Cartilage-hair hypoplasia
Cartilage-hair hypoplasia also known as McKusick’s metaphyseal chondrodysplasia syndrome, is a rare autosomal recessive chondrodysplasia, with combined immunodeficiency, short stature, hair hypoplasia, anemia, increased risk of malignancies, and Hirschsprung disease 1. Cartilage-hair hypoplasia is a disorder of bone growth characterized by short stature (dwarfism) with other skeletal abnormalities; fine, sparse hair (hypotrichosis); and abnormal immune system function (immune deficiency) that can lead to recurrent infections.
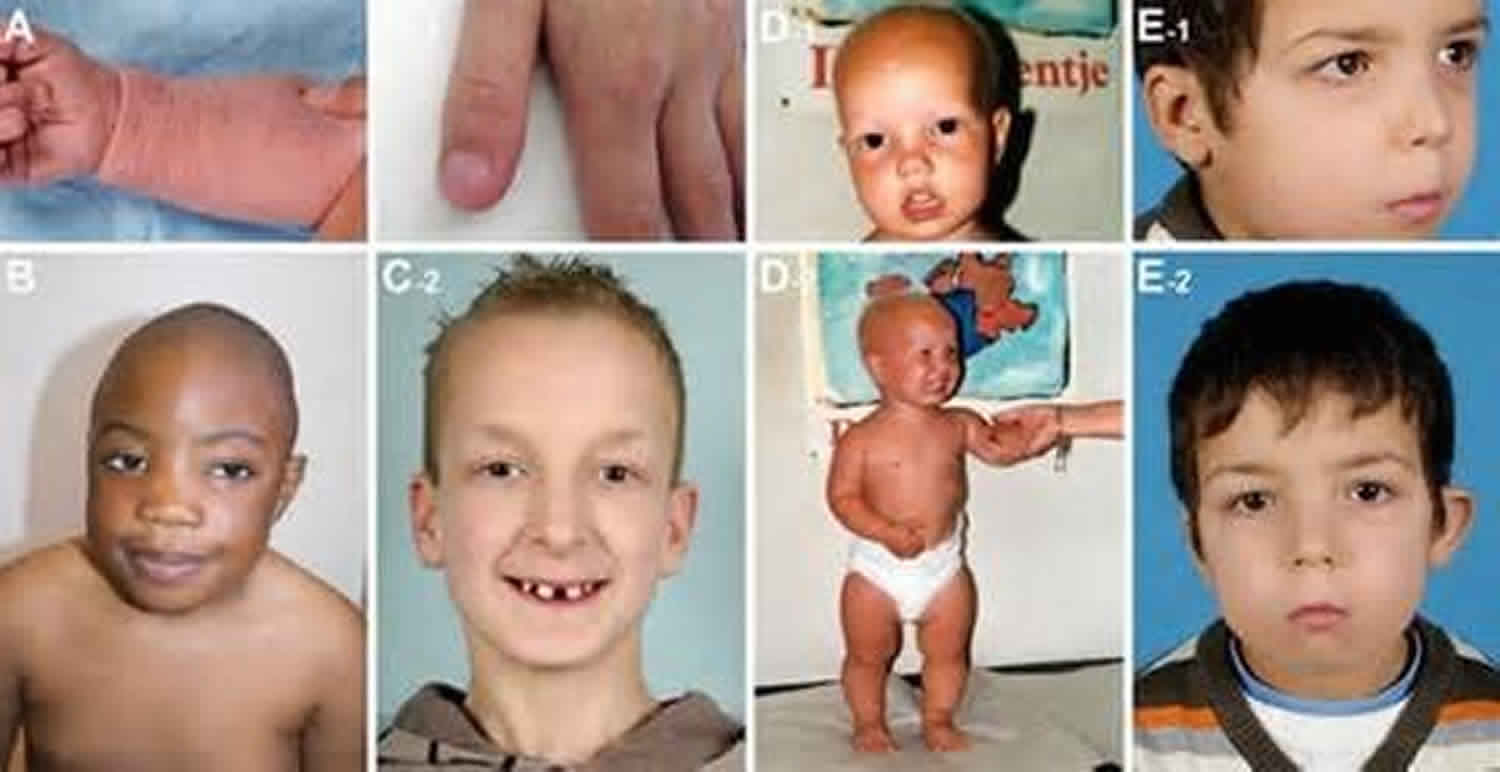
People with cartilage-hair hypoplasia have unusually short limbs and short stature from birth. They typically have malformations in the cartilage near the ends of the long bones in the arms and legs (metaphyseal chondrodysplasia), which then affects development of the bone itself. Most people with cartilage-hair hypoplasia are unusually flexible in some joints, but they may have difficulty extending their elbows fully.
