What is sulforaphane
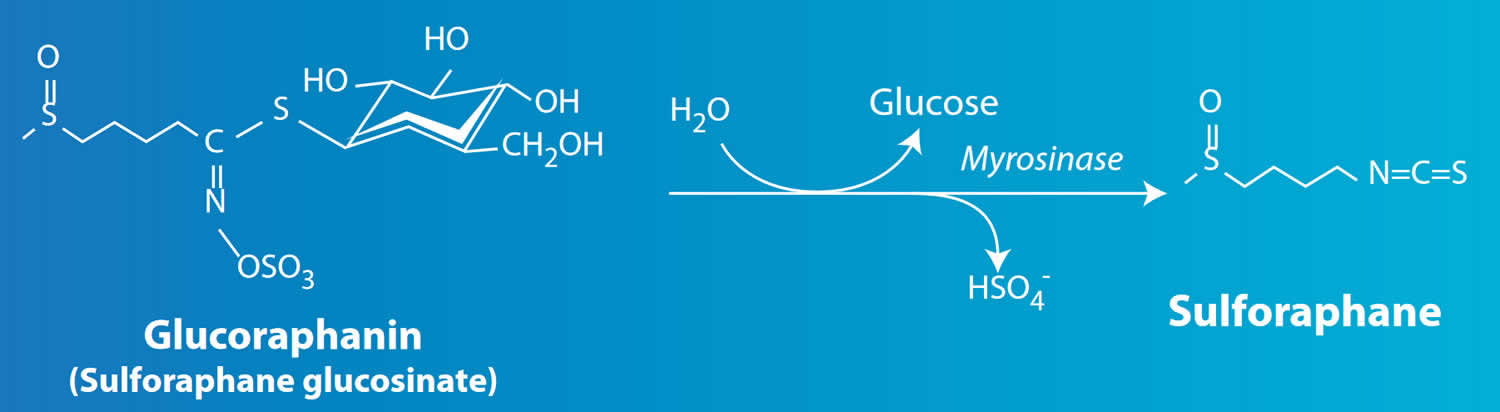
Sulforaphane also known as 1-isothiocyanato-4-(methylsulfinyl)-butane, is a phytochemical extract derived from cruciferous vegetables such as broccoli, cabbage and cauliflower and radish 1. Glucoraphanin in broccoli is enzymatically hydrolyzed by myrosinase (thioglucoside glucohydrolase), an enzyme that is released when the plant is chewed or processed (Figure 1) 2. Heating broccoli partially denatures and inactivates myrosinase, leaving the glucoraphanin at least partially intact. In the gut of healthy individuals any intact glucoraphanin is then metabolized by myrosinase-producing bacteria 3. Because broccoli sprout or seed extracts taken orally contain no myrosinase to hydrolyze the glucoraphanin, transformation to sulforaphane must be carried out by the gut microflora 4. In individuals with compromised intestinal flora and low myrosinase activity, it is unclear if glucoraphanin exerts the same systemic effects as observed in individuals with normal intestinal flora 5. Sulforaphane is unstable in aqueous medium and at high temperature; thus, the stability of sulforaphane during storage is the focus of its biological activity research. Studies have shown that −20°C and 4°C are the best storage temperatures for sulforaphane 6.
Sulforaphane is often used as an anticancer and/or anti-inflammatory drug in traditional Chinese herbal medicine 7. Sulforaphane is known to upregulate genes that fight oxidative stress, inflammation and DNA damage, all of which are believed to be involved in autism spectrum disorder symptoms that lead to stimming. Sulforaphane has reported anti-cancer effects through cell cycle arrest and apoptosis in various cancer cells, such as prostate, lung, breast, and colon cancers 8. The chemoprevention properties of sulforaphane against cancer are through both “blocking” and “suppressing” effects 9. The “blocking” function of sulforaphane is achieved through inhibiting Phase 1 metabolism enzymes that convert procarcinogens to carcinogens and inducing Phase 2 metabolism enzymes that promote excretion of carcinogens 9. Subsequent studies revealed the “suppressing” effects of sulforaphane in modulating diverse cellular activities to inhibit the growth of transformed cells 10. The ability of sulforaphane to induce apoptosis and cell cycle arrest is associated with regulation of many molecules including Bcl-2 family proteins, caspases, p21, cyclins and cdks 10. Sulforaphane was also shown to suppress angiogenesis and metastasis by down-regulating VEGF, HIF-1α, MMP-2 and MMP-9 10.
Currently, it is well known that evaluation of the bioavailability of natural compounds is one challenge in the design of clinical trials for studying their biological activity. Recently, Fahey et al. 11 identified that changes of inflammatory-related genes in peripheral blood mononuclear cells have significant influence on the sulforaphane bioavailability in 20 healthy participants. Similarly, another research has been carried out to evaluate the bioavailability of sulforaphane in 14 women and found that repeated dosing of sulforaphane could not result in the accumulation of toxic metabolites in urine over time 12. Moreover, sulforaphane-loaded nanostructured lipid carriers were developed and optimized to effectively improve its bioavailability and cytotoxicity efficacy against cancers 13. These findings provide valuable recommendation to better design the clinical trials to study the sulforaphane functionality in the future. To date, a preliminary randomized controlled trial was performed to demonstrate that pretreatment with broccoli sprout extract could improve the bioavailability and chemopreventive activity of sulforaphane, together with downregulation of several prostate cancer development-associated genes in the biopsy from 98 men 14.
Figure 1. Glucoraphanin metabolism into sulforaphane
[Source 15 ]Sulforaphane health benefits
Sulforaphane is one of the most frequently studied plant-derived isothiocyanate organosulfur compounds 16. Sulforaphane has been reported to exhibit a wide range of biological effects including antioxidant 17, antibacterial 18, anticancer 19, anti-inflammatory 20, anti-aging 21, neuroprotective 22 and antidiabetic 23.
An additional benefit of sulforaphane is that it plays a major role in energy metabolism and can mitigate obesity by increasing energy expenditure 24. In an animal model, sulforaphane or its precursor (glucoraphanin) enhanced reduction of food intake, adiposity, and weight gain caused by intraperitoneal injection of leptin 25.
Table 1. Summary of clinical trials using broccoli and sulforaphane for disease indications.
