Chronic wound
A chronic wound is one that has failed to progress through the phases of healing in an orderly set of stages and timely fashion and has shown no significant progress toward healing in 30 days 1. Chronic wounds include, but are not limited, to diabetic foot ulcers, venous leg ulcers, and pressure ulcers 2. Chronic wounds may never heal or may take years to do so. These wounds cause patients severe emotional and physical stress and create a significant financial burden on patients and the whole healthcare system 3. Chronic wounds can be classified as vascular ulcers (e.g., venous and arterial ulcers), diabetic ulcers, and pressure ulcers 4. Some common features shared by each of these wounds include prolonged or excessive inflammation, persistent infections, formation of drug-resistant microbial biofilms, and the inability of dermal and/or epidermal cells to respond to reparative stimuli 5. In aggregate, these pathophysiologic phenomena result in the failure of these wounds to heal. The underlying pathologies, however, differ among various types of chronic wounds.
Factors contributing to the chronicity of the wound may include:
- Pressure, trauma and/or lower extremity wounds
- Increased bacterial load
- Excessive proteases: Degraded growth factors, matrix metalloproteinases (MMPs), degraded cell surface structures
- Senescent/Aberrant cells
- Inappropriate treatment
Chronic lower extremity ulcers are those that do not progress through the healing process in a timely manner and have become a major challenge to healthcare systems worldwide. In the United States alone, chronic leg wounds affect an estimated 2.4–4.5 million people 6. Chronic leg and foot ulcers occur in many adults with vascular disease or diabetes and are attributed to chronic venous insufficiency, arterial disease, prolonged pressure, or neuropathy 7. These ulcers last on average 12 to 13 months, recur in up to 60% to 70% of patients, can lead to loss of function and decreased quality of life, and are a significant cause of morbidity 8. Predominantly a condition of the elderly, chronic wounds are becoming more prevalent and more difficult to treat and are associated with high treatment costs 9. The care of chronic wounds has become its own specialty, with providers often using advanced therapies, including growth factors, extracellular matrices, engineered skin, and negative pressure wound therapy 10. Care for chronic non healing wounds has been reported to cost 2% to 3% of the healthcare budgets in developed countries 7.
How wounds heal
A skin wound results from the breakdown of the epidermal layer integrity 11. Any tissue injury with anatomical integrity disruption with functional loss can be described as a wound. Wound healing mostly means healing of the skin. The wound healing begins immediately after an injury to the epidermal layer and might take years. This dynamic process includes the highly organized cellular, humoral, and molecular mechanisms 12. Wound healing has 3 overlapping phases which are inflammation, proliferation, and remodeling 13. Any disruption leads to abnormal wound healing 14.
Wound healing is occasionally classified as primary healing and secondary healing. Uncomplicated healing of a noninfected, well-approximated wound is defined as primary healing. Surgical wounds are the best example for primary healing. If the wound healing course in this wound is disrupted by infection, dehiscence, hypoxia or immune dysfunction, secondary healing stage begins. During secondary healing, granulation tissue formation and epithelization over this new tissue take place. These type of wounds are more susceptible to infections and poor healing 15.
Stages of wound healing
Wounds heal in stages. The smaller the wound, the quicker it will heal. The larger or deeper the wound, the longer it takes to heal. When you get a cut, scrape, or puncture, the wound will bleed.
- The blood will start to clot within a few minutes or less and stop the bleeding.
- The blood clots dry and form a scab, which protects the tissue underneath from germs.
Not all wounds bleed. For example, burns, some puncture wounds, and pressure sores do not bleed.
Once the scab forms, your body’s immune system starts to protect the wound from infection.
- The wound becomes slightly swollen, red or pink, and tender.
- You also may see some clear fluid oozing from the wound. This fluid helps clean the area.
- Blood vessels open in the area, so blood can bring oxygen and nutrients to the wound. Oxygen is essential for healing.
- White blood cells help fight infection from germs and begin to repair the wound.
- This stage takes about 2 to 5 days.
Tissue growth and rebuilding occur next.
- Over the next 3 weeks or so, the body repairs broken blood vessels and new tissue grows.
- Red blood cells help create collagen, which are tough, white fibers that form the foundation for new tissue.
- The wound starts to fill in with new tissue, called granulation tissue.
- New skin begins to form over this tissue.
- As the wound heals, the edges pull inward and the wound gets smaller.
A scar forms and the wound becomes stronger.
- As healing continues, you may notice that the area itches. After the scab falls off, the area may look stretched, red, and shiny.
- The scar that forms will be smaller than the original wound. It will be less strong and less flexible than the surrounding skin.
- Over time, the scar will fade and may disappear completely. This can take as long as 2 years. Some scars never go away completely.