Affected individuals have hair that is lighter in color than that of other family members because the core of each hair, which contains some of the pigment that contributes the hair’s color, is missing. The missing core also makes each strand of hair thinner, causing the hair to have a sparse appearance overall. Unusually light-colored skin (hypopigmentation), malformed nails, and dental abnormalities may also be seen in this disorder.
The extent of the immune deficiency in cartilage-hair hypoplasia varies from mild to severe. Affected individuals with the most severe immune problems are considered to have severe combined immunodeficiency (SCID). People with severe combined immunodeficiency lack virtually all immune protection from bacteria, viruses, and fungi and are prone to repeated and persistent infections that can be very serious or life-threatening. These infections are often caused by “opportunistic” organisms that ordinarily do not cause illness in people with a normal immune system. Most people with cartilage-hair hypoplasia, even those who have milder immune deficiency, experience infections of the respiratory system, ears, and sinuses. In particular, the chicken pox virus (varicella) often causes dangerous infections in people with this disorder. Autoimmune disorders, which occur when the immune system malfunctions and attacks the body’s tissues and organs, occur in some people with cartilage-hair hypoplasia. Affected individuals are also at an increased risk of developing cancer, particularly certain skin cancers (basal cell carcinomas), cancer of blood-forming cells (leukemia), and cancer of immune system cells (non-Hodgkin lymphoma) 2.
Increased mortality in cartilage hair hypoplasia relates to infections in childhood and malignancies in young adulthood 3. In addition, lung disease related to bronchiectasis is an important contributor to morbidity 4.
Some people with cartilage-hair hypoplasia experience gastrointestinal problems. These problems may include an inability to properly absorb nutrients or intolerance of a protein called gluten found in wheat and other grains (celiac disease). Affected individuals may have Hirschsprung disease, an intestinal disorder that causes severe constipation, intestinal blockage, and enlargement of the colon. Narrowing of the anus (anal stenosis) or blockage of the esophagus (esophageal atresia) may also occur.
Cartilage-hair hypoplasia occurs most often in the Old Order Amish population, where it affects about 1 in 1,300 newborns 5. In people of Finnish descent, its incidence is approximately 1 in 20,000 6. Outside of these populations, the condition is rare, and its specific incidence is not known. It has been reported in individuals of European and Japanese descent.
Cartilage hair hypoplasia causes
Cartilage-hair hypoplasia is caused by mutations in the RMRP gene located on chromosome 9p13.3. Unlike many genes, the RMRP gene does not contain instructions for making a protein. Instead, a molecule called a noncoding RNA, a chemical cousin of DNA, is produced from the RMRP gene. This RNA attaches (binds) to several proteins, forming an enzyme complex called mitochondrial RNA-processing endoribonuclease, or RNase MRP.
The RNase MRP enzyme is thought to be involved in several important processes in the cell. For example, it likely helps copy (replicate) the DNA found in the energy-producing centers of cells (mitochondria). The RNase MRP enzyme probably also processes ribosomal RNA, which is required for assembling protein building blocks (amino acids) into functioning proteins. In addition, this enzyme helps control the cell cycle, which is the cell’s way of replicating itself in an organized, step-by-step fashion.
