Centella asiatica
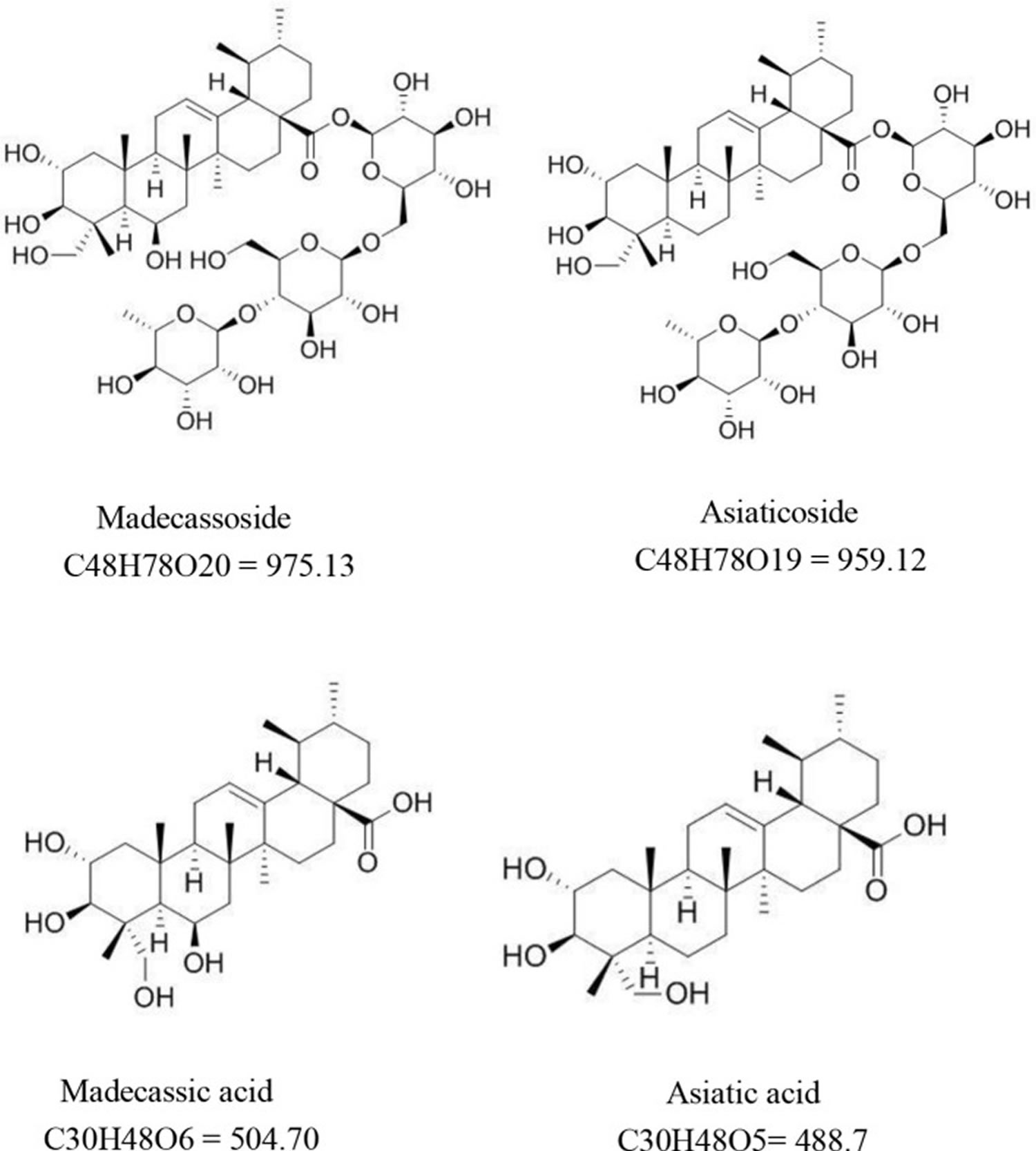
Centella asiatica is also known as Gotu kola, Hydrocotyle erecta, Hydrocotyle asiatica, Indian Pennywort or Asian Pennywort, is a small perennial herbaceous creeper with kidney-shaped leaves that grows in moist, tropical and sub-tropical regions throughout the world such as India, Sri Lanka, Madagascar, South Africa, Australia, China, and Japan 1. Centella asiatica should not be confused with kolanut. Centella asiatica does not contain any caffeine and has not been shown to have stimulant properties. Phytochemicals identified from Centella asiatica to date include isoprenoids (sesquiterpenes, plant sterols, pentacyclic triterpenoids and saponins) and phenylpropanoid derivatives (eugenol derivatives, caffeoylquinic acids, and flavonoids) 2. The main chemical components responsible for Centella asiatica pharmacological activity are triterpenes, mostly asiaticoside, asiatic acid, madecassoside, and madecassic acid (see Figure 1). Madecassoside is a pentacyclic triterpene saponin from Centella asiatica with multiple pharmaceutical activities. Madecassoside has a molecular formula of C48H78O20 and a molecular weight of 975.1 g/mol. It is widely distributed in the heart, liver, spleen, lung, brain, stomach, skin, and kidney through oral dosing, reaching maximum levels within 5–15 min after oral administration 3. Asiaticoside has a molecular formula of C48H78O19 and a molecular weight of 959.1 g/mol. Asiaticoside also reaches maximum levels within 5–15 min after oral administration. Asiaticoside is extensively distributed in the brain, stomach, and skin within 1 hour after dosing 3. Madecassic acid has a molecular formula of C30H48O6 and a molecular weight of 504.7 g/mol. Madecassic acid is a pentacyclic triterpenoid in which ursane is substituted by a carboxy group at position 28 and hydroxy groups at positions 2, 3, 6, and 23 (the 2 alpha, 3 beta, 6 beta stereoisomer). A previous study confirmed that madecassic acid was found in the plasma, brain, heart, liver, kidney, colon, and bladder after oral administration 4. The molecular formula of asiatic acid is C30H48O5, and the degree of solubility in water is 5.98 × 10−2 mg/L at 25°C. Although asiatic acid is mainly absorbed in the jejunum 5, it is also distributed in the plasma, brain, heart, liver, kidney, colon, and bladder 4. Ojha et al. 6 pointed out that for the physicochemical properties of asiatic acid, it is hardly soluble in water, but stable in saline. Moreover, preclinical and clinical pharmacokinetic data demonstrated that asiatic acid could be distributed in many tissues by binding with albumin. Although the bioavailability of asiatic acid is poor, derivatives of asiatic acid showed multiple therapeutic values. Furthermore, chemical modification of the asiatic acid’s backbone improved its bioavailability and biological activity 7. Asiatic acid and madecassic acid are biologically active ingredients of glycosides. Although in tissues and plasma their concentration is low, they can be detected in feces within 48 hour after oral administration of Centella asiatica extract. It suggests that triterpenoid glycosides are mainly metabolized in the intestine 3. In summary, madecassoside, asiaticoside, madecassic acid, and asiatic acid are widely distributed in the body and madecassoside, asiaticoside may exert their biological activity through converted into aglycone (madecassic acid, and asiatic acid).
Centella asiatica has several pharmacological actions, based primarily on animal experiments. After oral and topical Centella asiatica administration in rats, increased cellular hyperplasia and collagen production were noted at the site of injury, measured by increased granulation tissue levels of DNA, protein, total collagen, and hexosamine 8. More rapid maturation and cross-linking of collagen were seen in animals treated with Centella asiatica extract, as evidenced by elevated stability of acid-soluble collagen and increases in aldehyde content and tensile strength. Compared to control wounds, rats treated with Centella asiatica had a higher degree of epithelialization and a significantly more rapid rate of wound contraction 9. In addition to improving wound healing, Centella asiatica may also have an effect on connective tissue of varicosities. After receiving 30 mg total triterpenoid fraction of Centella asiatica asiatica twice daily for three months, individuals with varicose veins had significantly reduced serum enzymes involved in mucopolysaccharide metabolism (beta-glucuronidase, beta-N-acetylglucosaminidase, and arylsulfatase) compared to baseline values 10. The capacity to regenerate axons is an important component of healing following nerve damage. Rats given Centella asiatica extract in their drinking water recovered more quickly after nerve damage than controls, with increased axonal regeneration and more rapid functional recovery 11. The fresh juice extract of Centella asiatica at 200 and 600 mg/kg twice per day has proven to be protective against aspirin- and ethanol-induced gastric ulcers 12, with similar effects as the medication sucralfate 13. Centella asiatica significantly induced gastric mucin secretion and mucosal cell glycoprotein production, markers of increased gastric mucosal defense factors 13.
Clinical studies have found that Centella asiatica effectively improved the cognitive function of stroke patients 14. Patients were divided into 3 groups and administered with 1,000 mg/day, 750 mg/day Centella asiatica extract, and 3 mg/day folic acid, respectively 14. The patients were treated at the acute phase of stroke infarction for 6 weeks. The cognitive function of the patients was evaluated by the MoCA-Ina test. The 1,000 mg/day treatment group scored highest among the three 14. No significant difference was noticed in aspartate aminostransferase (AST) and alanine aminotransferase (ALT) levels when compared with baselines. A randomized, controlled, double-blind clinical trial found that Centella asiatica have the potential to reduce diabetic neuropathy 15. Patients were administered capsules containing Centella asiatica selected triterpenes for 52 weeks, a significant reduction was observed in their total symptom score. However, almost 67% of the patients in the treatment group experienced at least one adverse event, including transient liver, kidney or gastrointestinal dysfunction, but these symptoms resolved on their own 15. Another randomized controlled double-blind trial found that Centella asiatica cream containing 5.12% asiaticoside and 5.1% madecassoside can be completely absorbed by the skin and effectively improve pigmentation and may be used in treating hypertrophic scars 16. In a 4-week study of a group of 25 volunteers, a cosmetic formula containing the Centella asiatica extract was applied on the forearm twice a day, which were prepared into emulsion and hydrogel preparations containing 2.5 and 5% Centella asiatica extract respectively. Revealing that this formula increased the hydration status of skin surface, reduced epidermal water loss, and exerted an anti-inflammatory effect. In addition, the hydration and epidermal barrier function of the subjects in emulsion formulation group was better than that of hydrogel formulation group. Therefore, Centella asiatica can be used in moisturizing cosmetic formulations 17. Clinical studies also confirmed the effect of Centella asiatica against generalized anxiety disorder (GAD), but the level of evidence was low and the number of patients was rather small 18. In addition, a 21-day prospective randomized control study found that Centella asiatica extract can effectively promote wound healing in diabetic patients without any serious side effects 19. Above all, clinical trials have found that Centella asiatica can improve cognitive function, relieve anxiety, promote wound healing, and has effect for skin care, but these effects require more clinical studies with higher levels of evidence need to be performed urgently to validate the findings 20.
A pharmacokinetic study suggests the active ingredients in triterpenoid fraction of Centella asiatica are well absorbed in human volunteers 21. After single oral administration of 30 and 60 mg of Centella asiatica extract, maximum plasma levels of asiatic acid were reached at 4.5 and 4.2 hours, respectively. Plasma half-lives were 2.2 hours in the 30-mg dose and 3.4 hours in the 60-mg dose, with no detectable levels of the saponin present 24 hours after single dosing. Seven-day treatment with the Centella asiatica herb at the same dosing schedule resulted in higher peak plasma concentrations, longer half-lives, and greater area-under-the-curve values 21.
Table 1. Common names for Centella asiatica in different regions or languages of the world
| Region/language | Names | Reference |
| Arabic | Artaniyal-hindi, zarnab. | 22 |
| Chinese | Tungchian, luei gong gen, ji xue cao. | 23 |
| English | Indian Pennywort, Asiatic pennywort, marsh pennywort, pennyweed, sheeprot, ndian water navelwort. | 24 |
| French | Hydrocotyle Asiatique, écuelle d’eau. | 23 |
| German | Indischer wassernabel, Asiatisches Sumpfpfennigkraut. | 23 |
| India (language given in brackets after name) | Brahmi, mandukaparni, cheka parni (Sanskrit); khulakudi, brahma-manduki (Hindi); karivana, karinga, undri (Marathi); thulkuri, brahma-manduki (Bengali); karbrahmi (Gujarati); saraswathi aku, manduka, brahmakuraku (Telegu); vellarai, babassa (Tamil); kutakam (Malayalam); ekpanni (Konkani). | 25 |
| Indonesia | Kaki kuda, pegagan, anantan gede, gagan-gagan, gang-gagan, kirok gatok, panegowan, rending, calingan rambat, kos tekosan, pagaga, tungke-tungke, papaiduh, pepiduh, piduh, puhe beta, kaki kuta, tete karo, tete kadho. | 24 |
| Italian | Idrocotyle. | 24c |
| Japan | Tsubo-kusa, tsubokura. | 22 |
| Malagasy | Talapetraka. | 26 |
| Malaysia | Dawoopungah-gah, pegaga. | 27 |
| Mauritius | Bavilaaqua. | 28 |
| Myanmar | Minkhuabin. | 25 |
| Nepal | Kholachagya. | 22 |
| Persian | Sard Turkistan. | 22 |
| Spanish | Sombrerito, hierba de clavo. | 23 |
| Sri Lanka (Sinhalese) | Gotu kola; hingolukola. | 22 |
| Swedish | Sallatsspikblad. | 23 |
| Thailand | Bau-bog. | Botanica B (2017) Gotu-Kola. |
| Tibetan | Sin-nmar. | 22 |
Figure 1. Centella asiatica bioactive compounds
[Source 20 ]Centella asiatica mechanism of action
Triterpenoids have been identified as active constituents in Centella asiatica. Asiaticoside demonstrates anti-inflammatory effects by inhibiting lipopolysaccharide-induced fever and inflammatory response, including production of serum tumor necrosis factor-alpha, interleukin-6, prostaglandin E2, liver myeloperoxidase activity, and expression of brain cyclooxygenase-2 protein 29. Asiaticoside also promotes wound healing by stimulating collagen and glycosaminoglycan synthesis, and angiogenesis 30. Another study showed that a Centella asiatica extract may regulate stress-induced premature senescence by preventing repression of DNA replication and mitosis-related gene expression 31.
