kim DH, Kwon KA, Kim YJ, Yang D, Tchah H, Hahm KB. BPC157 as Potential Agent Rescuing from Cancer Cachexia. Curr Pharm Des. 2018;24(18):1947-1956. doi: 10.2174/1381612824666180614082950
- Gjurasin M, Miklic P, Zupancic B, Perovic D, Zarkovic K, Brcic L, et al. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept. 2010;160(1–3):33–41.
- Cerovecki T, Bojanic I, Brcic L, Radic B, Vukoja I, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 (PL 14736) improves ligament healing in the rat. J Orthop Res. 2010 Sep;28(9):1155-61. doi: 10.1002/jor.21107
- Krivic A, Anic T, Seiwerth S, Huljev D, Sikiric P. Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation. J Orthop Res. 2006 May;24(5):982-9. doi: 10.1002/jor.20096
- Sikiric P, Seiwerth S, Brcic L, Blagaic AB, Zoricic I, Sever M, Klicek R, Radic B, Keller N, Sipos K, Jakir A, Udovicic M, Tonkic A, Kokic N, Turkovic B, Mise S, Anic T. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006 Dec;14(5-6):214-21. doi: 10.1007/s10787-006-1531-7
- Veljaca M, Sladoljev P, Mildner B (2003) Tolerability and pharmacokinetics of PL 14736, a novel agent for treatment of ulcerative colitis, in healthy male volunteers. Gut 3:A309
- PCO-02 – Safety and Pharmacokinetics Trial. Phase I clinical trial in healthy volunteers to study safety and pharmacokinetics of BPC-157, a pentadecapeptide from gastric source. https://clinicaltrials.gov/ct2/show/NCT02637284
- Staresinic M, Sebecic B, Patrlj L, Jadrijevic S, Suknaic S, Perovic D, Aralica G, Zarkovic N, Borovic S, Srdjak M, Hajdarevic K, Kopljar M, Batelja L, Boban-Blagaic A, Turcic I, Anic T, Seiwerth S, Sikiric P. Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. J Orthop Res. 2003 Nov;21(6):976-83. doi: 10.1016/S0736-0266(03)00110-4
- Waters RV, Gamradt SC, Asnis P, Vickery BH, Avnur Z, Hill E, Bostrom M. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop Scand. 2000 Jun;71(3):316-21. doi: 10.1080/000164700317411951
- Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985). 2011 Mar;110(3):774-80. doi: 10.1152/japplphysiol.00945.2010
- Gwyer, D., Wragg, N.M. & Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res 377, 153–159 (2019). https://doi.org/10.1007/s00441-019-03016-8
- Staresinic M, Petrovic I, Novinscak T, Jukic I, Pevec D, Suknaic S, Kokic N, Batelja L, Brcic L, Boban-Blagaic A, Zoric Z, Ivanovic D, Ajduk M, Sebecic B, Patrlj L, Sosa T, Buljat G, Anic T, Seiwerth S, Sikiric P. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. J Orthop Res. 2006 May;24(5):1109-17. doi: 10.1002/jor.20089
- Novinscak T, Brcic L, Staresinic M, Jukic I, Radic B, Pevec D, Mise S, Tomasovic S, Brcic I, Banic T, Jakir A, Buljat G, Anic T, Zoricic I, Romic Z, Seiwerth S, Sikiric P. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg Today. 2008;38(8):716-25. doi: 10.1007/s00595-007-3706-2
- Farges MC, Balcerzak D, Fisher BD, Attaix D, Béchet D, Ferrara M, Baracos VE. Increased muscle proteolysis after local trauma mainly reflects macrophage-associated lysosomal proteolysis. Am J Physiol Endocrinol Metab. 2002 Feb;282(2):E326-35. doi: 10.1152/ajpendo.00345.2001
- Pevec D, Novinscak T, Brcic L, Sipos K, Jukic I, Staresinic M, Mise S, Brcic I, Kolenc D, Klicek R, Banic T (2010) Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med Sci Monit 16(3):BR81-8