Childhood acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is also known as acute lymphocytic leukemia or acute lymphoid leukemia, is a cancer of the blood and bone marrow — in which the bone marrow makes too many abnormal, immature lymphocytes called “blasts” or lymphoblasts (immature lymphocytes). These blasts (lymphoblasts) do not become healthy white blood cells. Instead, they build up in the bone marrow, so there is less room for healthy white blood cells, red blood cells, and platelets. In addition, these abnormal cells are unable to fight off infection. Acute lymphoblastic leukemia (ALL) usually develops quickly over days or weeks and the cancer usually gets worse quickly if it is not treated. The word “acute” in acute lymphocytic leukemia comes from the fact that the disease progresses rapidly and creates immature blood cells (lymphoblasts or immature lymphocytes), rather than mature ones. The word “lymphocytic” in acute lymphocytic leukemia refers to the white blood cells called lymphocytes, which acute lymphoblastic leukemia (ALL) affects.
Lymphocyte is a type of white blood cell that is made in the bone marrow and is found in the blood and in lymph tissue. Lymphocytes are involved in your immune response which produce antibodies that seek out and immobilize bacteria, viruses, and toxins which invade your body. There are two types of lymphocytes:
- B-cells (formed in your bone marrow)
- T-cells (formed in your thymus gland, behind the sternum) which destroy the invading organisms that have been tagged by the B-cells as well as any cells that have become cancerous.
In children with acute lymphoblastic leukemia (childhood ALL):
- 80% to 85% of acute lymphoblastic leukemia consists of early B-cells (also called precursor B-cell)
- 15% are early T-cells. This subtype of acute lymphoblastic leukemia originates in immature cells that would normally develop into T-cell lymphocytes. This subtype is less common than B-cell acute lymphoblastic leukemia and occurs more often in adults than in children.
- Approximately 2% are mature B-cells
Acute lymphocytic leukemia (acute lymphoblastic leukemia) starts in your bone marrow (the soft inner part of certain bones, where new blood cells are made). Most often, the leukemia cells invade your blood fairly quickly. They can also sometimes spread to other parts of your body, including the lymph nodes, liver, spleen, central nervous system (brain and spinal cord), and testicles (in males).
Other types of cancer that start in lymphocytes are known as lymphomas (either non-Hodgkin lymphoma or Hodgkin lymphoma). While leukemias like acute lymphocytic leukemia (acute lymphoblastic leukemia) mainly affect the bone marrow and the blood, lymphomas mainly affect the lymph nodes or other organs (but may also involve the bone marrow). Sometimes it can be hard to tell if a cancer of lymphocytes is a leukemia or a lymphoma. Usually, if at least 20% of the bone marrow is made up of cancerous lymphocytes (called lymphoblasts, or just blasts), the disease is considered leukemia.
Acute lymphocytic leukemia (acute lymphoblastic leukemia) is the most common type of cancer in children (around 85% of cases of acute lymphoblastic leukemia occur in children), representing approximately 25% of cancer diagnoses among children younger than 15 years (with a peak from two to five years of age) 1 and treatments result in a good chance for a cure. Acute lymphocytic leukemia can also occur in adults (15% of acute lymphoblastic leukemia cases), , mainly aged over 50 years, though the chance of a cure is greatly reduced and most deaths from acute lymphoblastic leukemia (about 4 out of 5) occur in adults. In children, although complete remission occurs in 97 to 99% of patients following initial multi-agent chemotherapy treatment, between 15 and 20% will have a relapse. In adults, despite complete remission rates of between 78% and 93%, relapse will occur in 60-70% of patients. Five-year survival is approximately 90% in children, but only 30% to 40% in adults and elderly patients 2.
The American Cancer Society’s estimates for acute lymphoblastic leukemia (acute lymphocytic leukemia) in the United States for 2021 (including both children and adults) are 3:
- About 5,690 new cases of acute lymphoblastic leukemia (3,000 in males and 2,690 in females).
- About 1,580 deaths from acute lymphoblastic leukemia (900 in males and 680 in females).
- The risk for developing acute lymphoblastic leukemia is highest in children younger than 5 years of age. The risk then declines slowly until the mid-20s, and begins to rise again slowly after age 50. Overall, about 4 of every 10 cases of acute lymphoblastic leukemia are in adults.
- Acute lymphoblastic leukemia is not a common cancer, accounting for less than half of 1% of all cancers in the United States. The average person’s lifetime risk of getting acute lymphoblastic leukemia is about 1 in 1,000. The risk is slightly higher in males than in females, and higher in whites than in African Americans.
In the United States, acute lymphoblastic leukemia (acute lymphocytic leukemia) occurs at an annual rate of approximately 41 cases per 1 million people aged 0 to 14 years and approximately 17 cases per 1 million people aged 15 to 19 years 4. There are approximately 3,100 children and adolescents younger than 20 years diagnosed with acute lymphoblastic leukemia each year in the United States 5. Since 1975, there has been a gradual increase in the incidence of acute lymphoblastic leukemia 6.
A sharp peak in acute lymphoblastic leukemia incidence is observed among children aged 2 to 3 years (>90 cases per 1 million per year), with rates decreasing to fewer than 30 cases per 1 million by age 8 years 7. The incidence of acute lymphoblastic leukemia among children aged 2 to 3 years is approximately fourfold greater than that for infants and is likewise fourfold to fivefold greater than that for children aged 10 years and older 7.
The incidence of acute lymphoblastic leukemia appears to be highest in Hispanic children (43 cases per 1 million) 8. The incidence is substantially higher in White children than in Black children, with a nearly threefold higher incidence of acute lymphoblastic leukemia from age 2 to 3 years in White children than in Black children 9.
Acute lymphoblastic leukemia in infants
Infant acute lymphoblastic leukemia generally refers to cases of acute lymphoblastic leukemia diagnosed in children younger than age 1. Leukemia is very rare in infants. There are only approximately 90 cases of infant acute lymphoblastic leukemia per year in the United States.
Lower survival rates and poorer outcomes are seen in infants with acute lymphoblastic leukemia than in older children with the disease. Most infants with acute lymphoblastic leukemia present with aggressive features, including high white blood cell counts, central nervous system involvement and presence of leukemia cells in the skin (a condition called “leukemia cutis”). As a result, infant patients typically need to be treated with intensive chemotherapy regimens. However, infants are very vulnerable to treatment-related toxicities, and newer, less-toxic treatments continue to be studied in clinical trials.
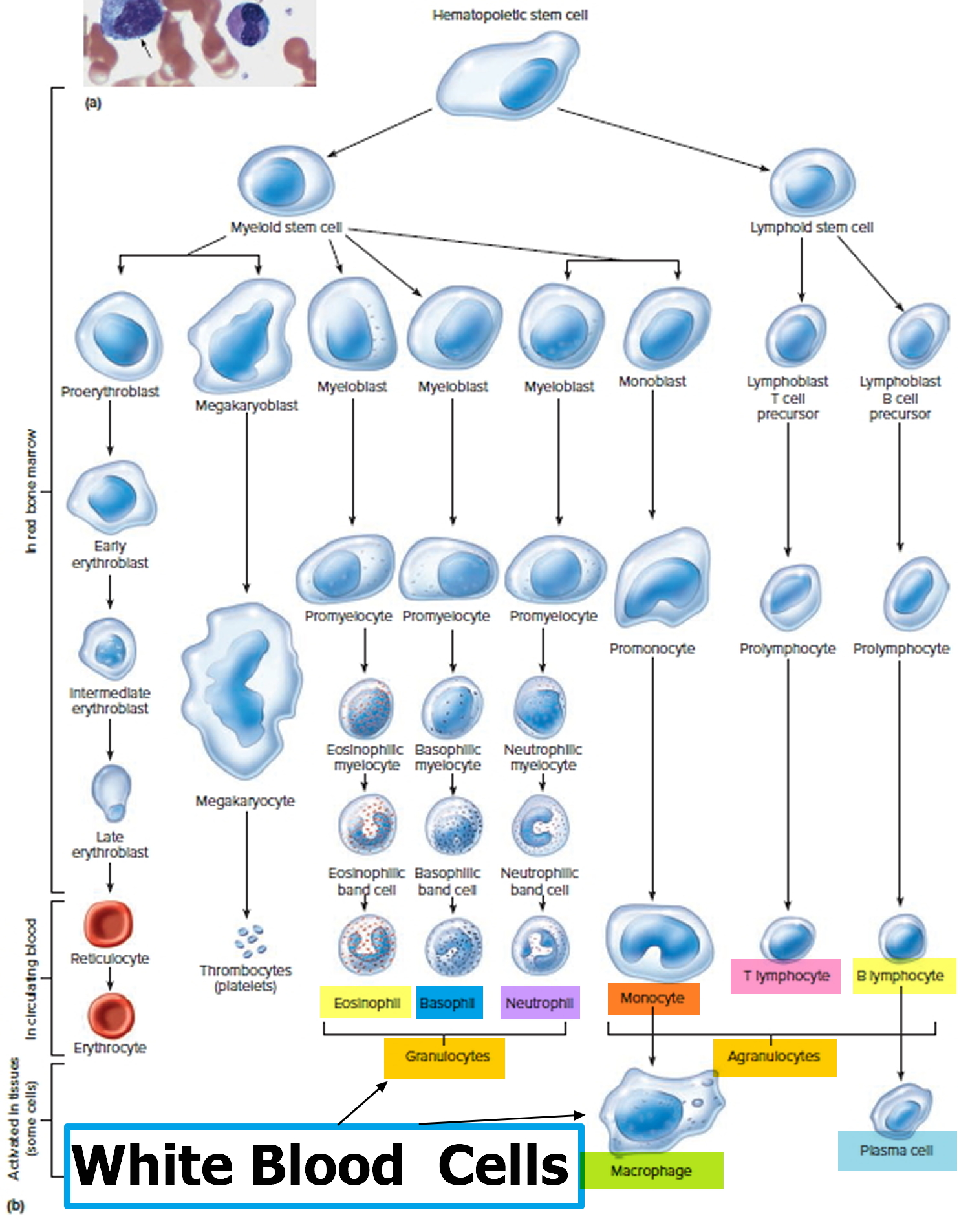
Normal bone marrow, blood, and lymph tissue
To understand leukemia, it helps to know about the bone marrow, blood, and lymph systems.
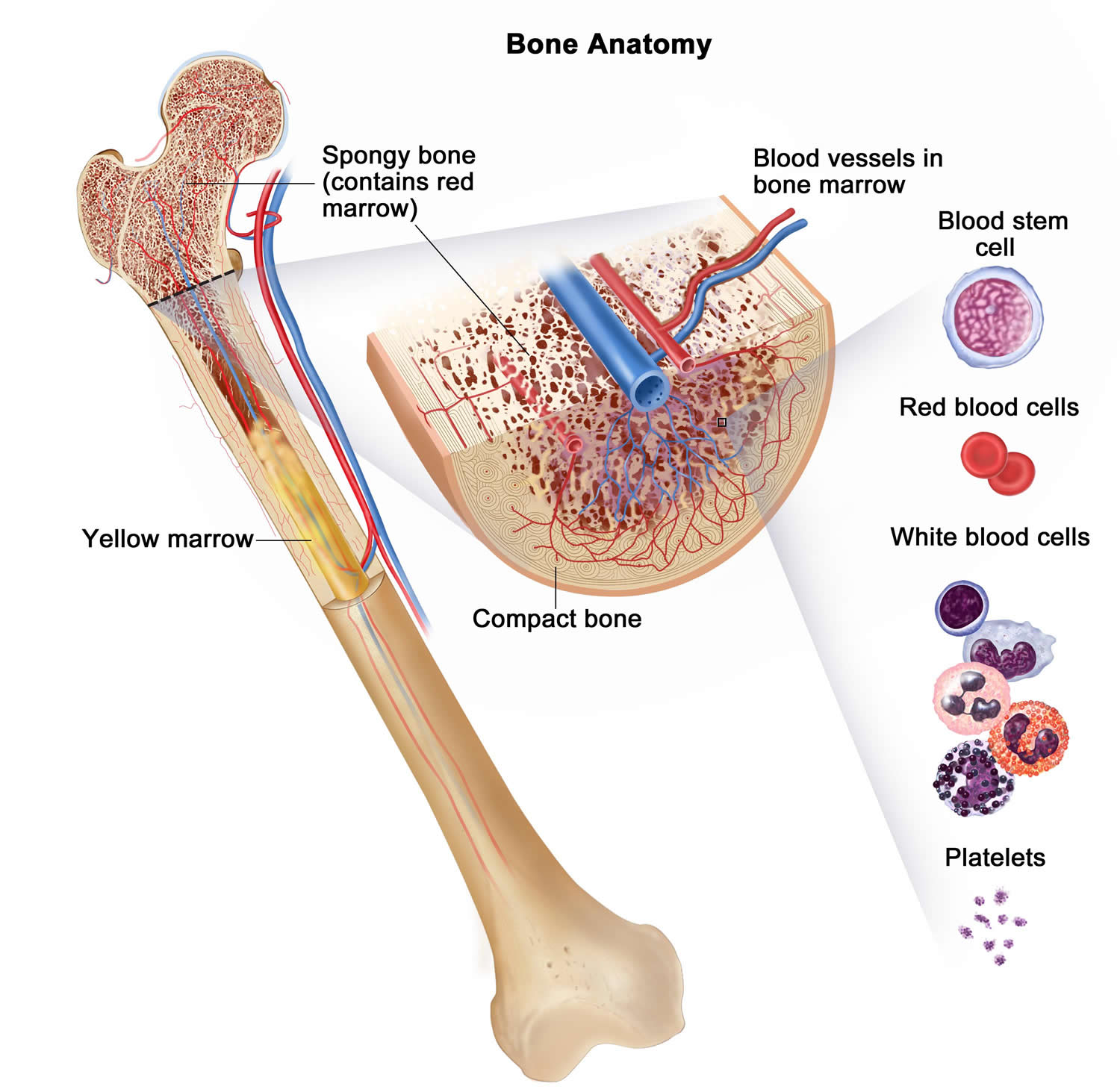
Bone marrow
Bone marrow is the soft inner part of certain bones. It is made up of blood-forming cells, fat cells, and supporting tissues. A small fraction of the blood-forming cells are blood stem cells.
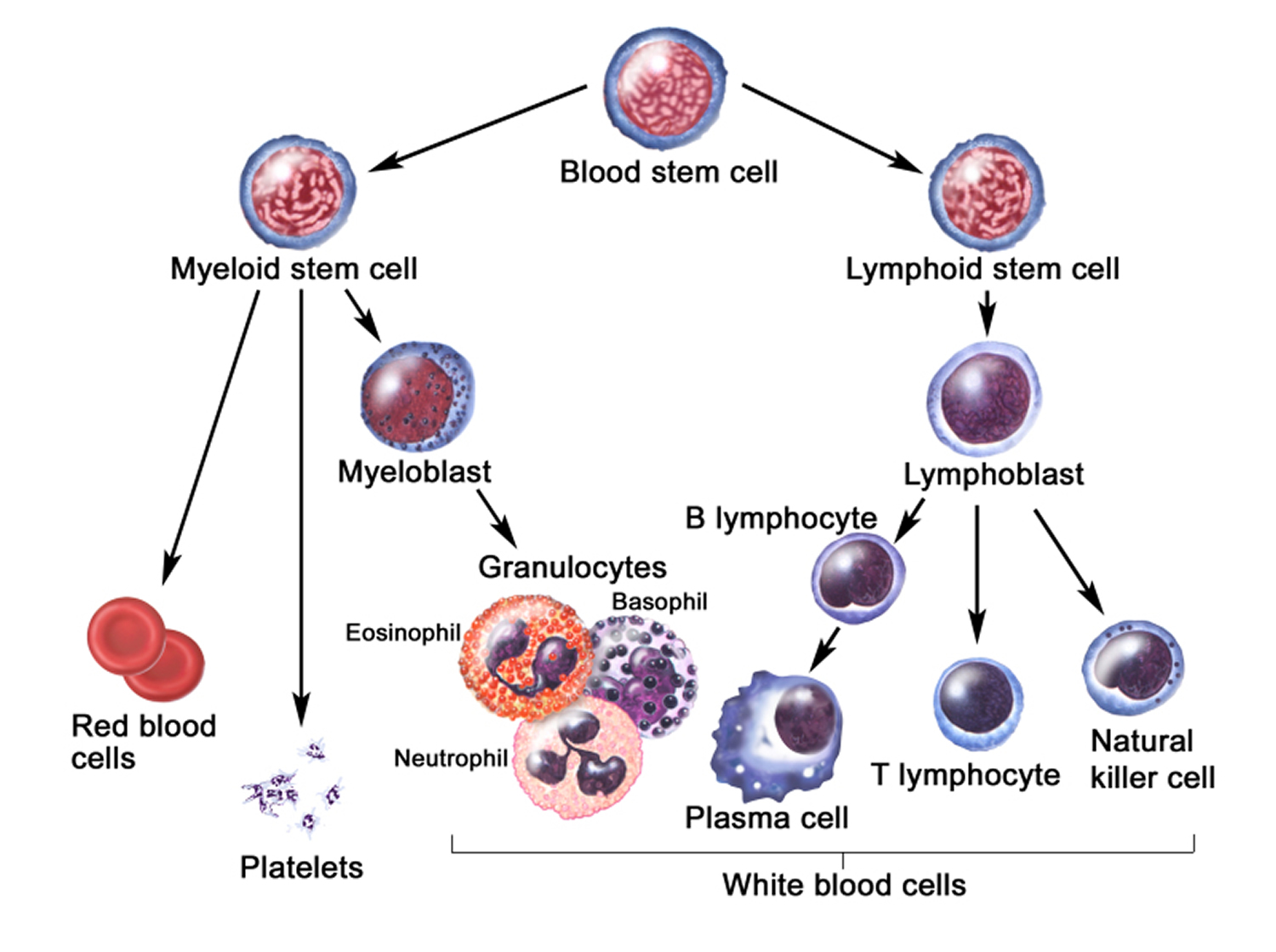
Inside the bone marrow, blood stem cells develop into new blood cells. During this process, the cells become either lymphocytes (a kind of white blood cell) or other blood-forming cells, which are types of myeloid cells. Myeloid cells can develop into red blood cells, white blood cells (other than lymphocytes), or platelets. These myeloid cells are the ones that are abnormal in acute myeloid leukemia.