A water extract of Centella asiatica prevented the formation of intracellular beta-amyloid aggregates in a mouse model of Alzheimer’s disease with high amounts of beta-amyloid 32.
Centella asiatica health benefits
Centella asiatica is an herb used in traditional Chinese medicine in China and Southeast Asian countries to treat a variety of diseases. The earliest records of Centella asiatica in China can be retraced back to “su wen shi” and “zheng lei ben cao” in Song Dynasty 20. It is described as follows: “Centella asiatica, bitter, cold, nontoxic. Suitable for use in fever and skin conditions.” Another source “ming jia fang xuan” lists Centella asiatica for use in treating Gilles de la Tourette syndrome in children. A large number of animal and cell experiments have been performed on Centella asiatica and its active components. Centella asiatica contains several pentacyclic triterpenoids, including asiaticoside, brahmoside, and madecassic acid, along with other constituents such as centellose, centelloside, and madecassoside 33. Preclinical studies on Centella asiatica and its triterpenoids reported positive effects on diseases listed in Table 2, including (1) protecting retinal blood vessels, (2) reducing toxic and side effects of drugs, (3) reducing drug resistance, and (4) promoting periodontal tissue regeneration. Centella asiatica could also alleviate oral submucous fibrosis, sepsis, migraine, glaucoma, periodontitis, leukemia, and osteolytic bone diseases.
Moreover, the Centella asiatica extract has a positive significance for leukemia, oral submucous fibrosis, migraine, and toxic side effects. Leukemia is mainly affected by increased activity of oxidative scavengers. In vitro experiments found that the activity of leukemic THP-1 cells treated with Centella asiatica ethanolic leaf extract decreased by 28.404%. Also, the levels of IL-1β and IL-6 decreased, but the level of IL-10 increased, which may reduce the cytokine-induced tumor immunosuppressive activity, cancer progression and cancer cachexia syndrome. Moreover, Centella asiatica can also activate exogenous apoptosis pathways in THP-1 cells, and may reduce the proliferation of THP-1 cells by cutting down the levels of ATP. Therefore, it may be effective in treating leukemia cachexia 34. In addition, Centella asiatica was found to be effective in reversing the hyperalgesia and 5-hydroxytryptophan levels in the brain of migraine animal models. Compared with the positive control (sumatriptan, 42 mg/kg), the oral treatment agent [asiaticoside-based standardized Centella asiatica extract (30 mg/kg, 7 days)] was effective in reducing nociception in rats 35. Furthermore, an in vitro experiment showed that Centella asiatica also downregulated fibrotic markers (TGFb1 ↓, COL1A2 ↓, COL3A1 ↓) to reverse oral mucosal fibrosis caused by arecoline. Therefore, Centella asiatica might have an anti-fibrotic effect 36. Finally, an in vivo experiment found that Centella asiatica at 100 mg/kg bw effectively reduced the side effects of isoniazid in the treatment of pulmonary tuberculosis. This oral dose restored abnormal indicators to normal or even close to normal levels as reflected by liver and kidney functions 37.
Asiatic acid can improve the side effects caused by antibiotics, reverse multidrug resistance (MDR), and reduce sepsis. It also has therapeutic potential for periodontitis and glaucoma. An in vivo experiment showed that asiatic acid had a visible effect on doxorubicin (DXR)-induced organ toxicities, showing the best effect at 20 mg/kg by affecting the expression of Nrf2 38. Doxorubicin causes the peroxidation of organs and reduces the activity of innate antioxidant factors 39 while upregulating the expression of Nrf2 effectively promotes antioxidant activity and protects cells from the damage caused by oxidative stress 40. In vivo and in vitro experiments also found that asiatic acid reduced the levels of inflammatory factors (IL-1β ↓, IL-6 ↓) by affecting the Notch signaling pathway, weakened liver and kidney damage, and improved the survival rate in the experimental sepsis mice model 41. Asiatic acid may also provide therapeutic latent energy for periodontitis through influencing the NF-κB signaling pathway and reducing the levels of related inflammatory factors (IL-8 ↓, IL-6 ↓, p-p65 ↓, p-IκBα ↓) 42. Finally, asiatic acid also has a potential therapeutic effect on glaucoma; it can improve the survival rate of retinal ganglion cells (RGCs) in the glaucoma rat model. More importantly, it can increase the level of anti-apoptotic factors (Bcl-2 ↑) while reducing the level of apoptotic factors (Bax ↓, caspase-3 ↓) 43.
Madecassic acid can ameliorate ischemic retinopathy. A cell experiment showed that madecassic acid reduced apoptosis and endoplasmic reticulum stress for hypoxia-induced human retinal microvascular endothelial cells 44. It also reversed cell dysfunction through affecting the oxidative stress of cells under hypoxic conditions 44.
Interestingly, in vitro experiments found that asiaticoside effectively promoted the osteogenic differentiation of human periodontal ligament and inhibited receptor activator of nuclear factor kappa B ligand (RANKL)-induced formation of osteoclasts, indicating its therapeutic potential for periodontal tissue regeneration and osteolytic diseases 45.
In summary, Centella asiatica and its triterpenoids have broad therapeutic potential. The specific mechanism mainly involves the following four aspects: (1) anti-inflammatory; (2) antioxidant; (3) anti-apoptosis; and (4) anti-fibrosis 20.
Table 2. Centella asiatica effects on disease models
| Disease model | Type | Solvent composition of extract | Animal/cell | signaling pathway | Major findings | References |
| Leukemia | In vitro | C. asiatica ethanolic leaf extract | THP-1 cells | – | Cell viability ↓, TNF-α ↑, IL-1β ↓, IL-6 ↓, IL-10 ↑, GSH ↑, caspase-9, caspase-3/7) ↓, ATP ↓ | 34 |
| Ischemic retinopathies | In vitro | Madecassic acid | hRMECs | PERK/eIF2a | Cell viability ↑, SOD ↑, GSH-PX ↑, LDH ↓, MDA ↓, caspase-3 ↓, caspase-9 ↓, Bax/Bcl-2 ↓, GRP78 ↓, CHOP ↓, ATF6 ↓, IRE1a ↓, PERK ↓, eIF2a ↓ | 44 |
| Doxorubicin (DXR)-induced organ toxicities | In vivo | Asiatic acid | Wistar rats | – | CK-MB ↓, LDH ↓, SGPT ↓, SGOT ↓, BUN ↓, creatinine ↓, SOD ↑, GSH ↑, LPO ↓, Nrf2 ↑ | 38 |
| Drug resistance | In vitro | Asiatic acid | Cisplatin-resistant A549/DDP cells | NF-kB, MAPK/ERK | DDP resistance ↓, DDP sensitivity ↑, MDR1 ↓, p65 ↓, Akt ↓, YB1 ↓, p-ERK1/2 ↓, p-JNK ↓, p-p38 ↓, p-IkBα↓ | 46 |
| Oral submucous fibrosis | In vitro | Ethanolic extract of CA, Asiatic acid | Primary human buccal fibroblasts(HBF) | TGFβ/Smad | TGFβ1 ↓, COL1A2 ↓, COL3A1 ↓, extracellular matrix (ECM) deposition ↓ | 36 |
| – | In vitro | ECa 233 | RAW264.7 macrophages | MAPK | ROS ↓, NO ↓, PGE2 ↓, TNF-α ↓, IL-1β ↓, COX-2 ↓, iNOS ↓ | 47 |
| – | In vitro | Asiatic acid | HepG2 cells | Nrf2 | ROS↓, apoptosis↓, Nrf2↑, Keap1↓, HO-1↑, NQO-1↑, GCLC↑ | 48 |
| Sepsis | In vivo + In vitro | Asiatic acid | BALB/c mice, RAW264.7 cells | Notch | neutrophil infiltration↓, ALT ↓, BUN ↓, IL-1β ↓, IL-6 ↓, NO ↓, Notch3 ↓, Dll4 ↓, ROS ↓, MMP ↓, ATP ↓ | 41 |
| Migraine | In vivo | Asiaticoside (AS)-based standardized extract of Centella asiatica | Wistar rats | – | Hyperalgesia ↓, 5-HT ↑, vocalization ↓, pain latency ↑ | 35 |
| Glaucoma | In vivo | Asiatic acid | Wistar rats, | – | RGCs ↑, Bcl-2 ↑, Bax ↓, caspase-3 ↓ | 43 |
| – | In vitro + in vivo | Methanol extract of CA | Swiss albino male mice | MAPK | Lipid peroxide ↓, superoxide ↓, hydroxyl radicals ↓, RNS ↓, IL-10 ↑, IL-1 ↓, MCP-1 ↓, INF-γ ↓, TNF-β ↓, MAPK 14 ↓ | 49 |
| Periodontitis | In vivo + in vitro | Asiatic acid | Male SD rats, HGFs, RAW264.7 cells | NF-κB | PGE2 ↓, NO ↓, IL-8 ↓, IL-6 ↓, p-p65 ↓, p-IκBα ↓, PPAR-γ ↑ | 42 |
| – | In vitro | Asiaticoside | hPDL cells | Wnt/β-catenin | OSX ↑, WNT3A ↑, AXIN2 ↓, DMP 1↑ | 50 |
| Toxic side effects | In vivo | Ethanolic extraction of CA | Male Wistar albino rats | – | WBC ↓, TBARS ↓, SOD ↑, CAT ↑, GSH ↑, TSP ↑, albumin ↑, ALT ↓, AST ↓, ALP ↓, globulin ↓, total bilirubin ↓, urea ↓, creatinine ↓ | 37 |
| Osteolytic bone diseases | In vitro | Asiaticoside | Bone marrow macrophages (BMMs) | NF‐κB | TRAcP-positive cells ↓, Ctsk ↓, Nfatc1 ↓,V‐ATPase d2 ↓, NFAT ↓, cFos↓, Ca2+ oscillation ↓, IκB‐α ↑, p-ERK ↓ | 45 |
Abbreviations: GRP78 = glucose-regulated protein 78; CHOP = C/EBP homologous transcription factor; ATF6 = activating transcription factor 6; IRE1a = inositolrequiring kinase/endonuclease 1 alpha; PERK = phosphorylation of pancreatic ER stress kinase; eIF2a = eukaryotic initiation factor 2 alpha; CK-MB = Creatine kinase-MB; BUN = blood urea nitrogen; SGPT = serum glutamic pyruvic transaminase; SGOT = serum glutamic-oxaloacetic transaminase; DDP = cisplatin; MDR = multidrug resistance; YB1 = Y-box binding protein 1; COL1A2 = collagen 1A2; COL3A1, = collagen 3A1; NQO-1, NAD(P)H = quinone oxidase; GCLC = glutamyl cysteine ligase catalytic subunit; Dll4 = delta-like ligand; 5-HT = 5-hydroxytryptophan; RGCs = retinal ganglion cells; RNS = reactive nitrogen species; OSX = osterix; ALP, AXIN2 = axis inhibition protein 2;DMP 1 = dentin matrix protein1; TSP = total serum protein; ALP = alkaline phosphatase; TRAcP = tartrate resistant acid phosphatase; Ctsk = cathepsin K; Nfatc1, V‐ATPase d2 = vacuolar‐type H+‐ATPase V0 subunit d2; NFAT = nuclear factor of activated T-cells.
Centella asiatica effects on neurological diseases
Centella asiatica enhances the function of the nervous system 20. It dissolves in methanol, ethanol, and water. Relevant literature on the nervous system demonstrated that Centella asiatica and its triterpenes could be used to relieve a variety of neurological diseases, but the most researched are improve Alzheimer’s disease 51 and Parkinson’s disease 52. The pathogenesis of Alzheimer’s disease and Parkinson’s disease involve neuroinflammatory activities 53, oxidative stress 54, mitochondrial dysfunction 55, and dysfunction in brain-derived neurotrophic factor 56.
Increased production of reactive oxygen species (ROS) can directly affect neuronal synaptic activity and neurotransmission, leading to cognitive dysfunction. Under normal conditions, superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase can act as free radical scavengers, affecting the level of ROS. The activation of nuclear factor erythroid-2-related factor 2 (Nrf2) prevents oxidative stress 57. Previous studies found that Centella asiatica and its triterpenoids could effectively increase SOD and GPX activities, activate nuclear factor erythroid-2-related factor 2, improve the cognitive impairment of animals, and then alleviate the symptoms of related diseases 58.
Mitochondria are the main place where cells carry out aerobic respiration. Mitochondrial dysfunction is closely related to the occurrence of Alzheimer’s disease and Parkinson’s disease. The signaling pathway of cell death can be activated by mitochondrial ROS. Hence, restoring mitochondrial dysfunction can recover neuronal function in Alzheimer’s disease and Parkinson’s disease 59. The results showed that Centella asiatica and its triterpenoids could reduce ROS production 60.
