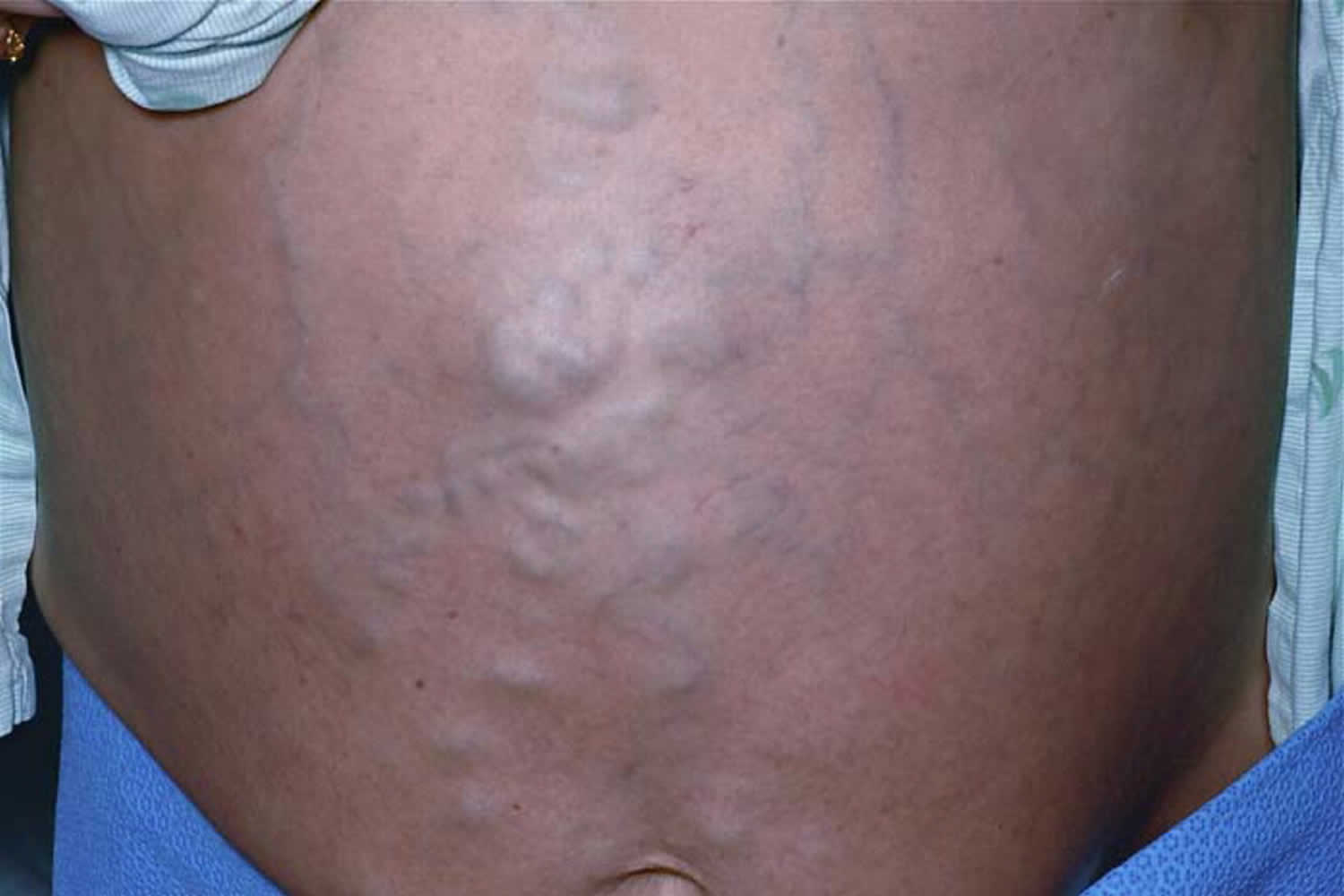
Caput medusae
Caput medusae describes the appearance of distended and engorged paraumbilical veins, which are seen radiating from the umbilicus across the abdomen to join the systemic veins 1. Caput medusae is one of the cardinal features of portal hypertension 2. Caput medusae is due to cutanous portosystemic collateral formation between distended and engorged paraumbilical veins that radiate from the umbilicus across the abdomen to join systemic veins. Blood from the portal venous system is shunted through the umbilical veins into the abdominal wall veins, which manifest as the caput medusae. No specific treatment is required for this condition. It is rarely encountered in clinical practice nowadays due to earlier diagnosis and treatment of portal hypertension.