Types of blood cells
There are 3 main types of blood cells:
- Red blood cells (RBCs) carry oxygen from the lungs to all other tissues in the body, and take carbon dioxide back to the lungs to be removed.
- Platelets are actually cell fragments made by a type of bone marrow cell called the megakaryocyte. Platelets are important in stopping bleeding. They help plug up holes in blood vessels caused by cuts or bruises.
- White blood cells (WBCs) help the body fight infections.
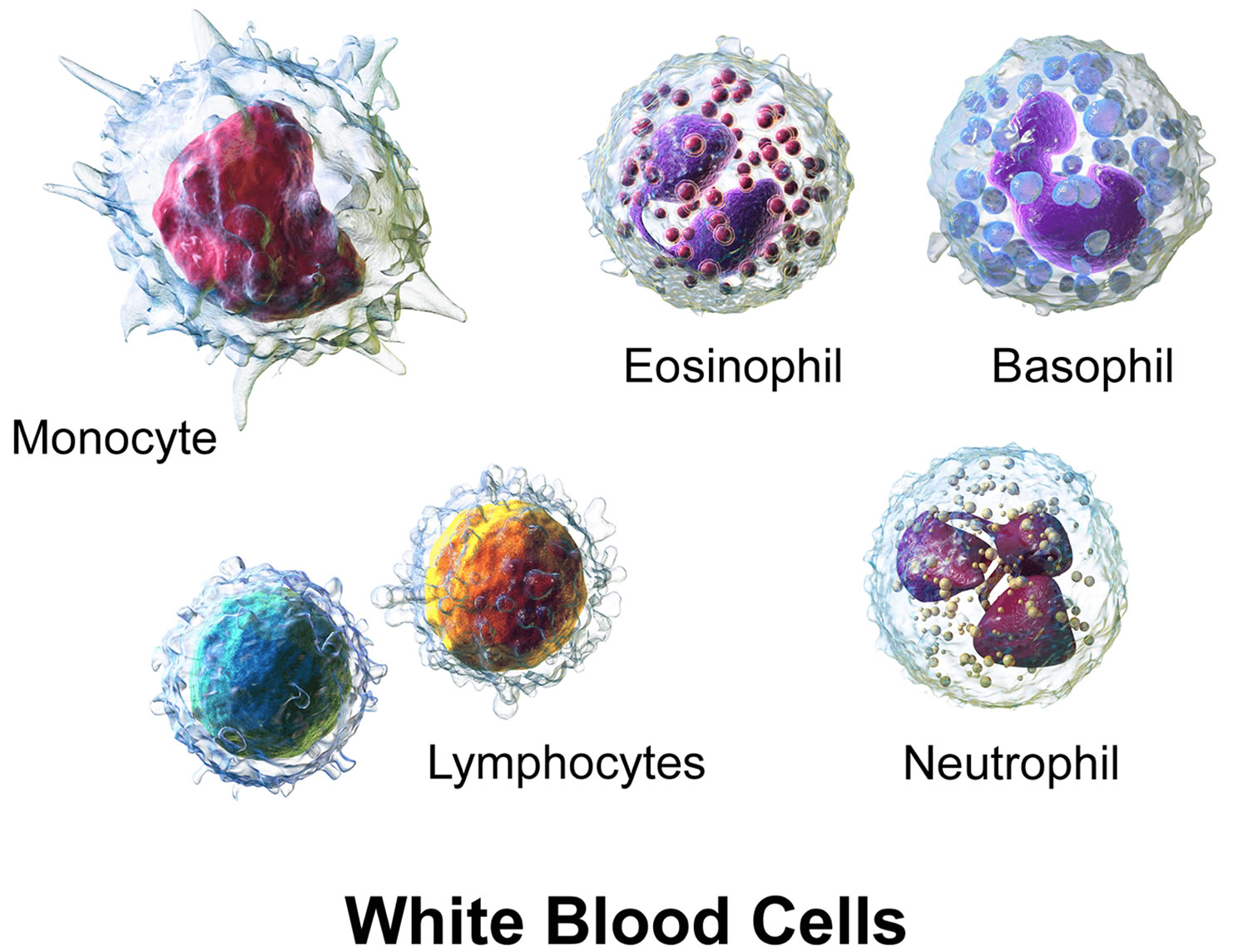
There are different types of white blood cells (WBCs):
- Granulocytes are mature white blood cells that develop from myeloblasts, a type of blood-forming cell in the bone marrow. Granulocytes have granules that show up as spots under the microscope. These granules contain enzymes and other substances that can destroy germs, such as bacteria. The 3 types of granulocytes – neutrophils, basophils, and eosinophils – are distinguished by the size and color of their granules.
- Monocytes are white blood cells that develop from blood-forming monoblasts in the bone marrow. After circulating in the bloodstream for about a day, monocytes enter body tissues to become macrophages, which can destroy some germs by surrounding and digesting them. Macrophages also help lymphocytes recognize germs and make antibodies to fight them.
- Lymphocytes are mature white blood cells that develop from lymphoblasts in the bone marrow. Lymphocytes are the main cells that make up lymph tissue, a major part of the immune system. Lymph tissue is found in lymph nodes, the thymus (a small organ behind the breast bone), the spleen, the tonsils and adenoids, and is scattered throughout the digestive and respiratory systems and the bone marrow. The 2 main types of lymphocytes are B cell and T cells.
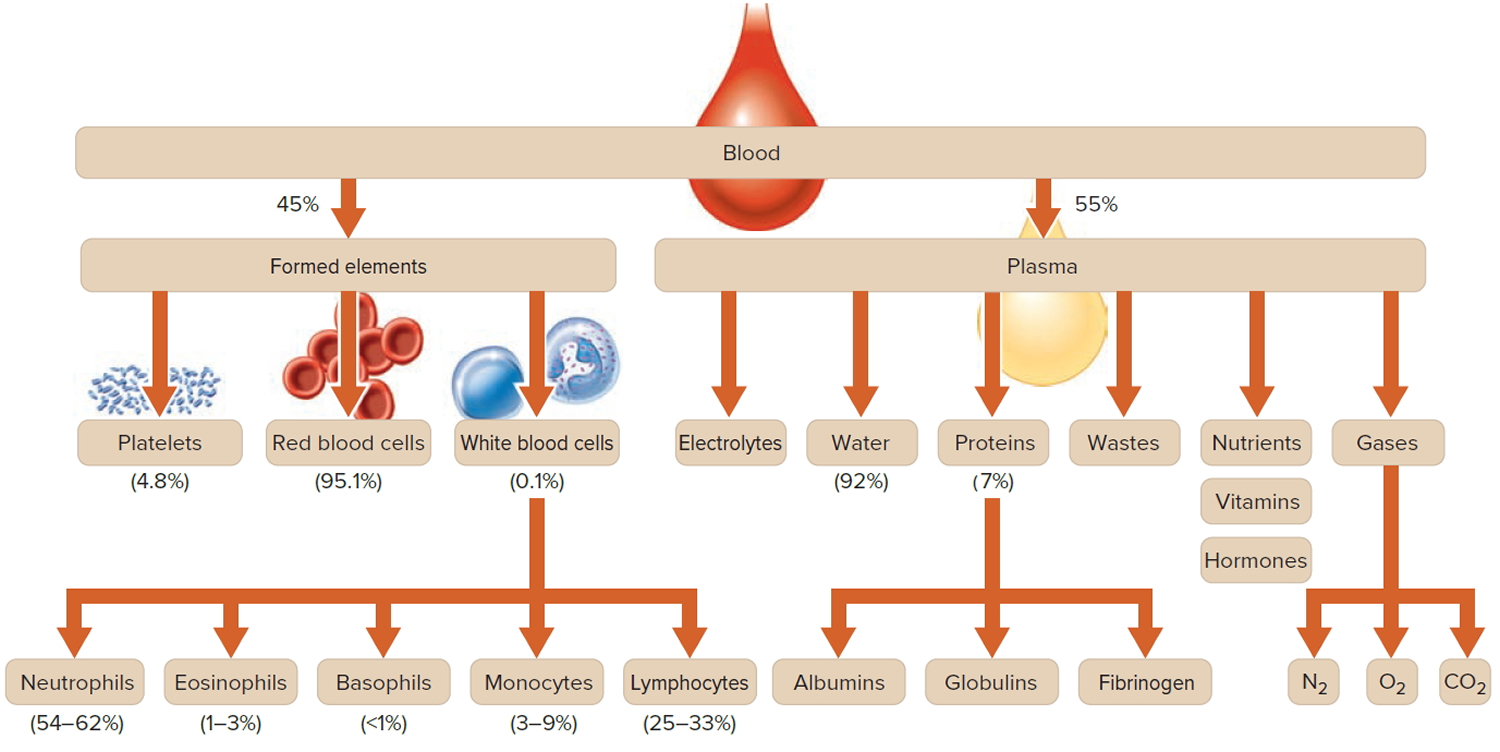
Figure 1. Blood composition
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 2. Bone marrow anatomy
Figure 3. White blood cells development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. White blood cells development
Figure 5. White blood cells
Causes of acute lymphoblastic leukemia in childhood
Acute lymphoblastic leukemia (acute lymphocytic leukemia) results from either an acquired or a genetic injury to the DNA (deoxyribonucleic acid) genetic material of a developing stem cell in the bone marrow. Stem cells form blood cells (white cells, red cells and platelets). Doctors don’t know why some stem cells become leukemic cells and others don’t. Usually DNA mutations associated with acute lymphoblastic leukemia occur during a person’s lifetime rather than being inherited from a parent. For most people who have acute lymphoblastic leukemia (ALL), there are no obvious reasons why they developed the disease.
Although acute lymphoblastic leukemia starts in a stem cell in the bone marrow, it can spread to other areas such as the central nervous system, the lymph nodes and, more rarely, the testes.
Great progress has been made in understanding how certain changes in the DNA in normal bone marrow cells can cause them to become leukemia cells. The DNA inside your cells makes up your genes, which control how your cells function.
Some genes control when your cells grow, divide to make new cells, and die at the right time:
- Certain genes that help cells grow, divide, or stay alive are called oncogenes.
- Genes that keep cell growth and division under control or make cells die at the right time are called tumor suppressor genes.
Each time a cell divides into 2 new cells, it must make a new copy of its chromosomes (long strands of DNA). This process isn’t perfect, and errors can occur that can affect genes within the chromosomes. Cancers (including acute lymphocytic leukemia) can be caused by mutations (changes) that turn on oncogenes or turn off tumor suppressor genes. These types of changes can stop bone marrow cells from maturing the way they normally would, or help the cells grow out of control.
Mutations in many different genes can be found in acute lymphocytic leukemia cells, but larger changes in one or more chromosomes are also common. Even though these changes involve larger pieces of DNA, their effects are still likely to be due to changes in just one or a few genes that are on that part of the chromosome.
Several types of chromosome changes may be found in acute lymphocytic leukemia cells:
Translocations are the most common type of chomosome change that can lead to leukemia. A translocation means that DNA from one chromosome breaks off and becomes attached to a different chromosome. The point on the chromosome where the break occurs can affect nearby genes – for example, it can turn on oncogenes or turn off genes that would normally help a cell mature.
The most common translocation in acute lymphocytic leukemia in adults is known as the Philadelphia chromosome, which is a swap of DNA between chromosomes 9 and 22, abbreviated as t(9;22). Many other, less common translocations, can occur as well, including those between chromosomes 4 and 11, t(4;11), or 8 and 14, t(8;14).
In Philadelphia chromosome positive acute lymphoblastic leukemia (also known as “Ph+ ALL” or “Ph-positive ALL”), an abnormal change happens to chromosomes 9 and 22. Part of chromosome 9 breaks off where the gene ABL1 is located and part of chromosome 22 breaks off where the BCR gene is located. The broken parts swap places creating a new gene called BCR-ABL1 on chromosome 22, which causes the cell to make too much of a protein called tyrosine kinase. This protein encourages leukaemia cells to grow and multiply out of control. Doctors treat Philadelphia positive acute lymphoblastic leukemia with a targeted cancer drug called imatinib (tyrosine kinase blocker), which blocks this protein. You take them as tablets.
About 15 percent of children with acute lymphoblastic leukemia have a subtype of B-cell ALL called Philadelphia chromosome-like ALL (“Ph-like ALL”). This is a high-risk subtype of acute lymphoblastic leukemia in children that seems to peak in adolescents and young adults and is more likely to be seen in males and patients with Down syndrome. It is associated with an unfavorable prognosis. Ph-like ALL has genetic features similar to Ph+ ALL, but without the BCR-ABL1 fusion gene that defines Ph+ ALL. Instead, patients have a highly diverse range of genetic mutations that activate tyrosine kinase signaling.
Other chromosome changes such as deletions (the loss of part of a chromosome) and inversions (the rearrangement of the DNA within part of a chromosome) are also sometimes found in acute lymphocytic leukemia cells, although they are less common. In many cases of acute lymphocytic leukemia, the gene changes that lead to the leukemia are not known.
Doctors are trying to figure out why these changes occur and how each of them might lead to leukemia. But there are different subtypes of acute lymphocytic leukemia, and even within a subtypes, not all cases of acute lymphocytic leukemia have the same gene or chromosome changes. Some changes are more common than others, and some seem to have more of an effect on a person’s prognosis (outlook) than others.
Risk factors for developing acute lymphoblastic leukemia
Few factors associated with an increased risk of acute lymphoblastic leukemia have been identified. The primary accepted risk factors for acute lymphoblastic leukemia and associated genes (when relevant) include the following:
- Prenatal exposure to x-rays. If a fetus is exposed to radiation within the first months of development, there may also be an increased risk of childhood leukemia, but the extent of the risk is not clear.
- Postnatal exposure to high doses of radiation (e.g., therapeutic radiation as previously used for conditions such as tinea capitis and thymus enlargement). Exposure to high levels of radiation is a risk factor for childhood leukemia. Japanese atomic bomb survivors had a greatly increased risk of developing acute myeloid leukemia (AML). The possible risks from fetal or childhood exposure to lower levels of radiation, such as from x-ray tests or CT scans, are not known for sure. Some studies have found a slight increase in risk, while others have found no increased risk. Any risk increase is likely to be small, but to be safe, most doctors recommend that pregnant women and children not get these tests unless they are absolutely needed.
- Previous treatment with chemotherapy. Children and adults treated for other cancers with certain chemotherapy drugs have a higher risk of getting a second cancers, usually acute myeloid leukemia (AML), later in life. Drugs such as cyclophosphamide, doxorubicin, etoposide, and teniposide have been linked to a higher risk of leukemia. These leukemias usually develop within 5 to 10 years of treatment, and they tend to be hard to treat.
- Genetic conditions that include the following:
- Down syndrome (trisomy 21): Children with Down syndrome have an extra (third) copy of chromosome 21. They are many times more likely to develop either acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML) than are other children, with an overall risk of about 2% to 3%. Down syndrome has also been linked with transient leukemia (also known as transient myeloproliferative disorder) – a leukemia-like condition within the first month of life, which often resolves on its own without treatment.
- Neurofibromatosis (NF1) 10
- Bloom syndrome (BLM) 11
- Fanconi anemia (multiple genes; acute lymphoblastic leukemia is observed much less frequently than acute myeloid leukemia [AML]) 12
- Ataxia telangiectasia (ATM) 13
- Li-Fraumeni syndrome (TP53). This is a rare inherited condition caused by a change in the TP53 gene. People with this change have a higher risk of developing several kinds of cancer, including leukemia, bone or soft tissue sarcomas, breast cancer, adrenal gland cancer, and brain tumors. 14
- Constitutional mismatch repair deficiency (biallelic mutation of MLH1, MSH2, MSH6, and PMS2) 15
- Low- and high-penetrance inherited genetic variants 16
- Carriers of a constitutional Robertsonian translocation that involves chromosomes 15 and 21 are specifically and highly predisposed to developing intrachromosomal amplification of chromosome 21 (iAMP21) acute lymphoblastic leukemia 17.
- Having a brother or sister with leukemia. Siblings (brothers and sisters) of children with leukemia have a slightly increased chance of developing leukemia, but the overall risk is still low. The risk is much higher among identical twins. If one twin develops childhood leukemia, the other twin has about a 1 in 5 chance of getting leukemia as well. This risk is much higher if the leukemia develops in the first year of life.
- Exposure to chemicals such as benzene (a solvent used in the cleaning industry and to manufacture some drugs, plastics, and dyes) may cause acute leukemia in adults and, rarely, in children. Chemical exposure is more strongly linked to an increased risk of AML than to acute lymphoblastic leukemia.
- Exposure to pesticides. Several studies have found a possible link between childhood leukemia and household exposure to pesticides, either during pregnancy or early childhood. Some studies have also found a possible increased risk among mothers with workplace exposure to pesticides before their child is born. However, most of these studies had serious limitations in the way they were done. More research is needed to try to confirm these findings and to provide more specific information about the possible risks.
- Immune system suppression. Children who are getting intensive treatment to suppress their immune system (mainly children who have had organ transplants) have an increased risk of certain cancers, such as lymphoma and acute lymphoblastic leukemia.
Symptoms of acute lymphoblastic leukemia in childhood
Acute lymphoblastic leukemia (acute lymphocytic leukemia) can cause many different signs and symptoms. Acute lymphoblastic leukaemia usually starts slowly before rapidly becoming severe as the number of immature white blood cells in your blood increases. Most of the symptoms are caused by the lack of healthy blood cells (red cells, white cells and platelets) in your blood supply.
Symptoms of a low red blood cell count (anemia) include:
- Fatigue
- Shortness of breath during normal physical activities
- Dizziness
- Pale complexion
Symptoms of a low white blood count (leukopenia or neutropenia) include:
- Frequent infections
- Recurrent fevers
Symptoms of a low platelet count (thrombocytopenia) include:
- Bruising easily
- Prolonged bleeding from minor cuts
- The appearance of pinhead-sized red spots on the skin, called “petechiae”
- Frequent or severe nosebleeds
- Bleeding gums
- Heavier or more frequent menstrual periods in females.
Symptoms may also be related to leukemia cells collecting in other parts of the body. These symptoms may include:
- Unexplained weight loss or loss of appetite
- Pain in bones and joints
- Swollen lymph nodes
- Enlarged spleen or liver
- Abdominal pain
- Wheezing, coughing or painful breathing
The symptoms listed above are common symptoms of acute lymphoblastic leukemia, but do not include all possible symptoms, as children may experience symptoms differently. It is also important to note that the symptoms of acute lymphoblastic leukemia may be similar to those of other blood disorders or medical conditions. Speak with your doctor if your child has any of the above symptoms to ensure proper diagnosis and treatment.