Brain-derived neurotrophic factor (BDNF) is closely related to neuron maintenance, neuron survival, and neurotransmitter regulation. The concentration of brain-derived neurotrophic factor (BDNF) is reduced in the brain of patients with neurodegenerative diseases 61. Centella asiatica extract, asiatic acid, and asiaticoside could effectively increase the content of brain-derived neurotrophic factor (BDNF) 62.
Centella asiatica and its triterpenoids affect neurological diseases possibly through the mitogen-activated protein kinase (MAPK) signaling pathway, phosphotidylinositol 3 kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathway 20. The MAPK signaling pathway is activated by a variety of extracellular stimuli, including growth factors, mitogens, hormones, cytokines, and different cellular stress factors (such as oxidative stress). Also, the p38 MAPK signaling pathway can modulate various events regarding Alzheimer’s disease, such as tau phosphorylation, neurotoxicity, neuroinflammation, and synaptic dysfunction 63. The PI3K/Akt/mTOR pathway is a major intracellular signaling pathway that regulates the cell cycle. It is directly related to cellular quiescence, proliferation, and longevity. An in vivo study found that the inhibition of the PI3K/Akt/mTOR signaling pathway led to a decrease in the expression of c-Jun N-terminal kinase-p53-Bax 3(JNK3), thus protecting dopaminergic neurons and improving Parkinson’s disease 64. Moreover, ROS can mediate the PI3K/Akt/mTOR signaling pathway to exert related effects 65. NF-κB is a protein complex that controls cytokine production, cell survival, and transcription of DNA. This signaling pathway is implicated in the process of many diseases of the brain 66.
In summary, Centella asiatica and its extracts had a positive effect on diseases of the nervous system. More importantly, Centella asiatica and its extracts improved neurological diseases by reducing inflammatory factors, balancing oxidative stress, repairing abnormal expression of mitochondrial-related proteins, and improving the content of BDNF. In addition, they reduced related nerve cell apoptosis, increased synaptic density, and improved the survival rate of neural cells 67.
Anxiety
Centella asiatica is used in Ayurvedic medicine for the treatment of anxiety. A 2000 study supports this ancient claim 68. After assessing baseline measurements of acoustic startle response (ASR), mood self-rating scale, heart rate, and blood pressure, 40 healthy subjects (21 males, 19 females; ages 18-45 years) were randomized to receive 12 g non-standardized Centella asiatica dissolved in 300 mL grape juice or placebo. Evaluations were re-corded at 30, 60, 90, and 120 minutes after beginning therapy. Centella asiatica significantly decreased acoustic startle response amplitude compared to placebo at 30 and 60 minutes; heart rate, blood pressure, and mood did not change 68.
Centella asiatica effects on endocrine diseases
Centella asiatica extracts are promising in treating endocrine diseases, especially type 2 diabetes and obesity. As for specific compounds, asiatic acid was effective in obesity 69 and madecassoside might be a potential candidate for treating osteolytic bone diseases 70.
Type 2 diabetes mellitus is a form of diabetes characterized by high blood glucose, insulin resistance, and a weaker insulin-stimulated response in the presence of high blood glucose level 71. Oxidative stress is mainly caused by lipid peroxidation and has been considered as the main indicator of the pathogenesis and development of type 2 diabetes. Oxidative stress causes microvascular and macrovascular complications 72. In addition, the inflammation response may cause the occurrence of type 2 diabetes by inducing insulin resistance. The inflammation response is exacerbated in the presence of hyperglycemia and can, in turn, worsen hyperglycemia. Hence, targeting the inflammation pathway may be a potential strategy to prevent and control diabetes 73. In 2015, the World Health Organization (WHO) defined the body mass index more than 30 kg/m² as obesity and 25–30 kg/m² as overweight 74. Obesity is a risk factor for many diseases, including cardiovascular disease, musculoskeletal muscle disease, and even cancer 75. Furthermore, obesity causes chronic inflammation of the body and inflammation involving multiple organs (e.g., liver, heart, skeletal muscle, and brain) 76. Osteoporosis is a bone metabolism disease, manifesting itself in the form of bone loss and structure degradation. Main targets are the middle-aged and elderly people over the age of 50. The occurrence of osteoporosis can be linked to other endocrine morbidities, like diabetes, obesity, thyroid hormone disease 77.
Based on the aforementioned pathological mechanisms, the potential mechanism of action of Centella asiatica and its triterpenes on the diseases involving the endocrine system was elaborated from two aspects: reduced oxidative stress and exerted anti-inflammatory effect. First, the Centella asiatica extract seemed to improve the oxidative stress. Both the diabetic animal model and the obesity animal model demonstrated that the Centella asiatica extract increased the GSH, CAT, and SOD activities, thereby improving the enzyme antioxidant system 78. Second, the results of animal experiments showed that the Centella asiatica extract could effectively decrease related inflammation factors (TNF-α, IL-1β, and IL-4). At the same time, it also reduced blood glucose and blood lipid levels 79. Besides, the results showed that the extracts of Centella asiatica lowered food and water intake and body weight, which suggested that Centella asiatica extract may affect obesity by influencing the feeding center controlled by central nervous system 80. Moreover, the potential of asiatic acid as an anti-obesity agent can be proved from the facts that it suppresses weight gain, and enhance the sensitivity of leptin and insulin. At the molecular level, asiatic acid can increase the level of enzymatic antioxidants (CAT, GPx and SOD), reverse the expression of CPT-1 and UCP-2 that are suppressed by high-fat diet. Therefore, it can be deduced that asiatic acid can repair oxidative stress damage caused by obesity, and can also suppress weight gain by promoting fatty acid oxidation 69. The results of madecassoside intervention in a mouse model of osteoporosis, caused by estrogen deficiency and bone marrow monocytes showed that it can inhibit the expression of related genes by affecting the NF-κB and MAPK signaling pathways (NFATc1, c-Fos, Acp5, CTSK, VATPase-d2), inhibits the generation of osteoclasts, weaken the absorption activity of osteoclasts. It can be inferred that madecassoside can be a potential candidate for the treatment of osteoporosis 70.
In summary, available evidence showed that the Centella asiatica extract and asiatic acid could (1) lower blood glucose levels, (2) improve insulin resistance, (3) inhibit weight gain (4) ameliorate inflammation, and (5) improve oxidative stress 20. Besides, madecassoside could improve osteoporosis by weakening the absorption of osteoclasts and reducing osteoclast formation. These results show that the prospects of Centella asiatica extract and related components (asiatic acid, madecassoside) for the treatment of endocrine diseases such as diabetes, obesity and osteoporosis are excellent.
Centella asiatica effects on skin diseases
The Centella asiatica extract and its triterpenoids had certain therapeutic and relieving effects on acne, baldness, vitiligo, atopic dermatitis, and wounds 81. Acne is one of the most common skin disorders. Studies have pointed out that the activation of vascular endothelial cells and the involvement of inflammation responses are essential for the early stages of the development of acne lesions 82. Vitiligo is an acquired depigmenting disorder of the skin, and one of the most common skin diseases. Some studies suggested that oxidative stress may be the initial cause of this disease. The main targets of ROS are mitochondria, causing structural and functional changes 83. Atopic dermatitis, also known as atopic eczema, is a chronic relapsing inflammatory skin condition 84. This disease occurs due to skin barrier dysfunction, alterations in cell-mediated immune responses, and Immunoglobulin E(IgE)-mediated hypersensitivity 85. Skin is the largest organ of the human body playing a vital role in maintaining the body’s physiological homeostasis. The appearance of wounds can lead to an imbalance in physiological homeostasis. The stages of wound healing comprise inflammation, proliferation, epithelialization, angiogenesis, remodeling, and scarring 86.
According to the summary report by 1998 committee for veterinary medicinal products, the transdermal absorption of the active ingredients in Centella asiatica in rats showed that madecassic acid can quickly penetrate the skin barrier, but the dose measured at the drug application point on the skin after 24 hour was only 0.06% concentrated as compared to the original dose. The results of asiatic acid were similar to madecassic acid. The high concentration of asiaticoside administered transdermally did not cause any systemic toxicity, but it could cause excessive keratinization of the skin at the application site. Some reports also suggested allergic dermatitis over using Centella asiatica externally, but none of them reports the exact dose. Centella asiatica and its triterpenoids are made into different formulations to explore the treatment options for skin diseases, and it has been established that they have a potential role in wound healing and skin inflammation.
For wound healing, the present study found that Centella asiatica and its triterpenoids had a direct wound-healing function 87. Centella asiatica extracted with methanol contains 0.12% asiatic acid, 0.54% madecassic acid, 0.25% asiaticoside and 1.02% madecassoside, and was made into a spray with hydroxypropyl-β-cyclodextrin, Eudragit E100, glycerin, PEG 400, etc., and the spray of triterpenes content are close to 100% compared with Centella asiatica extract, the wound was healed completely without any skin irritation 87. Compared with ordinary gauze, the electrospun gelatin membranes containing Centella asiatica can promote the wound repair process by affecting the proliferation of fibroblasts and collagen synthesis, and are antibacterial as well 88. The asiaticoside-rich hydrogel formulation exhibited 40% fast wound healing without any skin irritation as compared to untreated group. Thick epithelial layer and keratin formation can be found, while granulation tissue, fibroblasts and collagen were formed moderately 89. Cells studies have found that Centella asiatica standard extract (ECa 233) can affect the formation of filopodia and promote wound healing by activating the FAK, Akt and MAPK signaling pathways 90. In above studies, though the vehicles were different, but animal and cell experiments have found that Centella asiatica and its triterpenoids improved the degree of re-epithelialization, increased the collagen synthesis, reduced the inflammation around wounds and cause no obvious skin irritation.
For the treatment of atopic dermatitis, Centella asiatica significantly reduced the inflammation response (TNF-α ↓, IL-1β ↓, IL-8 ↓, IL-4 ↓, and IL-13 ↓), and also the local immune response (IgE ↓). Whether titrated extract of Centella asiatica or ethanol extract of Centella asiatica, both seems to inhibit hyperkeratosis, mast cell and inflammatory cell infiltration. Both of them can inhibit the expression of iNOS and COX-2 and NF -κB activity, it confirms that Centella asiatica extract may be a promising therapeutic for the treatment of atopic dermatitis 91. The effect of madecassoside in the treatment of dermatitis is reflected in reducing the pro-inflammatory cytokines (IL-1β, TLR2), moreover, it can promote the secretion of AQP3, LOR, IVL in HaCaT keratinocytes and the secretion of HA in human skin fibroblasts, thus can significantly enhance skin hydration 81.
Madecassoside, a specific component of Centella asiatica, had a certain improvement effect on vitiligo, and the possible mechanism of action was to reduce the oxidative stress response and weaken the damage to mitochondria by oxidative stress [matrix metalloproteinase (MMP) ↑ and [Ca2+]i ↓]. In addition, it was found that the LC3-II/LC3-I ratio of melanocytes treated with madecassoside increased significantly, suggesting that it enhances the autophagy activation of the cells, thereby protecting skin cells from physiological and pathological aging damage 92. Lastly, Centella asiatica also demonstrated a positive activation effect on dermal papilla, improved the viability of dermal papilla cells and increased the expression of characteristic genes related to hair growth in the cells, thus providing good application prospects for baldness 93.
Although Centella asiatica and its triterpenoids have low transdermal absorption rate, current animal experiments and cell experiments have found that they can effectively promote wound healing, reduce skin inflammatory diseases, and seem to have a certain effect on vitiligo and baldness 20. The mechanism of action of Centella asiatica and its ingredients in the treatment of skin diseases is mainly anti-inflammation, anti-oxidation, and weakening of the damage to mitochondria by oxidative stress, which was consistent with the pathogenesis of these diseases 20.
Keloid and scar management
Centella asiatica has long been recommended for the treatment of keloids and/or hypertrophic scars. In one open clinical trial, 227 patients were divided into two groups and treated with oral Centella asiatica alone or surgical scar revision plus Centella asiatica at doses of 60-150 mg daily for up to 18 months 94. In the Centella asiatica-only group, 116 of 139 patients (82%) experienced relief of symptoms and disappearance of inflammation. In the 88 subjects in the combined surgery and Centella asiatica group, 72 percent demonstrated improvement 94. In addition to its oral use, Centella asiatica has been used as a topical cream in a com-prehensive scar management program. Observationally, it was found to improve scar maturity from an average of six months without treatment to three months with treatment 95.
Centella asiatica effects on cardiovascular diseases
Centella asiatica has a positive effect on cardiovascular diseases. The main components that affect the cardiovascular system are asiaticoside and asiatic acid. Hypertension and atherosclerosis are the mostly studied diseases in involved articles 20.