| Agent | Disease/ condition | Participants, agent, dose and schedule | outcome | Reference |
| Broccoli/ sulforaphane | Cancer – breast | 54 breast biopsy candidates; Broccoli seed extract; 514 μmol glucoraphanin rich/ day; 56 days) | Lower Ki67, lower HDAC3 in benign tissue, lower HDAC in peripheral blood mononuclear cells compared to placebo | 26 |
| Cancer – lung | 30 healthy, young smokers; steam-cooked broccoli; 250 g per day; 10 days | Increased DNA repair activity in peripheral blood mononuclear cells compared to control diet | 27 | |
| 291 healthy participants; broccoli sprout beverages; glucoraphanin (600 μmol) and sulforaphane (40 μmol); 84 days | Rapid and sustained increases in urinary excretion of benzene (61%) and acrolein (23%) | 28 | ||
| 50 healthy participants; glucoraphanin- or sulforaphane-rich broccoli sprout beverage; 7 days | 20-50% increase in excretion levels of glutathione conjugates of acrolein, crotonaldehyde and benzene in glucoraphanin, sulforaphane or both compared to baseline | 29 | ||
| Cancer – gastrointestinal tract | 40 H. pylori-infected subjects; broccoli sprouts; 70 g/ day/ 8 weeks | Reduced urease, inflammation and bacterial colonization in broccoli intervention group compared to alfalfa control group | 30 | |
| Cancer – prostate | 90 men with biochemical recurrence after radial prostatectomy; 60 mg of prostaphane daily; 6 months | Significantly lower log prostate-specific antigen (PSA) slope compared to placebo | 31 | |
| Diabetes | 103 Scandinavian type 2 diabetes patients; broccoli sprout extract; 150 μmol sulforaphane per dose; 12 weeks | Improved fasting glucose and hemoglobin A1C (HbA1C) in obese participants | 32 | |
| 81 type 2 diabetes patients; broccoli sprout powder (22.5 μmol /g sulforaphane) ; 5 g or 10 g per day; 4 weeks | Reduced fasting glucose, reduced inflammatory markers and serum insulin compared to placebo | 33 | ||
| Skin disorders | 5 subjects with Epidermolysis bullosa simplex; topical application of broccoli sprout extract (500 nmol sulforaphane/ ml); 1 week | Increase in K17 expression, variable but induced expression on K6 and K16 | 34 | |
| 6 volunteers; topical application with broccoli sprout extract (200 or 400 nmol sulforaphane); 3 doses every 24 hours | Reduced erythema (mean = 37.7%) caused by UV radiation | 35 | ||
| Heart and vascular disease | 37 subjects with high cardiovascular disease risk; standard or high glucoraphanin rich broccoli; 400 g per week; 12 weeks | Reduced plasma LDL-C (low density lipoprotein or “bad” cholesterol) in high glucoraphanin rich group | 36 | |
| 77 type 2 diabetes patients with a positive H.pylori stool antigen test; broccoli sprout powder or in combination with standard therapy; 6 g/ day; 28 days | Improvement in systolic and diastolic blood pressure in the combination group | 37 | ||
| 14 adults with sickle cell disease; broccoli sprout homogenate; 50-150 μmol dose escalation for 21 days | Increase in whole blood mRNA levels of heme oxygenase 1 and trend for same with subunit of fetal hemoglobin | 38 | ||
| Developmental/ behavioral disorders | 27 young males with moderate to severe autism spectrum disorder; sulforaphane derived from lyophilized broccoli sprout; 50-150 μmol sulforaphane; 18 weeks | Improvement in social interaction, abnormal behavior and verbal communication | 39 | |
| 10 schizophrenia patients; broccoli seed extract; 69 μmol glucoraphanin rich (3 tablets, daily); 54 days | Improvement in cognitive function tests | 40 | ||
| Respiratory conditions | 29 subjects inoculated with FluMist live attenuated influenza virus; broccoli sprout homogenate; 100 μmol sulforaphane; 21 days | Increase in peripheral blood NK cell expression and reduction in circulating influenza RNA | 41 | |
| 16 young, healthy smokers; broccoli sprout homogenate; 200 g per day; 4 days | Reduction in virus-induced inflammation; reduction in influenza sequences in nasal lavage fluid from smokers | 42 | ||
| 45 moderate asthmatics; sulforaphane; 100 μmol daily; 14 days | Reduction in bronchoconstrictor effects of methacholine; reduction in airway resistance | 43 | ||
| 29 healthy subjects who tested positive for cat allergens; sulforaphane-rich broccoli sprout extract; 100 μmol per day; 4 days | 54% reduction in diesel exhaust particle-induced nasal white blood cell counts | 44 |
Sulforaphane and cancer
Sulforaphane exerts anti-cancer activity against multiple cancer types 16. Epidemiological studies have also shown that sulforaphane consumption is associated with reduced risk of various human cancers, including lung, colon, breast, stomach, and prostate 45. The chemopreventive activity of sulforaphane involves inhibition of phase 1 enzymes (thereby preventing the conversion of procarcinogens to carcinogens), activation of phase 2 enzymes (leading carcinogens detoxification and excretion from the body), and suppression of pro-inflammatory responses 46. Sulforaphane also exerts direct effects on cancer cells by inhibition of growth, induction of cell cycle arrest, and activation of apoptosis in various types of cancer cells 47. Also, sulforaphane modulates epigenetic changes that regulate various molecular targets involved in cell proliferation, differentiation, apoptosis, or cell cycle, and viability of cancer stem cells 48. The chemopreventive and therapeutic effects of sulforaphane have been comprehensively investigated in various cancers in vitro and in vivo 46. Therefore, sulforaphane is a rational candidate for development as a therapeutic agent for endometrial cancer 49. The safety of sulforaphane and sulforaphane-rich broccoli sprout extract has been tested clinically 50 and multiple phase I and phase II trials have been completed evaluating the efficacy of sulforaphane amongst patients with prostate cancer and breast cancer. These promising activities suggest that sulforaphane may also have value for use in endometrial cancer treatment regimens. However, further additional investigation, mainly well-designed clinical trials, are required to establish correlations and allow to further verify the efficacy, safety, and possible adverse reactions of sulforaphane products.
Table 2. Mechanism of action of sulforaphene in human tumors
| Tumors | Action | Outcome | Model Used | Reference |
| Breast cancer | Akt–mTOR–S6K kinase pathway↓ | Reversal multidrug resistance, apoptosis↑ | SKBR-3, BT-474 | 51 |
| Triple-negative breast cancer | Hedgehog↓, MMP-2↓, MMP-9↓ | Migration and invasion↓, apoptosis↑, proliferation↓ | MCF7, T47D, MCF10A, MCF10AT1, MCF10CA1a, SUM159 | 52 |
| Triple-negative breast cancer | EGR1 ↑, cyclinB1↓, Cdc2↓ | Apoptosis↑, cell cycle G2/M phase arrest | MDA-MB-231, MDA-MB-453, MDA-MB-436, MDA-MB-468 | 53 |
| Hepatocellular carcinoma | caspases -3/7 and -9↑, caspase-8↓ | Apoptosis↑, cell cycle G0/G1 phase arrest | MFC-7, HT-29 | 54 |
| Hepatocellular carcinoma | ROS↑, microtubule polymerization↑ | Apoptosis↑, radiation-induced cell death↑ | HB-8065 | 55 |
| Hepatocellular carcinoma | NF-κB↓ | Apoptosis↑, proliferation↓ | HepG2, Hep3B | 56 |
| Lung cancer | PI3K-Akt↓, PTEN↓ | Apoptosis↑, migration and invasion↓, proliferation↑ | A549, H460, H446, HCC827, H1975, H1299 | 57 |
| Non-small cell lung carcinoma | ROS↑, Bcl-2↓, Bax↓, cytochrome C↑, caspase 9/3↑ | Apoptosis↑, proliferation↓ | A549 | 58 |
| Cervical cancer | Caspase 3↑, caspase 9↑, EGFR↑ | Apoptosis↑, proliferation↓ | HeLa | 59 |
| Ovarian cancer | ROS↑, mitochondrial membrane depolarization | Apoptosis↑, proliferation↓ | SKOV 3, SNU 8 | 60 |
| Colon cancer | p38, CDK1, CDC25B | Apoptosis↑, cell cycle G2/M phase arrest | HCT116, HT-29, DLD1, KM12 | 61 |
| Gastric cancer | ROS↑, cytochrome c↑, Casp-3↑, Casp-8↑, PARP-1↑ | Apoptosis↑, migration and invasion↓ | AGS | 62 |
| Lymphoma | CRM1, p62↑, AMPK↑ | Apoptosis↑ | U937, HUT78, Raji, JeKo-1, U2932 | 63 |
| Thyroid cancer | Ras↑, MEK↑, ERK↑, B-Raf↑ | Apoptosis↑, proliferation↓ | FRO | 64 |
Abbreviations: MMP = matrix metalloproteinases; EGR1 = early growth response 1; Cdc2 = cell division cycle gene 2; ROS = reactive oxygen species; PTEN = phosphatase and tensin homolog; Bcl-2 = B-cell lymphoma 2; CDK1 = cyclin dependent kinase 1; CDC25B = cell division cycle 25 B; CRM1 = chromosome-region-maintenance-1; AMPK = AMP-activated protein kinase; MEK = mitogen-activated protein kinase; ERK = extracellular signal-regulated kinase. ↑ = activation/up regulation; ↓ = suppression/down regulation.