Symptoms caused by low numbers of blood cells
Most signs and symptoms of acute lymphocytic leukemia are the result of shortages of normal blood cells (red cells, white cells and platelets), which happen when the leukemia cells crowd out the normal blood-making cells in the bone marrow. These shortages show up on blood tests, but they can also cause symptoms, including:
- Feeling tired
- Feeling weak
- Feeling dizzy or lightheaded
- Shortness of breath
- Pale skin
- Infections that don’t go away or keep coming back
- Bruises (or small red or purple spots) on the skin
- Bleeding, such as frequent or severe nosebleeds, bleeding gums, or heavy menstrual bleeding in women
General symptoms
Patients with acute lymphocytic leukemia also often have several non-specific symptoms. These can include:
- Weight loss
- Fever
- Night sweats
- Loss of appetite
Of course, these are not just symptoms of acute lymphocytic leukemia and are more often caused by something other than leukemia.
Swelling in the abdomen
Leukemia cells may build up in the liver and spleen, making them larger. This might be noticed as a fullness or swelling of the belly, or feeling full after eating only a small amount. The lower ribs usually cover these organs, but when the organs are enlarged the doctor can feel them.
Enlarged lymph nodes
Acute lymphocytic leukemia that has spread to lymph nodes close to the surface of the body (such as on the sides of the neck, in the groin, or in underarm areas), might be noticed as lumps under the skin. Lymph nodes inside the chest or abdomen may also swell, but these can be detected only by imaging tests such as CT or MRI scans.
Bone or joint pain
Sometimes leukemia cells build up near the surface of the bone or inside the joint, which can lead to bone or joint pain.
Spread to other organs
Less often, acute lymphocytic leukemia spreads to other organs:
- If acute lymphocytic leukemia spreads to the brain and spinal cord it can cause headaches, weakness, seizures, vomiting, trouble with balance, facial muscle weakness or numbness, or blurred vision.
- Acute lymphocytic leukemia may spread inside the chest, where it can cause fluid buildup and trouble breathing.
- Rarely, acute lymphocytic leukemia may spread to the skin, eyes, testicles, ovaries, kidneys, or other organs.
Symptoms from an enlarged thymus
The T-cell subtype of acute lymphocytic leukemia often affects the thymus, which is a small organ in the middle of the chest behind the sternum (breastbone) and in front of the trachea (windpipe). An enlarged thymus can press on the trachea, which can lead to coughing or trouble breathing.
The superior vena cava, a large vein that carries blood from the head and arms back to the heart, passes next to the thymus. If the thymus is enlarged, it may press on the superior vena cava, causing the blood to “back up” in the veins. This is known as superior vena cava syndrome. It can cause:
- Swelling in the face, neck, arms, and upper chest (sometimes with a bluish-red color)
- Headaches
- Dizziness
- Change in consciousness if it affects the brain
The superior vena cava syndrome can be life-threatening, and needs to be treated right away.
Childhood acute lymphoblastic leukemia prevention
There are very few known lifestyle-related or environmental causes of childhood leukemias, so it is important to know that in most cases there is nothing these children or their parents could have done to prevent these cancers.
Childhood acute lymphoblastic leukemia prognosis
Among children with acute lymphoblastic leukemia, approximately 98% attain remission 18. Approximately 85% of patients aged 1 to 18 years with newly diagnosed acute lymphoblastic leukemia treated on current regimens are expected to be long-term event-free survivors, with over 90% surviving at 5 years 19. Cytogenetic and genomic findings combined with minimal residual disease results can define subsets of acute lymphoblastic leukemia with event-free survival rates exceeding 95% and, conversely, subsets with event-free survival rates of 50% or lower 18.
Despite the treatment advances in childhood acute lymphoblastic leukemia, numerous important biologic and therapeutic questions remain to be answered before the goal of curing every child with acute lymphoblastic leukemia with the least associated toxicity can be achieved 18. The systematic investigation of these issues requires large clinical trials, and the opportunity to participate in these trials is offered to most patients and families.
Clinical trials for children and adolescents with acute lymphoblastic leukemia are generally designed to compare therapy that is currently accepted as standard with investigational regimens that seek to improve cure rates and/or decrease toxicity 18. In certain trials in which the cure rate for the patient group is very high, therapy reduction questions may be asked. Much of the progress made in identifying curative therapies for childhood acute lymphoblastic leukemia and other childhood cancers has been achieved through investigator-driven discovery and tested in carefully randomized, controlled, multi-institutional clinical trials. Information about ongoing clinical trials is available from the National Cancer Institute website (https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/advanced).
Childhood acute lymphoblastic leukemia prognostic factors
Childhood acute lymphoblastic leukemia risk factors at initial presentation, which can also be used to inidicate a poor outcome or the chances of a relapse occurring, include the following:
- Age at diagnosis. Younger than 6 months and older than 60 years
- High white blood cell (WBC) count at diagnosis. High white blood cell count of greater than 300,000 white blood cells x 106 cells/L
- T-cell lymphocyte acute lymphoblastic leukemia (T-ALL) rather than B-cell lymphocyte acute lymphoblastic leukemia (B-ALL).
- Central nervous system (CNS) involvement at diagnosis.
- Testicular involvement at diagnosis.
- Down syndrome (trisomy 21).
- Male sex, possibly because of the impact of a major relapse site being in the testicles
- Race and ethnicity.
- Weight at diagnosis and during treatment.
- Certain abnormal gene rearrangements can also act as prognostic risk factors. These include:
- t(9;22) BCR-ABL1 (Philadelphia chromosome)
- MLL (KMT2A) translocations
- t(17;19) TCF3-HLF
- Near haploidy (24-30 chromosomes). Haploidy is having only half of the normal number of 46 chromosomes
- Low hypodiploidy (31–39 chromosomes). Hypodiploidy is having less than the normal number of 46 chromosomes
Age at diagnosis
Age at diagnosis has strong prognostic significance, reflecting the different underlying biology of acute lymphoblastic leukemia in different age groups 20.
Infants (younger than 1 year)
Infants with acute lymphoblastic leukemia have a particularly high risk of treatment failure. Treatment failure is most common in the following groups:
- Infants younger than 6 months (with an even poorer prognosis for those aged ≤90 days) 21.
- Infants with extremely high presenting leukocyte counts (>200,000–300,000 × 109/L) 22.
- Infants with a poor response to a prednisone prophase 22.
- Infants with a KMT2A (MLL-R) gene rearrangement (rearranged MPAL with KMT2A) 23.
Up to 80% of infants with acute lymphoblastic leukemia have a translocation of 11q23 with numerous chromosome partners generating a KMT2A gene rearrangement 21. The most common rearrangement is KMT2A-AFF1 (t(4;11)(q21;q23)), but KMT2A rearrangements with many other translocation partners are observed.
The rate of KMT2A gene rearrangements is extremely high in infants younger than 6 months; from 6 months to 1 year, the incidence of KMT2A rearrangements decreases but remains higher than that observed in older children.[9,15] Black infants with acute lymphoblastic leukemia are significantly less likely to have KMT2A rearrangements than are White infants 24.
Infants with leukemia and KMT2A rearrangements typically have very high white blood cell (WBC) counts and an increased incidence of central nervous system (brain & spinal cord) involvement. Event-free survival and overall survival are poor, with 5-year event-free survival and overall survival rates of only 35% to 40% for infants with KMT2A-rearranged acute lymphoblastic leukemia 22. A comparison of the landscape of somatic mutations in infants and children with KMT2A-rearranged acute lymphoblastic leukemia revealed significant differences between the two groups, suggesting distinctive age-related biological behaviors for KMT2A-rearranged acute lymphoblastic leukemia that may relate to the significantly poorer outcome for infants 25.
Blasts from infants with KMT2A rearrangements are often CD10 negative and express high levels of FLT3.[9,10,14,18] Conversely, infants whose leukemic cells show a germline KMT2A gene configuration frequently present with CD10-positive precursor-B immunophenotype. These infants have a significantly better outcome than do infants with acute lymphoblastic leukemia characterized by KMT2A rearrangements 26.
Young children (aged 1 to <10 years)
Young children (aged 1 to <10 years) have a better disease-free survival than older children, adolescents, and infants 27. The improved prognosis in young children is at least partly explained by the more frequent occurrence of favorable cytogenetic features in the leukemic blasts, including hyperdiploidy with 51 to 65 chromosomes and/or favorable chromosome trisomies, or the ETV6-RUNX1 fusion (t(12;21)(p13;q22), also known as the TEL-AML1 translocation) 28.
Adolescents and young adults (aged ≥10 years)
In general, the outcome of patients aged 10 years and older is inferior to that of patients aged 1 to younger than 10 years. However, the outcome for older children, especially adolescents, has improved significantly over time 29. Five-year survival rates for adolescents aged 15 to 19 years increased from 36% (1975–1984) to 72% (2003–2009) 1.
Multiple retrospective studies have established that adolescents aged 16 to 21 years have a better outcome when treated on pediatric versus adult protocols 30.
White blood cell count at diagnosis
A white blood cell (WBC) count of 50,000/µL is generally used as an operational cut point between better and poorer prognosis, although the relationship between white blood cell count and prognosis is a continuous function rather than a step function 31. Patients with B cell acute lymphoblastic leukemia (B-ALL) and high white blood cell counts at diagnosis have an increased risk of treatment failure compared with patients with low initial white blood cell counts 32.
The median white blood cell count at diagnosis is much higher for T cell acute lymphoblastic leukemia (T-ALL) (>50,000/µL) than for B cell acute lymphoblastic leukemia (B-ALL) (<10,000/µL), and there is no consistent effect of white blood cell count at diagnosis on prognosis for T-ALL 33.
Central nervous system (CNS) involvement at diagnosis
The presence or absence of central nervous system (CNS or brain & spinal cord) leukemia at diagnosis has prognostic significance. Patients who have a nontraumatic diagnostic lumbar puncture may be placed into one of three categories according to the number of white blood cell/µL and the presence or absence of blasts on cytospin as follows:
- CNS1: Cerebrospinal fluid (CSF) that is cytospin negative for blasts regardless of white blood cell count.
- CNS2: CSF with fewer than 5 white blood cell/µL and cytospin positive for blasts.
- CNS3 (CNS disease): CSF with 5 or more white blood cell/µL and cytospin positive for blasts.
Children with ALL who present with CNS disease (CNS3) at diagnosis are at a higher risk of treatment failure (both within the CNS and systemically) than are patients who are classified as CNS1 or CNS2 34. Some studies have reported increased risk of central nervous system (CNS) relapse and/or inferior event-free survival in CNS2 patients, compared with CNS1 patients 35, while others have not 36, 37.
A traumatic lumbar puncture (≥10 erythrocytes/µL) that includes blasts at diagnosis has also been associated with increased risk of CNS relapse and overall poorer outcome in some studies 38, but not others 35. Patients with CNS2, CNS3, or traumatic lumbar puncture have a higher frequency of unfavorable prognostic characteristics than do those with CNS1, including significantly higher white blood cell counts at diagnosis, older age at diagnosis, an increased frequency of the T-ALL phenotype, and KMT2A gene rearrangements 36.
Most clinical trial groups have approached the treatment of CNS2 and traumatic lumbar puncture patients by utilizing more intensive therapy, primarily additional doses of intrathecal therapy during induction 39.
To determine whether a patient with a traumatic lumbar puncture (with blasts) should be treated as CNS3, the Children’s Oncology Group uses an algorithm relating the white blood cell and red blood cell counts in the spinal fluid and the peripheral blood 40.
Testicular involvement at diagnosis
Overt testicular involvement at the time of diagnosis occurs in approximately 2% of males 41, with its frequency being higher in patients with T cell acute lymphoblastic leukemia (T-ALL) than in patients with B cell acute lymphoblastic leukemia (B-ALL) 42.
In early acute lymphoblastic leukemia trials, testicular involvement at diagnosis was an adverse prognostic factor. With more aggressive initial therapy, however, it does not appear that testicular involvement at diagnosis has prognostic significance 42. For example, the European Organization for Research and Treatment of Cancer (EORTC [EORTC-58881]) reported no adverse prognostic significance for overt testicular involvement at diagnosis 42.
The role of radiation therapy for testicular involvement is unclear. A study from St. Jude Children’s Research Hospital suggests that a good outcome can be achieved with aggressive conventional chemotherapy without radiation 41. The Children’s Oncology Group has also adopted this strategy for boys with testicular involvement that resolves completely by the end of induction therapy. The Children’s Oncology Group considers patients with testicular involvement to be high risk regardless of other presenting features, but most other large clinical trial groups in the United States and Europe do not consider testicular disease to be a high-risk feature.
Down syndrome (trisomy 21)
Outcomes in children with Down syndrome and acute lymphoblastic leukemia have often been reported as somewhat inferior to outcomes observed in children who do not have Down syndrome 43, although on some studies, patients with Down syndrome appear to fare as well as patients without Down syndrome 44. The lower event-free survival and overall survival of children with Down syndrome appear to be related to increased frequency of treatment-related mortality, as well as higher rates of induction failure and relapse 45. The inferior anti-leukemic outcome may be due, in part, to the decreased prevalence of favorable biological features such as ETV6-RUNX1 or hyperdiploidy (51–65 chromosomes) with trisomies of chromosomes 4 and 10 in Down syndrome acute lymphoblastic leukemia patients 45.
- In a large retrospective study that included 653 patients with Down syndrome and acute lymphoblastic leukemia, Down syndrome patients had a lower complete remission rate (97% vs. 99%), higher cumulative incidence of relapse (26% vs. 15%) and higher treatment-related mortality (7% vs. < 1%) compared with non-Down syndrome patients 45. Among the patients with Down syndrome, age younger than 6 years, white blood cell (WBC) count of less than 10,000/µL, and the presence of the ETV6-RUNX1 fusion (observed in 8% of patients) were independent predictors of favorable event-free survival.
- In a report from the Children’s Oncology Group, among patients with B-ALL who lacked KMT2A rearrangements, BCR-ABL1, ETV6-RUNX1, and hyperdiploidy with trisomies of chromosomes 4 and 10, the event-free survival and overall survival rates were similar in children with and without Down syndrome 46.
- Certain genomic abnormalities, such as IKZF1 deletions, CRLF2 aberrations, and JAK mutations are seen more frequently in acute lymphoblastic leukemia arising in children with Down syndrome than in those without Down syndrome 47. Studies of children with Down syndrome and acute lymphoblastic leukemia suggest that the presence of IKZF1 deletions (but not CRLF2 aberrations or JAK mutations) is associated with an inferior prognosis 48.
Sex
In some studies, the prognosis for girls with acute lymphoblastic leukemia is slightly better than it is for boys with acute lymphoblastic leukemia 49. One reason for the better prognosis for girls is the occurrence of testicular relapses among boys, but boys also appear to be at increased risk of bone marrow and CNS relapse for reasons that are not well understood 49. While some reports describe outcomes for boys as closely approaching those of girls 50, larger clinical trial experiences and national data continue to show somewhat lower survival rates for boys 51.
Race and ethnicity
Over the last several decades in the United States, survival rates in Black and Hispanic children with acute lymphoblastic leukemia have been somewhat lower than the rates in White children with acute lymphoblastic leukemia 52.
The following factors associated with race and ethnicity influence survival:
- Acute lymphoblastic leukemia subtype. The reason for better outcomes in White and Asian children than in Black and Hispanic children is at least partially explained by the different spectrum of acute lymphoblastic leukemia subtypes. For example, Black children have a higher relative incidence of T-cell acute lymphoblastic leukemia (T-ALL) and lower rates of favorable genetic subtypes of B-cell acute lymphoblastic leukemia (B-ALL).
- Treatment adherence. Differences in outcome may also be related to treatment adherence, as illustrated by a study of adherence to oral mercaptopurine (6-MP) in maintenance therapy. In the first report from the study, there was an increased risk of relapse in Hispanic children compared with non-Hispanic White children, depending on the level of adherence, even when adjusting for other known variables. However, even with adherence rates of 90% or more, Hispanic children continued to demonstrate increased rates of relapse 53. In the second report from the study, adherence rates were shown to be significantly lower in Asian American and African American patients than in non-Hispanic White patients. A greater percentage of patients in these ethnic groups had adherence rates of less than 90%, which was associated with a 3.9-fold increased risk of relapse 54.
- Ancestry-related genomic variations. Ancestry-related genomic variations may also contribute to racial and ethnic disparities in both the incidence and outcome of acute lymphoblastic leukemia 55. For example, the differential presence of specific host polymorphisms in different racial and ethnic groups may contribute to outcome disparities, as illustrated by the occurrence of single nucleotide polymorphisms in the ARID5B gene that occur more frequently among Hispanic patients and are linked to both acute lymphoblastic leukemia susceptibility and to relapse hazard 56.
Weight at diagnosis and during treatment
Studies of the impact of obesity on the outcome of acute lymphoblastic leukemia have had variable results. In most of these studies, obesity is defined as weight above the 95th percentile for age and height.
- Three studies did not demonstrate an independent effect of obesity on event-free survival 57.
- Two studies showed obesity to be an independent prognostic factor only in patients older than 10 years or in patients with intermediate-risk or high-risk disease 58.
- The Children’s Oncology Group reported on the impact of obesity on outcome in 2,008 children, 14% of whom were obese, who were enrolled on a high-risk acute lymphoblastic leukemia trial 59. Obesity was found to be an independent variable for inferior outcome compared with nonobese patients (5-year event-free survival rates, 64% vs. 74%). However, obese patients at diagnosis who then normalized their weight during the premaintenance period of treatment had outcomes similar to patients with normal weight at diagnosis.
- In a retrospective study of patients treated at a single institution, obesity at diagnosis was linked to an increased risk of having minimal residual disease at the end of induction and an inferior event-free survival 60.
- In a different retrospective study of 373 patients treated at a single institution, body mass index (BMI) at diagnosis was not associated with minimal residual disease at days 19 and 46, cumulative incidence of relapse, or event-free survival. overall survival was lower in patients with a high BMI, primarily resulting from treatment-related mortality and inferior salvage after relapse 61.
In a study of 762 pediatric patients with acute lymphoblastic leukemia (aged 2–17 years), the Dutch Childhood Oncology Group found that those who were underweight at diagnosis (8% of the population) had an almost twofold higher risk of relapse compared with patients who were not underweight (after adjusting for risk group and age), although this did not result in a difference in event-free survival or overall survival. Patients with a decrease in BMI during the first 32 weeks of treatment had similar rates of relapse as other patients, but had significantly worse overall survival, primarily because of poorer salvage rates after relapse 62.