Hypoxic pulmonary hypertension can cause pulmonary arterial changes, including pulmonary arterial stiffness and narrowing. Ventricular changes caused by right ventricular hypertrophy and right ventricular fibrosis affect ventricular function 96. Renovascular hypertension is one of the common causes of secondary hypertension. About 90% of cases are due to atherosclerotic renal artery stenosis and often accompanied by severe occlusive diseases of other blood vessels, accounting for poor prognosis 97. The Ang II/AT1R signaling pathway can regulate a series of intracellular pathways to improve cardiac insufficiency and myocardial remodeling, which is closely associated with the occurrence and development of renal hypertension 98. The transverse aortic constriction contributes to the occurrence of cardiac hypertrophy and failure. The common pathological changes are systolic dysfunction and cardiac fibrosis of the heart. Pressure overload triggers the expression of inflammation genes. Inhibiting early inflammation reactions can reduce cardiac remodeling and improve heart function 99. Fibrosis is a pivotal player in the development and progression of heart failure, which is controlled by the TGF-β/Smads pathway. Smad2 and Smad3 are the two main downstream regulators of TGF-β1-mediated tissue fibrosis, and Smad7 is a negative feedback regulator 100. Cardiovascular diseases can cause a variety of pathological changes and affect the development of related pathology by affecting Ang II/AT1R and TGF-β/Smads signaling pathways. Wang X. B. et al. 101 found that asiaticoside reduced mean pulmonary artery pressure and right ventricular hypertrophy by inhibiting the overexpressed TGF-β1/Smad2/3 signaling pathway in the hypoxia-induced pulmonary hypertension rat model. In 2017, Wang et al. 102 further confirmed that asiaticoside effectively reduced the apoptotic factor (caspase-3), increasing the production of NO by activating the Akt/eNOS pathway. They confirmed that asiaticoside protected pulmonary hypertension by affecting endothelial cell function effect 102. And asiatic acid has anti-hypertensive and anti-inflammatory effects. In animal models of renovascular hypertension, it can play the role of angiotensin-converting enzyme (ACE)by inhibiting the Ang II–AT1R–Nicotinamide adenine dinucleotide phosphate (NADPH) signaling pathway. Moreover, it can reduce the inflammatory response (TNF-α ↓, phospho-NF-κB ↓, IL-6 ↓) 103. A clinical prospective, placebo-controlled, randomized, dose range trial found that after 4 weeks of treatment with Centella asiatica total triterpenes, the capillary filtration rate, ankle circumference and ankle edema of patients with venous hypertension were improved, and the dose range showed that 180 mg/day was most effective in symptoms improvement 104.
Atherosclerosis is a disease in which the inside of arteries narrows due to the buildup of plaque, leading to some serious problems such as heart attack, stroke, or even death. Maintaining arterial integrity and retaining endothelial barrier function and normal contraction of smooth muscle can limit the development of atherosclerotic disease 105. Asiaticoside has been found to reduce endothelial permeability; it can effectively protect the occurrence of atherosclerosis by lowering the levels of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin. Moreover, it can also reduce the levels of related inflammation factor (IL-18) and has anti-inflammation effects 106. A cell experiment found that asiatic acid reduced atherosclerosis by inhibiting the redistribution of occludin and zona occludens -1(ZO-1). Furthermore, it decreased F-actin rearrangement and myosin light chain dephosphorylation 107. Clinical studies have shown that after 4 years of intervention in patients with Pycnogenol® 100 mg/day plus Centella asiatica (100 mg/day), the combined treatment group has reduced plaque progression, reduced oxidative stress, and mild transient brain deficiency as compared to the control group. The incidence of angina events in combined treatment group was less than 3%, while the control group it was 6.25%. Therefore, it can be established that Centella asiatica may have a role in preventing preclinical atherosclerosis 108. For improving the inflammation response, reducing oxidative stress and retaining the endothelial barrier function have beneficial effects on the occurrence and development of atherosclerosis.
In myocardial ischemic disease, apoptosis is the main cause of cardiomyocyte death 109 and asiatic acid could reduce the levels of apoptotic factors (Bax/Bcl-2, caspase-9, caspase-3) and improve cardiomyocyte apoptosis 110. It can also improve the fibrotic changes caused by myocardial dysfunction by affecting the TGF-b1-Smad2/3 signaling pathway. In addition, Rosdah et al. 111 pointed out that after administering Centella asiatica extract to rats at doses 200mg/kg and 400mg/kg for 21 days, the content of acetylcholine (ACh) in heart was modulated significantly, which might contribute to its cardioprotective effect. A summary of the related literature showed that asiatic acid and asiaticoside had beneficial effects on cardiovascular diseases. Basic experiments confirmed that these two triterpenoids effectively improved hypertension, atherosclerosis, and myocardial ischemia.
Venous insufficiency
In a double-blind study 112, 94 patients (86 men, 8 women; ages 20-80) with venous insufficiency of the lower extremities for an average of 14 years, were ran-domized to one of three treatment groups: a triterpenoid extract of Centella asiatica asiatica at a daily dose of 60 or 120 mg or placebo for three months. Individuals who took Centella asiatica at either dose demonstrated significant clinical improvements in limb heaviness, edema and global evaluation of efficacy. Venous distension, measured by plethysmography, was significantly better in the active group at 40 mmHg, 50 mmHg and 60 mmHg, compared to deteriorating placebo values 112.
Venous hypertensive angiopathy
Forty patients (21 males, 19 females; mean age 48 years) with severe venous hypertension, ankle swelling, and lipodermatosclerosis, were randomized to receive triterpenoid fraction of Centella asiatica 60 mg twice daily or placebo for eight weeks; patients in the study did not wear compression stockings 113. After trial conclusion, patients taking the herbal extract experienced a significant decrease in skin flux and rate of ankle swelling compared to baseline values 113. In addition, patients in the active group reported rapid clinical improvement, reflected by a reduction in the analogue scale line score (e.g., symptoms of edema, pain, restless limbs, swelling, and change in skin condition/color) from 9.5 at baseline to 4.5 after eight weeks 113. In another study using Laser doppler evaluation, subjects taking 60 mg triterpenoid fraction of Centella asiatica twice daily for six weeks demonstrated a 29-percent decrease in resting flux, 52-percent increase in venoar-teriolar response and 66-mL reduction in leg volume 114. Similarly, those utilizing the herbal extract demonstrated 7.2-percent increase in pO2 and 9.6-per-cent reduction in pCO2 114.
Airline flight microangiopathy
Physical consequences of long-distance flights range from simple swelling of the lower limbs to the formation of dangerous blood clots. Centella asiatica’s effectiveness was evaluated in 66 flight passengers (33 men, 33 women; mean age 38) traveling in economy class for 3-12 hours 115. Subjects were randomized to receive 60 mg triterpenoid fraction of Centella asiatica three times per day or a placebo two days before, the day of, and two days after the flight. Results showed significant improvements in microcirculatory function (transcutaneous pO2 and pCO2, laser doppler flowmetry, venoarteriolar response, rate of ankle swelling, and edema) in those utilizing triterpenoid fraction of Centella asiatica, with edema and rate of ankle swelling approached nor-mal values in those given triterpenoid fraction of Centella asiatica 115.
Diabetic microangiopathy
Diabetes is characterized by increased skin blood flow and decreased venous return, resulting in blood pooling. Forty-eight patients with diabetic microangiopathy were randomized to one of three treatment groups for six months: 60 mg triterpenoid fraction of Centella asiatica twice daily, placebo, or no treatment 116. Using laser doppler flowmetry measurements, the researchers concluded those taking triterpenoid fraction of Centella asiatica had significant reductions in skin blood flow at rest after three and six months compared to baseline values 116. In addition, VAR scores (decrease of skin blood flow on standing) increased significantly from 6.4 percent to 23.9 percent at three months and 25.9 percent at six months. During the investigation period, pO2 increased while pCO2 values decreased significantly in the Centella asiatica group. Fasting blood sugar and hemoglobin A1C values did not change 116.
Echolucency in carotid and femoral plaques
Carotid artery plaques that are echolucent on ultrasound have greater amounts of certain physiological components (e.g., lipids, blood elements) and limited amounts of collagen, making plaque inherently weaker and increasing the risk of embolization. This unstable plaque is associated with a higher clinical risk of stroke and asymptomatic cerebral lesions 117.
Asymptomatic patients (49 men, 38 women; mean age 56) with high-risk, echolucent carotid artery plaques were randomized to receive 60 mg triterpenoid fraction of Centella asiatica or placebo three times daily for one year; patients also took platelet anti-aggregating medication throughout the trial. After 12 months, sonographic evaluation in-dicated a significant decrease in plaque echolucency in the triterpenoid fraction of Centella asiatica group. Incidence of positive MRI images indicating cerebral ischemic lesions was seven percent in the triterpenoid fraction of Centella asiatica group and 17 percent in the control group 117.
In a second study testing a similar dose of Centella asiatica, patients with femoral plaques demonstrated a decrease in plaque echolucency after 12 months of therapy, compared to no change in the control group. Degree of stenosis and walking distance did not change in the two groups 118.
Centella asiatica effects on digestive diseases
Centella asiatica and its triterpenoids also have therapeutic effects on digestive disorders, which is mainly reflected by improved liver fibrosis, colitis, and gastric mucosal damage; and even reduced Helicobacter pylori gastric colonization 20.
Chronic and recurrent liver injuries are often accompanied by inflammatory reactions and often develop into liver fibrosis. Therefore, treating chronic and uncontrolled inflammation is a strategy to prevent liver injury and fibrosis 119. The pathological mechanism of gastric mucosal injury is complex, and nonsteroidal anti-inflammatory drugs are relatively common causes 120. Prostaglandin biosynthesis is one of the basic components that maintain the integrity of gastric mucosa, and cyclooxygenase is essential in the process of prostaglandin synthesis. The malondialdehyde level can reflect the ROS level 121. Therefore, an oxidative stress response is also crucial in the process of gastric mucosal injury. The present study found that the Centella asiatica extract effectively ameliorated the drug-induced liver toxicity, improved gastric mucosal injury, and reduced H. pylori infection. The mechanisms involved were as follows: the reduction of related inflammation factors (IL−1β ↓, IL−2 ↓, IL−6 ↓, IL−10 ↓, IL−12 ↓, and TNF−α ↓) and the increase in the level of antioxidant stress factors (SOD ↑, CAT ↑, and GPx ↑). Furthermore, evidence showed that Centella asiatica could also reduce malondialdehyde and COX-2 levels, thereby ameliorating gastric mucosal damage 122. The pharmacological effect of asiatic acid is mainly reflected in the improvement in liver fibrosis and acute pancreatitis. It also has a certain therapeutic effect on gastrointestinal tumors. Three mechanisms are reported in the studies: asiatic acid can reduce the level of pro-apoptotic factors (B-cell lymphoma 2(Bcl-2) ↑, Bcl-2-associated X protein(Bax) ↓, caspase-3 ↓), and related inflammation factors (TGF-β1 ↓, TNF-α ↓, IL-6 ↓, IL-1β ↓), and increase the level of anti-oxidative stress factors (SOD ↑, GSH-Px ↑, CAT ↑, GST ↑, GSH ↑) 123. The study on madecassoside found that it could ameliorate drug-induced acute liver failure by reducing inflammation (TNF-α ↓, IL-1β ↓, IL-6 ↓, iNOS ↓, COX-2 ↓) and oxidative stress (SOD ↑, CAT ↑, GPx ↑, Nrf2 ↑, HO-1 ↑) 124. Oral administration of the four components of Centella asiatica could attenuate colitis in mice, but it’s mainly madecassoside acid, the active form of madecassoside, when topically administered in the colon, weakened colitis by regulating Th17/Treg balance via affecting the PPARγ/AMPK/ACC1 pathway 125.
Colon cancer and primary liver cancer are common types of cancers in the digestive system 126. The expression of E-cadherin and vimentin is considered of high reference value in the prognosis of colon cancer. The mitochondrial morphology and the cytosolic calcium level [Ca2+] are indicators of the pathological development of hepatocellular carcinoma 127. Asiatic acid can also affect the expression of epithelial-mesenchymal transition marker proteins in colon cancer cells (E cadherin↑, vimentin ↓, N-cadherin↓), achieved this anti-cancer potential by regulating PI3K/Akt/mTOR/p70S6K signaling pathway. In addition, asiatic acid can induce the dissipation of mitochondrial membrane potential (MMP), ATP depletion, release of cytochrome c from mitochondria into the cytosol of HepG2 cells, which may induce the death of liver cancer cells by directly affecting mitochondrial function, it may be a potential therapeutic drug for liver cancer and colon cancer 128.
Based on the aforementioned evidence, it was concluded that Centella asiatica maybe can improve liver, colon and stomach related digestive disorders by reducing inflammation, ameliorating oxidative stress, and improving mitochondrial function 20.
Centella asiatica effects on respiratory diseases
The effects of Centella asiatica on respiratory diseases is mainly reflected in its ability to improve pulmonary fibrosis, ameliorate chronic obstructive pulmonary disease, and decrease lung injury and certain anti-lung cancer effects 20.
Pulmonary fibrosis can be induced by a variety of injuries to the lung. It is characterized by fibroblast/myofibroblast activation and excessive extracellular matrix accumulation, leading to a progressive organ dysfunction mainly including varying degrees of inflammation and fibrosis 129. By reducing collagen accumulation, pulmonary fibrosis can be effectively improved through TGF-β1 and NLRP3 pathways and decreasing the levels of inflammation factors 130. Chronic obstructive pulmonary disease (COPD) is a frequently progressive inflammatory disease of the respiratory tract, alveoli, and microvasculature. The pathological mechanism of COPD is that airway epithelial cell damage triggers nonspecific inflammatory responses by releasing endogenous intracellular molecules or molecular patterns associated with danger. Impaired immune regulation may play a major role in COPD 131. For the treatment of COPD, it is generally recommended to use appropriate long-acting maintenance bronchodilators and inhaled corticosteroids; pulmonary rehabilitation can also relieve symptoms 132. However, corticosteroid treatment has certain side effects. Lung cancer is the most common cause of cancer-related mortality in the world. Different treatment methods for lung cancer are generally selected according to the stages, such as surgery, radiation therapy, molecular targeted therapy, and immunotherapy 133. Acute lung injury (ALI) is a systemic inflammation of the lungs manifested as hypoxia, edema, and pulmonary infiltrates present in the chest cavity. Acute lung injury is characterized by (1) epithelial and vascular permeability increased, (2) hypercoagulation and insufficient fibrinolysis, and (3) inflammation and immune regulation 134.