Mutations in the RMRP gene likely result in the production of a noncoding RNA that is unstable. This unstable molecule cannot bind to some of the proteins needed to make the RNase MRP enzyme complex. These changes are believed to affect the activity of the enzyme, which interferes with its important functions within cells. Disruption of the RNase MRP enzyme complex causes the signs and symptoms of cartilage-hair hypoplasia.
Cartilage hair hypoplasia inheritance pattern
Cartilage hair hypoplasia is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Cartilage hair hypoplasia autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Cartilage hair hypoplasia symptoms
The clinical findings in cartilage hair-hypoplasia are outlined below 7. The predominant features include disproportionate short-limbed stature, hair hypoplasia, and immunodeficiency.
The frequencies of reported features are as follows 8:
- Short limbed/short stature – 100%
- Hair hypoplasia – 93%
- Immunodeficiency in 86% of patients
- T cell immunodeficiency, 58% in vitro immunodeficiency
- B cell immunodeficiency, especially specific pneumococcal antibody deficiency
- Recurrent infections – 56% of patients
- Varicella most common severe infection
- Bronchiectasis
- Hypoplastic childhood anemia – 79%
- Gastrointestinal dysfunction – 18% ( Hirschsprung disease – 9%)
- Defective spermatogenesis – 100%
- Metaphyseal chondrodysplasia – 100%
- Risk of malignancies – 6.9% (Non-Hodgkin lymphoma – 90%; basal cell carcinoma – 35%)
Disproportionate short-limbed dwarfism is the most prominent feature in cartilage hair-hypoplasia; it is due to metaphyseal dysplasia. The limbs and ribs are most affected, with sparing of the spine and skull. Radiographic studies reveal short and thick tubular bones, with splaying and irregular metaphyseal borders of the growth plates. The costochondral junctions are similarly affected. These radiographic abnormalities develop by age 6–9 months and are diagnostic. The hair of the scalp, eyebrows and eyelashes is characteristic in cartilage hair hypoplasia; it is fair, thin, sparse, and white or yellow in color, beginning in the newborn period. In addition, the hair is thinner due to decreased central pigment core core. In addition, the hair is characteristic in cartilage hair-hypoplasia; it is fair, thin, and sparse, beginning in the newborn period. Gastrointestinal problems occur in approximately 18% of patients with cartilage hair-hypoplasia. Hirschsprung disease is the most common disorder.
Recently, defective spermatogenesis that affects the number and function of sperm has been identified in all 11 patients with cartilage hair-hypoplasia in one study. Hypoplastic anemia of childhood has been reported in approximately 79% of patients with cartilage hair-hypoplasia and may be life-threatening. It usually resolves by age 2–3 years.
Most individuals with cartilage hair-hypoplasia have limited susceptibility to infections. However, life-threatening varicella infections may occur. Individuals with cartilage hair-hypoplasia occasionally have infections with common pathogens observed in T-cell immunodeficiency, such as Candida species, Pneumocystis carinii, and cytomegalovirus (CMV). In these patients with cartilage hair-hypoplasia, the immunodeficiency may resemble severe combined immunodeficiency (SCID) or Omenn syndrome 9. Omenn syndrome is characterized as severe combined immunodeficiency associated with generalized erythroderma, lymphadenopathy, hepatosplenomegaly, and eosinophilia. Individuals with severe combined T- and B-cell immunodeficiency have more serious infections and are susceptible to graft versus host disease.
In some patients with cartilage hair-hypoplasia, a predominant B-cell immunodeficiency with increased susceptibility to bacterial sinopulmonary infections is reported 10. Toivianen-Salo et al 11 reported that cartilage hair-hypoplasia patients are also at risk of developing bronchiectasis. Autoimmune cytopenia, such as hemolytic anemia and autoimmune neutropenia, and hypothyroidism have also been reported. Moshous et al reported epithelial cell granulomatous lesions in the skin and internal organs of 4 patients with cartilage hair-hypoplasia.