Leukemic characteristics
Leukemic cell characteristics affecting prognosis include the following:
- Immunophenotype.
- Cytogenetics/genomic alterations.
Immunophenotype
The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia classifies acute lymphoblastic leukemia as either B-lymphoblastic leukemia (B-ALL) or T-lymphoblastic leukemia (T-ALL), with further subdivisions based on molecular characteristics 63.
Either B- or T-lymphoblastic leukemia can coexpress myeloid antigens. These cases need to be distinguished from leukemia of ambiguous lineage.
B-ALL (WHO B-lymphoblastic leukemia)
Before 2008, the WHO classified B-lymphoblastic leukemia as precursor B-lymphoblastic leukemia, and this terminology is still frequently used in the literature of childhood acute lymphoblastic leukemia to distinguish it from mature B-cell acute lymphoblastic leukemia. Mature B-cell acute lymphoblastic leukemia is now termed Burkitt leukemia and requires different treatment than has been given for B-ALL (precursor B-cell acute lymphoblastic leukemia).
B-ALL, defined by the expression of CD19, HLA-DR, cytoplasmic CD79a, and other B-cell–associated antigens, accounts for 80% to 85% of childhood acute lymphoblastic leukemia. Approximately 90% of B-ALL cases express the CD10 surface antigen (formerly known as common acute lymphoblastic leukemia antigen [cALLa]). Absence of CD10 is usually associated with KMT2A rearrangements, particularly t(4;11)(q21;q23), and a poor outcome 64. It is not clear whether CD10-negativity has any independent prognostic significance in the absence of a KMT2A gene rearrangement 65.
The major immunophenotypic subtypes of B-ALL are as follows:
- Common B-ALL (CD10 positive and no surface or cytoplasmic immunoglobulin [Ig]). Approximately three-quarters of patients with B-ALL have the common precursor B-cell immunophenotype and have the best prognosis. Patients with favorable cytogenetics almost always show a common precursor B-cell immunophenotype.
- Pro-B ALL (CD10 negative and no surface or cytoplasmic Ig). Approximately 5% of patients have the pro-B immunophenotype. Pro-B is the most common immunophenotype seen in infants and is often associated with KMT2A gene rearrangements.
- Pre-B ALL (presence of cytoplasmic Ig). The leukemic cells of patients with pre-B ALL contain cytoplasmic Ig, and 25% of patients with pre-B ALL have the t(1;19)(q21;p13) translocation with the TCF3-PBX1 (previously known as E2A-PBX1) fusion 66. Approximately 3% of patients have transitional pre-B ALL with expression of surface Ig heavy chain without expression of light chain, MYC gene involvement, or L3 morphology. Patients with this phenotype respond well to therapy used for B-ALL 67.
- Mature B-ALL (Burkitt lymphoma/leukemia). Approximately 2% of patients present with mature B-cell leukemia (surface Ig expression, generally with French-American-British criteria L3 morphology and an 8q24 translocation involving MYC), also called Burkitt leukemia. The treatment for mature B-cell acute lymphoblastic leukemia is based on therapy for non-Hodgkin lymphoma and is completely different from the treatment for B-ALL. Rare cases of mature B-cell leukemia that lack surface Ig but have L3 morphology with MYC gene translocations should also be treated as mature B-cell leukemia 67. A small number of cases of IG-MYC-translocated leukemias with precursor B-cell immunophenotype (e.g., absence of CD20 expression and surface Ig expression) have been reported 68. These cases presented in both children and adults. Like Burkitt lymphoma/leukemia, they had a male predominance and most patients showed L3 morphology. The cases lacked mutations in genes recurrently altered in Burkitt lymphoma (e.g., ID3, CCND3, or MYC), whereas mutations in RAS genes (frequently altered in B-ALL) were common. The clinical significance of IG-MYC–translocated leukemias with precursor B-cell phenotype and molecular characteristics requires further study.
T-ALL
T-ALL is defined by expression of the T-cell–associated antigens (cytoplasmic CD3, with CD7 plus CD2 or CD5) on leukemic blasts. T-ALL is frequently associated with a constellation of clinical features, including the following 69.:
- Male sex.
- Older age.
- Leukocytosis.
- Mediastinal mass.
While not true historically, with appropriately intensive therapy, children with T-ALL now have an outcome approaching that of children with B-lineage acute lymphoblastic leukemia 70.
There are few commonly accepted prognostic factors for patients with T-ALL. Conflicting data exist regarding the prognostic significance of presenting leukocyte counts in T-ALL 71. The presence or absence of a mediastinal mass at diagnosis has no prognostic significance. In patients with a mediastinal mass, the rate of regression of the mass lacks prognostic significance 72.
- Early T-cell precursor (ETP) acute lymphoblastic leukemia (ALL)
- ETP acute lymphoblastic leukemia, a distinct subset of childhood T-ALL, was initially defined by identifying T-ALL cases with gene expression profiles highly related to expression profiles for normal early T-cell precursors.[106] The subset of T-ALL cases, identified by these analyses represented 13% of all cases and they were characterized by a distinctive immunophenotype (CD1a and CD8 negativity, with weak expression of CD5 and coexpression of stem cell or myeloid markers).
- Initial reports describing ETP acute lymphoblastic leukemia suggested that this subset of patients has a poorer prognosis than other patients with T-ALL 73. In addition, some studies have reported that these patients have a slower early response and higher frequency of induction failure than other patients with T-ALL. Other studies have observed a more favorable outcome for patients with ETP acute lymphoblastic leukemia, including one study from the U.K. Medical Research Council that showed that the ETP acute lymphoblastic leukemia subgroup of patients had nonsignificantly inferior 5-year event-free survival rates compared with non–ETP patients (76% vs. 84%) 74. Similarly, in the Children’s Oncology Group AALL0434 [NCT00408005] trial 75, ETP status did not have a statistically significant impact on disease-free survival (hazard ratio, 0.99) on multivariable analysis 76. Further study in additional patient cohorts is needed to firmly establish the prognostic significance of early T-cell precursor acute lymphoblastic leukemia, but most acute lymphoblastic leukemia treatment groups do not change patient treatment on the basis of early T-cell precursor status.
Myeloid antigen expression
Up to one-third of childhood acute lymphoblastic leukemia patients have leukemia cells that express myeloid-associated surface antigens. Myeloid-associated antigen expression appears to be associated with specific acute lymphoblastic leukemia subgroups, notably those with KMT2A rearrangements, ETV6-RUNX1, and BCR-ABL1 77. Patients with B-ALL who have gene rearrangements involving ZNF384 also commonly show myeloid antigen expression 78. No independent adverse prognostic significance exists for myeloid-surface antigen expression 79.
Response to initial treatment
The rapidity with which leukemia cells are eliminated after initiation of treatment and the level of residual disease at the end of induction are associated with long-term outcome. Because treatment response is influenced by the drug sensitivity of leukemic cells and host pharmacodynamics and pharmacogenomics, early response has strong prognostic significance 80.
Relapsed childhood acute lymphoblastic leukaemia
Relapsed acute lymphoblastic leukemia or relapsed ALL, refers to the return of acute lymphoblastic leukemia (acute lymphoblastic leukemia) in patients who have already undergone treatment for the disease and reached complete remission 2. Between 15 and 20 percent of children who are treated for acute lymphoblastic leukemia and achieve an initial complete remission will have the disease return or relapse 81. Relapse of acute lymphoblastic leukemia
generally occurs within two years of initial treatment, although it may occur several months to years after the initial remission.
For complete remission to have occurred, the following conditions will have been met 2:
- Blood cell counts returned to normal
- Less than 5% of blasts (abnormal, immature, early lymphocytes or lymphoblasts) are still present in the bone marrow
- There is no leukaemia present elsewhere in the body
In relapsed acute lymphoblastic leukemia — as with newly diagnosed acute lymphoblastic leukemia — lymphocyte stem cells (a type of blood stem cell) become immature white blood cells called lymphoblasts or “blasts.” These blasts do not become healthy white blood cells. Instead, they build up in the bone marrow again, reaching high levels beyond levels considered appropriate for
remission so there is less room for healthy white blood cells, red blood cells, and platelets. This is also called a recurrence. In addition, these abnormal cells are unable to fight off infection.
While a large number of patients go into remission after induction therapy, there are subsets of patients who do not at all, and still have numerous blast cells in their bone marrow after treatment. This is called refractory acute lymphoblastic leukemia.
Patients with relapsed acute lymphoblastic leukemia remain curable despite the failure of the initial course of treatment. The treatment strategies for children with acute lymphoblastic leukemia are similar to those for adult patients with acute lymphoblastic leukemia.
The symptoms of relapsed acute lymphoblastic leukemia are the same as those for newly diagnosed acute lymphoblastic leukemia, including:
- Anemia
- Bone and joint pain
- Bruising or petechiae (small red spots on the skin)
- Fever
- Recurrent infections
- Abdominal pain
- Swollen lymph nodes
- Dyspnea or difficulty breathing
The prognosis for children with relapsed acute lymphoblastic leukemia depends on a number of factors, including:
- The site of relapse (i.e., bone marrow, central nervous system, testicles)
- The length of time between initial diagnosis and relapse
- Age of child at initial diagnosis
- Response after the first month of reinduction treatment
- Biological features of the relapsed cells
- How many relapses your child has experienced (first, second, etc.).
To make a diagnosis of pediatric relapsed acute lymphoblastic leukemia, a doctor may order a variety of different tests including:
- Complete blood count (CBC)
- Bone marrow aspiration and biopsy
- Lumbar puncture (spinal tap)
- X-ray
- Chromosomal analysis, which may help determine the way the leukemia is treated
After all tests are completed, doctors will be able to outline the best treatment options.
The mainstay of the treatment for relapse acute lymphoblastic leukemia is chemotherapy, often given with steroids to improve the effectiveness. If required, novel target therapy drugs which attack specific components of the leukaemia cells can be given. High-risk patients are frequently offered an allogeneic stem cell transplant because the likelihood of a cure with chemotherapy alone is very low.
Childhood acute lymphoblastic leukemia relapse rate
Between 15 and 20 percent of children who are treated for acute lymphoblastic leukemia and achieve an initial complete remission will have the disease return 81.
Causes of relapsed acute lymphoblastic leukemia in childhood
Patients with acute lymphoblastic leukemia are known to have a number of characteristics that make them more likely to relapse. Patients with a likelihood or risk that they will have a relapse after treatment can be subdivided into well-defined risk groups according to these characteristics. By way of an example, there is a well-established risk stratification for acute lymphoblastic leukemia patients, which is used by many doctors. This risk stratification is for children since the majority of patients with acute lymphoblastic leukemia are children.
Both the National Cancer Institute and Rome criteria uses only patients’ ages and white blood cell counts to determine their risk and predict relapse and outcome.
Standard risk
- Both of the following criteria must be present:
- White blood cell count less than 50 x 109 cells/L
- Age of patient between one and nine years
High risk
- Both of the following criteria must be present:
- White blood cell count greater than 50 x 109 cells/L
- Age of patient younger than one year or older than nine years
Since this risk classification was established, numerous other risks factors for relapse have been found, none more so than the implications of faulty chromosomes and genes.
Chromosomes are thread-like structures which carry the genes and are located in the nuclei of every cell. Genes are made up of DNA (deoxyribonucleic acid) which stores the genetic information required to make human proteins. The presence of abnormal gene rearrangements alone cannot predict a relapse rate in patients, but the relationship between genetic rearrangements and the prognosis of the first occurrence of acute lymphoblastic leukemia can.
Although not having any of the prognostic risk factors at initial presentation predicts less chance of relapse and a better outcome, there is always a risk of relapse irrespective of a patient’s age or prognostic factors.
Symptoms of relapsed acute lymphoblastic leukemia in childhoood
The symptoms of relapsed acute lymphoblastic leukemia are the same as those for newly diagnosed acute lymphoblastic leukemia, and include:
- Anemia
- Bruising or petechiae (small red spots on the skin)
- Fever
- Recurrent infections
- Abdominal pain
- Bone and joint pain
- Swollen lymph nodes (showing up as lumps and bumps on your neck, armpits and groin)
- Dyspnea (difficulty in breathing)
Relapsed acute lymphoblastic leukemia in childhoood diagnosis
To make a diagnosis of relapsed acute lymphoblastic leukemia, your doctor will carry out the following tests:
- Complete blood count (CBC). This will show the number of red blood cells, white blood cells and platelets. In a relapse, acute lymphoblastic leukemia patients have lower-than-expected red blood cells and platelets.
- A peripheral blood smear. A sample of blood is viewed under a microscope to count different circulating blood cells and to see whether the cells look normal. In relapsed acute lymphoblastic leukemia patients, there are too many blast cells.
- Bone marrow aspiration and biopsy. The aspiration procedure removes a liquid marrow sample and the biopsy removes a small amount of bone filled with marrow. Medication is given to numb the area, or a general anaesthetic is performed, to remove a sample from the hip bone. The following can be examined:
- Percentage of acute lymphoblastic leukemia cells are in your bone marrow
- Any abnormalities of the acute lymphoblastic leukemia cells
- Immunophenotyping. This procedure identifies the types of proteins on the surface of the cell to find out if the acute lymphoblastic leukemia cells are B-cells or T-cells.
- Lumbar puncture. This will determine if the acute lymphoblastic leukemia cells are in your central nervous system (CNS).
- Chromosomal analysis (cytogenetic analysis). The blood smear sample can also be used to identify certain changes in the number and size of chromosomes within cells that might have led to the relapse.
- Other tests and scans. X-rays are used to monitor the presence of acute lymphoblastic leukemia in any organs.
Treatment for relapsed acute lymphoblastic leukemia in childhood
Treatment of relapsed acute lymphoblastic leukemia is typically more intensive than for newly diagnosed acute lymphoblastic leukemia 81. At the time of first relapse, children and adolescents receive reinduction therapy — a treatment course intended to achieve another complete remission. Reinduction therapy typically consists of chemotherapy given by vein (intravenous), by mouth (oral), and into the spinal fluid (intrathecal). Often, it is similar to treatment that was given when the acute lymphoblastic leukemia was first diagnosed.
After achieving a second complete remission, treatment options include:
- 1) chemotherapy with or without radiation therapy and
- 2) stem cell (bone marrow) transplantation.
The treatment strategy recommended for your child will depend on several factors, including:
- The site of relapse (i.e., bone marrow, central nervous system, testicles)
- The length of time between initial diagnosis and relapse
- The type of acute lymphoblastic leukemia for which your child was initially treated (B-cell versus T-cell)
- How well the leukemia responded to the first month of treatment (reinduction therapy)
Patients who relapse in their marrow during or just after completing initial treatment may benefit from a stem cell transplant. Patients who relapse six months or more after initial treatment can often be re-treated with more intensive chemotherapy without a stem cell transplant.
Relapses most often occur in the bone marrow. Less commonly, acute lymphoblastic leukemia will relapse in the central nervous system (CNS; the brain and spinal fluid) or, in boys, in the testicles, without any bone marrow involvement. As with bone marrow relapses, such cases are treated with aggressive chemotherapy — including, in CNS relapses, intrathecal chemotherapy (treatment delivered to the spinal canal), but with the addition of radiation therapy targeted to the site of relapse.
Sometimes relapsed acute lymphoblastic leukemia does not respond to standard chemotherapy agents. For patients whose leukemia persists (does not go into remission) despite standard treatment approaches, or relapses again (second or greater relapse), the Childhood Hematologic Malignancy Center offers clinical trials of many new agents and treatment approaches.
These treatment approaches include:
- New chemotherapy drugs
- Novel combinations of chemotherapy drugs or combinations incorporating new agents to other known active agents
- Antibodies directed against the leukemia
- Drugs that stimulate the body’s immune system to attack the leukemia
- Chimeric antigen receptor (CAR) T-cell therapy, which involves genetically engineering a child’s own immune cells (T-cells) to target and kill leukemia cells.
A major challenge in the future treatment of acute lymphoblastic leukemia will be to devise less toxic regimens for patients with low-risk disease and high cure potential, with the ultimate goal of improving their quality of life.
Chemotherapy
Chemotherapy for acute lymphoblastic leukemia normally consists of induction, consolidation, and long-term maintenance therapy, with CNS (central nervous system) prophylaxis treatment to prevent blast cells entering the brain or spinal cord, often given during the first year of treatment.
After a first relapse, patients should receive re-induction therapy to try and achieve another complete remission. The treatment of relapsed acute lymphoblastic leukemia is normally more intensive than for newly diagnosed acute lymphoblastic leukemia.
Treatment outcome depends on the time of the patient’s relapse and the type of acute lymphoblastic leukemia:
- For patients who relapse during or just after finishing chemotherapy, another course of chemotherapy is unlikely to achieve a cure. An allogeneic stem cell transplant in the second remission period is the only way to cure these patients with acute lymphoblastic leukemia, and should be the main focus whenever possible.
- For patients who relapse six months or longer after finishing treatment, many patients can achieve a second remission with therapy similar to that used in initial treatment.
- For patients with B-cell acute lymphoblastic leukemia (B-ALL) with a late first bone marrow relapse and low levels of minimal residual disease, chemotherapy can achieve a positive outcome. In addition, the dual specific antibody, blinatumumab, is known to inactivate the T-cell immune response against B-cells and directly activate T-cells against the acute lymphoblastic leukemia blasts. Blinatumumab is recommended by the National Institute for Health and Care Excellence (NICE) as an option for treating Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia in adults. A recent study of 113 adults with B-cell precursor acute lymphoblastic leukemia in complete hematological remission showed that blinatumumab achieved a complete minimal residual disease response in 78% of patients. However, an allogeneic stem cell transplantation, particularly for those patients with unfavourable genetic factors and/or who are minimal residual disease-positive, offers a significant benefit.
Chemotherapy for T-cell acute lymphoblastic leukemia, paying special attention to the patient’s responses to previous treatments, has also resulted in good survival rates. Patients with mature T-cell acute lymphoblastic leukemia have a better outcome than those with early T-cell acute lymphoblastic leukemia
Discovery of abnormal gene rearrangements in patients with acute lymphoblastic leukemia will also influence the choice of treatment. The Philadelphia chromosome (BCR-ABL) is the most common genetic abnormality associated with adult acute lymphoblastic leukemia and has a very poor prognosis for both children and adults. It only occurs in 3% to 5% of patients with acute lymphoblastic leukemia, but less than 40% of them are cured with intensive chemotherapy.
