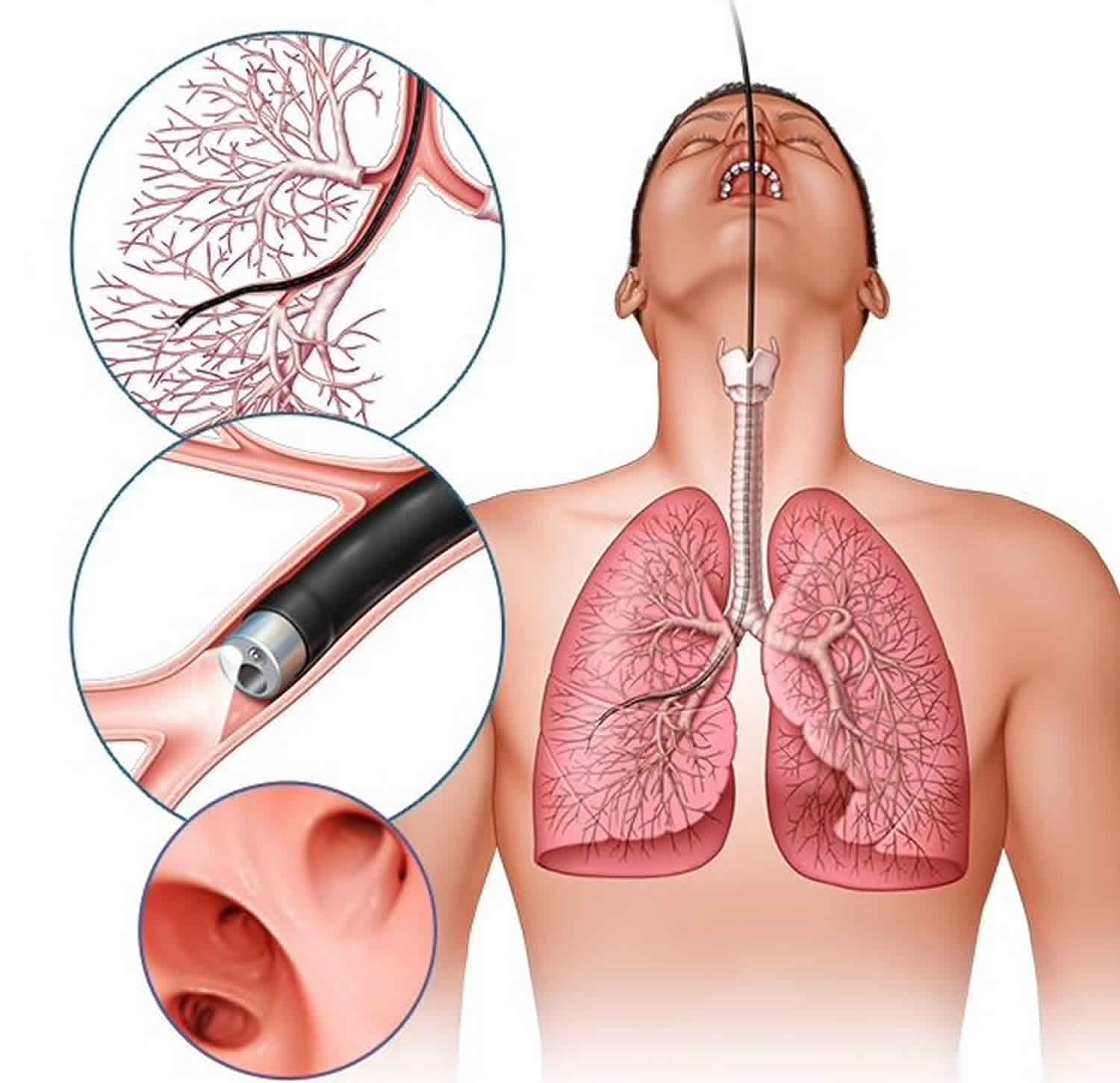
Bronchoalveolar lavage
Bronchoalveolar lavage also known as BAL or bronchoalveolar washing, is a minimally invasive procedure and a diagnostic tool of the lower respiratory system in which a flexible bronchoscope is passed through the mouth or nose into a sub-segment of the lung, with instillation of sterile normal saline into a subsegment of the lung, followed by suction and collection of the instillation for analysis 1. Analysis of the bronchoalveolar lavage cytology reflects the cellular composition within the alveoli making BAL part of diagnostic workup for suspected lung diseases. Bronchoalveolar lavage serves predominantly as a diagnostic tool for the evaluation of lower respiratory tract pathology and in some uncommon conditions, it also has therapeutic utility 2.