Sulforaphane in breast cancer
Breast cancer is one of the most common malignant tumors in the world, especially in women 65. According to statistics, among women younger than 45, breast cancer is undoubtedly the leading cause of cancer-related death 66. At present, the treatment of breast cancer mainly includes surgical resection, radiotherapy, and chemotherapy. Previous research found that pretreatment with sulforaphane, at as low concentration as 5 μM, inhibited cell clonogenicity by nearly 70% in breast cancer cells, when compared to untreated cells. Human epidermal growth factor receptor 2 (HER-2) is known to be involved in the proliferation and division of breast cancer cells 67, specifically through the Akt–mTOR–S6K kinase pathway 51. The anti-HER2-targeted drug lapatinib is often used in breast cancer patients with HER2 overexpression 68. Studies have found that the combination of sulforaphane (2.5 μM) and lapatinib (100 nM) could effectively induce cell apoptosis and decrease cell viability mainly by inhibiting the Akt–mTOR–S6K pathway in breast cancer cells, thus improving the therapeutic effect of lapatinib 69. Triple-negative breast cancer is a common subtype of breast cancer lacking estrogen receptor, progesterone receptor, and HER2 gene overexpression 70. sulforaphane also has the significant therapeutic potential against triple-negative breast cancer. In recent years, the Hedgehog (Hh) pathway has been identified as a key signaling pathway that drives tumorigenesis in triple-negative breast cancer 52. Downregulation of the Hedgehog (Hh) signaling pathway by inhibitors can reduce cell migration and invasion 71. Sulforaphane can significantly inhibit the Hedgehog (Hh) pathway, thereby reducing the activity of the downstream signal modulators matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) and inhibiting the invasion of human triple-negative breast cancer cells 72. Early growth response 1 (EGR1) is an immediate early gene induced by estrogen, growth factor, or stress signal that can exert both cancer-suppressive and -promoter activities 73. At the same time, EGR1 was successfully verified as a uniformly activated marker after sulforaphane treatment in triple-negative breast cancer cell lines MDA-MB453 and MDA-MB-436. The data indicated that sulforaphane could inhibit the expression of cyclin B1 and phosphorylated Cdc2 by mediating tumor suppressor EGR1, thus inducing G2/M phase arrest of triple-negative breast cancer cells 54.
Sulforaphane in hepatocellular carcinoma
Hepatocellular carcinoma (liver cancer) is one of the deadliest and most common cancers in humans. The treatment of liver cancer mostly involves surgical resection, transplantation, and ablation, but the therapeutic effect is not good 55. Some researchers have found that sulforaphane can promote apoptosis of hepatocellular carcinoma cells, which is morphologically manifested as cell contraction, blistering, chromatin condensation, and nuclear fragmentation. They also found that sulforaphane was most toxic in HepG2 cells. sulforaphane exhibited an IC50 value of 33.8 μM when incubated with HepG2 cells for 72 hours. An annexin V assay found that the same treatment increased caspases 3/7 and 9 activities, while caspase 8 activity decreased 74. Oxidative reactive oxygen species (ROS), which are responsible for killing cancer cells, also affect secondary signaling networks 56. Sulforaphane can induce the generation of intracellular ROS and inhibit the polymerization of microtubules, leading to the apoptosis and necrosis of hepatocellular carcinoma cells 75. The transcription factor nuclear factor-κB (NF-κB) is a key transcriptional regulator in the inflammatory response. The NF-κB pathway is one of the important pathways activated during liver injury and inflammation and has been widely studied in the development of liver cancer 76. Sulforaphane can inhibit NF-κB activity and downstream gene expression of the NF-κB pathway in hepatocellular carcinoma cells. sulforaphane can increase the radiation sensitivity of hepatocellular carcinoma by blocking the NF-κB pathway 77.
Sulforaphane in lung cancer
Lung cancer is the leading cause of cancer death in the world 78. Non-small cell lung cancer (NSCLC), the most frequent subtype of lung cancer, has increased in both incidence and mortality 79. At present, research advancement in the field has revealed the tumor promotion roles of PI3K–Akt overactivation in non-small cell lung cancer 57. The PI3K–Akt pathway promotes proliferation, migration, invasion, and resistance to treatment by activating a variety of mechanisms, including the loss of the negative regulator phosphatase and tensin homolog (PTEN) and/or Akt1 itself 58. Sulforaphane-treated non-small cell lung cancer cells have significant inhibitory effects on the PI3K–Akt signaling pathway, including inhibition of PTEN expression and inhibition of Akt phosphorylation 80. Sulforaphane (7.5 μM) combined with the chemotherapy drug carboplatin (20 μM) can significantly induce mitochondrial membrane potential and intracellular ROS depolarization. By activating caspases, destroying matrix metalloproteinases and arresting the cell cycle, combination treatment with sulforaphane and carboplatin synergistically promotes the apoptosis and antiproliferative effects of human non-small cell lung cancer cells A549d and enhances the tumor toxicity effect of conventional therapy alone 81.
Sulforaphane in cervical cancer
Cervical cancer remains the third most common cancer in developing countries, despite a wide range of screening procedures 59. The therapeutic effects of photodynamic therapy in cervical intraepithelial neoplasia (CIN) and cervical cancer have been extensively studied 82. Effects of photodynamic therapy with a very low dose of sulforaphane (2.0 μg/ml) and radachlorin (0.5 μg/ml) at a fluence of 27 J/cm² (30 mW/cm², λmax ∼ 670 ± 3 nm) on human cervical cancer cells HeLa has shown a synergistic effect in inducing cell apoptosis. This combination therapy activates the mitochondrial apoptotic pathway primarily through upregulating the levels of caspase 3 and caspase 9. This therapeutic strategy also activates the caspase 8-dependent death receptor pathway and inhibits cell proliferation by downregulating epidermal growth factor receptor (EGFR) 60.
Sulforaphane in gastrointestinal cancer
In the Netherlands Cohort Study 83, it was observed that consumption of Brassica vegetables was inversely associated with development of gastric cardia adenocarcinoma. In an ecologic study where colorectal cancer rates between Maori and non-Maori people in New Zealand were compared, the authors attributed the low prevalence of cancer amongst Maori people to the consumption of vegetables like watercress 84, in spite of their high red meat consumption. Interestingly, another study showed that consumption of cruciferous vegetables increased the urinary excretion of PhIP, a DNA adduct forming carcinogen present in cooked meat 85.
Reduced urease and inflammation was seen in Helicobacter pylori-infected humans fed a broccoli sprout-rich diet indicating that isothiocyanates could likely be used to block stomach carcinogenesis in high-risk individuals 30. Similar encouraging results have been shown in smaller trials with a lower number of participants 86. However, in another trial using 250 mg standardized broccoli sprout yielding 1000 μg sulforaphane twice daily for four weeks, no significant changes were observed in urea breath test and gastric juice ammonia concentrations but modest changes were seen in lipid peroxidation in the gastric mucosa compared to placebo 87.
Sulforaphane in prostate cancer
The efficacy of sulforaphane against prostate cancer has been tested in a few small clinical trials. Treating 20 prostate cancer patients with sulforaphane-rich extracts (200 μmol/ day) for 20 weeks did not result in a significant decline (≥50%) in prostate-specific antigen (PSA) levels 88. However, in another study, men with biochemical recurrence after radical prostatectomy showed promising results after daily ingestion of 60 mg (~340 μmol) of stabilized free sulforaphane in a commercial dietary supplement for 6 months 89.