This review found that pretreatment with asiatic acid can inhibit bleomycin-induced lung injury and fibrosis in mice 135. It can down-regulate the expression of pro-inflammatory factors, inhibit inflammatory cells infiltration and expression of transforming growth factor-β1. In a mouse model, lung inflammation was induced by exposure to cigarette smoke, oral administration of asiatic acid reduced the excessive production of mucus in lung tissues, inhibited the release of pro-inflammatory factors, and induce the expression of HO-1, which may become a potential drug for the treatment of COPD by regulating key progressions (Lee et al., 2016; Dong et al., 2017). The common possible mechanism was that asiatic acid could reduce the level of related inflammation factors (IL-6 ↓, TNF-a ↓). In addition, asiatic acid can inhibit collagen deposition in lung fibrosis diseases 135. Cell and animal experiments found that asiatic acid could reduce tumor volume, tumor migration, and differentiation. Furthermore, it also has the ability to promote tumor cell apoptosis (caspase-9 ↑, caspase-3 ↑) 136. Therefore, asiatic acid may be a potential therapeutic drug for lung cancer. As for asiaticoside, the review found that it can reduce the inflammatory infiltration caused by lipopolysaccharide in a dose-dependent manner, and inhibit the inflammatory response in lung tissue by inhibiting the NF-κB signaling pathway(TNF-α ↓, IL-6 ↓, p-IκB ↓, p-p65 ↓), which can be an effective preventive agent for acute lung injury 137.
Therefore, the present study showed that the effective components of Centella asiatica on respiratory diseases were asiatic acid and asiaticoside, and the major mechanism was anti-inflammation; this was also consistent with the pathological mechanism of the aforementioned diseases 20. Moreover, it is worth paying close attention to the potential therapeutic effect of Centella asiatica and asiatic acid on lung cancer. Also, the mechanisms of promoting apoptosis and inhibiting differentiation of tumor cells are worthy of further exploration.
Centella asiatica effects on gynecological diseases
Centella asiatica can effectively improve endometriosis and relief pelvic inflammatory disease (PID), as well as exert anti-ovarian cancer and anti-breast cancer functions.
Pelvic inflammatory disease is a microbial infection of the upper reproductive tract. Its major complications are infertility, chronic pelvic pain, rupture of a renal tubular ovarian abscess, and ectopic pregnancy. Western medicine uses antibiotics to effectively control the symptoms 138. However, no conclusive evidence indicated that antibiotic treatment for the pelvic inflammatory disease was safer or more effective than other methods 139. Endometriosis is a common inflammatory disease often accompanied by pelvic pain and infertility. Generally, surgeries are conducted to treat endometriosis, but accumulating evidence suggest the use of plant-based drugs for the treatment, and these medicines usually alleviate the symptoms via their anti-inflammatory, anti-oxidant, anti-proliferation, and anti-apoptotic effects 140. Ovarian cancer and breast cancer are the top two cancers in women 141. Surgery, radiotherapy, chemotherapy, and other treatments are often associated with various complications. Therefore, exploring new therapeutic agents is particularly important.
Asiatic acid can efficaciously treat pelvic inflammation. Asiatic acid has potential therapeutic effects on endometriosis and ovarian cancer 142. The main mechanism is reduction in the production of inflammatory body NLRP3 and inflammatory factors (IL−1 β, IL−6, TNF−α), inhibition of the NF−κB signaling pathway, which regulates the production of inflammatory factors, and thus alleviation of pelvic inflammation. Cell experiments confirmed that asiatic acid can effectively improve the symptoms of endometriosis. The main mechanism is inhibition of the NF-κB pathway to reduce the production of inflammatory factors (TNF-α ↓, IL-1β ↓, p-IκBα ↓, p- p65 ↓) 143. The potential therapeutic value of asiatic acid for ovarian cancer is mainly reflected in that it can promote the apoptosis of ovarian cancer cells and inhibit the growth of ovarian cancer cells by affecting the cell cycle progression 144. In addition, studies found that asiatic acid improved the developmental ability of early embryos in pigs; the main underlying mechanism was amelioration of oxidative stress and downregulation of the expression of apoptosis-related genes 145. The potential therapeutic value of Centella asiatica for breast cancer mainly reflected in the promotion of apoptosis of breast cancer cells 146.
Therefore, this study concluded that the therapeutic effect of Centella asiatica on the gynecological diseases mainly reflected in the improvement in inflammation. The main research component was asiatic acid, which worked by affecting apoptosis, reducing the production of inflammatory factors and influencing the cell cycle progression. Therefore, asiatic acid may be a potential agent in the treatment of gynecological diseases, and further clinical trials are needed to verify its efficacy 20.
Centella asiatica effects on rheumatoid arthritis
Animal and cell experiments confirmed that madecassoside exerted an anti-rheumatoid effect 20. Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that usually affects women and elderly people; the main pathological change is persistent synovitis. If not controlled well, it can lead to joint deformities and other diseases and decrease patients’ quality of life 147. Studies confirmed that TNF-α is a powerful proinflammatory cytokine overexpressed in the synovium of patients with rheumatoid arthritis and reducing TNF-α production can effectively improve the symptoms of rheumatoid arthritis 148. In addition, matrix metalloproteinase (MMP)-13 is a specific protein associated with rheumatoid arthritis and may be involved in the physiological remodeling of synovial tissue 149.
The pharmacological study on madecassoside demonstrated that it can effectively lessen the related inflammatory factors in arthritis model rats (TNF-α ↓, IL-1b ↓, IL-6 ↓, IFN-γ ↓, IL-17 ↓). Animal experiments have confirmed that oral madecassoside (30 mg/kg) can significantly reduce the symptoms of arthritis, and can inhibit the secretion of inflammatory cytokines. However, in vitro experiments have found that madecassoside and madecassic acid, the main metabolite of madecassoside, cannot influence the secretion of inflammatory cytokines. It was subsequently suggested that madecassoside may exhibits anti-arthritis potency through affecting the secretion of IL-10 from Foxp3+ cells in lamina propria of intestine, thus regulates the immune function of rats with collagen-induced arthritis 150. The results of madecassoside pharmacokinetics experiments are poor, but it has significant bioavailability, can effectively reverse adjuvant-induced arthritis, and inhibit the migration and invasion of fibroblast-like synovial cells, however, it has no effect on cell proliferation. Wei-GuangYU et al. pointed out that madecassoside may have anti-arthritis activity by inhibiting the NF-κB/MMP-13 pathway 151.
In summary, madecassoside as a triterpene component of Centella asiatica, may have anti-arthritis effect, and the underlying mechanism is reduction in the level of inflammatory factors.
Centella asiatica summary
Centella asiatica is an evergreen perennial plant that is prevalent in East Asia and many parts of South Africa. Extracts from the Centella asiatica leaf and the whole plant are used for a variety of conditions including venous insufficiency, varicose veins, wound healing, scleroderma, and scars. In vitro and in vivo analyses indicate that Centella asiatica has neuroprotective 152 and chemopreventive properties 153 and also protects against cognitive impairment 154. Other laboratory studies suggest the active constituent madecassoside has antiarthritic 155 and cardioprotective properties 156 and that topical application of an asiaticoside extracted from Centella asiatica may enhance burn wound healing 30. However, there is insufficient evidence to support Centella asiatica use for any of these conditions.
Only a few studies have been conducted in humans. Data show a reduction in lower extremity edema with Centella asiatica compared to placebo in patients with chronic venous insufficiency 157, 158, 159. With respect to wounds, one study suggests an oral Centella asiatica extract may speed healing in diabetic patients 19. In another study, treatment with a topical Centella asiatica ointment improved both objective and subjective symptoms in burn wound patients compared with silver sulfadiazine 160. A few small studies suggest that supplementation with Centella asiatica improved cognitive function and mood in the elderly 161, and alleviated generalized anxiety disorder 18, although a meta-analysis did not find strong enough evidence to support the use of Centella asiatica for cognitive function improvement 162. Larger well-designed studies are needed to confirm these effects.
Centella asiatica dosage
In adults, the recommended daily dose of triterpenoid fraction of Centella asiatica extract or triterpenoid extract of Centella asiatica standardized for asiaticoside, asiatic acid, and madecassic acid is 60-120 mg. The recommended daily dosages of crude Centella asiatica herb and 1:5 tincture are 0.5-6 g and 10-20 mL, respectively 163.
Centella asiatica side effects
As for the safety of Centella asiatica extract, Phanit Songvut et al. 164 in healthy volunteers from Thailand, pointed out that daily oral Centella asiatica doses (single or multiple) of 250 mg and 500 mg are safe for patients. Patients reported just mild to moderate degrees of side effects, such as constipation, skin itching and abdominal distension. Topical use of Centella asiatica extract has led to reports of rash 165. Three cases of jaundice with elevated liver enzymes were reported in Argentina following dosing of Centella asiatica. Patients had taken Centella asiatica (standardization and dose unknown) for 20-60 days, and recovered on discontinuation of the herb 166.
Modern pharmacological tests showed that the interaction potential of Centella asiatica biologically active compounds with CYP isoenzymes is negligible and the heavy metal content in the Centella asiatica extract is within the allowable range 167. Animal experiments have found that Centella asiatica extract has anti-spermogenic and anti-fertility effects on the reproductive system of male rats 168. Hematological parameters and histopathology in acute oral toxicity study, sub-chronic toxicity study and mutagenicity study have confirmed that Centella asiatica extract is safe in rats. Also, Centella asiatica extract did not show any dose-related adverse effects in Ames test 169. However, there are case reports that three women developed jaundice after taking Centella asiatica for 30, 20, and 60 days, they were clinically diagnosed with granulomatous hepatitis, and their symptoms improved after the drug was stopped 170. About the triterpenoids of Centella asiatica, the previous clinical trials have showed that emulsions and capsules, which contain several major triterpenoids have different degrees of side effects for patients, but they all relieve without any medical interventions.
Drug interactions
Cytochrome P450 substrates: In vitro studies suggest Centella asiatica inhibits CYP 1A2, 2C9 171, CYP 2D6, CYP 3A4 172 and CYP 2C19 173 and may affect the intracellular concentration of drugs metabolized by these enzymes. Do not take Centella asiatica if you are taking drugs that are substrates of cytochrome P450 1A2, 2C9, 2D6, 3A4, and 2C19 enzymes. Lab studies suggest that Centella asiatica may increase the risk of side effects of these drugs. Clinical relevance has yet to be determined.
Warnings and contraindications
Centella asiatica should be avoided during pregnancy, due to its emmenagogue (stimulates or increases menstrual flow even when it is not due) action 174.
References- Bhavan BV. Selected Medicinal Plants of India. Bombay, India; Tata Press; 1992.
- Gray, N. E., Alcazar Magana, A., Lak, P., Wright, K. M., Quinn, J., Stevens, J. F., Maier, C. S., & Soumyanath, A. (2018). Centella asiatica – Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochemistry reviews : proceedings of the Phytochemical Society of Europe, 17(1), 161–194. https://doi.org/10.1007/s11101-017-9528-y
- Anukunwithaya T, Tantisira MH, Tantisira B, Khemawoot P. Pharmacokinetics of a Standardized Extract of Centella asiatica ECa 233 in Rats. Planta Med. 2017 May;83(8):710-717. doi: 10.1055/s-0042-122344
- Yin MC, Lin MC, Mong MC, Lin CY. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J Agric Food Chem. 2012 Aug 8;60(31):7697-701. doi: 10.1021/jf302529x
- Yuan Y, Zhang H, Sun F, Sun S, Zhu Z, Chai Y. Biopharmaceutical and pharmacokinetic characterization of asiatic acid in Centella asiatica as determined by a sensitive and robust HPLC-MS method. J Ethnopharmacol. 2015 Apr 2;163:31-8. doi: 10.1016/j.jep.2015.01.006
- Nagoor M. M., Goyal S. N., Suchal K., Sharma C., Patil C. R., Ojha S. K. (2018). Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. [Journal Article; Review]. Front. Pharmacol. 9, 892. 10.3389/fphar.2018.00892
- Lv J, Sharma A, Zhang T, Wu Y, Ding X. Pharmacological Review on Asiatic Acid and Its Derivatives: A Potential Compound. SLAS Technol. 2018 Apr;23(2):111-127. doi: 10.1177/2472630317751840
- Centella asiatica. Alternative Medicine Review Volume 12, Number 1, March 2007. https://altmedrev.com/wp-content/uploads/2019/02/v12-1-69.pdf
- Shetty BS, Udupa SL, Udupa AL, Somayaji SN. Effect of Centella asiatica L (Umbelliferae) on normal and dexamethasone-suppressed wound healing in Wistar Albino rats. Int J Low Extrem Wounds 2006;5:137-143.
- Arpaia MR, Ferrone R, Amitrano M, et al. Effects of Centella asiatica extract on mucopolysaccharide metabolism in subjects with varicose veins. Int J Clin Pharmacol Res 1990;10:229-233.
- Soumyanath A, Zhong YP, Gold SA, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in vitro. J Pharm Pharmacol 2005;57:1221-1229.
- Cheng CL, Koo MW. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci 2000;67:2647-2653.
- Sairam K, Rao CV, Goel RK. Effect of Centella asiatica Linn on physical and chemical factors induced gastric ulceration and secretion in rats. Indian J Exp Biol 2001;39:137-142.