Bronchoalveolar lavage is an excellent method of obtaining specimens to rule out opportunistic infections in immunocompromised individuals. It is important to combine culture results with cytology which may show viral intranuclear or intracytoplasmic inclusion bodies on examination of pulmonary epithelial cells. this may help identify viruses like herpes simplex and cytomegalovirus (CMV). Bronchoalveolar lavage is also very useful for detection of fungi and mycobacteria which may not always be identified in blood. The one big disadvantage of bronchoalveolar lavage is that many potentially pathogenic microorganism may have colonized the airways in the absence of any clinical disease and hence their recovery may not be meaningful 3.
The use of bronchoalveolar lavage has been extensively studied in patients with interstitial lung disorders like hypersensitivity pneumonitis, sarcoidosis and idiopathic pulmonary fibrosis. Unfortunately, the findings of bronchoalveolar lavage are frequently not specific for these lung disorders and thus, the physician must also incorporate the clinical and imaging findings before making the diagnosis 1. In some disorders like acute eosinophilic pneumonia, bronchoalveolar lavage has saved the patient from an unnecessary lung biopsy
Bronchoalveolar lavage is also proving to be useful in evaluating individuals with occupational exposures to asbestos and other silicates. These macrophage-ingested occupational dust particles is usually visible in the fluid under polarized light. Bronchoalveolar lavage cytology is also useful for evaluating patients with malignancies of the airways.
For the most part, bronchoalveolar lavage is performed for diagnostic purposes. However, bronchoalveolar lavage is uniquely used for the treatment of pulmonary alveolar proteinosis (PAP). This involves instilling 30 to 50 liters of sterile saline through a double lumen endotracheal tube while the patient is under general anesthesia.
Bronchoalveolar lavage indications
The American Thoracic Society and British Thoracic Society have published guidelines on the performance of bronchoalveolar lavage 4. Common indications for the use of bronchoalveolar lavage include work up for opportunistic and atypical respiratory infections in immunocompromised patients, unexplained radiographic pulmonary infiltrates or hypoxemia. Bronchoalveolar lavage can also provide clues to support the diagnosis of some noninfectious conditions such as Diffuse Alveolar Hemorrhage (DAH), Pulmonary Alveolar Proteinosis (PAP), Eosinophilic Pneumonia, Hypersensitivity Pneumonitis, Interstitial Lung Diseases, chronic berylliosis, the presence of malignant cells and asbestos exposure. Pulmonary alveolar proteinosis (PAP) is an uncommon disease in which alveoli are progressively filled with surfactant-related material. Although a definitive diagnosis is usually made by an open lung biopsy, bronchoalveolar lavage (BAL) cytology may play a decisive role in the clinical work-up of these patients, and, in some cases, may spare a patient a more invasive diagnostic procedure.
Bronchoalveolar lavage contraindications
Bronchoalveolar lavage contraindications include myocardial infarction within the past four weeks or unstable angina, hemodynamic instability, uncontrolled arrhythmias and refractory hypoxemia.