Sulforaphane in other tumors
Sulforaphane obviously also has cytotoxic effects on other human malignant tumor models. For example, cisplatin is a first-line chemotherapy drug for a variety of cancers, including ovarian cancer 61. Sulforaphane can sensitize cisplatin by enhancing ROS and mitochondrial membrane depolarization and can activate multiple apoptotic pathways to synergistically inhibit the proliferation of ovarian cancer SKOV3 and SNU8 cells and induce apoptosis. Therefore, sulforaphane could be used as a promising chemotherapy sensitizer to improve the efficacy of cisplatin in ovarian cancer 63. Sulforaphane can also reduce the viability of gastric cancer cells and induce apoptosis 64. In addition, sulforaphane can induce cell cycle arrest and apoptosis in the G2/M phase of colon cancer cells, accompanied by the phosphorylation of CDK1 and CDC25B inhibitory sites and the upregulation of the p38 and JNK pathways 90. Surprisingly, sulforaphane can selectively clear lymphoma cells via CRM1-mediated SQSTM1/p62 overexpression and AMPK activation. At the same time, sulforaphane protects normal lymphocytes by inducing cophage and apoptosis 91. Sulforaphane and photosensitive fiber-mediated photodynamic therapy can induce the apoptosis of thyroid cancer cells via significantly upregulating Ras, mitogen-activated protein kinase (MEK), extracellular signal-regulated kinase (ERK), and B-Raf protein expression levels. After combined treatment, their proapoptosis and antiproliferative effects were both significantly enhanced to a much higher level than the single dose 92.
Sulforaphane and autism
Sulforaphane treatment (50-150 μmol daily, for 18 weeks followed by 4 weeks without treatment) improved autism spectrum disorder-related outcomes (largely based on the Social Responsiveness Scale) in young, male patients 39. This finding was particularly important because sulforaphane potentially addressed the pathophysiological hallmarks of autism spectrum disorder (oxidative stress and antioxidant deficiency) instead of treating symptoms of autism spectrum disorder as done by standard therapy 93. Such results are in alignment with the findings from a recent report that showed that sulforaphane was able to reduce damage caused to mouse cortical cultures by chemicals that mimicked the action in several brain disorders, including autism spectrum disorder 94. There are multiple clinical trials in the pipeline evaluating the utility of sulforaphane/ broccoli preparations in autism spectrum disorder and findings from these studies would add valuable insight to incorporating these compounds into autism spectrum disorder treatment methods. Dietary broccoli sprout extract also improved outcomes related to schizophrenia in patients 95, suggesting that isothiocyanates may collectively have a very important role to play in treating neurological and developmental conditions.
Sulforaphane and diabetes
In a randomized, double-blind, placebo-controlled study, 103 Scandinavian type 2 diabetes patients were given daily oral broccoli sprout extract (containing 150 μmol sulforaphane per dose) for 12 weeks, and improved fasting glucose and hemoglobin A1C (HbA1C) was observed with the most robust improvement occurring in dysregulated, obese participants 32. Most of these patients were concurrently on metformin treatment, therefore the therapeutic effect of sulforaphane alone in type 2 diabetes is unknown from this study. Previous studies in Iran showed that broccoli sprout powder consumption (112 or 225 μmol/day of sulforaphane equivalents given as a commercial glucoraphanin-rich dietary supplement) for 4 weeks, reduced serum insulin concentration in type 2 diabetes patients 96. The same intervention resulted in favorable lipid profiles in type 2 diabetes patients suggesting that isothiocyanates have utility in reducing diabetes-related complications too 33. Whether or not sulforaphane and other isothiocyanates could replace or complement current diabetes medications such as metformin requires further investigation.
Sulforaphane and skin disorders
Topical application of broccoli sprout extract for keratin-based disorders was tested by Kerns and colleagues in a small study 97. Here, five subjects with epidermolysis bullosa simplex (caused by mutations in keratin 14 or 5) applied the extract (500 nmol sulforaphane/ mL) daily. Variable but induced expression of keratins 16 and 6 were observed after application indicating the potential of broccoli sprout extract to be used in similar keratin-associated disorders. sulforaphane-rich broccoli sprout extract application was shown to protect skin of volunteers against erythema caused by ultraviolet radiation 98. However, more extensive studies with larger sample sizes are needed to understand the broad spectrum of possible uses of isothiocyanates in skin disease.
Sulforaphane and cardiovascular disease
In 2015, a study showed that a broccoli diet containing high levels of glucoraphanin reduced plasma LDL cholesterol (low-density lipoprotein or “bad” cholesterol) levels significantly 99. Cardiovascular disease risk in Helicobacter pylori-infected type 2 diabetes patients was shown to be reduced after administration of broccoli sprouts powder 100. However, in 40 hypertension patients consuming 10 g of dried broccoli sprouts for 4 weeks, no changes in blood pressure or flow mediated dilation were detected 101. The exact mechanism by which sulforaphane/ broccoli is able to protect against heart and vascular disease is currently unknown but is likely related to redox changes associated with NRF2 signaling 102. This is an active area of research that warrants more clinical studies. In a phase 1 study that used sulforaphane-containing broccoli sprout homogenate (50-150 μmol dose escalation for 14 days) in adults with sickle cell disease, increases in whole blood mRNA levels of heme oxygenase1 and subunit of fetal hemoglobin were observed 103.
Sulforaphane and respiratory conditions
A broccoli sprout homogenate was shown to reduce influenza-related outcomes in human volunteers 104. Influenza virus-induced markers of inflammation were significantly lower in smokers after consumption of broccoli sprout homogenates 105. In another study, daily 100 μmol sulforaphane for 14 days was shown to improve the bronchoprotective response in asthmatics 106. Broccoli sprout extract (dose equivalent to consumption of 100 – 200 g broccoli) was also shown to reduce the nasal allergic response to diesel exhaust particles in human subjects 107. When 25 or 150 μmol sulforaphane was orally administered to smokers with chronic obstructive pulmonary disorder (COPD) for four weeks, no significant changes in inflammatory markers were observed compared to placebo 108. This outcome may be related to the fact that baseline oxidative stress and inflammation is already very high in such a patient population and cannot be reversed by a dietary agent.
Sulforaphane foods
Foods high in sulforaphane include cruciferous vegetables, such as broccoli, watercress, kale, cabbage, collard greens, brussels sprouts, bok choy, mustard greens, and cauliflower 109. In cruciferous vegetables, sulforaphane is present as its precursor glucosinolate glucoraphanin 110. When the tissues of cruciferous plants are processed by cutting, cooking, freezing, or mastication, glucoraphanine and an enzyme called myrosinase in the cruciferous vegetables are exposed to each other, which removes the glucose moiety on glucoraphanine and hydrolyzes glucoraphanine to sulforaphane 111. Beta-thioglucosidases occurring in the human gastrointestinal microbiome also convert precursor glucoraphanine into bioactive sulforaphane 112.
Critical to the formation of sulforaphane is the plant enzyme myrosinase (thioglucoside glucohydrolase) and beta-thioglucosidases occurring in the human gastrointestinal microbiome 113, which convert precursor glucosinolate glucoraphanin into bioactive sulforaphane. A human homolog of myrosinase has not been described. Directly administered sulforaphane has much higher bioavailability than glucosinolate glucoraphanin 114, reflecting their intrinsic lipophilicity, but more importantly reflecting the need for ingested glucosinolates to first be converted to isothiocyanates prior to absorption and further metabolism. Measurement of urinary excretion of sulforaphane and sulforaphane metabolites has been used routinely to determine oral bioavailability in various trials. In a randomized, cross-over clinical trial where participants were administered either sulforaphane- or glucoraphanin-rich beverages, it was observed that participants who received sulforaphane-rich beverage had substantially higher rates of urinary excretion of sulforaphane metabolites than those who received glucoraphanin-rich beverage 115. Only 5% of the administered glucoraphanin-rich was recovered as sulforaphane metabolites as compared to 70% when sulforaphane was administered. Egner and colleagues 116 also showed in this cohort of healthy Chinese adults that there was rapid clearance of urinary metabolites of sulforaphane, a finding that had been reported by others previously. Four healthy volunteers who were fed an extract of 3-day old broccoli sprouts that had been hydrolyzed with myrosinase (mean dose of isothiocyanates ~201 μmol) showed rapid excretion rates of isothiocyanates (mean cumulative 8 hour excretion ~117 μmol; about 58% of the administered dose) 117.