Individuals with cartilage hair-hypoplasia are at increased risk for leukemia and lymphoma. Both Hodgkin lymphoma and non-Hodgkin lymphoma have been reported.
Physical findings
Abnormal physical findings of cartilage hair-hypoplasia are present at birth 12. Head size is within the normal reference range, hands are short and pudgy, and skin forms redundant folds around the neck and extremities.
Physical findings include the following:
- Growth – Short-limb dwarfism, average adult height 107–157 cm (40–60 in)
- Skin – Hypopigmentation
- Nails – Dysplasia
- Hair – Fine, sparse, light-colored hair on the scalp, eyebrows, and eyelashes; body hair similarly affected; hair darkens with age. Hair of the scalp, eyebrows, and eyelashes at birth is light in color, fine, and sparse and lacks a central pigmented core.
- Teeth – Notched incisor, microdontia, doubling of lower premolar lingual cusps
- Limbs – Short hands, brachydactyly, bowleg
- Joints – Hypermobility, hyperflexibility, possible limitation of motion affecting elbow extension
- Spine – Mild platyspondylia, lumbar lordosis
- Thorax – Flaring of lower rib cage, Harrison grooves
- Gastrointestinal -Malabsorption, celiac syndrome, Hirschsprung disease, anal stenosis, esophageal atresia
Cartilage hair hypoplasia diagnosis
Laboratory studies
T-cell immunodeficiency
Immunologic dysfunction occurs in approximately 86% of patients with cartilage hair-hypoplasia 13. Notarangelo et al 9 and Ip et al 14 reported on the heterogeneity of the immunodeficiency. The immunodeficiency predominantly affects T-cell immunity.
Lymphopenia and decreased CD3+, CD4+, and CD8+ T cells present in early infancy. There may be a selective decrease of CD8+ T cells. Skewing of TCRab repertoire may be present.
T cell lymphoproliferative responses to mitogens such as phytohemagglutinin ([PHA], concanavalin A [Con A], and pokeweed mitogen [PWM] and to antigens such as Candidaalbicans and tetanus toxoid may be decreased.
Delayed-type hypersensitivity responses to recall antigens are absent, anergic. de la Fuente et al 15 reported decreased CD4+ CD45RA+ CD31+ –naïve T cells in patients with cartilage hair-hypoplasia. In addition, T-cell receptor rearrangement circles (TRECs) were reduced in cartilage hair-hypoplasia patients, indicating decreased thymopoiesis.
T cells from cartilage hair-hypoplasia patients also demonstrated defects in cell cycle control with reduced cell divisions and increased apoptosis.
Previous studies demonstrated decreased stimulated T-cell interleukin 2 and interferon-γ synthesis and defective CD25 expression. There also appears to be increased T cell apoptosis associated with increased expression of Fas, FAS ligand (FasL), proapoptotic Bax molecules, whereas expression of antiapoptotic bcl-2 and inhibitory of apoptosis molecules are reduced.
B-cell immunodeficiency
Serum immunoglobulin levels and antibody responses to immunizations are usually normal, although a few patients with antibody deficiency have been described. Approximately 35% of patients have abnormal humoral immunity, consisting of immunoglobulin A (IgA) and/or immunoglobulin G2 (IgG2) or immunoglobulin G4 (IgG4) deficiency 16. Although earlier studies reported that antibody responses to protein immunization were normal, data regarding bacterial polysaccharide antigens must be obtained. Occasionally, hypogammaglobulinemia consistent with common variable immunodeficiency has been described. CD27+ IgD-switched B cells are normal 15. The T- and B-cell immune function should be closely monitored, perhaps yearly.
Anemia
Cyclic neutropenia is occasionally associated with cartilage-hair hypoplasia 17. Megaloblastic anemia unrelated to folate and vitamin B-12 deficiency has been reported. The anemia is related to insulinlike growth factor. Fetal hemoglobulin is increased, correlating with the severity of the anemia. Over time, both the anemia and neutropenia appear to decrease in severity. Routine bone marrow examination is unnecessary. Anemia is observed in more than 80% of patients with cartilage-hair hypoplasia. Although usually mild and self-limited, some patients (9%) have severe anemia, which is permanent in one half of these patients 18.