Portal hypertension is an increase in pressure in the portal vein and its tributaries 3. Portal hypertension is defined as a portal pressure gradient (the difference in pressure between the portal vein and the hepatic veins) greater than 5 mm Hg. Although this gradient defines portal hypertension, a gradient of 10 mm Hg or greater defines clinically significant portal hypertension, because this pressure gradient predicts the development of varices 4, decompensation of cirrhosis 5 and hepatocellular carcinoma 6. The most direct consequence of portal hypertension is the development of gastroesophageal varices that may rupture and lead to the development of variceal hemorrhage.
Caput medusae sign also refers to developmental venous anomalies of the brain, where a number of veins drain centrally towards a single drain vein 7. Caput medusae sign is seen on both CT and MRI when contrast is administered. Angiographically the caput medusae appearance is seen only in the venous phase.
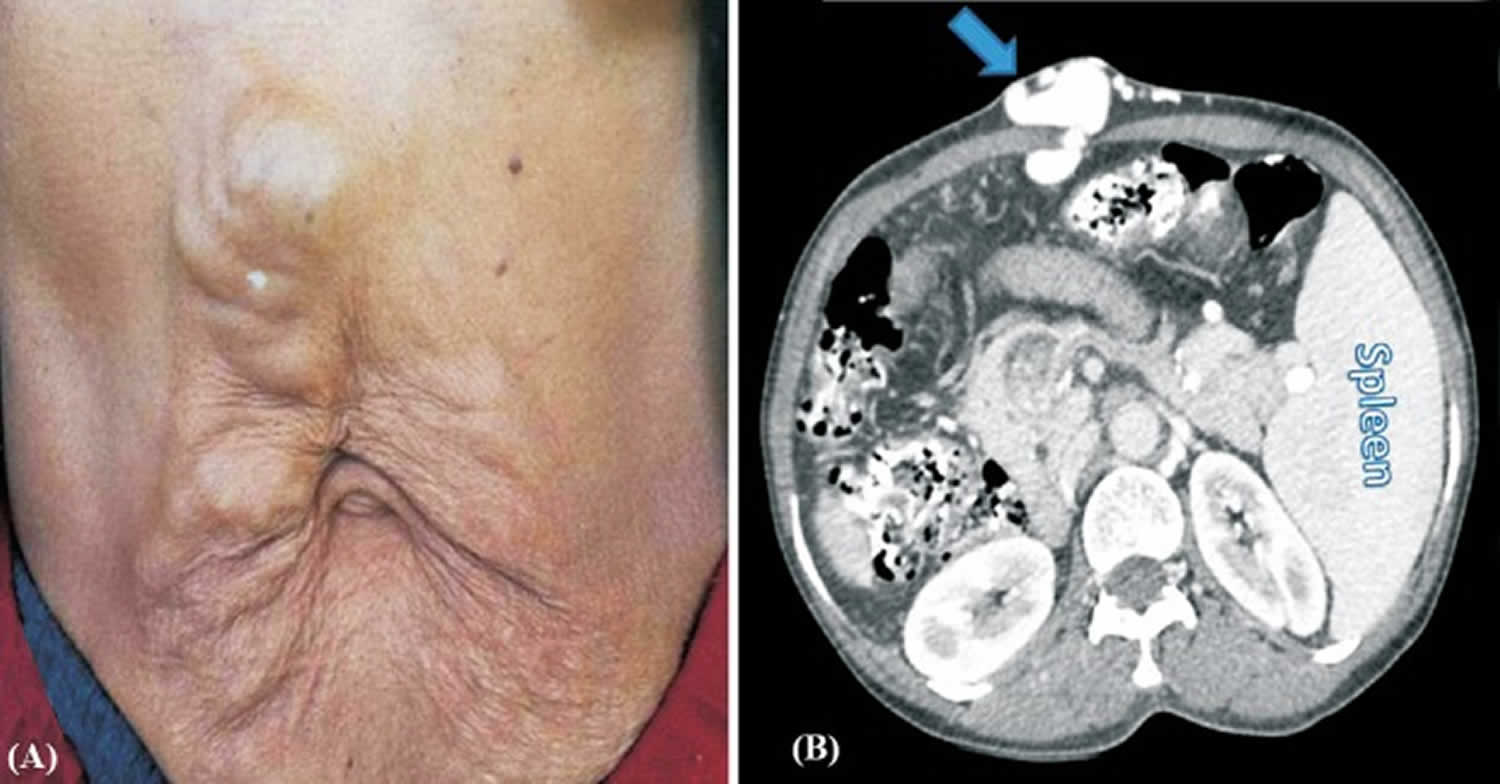
Figure 1. Periumbilical caput medusae (large caput medusae over anterior abdomen wall)
Footnote:Large caput medusae over anterior abdomen wall. (B). Axial computed tomography (CT) abdomen showing subcutaneous collateral veins on the anterior abdominal wall communicating with umbilical vein opacified by radiocontrast (arrow). Also seen is enlarged spleen on left side.
[Source 2 ]Portal hypertension pathophysiology
Anatomically, the portal vein is formed by the union of the superior mesenteric vein and the splenic vein. The mesenteric vein collects blood from the splanchnic circulation. Thus, portal venous inflow is determined by the state of constriction or dilatation of splanchnic arterioles.