Others have shown that consumption of fresh broccoli sprouts resulted in significantly higher plasma and urinary concentrations of sulforaphane than achieved following consumption of a commercial dietary supplement claiming to be rich in glucoraphanin, but lacking myrosinase 118. In another small clinical study, a different commercial supplement rich in glucoraphanin but without myrosinase, provided identical crude pharmacokinetics as a laboratory-prepared, freeze-dried broccoli sprout extract 119, indicating inter-individual variability observed in glucoraphanin delivery. Similarly, it was shown that high myrosinase activity corresponded with higher bioavailability of sulforaphane and somewhat shorter excretion half lives (2.2 hours for high activity compared with 3.1 hours for low activity) of urinary sulforaphane conjugates compared to heat-applied broccoli florets with low myrosinase activity 120.
The finding that myrosinase-containing preparations of broccoli have higher bioavailability than those that have no myrosinase has been echoed by other studies 119. A comparison of fresh broccoli sprouts, glucosinolate-rich broccoli powder lacking myrosinase and a combination of both, yielded the highest urinary sulforaphane recovery from the sprouts followed by the combination and the sulforaphane supplement powder, respectively 121. In this study, appearance of sulforaphane metabolites in urine and plasma was delayed after consumption of the sulforaphane supplement powders compared to that from consumption of broccoli sprouts, likely due to the lack of active and readily available, ingested myrosinase and a dependence on the gut microbiome to supply it. Not surprisingly, methods of cooking broccoli and other cruciferous vegetables can have a significant impact on the formation and content of isothiocyanates. Fresh broccoli yields approximately 3 times higher levels of isothiocyanates compared to cooked broccoli 122, leading to the clear suggestion that retention of endogenous myrosinase activity by avoiding heat is an important consideration for sulforaphane delivery from cruciferous vegetables. These concerns are supported by epidemiologic data reviewed by Tang and colleagues 123.
Sulforaphane supplement
In the United States, both broccoli and watercress are available in the form of dietary supplement capsules. Many of these products have not been tested for efficacy in randomized clinical trials or monitored for bioactive content, and therefore present areas of research or manufacturing that require more robust work. While most manufacturers don’t directly make health or disease treatment claims on the labels of these products, some do so overtly. The Food and Drug Administration (FDA) recently issued a series of warnings to companies that produce supplements that claim cancer preventive and therapeutic properties 124. Amongst the common statements found on broccoli and watercress supplements are claims to support detoxification – a finding that has been essentially extrapolated from the larger body of research on these plants and their bioactives and not necessarily by direct testing the respective products. Hence, it is important not only to conduct rigorous scientific experimentation on these products but also to raise public awareness so that people know what to consume, how and when.
Advances have been made to circumvent some of the challenges associated with sulforaphane delivery. To account for the variability – or lack – in myrosinase activity in preparations or individuals that could lead to lower or higher sulforaphane concentrations, tablets containing both the glucoraphanin as well as active myrosinase have been manufactured and are sold in the US and internationally. Additionally, cruciferous crops that produce higher content of glucoraphanin can been selected-for and emerging technologies in the field are evaluating how bacteria can be engineered to bind specific cells and secrete myrosinase in a targeted chemoprevention approach 125. A recent study compared the delivery efficiency of an alpha-cyclodextrin inclusion sulforaphane preparation with a commercial sulforaphane-rich nutritional supplement 126.
Sulforaphane dosage
Based on available research, typical dosage for broccoli sprout and seed extracts is 50-100 mg sulforaphane glucosinolate (glucoraphanin) daily in divided doses 15.
Sulforaphane side effects
Because of sulforaphane’s toxicity to certain cells, it is of great significance to evaluate the clinical safety of sulforaphane 127. Some researchers have tested sulforaphane in acute toxicity analyses. After fasting overnight, 48 mice were given five different doses of sulforaphane at 400, 300, 225, 168.8, and 126.6 mg/kg (8 in each group), and any serious effects or mortality were carefully observed after administration. After 14 days, all eight mice treated with 126.6 mg/kg sulforaphane survived during treatment. However, eight, seven, four, or two animals treated with 400, 300, 225, or 168.8 mg/kg sulforaphane died within 24 hours of dosing 128. In addition, one mouse treated with 225 or 168.8 mg/kg sulforaphane died within 48 hours. For the 126.6 mg/kg sulforaphane group, no physical or abnormal changes were observed in sleep patterns, behavior patterns, fur, skin, eyes, mucous membranes, tremors, or salivation 80. In another study, scientists implanted lymphoma cells in nude mouse xenografts and administered sulforaphane to them twice a week, 100 mg/kg each time. After 10 days, there was no significant change in body weight compared with the control group, indicating that sulforaphane is less toxic 91. Thus, the dose-associated superiority of sulforaphane in reducing adverse reactions is obvious in current preclinic research. In addition, the findings from Li et al. 129 have shown that sulforaphane could be able to evidently restrain the pathological process of diseases in C57BL/6J mice associated with increased intestinal inflammatory factors. They demonstrated no apparent toxicity to animals induced by sulforaphane administration.
Several studies have been conducted to assess the safety of sulforaphane in humans. A randomized, placebo-controlled, double-blind study showed broccoli sprout extracts were without significant side effects at doses of 25 and 100 μmol glucoraphanin for seven days 15. Another randomized, placebo-controlled study involving 200 healthy adults consuming broccoli sprout infusions daily for two weeks (400 μmol or approximately 175 mg glucoraphanin) showed no adverse effects 130. In a dose escalation safety study, broccoli sprout extracts containing sulforaphane doses as high as 340 nmol were topically applied three consecutive times to forearm skin. Researchers reported significant induction of phase II enzyme activity in biopsied tissue without any adverse reactions 15.
References- Mi, L., Hood, B. L., Stewart, N. A., Xiao, Z., Govind, S., Wang, X., Conrads, T. P., Veenstra, T. D., & Chung, F. L. (2011). Identification of potential protein targets of isothiocyanates by proteomics. Chemical research in toxicology, 24(10), 1735–1743. https://doi.org/10.1021/tx2002806
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry2001;56:5-51.
- Conaway CC, Getahun SM, Liebes LL, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 2000;38:168-178.
- Bheemreddy RM, Jeffery EH. The metabolic fate of purified glucora-phanin in F344 rats. J Agric Food Chem2007;55:2861-2866.
- Zhang Y, Kensler TW, Cho CG, et al. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocya-nates. Proc Natl Acad Sci 1994;91:3147-3150.
- Tian G, Li Y, Cheng L, Yuan Q, Tang P, Kuang P, Hu J. The mechanism of sulforaphane degradation to different water contents. Food Chem. 2016;194:1022–7.
- Kim KH, Moon E, Kim SY, Choi SU, Lee JH, Lee KR. 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. J Ethnopharmacol. 2014;151(1):503-8. doi: 10.1016/j.jep.2013.11.003
- Li, Y., Zhang, T., Korkaya, H., Liu, S., Lee, H. F., Newman, B., Yu, Y., Clouthier, S. G., Schwartz, S. J., Wicha, M. S., & Sun, D. (2010). Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clinical cancer research : an official journal of the American Association for Cancer Research, 16(9), 2580–2590. https://doi.org/10.1158/1078-0432.CCR-09-2937
- Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008 Oct 8;269(2):291-304. doi: 10.1016/j.canlet.2008.04.018
- Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007 Sep;28(9):1343-54. doi: 10.1111/j.1745-7254.2007.00679.x
- Fahey JW, Wade KL, Stephenson KK, Panjwani AA, Liu H, Cornblatt G, Cornblatt BS, Ownby SL, Fuchs E, Holtzclaw WD, Cheskin LJ. Bioavailability of sulforaphane following ingestion of glucoraphanin-rich broccoli sprout and seed extracts with active myrosinase: A pilot study of the effects of proton pump inhibitor administration. Nutrients 2019;11(7).
- Baenas N, Suarez-Martinez C, Garcia-Viguera C, Moreno DA. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res Int. 2017;100(Pt 1):497–503.
- Soni K, Rizwanullah M, Kohli K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif Cells Nanomed Biotechnol. 2018;46(Supp1):15–31.