Imaging Studies
Radiography reveals bony scalloping, irregular sclerosis, cystic changes of the widened metaphyses, and metaphysial dysplasia in cartilage-hair hypoplasia 19. Ribs display splaying of the ends at the costochondral junctions, reminiscent of vitamin D deficiency and adenosine deaminase deficiency.
Hirschsprung disease is more common in individuals with cartilage-hair hypoplasia. Appropriate radiographic studies are performed as the clinical symptoms warrant.
Cartilage hair hypoplasia treatment
The treatment of the immunodeficiency depends on whether an isolated T-cell defect, isolated B-cell defect, or a combined T-cell and B-cell immunodeficiency is present. Some patients with cartilage-hair hypoplasia have only a limited susceptibility to infections, thus need no specific treatment 20.
Patients with a severe T-cell immunodeficiency with or without concomitant B-cell immunodeficiency are given the same treatment as patients with severe combined immunodeficiency (SCID).
Thus, T-cell immune reconstitution using bone marrow transplantation (BMT) is performed. Bone marrow transplantation corrects the immunodeficiency but not the skeletal abnormalities 21. Bone marrow transplantation can prevent lymphoma. Bordon et al 22 reported on the outcome of 16 patients with cartilage hair-hypoplasia who received bone marrow transplantation. Thirteen patients were transplanted in early childhood (~2.5 years) and 3 patients were transplanted at adolescent age. Ten patients, 62.5%, were long-term survivors; T-cell numbers and function were normal. Kavadas et al 23 reported an additional 4 patients with cartilage hair-hypoplasia who had severe T-cell immunodeficiency successfully transplanted with matched unrelated donor stem cells during infancy.
Individuals with an isolated T-cell immunodeficiency have an increased susceptibility to infections, and varicella is the most common, severe, life-threatening infection. Acyclovir is recommended in the treatment of varicella infections. In patients exposed to varicella, prophylaxis with varicella-zoster immune globulin (VZIG), acyclovir, or both can be administered. In the United States, varicella-zoster immune globulin was discontinued by the manufacturer. An investigational product (VariZIG) is currently available via investigational new drug protocol. However, prophylaxis with acyclovir in other patients with T-cell impairment who are exposed to varicella may not prevent varicella infection.
The measles mumps rubella (MMR) vaccine may be given in the second year of life in patients with cartilage-hair hypoplasia without severe combined immunodeficiency. Rotavirus vaccine, a live-viral vaccine given in the first year of life, should be avoided.
An attenuated varicella vaccine has been developed as a routine part of childhood immunizations. Some investigators have recommended this vaccine in patients with near-normal T-cell function and normal B-cell function. In this situation, the varicella vaccine may have some protective role in patients with cartilage-hair hypoplasia. However, because it is a live vaccine, it may result in vaccine-related varicella infection. Guidelines for the administration of the vaccine have been established by the Centers for Disease Control and Prevention (CDC) 24.
In patients with cartilage-hair hypoplasia with antibody immunodeficiency and recurrent bacterial infections, antibody replacement therapy in the form of intravenous immunoglobulin (IVIG) or, alternatively, subcutaneous gammaglobulin, therapy is indicated
Treatment of neutropenia with granulocyte colony-stimulating factor (G-CSF) has been successful in patients with cartilage-hair hypoplasia 10. Neutropenia is a common feature in individuals with cartilage-hair hypoplasia, occurring as frequently as 27% in a group of 79 Finnish children. The typical mechanism is maturation arrest, but autoimmune neutropenia also occurs. The severity of the neutropenia correlates with the severity of the immunodeficiency and, therefore, contributes to the increased frequency and severity of infections in patients with cartilage-hair hypoplasia. Ammann et al 10 reported that a 3-year-old Japanese boy with cartilage-hair hypoplasia and autoimmune anti-FcgRIIIb (NA 1/2) neutropenia was treated with G-CSF, which improved the boy’s peripheral neutrophil numbers and reduced recurrent bacterial infections.