- Farhana K. M., Malueka R. G., Wibowo S., Gofir A. (2016). Effectiveness of Gotu Kola Extract 750 mg and 1000 mg Compared with Folic Acid 3 mg in Improving Vascular Cognitive Impairment after Stroke. Evidence-Based Complement. Altern. Med. 2016, 2795915. 10.1155/2016/2795915
- Lou JS, Dimitrova DM, Murchison C, Arnold GC, Belding H, Seifer N, Le N, Andrea SB, Gray NE, Wright KM, Caruso M, Soumyanath A. Centella asiatica triterpenes for diabetic neuropathy: a randomized, double-blind, placebo-controlled, pilot clinical study. Esper Dermatol. 2018 Jun;20(2 Suppl 1):12-22. doi: 10.23736/S1128-9155.18.00455-7
- Jenwitheesuk K, Rojsanga P, Chowchuen B, Surakunprapha P. A Prospective Randomized, Controlled, Double-Blind Trial of the Efficacy Using Centella Cream for Scar Improvement. Evid Based Complement Alternat Med. 2018 Sep 17;2018:9525624. doi: 10.1155/2018/9525624
- Ratz-Łyko A, Arct J, Pytkowska K. Moisturizing and Antiinflammatory Properties of Cosmetic Formulations Containing Centella asiatica Extract. Indian J Pharm Sci. 2016 Jan-Feb;78(1):27-33. doi: 10.4103/0250-474x.180247
- Jana U, Sur TK, Maity LN, Debnath PK, Bhattacharyya D. A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal Med Coll J. 2010 Mar;12(1):8-11.
- Paocharoen V. The efficacy and side effects of oral Centella asiatica extract for wound healing promotion in diabetic wound patients. J Med Assoc Thai. 2010 Dec;93 Suppl 7:S166-70.
- Sun, B., Wu, L., Wu, Y., Zhang, C., Qin, L., Hayashi, M., Kudo, M., Gao, M., & Liu, T. (2020). Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Frontiers in pharmacology, 11, 568032. https://doi.org/10.3389/fphar.2020.568032
- Grimaldi R, De Ponti F, D’Angelo L, et al. Pharmacokinetics of the total triterpenic fraction of Centella asiatica after single and multiple administrations to healthy volunteers. A new assay for asiatic acid. J Ethnopharmacol 1990;28:235-241.
- Kapoor L (1990) Handbook of Ayurvedic medicinal plants. CRC Press, Boca Raton
- USDA (2016) National Plant Germplasm System, Germplasm Resources Information Network (GRIN database).
- Brinkhaus B, Lindner M, Schuppan D, Hahn EG (2000) Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7: 427–448.
- Nadkarni AK (1976) Dr K.M. Nadkarni’s Indian Materia Medica. Popular Prakashan PVT Ltd
- Rahajanirina V, Rakotondralambo Raoseta SO, Roger E, Razafindrazaka H, Pirotais S, Boucher M, Danthu P (2012) The influence of certain taxonomic and environmental parameters on biomass production and triterpenoid content in the leaves of Centella asiatica (L.) Urb. from Madagascar. Chemistry and Biodiversity 9: 298–308
- Maulidiani H, Abas F, Khatib A, Shaari K, Lajis NH (2014) Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Industrial Crops and Products 55: 238–247
- Brinkhaus B, Lindner M, Schuppan D, Hahn EG (2000) Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 7: 427–448
- Wan J, Gong X, Jiang R, Zhang Z, Zhang L. Antipyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phytother Res. 2013 Aug;27(8):1136-42. doi: 10.1002/ptr.4838
- Kimura Y, Sumiyoshi M, Samukawa K, Satake N, Sakanaka M. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur J Pharmacol. 2008 Apr 28;584(2-3):415-23. doi: 10.1016/j.ejphar.2008.02.036
- Kim YJ, Cha HJ, Nam KH, Yoon Y, Lee H, An S. Centella asiatica extracts modulate hydrogen peroxide-induced senescence in human dermal fibroblasts. Exp Dermatol. 2011 Dec;20(12):998-1003. doi: 10.1111/j.1600-0625.2011.01388.x
- Soumyanath A, Zhong YP, Henson E, Wadsworth T, Bishop J, Gold BG, Quinn JF. Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. Int J Alzheimers Dis. 2012;2012:381974. doi: 10.1155/2012/381974
- Murray M. T., Pizzorno J. E., Jr (2012). Textbook of natural medicine. 4th ed. Eds. Pizzorno J. E., Jr., Michael T. (Edinburgh: Churchill Livingstone; ), 650 ISBN 9781437723335
- Naidoo D. B., Chuturgoon A. A., Phulukdaree A., Guruprasad K. P., Satyamoorthy K., Sewram V. (2017. a). Centella asiatica modulates cancer cachexia associated inflammatory cytokines and cell death in leukaemic THP-1 cells and peripheral blood mononuclear cells (PBMC’s). BMC Complement. Altern. Med. 17 (1), 1–11. 10.1186/s12906-017-1865-2
- Bobade V., Bodhankar S. L., Aswar U., Vishwaraman M., Thakurdesai P. (2015). Prophylactic effects of asiaticoside-based standardized extract of Centella asiatica (L.) Urban leaves on experimental migraine: Involvement of 5HT1A/1B receptors. Chin. J. Natural Medicines 13 (4), 274–282. 10.1016/S1875-5364(15)30014-5
- Adtani P. N., Narasimhan M., Punnoose A. M., Kambalachenu H. R. (2017). Antifibrotic effect of Centella asiatica Linn and asiatic acid on arecoline-induced fibrosis in human buccal fibroblasts. J. Invest. Clin. Dent. 8 (2), 1–9. 10.1111/jicd.12208
- Ghosh K., Indra N., Jagadeesan G. (2017). The ameliorating effect of Centella asiatica ethanolic extract on albino rats treated with isoniazid. J. Basic Clin. Physiol. Pharmacol. 28 (1), 67–77. 10.1515/jbcpp-2016-0059
- Kamble S. M., Patil C. R. (2018). Asiatic Acid Ameliorates Doxorubicin-Induced Cardiac and Hepato-Renal Toxicities with Nrf2 Transcriptional Factor Activation in Rats. Cardiovasc. Toxicol. 18 (2), 131–141. 10.1007/s12012-017-9424-0
- Sonawane V. K., Mahajan U. B., Shinde S. D., Chatterjee S., Chaudhari S. S., Bhangale H. A., et al. (2018). A Chemosensitizer Drug: Disulfiram Prevents Doxorubicin-Induced Cardiac Dysfunction and Oxidative Stress in Rats. Cardiovasc. Toxicol. 18 (5), 459–470. 10.1007/s12012-018-9458-y
- Delgado-Wicke P., Rodríguez-Luna A., Ikeyama Y., Honma Y., Kume T., Gutierrez M., et al. (2020). Fernblock® Upregulates NRF2 Antioxidant Pathway and Protects Keratinocytes from PM2.5-Induced Xenotoxic Stress. Oxid. Med. Cell. Longevity. 2020, 2908108. 10.1155/2020/2908108
- Yuyun X., Xi C., Qing Y., Lin X., Ke R., Bingwei S. (2018). Asiatic acid attenuates lipopolysaccharide-induced injury by suppressing activation of the Notch signaling pathway. Oncotarget 9 (19), 15036–15046. 10.18632/oncotarget.24542
- Hao C., Wu B., Hou Z., Xie Q., Liao T., Wang T., et al. (2017). Asiatic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts. Int. Immunopharmacol. 50, 313–318. 10.1016/j.intimp.2017.07.005
- Huang W., Gao F., Hu F., Huang J., Wang M., Xu P., et al. (2018). Asiatic acid prevents retinal ganglion cell apoptosis in a rat model of glaucoma. Front. Neurosci. 12, 489. 10.3389/fnins.2018.00489
- Yang B., Xu Y., Hu Y., Luo Y., Lu X., Tsui C. K., et al. (2016). Madecassic Acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed. Pharmacother. 84, 845–852. 10.1016/j.biopha.2016.10.015
- He L., Hong G., Zhou L., Zhang J., Fang J., He W., et al. (2019). Asiaticoside, a component of Centella asiatica attenuates RANKL-induced osteoclastogenesis via NFATc1 and NF-κB signaling pathways. J. Cell. Physiol. 234 (4), 4267–4276. 10.1002/jcp.27195
- Cheng Q., Liao M., Hu H., Li H., Wu L. (2018). Asiatic Acid (AA) Sensitizes Multidrug-Resistant Human Lung Adenocarcinoma A549/DDP Cells to Cisplatin (DDP) via Downregulation of P-Glycoprotein (MDR1) and Its Targets. Cell. Physiol. Biochem. 47 (1), 279–292. 10.1159/000489806
- Sukketsiri W., Tanasawet S., Moolsap F., Tantisira M. H., Hutamekalin P., Tipmanee V. (2019). ECa 233 suppresses LPS-induced proinflammatory responses in macrophages via suppressing ERK1/2, p38 MAPK and Akt pathways. Biol. Pharm. Bull. 42 (8), 1358–1365. 10.1248/bpb.b19-00248
- Qi Z., Ci X., Huang J., Liu Q., Yu Q., Zhou J., et al. (2017). Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed. Pharmacother. 88, 252–259. 10.1016/j.biopha.2017.01.067
- Viswanathan G., Dan V. M., Radhakrishnan N., Nair A. S., Rajendran Nair A. P., Baby S. (2019). Protection of mouse brain from paracetamol-induced stress by Centella asiatica methanol extract. J. Ethnopharmacol. 236, 474–483. 10.1016/j.jep.2019.03.017
- Fitri A. R., Pavasant P., Chamni S., Sumrejkanchanakij P. (2018). Asiaticoside induces osteogenic differentiation of human periodontal ligament cells through the Wnt pathway. J. Periodontol. 89 (5), 596–605. 10.1002/JPER.17-0471
- Song D., Jiang X., Liu Y., Sun Y., Cao S., Zhang Z. (2018). Asiaticoside attenuates cell growth inhibition and apoptosis induced by Aβ1-42 via inhibiting the TLR4/NF-κB signaling pathway in human brain microvascular endothelial cells. Front. Pharmacol. 9, 28 (JAN). 10.3389/fphar.2018.00028
- Nataraj J, Manivasagam T, Justin Thenmozhi A, Essa MM. Neurotrophic Effect of Asiatic acid, a Triterpene of Centella asiatica Against Chronic 1-Methyl 4-Phenyl 1, 2, 3, 6-Tetrahydropyridine Hydrochloride/Probenecid Mouse Model of Parkinson’s disease: The Role of MAPK, PI3K-Akt-GSK3β and mTOR Signalling Pathways. Neurochem Res. 2017 May;42(5):1354-1365. doi: 10.1007/s11064-017-2183-2
- Gelders G, Baekelandt V, Van der Perren A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J Immunol Res. 2018 Apr 16;2018:4784268. doi: 10.1155/2018/4784268
- Jiang T, Sun Q, Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol. 2016 Dec;147:1-19. doi: 10.1016/j.pneurobio.2016.07.005
- Morais VA, De Strooper B. Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis. 2010;20 Suppl 2:S255-63. doi: 10.3233/JAD-2010-100345
- Mohammadi A, Amooeian VG, Rashidi E. Dysfunction in Brain-Derived Neurotrophic Factor Signaling Pathway and Susceptibility to Schizophrenia, Parkinson’s and Alzheimer’s Diseases. Curr Gene Ther. 2018;18(1):45-63. doi: 10.2174/1566523218666180302163029
- Tönnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1105-1121. doi: 10.3233/JAD-161088
- Welbat J. U., Chaisawang P., Pannangrong W., Wigmore P. (2018). Neuroprotective properties of asiatic acid against 5-fluorouracil chemotherapy in the hippocampus in an adult rat model. Nutrients 10 (8), 1053. 10.3390/nu10081053
- Onyango I. G., Khan S. M., Bennett J. P., Jr. (2017). Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases. Front. Biosci. (Landmark Ed). 22, 854–872. 10.2741/4521
- Nataraj J., Manivasagam T., Justin Thenmozhi A., Essa M. M. (2017. a). Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr. Neurosci. 20 (6), 351–359. 10.1080/1028415X.2015.1135559
- Lima Giacobbo B., Doorduin J., Klein H. C., Dierckx R. A. J. O., Bromberg E., de Vries E. F. J. (2019). Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 56 (5), 3295–3312. 10.1007/s12035-018-1283-6
- Boondam Y., Songvut P., Tantisira M. H., Tapechum S., Tilokskulchai K., Pakaprot N. (2019). Inverted U-shaped response of a standardized extract of Centella asiatica (ECa 233) on memory enhancement. Sci. Rep. 9 (1), 1–11. 10.1038/s41598-019-44867-z
- Lee JK, Kim NJ. Recent Advances in the Inhibition of p38 MAPK as a Potential Strategy for the Treatment of Alzheimer’s Disease. Molecules. 2017 Aug 2;22(8):1287. doi: 10.3390/molecules22081287
- Chen Y., Zheng X., Wang Y., Song J. (2018). Effect of PI3K/Akt/mTOR signaling pathway on JNK3 in Parkinsonian rats. Exp. Ther. Med. 17 (3), 1771–1775. 10.3892/etm.2018.7120
- Chen L., Liu P., Feng X., Ma C. (2017). Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 21 (12), 3178–3189. 10.1111/jcmm.12871
- Caviedes A., Lafourcade C., Soto C., Wyneken U. (2017). BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr. Pharm. Des. 23 (21), 3154–3163. 10.2174/1381612823666170111141915
- Ahmad Rather M, Justin Thenmozhi A, Manivasagam T, Nataraj J, Essa MM, Chidambaram SB. Asiatic acid nullified aluminium toxicity in in vitro model of Alzheimer’s disease. Front Biosci (Elite Ed). 2018 Jan 1;10:287-299. doi: 10.2741/e823
- Bradwejn J, Zhou Y, Koszycki D, Shlik J. A double-blind, placebo-controlled study on the effects of Gotu Kola (Centella asiatica) on acoustic startle response in healthy subjects. J Clin Psychopharmacol 2000;20:680-684.