- Zhang Z, Garzotto M, Davis EW 2nd, Mori M, Stoller WA, Farris PE, Wong CP, Beaver LM, Thomas GV, Williams DE, Dashwood RH, Hendrix DA, Ho E, Shannon J. Sulforaphane bioavailability and chemopreventive activity in men presenting for biopsy of the prostate gland: A randomized controlled trial. Nutr Cancer 2020;72(1):74–87.
- Sulforaphane Glucosinolate Monograph. https://altmedrev.com/wp-content/uploads/2019/02/v15-4-352.pdf
- Rai, R., Gong Essel, K., Mangiaracina Benbrook, D., Garland, J., Daniel Zhao, Y., & Chandra, V. (2020). Preclinical Efficacy and Involvement of AKT, mTOR, and ERK Kinases in the Mechanism of Sulforaphane against Endometrial Cancer. Cancers, 12(5), 1273. https://doi.org/10.3390/cancers12051273
- Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999 Sep-Oct;37(9-10):973-9. doi: 10.1016/s0278-6915(99)00082-4
- Johansson NL, Pavia CS, Chiao JW. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008 Jun;74(7):747-50. doi: 10.1055/s-2008-1074520
- Amjad AI, Parikh RA, Appleman LJ, Hahm ER, Singh K, Singh SV. Broccoli-Derived Sulforaphane and Chemoprevention of Prostate Cancer: From Bench to Bedside. Curr Pharmacol Rep. 2015 Nov 1;1(6):382-390. doi: 10.1007/s40495-015-0034-x
- Greaney AJ, Maier NK, Leppla SH, Moayeri M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J Leukoc Biol. 2016 Jan;99(1):189-99. doi: 10.1189/jlb.3A0415-155RR
- Sikdar S, Papadopoulou M, Dubois J. What do we know about sulforaphane protection against photoaging? J Cosmet Dermatol. 2016 Mar;15(1):72-7. doi: 10.1111/jocd.12176
- Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev. 2013;2013:415078. doi: 10.1155/2013/415078
- Lee JH, Moon MH, Jeong JK, Park YG, Lee YJ, Seol JW, Park SY. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochem Biophys Res Commun. 2012 Oct 5;426(4):492-7. doi: 10.1016/j.bbrc.2012.08.107
- Nagata N., Xu L., Kohno S., Ushida Y., Aoki Y., Umeda R., Fuke N., Zhuge F., Ni Y., Nagashimada M., et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes. 2017;66:1222–1236. doi: 10.2337/db16-0662
- Shawky N.M., Segar L. Sulforaphane improves leptin responsiveness in high-fat high-sucrose diet-fed obese mice. Eur. J. Pharmacol. 2018;835:108–114. doi: 10.1016/j.ejphar.2018.07.050
- Atwell LL, Zhang Z, Mori M, Farris P, Vetto JT, Naik AM, Oh KY, Thuillier P, Ho E, Shannon J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev Res (Phila) 2015;8:1184–1191.
- Riso P, Martini D, Moller P, Loft S, Bonacina G, Moro M, Porrini M. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis 2010;25:595–602.
- Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 2014;7:813–823.
- Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, Egner PA, Chen JG, Qian GS, Chen TY, Fahey JW, Talalay P, Groopman JD, Yuan JM, Hecht SS. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 2012;33:101–107.
- Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009;2:353–360.
- Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della NE, Corbel L, Le SR, Azzouzi AR, Mottet N. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila) 2015;8:712–719.
- Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JMJ, Wollheim CB, Wierup N, Haymond MW, Friend SH, Mulder H, Rosengren AH. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 2017;9 DOI:10.1126/scitranslmed.aah4477
- Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract 2012;96:348–354.
- Kerns ML, Guss L, Fahey J, Cohen B, Hakim JM, Sung S, Lu RG, Coulombe PA. Randomized, split-body, single-blinded clinical trial of topical broccoli sprout extract: Assessing the feasibility of its use in keratin-based disorders. J Am Acad Dermatol 2017;76:449–453.
- Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A 2007;104:17500–17505.
- Armah CN, Derdemezis C, Traka MH, Dainty JR, Doleman JF, Saha S, Leung W, Potter JF, Lovegrove JA, Mithen RF. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: evidence from randomised controlled trials. Mol Nutr Food Res 2015;59:918–926.
- Mirmiran P, Bahadoran Z, Golzarand M, Zojaji H, Azizi F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H.pylori eradication: a randomized clinical trial in patients with type 2 diabetes. J Diabetes Metab Disord 2014;13:64.
- Doss JF, Jonassaint JC, Garrett ME, Ashley-Koch AE, Telen MJ, Chi JT. Phase 1 study of a sulforaphane-containing broccoli sprout homogenate for sickle cell disease. PLoS One 2016;11:e0152895
- Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, Zimmerman AW. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci U S A. 2014 Oct 28;111(43):15550-5. doi: 10.1073/pnas.1416940111
- Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, Yoshida T, Iyo M, Hashimoto K. An open Study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharmacol Neurosci 2015;13:62–67.
- Muller L, Meyer M, Bauer RN, Zhou H, Zhang H, Jones S, Robinette C, Noah TL, Jaspers I. Effect of broccoli sprouts and live attenuated influenza virus on peripheral blood natural killer cells: a randomized, double-blind study. PLoS One 2016;11:e0147742
- Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN, Meyer M, Murphy PC, Jones S, Letang B, Robinette C, Jaspers I. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One 2014;9:e98671
- Brown RH, Reynolds C, Brooker A, Talalay P, Fahey JW. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res 2015;16:106.
- Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, Krak M, Zhang Y, Nel A. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct 2014;5:35–41.
- Royston K.J., Tollefsbol T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharmacol. Rep. 2015;1:46–51. doi: 10.1007/s40495-014-0003-9
- Lenzi M., Fimognari C., Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat. Res. 2014;159:207–223. doi: 10.1007/978-3-642-38007-5_12
- Dos Santos P., Machado A.R.T., De Grandis R.A., Ribeiro D.L., Tuttis K., Morselli M., Aissa A.F., Pellegrini M., Antunes L.M.G. Transcriptome and DNA methylation changes modulated by sulforaphane induce cell cycle arrest, apoptosis, DNA damage, and suppression of proliferation in human liver cancer cells. Food Chem. Toxicol. 2020;136:111047. doi: 10.1016/j.fct.2019.111047
- Clarke J.D., Dashwood R.H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018
- Yao A., Shen Y., Wang A., Chen S., Zhang H., Chen F., Chen Z., Wei H., Zou Z., Shan Y., et al. Sulforaphane induces apoptosis in adipocytes via Akt/p70s6k1/Bad inhibition and ERK activation. Biochem. Biophys. Res. Commun. 2015;465:696–701. doi: 10.1016/j.bbrc.2015.08.049
- Pore S.K., Hahm E.R., Kim S.H., Singh K.B., Nyiranshuti L., Latoche J.D., Anderson C.J., Adamik J., Galson D.L., Weiss K.R., et al. A Novel Sulforaphane-Regulated Gene Network in Suppression of Breast Cancer-Induced Osteolytic Bone Resorption. Mol. Cancer Ther. 2020;19:420–431. doi: 10.1158/1535-7163.MCT-19-0611
- Cadona FC, Rosa JL, Schneider T, Cubillos-Rojas M, Sanchez-Tena S, Azzolin VF, Assmann CE, Machado AK, Ribeiro EE, da Cruz IBM. Guarana, a highly caffeinated food, presents in vitro antitumor activity in colorectal and breast cancer cell lines by inhibiting AKT/mTOR/S6K and MAPKs pathways. Nutr Cancer 2017;69(5):800–10.
- Habib JG, O’Shaughnessy JA. The hedgehog pathway in triple-negative breast cancer. Cancer Med. 2016;5(10):2989–3006.