A young person who is Philadelphia chromosome-positive will be a candidate for an allogeneic stem cell transplantation in the first remission. Patients who relapse but achieve a second complete remission may also benefit from an allogeneic stem cell transplantation. However, the success of tyrosine kinase inhibitors in chronic myeloid leukaemia (CML) has encouraged their use in Philadelphia chromosome-positive acute lymphoblastic leukemia patients. The tyrosine kinase inhibitor, imatinib, has achieved complete remission rates of 90%-100% for patients who have Philadelphia chromosome-positive acute lymphoblastic leukemia with relatively low toxicity. However, the combination of a tyrosine kinase inhibitor with standard chemotherapy has led to a longer survival in both adults and children.
Relapses can result from a lack of response to standard chemotherapy agents. In patients with refractory acute lymphoblastic leukemia, or patients who have had several relapses, multidrug treatment is often used with the aim of eliminating blast cells and targeting therapy-resistant cells that could cause further episodes of relapse.
Introduction of new treatments from trials for high-risk patients is an option for improving outcome. Other possibilities which can also be explored include the following:
- Ground-breaking combinations of chemotherapy drugs
- Antibodies directed against the leukemia
- Drugs that stimulate the body’s immune system to attack the leukaemia
Chimeric Antigen Receptor (CAR) T-cell therapy
This new cancer treatment works by removing the patient’s own immune cells, genetically modifying them to recognize the tumor cells, and then re-infusing them back into the patient so they can target the cancer cells.
Clinical trials of CAR T-cell therapy for relapsed or refractory B-cell cancers, such as B-cell leukemia and lymphoma, and several solid tumors, have shown promising results. The anti-CD19 CAR T-cell therapy has achieved remission in up to 90% of patients with B-cell acute lymphoblastic leukemia (B-ALL). CD19 is a B-cell receptor associated protein present on the surface of B-cells. However, relapse after CAR T-cell therapy is still a problem, often because the acute lymphoblastic leukemia cells lose expression of CD19.
The anti-CD19 agent tisagenlecleucel (Kymriah) is the first CAR T-cell therapy to be approved in the United States for the treatment of patients up to 25 years of age with B-cell acute lymphoblastic leukemia that is refractory or in second or later relapse. In Europe, this anti-CD19 CAR T-cell drug has been approved by the European Medicines Agency.
Allogeneic stem cell transplantation
An allogeneic stem cell transplantation uses stem cells from a matched or partially matched healthy donor. The donor may be a brother or sister. Or, the donor can be an unrelated person with stem cells that “match” the patient’s. Stem cells may also come from a cord blood unit (the blood in the umbilical cord after a baby’s birth). High-risk patients are frequently offered an allogeneic stem cell transplantation because the likelihood of a cure with chemotherapy alone is very low. Currently an allogeneic stem cell transplantation is an established treatment for high-risk acute leukemia.
Patients who have a bone marrow relapse following initial chemotherapy treatment may benefit from a stem cell transplant; however, this is not uniformly recommended. General procedure for allogeneic stem cell transplantation in acute lymphoblastic leukemia patients involves total body irradiation, because outcomes are improved in patients who undergo transplant after achieving a low minimal residual disease status.
Careful selection of stem cell transplantation for acute lymphoblastic leukemia patients is important to achieve the best outcomes. Human leukocyte antigen (HLA)-matched sibling donors are recognized as the best option, but this is only available for 30% of patients. Alternative sources of stem cells for the remaining 70% of patients include matched unrelated adult volunteer donor, a haploidentical donor or an umbilical cord blood unit.
Allogeneic stem cell transplantations are increasingly performed due to the improved availability of alternative donors and refinement of indications. In addition, the advent of the haploidentical stem cell transplantation (HID-stem cell transplantation), where the donor matches exactly half of the HLA, offers another option for patients.
Adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in their first complete remission will benefit from an HLA-matched donor allogeneic stem cell transplantation or a HID-stem cell transplantation. Moreover, children with T-cell acute lymphoblastic leukemia benefit from allogeneic stem cell transplantations, including HID-stem cell transplantation.
Philadelphia chromosome-positive acute lymphoblastic leukemia patients who have received a HLA-matched donor allogeneic stem cell transplantation compared to those who had a HID-stem cell transplantation showed similar results in studies with children and adults. In a study of 82 Philadelphia chromosome-positive acute lymphoblastic leukemia Chinese patients, HID-stem cell transplantation was associated with a meaningful lower relapse rate compared with HLA-matched donor allogeneic stem cell transplantation (44.8 vs. 19.1%, respectively), although overall survival times were the same.
If an HLA-identical sibling donor is not available, the probability of finding a fully matched unrelated donor should be estimated to help inform the decision on whether to search for an unrelated donor or find an alternative source of hematopoietic stem cells (haploidentical donor or cord blood unit).
Acute lymphoblastic leukemia survival rate childhood
The 5-year survival rate tells you what percent of children and teens live at least 5 years after the cancer is found. Percent means how many out of 100. The 5-year survival rate for children 0 to 14 is 91%. The 5-year survival rate for people ages 15 to 19 is 75%. For children diagnosed with acute leukemia, those who remain free from the disease after 5 years are generally considered “cured” because it is rare for acute leukemia to recur after this amount of time.
It is important to remember that statistics on the survival rates for children and teens with acute lymphoblastic leukemia are an estimate. The estimate comes from annual data based on the number of children and teens with this cancer in the United States. Also, experts measure the survival statistics every 5 years. So the estimate may not show the results of better diagnosis or treatment available for less than 5 years. Talk with your child’s doctor if you have any questions about this information.
Childhood acute lymphoblastic leukemia diagnosis
Certain signs and symptoms can suggest that a person might have acute lymphoblastic leukemia (acute lymphocytic leukemia), but tests are needed to confirm the diagnosis.
In children, a diagnosis of acute lymphoblastic leukemia generally requires a finding that 25 percent or more of the cells in the bone marrow are leukemic blasts of lymphoid origin (lymphoblasts). The acute lymphoblastic leukemia subtype is determined based on a patient’s lab test results.
Medical history and physical exam
If you have signs and symptoms that suggest you might have leukemia, the doctor will want to get a thorough medical history, including how long you have had symptoms and if you have possibly been exposed to anything considered a risk factor.
During the physical exam, the doctor will probably focus on any enlarged lymph nodes, areas of bleeding or bruising, or possible signs of infection. The eyes, mouth, and skin will be looked at carefully, and a thorough nervous system exam may be done. Your abdomen will be felt for spleen or liver enlargement.
If there is reason to think low levels of blood cells might be causing your symptoms (anemia, infections, bleeding or bruising, etc.), the doctor will most likely order blood tests to check your blood cell counts. You might also be referred to a hematologist, a doctor who specializes in diseases of the blood (including leukemia).
Tests used to diagnose and classify acute lymphocytic leukemia
If your doctor thinks you might have leukemia, he or she will need to check samples of cells from your blood and bone marrow to be sure. Other tissue and cell samples may also be taken to help guide treatment.
Blood tests
Blood samples for acute lymphocytic leukemia tests are generally taken from a vein in the arm.
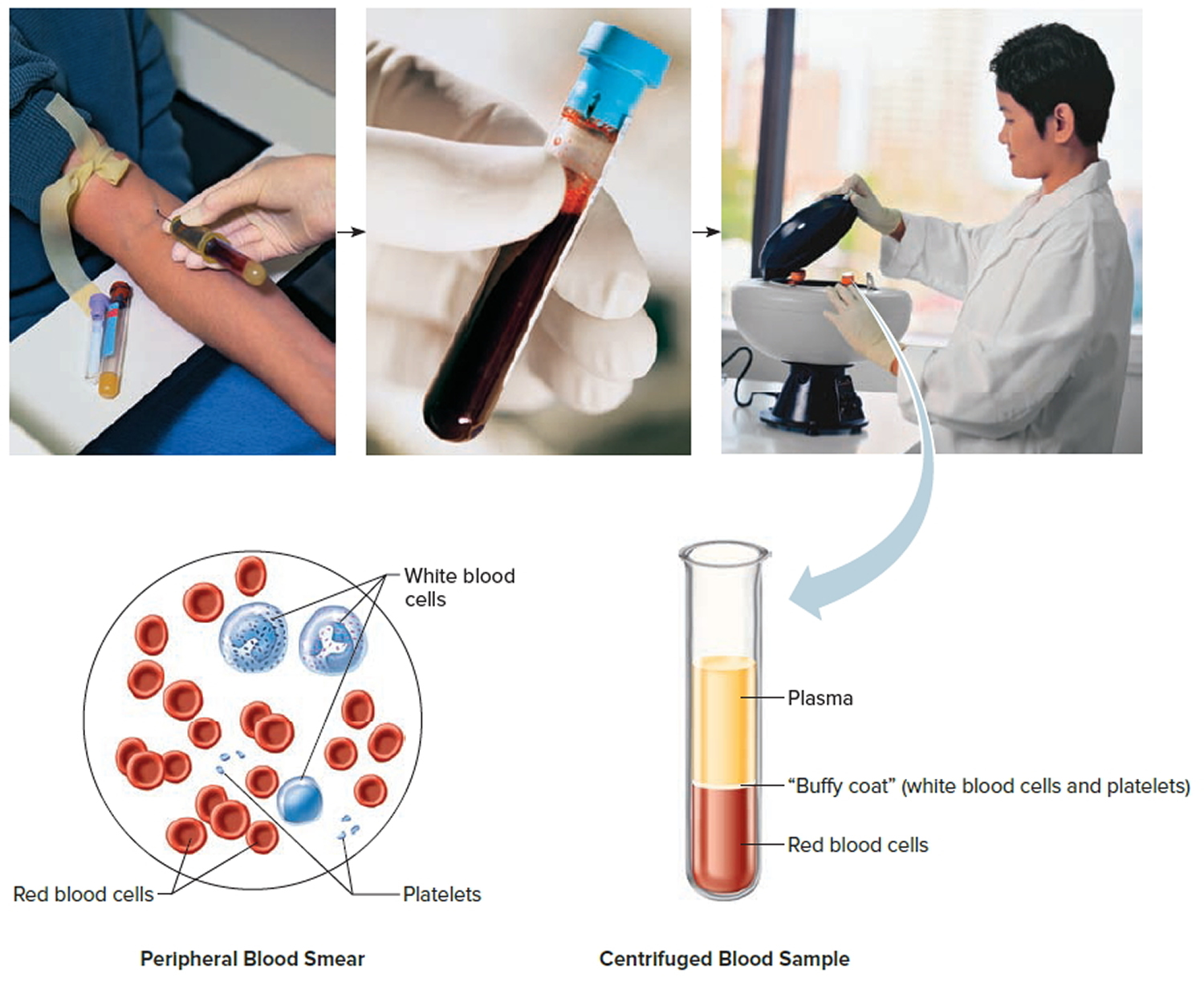
Complete blood count (CBC) and peripheral blood smear
The complete blood count (CBC) measures the numbers of red blood cells, white blood cells, and platelets. This test is often done along with a differential (or diff) which looks at the numbers of the different types of white blood cells. These tests are often the first ones done on patients with a suspected blood problem.
For the peripheral blood smear (sometimes just called a smear), a drop of blood is smeared across a slide and then looked at under a microscope to see how the cells look. Changes in the numbers and the appearance of the cells often help diagnose leukemia.
Most patients with acute lymphocytic leukemia have too many immature white cells called lymphoblasts (or just blasts) in their blood, and not enough red blood cells or platelets. Lymphoblasts are not normally found in the blood, and they don’t function like normal, mature white blood cells.
Even though these findings may suggest leukemia, the disease usually is not diagnosed without looking at a sample of bone marrow cells.
Blood chemistry tests
Blood chemistry tests measure the amounts of certain chemicals in the blood, but they are not used to diagnose leukemia. In patients already known to have acute lymphocytic leukemia, these tests can help detect liver or kidney problems caused by spreading leukemia cells or the side effects of certain chemotherapy drugs. These tests also help determine if treatment is needed to correct low or high blood levels of certain minerals.
Coagulation tests
Blood coagulation tests may be done to make sure the blood is clotting properly.
Bone marrow tests
Leukemia starts in the bone marrow, so checking the bone marrow for leukemia cells is a key part of testing for it.
Bone marrow aspiration and biopsy
Bone marrow samples are obtained by bone marrow aspiration and biopsy – tests usually done at the same time. The samples are usually taken from the back of the pelvic (hip) bone, although in some cases they may be taken from the sternum (breastbone) or other bones.
In bone marrow aspiration, you lie on a table (either on your side or on your belly). After cleaning the skin over the hip, the doctor numbs the skin and the surface of the bone by injecting a local anesthetic, which may cause a brief stinging or burning sensation. A thin, hollow needle is then inserted into the bone and a syringe is used to suck out a small amount of liquid bone marrow. Even with the anesthetic, most patients still have some brief pain when the marrow is removed.
A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is pushed down into the bone. With local anesthetic, most patients just feel some pressure and tugging from the biopsy, but some may feel a brief pain. Once the biopsy is done, pressure will be applied to the site to help prevent bleeding.
These bone marrow tests are used to help diagnose leukemia. They may also be done again later to tell if the leukemia is responding to treatment.
Lab tests used to diagnose and classify acute lymphocytic leukemia
One or more of the following lab tests may be done on the samples to diagnose acute myeloid leukemia (AML) and/or to determine the specific subtype of acute lymphocytic leukemia.
Routine exams with a microscope
The bone marrow (and sometimes blood) samples are looked at with a microscope by a pathologist (a doctor specializing in lab tests) and may be reviewed by the patient’s hematologist/oncologist (a doctor specializing in cancer and blood diseases).
The doctors will look at the size, shape, and other traits of the white blood cells in the samples to classify them into specific types. A key factor is whether the cells look mature (like normal blood cells), or immature (lacking features of normal blood cells). The most immature cells are called lymphoblasts (or just blasts).
Determining what percentage of cells in the bone marrow are blasts is particularly important. A diagnosis of acute lymphocytic leukemia generally requires that at least 20% of the cells in the bone marrow are blasts. Under normal circumstances, blasts don’t make up more than 5% of bone marrow cells. Sometimes just counting and looking at the cells doesn’t provide a definite diagnosis, and other lab tests are needed.
Cytochemistry
In cytochemistry tests, cells are put on a slide and exposed to chemical stains (dyes) that react only with some types of leukemia cells. These stains cause color changes that can be seen under a microscope, which can help the doctor determine what types of cells are present. For instance, one stain will turn parts of acute myeloid leukemia (AML) cells black, but has no effect on acute lymphocytic leukemia cells.
Flow cytometry and immunohistochemistry
For both flow cytometry and immunohistochemistry, samples of cells are treated with antibodies, which are proteins that stick only to certain other proteins on cells. For immunohistochemistry, the cells are examined under a microscope to see if the antibodies stuck to them (meaning they have those proteins), while for flow cytometry a special machine is used.
These tests are used for immunophenotyping – classifying leukemia cells according to proteins on or in the cells. This kind of testing is very helpful in determining the exact type of leukemia. For diagnosing leukemia, it is most often done on cells from bone marrow, but it can also be done on cells from the blood, lymph nodes, and other body fluids.
For acute lymphocytic leukemia, these tests are most often used to help determine the exact subtype of in someone already thought to have acute lymphocytic leukemia based on other tests.
Chromosome tests
These tests look at the chromosomes (long strands of DNA) inside the cells. Normal human cells contain 23 pairs of chromosomes (bundles of DNA). In acute lymphocytic leukemia, the cells sometimes have chromosome changes. Recognizing these changes can help identify certain types of acute lymphocytic leukemia, and it can be important in determining a patient’s outlook and likely response to some treatments. For this reason, chromosome testing is a standard part of the work-up for acute lymphocytic leukemia.
The most common chromosome change in acute lymphocytic leukemia is a translocation, in which, 2 chromosomes swap some of their DNA, so that part of one chromosome becomes attached to part of a different chromosome. The most common chromosome change in adult acute lymphocytic leukemia is a translocation that results in a shortened chromosome 22 (called the Philadelphia chromosome). About 1 out of 4 adults with acute lymphocytic leukemia have this abnormality in their leukemia cells. This change is especially important because it can be targeted with certain drugs.
Cytogenetics
For this test, the cells are grown in lab dishes until they start dividing. Then the chromosomes are looked at under a microscope to detect any changes. Because it takes time for the cells to start dividing, cytogenetic testing often takes about 2 to 3 weeks. Not all chromosome changes can be seen under a microscope. Other lab tests can often help find these changes.
Fluorescent in situ hybridization (FISH)
This is another way to look at chromosomes and genes. It uses special fluorescent dyes that only attach to specific genes or parts of particular chromosomes. Fluorescent in situ hybridization (FISH) can find most chromosome changes (such as translocations) that are visible under a microscope in standard cytogenetic tests, as well as some changes too small to be seen with usual cytogenetic testing.
Fluorescent in situ hybridization (FISH) can be used on regular blood or bone marrow samples. Because the cells don’t have to be able to divide for this test, it can also be used to look at cells from other tissues, like lymph node samples. It is very accurate and can usually provide results within a couple of days. But because fluorescent in situ hybridization (FISH) only tests for certain gene changes (and doesn’t look at the chromosomes overall), it is best for looking for the changes that are important based on the kind of leukemia a person has.
Polymerase chain reaction (PCR)
This is a very sensitive DNA test that can also find certain gene and chromosome changes too small to be seen with a microscope, even if very few leukemia cells are present in a sample. Like fluorescent in situ hybridization (FISH), it is used to find particular gene changes and not to look at the chromosomes overall.
If the leukemia cells have a particular gene (or chromosome) change, polymerase chain reaction (PCR) can be used after treatment to try to find small numbers of leukemia cells that may not be visible with a microscope.
Other molecular and genetic tests
Other, newer types of lab tests can also be done on the samples to look for specific gene or other changes in the leukemia cells.
Lumbar puncture (spinal tap)
Acute lymphocytic leukemia can spread to the area around the brain and spinal cord. To check for this spread, doctors remove a sample of the fluid from that area (cerebrospinal fluid or CSF) for testing. You may lay on your side or sit up for this test. The doctor first numbs an area in the lower part of the back over the spine. A small, hollow needle is then placed between the bones of the spine and into the area around the spinal cord to collect some fluid.