Bronchoalveolar lavage procedure
Bronchoalveolar lavage is typically performed after introduction of the bronchoscope into the tracheobronchial tree and inspection of the airways but before any biopsies or brushings are collected 1. This minimizes the potential introduction of bronchial wall debris and additional red blood cells into the most distal airways, which could alter the composition of the lavage fluid. The outer diameter of adult flexible bronchoscope tips range between approximately 3 mm-6 mm, and the scope is guided into the subsegment of the lung that is to undergo bronchoalveolar lavage and advanced until the tip is wedged into a bronchiole.
Depending on local practice, anywhere from between 20 – 60 ml of room temperature, sterile normal saline is injected via handheld syringe and then gradually withdrawn back into the syringe. This is repeated 3-5 times, and a total of up to 300 ml is instilled. If only 5% of each aliquot injected returns – indicating that most of the injected fluid is being retained, the procedure should be aborted. A return sample yield of 30% or more of the instillate is considered an adequate return, of which at least 10 ml – 20 ml is required for cellular and infectious workup.
If a localized disease process predominates, usually identified on radiological imaging (e.g., hence a Chest X-ray or Computed Tomography (CT) of the Chest), then the bronchoscope is guided to that specific region and wedged into the subsegment with visualization of the distal airway ideally in the center of the image. If diffuse, (the heterogeneous disease is present on imaging), the most commonly preferred locations for lavage would be the right middle lobe or lingula. As bronchoscopy is usually performed with the patient lying supine, the anteriorly projected location of these segments allows gravity to assist with maximal bronchoalveolar lavage. If these lung segments are not accessible, the superior or anterior segment of either lower lobe may also be used. In theory, this process enables lavage of up to 1 million alveoli 5.
Bronchoalveolar lavage results
Even though the collection of bronchoalveolar lavage samples is not standardized, experts suggest following a protocol to minimize contamination and artifacts. The recovered lavage fluid should be pooled, mixed, and the volume recorded. The fluid should be sent to the laboratory while stored on ice if the transportation is going to be delayed by more than 1 hour. Studies show that cells in bronchoalveolar lavage fluid can remain viable for 4 hours at room temperature. The fluid is usually analyzed for white and red cells. To distinguish immature macrophages from large lymphocytes, esterase staining is employed. Other stains may evaluate for the presence of iron, malignant cells, inorganic dust, and microorganism. In general, if the bronchoalveolar lavage specimen contain less than 2 million total cells or has fewer than ten alveolar macrophages per high-power field or contains a high number of red cells (due to trauma) or there are degenerative changes, the specimen should not be relied upon to make any valid diagnosis 1. Of note, the average number of cells recovered from bronchoalveolar lavage fluid in healthy non-smoking adults varies from 100-150,000/ml. Smoking increases the number of cells by 4-6 fold, of which most are macrophages 6. The macrophage count obtained by bronchoalveolar lavage is found to be four to six times greater in smokers than nonsmokers 7. Apart from this, alveolar macrophages are morphologically different and contain a higher amount of harmful pigment and free radicals in smokers than in non-smokers 8.
Bronchoalveolar lavage fluid
The American Thoracic Society 4 and the European Respiratory Society 9 published practical guidelines on bronchoalveolar lavage including normal values for bronchoalveolar lavage differential cell count leading to standardization of bronchoalveolar lavage procedure and interpretation. Normal values were specified based on these studies with alveolar macrophages > 85% of total cell count, lymphocytes 10–15%, neutrophils < 3% and eosinophils < 1% 4 based on different studies and a meta‐analysis investigating bronchoalveolar lavage cellular composition in healthy individuals 10. Procedural differences in bronchoalveolar lavage within these studies limit however the comparability of results. Furthermore, the overall impact of these normal values to discriminate between healthy and diseased individuals has only been addressed for specific diseases (e.g. hypersensitivity pneumonitis, systemic scleroderma associated interstitial lung disease) 11. Their importance for assessing individuals without presumptive diagnosis has never been investigated. The paucity of good evidence to support specific values for a normal bronchoalveolar lavage, leaves doctors to pay attention to the specific cell line cut offs that certain support diagnoses such as:
- Increased Neutrophils (>5%)
- Idiopathic Pulmonary Fibrosis, Acute Respiratory Distress Syndrome (ARDS), infection, connective tissue disorders.