The initial mechanism in the genesis of portal hypertension is an increase in vascular resistance that can occur at any level within the portal venous system. Portal hypertension is therefore classified as prehepatic (portal or splenic vein thrombosis); intrahepatic (cirrhosis), and posthepatic (Budd-Chiari syndrome). The most common cause of portal hypertension is cirrhosis. In cirrhosis, the increased resistance is mostly caused by hepatic architectural distortion (fibrosis and regenerative nodules) but about a third of the increased resistance is caused by intrahepatic vasoconstriction, amenable to vasodilators 8. This is caused by the activation of stellate cells with active contraction of myofibroblasts and vascular smooth muscle cells in portal venules 9, which in turn is caused by increased endogenous vasoconstrictors, such as endothelin, and reduced nitric oxide bioavailability 10.
Portosystemic collaterals develop as a consequence of the high pressure in the portal vein and ameliorate the increased resistance. However, even when portal blood flow is entirely diverted through collaterals, portal hypertension persists because of a concomitant increase in portal venous inflow, which in turn is caused by splanchnic vasodilatation,9 mostly mediated by an increase in nitric oxide 11.
The most important collaterals are those that constitute gastroesophageal varices. Although the formation of collaterals had been assumed to be the result of dilatation of preexisting vascular channels, recent studies have implicated a process of neoangiogenesis. This process has been shown to contribute not only to portal-systemic collaterals but also to increased splanchnic blood flow (arteriolar-capillary network) 12.
Caput medusae treatment
No specific treatment is required for caput medusae. It is rarely encountered in clinical practice nowadays due to earlier diagnosis and treatment of portal hypertension.
Portal hypertension
Treatment is directed at the cause of portal hypertension. Gastroesophageal variceal hemorrhage is the most dramatic and lethal complication of portal hypertension. Medical care includes emergent treatment, primary and secondary prophylaxis, and surgical intervention.