A lumbar puncture can also be used to put chemotherapy drugs into the CSF to try to prevent or treat the spread of leukemia to the spinal cord and brain.
Lymph node biopsy
A lymph node or part of a lymph node is often removed to help diagnose lymphomas, but this is only rarely needed with leukemia because the diagnosis is usually made looking at blood and bone marrow. In this procedure, a surgeon cuts through the skin to remove all or part of a lymph node. If the node is just under the skin, this is a simple operation that can often be done with local anesthesia, but if the node is inside the chest or abdomen, general anesthesia is used to keep you asleep during the biopsy.
When the entire lymph node is removed, it is called an excisional lymph node biopsy. If only part of the lymph node is removed, it is called an incisional lymph node biopsy.
Imaging tests
Imaging tests use x-rays, sound waves, magnetic fields, or radioactive particles to create pictures of the inside of the body. Leukemia does not usually form tumors, so imaging tests aren’t as useful as they are for other types of cancer. Imaging tests might be done in people with acute lymphocytic leukemia to help determine the extent of the disease, if it is thought to have spread beyond the bone marrow and blood. They might also be done to look for infections or other problems. .
X-rays
Chest x-rays may be done if the doctor suspects a lung infection. They may also be done to look for enlarged lymph nodes in the chest.
Computed tomography (CT) scan
The CT scan uses x-rays to make detailed, cross-sectional images of your body. The CT scan can show if any lymph nodes or organs in your body are enlarged. It isn’t usually needed to diagnose acute lymphocytic leukemia, but it may be done if your doctor suspects leukemia cells are growing in an organ, like your spleen.
Sometimes a test that combines the CT scan with a PET (positron emission tomography) scan (PET/CT scan) is done. This is not often needed for patients with acute lymphocytic leukemia.
Magnetic resonance imaging (MRI) scan
MRI scans make detailed images of the body using radio waves and strong magnets instead of x-rays. They are very helpful in looking at the brain and spinal cord. This test might be done if a lumbar puncture finds leukemia cells in the cerebrospinal fluid (CSF), or if a person is having symptoms that could mean the acute lymphocytic leukemia has spread to the area around the brain.
Ultrasound
This is an easy test to have, and it uses no radiation. Ultrasound can be used to look at lymph nodes near the surface of the body or to look for enlarged organs inside the abdomen such as the kidneys, liver, and spleen. It can also be used to look at the testicles, if needed.
Acute lymphoblastic leukemia subtypes
Different systems have been used to classify acute lymphoblastic leukemia into subtypes. The subtypes of acute lymphoblastic leukemia are identified based on certain features of the leukemia cells. Determining the acute lymphoblastic leukemia subtype is an important factor in treatment planning. The doctor will discuss with you which drug combinations and “protocols” are indicated based on your child’s acute lymphoblastic leukemia subtype. In medicine, protocols are detailed plans of treatments and procedures. The doctor may also talk about whether a clinical trial may be an appropriate treatment option.
Leukemia cells can be classified by the antigens, known as “immunophenotypes,” found on their surfaces.
In the 1970s, a group of French, American, and British (FAB) leukemia experts divided acute lymphoblastic leukemia into 3 subtypes (L1, L2, and L3), based on the way the leukemia cells looked under the microscope after routine staining. This system, known as the FAB classification, has largely been replaced, as newer lab tests now allow doctors to classify acute lymphoblastic leukemia more accurately.
Doctors have found that cytogenetic tests, flow cytometry, and other lab tests provide more detailed information about the subtype of acute lymphoblastic leukemia and the patient’s prognosis. These tests help divide acute lymphoblastic leukemia into groups based on the gene and chromosome changes in the leukemia cells.
The World Health Organization (WHO) system, most recently updated in 2016, includes some of these factors to try to better classify acute lymphoblastic leukemia. The World Health Organization (WHO) classifies acute lymphoblastic leukemia based on the immunophenotype of the leukemia cell in the following ways:
- B-cell acute lymphoblastic leukemia with certain genetic abnormalities (gene or chromosome changes)
- B-cell ALL with hypodiploidy (the leukemia cells have fewer than 44 chromosomes [normal cells have 46])
- B-cell ALL with hyperdiploidy (the leukemia cells have more than 50 chromosomes)
- B-cell ALL with a translocation between chromosomes 9 and 22 [t(9;22)] (the Philadelphia chromosome, which creates the BCR-ABL1 fusion gene)
- B-cell ALL with a translocation between chromosome 11 and another chromosome
- B-cell ALL with a translocation between chromosomes 12 and 21 [t(12;21)]
- B-cell ALL with a translocation between chromosomes 1 and 19 [t(1;19]
- B-cell ALL with a translocation between chromosomes 5 and 14 [t(5;14)]
- B-cell ALL with amplification (too many copies) of a portion of chromosome 21 (iAMP21)*
- B-cell ALL with translocations involving certain tyrosine kinases or cytokine receptors (also known as “BCR-ABL1–like ALL”)*
- B-cell ALL, not otherwise specified
- T-cell acute lymphoblastic leukemia
- Early T-cell precursor lymphoblastic leukemia*
- * It’s not yet clear if there’s enough evidence that it’s a unique group (meaning it is still a “provisional entity”)
- Mixed lineage acute leukemias
- A small number of acute leukemias have both lymphocytic and myeloid features. Sometimes the leukemia cells have both myeloid and lymphocytic traits in the same cells. In other cases, a person may have some leukemia cells with myeloid features and others with lymphocytic features. These types of leukemias may be called mixed lineage leukemia, acute undifferentiated leukemia, or, or mixed phenotype acute leukemia (MPAL). Most studies suggest these leukemias tend to have a poorer outlook than standard subtypes of acute lymphoblastic leukemia (ALL) or acute myloid leukemia (AML). Not all doctors agree on the best way to treat them. Intensive treatment (such as a stem cell transplant) is often used when possible, as there is a high risk of recurrence after treatment.
B-cell lymphoblastic leukemia or lymphoma
This subtype begins in immature cells that would normally develop into B cells. In children, if the bone marrow has 25 percent or more lymphoblasts, the disease is called B-cell lymphoblastic leukemia (B-cell ALL). If the lymphoblasts are restricted to a mass in a lymph node or other lymph tissue and less than 25 percent of the bone marrow cells are lymphoblasts, it is called B-cell lymphoblastic lymphoma. Patients with lymphoblastic lymphoma generally benefit from treatment with acute lymphoblastic leukemia-like regimens rather than traditional lymphoma therapy.
B-cell acute lymphoblastic leukemia is the most common acute lymphoblastic leukemia subtype, accounting for approximately 80 percent of cases among children with acute lymphoblastic leukemia. Within the B-cell lineage, the cell surface markers (proteins) differ according to the stage of cell maturation.
Before 2008, the WHO classified B-cell lymphoblastic leukemia as “precursor B-lymphoblastic leukemia.” This older term is sometimes used to distinguish B-cell acute lymphoblastic leukemia from mature B-cell acute lymphoblastic leukemia. Mature B-cell acute lymphoblastic leukemia is now referred to as “Burkitt leukemia.” The treatment for Burkitt leukemia is based on therapy for
non-Hodgkin lymphoma and is very different than the treatment used for acute lymphoblastic leukemia.
T-cell lymphoblastic leukemia or lymphoma
This subtype begins in immature cells that would normally develop into T cells. If the bone marrow has 25 percent or more lymphoblasts, the disease is called T-cell lymphoblastic leukemia (T-cell ALL). If the bone marrow has less than 25 percent lymphoblasts and the lymph nodes are enlarged, it is call T-cell lymphoblastic lymphoma. This subtype is less common than B-cell ALL and occurs more often in adults than in children. T-cell ALL accounts for approximately 15 to 20 percent of ALL cases in children.
Genetic changes
In addition to classifying acute lymphoblastic leukemia as either B-cell or T-cell, it can be further classified based on changes to certain chromosomes and genes. This identification of specific genetic abnormalities is critical for disease evaluation, risk stratification, treatment planning and can affect prognosis.
One type of genetic change that may occur in acute lymphoblastic leukemia is the result of numerical abnormalities. A numerical abnormality is either a gain or loss in the number of chromosomes from the normal total of 46. A change in the number of chromosomes can affect growth, development and the functioning of body systems.
Another type of genetic change associated with acute lymphoblastic leukemia is a translocation. In a translocation, the DNA from one chromosome breaks off and becomes attached to a different chromosome.
About 75 percent of childhood cases of acute lymphoblastic leukemia can be classified into subgroups based on chromosomal abnormalities and genetic mutations. Not all patients who have acute lymphoblastic leukemia exhibit the same genetic changes. Some changes are more common than others, and some have a greater effect on the patient’s prognosis. For example, patients tend to have a poorer outcome if the leukemia cells have:
- The Philadelphia chromosome (a translocation between chromosomes 9 and 22), although this outlook has improved with modern targeted therapy drugs
- A translocation between chromosomes 4 and 11
- A translocation involving chromosome 14
- Amplification (too many copies) of part of chromosome 21
- Fewer than 44 chromosomes (hypodiploidy)
- 5 or more chromosome changes (complex karyotype)
On the other hand, people tend to have a better outlook if the leukemia cells have:
- A translocation between chromosomes 12 and 21
- More than 50 chromosomes (hyperdiploidy)
Treatment of acute lymphoblastic leukemia in childhood
Once you learn that your child has acute lymphoblastic leukemia, you need to decide where to go for treatment. Going to a specialized children’s cancer hospital helps ensure that your child gets
the best available treatment. Most children with cancer receive treatment at hospitals that specialize in treating children with cancer. The doctors and other healthcare providers at these centers have special training and expertise in giving comprehensive care to children. These centers are often members of the Children’s Oncology Group (COG). This is the world’s largest organization devoted to clinical research to improve the care and treatment of children with cancer.
Most children with acute lymphoblastic leukemia are cared for by a pediatric hematologist-oncologist. A pediatric doctor (pediatrician) specializes in the treatment of children. A hematologist is a doctor who has special training in disorders of the blood, and an oncologist is a doctor who has special training in cancer. A pediatric hematologist-oncologist specializes in blood cancers in children.
Children who are diagnosed with acute lymphoblastic leukemia usually need to start treatment as soon as possible after diagnosis. Some families may wish to seek a second opinion, particularly if their child has a high-risk subtype of acute lymphoblastic leukemia or if the acute lymphoblastic leukemia has come back (relapsed) after initial treatment. A second opinion may help you feel more confident about your child’s treatment plan. The second opinion should come from a pediatric hematologist-oncologist, preferably one who specializes in childhood acute lymphoblastic leukemia. This doctor will usually have the most knowledge and experience regarding the latest treatment options.
If you are unsure or feel uncomfortable about how to tell your child’s doctor you are getting a second opinion, call our Information Specialists to discuss a way that makes you comfortable. You may also want to check with your insurance company to be sure that a second opinion will be covered.
The main treatment for children with acute lymphoblastic leukemia (ALL) is chemotherapy, which is usually given in 3 main phases:
- Induction
- Consolidation (also called intensification)
- Maintenance
The entire length of treatment is typically about 2 to 3 years, with the most intense treatment in the first few months.
Children with acute lymphoblastic leukemia are typically classified by risk group to make sure that the correct types and doses of drugs are given. Treatment may be more or less intense, depending on the risk group.
Some treatment plans may also include targeted agents and stem cell transplantation. Treatment regimens for acute lymphoblastic leukemia include central nervous system (CNS) prophylaxis to prevent leukemia cells from spreading to the area around the brain and spinal cord. Central nervous system (CNS) prophylaxis is typically given to children throughout all phases of ALL treatment.
Induction therapy
The goal of induction therapy is to achieve a remission. This means that leukemia cells are no longer found in bone marrow samples, the normal marrow cells return, and the blood counts become normal. (A remission is not necessarily a cure.) More than 95% of children with acute lymphoblastic leukemia enter remission after 1 month of induction treatment.
Induction chemotherapy lasts for 4 weeks. The specific drugs, dosages and timing of administration depend on several factors, including the patient’s age, the specific features of the leukemia and the overall health of the patient.
This first month is intense and requires prolonged hospital stays for treatment and frequent visits to the doctor. Your child may spend some or much of this time in the hospital, because serious infections or other complications can occur. It is very important to take all medicines as prescribed. Sometimes complications can be serious enough to be life-threatening, but in recent years, advances in supportive care (nursing care, nutrition, antibiotics, red blood cell and platelet transfusions as needed, etc.) have made these much less common than in the past.
Children with standard-risk acute lymphoblastic leukemia often receive 3 drugs for the first month of treatment. These include the chemotherapy drugs L-asparaginase and vincristine, and a steroid drug (such as dexamethasone). For children in high-risk groups, a fourth chemo drug in the anthracycline class (most often daunorubicin) is typically added. Other drugs that may be given early are methotrexate and/or 6-mercaptopurine.
Children with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) may benefit from the addition of a tyrosine kinase inhibitor (TKI) medication, such as imatinib (Gleevec) or dasatinib (Sprycel). Some children with Philadelphia Chromosome-like (Ph-like ALL) acute lymphoblastic leukemia may be treated with a tyrosine kinase inhibitor (TKI) called ruxolitinib (Jakafi), usually in a clinical trial.
Central Nervous System (CNS) prophylaxis
Pediatric regimens typically include treatment to prevent the spread of leukemia cells to the central nervous system (CNS) and to kill any leukemia cells that may already be present in the brain and spinal cord. It is uncommon for leukemia cells to be present in the cerebrospinal fluid (CSF) at the time of diagnosis, occurring in only 3 to 7 percent of cases. However, without the routine administration of a therapy targeting the central nervous system (referred to as “CNS prophylaxis”), leukemia cells will eventually spread to the cerebrospinal fluid in a large percentage of patients (50 percent or more). The CNS-directed therapy is typically given to all patients throughout the entire course of childhood acute lymphoblastic treatment. It begins during the induction phase and continues throughout the rest of the treatment regimen.
Central nervous system-directed therapy may include:
Intrathecal chemotherapy
All children also get chemo into their cerebrospinal fluid (CSF) to kill any leukemia cells that might have spread to the brain and spinal cord. This treatment, known as intrathecal chemotherapy, is given through a lumbar puncture (spinal tap). It is usually given twice (or more if the leukemia is high risk or leukemia cells have been found in the CSF) during the first month and several times during the next 1 or 2 months. It is then repeated less often during the rest of treatment.
Usually, methotrexate is the drug used for intrathecal chemo. Hydrocortisone (a steroid) and cytarabine (ara-C) may be added, particularly in high-risk children.
A possible side effect of intrathecal chemo is seizures during treatment, which happen in a small percentage of children. Children who develop seizures are treated with drugs to prevent them.
High-dose systemic chemotherapy
High-dose systemic chemotherapy, in which anti-cancer drugs are injected into a vein and travel through the blood to cells throughout the body. High-dose methotrexate is the most common drug used for this treatment in children with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
Cranial irradiation
Along with intrathecal chemo, some high-risk patients (for example, those with T-cell acute lymphoblastic leukemia) and those with many leukemia cells in their CSF when the leukemia is diagnosed may be given radiation therapy to the brain. This was more common in the past, but recent studies have found that many children even with high-risk acute lymphoblastic leukemia may not need radiation therapy if they are given more intensive chemo. Doctors try to avoid giving radiation to the brain if possible, especially in younger children, because no matter how low the dose is kept, it can increase a child’s risk of developing long-term and late effects, such as problems with thinking, growth and development. Cranial radiation is almost never used in very young children.
Cranial radiation is no longer routinely used in children with acute lymphoblastic leukemia, except in those with leukemia cells in their cerebrospinal fluid at the time of diagnosis or those with CNS relapse. Cranial irradiation is very effective in the treatment of CNS leukemia, when indicated.
Assessing treatment response
At the end of the month of induction chemo, your child will have another bone marrow aspiration performed. The bone marrow sample is examined under a microscope. Children with acute lymphoblastic lymphoma may not need a bone marrow test and may instead require imaging studies only; see page 12 for information about acute lymphoblastic lymphoma. These tests or imaging studies are to check whether your child’s leukemia or lymphoma is in complete remission.
A complete remission is achieved when:
- No leukemic blast cells are detected in the bone marrow (with a microscope)
- No more than 5 percent of cells in the bone marrow are blast cells
- No blast cells are in the bloodstream
- Blood cell counts are back to normal
- All signs and symptoms of acute lymphoblastic leukemia are gone
Over 95 percent of children achieve a remission at the end of induction therapy. However, remission does not mean that your child is cured. Your child still needs more treatment to ensure that the disease does not come back (relapse). Even when a complete remission is achieved, some leukemia cells that cannot be seen with a microscope may remain in the body. The presence of these cells is referred to as minimal/measurable residual disease (MRD). Patients who have achieved remission after initial treatment but have minimal/measurable residual disease are at increased risk of disease relapse. Testing for minimal residual disease can help your child’s doctor re-evaluate your child’s acute lymphoblastic leukemia risk category and determine whether your child may benefit from further intensified therapies.
It is important for your child to get tested for minimal residual disease even after achieving remission. There are very sensitive tests to detect minimal residual disease. The most widely used tests are flow cytometry, polymerase chain reaction (PCR) and next-generation sequencing. These tests use samples of bone marrow cells.
It is often recommended that minimal residual disease testing be done after the completion of induction therapy. Recommendations for additional minimal residual disease testing depend on the treatment regimen that is used.
If your child tests negative for minimal residual disease, that indicates that the tests did not detect residual leukemia cells. If your child tests positive for minimal residual disease, there are still detectable leukemia cells inside the bone marrow. Children who are minimal residual disease-positive after induction therapy are categorized as having high-risk or very high-risk acute lymphoblastic leukemia. Depending on the amount of minimal residual disease, your child’s doctor may change the treatment plan. Your child may undergo more intense treatment, or the doctor may recommend a stem cell transplant.
If your child is in remission but tests positive for minimal residual disease, the doctor may prescribe a drug called blinatumomab (Blincyto). Blinatumomab is approved by the US Food and Drug Administration (FDA) to treat adults and children with:
- B-cell acute lymphoblastic leukemia and are in first or second complete remission with minimal residual disease (minimal residual disease) greater than or equal to 0.1%.
- Relapsed or refractory B-cell acute lymphoblastic leukemia.
For patients who do not achieve remission after the first course of induction chemotherapy, a second course of chemotherapy is given, usually using different chemotherapy drugs.