- Benvenuto M, Masuelli L, De Smaele E, Fantini M, Mattera R, Cucchi D, Bonanno E, Di Stefano E, Frajese GV, Orlandi A, Screpanti I, Gulino A, Modesti A, Bei R. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget 2016;7(8):9250–70.
- Yang M, Teng W, Qu Y, Wang H, Yuan Q. Sulforaphane inhibits triple negative breast cancer through activating tumor suppressor Egr1. Breast Cancer Res Treat. 2016;158(2):277–86.
- Tahmasebi Birgani M, Carloni V. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy. Int J Mol Sci. 2017;18(2).
- Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res. 2015;100:170–4.
- De Marco C, Laudanna C, Rinaldo N, Oliveira DM, Ravo M, Weisz A, Ceccarelli M, Caira E, Rizzuto A, Zoppoli P, Malanga D, Viglietto G. Specific gene expression signatures induced by the multiple oncogenic alterations that occur within the PTEN/PI3K/AKT pathway in lung cancer. PLoS One 2017;12(6):e0178865
- Zhao ZQ, Yu ZY, Li J, Ouyang XN. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncol Lett. 2016;12(1):63–8.
- Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, Bothou A, Galazios G. Cervical cancer: Screening, diagnosis and staging. J BUON. 2016;21(2):320–5.
- Biswas R, Mondal A, Chatterjee S, Ahn JC. Evaluation of synergistic effects of sulforaphane with photodynamic therapy in human cervical cancer cell line. Lasers Med Sci. 2016;31(8):1675–82.
- Choi BY, Joo JC, Lee YK, Jang IS, Park SJ, Park YJ. Anti-cancer effect of Scutellaria baicalensis in combination with cisplatin in human ovarian cancer cell. BMC Complement Altern Med. 2017;17(1):277.
- Samuel P, Pink RC, Brooks SA, Carter DR. miRNAs and ovarian cancer: A miRiad of mechanisms to induce cisplatin drug resistance. Expert Rev Anticancer Ther. 2016;16(1):57–70.
- Biswas R, Ahn JC, Kim JS. Sulforaphane synergistically sensitizes cisplatin via enhanced mitochondrial dysfunction and PI3K/PTEN modulation in ovarian cancer cells. Anticancer Res. 2015;35(7):3901–8.
- Mondal A, Biswas R, Rhee YH, Kim J, Ahn JC. Sulforaphane promotes Bax/Bcl2, MAPK-dependent human gastric cancer AGS cells apoptosis and inhibits migration via EGFR, p-ERK1/2 down-regulation. Gen Physiol Biophys. 2016;35(1):25–34.
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51.
- Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: An overview. Updates Surg. 2017;69(3):313–7.
- Asif HM, Sultana S, Ahmed S, Akhtar N, Tariq M. HER-2 positive breast cancer—A mini-review. Asian Pac J Cancer Prev. 2016;17(4):1609–15.
- Oh DY, Bang YJ. HER2-targeted therapies—A role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48.
- Kaczyńska A, Świerczyńska J, Herman-Antosiewicz A. Sensitization of HER2 Positive Breast Cancer Cells to Lapatinib Using Plants-Derived Isothiocyanates. Nutr Cancer. 2015;67(6):976-86. doi: 10.1080/01635581.2015.1053498
- Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–69.
- Miele E, Po A, Begalli F, Antonucci L, Mastronuzzi A, Marras CE, Carai A, Cucchi D, Abballe L, Besharat ZM, Catanzaro G, Infante P, Di Marcotullio L, Canettieri G, De Smaele E, Screpanti I, Locatelli F, Ferretti E. β-Arrestin1-mediated acetylation of Gli1 regulates Hedgehog/Gli signaling and modulates self-renewal of SHH medulloblastoma cancer stem cells. BMC Cancer 2017;17(1):488.
- Bao C, Kim MC, Chen J, Song J, Ko HW, Lee HJ. Sulforaphane interferes with human breast cancer cell migration and invasion through inhibition of hedgehog signaling. J Agric Food Chem. 2016;64(27):5515–24.
- Shajahan-Haq AN, Boca SM, Jin L, Bhuvaneshwar K, Gusev Y, Cheema AK, Demas DD, Raghavan KS, Michalek R, Madhavan S, Clarke R. EGR1 regulates cellular metabolism and survival in endocrine resistant breast cancer. Oncotarget 2017;8(57):96865–84.
- Kntayya SB, Ibrahim MD, Mohd Ain N, Iori R, Ioannides C, Abdull Razis AF. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphane in human hepatocarcinoma HepG2 cells. Nutrients 2018;10(6).
- Pocasap P, Weerapreeyakul N, Thumanu K. Structures of isothiocyanates attributed to reactive oxygen species generation and microtubule depolymerization in HepG2 cells. Biomed Pharmacother. 2018;101:698–709.
- He G, Karin M. NF-kappaB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011;21(1):159–68.
- Ren K, Li Z, Li Y, Zhang W, Han X. Sulforaphane enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-kappaB pathway. J Biochem Mol Toxicol. 2017;31(8).
- Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–73.
- Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–9.
- Yang M, Wang H, Zhou M, Liu W, Kuang P, Liang H, Yuan Q. The natural compound sulforaphane, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget 2016;7(47):76656–66.
- Chatterjee S, Rhee YH, Ahn JC. Sulforaphane–carboplatin combination synergistically enhances apoptosis by disruption of mitochondrial membrane potential and cell cycle arrest in human non-small cell lung carcinoma. J Med Food 2016;19(9):860–9.
- Chizenga EP, Chandran R, Abrahamse H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019;10(43):4380–96.
- Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer. 2011 Dec 1;129(11):2681-93. doi: 10.1002/ijc.25928
- Thomson B, Shaw I. A Comparison of risk and protective factors for colorectal cancer in the diet of New Zealand Maori and non-Maori. Asian Pac J Cancer Prev 2002;3:319–324.
- Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG. Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Carcinogenesis 2004;25:1659–1669.
- Galan MV, Kishan AA, Silverman AL. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci 2004;49:1088–1090.
- Chang YW, Jang JY, Kim YH, Kim JW, Shim JJ. The Effects of Broccoli Sprout Extract Containing Sulforaphane on Lipid Peroxidation and Helicobacter pylori Infection in the Gastric Mucosa. Gut Liver 2015;9:486–493.
- Alumkal JJ, Slottke R, Schwartzman J, Cherala G, Munar M, Graff JN, Beer TM, Ryan CW, Koop DR, Gibbs A, Gao L, Flamiatos JF, Tucker E, Kleinschmidt R, Mori M. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest New Drugs. 2015 Apr;33(2):480-9. doi: 10.1007/s10637-014-0189-z
- Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E, Corbel L, Le Scodan R, Azzouzi AR, Mottet N. Effect of Sulforaphane in Men with Biochemical Recurrence after Radical Prostatectomy. Cancer Prev Res (Phila). 2015 Aug;8(8):712-9. doi: 10.1158/1940-6207.CAPR-14-0459
- Byun S, Shin SH, Park J, Lim S, Lee E, Lee C, Sung D, Farrand L, Lee SR, Kim KH, Dong Z, Lee SW, Lee KW. Sulforaphane suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol Nutr Food Res. 2016;60(5):1068–78.
- Wang H, Wang F, Wu S, Liu Z, Li T, Mao L, Zhang J, Li C, Liu C, Yang Y. Traditional herbal medicine-derived sulforaphane promotes mitophagic cell death in lymphoma cells through CRM1-mediated p62/SQSTM1 accumulation and AMPK activation. Chem Biol Interact. 2018;281:11–23.
- Chatterjee S, Rhee Y, Chung PS, Ge RF, Ahn JC. Sulforaphane enhances the efficacy of photodynamic therapy in anaplastic thyroid cancer through Ras/RAF/MEK/ERK pathway suppression. J Photochem Photobiol B 2018;179:46–53.