Pharmacologic therapy for portal hypertension includes the use of beta-blockers, most commonly propranolol and nadolol. Brazilian investigators have suggested that the use of some statins (eg, simvastatin) may lower portal pressure and potentially improve the liver function 13. In a 3-month prospective, triple-blind randomized trial with simvastatin 40 mg/day and placebo in 34 patients with cirrhotic portal hypertension, 55% of those who received simvastatin showed a clinically relevant decrease in hepatic venous pressure gradient compared to none in the placebo group. Moreover, simvastatin response rates were greater in those with medium to large esophageal varices and previous variceal bleeding 13.
Endoscopic procedures such as sclerotherapy and variceal ligation can be used to prevent the recurrence of variceal hemorrhage. Surgical care includes the use of decompressive shunts, devascularization procedures, and liver transplantation. Decompressive shunts and devascularization procedures are mainly rescue therapies.
Management of patients with liver cirrhosis and ascites but without variceal hemorrhage includes a low-sodium diet and diuretics.
Nasogastric tube
In patients with hemodynamically significant upper gastrointestinal (GI) tract bleeding, a nasogastric tube should remain in place for 24 hours to assist in identifying any rebleeding. Gastric lavage may be performed frequently through the nasogastric tube, and the volume and appearance of material aspirated from the stomach should be recorded. Do not allow any food by mouth.
Control of bleeding
Bleeding from esophageal varices is a medical emergency. Drugs such as vasopressin or octreotide may be given intravenously to make the bleeding veins contract and thus slow the bleeding. Blood transfusions are given to replace lost blood. Doctors usually use a flexible viewing tube (endoscope), inserted through the mouth into the esophagus to confirm that the bleeding is from varices. Working through the endoscope, doctors can use rubber bands to tie off the veins.
To reduce the risk of bleeding from esophageal varices, doctors may try to reduce pressure in the portal vein. One way is to give drugs such as timolol, propranolol, nadolol, or carvedilol.
Doctors regularly monitor people who have had bleeding from varices because bleeding may recur.
Portosystemic shunting
If the bleeding continues or recurs repeatedly, a procedure called portosystemic shunting may be done to connect the portal vein or one of its branches to a vein in the general circulation. This procedure reroutes most of the blood that normally goes to the liver so that it bypasses the liver. This bypass (called a shunt) lowers pressure in the portal vein because pressure is much lower in the general circulation.
There are various types of portosystemic shunt procedures. In one type, called transjugular intrahepatic portosystemic shunting (TIPS), doctors, using x-rays for guidance, insert a catheter with a needle into a vein in the neck and thread it to veins in the liver. The catheter is used to create a passage (shunt) that connects the portal vein (or one of its branches) directly with one of the hepatic veins. Less commonly, portosystemic shunts are created surgically.
Shunt procedures are usually successful in stopping the bleeding but pose certain risks, particularly hepatic encephalopathy. The procedure may have to be repeated because the shunt may become blocked.
Liver transplantation
Some people require liver transplantation.
References- Varices in portal hypertension: evaluation with CT. Radiographics. 1995 May;15(3):609-22. https://pubs.rsna.org/doi/pdf/10.1148/radiographics.15.3.7624566
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141(4):494. doi:10.4103/0971-5916.159322 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4510739
- Miñano C, Garcia-Tsao G. Clinical pharmacology of portal hypertension. Gastroenterol Clin North Am. 2010;39(3):681–695. doi:10.1016/j.gtc.2010.08.015 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000670
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–61.
- Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–8.
- Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923–8.
- The caput medusae sign. Radiology. 1998 Jun;207(3):599-600. https://doi.org/10.1148/radiology.207.3.9609879
- Bhathal PS, Grossman HJ. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J Hepatol. 1985;1:325–37.
- Pinzani M, Gentilini P. Biology of hepatic stellate cells and their possible relevance in the pathogenesis of portal hypertension in cirrhosis. Semin Liver Dis. 1999;19:397–410.
- Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927–34.
- Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478–91.
- Fernandez M, Vizzutti F, Garcia-Pagan JC, et al. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–94.
- Pollo-Flores P, Soldan M, Santos UC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015 Nov. 47(11):957-63.