Postremission therapy
“Postremission therapy” refers to treatments given to patients after their disease is in complete remission. Even when minimal residual disease test results are negative, undetectable residual cancer cells are believed to remain in the body. Because of this, patients with acute lymphoblastic leukemia require additional treatment after they achieve remission. Most of these phases are given in an outpatient setting, while some phases require a brief hospitalization for chemotherapy administration. During this period, children also continue to receive CNS prophylaxis therapy.
Consolidation (intensification) therapy
The next, and usually more intense, consolidation phase of chemo starts once the leukemia is in remission and typically lasts for several months. This phase further reduces the number of leukemia cells still in the body. Several chemo drugs are combined to help prevent the remaining leukemia cells from developing resistance. Intrathecal chemo (as described above) is continued at this time.
Children with standard-risk acute lymphoblastic leukemia are usually treated with drugs such as methotrexate, 6-mercaptopurine (6-MP), vincristine, L-asparaginase, and/or prednisone, but regimens differ among cancer centers.
Children with high-risk leukemia (because of gene or chromosome changes in the leukemia cells, for example, or because there is still minimal residual disease after induction) generally get more intense chemo. Extra drugs such as L-asparaginase, doxorubicin (Adriamycin), etoposide, cyclophosphamide, and cytarabine (ara-C) are often used, and dexamethasone is substituted for prednisone.
There may be a second round of intense chemotherapy as part of consolidation. (This is known as delayed intensification.)
Children with Philadelphia chromosome-positive acute lymphoblastic leukemia may benefit from the addition of a targeted drug such as imatinib (Gleevec).
For some children in high-risk groups, a stem cell transplant might be an option at this time once the leukemia is in remission.
Interim maintenance
After consolidation chemotherapy, there is a recovery period called “interim maintenance.” Interim maintenance is typically given for up to 8 weeks, depending on your child’s treatment plan. This phase aims to maintain the remission, but also allows the bone marrow to recover from the effects of therapy. Interim maintenance typically involves non-myelosuppressive chemotherapy (chemotherapy that does not cause decreased blood cell counts). Patients receive methotrexate in combination with other chemotherapy agents. Methotrexate is given intravenously. If lower doses are prescribed, it may be given in a clinic. Higher doses may require a 2-3 day stay in the hospital.
Delayed intensification
The goal of the delayed intensification phase of treatment is to eliminate residual, drug-resistant leukemia cells from the body. It typically lasts 8 weeks and includes chemotherapy combinations similar to those used in the induction and consolidation phases. The exact timing of the doses and the specific drugs given will depend on the individual characteristics of your child’s disease. Some of the drugs that are frequently incorporated into delayed intensification regimens include:
- Vincristine
- Dexamethasone
- Pegaspargase
- Doxorubicin
- 6-mercaptopurine
- Cyclophosphamide
- Cytarabine
Delayed intensification does not usually require a hospital stay, but children are sometimes admitted to the hospital for complications, such as fever and infection.
Maintenance therapy
If the leukemia remains in remission after induction and consolidation chemo, maintenance therapy can begin. Maintenance chemotherapy is the last and longest phase of treatment. The goal of maintenance chemotherapy is to prevent disease relapse. Most treatment plans use daily 6-mercaptopurine (6-MP) and weekly methotrexate, given as pills, often along with vincristine, which is given into a vein (IV), and a steroid (prednisone or dexamethasone). These latter 2 drugs are given for brief periods every 4 to 8 weeks. Other drugs may be added depending on the type of acute lymphoblastic leukemia and the risk of recurrence.
Because some of these medications are taken orally, at home, it is extremely important that a parent or caretaker ensure that the child takes the medication as prescribed by the doctor. Not taking the medication as prescribed by the doctor can increase the chance that the cancer will come back.
Children receive lower doses of chemotherapy during the maintenance phase and, as a result, tend to have less-severe side effects. Some children at higher risk may get more intense maintenance chemo and intrathecal therapy.
Maintenance chemotherapy usually lasts 2 to 3 years.
Treatment of residual disease
The treatment plans may change if the leukemia doesn’t go into remission during induction or consolidation. The doctor will probably check the child’s bone marrow soon after treatment starts to see if the leukemia is going away. If not, treatment might need to be more intense or prolonged.
If standard lab tests show the leukemia seems to have gone away, the doctor may use more sensitive tests to look for even small numbers of remaining leukemia cells (known as minimal residual disease, or MRD). If any are found, chemotherapy again might need to be intensified or prolonged.
Philadelphia chromosome-type acute lymphoblastic leukemia
For children with certain types of acute lymphoblastic leukemia, such as those with the Philadelphia chromosome, standard chemotherapy for acute lymphoblastic leukemia (as outlined above) might not be as effective. A stem cell transplant may be advised if induction treatment puts the leukemia in remission and a suitable stem cell donor is available.
Newer, targeted drugs such as imatinib (Gleevec) and dasatinib (Sprycel) are designed to kill leukemia cells that have the Philadelphia chromosome. These drugs are taken as pills. Adding these drugs to chemotherapy throughout treatment seems to help improve outcomes, according to studies done so far.
Treatment of recurrent acute lymphoblastic leukemia
If the acute lymphoblastic leukemia recurs (comes back) during or after treatment, the child will most likely be treated again with chemotherapy. Much of the treatment strategy depends on how soon the leukemia returns after the first treatment. If the relapse occurs after a long time, the same drugs might still be effective, so the same or similar treatment may be used to try to get the leukemia into a second remission.
If it comes back after a shorter time interval, more aggressive chemo with other drugs may be needed. The most commonly used chemo drugs are vincristine, L-asparaginase, anthracyclines (doxorubicin, daunorubicin, or mitoxantrone), cyclophosphamide, cytarabine (ara-C), and either etoposide or teniposide. The child will also receive a steroid (prednisone or dexamethasone). Intrathecal chemo will also be given.
For children whose leukemia comes back sooner after starting treatment, or for children with T-cell acute lymphoblastic leukemia who relapse, a stem cell transplant may be considered, especially if the child has a brother or sister who is a good tissue type match. Stem cell transplants may also be used for children who relapse after a second course of chemotherapy.
Some children have an extramedullary relapse, meaning that leukemia cells are found in one part of the body (such as the cerebrospinal fluid [CSF] or the testicles) but are not detectable in the bone marrow. In addition to intensive chemotherapy as described above, children with spread to the CSF may get more intense intrathecal chemotherapy, sometimes with radiation to the brain and spinal cord (if that area had not been already treated with radiation). Boys with relapse in a testicle may get radiation to the area.
If acute lymphoblastic leukemia doesn’t go away completely or if it comes back after a stem cell transplant, it can be very hard to treat. For some children, newer types of immunotherapy, such as CAR T-cell therapy or blinatumomab (a monoclonal antibody) might be helpful.
References- Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, 2013, Section 29. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/sect_29_childhood_cancer_iccc.pdf
- Relapse in Acute Lymphoblastic Leukaemia (ALL). https://media.leukaemiacare.org.uk/wp-content/uploads/Relapse-in-Acute-Lymphoblastic-Leukaemia-ALL-Web-Version.pdf
- Key Statistics for Acute Lymphocytic Leukemia (ALL). https://www.cancer.org/cancer/acute-lymphocytic-leukemia/about/key-statistics.html
- Howlader N, Noone AM, Krapcho M: SEER Cancer Statistics Review (CSR) 1975-2013. Bethesda, Md: National Cancer Institute, 2015. https://seer.cancer.gov/archive/csr/1975_2013
- Special section: cancer in children and adolescents. In: American Cancer Society: Cancer Facts and Figures 2014. American Cancer Society, 2014, pp 25-42. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2014/special-section-cancer-in-children-and-adolescents-cancer-facts-and-figures-2014.pdf
- Shah, A., & Coleman, M. P. (2007). Increasing incidence of childhood leukaemia: a controversy re-examined. British journal of cancer, 97(7), 1009–1012. https://doi.org/10.1038/sj.bjc.6603946
- Childhood cancer. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, 2013, Section 28. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/sect_28_childhood_cancer.pdf
- Barrington-Trimis, J. L., Cockburn, M., Metayer, C., Gauderman, W. J., Wiemels, J., & McKean-Cowdin, R. (2015). Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. Blood, 125(19), 3033–3034. https://doi.org/10.1182/blood-2015-03-634006
- Smith MA, Ries LA, Gurney JG, et al.: Leukemia. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 17-34. https://seer.cancer.gov/archive/publications/childhood/childhood-monograph.pdf
- Stiller CA, Chessells JM, Fitchett M. Neurofibromatosis and childhood leukaemia/lymphoma: a population-based UKCCSG study. Br J Cancer. 1994 Nov;70(5):969-72. doi: 10.1038/bjc.1994.431
- Passarge E. Bloom’s syndrome: the German experience. Ann Genet. 1991;34(3-4):179-97.
- Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer. 2003 Jan 15;97(2):425-40. doi: 10.1002/cncr.11046
- Taylor AM, Metcalfe JA, Thick J, Mak YF. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996 Jan 15;87(2):423-38.
- Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L, Payne-Turner D, Churchman M, Andersson A, Chen SC, McCastlain K, Becksfort J, Ma J, Wu G, Patel SN, Heatley SL, Phillips LA, Song G, Easton J, Parker M, Chen X, Rusch M, Boggs K, Vadodaria B, Hedlund E, Drenberg C, Baker S, Pei D, Cheng C, Huether R, Lu C, Fulton RS, Fulton LL, Tabib Y, Dooling DJ, Ochoa K, Minden M, Lewis ID, To LB, Marlton P, Roberts AW, Raca G, Stock W, Neale G, Drexler HG, Dickins RA, Ellison DW, Shurtleff SA, Pui CH, Ribeiro RC, Devidas M, Carroll AJ, Heerema NA, Wood B, Borowitz MJ, Gastier-Foster JM, Raimondi SC, Mardis ER, Wilson RK, Downing JR, Hunger SP, Loh ML, Mullighan CG. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet. 2013 Mar;45(3):242-52. doi: 10.1038/ng.2532
- Ripperger T, Schlegelberger B. Acute lymphoblastic leukemia and lymphoma in the context of constitutional mismatch repair deficiency syndrome. Eur J Med Genet. 2016 Mar;59(3):133-42. doi: 10.1016/j.ejmg.2015.12.014
- Moriyama T, Relling MV, Yang JJ. Inherited genetic variation in childhood acute lymphoblastic leukemia. Blood. 2015 Jun 25;125(26):3988-95. doi: 10.1182/blood-2014-12-580001
- Li Y, Schwab C, Ryan S, Papaemmanuil E, Robinson HM, Jacobs P, Moorman AV, Dyer S, Borrow J, Griffiths M, Heerema NA, Carroll AJ, Talley P, Bown N, Telford N, Ross FM, Gaunt L, McNally RJQ, Young BD, Sinclair P, Rand V, Teixeira MR, Joseph O, Robinson B, Maddison M, Dastugue N, Vandenberghe P, Stephens PJ, Cheng J, Van Loo P, Stratton MR, Campbell PJ, Harrison CJ. Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature. 2014 Apr 3;508(7494):98-102. doi: 10.1038/nature13115
- Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. Rob Pieters, Hester de Groot-Kruseman, Vincent Van der Velden, Marta Fiocco, Henk van den Berg, Evelien de Bont, R. Maarten Egeler, Peter Hoogerbrugge, Gertjan Kaspers, Ellen Van der Schoot, Valerie De Haas, and Jacques Van Dongen. Journal of Clinical Oncology 2016 34:22, 2591-2601. https://ascopubs.org/doi/10.1200/JCO.2015.64.6364
- Möricke A, Zimmermann M, Reiter A, Gadner H, Odenwald E, Harbott J, Ludwig WD, Riehm H, Schrappe M. Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr. 2005 Nov-Dec;217(6):310-20. doi: 10.1055/s-2005-872515 https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-2005-872515
- Dreyer, Z. E., Hilden, J. M., Jones, T. L., Devidas, M., Winick, N. J., Willman, C. L., Harvey, R. C., Chen, I. M., Behm, F. G., Pullen, J., Wood, B. L., Carroll, A. J., Heerema, N. A., Felix, C. A., Robinson, B., Reaman, G. H., Salzer, W. L., Hunger, S. P., Carroll, W. L., & Camitta, B. M. (2015). Intensified chemotherapy without SCT in infant ALL: results from COG P9407 (Cohort 3). Pediatric blood & cancer, 62(3), 419–426. https://doi.org/10.1002/pbc.25322
- Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A, Janka G, Rubnitz J, Silverman L, Stary J, Campbell M, Li CK, Mann G, Suppiah R, Biondi A, Vora A, Valsecchi MG. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007 Jul 21;370(9583):240-250. doi: 10.1016/S0140-6736(07)61126-X
- Reaman GH, Sposto R, Sensel MG, Lange BJ, Feusner JH, Heerema NA, Leonard M, Holmes EJ, Sather HN, Pendergrass TW, Johnstone HS, O’Brien RT, Steinherz PG, Zeltzer PM, Gaynon PS, Trigg ME, Uckun FM. Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trials of the Children’s Cancer Group. J Clin Oncol. 1999 Feb;17(2):445-55. doi: 10.1200/JCO.1999.17.2.445
- Sam, T. N., Kersey, J. H., Linabery, A. M., Johnson, K. J., Heerema, N. A., Hilden, J. M., Davies, S. M., Reaman, G. H., & Ross, J. A. (2012). MLL gene rearrangements in infant leukemia vary with age at diagnosis and selected demographic factors: a Children’s Oncology Group (COG) study. Pediatric blood & cancer, 58(6), 836–839. https://doi.org/10.1002/pbc.23274
- Andersson, A. K., Ma, J., Wang, J., Chen, X., Gedman, A. L., Dang, J., Nakitandwe, J., Holmfeldt, L., Parker, M., Easton, J., Huether, R., Kriwacki, R., Rusch, M., Wu, G., Li, Y., Mulder, H., Raimondi, S., Pounds, S., Kang, G., Shi, L., … St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project (2015). The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nature genetics, 47(4), 330–337. https://doi.org/10.1038/ng.3230
- De Lorenzo, P., Moorman, A. V., Pieters, R., Dreyer, Z. E., Heerema, N. A., Carroll, A. J., Hunger, S. P., Harvey, R., Willman, C. L., Devidas, M., Valsecchi, M. G., & Harrison, C. J. (2014). Cytogenetics and outcome of infants with acute lymphoblastic leukemia and absence of MLL rearrangements. Leukemia, 28(2), 428–430. https://doi.org/10.1038/leu.2013.280
- Place AE, Stevenson KE, Vrooman LM, Harris MH, Hunt SK, O’Brien JE, Supko JG, Asselin BL, Athale UH, Clavell LA, Cole PD, Kelly KM, Laverdiere C, Leclerc JM, Michon B, Schorin MA, Welch JJ, Lipshultz SE, Kutok JL, Blonquist TM, Neuberg DS, Sallan SE, Silverman LB. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015 Dec;16(16):1677-90. doi: 10.1016/S1470-2045(15)00363-0
- Dastugue N, Suciu S, Plat G, Speleman F, Cavé H, Girard S, Bakkus M, Pagès MP, Yakouben K, Nelken B, Uyttebroeck A, Gervais C, Lutz P, Teixeira MR, Heimann P, Ferster A, Rohrlich P, Collonge MA, Munzer M, Luquet I, Boutard P, Sirvent N, Karrasch M, Bertrand Y, Benoit Y. Hyperdiploidy with 58-66 chromosomes in childhood B-acute lymphoblastic leukemia is highly curable: 58951 CLG-EORTC results. Blood. 2013 Mar 28;121(13):2415-23. doi: 10.1182/blood-2012-06-437681
- Pui, C. H., Pei, D., Campana, D., Bowman, W. P., Sandlund, J. T., Kaste, S. C., Ribeiro, R. C., Rubnitz, J. E., Coustan-Smith, E., Jeha, S., Cheng, C., Metzger, M. L., Bhojwani, D., Inaba, H., Raimondi, S. C., Onciu, M., Howard, S. C., Leung, W., Downing, J. R., Evans, W. E., … Relling, M. V. (2011). Improved prognosis for older adolescents with acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 29(4), 386–391. https://doi.org/10.1200/JCO.2010.32.0325
- Stock, W., La, M., Sanford, B., Bloomfield, C. D., Vardiman, J. W., Gaynon, P., Larson, R. A., Nachman, J., Children’s Cancer Group, & Cancer and Leukemia Group B studies (2008). What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood, 112(5), 1646–1654. https://doi.org/10.1182/blood-2008-01-130237
- Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reamon G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996 Jan;14(1):18-24. doi: 10.1200/JCO.1996.14.1.18