- Rameshreddy P., Uddandrao V. V. S., Brahmanaidu P., Vadivukkarasi S., Ravindarnaik R., Suresh P., et al. (2018). Obesity-alleviating potential of asiatic acid and its effects on ACC1, UCP2, and CPT1 mRNA expression in high fat diet-induced obese Sprague–Dawley rats. Mol. Cell. Biochem. 442 (1–2), 143–154. 10.1007/s11010-017-3199-2
- Wang Q., Yao L., Xu K., Jin H., Chen K., Wang Z., et al. (2019). Madecassoside inhibits estrogen deficiency-induced osteoporosis by suppressing RANKL-induced osteoclastogenesis. J. Cell. Mol. Med. 23 (1), 380–394. 10.1111/jcmm.13942
- Zheng Y., Ley S. H., Hu F. B. (2018). Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14 (2), 88–98. 10.1038/nrendo.2017.151
- Rehman K., Akash M. S. H. (2017). Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 118 (11), 3577–3585. 10.1002/jcb.26097
- Lontchi-Yimagou E., Sobngwi E., Matsha T. E., Kengne A. P. (2013). Diabetes mellitus and inflammation. Curr. Diabetes Rep. 13 (3), 435–444. 10.1007/s11892-013-0375-y
- World Health Organisation (2013). “Obesity and overweight. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- Collins K. H., Herzog W., MacDonald G. Z., Reimer R. A., Rios J. L., Smith I. C., et al. (2018). Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 112–112. 10.3389/fphys.2018.00112
- Saltiel A. R., Olefsky J. M. (2017). Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127 (1), 1–4. 10.1172/JCI92035
- Delitala A. P., Scuteri A., Doria C. (2020). Thyroid Hormone Diseases and Osteoporosis. [Journal Article; Review]. J. Clin. Med. 9 (4), 1034. 10.3390/jcm9041034
- Masola B., Oguntibeju O. O., Oyenihi A. B. (2018). Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed. Pharmacother. 101 (October 2017), 447–457. 10.1016/j.biopha.2018.02.115
- Oyenihi A. B., Chegou N. N., Oguntibeju O. O., Masola B. (2017). Centella asiatica enhances hepatic antioxidant status and regulates hepatic inflammatory cytokines in type 2 diabetic rats. Pharm. Biol. 55 (1), 1671–1678. 10.1080/13880209.2017.1318293
- Blanco M. D. M. P., Gonzalez C. R., Saha A. K., Martins L., Dieguez C., Vidal-Puig A., et al. (2011). Hypothalamic AMP-activated protein kinase as a mediator of whole body energy balance. [Journal Article; Research Support, Non-U.S. Gov’t; Review]. Rev. Endocr. Metab. Disord. 12 (3), 127–140. 10.1007/s11154-011-9165-5
- Shen X., Guo M., Yu H., Liu D., Lu Z., Lu Y. (2019). Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Biosci. Biotechnol. Biochem. 83 (3), 561–568. 10.1080/09168451.2018.1547627
- Kircik L. H. (2016). Advances in the Understanding of the Pathogenesis of Inflammatory Acne. J. Drugs Dermatol. 15 (1 Suppl 1), s7–s10.
- Glassman S. J. (2011). Vitiligo, reactive oxygen species and T-cells. Clin. Sci. (Lond). 120 (3), 99–120. 10.1042/CS20090603
- Avena-Woods C. (2017). Overview of atopic dermatitis. Am. J. Managed Care. 23 (8 Suppl), S115–S123. 10.5415/apallergy.2013.3.2.79
- David Boothe W., Tarbox J. A., Tarbox M. B. (2017). Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 1027, 21–37. 10.1007/978-3-319-64804-0_3
- Sorg H., Tilkorn D. J., Hager S., Hauser J., Mirastschijski U. (2017). Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 58 (1–2), 81–94. 10.1159/000454919
- Sawatdee S., Choochuay K., Chanthorn W., Srichana T. (2016). Evaluation of the topical spray containing Centella asiatica extract and its efficacy on excision wounds in rats. Acta Pharm. 66 (2), 233–244. 10.1515/acph-2016-0018
- Yao C. H., Yeh J. Y., Chen Y. S., Li M. H., Huang C. H. (2017). Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 11 (3), 905–915. 10.1002/term.1992
- Sh Ahmed A., Taher M., Mandal U. K., Jaffri J. M., Susanti D., Mahmood S., et al. (2019). Pharmacological properties of Centella asiatica hydrogel in accelerating wound healing in rabbits. BMC Complement. Altern. Med. 19 (1), 1–7. 10.1186/s12906-019-2625-2
- Singkhorn S., Tantisira M. H., Tanasawet S., Hutamekalin P., Wongtawatchai T., Sukketsiri W. (2018). Induction of keratinocyte migration by ECa 233 is mediated through FAK/Akt, ERK, and p38 MAPK signaling. Phytother. Res. 32 (7), 1397–1403. 10.1002/ptr.6075
- Ju Ho P., Jun Sung J., Ki Cheon K., Jin Tae H. (2018). Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 43, 110–119. 10.1016/j.phymed.2018.04.013
- Ling Y., Gong Q., Xiong X., Sun L., Zhao W., Zhu W., et al. (2017). Protective effect of madecassoside on H2O2-induced oxidative stress and autophagy activation in human melanocytes. Oncotarget 8 (31), 51066–51075. 10.18632/oncotarget.17654
- Choi Y. M., An S., Lee J., Lee J. H., Lee J. N., Kim Y. S., et al. (2017). Titrated extract of Centella asiatica increases hair inductive property through inhibition of STAT signaling pathway in three-dimensional spheroid cultured human dermal papilla cells. Biosci. Biotechnol. Biochem. 81 (12), 2323–2329. 10.1080/09168451.2017.1385383
- Bosse JP, Papillon J, Frenette G, et al. Clinical study of a new antikeloid agent. Ann Plast Surg 1979;3:13-21.
- Widgerow AD, Chait LA, Stals R, Stals PJ. New innovations in scar management. Aesthetic Plast Surg 2000;24:227-234.
- Wang Z., Chesler N. C. (2013). Pulmonary vascular mechanics: Important contributors to the increased right ventricular afterload of pulmonary hypertension. Exp. Physiol. 98 (8), 1267–1273. 10.1113/expphysiol.2012.069096
- Matuszkiewicz-Rowińska J., Wieliczko M. (2015). Nadciśnienie naczyniowo-nerkowe [Renovascular hypertension]. Wiad Lek. 68 (4 Pt 2), 623–625.
- Liu S. Y., Duan X. C., Jin S., Teng X., Xiao L., Xue H. M. (2017). Hydrogen Sulfide Improves Myocardial Remodeling via Downregulated Angiotensin II/AT1R Pathway in Renovascular Hypertensive Rats. Am. J. Hypertens. 30 (1), 67–74. 10.1093/ajh/hpw104
- Richards D. A., Aronovitz M. J., Calamaras T. D., Tam K., Martin G. L., Liu P., et al. (2019). Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci. Rep. 9 (1), 5844. 10.1038/s41598-019-42209-7
- de Boer R. A., De Keulenaer G., Bauersachs J., Brutsaert D., Cleland J. G., Diez J., et al. (2019). Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. In Eur. J. Heart Failure 21 (3), 272–285. 10.1002/ejhf.1406
- Wang T., Wei Z., Dou Y., Yang Y., Leng D., Kong L., et al. (2015). Intestinal interleukin-10 mobilization as a contributor to the anti-arthritis effect of orally administered madecassoside: A unique action mode of saponin compounds with poor bioavailability. Biochem. Pharmacol. 94 (1), 30–38. 10.1016/j.bcp.2015.01.004
- Wang X., Cai X., Wang W., Jin Y., Chen M., Huang X., et al. (2018). Effect of asiaticoside on endothelial cells inhypoxia-induced pulmonary hypertension. Mol. Med. Rep. 17 (2), 2893–2900. 10.3892/mmr.2017.8254
- Maneesai P., Bunbupha S., Kukongviriyapan U., Senggunprai L., Kukongviriyapan V., Prachaney P., et al. (2017). Effect of asiatic acid on the Ang II-AT1R-NADPH oxidase-NF-κB pathway in renovascular hypertensive rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 390 (10), 1073–1083. 10.1007/s00210-017-1408-x
- De Sanctis M. T., Belcaro G., Incandela L., Cesarone M. R., Griffin M., Ippolito E., et al. (2001). Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: a clinical, prospective, placebo-controlled, randomized, dose-ranging trial. [Clinical Trial; Journal Article; Randomized Controlled Trial]. Angiology 52 (Suppl 2), S55–S59.
- Döring Y., Noels H., van der Vorst E. P. C., Neideck C., Egea V., Drechsler M., et al. (2017). Vascular CXCR4 Limits Atherosclerosis by Maintaining Arterial Integrity: Evidence From Mouse and Human Studies. Circulation 136 (4), 388–403. 10.1161/CIRCULATIONAHA.117.027646
- Jing L., Haitao W., Qiong W., Fu Z., Nan Z., Xuezheng Z. (2018). Anti inflammatory effect of asiaticoside on human umbilical vein endothelial cells induced by ox-LDL. Cytotechnology 70 (2), 855–864. 10.1007/s10616-018-0198-4
- Fong L. Y., Ng C. T., Yong Y. K., Hakim M. N., Ahmad Z. (2019). Asiatic acid stabilizes cytoskeletal proteins and prevents TNF-α-induced disorganization of cell-cell junctions in human aortic endothelial cells. Vasc. Pharmacol. 117, 15–26. 10.1016/j.vph.2018.08.005
- Belcaro G., Dugall M., Ippolito E., Hosoi M., Cornelli U., Ledda A., et al. (2017). Pycnogenol(R) and Centella asiatica to prevent asymptomatic atherosclerosis progression in clinical events. Minerva Cardioangiol. 65 (1), 24–31. 10.23736/S0026-4725.16.04008-1
- Li Q., Yang J., Zhang J., Liu X. W., Yang C. J., Fan Z. X., et al. (2020). Inhibition of microRNA-327 ameliorates ischemia/reperfusion injury-induced cardiomyocytes apoptosis through targeting apoptosis repressor with caspase recruitment domain. J. Cell Physiol. 235 (4), 3753–3767. 10.1002/jcp.29270
- Huang X., Zuo L., Lv Y., Chen C., Yang Y., Xin H., et al. (2016). Asiatic acid attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β/HIF-1α signaling in rat H9c2 cardiomyocytes. Molecules 21 (9), 1–14. 10.3390/molecules21091248
- Rosdah A. A., Lusiana E., Reagan M., Akib A., Khairunnisa F., Husna A. (2019). A preliminary study: Centella asiatica extract modulates acetylcholine in the heart. J. Phys.: Conf. Ser. 1246, 12048. 10.1088/1742-6596/1246/1/012048
- Pointel JP, Boccalon H, Cloarec M, et al. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology 1987;38:46-50.
- Cesarone MR, Belcaro G, De Sanctis MT, et al. Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Angiology 2001;52:S15-S18.
- Cesarone MR, Belcaro G, Rulo A, et al. Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: measurement by laser Doppler, TcPO2-CO2, and leg volumetry. Angiology 2001;52:S45-S48.
- Cesarone MR, Incandela L, De Sanctis MT, et al. Flight microangiopathy in medium- to long-distance flights: prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology 2001;52:S33-S37.
- Cesarone MR, Incandela L, De Sanctis MT, et al. Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centella asiatica: a clinical prospective randomized trial with a microcirculatory model. Angiology 2001;52:S49-S54.
- Cesarone MR, Belcaro G, Nicolaides AN, et al. Increase in echogenicity of echolucent carotid plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, placebo-controlled, randomized trial. Angiology 2001;52:S19-S25.
- Incandela L, Belcaro G, Nicolaides AN, et al. Modification of the echogenicity of femoral plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, randomized, placebo-controlled trial. Angiology 2001;52:S69-S73.
- Nguyen-Lefebvre A. T., Ajith A., Portik-Dobos V., Horuzsko D. D., Arbab A. S., Dzutsev A., et al. (2018). The innate immune receptor TREM-1 promotes liver injury and fibrosis. J. Clin. Investi. 128 (11), 4870–4883. 10.1172/JCI98156
- Soll A. H., Weinstein W. M., Kurata J., McCarthy D. (1991). Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann. Internal Med. 114 (4), 307–319. 10.7326/0003-4819-114-4-307
- Kwiecien S., Konturek P. C., Sliwowski Z., Mitis-Musiol M., Pawlik M. W., Brzozowski B., et al. (2012). Interaction between selective cyclooxygenase inhibitors and capsaicin-sensitive afferent sensory nerves in pathogenesis of stress-induced gastric lesions. Role of oxidative stress. J. Physiol. Pharmacol. 63 (2), 143–151.