- Liu H, Talalay P, Fahey JW. Biomarker-Guided Strategy for Treatment of Autism Spectrum Disorder (ASD). CNS Neurol Disord Drug Targets. 2016;15(5):602-13. doi: 10.2174/1871527315666160413120414
- Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun. 2016 Mar 31;7:11173. doi: 10.1038/ncomms11173
- Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, Yoshida T, Iyo M, Hashimoto K. An Open Study of Sulforaphane-rich Broccoli Sprout Extract in Patients with Schizophrenia. Clin Psychopharmacol Neurosci. 2015 Apr 30;13(1):62-7. doi: 10.9758/cpn.2015.13.1.62
- Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr 2012;63:767–771.
- Kerns ML, Guss L, Fahey J, Cohen B, Hakim JM, Sung S, Lu RG, Coulombe PA. Randomized, split-body, single-blinded clinical trial of topical broccoli sprout extract: Assessing the feasibility of its use in keratin-based disorders. J Am Acad Dermatol. 2017 Mar;76(3):449-453.e1. doi: 10.1016/j.jaad.2016.10.009
- Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007 Oct 30;104(44):17500-5. doi: 10.1073/pnas.0708710104
- Armah CN, Derdemezis C, Traka MH, Dainty JR, Doleman JF, Saha S, Leung W, Potter JF, Lovegrove JA, Mithen RF. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol Nutr Food Res. 2015 May;59(5):918-26. doi: 10.1002/mnfr.201400863
- Mirmiran P, Bahadoran Z, Golzarand M, Zojaji H, Azizi F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H.pylori eradication: a randomized clinical trial in patients with type 2 diabetes. J Diabetes Metab Disord. 2014 May 28;13:64. doi: 10.1186/2251-6581-13-64
- Christiansen B, Bellostas Muguerza N, Petersen AM, Kveiborg B, Madsen CR, Thomas H, Ihlemann N, Sørensen JC, Køber L, Sørensen H, Torp-Pedersen C, Domínguez H. Ingestion of broccoli sprouts does not improve endothelial function in humans with hypertension. PLoS One. 2010 Aug 27;5(8):e12461. doi: 10.1371/journal.pone.0012461
- Mann GE. Nrf2-mediated redox signalling in vascular health and disease. Free Radic Biol Med. 2014 Oct;75 Suppl 1:S1. doi: 10.1016/j.freeradbiomed.2014.10.595
- Doss JF, Jonassaint JC, Garrett ME, Ashley-Koch AE, Telen MJ, Chi JT. Phase 1 Study of a Sulforaphane-Containing Broccoli Sprout Homogenate for Sickle Cell Disease. PLoS One. 2016 Apr 12;11(4):e0152895. doi: 10.1371/journal.pone.0152895
- Müller L, Meyer M, Bauer RN, Zhou H, Zhang H, Jones S, Robinette C, Noah TL, Jaspers I. Effect of Broccoli Sprouts and Live Attenuated Influenza Virus on Peripheral Blood Natural Killer Cells: A Randomized, Double-Blind Study. PLoS One. 2016 Jan 28;11(1):e0147742. doi: 10.1371/journal.pone.0147742
- Noah TL, Zhang H, Zhou H, Glista-Baker E, Müller L, Bauer RN, Meyer M, Murphy PC, Jones S, Letang B, Robinette C, Jaspers I. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One. 2014 Jun 9;9(6):e98671. doi: 10.1371/journal.pone.0098671. Erratum in: PLoS One. 2014;9(9):e109513
- Brown RH, Reynolds C, Brooker A, Talalay P, Fahey JW. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res. 2015 Sep 15;16(1):106. doi: 10.1186/s12931-015-0253-z
- Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, Krak M, Zhang Y, Nel A. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 2014 Jan;5(1):35-41. doi: 10.1039/c3fo60277j
- Wise RA, Holbrook JT, Criner G, Sethi S, Rayapudi S, Sudini KR, Sugar EA, Burke A, Thimmulappa R, Singh A, Talalay P, Fahey JW, Berenson CS, Jacobs MR, Biswal S; Broccoli Sprout Extract Trial Research Group. Lack of Effect of Oral Sulforaphane Administration on Nrf2 Expression in COPD: A Randomized, Double-Blind, Placebo Controlled Trial. PLoS One. 2016 Nov 10;11(11):e0163716. doi: 10.1371/journal.pone.0163716. Erratum in: PLoS One. 2017 Mar 28;12 (3):e0175077.
- Palliyaguru, D. L., Yuan, J. M., Kensler, T. W., & Fahey, J. W. (2018). Isothiocyanates: Translating the Power of Plants to People. Molecular nutrition & food research, 62(18), e1700965. https://doi.org/10.1002/mnfr.201700965
- Thangstad OP, Winge P, Husebye H, Bones A. The myrosinase (thioglucoside glucohydrolase) gene family in Brassicaceae. Plant Mol Biol. 1993 Nov;23(3):511-24. doi: 10.1007/BF00019299
- Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. 2006 Jun;67(11):1053-67. doi: 10.1016/j.phytochem.2006.02.024
- Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila). 2012 Apr;5(4):603-11. doi: 10.1158/1940-6207.CAPR-11-0538
- Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila) 2012;5:603–611.
- Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev 2001;10:501–508.
- Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, Zhu J, Zhang YH, Chen YS, Friesen MD, Jacobson LP, Munoz A, Ng D, Qian GS, Zhu YR, Chen TY, Botting NP, Zhang Q, Fahey JW, Talalay P, Groopman JD, Kensler TW. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila) 2011;4:384–395.
- Hanlon N, Coldham N, Gielbert A, Sauer MJ, Ioannides C. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett 2009;284:15–20.
- Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta 2002;316:43–53.
- Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res 2011;64:456–463.
- Fahey JW, Holtzclaw WD, Wehage SL, Wade KL, Stephenson KK, Talalay P. Sulforaphane bioavailability from glucoraphanin-rich broccoli: control by active endogenous myrosinase. PLoS One 2015;10:e0140963.
- Oliviero T, Verkerk R, Vermeulen M, Dekker M. In vivo formation and bioavailability of isothiocyanates from glucosinolates in broccoli as affected by processing conditions. Mol Nutr Food Res 2014;58:1447–1456.
- Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer 2011;63:196–201.
- Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung FL. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer 2000;38:168–178.
- Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev 2010;19:1806–1811.
- FDA takes action against 14 companies for selling illegal cancer treatments. https://www.fda.gov/news-events/press-announcements/fda-takes-action-against-14-companies-selling-illegal-cancer-treatments
- Ho C, Tan H, Chua K, Kang A, Lim K, Ling K, Yew W, Lee Y, Thiery J, Chang M. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat Biomed Eng. 2018;2:27–37.
- Fahey JW, Wade KL, Wehage SL, Holtzclaw WD, Liu H, Talalay P, Fuchs E, Stephenson KK. Stabilized sulforaphane for clinical use: Phytochemical delivery efficiency. Mol Nutr Food Res. 2017 Apr;61(4). doi: 10.1002/mnfr.201600766
- Liu X, Wang Z, Xie R, Tang P, Yuan Q. Design, synthesis and biological evaluation of novel carbamodithioates as anti-proliferative agents against human cancer cells. Eur J Med Chem. 2018;157:1526–40.
- Wu, G., Yan, Y., Zhou, Y., Duan, Y., Zeng, S., Wang, X., Lin, W., Ou, C., Zhou, J., & Xu, Z. (2020). Sulforaphane: Expected to Become a Novel Antitumor Compound. Oncology research, 28(4), 439–446. https://doi.org/10.3727/096504020X15828892654385
- Li M, Gao J, Tang Y, Liu M, Wu S, Qu K, Long X, Li H, Liu M, Liu Y, Yuan J, Mao L, Liu Y, Zheng X, Wang E, Wang J, Yang Y. Traditional herbal medicine-derived sulforaphane LFS-01 reverses colitis in mice by selectively altering the gut microbiota and promoting intestinal gamma-delta T cells. Front Pharmacol. 2017;8:959.
- Kensler TW, Chen JG, Egner PA, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo Township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev 2005;14:2605-2613.