- Eosinophilia
- >25% eosinophils: suggesting eosinophilic lung diseases such as acute eosinophilic pneumonia, chronic eosinophilic pneumonia, and Churg-Strauss syndrome
- Lymphocytosis
- > 50% lymphocytes: Hypersensitivity Pneumonitis
- When greater than or equal to 15% Lymphocytes are present, CD4/CD8 ratios can be assessed.
- Elevated CD4/CD8: hypersensitivity pneumonitis (chronic or smoker), sarcoidosis, berylliosis, asbestosis, Crohn disease, connective tissue disorders
- Normal CD4/CD8: Tuberculosis, malignancies
- Low CD4/CD8: acute hypersensitivity pneumonitis, silicosis, drug-induced lung disease, HIV infection, bronchiolitis obliterans organizing pneumonia (BOOP)
Progressively darkening aliquots of bloody bronchoalveolar lavage fluid is highly suggestive of diffuse alveolar hemorrhage (DAH). Hemosiderin-laden macrophages can also be evident on bronchoalveolar lavage analysis.
If chronic berylliosis is suspected, a positive lymphocyte proliferation test will be positive.
Bronchoalveolar lavage complications
The most common risks of bronchoalveolar lavage procedure are similar to those seen in flexible bronchoscopy. They include transient hypoxemia, post-bronchoalveolar lavage fever (seen in up to 30% of patients), bronchospasm, and very rarely pneumothorax. Given the low incidence, routine chest x-ray after the procedure is not performed unless clinically indicated.
References- Patel PH, Antoine M, Ullah S. Bronchoalveolar Lavage. [Updated 2020 Apr 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430762
- Gibelin A, Parrot A, Fartoukh M, de Prost N. Rare respiratory diseases in the ICU: when to suspect them and specific approaches. Curr Opin Crit Care. 2019 Feb;25(1):29-36.
- Salzer HJF, Schäfer G, Hoenigl M, Günther G, Hoffmann C, Kalsdorf B, Alanio A, Lange C. Clinical, Diagnostic, and Treatment Disparities between HIV-Infected and Non-HIV-Infected Immunocompromised Patients with Pneumocystis jirovecii Pneumonia. Respiration. 2018;96(1):52-65.
- Meyer KC, Raghu G, Baughman RP et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012; 185: 1004–14.
- Pennington K, Wilson J, Limper AH, Escalante P. Positive Pneumocystis jirovecii Sputum PCR Results with Negative Bronchoscopic PCR Results in Suspected Pneumocystis Pneumonia. Can. Respir. J. 2018;2018:6283935
- Gharsalli H, Mlika M, Sahnoun I, Maalej S, Douik El Gharbi L, Mezni FE. The utility of bronchoalveolar lavage in the evaluation of interstitial lung diseases: A clinicopathological perspective. Semin Diagn Pathol. 2018 Sep;35(5):280-287.
- Naeem A, Rai SN, Pierre L. Histology, Alveolar Macrophages. [Updated 2020 Jun 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513313
- Kunz LI, Lapperre TS, Snoeck-Stroband JB, Budulac SE, Timens W, van Wijngaarden S, Schrumpf JA, Rabe KF, Postma DS, Sterk PJ, Hiemstra PS., Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study Group. Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir. Res. 2011 Mar 22;12:34.
- Report of the European Society of Pneumology Task Group. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Eur Respir J 1989; 2: 561–85.
- Balbi B, Pignatti P, Corradi M et al. Bronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: values in healthy adults. Eur Respir J 2007; 30: 769–81.
- Welker L, Jorres RA, Costabel U, Magnussen H. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. Eur Respir J 2004; 24: 1000–6.