- Wannasarit S., Puttarak P., Kaewkroek K., Wiwattanapatapee R. (2019). Strategies for Improving Healing of the Gastric Epithelium Using Oral Solid Dispersions Loaded with Pentacyclic Triterpene–Rich Centella Extract. AAPS PharmSciTech 20 (7), 277. 10.1208/s12249-019-1488-7
- Wei L., Chen Q., Guo A., Fan J., Wang R., Zhang H. (2018). Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int. Immunopharmacol. 60, 1–8. 10.1016/j.intimp.2018.04.016
- Wang W., Wu L., Li Q., Zhang Z., Xu L., Lin C., et al. (2018). Madecassoside prevents acute liver failure in LPS/D-GalN-induced mice by inhibiting p38/NF-κB and activating Nrf2/HO-1 signaling. Biomed. Pharmacother. 103, 1137–1145. 10.1016/j.biopha.2018.04.162
- Xu X., Wang Y., Wei Z., Wei W., Zhao P., Tong B., et al. (2017). Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. 8 (3), e2723. 10.1038/cddis.2017.150
- Chen H., Luo J., Guo J. (2020). Development and validation of a five-immune gene prognostic risk model in colon cancer. BMC Cancer 20 (1), 395. 10.1186/s12885-020-06799-0
- Huang Q., Cao H., Zhan L., Sun X., Wang G., Li J., et al. (2017). Mitochondrial fission forms a positive feedback loop with cytosolic calcium signaling pathway to promote autophagy in hepatocellular carcinoma cells. Cancer Lett. 403, 108–118. 10.1016/j.canlet.2017.05.034
- Hao Y., Huang J., Ma Y., Chen W., Fan Q., Sun X., et al. (2018). Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 15 (6), 8223–8230. 10.3892/ol.2018.841
- Thannickal V. J., Toews G. B., White E. S., Lynch III J. P., Martinez F. J. (2004). Mechanisms of Pulmonary Fibrosis. Annu. Rev. Med. 55, 395–417. 10.1146/annurev.med.55.091902.103810
- Tian R., Zhu Y., Yao J., Meng X., Wang J., Xie H., et al. (2017). NLRP3 participates in the regulation of EMT in bleomycin-induced pulmonary fibrosis. Exp. Cell Res. 357 (2), 328–334. 10.1016/j.yexcr.2017.05.028
- Rabe K. F., Watz H. (2017). Chronic obstructive pulmonary disease. Lancet. 389 (10082), 1931–1940. 10.1016/S0140-6736(17)31222-9
- Riley C. M., Sciurba F. C. (2019). Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA – J. Am. Med. Assoc. 321 (8), 786–797. 10.1001/jama.2019.0131
- Quaratino S., Forssmann U., Marschner J. P. (2017). New approaches in immunotherapy for the treatment of lung cancer. Curr. Topics Microbiol. Immunol. 405, 1–31. 10.1007/82_2014_428
- McVey M., Tabuchi A., Kuebler W. M. (2012). Microparticles and acute lung injury. Am. J. Physiol. – Lung Cell. Mol. Physiol. 303 (5), L364–L381. 10.1152/ajplung.00354.2011
- Dong S. H., Liu Y. W., Wei F., Tan H. Z., Han Z. D. (2017). Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways. Biomed. Pharmacother. 89, 1297–1309. 10.1016/j.biopha.2017.03.005
- Cui Q., Ren J., Zhou Q., Yang Q., Li B. (2019). Effect of asiatic acid on epithelial-mesenchymal transition of human alveolar epithelium A549 cells induced by TGF-β1. Oncol. Lett. 17 (5), 4285–4292. 10.3892/ol.2019.10140
- Qiu J., Yu L., Zhang X., Wu Q., Wang D., Wang X., et al. (2015). Asiaticoside attenuates lipopolysaccharide-induced acute lung injury via down-regulation of NF-κB signaling pathway. Int. Immunopharmacol. 26 (1), 181–187. 10.1016/j.intimp.2015.03.022
- Bugg C. W., Taira T. (2016). Pelvic Inflammatory Disease: Diagnosis And Treatment In The Emergency Department. Emergency Med. Pract. 18 (12), 1–24.
- Savaris R. F., Fuhrich D. G., Duarte R. V., Franik S., Ross J. (2017). Antibiotic therapy for pelvic inflammatory disease. Cochrane Database Syst. Rev. 4 (4), CD010285. 10.1002/14651858.CD010285.pub2
- Bina F., Soleymani S., Toliat T., Hajimahmoodi M., Tabarrai M., Abdollahi M., et al. (2019). Plant-derived medicines for treatment of endometriosis: A comprehensive review of molecular mechanisms. Pharmacol. Res. 76–90. 10.1016/j.phrs.2018.11.008
- Stewart C., Ralyea C., Lockwood S. (2019). Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 35 (2), 151–156. 10.1016/j.soncn.2019.02.001
- Kong D., Fu P., Zhang Q., Ma X., Jiang P. (2019). Protective effects of Asiatic acid against pelvic inflammatory disease in rats. Exp. Ther. Med. 17 (6), 4687–4692. 10.3892/etm.2019.7498
- Cao S., Wang W., Nan F., Liu Y., Wei S., Li F., et al. (2018). Asiatic acid inhibits LPS-induced inflammatory response in endometrial epithelial cells. Microbial. Pathogenesis 116, 195–199. 10.1016/j.micpath.2018.01.022
- Ren L., Cao Q. X., Zhai F. R., Yang S. Q., Zhang H. X. (2016). Asiatic acid exerts anticancer potential in human ovarian cancer cells via suppression of PI3K/Akt/mTOR signalling. Pharm. Biol. 54 (11), 2377–2382. 10.3109/13880209.2016.1156709
- Qi J. J., Li X. X., Diao Y. F., Liu P. L., Wang D. L., Bai C. Y., et al. (2020). Asiatic acid supplementation during the in vitro culture period improves early embryonic development of porcine embryos produced by parthenogenetic activation, somatic cell nuclear transfer and in vitro fertilization. Theriogenology 142, 26–33. 10.1016/j.theriogenology.2019.09.027
- Fard S. E., Tafvizi F., Torbati M. B. (2018). Silver nanoparticles biosynthesised using Centella asiatica leaf extract: Apoptosis induction in MCF-7 breast cancer cell line. IET Nanobiotechnol. 12 (7), 994–1002. 10.1049/iet-nbt.2018.5069
- Smolen J. S., Aletaha D., McInnes I. B. (2016). Rheumatoid arthritis. Lancet. 388 (10055), 2023–2038. 10.1016/S0140-6736(16)30173-8
- Marsal S., Juliá A. (2010). Rheumatoid arthritis pharmacogenomics. Pharmacogenomics. 11 (5), 617–619. 10.2217/pgs.10.53
- Konttinen Y. T., Ainola M., Vallcala H., Ma J., Ida H., Mandelin J., et al. (1999). Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: Different profiles in trauma and rheumatoid arthritis. Ann. Rheum. Dis. 58 (11), 691–697. 10.1136/ard.58.11.691
- Wang X. B., Wang W., Zhu X. C., Ye W. J., Cai H., Wu P. L., et al. (2015). The potential of asiaticoside for TGF-β1/Smad signaling inhibition in prevention and progression of hypoxia-induced pulmonary hypertension. Life Sci. 137, 56–64. 10.1016/j.lfs.2015.07.016
- Yu W. G., Shen Y., Wu J. Z., Gao Y. B., Zhang L. X. (2018). Madecassoside impedes invasion of rheumatoid fibroblast-like synoviocyte from adjuvant arthritis rats via inhibition of NF-κB-mediated matrix metalloproteinase-13 expression. Chin. J. Natural Medicines 16 (5), 330–338. 10.1016/S1875-5364(18)30064-5
- Shinomol GK, Muralidhara. Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology. 2008 Nov;29(6):948-57. doi: 10.1016/j.neuro.2008.09.009
- Tang XL, Yang XY, Jung HJ, Kim SY, Jung SY, Choi DY, Park WC, Park H. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol Pharm Bull. 2009 Aug;32(8):1399-405. doi: 10.1248/bpb.32.1399
- Kumar A, Prakash A, Dogra S. Centella asiatica Attenuates D-Galactose-Induced Cognitive Impairment, Oxidative and Mitochondrial Dysfunction in Mice. Int J Alzheimers Dis. 2011;2011:347569. doi: 10.4061/2011/347569
- Liu M, Dai Y, Yao X, Li Y, Luo Y, Xia Y, Gong Z. Anti-rheumatoid arthritic effect of madecassoside on type II collagen-induced arthritis in mice. Int Immunopharmacol. 2008 Nov;8(11):1561-6. doi: 10.1016/j.intimp.2008.06.011
- Bian GX, Li GG, Yang Y, Liu RT, Ren JP, Wen LQ, Guo SM, Lu QJ. Madecassoside reduces ischemia-reperfusion injury on regional ischemia induced heart infarction in rat. Biol Pharm Bull. 2008 Mar;31(3):458-63. doi: 10.1248/bpb.31.458
- Cesarone MR, Belcaro G, De Sanctis MT, Incandela L, Cacchio M, Bavera P, Ippolito E, Bucci M, Griffin M, Geroulakos G, Dugall M, Buccella S, Kleyweght S, Cacchio M. Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Angiology. 2001 Oct;52 Suppl 2:S15-18.
- Cesarone MR, Incandela L, De Sanctis MT, Belcaro G, Bavera P, Bucci M, Ippolito E. Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centella asiatica: a clinical prospective randomized trial with a microcirculatory model. Angiology. 2001 Oct;52 Suppl 2:S49-54.
- Pointel JP, Boccalon H, Cloarec M, Ledevehat C, Joubert M. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology. 1987 Jan;38(1 Pt 1):46-50. doi: 10.1177/000331978703800106
- Saeidinia A, Keihanian F, Lashkari AP, Lahiji HG, Mobayyen M, Heidarzade A, Golchai J. Partial-thickness burn wounds healing by topical treatment: A randomized controlled comparison between silver sulfadiazine and centiderm. Medicine (Baltimore). 2017 Mar;96(9):e6168. doi: 10.1097/MD.0000000000006168
- Wattanathorn J, Mator L, Muchimapura S, Tongun T, Pasuriwong O, Piyawatkul N, Yimtae K, Sripanidkulchai B, Singkhoraard J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol. 2008 Mar 5;116(2):325-32. doi: 10.1016/j.jep.2007.11.038
- Puttarak P, Dilokthornsakul P, Saokaew S, Dhippayom T, Kongkaew C, Sruamsiri R, Chuthaputti A, Chaiyakunapruk N. Effects of Centella asiatica (L.) Urb. on cognitive function and mood related outcomes: A Systematic Review and Meta-analysis. Sci Rep. 2017 Sep 6;7(1):10646. doi: 10.1038/s41598-017-09823-9
- Turton S. Centella asiatica. Aust J Herbalism 1993;5:60.
- Songvut P, Chariyavilaskul P, Tantisira MH, Khemawoot P. Safety and Pharmacokinetics of Standardized Extract of Centella asiatica (ECa 233) Capsules in Healthy Thai Volunteers: A Phase 1 Clinical Study. Planta Med. 2019 Apr;85(6):483-490. doi: 10.1055/a-0835-6671
- Eun HC, Lee AY. Contact dermatitis due to madecassol. Contact Dermatitis 1985;13:310-313.
- Jorge OA, Jorge AD. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev Esp Enferm Dig 2005;97:115-124.
- Kar A., Pandit S., Mukherjee K., Bahadur S., Mukherjee P. K. (2017). Safety assessment of selected medicinal food plants used in Ayurveda through CYP450 enzyme inhibition study. [Journal Article]. J. Sci. Food Agric. 97 (1), 333–340. 10.1002/jsfa.7739
- Yunianto I., Das S., Mat N. M. (2010). Antispermatogenic and antifertility effect of Pegaga (Centella asiatica L) on the testis of male Sprague-Dawley rats. [Journal Article; Research Support, Non-U.S. Gov’t] Clin. Ter. 161 (3), 235–239.
- Deshpande PO, Mohan V, Thakurdesai P. Preclinical Safety Assessment of Standardized Extract of Centella asiatica (L.) Urban Leaves. Toxicol Int. 2015 Jan-Apr;22(1):10-20. doi: 10.4103/0971-6580.172251
- Jorge OA, Jorge AD. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev Esp Enferm Dig. 2005 Feb;97(2):115-24. English, Spanish. doi: 10.4321/s1130-01082005000200006
- Savai J, Varghese A, Pandita N, Chintamaneni M. In vitro assessment of CYP1A2 and 2C9 inhibition potential of Withania somnifera and Centella asiatica in human liver microsomes. Drug Metab Pers Ther. 2015 Jun;30(2):137-41. doi: 10.1515/dmdi-2014-0035
- Pan Y, Abd-Rashid BA, Ismail Z, Ismail R, Mak JW, Pook PC, Er HM, Ong CE. In vitro modulatory effects on three major human cytochrome P450 enzymes by multiple active constituents and extracts of Centella asiatica. J Ethnopharmacol. 2010 Jul 20;130(2):275-83. doi: 10.1016/j.jep.2010.05.002
- Pan Y, Abd-Rashid BA, Ismail Z, Ismail R, Mak JW, Pook PC, Er HM, Ong CE. In vitro modulatory effects of Andrographis paniculata, Centella asiatica and Orthosiphon stamineus on cytochrome P450 2C19 (CYP2C19). J Ethnopharmacol. 2011 Jan 27;133(2):881-7. doi: 10.1016/j.jep.2010.11.026
- Brinker F. Herb Contraindications and Drug Interactions. 2nd ed. Sandy, OR: Eclectic Medical Publications; 1998:78.