Conflicting results have been reported in the use of growth hormone to treat 5 patients with cartilage-hair hypoplasia. In a 3-year-old Japanese boy who was treated with growth hormone for 7 years and underwent a leg-lengthening surgical procedure, the height improved from -4.2 standard deviations (SD) to -2.1 standard deviation 25. In another report of 4 patients with cartilage-hair hypoplasia, growth hormone was used to treat 4 patients, consisting of 2 pairs of siblings: a pair of 10-year-old twins (one boy, one girl) and a 7-year-old girl and her 4-year-old sister 26. The duration of growth hormone therapy was 5 years, 2 years, 5 years, and 6.5 years, respectively. Slight improvement of growth was reported during the first year of growth hormone treatment, varying from 0.2–0.8 SD, but the growth was not sustained, and no gain in final height was reported.
Obara-Moszynska et al 27 reported the use of recombinant human growth hormone in an 8-year-old girl with cartilage hair-hypoplasia. Recombinant growth hormone rhGH therapy was used for 4 years and 7 months and had a significant effect on height from -4.00 to -2.98 height SD score 27.
Various palliative bone reconstruction procedures have been performed in patients with other short-limb dwarfism disorders. Medical and surgical correction for scoliosis may be necessary. Arthroscopy and/or joint replacement surgery may be beneficial. These can also be performed in patients with cartilage-hair hypoplasia. However, the risk of infection in these patients is increased, and extra attention to preventing and treating infections is necessary.
References- Vakkilainen S, Taskinen M, Klemetti P, Pukkala E, Mäkitie O. A 30-Year Prospective Follow-Up Study Reveals Risk Factors for Early Death in Cartilage-Hair Hypoplasia. Front Immunol. 2019;10:1581. Published 2019 Jul 16. doi:10.3389/fimmu.2019.01581 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6646460
- Taskinen M, Ranki A, Pukkala E, Jeskanen L, Kaitila I, Makitie O. Extended follow-up of the Finnish cartilage-hair hypoplasia cohort confirms high incidence of non-Hodgkin lymphoma and basal cell carcinoma. Am J Med Genet A. (2008) 146A:2370–5. 10.1002/ajmg.a.32478
- Makitie O, Pukkala E, Kaitila I. Increased mortality in cartilage-hair hypoplasia. Arch Dis Child. (2001) 84:65–7. 10.1136/adc.84.1.65
- Kostjukovits S, Klemetti P, Fohr A, Kajosaari M, Valta H, Taskinen M, et al. . High prevalence of bronchiectasis in patients with cartilage-hair hypoplasia. J Allergy Clin Immun. (2017) 139:375–8. 10.1016/j.jaci.2016.07.023
- Cartilage-hair hypoplasia. https://ghr.nlm.nih.gov/condition/cartilage-hair-hypoplasia
- Makitie O. Cartilage-hair hypoplasia in Finland: epidemiological and genetic aspects of 107 patients. J Med Genet. (1992) 29:652–5. 10.1136/jmg.29.9.652
- Kostjukovits S, Klemetti P, Valta H, Martelius T, Notarangelo LD, Seppänen M, et al. Analysis of clinical and immunologic phenotype in a large cohort of children and adults with cartilage-hair hypoplasia. J Allergy Clin Immunol. 2017 Aug. 140 (2):612-614.e5.
- Abinun M, Kaitila I, Casanova J-L. Immunodeficiencies with associated manifestations of skin, hair, teeth, and skeleton. Ochs HS, Smith, CIE, Puck JM. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. 2nd ed. New York, NY: Oxford University Press, Inc; 2007. 513-24.