- Hastings, C., Gaynon, P. S., Nachman, J. B., Sather, H. N., Lu, X., Devidas, M., & Seibel, N. L. (2015). Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (≥200 × 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children’s Oncology Group. British journal of haematology, 168(4), 533–546. https://doi.org/10.1111/bjh.13160
- Burns MA, Place AE, Stevenson KE, Gutiérrez A, Forrest S, Pikman Y, Vrooman LM, Harris MH, Hunt SK, O’Brien JE, Asselin BL, Athale UH, Clavell LA, Cole PD, Gennarini LM, Kahn JM, Kelly KM, Laverdiere C, Leclerc JM, Michon B, Schorin MA, Sulis ML, Welch JJG, Neuberg DS, Sallan SE, Silverman LB. Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: Results from DFCI ALL Consortium Protocols 05-001 and 11-001. Pediatr Blood Cancer. 2021 Jan;68(1):e28719. doi: 10.1002/pbc.28719. Epub 2020 Oct 7. Erratum in: Pediatr Blood Cancer. 2021 Mar;68(3):e28885
- Vora, A., Andreano, A., Pui, C. H., Hunger, S. P., Schrappe, M., Moericke, A., Biondi, A., Escherich, G., Silverman, L. B., Goulden, N., Taskinen, M., Pieters, R., Horibe, K., Devidas, M., Locatelli, F., & Valsecchi, M. G. (2016). Influence of Cranial Radiotherapy on Outcome in Children With Acute Lymphoblastic Leukemia Treated With Contemporary Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 34(9), 919–926. https://doi.org/10.1200/JCO.2015.64.2850
- Winick, N., Devidas, M., Chen, S., Maloney, K., Larsen, E., Mattano, L., Borowitz, M. J., Carroll, A., Gastier-Foster, J. M., Heerema, N. A., Willman, C., Wood, B., Loh, M. L., Raetz, E., Hunger, S. P., & Carroll, W. L. (2017). Impact of Initial CSF Findings on Outcome Among Patients With National Cancer Institute Standard- and High-Risk B-Cell Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 35(22), 2527–2534. https://doi.org/10.1200/JCO.2016.71.4774
- Sirvent N, Suciu S, Rialland X, Millot F, Benoit Y, Plantaz D, Ferster A, Robert A, Lutz P, Nelken B, Plouvier E, Norton L, Bertrand Y, Otten J. Prognostic significance of the initial cerebro-spinal fluid (CSF) involvement of children with acute lymphoblastic leukaemia (ALL) treated without cranial irradiation: results of European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group study 58881. Eur J Cancer. 2011 Jan;47(2):239-47. doi: 10.1016/j.ejca.2010.10.019
- Gilchrist GS, Tubergen DG, Sather HN, Coccia PF, O’Brien RT, Waskerwitz MJ, Hammond GD. Low numbers of CSF blasts at diagnosis do not predict for the development of CNS leukemia in children with intermediate-risk acute lymphoblastic leukemia: a Childrens Cancer Group report. J Clin Oncol. 1994 Dec;12(12):2594-600. doi: 10.1200/JCO.1994.12.12.2594
- Bürger B, Zimmermann M, Mann G, Kühl J, Löning L, Riehm H, Reiter A, Schrappe M. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003 Jan 15;21(2):184-8. doi: 10.1200/JCO.2003.04.096
- Levinsen M, Taskinen M, Abrahamsson J, Forestier E, Frandsen TL, Harila-Saari A, Heyman M, Jonsson OG, Lähteenmäki PM, Lausen B, Vaitkevičienė G, Asberg A, Schmiegelow K; Nordic Society of Paediatric Haematology and Oncology (NOPHO). Clinical features and early treatment response of central nervous system involvement in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014 Aug;61(8):1416-21. doi: 10.1002/pbc.24981
- Cherlow JM, Sather H, Steinherz P, Gaynon P, Tubergen D, Trigg M, Novak L, Bleyer WA. Craniospinal irradiation for acute lymphoblastic leukemia with central nervous system disease at diagnosis: a report from the Children’s Cancer Group. Int J Radiat Oncol Biol Phys. 1996 Aug 1;36(1):19-27. doi: 10.1016/s0360-3016(96)00272-6
- Hijiya N, Liu W, Sandlund JT, Jeha S, Razzouk BI, Ribeiro RC, Rubnitz JE, Howard SC, Kyzer EP, Redd DS, Cheng C, Rivera GK, Hudson MM, Relling MV, Pui CH. Overt testicular disease at diagnosis of childhood acute lymphoblastic leukemia: lack of therapeutic role of local irradiation. Leukemia. 2005 Aug;19(8):1399-403. doi: 10.1038/sj.leu.2403843
- Sirvent N, Suciu S, Bertrand Y, Uyttebroeck A, Lescoeur B, Otten J. Overt testicular disease (OTD) at diagnosis is not associated with a poor prognosis in childhood acute lymphoblastic leukemia: results of the EORTC CLG Study 58881. Pediatr Blood Cancer. 2007 Sep;49(3):344-8. doi: 10.1002/pbc.20716
- Lundin, C., Forestier, E., Klarskov Andersen, M., Autio, K., Barbany, G., Cavelier, L., Golovleva, I., Heim, S., Heinonen, K., Hovland, R., Johannsson, J. H., Kjeldsen, E., Nordgren, A., Palmqvist, L., Johansson, B., Nordic Society of Pediatric Hematology Oncology (NOPHO), Swedish Cytogenetic Leukemia Study Group (SCLSG), & NOPHO Leukemia Cytogenetic Study Group (NLCSG) (2014). Clinical and genetic features of pediatric acute lymphoblastic leukemia in Down syndrome in the Nordic countries. Journal of hematology & oncology, 7, 32. https://doi.org/10.1186/1756-8722-7-32
- Matloub, Y., Rabin, K. R., Ji, L., Devidas, M., Hitzler, J., Xu, X., Bostrom, B. C., Stork, L. C., Winick, N., Gastier-Foster, J. M., Heerema, N. A., Stonerock, E., Carroll, W. L., Hunger, S. P., & Gaynon, P. S. (2019). Excellent long-term survival of children with Down syndrome and standard-risk ALL: a report from the Children’s Oncology Group. Blood advances, 3(11), 1647–1656. https://doi.org/10.1182/bloodadvances.2019032094
- Buitenkamp, T. D., Izraeli, S., Zimmermann, M., Forestier, E., Heerema, N. A., van den Heuvel-Eibrink, M. M., Pieters, R., Korbijn, C. M., Silverman, L. B., Schmiegelow, K., Liang, D. C., Horibe, K., Arico, M., Biondi, A., Basso, G., Rabin, K. R., Schrappe, M., Cario, G., Mann, G., Morak, M., … Zwaan, C. M. (2014). Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood, 123(1), 70–77. https://doi.org/10.1182/blood-2013-06-509463
- Maloney, K. W., Carroll, W. L., Carroll, A. J., Devidas, M., Borowitz, M. J., Martin, P. L., Pullen, J., Whitlock, J. A., Willman, C. L., Winick, N. J., Camitta, B. M., & Hunger, S. P. (2010). Down syndrome childhood acute lymphoblastic leukemia has a unique spectrum of sentinel cytogenetic lesions that influences treatment outcome: a report from the Children’s Oncology Group. Blood, 116(7), 1045–1050. https://doi.org/10.1182/blood-2009-07-235291
- Buitenkamp TD, Pieters R, Gallimore NE, van der Veer A, Meijerink JP, Beverloo HB, Zimmermann M, de Haas V, Richards SM, Vora AJ, Mitchell CD, Russell LJ, Schwab C, Harrison CJ, Moorman AV, van den Heuvel-Eibrink MM, den Boer ML, Zwaan CM. Outcome in children with Down’s syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia. 2012 Oct;26(10):2204-11. doi: 10.1038/leu.2012.84
- Hanada I, Terui K, Ikeda F, Toki T, Kanezaki R, Sato T, Kamio T, Kudo K, Sasaki S, Takahashi Y, Hayashi Y, Inukai T, Kojima S, Koike K, Kosaka Y, Kobayashi M, Imaizumi M, Mitsui T, Hori H, Hara J, Horibe K, Nagai J, Goto H, Ito E. Gene alterations involving the CRLF2-JAK pathway and recurrent gene deletions in Down syndrome-associated acute lymphoblastic leukemia in Japan. Genes Chromosomes Cancer. 2014 Nov;53(11):902-10. doi: 10.1002/gcc.22201
- Pui CH, Boyett JM, Relling MV, Harrison PL, Rivera GK, Behm FG, Sandlund JT, Ribeiro RC, Rubnitz JE, Gajjar A, Evans WE. Sex differences in prognosis for children with acute lymphoblastic leukemia. J Clin Oncol. 1999 Mar;17(3):818-24. doi: 10.1200/JCO.1999.17.3.818
- Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Arkin S, Declerck L, Cohen HJ, Sallan SE. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001 Mar 1;97(5):1211-8. doi: 10.1182/blood.v97.5.1211
- Hunger, S. P., Lu, X., Devidas, M., Camitta, B. M., Gaynon, P. S., Winick, N. J., Reaman, G. H., & Carroll, W. L. (2012). Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30(14), 1663–1669. https://doi.org/10.1200/JCO.2011.37.8018
- Kahn, J. M., Cole, P. D., Blonquist, T. M., Stevenson, K., Jin, Z., Barrera, S., Davila, R., Roberts, E., Neuberg, D. S., Athale, U. H., Clavell, L. A., Laverdiere, C., Leclerc, J. M., Michon, B., Schorin, M. A., Welch, J., Sallan, S. E., Silverman, L. B., & Kelly, K. M. (2018). An investigation of toxicities and survival in Hispanic children and adolescents with ALL: Results from the Dana-Farber Cancer Institute ALL Consortium protocol 05-001. Pediatric blood & cancer, 65(3), 10.1002/pbc.26871. https://doi.org/10.1002/pbc.26871
- Bhatia, S., Landier, W., Shangguan, M., Hageman, L., Schaible, A. N., Carter, A. R., Hanby, C. L., Leisenring, W., Yasui, Y., Kornegay, N. M., Mascarenhas, L., Ritchey, A. K., Casillas, J. N., Dickens, D. S., Meza, J., Carroll, W. L., Relling, M. V., & Wong, F. L. (2012). Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30(17), 2094–2101. https://doi.org/10.1200/JCO.2011.38.9924
- Bhatia, S., Landier, W., Hageman, L., Kim, H., Chen, Y., Crews, K. R., Evans, W. E., Bostrom, B., Casillas, J., Dickens, D. S., Maloney, K. W., Neglia, J. P., Ravindranath, Y., Ritchey, A. K., Wong, F. L., & Relling, M. V. (2014). 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood, 124(15), 2345–2353. https://doi.org/10.1182/blood-2014-01-552166
- Yang, J. J., Cheng, C., Devidas, M., Cao, X., Fan, Y., Campana, D., Yang, W., Neale, G., Cox, N. J., Scheet, P., Borowitz, M. J., Winick, N. J., Martin, P. L., Willman, C. L., Bowman, W. P., Camitta, B. M., Carroll, A., Reaman, G. H., Carroll, W. L., Loh, M., … Relling, M. V. (2011). Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nature genetics, 43(3), 237–241. https://doi.org/10.1038/ng.763
- Xu, H., Cheng, C., Devidas, M., Pei, D., Fan, Y., Yang, W., Neale, G., Scheet, P., Burchard, E. G., Torgerson, D. G., Eng, C., Dean, M., Antillon, F., Winick, N. J., Martin, P. L., Willman, C. L., Camitta, B. M., Reaman, G. H., Carroll, W. L., Loh, M., … Yang, J. J. (2012). ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30(7), 751–757. https://doi.org/10.1200/JCO.2011.38.0345
- Aldhafiri FK, McColl JH, Reilly JJ. Prognostic significance of being overweight and obese at diagnosis in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2014 Apr;36(3):234-6. doi: 10.1097/MPH.0000000000000056
- Gelelete CB, Pereira SH, Azevedo AM, Thiago LS, Mundim M, Land MG, Costa ES. Overweight as a prognostic factor in children with acute lymphoblastic leukemia. Obesity (Silver Spring). 2011 Sep;19(9):1908-11. doi: 10.1038/oby.2011.195
- Combination Chemotherapy in Treating Children With Acute Lymphocytic Leukemia. https://clinicaltrials.gov/ct2/show/NCT00002812
- Orgel E, Tucci J, Alhushki W, Malvar J, Sposto R, Fu CH, Freyer DR, Abdel-Azim H, Mittelman SD. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood. 2014 Dec 18;124(26):3932-8. doi: 10.1182/blood-2014-08-595389
- Eissa, H. M., Zhou, Y., Panetta, J. C., Browne, E. K., Jeha, S., Cheng, C., Relling, M. V., Campana, D., Pui, C. H., & Inaba, H. (2017). The effect of body mass index at diagnosis on clinical outcome in children with newly diagnosed acute lymphoblastic leukemia. Blood cancer journal, 7(2), e531. https://doi.org/10.1038/bcj.2017.11
- den Hoed, M. A., Pluijm, S. M., de Groot-Kruseman, H. A., te Winkel, M. L., Fiocco, M., van den Akker, E. L., Hoogerbrugge, P., van den Berg, H., Leeuw, J. A., Bruin, M. C., Bresters, D., Veerman, A. J., Pieters, R., & van den Heuvel-Eibrink, M. M. (2015). The negative impact of being underweight and weight loss on survival of children with acute lymphoblastic leukemia. Haematologica, 100(1), 62–69. https://doi.org/10.3324/haematol.2014.110668
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391-405. doi: 10.1182/blood-2016-03-643544
- Pui CH, Chessells JM, Camitta B, Baruchel A, Biondi A, Boyett JM, Carroll A, Eden OB, Evans WE, Gadner H, Harbott J, Harms DO, Harrison CJ, Harrison PL, Heerema N, Janka-Schaub G, Kamps W, Masera G, Pullen J, Raimondi SC, Richards S, Riehm H, Sallan S, Sather H, Shuster J, Silverman LB, Valsecchi MG, Vilmer E, Zhou Y, Gaynon PS, Schrappe M. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003 Apr;17(4):700-6. doi: 10.1038/sj.leu.2402883
- Möricke A, Ratei R, Ludwig WD, et al.: Prognostic factors in CD10 negative precursor b-cell acute lymphoblastic leukemia in children: data from three consecutive trials ALL-BFM 86, 90, and 95. [Abstract] Blood 104 (11): A-1957, 540a, 2004.
- Uckun FM, Sensel MG, Sather HN, Gaynon PS, Arthur DC, Lange BJ, Steinherz PG, Kraft P, Hutchinson R, Nachman JB, Reaman GH, Heerema NA. Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report from the Children’s Cancer Group. J Clin Oncol. 1998 Feb;16(2):527-35. doi: 10.1200/JCO.1998.16.2.527
- Koehler M, Behm FG, Shuster J, Crist W, Borowitz M, Look AT, Head D, Carroll AJ, Land V, Steuber P, et al. Transitional pre-B-cell acute lymphoblastic leukemia of childhood is associated with favorable prognostic clinical features and an excellent outcome: a Pediatric Oncology Group study. Leukemia. 1993 Dec;7(12):2064-8
- Wagener, R., López, C., Kleinheinz, K., Bausinger, J., Aukema, S. M., Nagel, I., Toprak, U. H., Seufert, J., Altmüller, J., Thiele, H., Schneider, C., Kolarova, J., Park, J., Hübschmann, D., Murga Penas, E. M., Drexler, H. G., Attarbaschi, A., Hovland, R., Kjeldsen, E., Kneba, M., … Siebert, R. (2018). IG-MYC+ neoplasms with precursor B-cell phenotype are molecularly distinct from Burkitt lymphomas. Blood, 132(21), 2280–2285. https://doi.org/10.1182/blood-2018-03-842088
- Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE, Asselin BL. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003 Oct 1;21(19):3616-22. doi: 10.1200/JCO.2003.10.116
- Winter, S. S., Dunsmore, K. P., Devidas, M., Wood, B. L., Esiashvili, N., Chen, Z., Eisenberg, N., Briegel, N., Hayashi, R. J., Gastier-Foster, J. M., Carroll, A. J., Heerema, N. A., Asselin, B. L., Gaynon, P. S., Borowitz, M. J., Loh, M. L., Rabin, K. R., Raetz, E. A., Zweidler-Mckay, P. A., Winick, N. J., … Hunger, S. P. (2018). Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children’s Oncology Group AALL0434 Methotrexate Randomization. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 36(29), 2926–2934. https://doi.org/10.1200/JCO.2018.77.7250
- Slack JL, Arthur DC, Lawrence D, Mrózek K, Mayer RJ, Davey FR, Tantravahi R, Pettenati MJ, Bigner S, Carroll AJ, Rao KW, Schiffer CA, Bloomfield CD. Secondary cytogenetic changes in acute promyelocytic leukemia–prognostic importance in patients treated with chemotherapy alone and association with the intron 3 breakpoint of the PML gene: a Cancer and Leukemia Group B study. J Clin Oncol. 1997 May;15(5):1786-95. doi: 10.1200/JCO.1997.15.5.1786
- Attarbaschi A, Mann G, Dworzak M, Wiesbauer P, Schrappe M, Gadner H. Mediastinal mass in childhood T-cell acute lymphoblastic leukemia: significance and therapy response. Med Pediatr Oncol. 2002 Dec;39(6):558-65. doi: 10.1002/mpo.10164
- Inukai T, Kiyokawa N, Campana D, Coustan-Smith E, Kikuchi A, Kobayashi M, Takahashi H, Koh K, Manabe A, Kumagai M, Ikuta K, Hayashi Y, Tsuchida M, Sugita K, Ohara A. Clinical significance of early T-cell precursor acute lymphoblastic leukaemia: results of the Tokyo Children’s Cancer Study Group Study L99-15. Br J Haematol. 2012 Feb;156(3):358-65. doi: 10.1111/j.1365-2141.2011.08955.x
- Patrick K, Wade R, Goulden N, Mitchell C, Moorman AV, Rowntree C, Jenkinson S, Hough R, Vora A. Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014 Aug;166(3):421-4. doi: 10.1111/bjh.12882
- Combination Chemotherapy in Treating Young Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or T-cell Lymphoblastic Lymphoma. https://clinicaltrials.gov/ct2/show/NCT00408005
- Dunsmore KP, Winter SS, Devidas M, Wood BL, Esiashvili N, Chen Z, Eisenberg N, Briegel N, Hayashi RJ, Gastier-Foster JM, Carroll AJ, Heerema NA, Asselin BL, Rabin KR, Zweidler-Mckay PA, Raetz EA, Loh ML, Schultz KR, Winick NJ, Carroll WL, Hunger SP. Children’s Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2020 Oct 1;38(28):3282-3293. doi: 10.1200/JCO.20.00256
- Corrente F, Bellesi S, Metafuni E, Puggioni PL, Marietti S, Ciminello AM, Za T, Sorà F, Fianchi L, Sica S, De Stefano V, Chiusolo P. Role of flow-cytometric immunophenotyping in prediction of BCR/ABL1 gene rearrangement in adult B-cell acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018 May;94(3):468-476. doi: 10.1002/cyto.b.21605
- Qian M, Zhang H, Kham SK, Liu S, Jiang C, Zhao X, Lu Y, Goodings C, Lin TN, Zhang R, Moriyama T, Yin Z, Li Z, Quah TC, Ariffin H, Tan AM, Shen S, Bhojwani D, Hu S, Chen S, Zheng H, Pui CH, Yeoh AE, Yang JJ. Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res. 2017 Feb;27(2):185-195. doi: 10.1101/gr.209163.116
- Pui CH, Rubnitz JE, Hancock ML, Downing JR, Raimondi SC, Rivera GK, Sandlund JT, Ribeiro RC, Head DR, Relling MV, Evans WE, Behm FG. Reappraisal of the clinical and biologic significance of myeloid-associated antigen expression in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998 Dec;16(12):3768-73. doi: 10.1200/JCO.1998.16.12.3768
- Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer. 2001 Nov;1(2):99-108. doi: 10.1038/35101056
- Relapsed Childhood Acute Lymphoblastic Leukemia. https://www.dana-farber.org/relapsed-childhood-acute-lymphoblastic-leukemia