- Notarangelo LD, Roifman CM, Giliani S. Cartilage-hair hypoplasia: molecular basis and heterogeneity of the immunological phenotype. Curr Opin Allergy Clin Immunol. December 2008. 8(6):534-539.
- Ammann RA, Duppenthaler A, Bux J, Aebi C. Granulocyte colony-stimulating factor-responsive chronic neutropenia in cartilage-hair hypoplasia. J Pediatr Hematol Oncol. 2004 Jun. 26(6):379-81.
- Toivianen-Salo S, Kajosaari M, Piilonen A,Mmakitie O. Patients with cartilage hypoplasia have an increased risk for bronchiectasis. J Pediatr. March 2008. 152:422-428.
- Makitie O, Kaitila I. Growth in diastrophic dysplasia. J Pediatr. 1997 Apr. 130(4):641-6.
- Makitie O, Kaitila I, Savilahti E. Susceptibility to infections and in vitro immune function in cartilage-hair hypoplasia. Eur J Pediatr. 1998. 157:816-820.
- Ip W, Gaspar HB, Kleta R, Chanudet E, Bacchella C, Pitts A, et al. Variable phenotype of severe immunodeficiencies associated with RMRP gene mutations. J Clin Immunol. Feb 2015. 35(2):147-157.
- de la Fuente MA, Recher M, Rider NL, Strauss KA, Morton DH, Adair M, et al. Reduced thymic output, cell cycle abnormalities, and increased apoptosis of T lymphocytes in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. July 2011. 128:139-146.
- Makitie O, Kaitila I, Savilahti E. Deficiency of humoral immunity in cartilage-hair hypoplasia. J Pediatr. 2000. 137:487-492.
- Lux SE, Johnston RB Jr, August CS, et al. Chronic neutropenia and abnormal cellular immunity in cartilage-hair hypoplasia. N Engl J Med. 1970 Jan 29. 282(5):231-6.
- Williams MS, Ettinger RS, Hermanns P, et al. The natural history of severe anemia in cartilage-hair hypoplasia. Am J Med Genet A. 2005 Sep 15. 138(1):35-40.
- Glass RB, Tifft CJ. Radiologic changes in infancy in McKusick cartilage hair hypoplasia. Am J Med Genet. 1999 Oct 8. 86(4):312-5.
- Cartilage-Hair Hypoplasia Treatment & Management. https://emedicine.medscape.com/article/885807-treatment
- Guggenheim R, Somech R, Grunebaum E, Atkinson A, Roifman CM. Bone marrow transplantation for cartilage-hair-hypoplasia. Bone Marrow Transplant. 2006 Dec. 38(11):751-6.
- Bordon V, Gennery AR, Slatter MA, Vandecruys E, Laureys G, Veys P, et al. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation. Blood. July 2010. 116(1):27-35.
- Kavadas FD, Giliani S, Gu Y, Mazzolari E, Bates A, Pegoiani E, et al. Variability of clinical and laboratory features among patients with ribonuclease mitochondrial RNA processing endoribonuclease gene mutations. J Allergy Clin Immunol. December 2008. 122(6):1178-1184.
- [Guideline] CDC. Update: recommendations from the Advisory Committee on Immunization Practices (ACIP) regarding administration of combination MMRV vaccine. MMWR Morb Mortal Wkly Rep. 2008 Mar 14. 57(10):258-60.
- Harada D, Yamanaka Y, Ueda K, et al. An effective case of growth hormone treatment on cartilage-hair hypoplasia. Bone. 2005 Feb. 36(2):317-22.
- Bocca G, Weemaes CM, van der Burgt I, Otten BJ. Growth hormone treatment in cartilage-hair hypoplasia: effects on growth and the immune system. J Pediatr Endocrinol Metab. 2004 Jan. 17(1):47-54.
- Obara-Moszynska M, Wielanowska W, Rojek A, Wolnik-Brzozowska D, Niedziela M. Treatment of cartilage-hair hypoplasia with recombinant human growth hormone. Pediatr Int. December 2013. 55(6):e162-164