- Scars form because the new tissue grows back differently than the original tissue. If you only injured the top layer of skin, you will probably not have a scar. With deeper wounds, you are more likely to have a scar.
Properly caring for your wound means keeping it clean and covered. This can help prevent infections and scarring.
- For minor wounds, clean your wound with gentle soap and water. Cover the wound with a sterile bandage or other dressing.
- For major wounds, follow your health care provider’s instructions on how to care for your injury.
- Avoid picking at or scratching the scab. This can interfere with healing and cause scarring.
- Once the scar forms, some people think it helps to massage it with vitamin E or petroleum jelly. However, this is not proven to help prevent a scar or help it fade. DO NOT rub your scar or apply anything to it without talking with your provider first.
Chronic wound causes
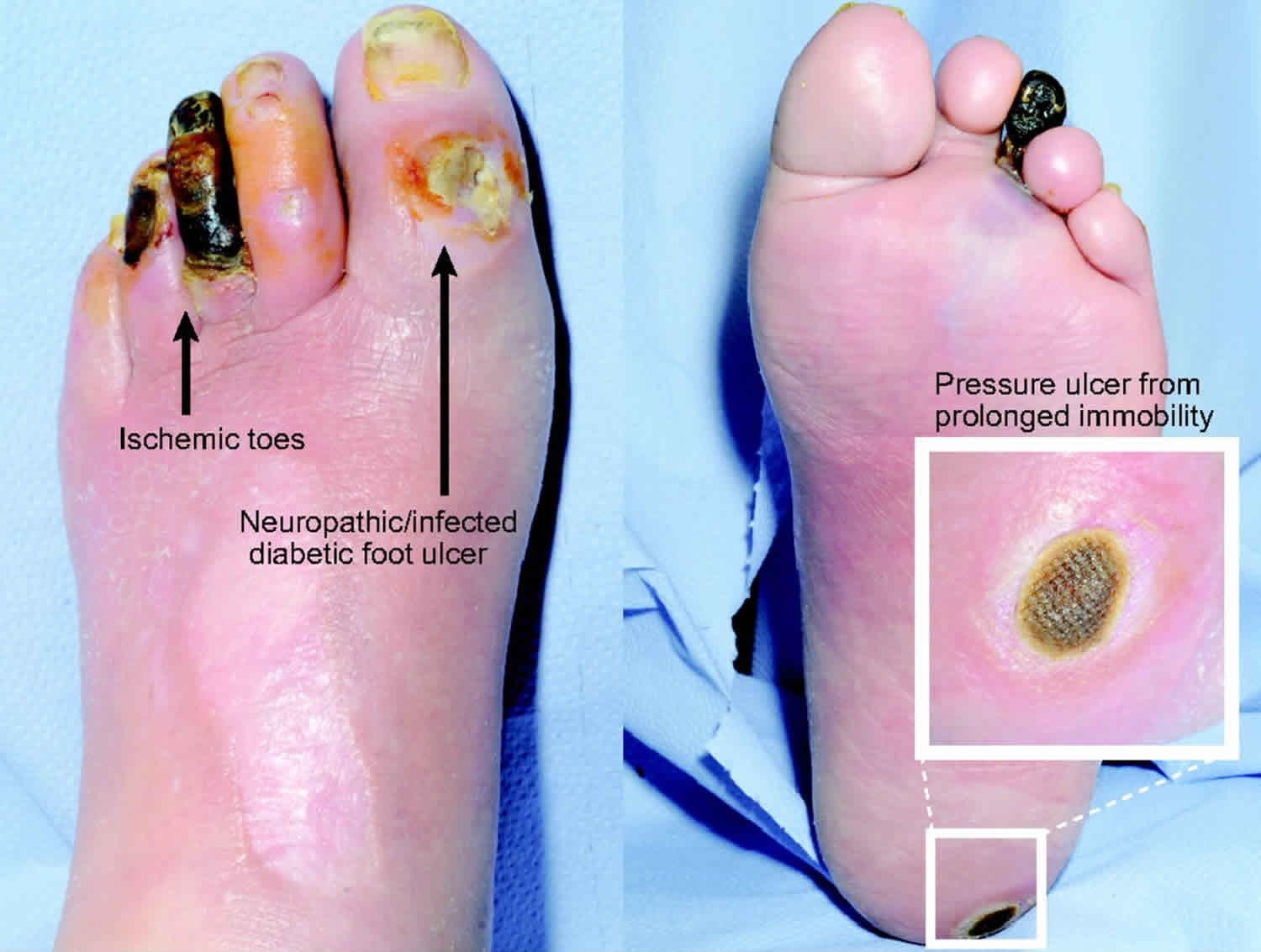
Types of chronic wounds may include, but are not limited to the following causes: venous ulcers, diabetic foot ulcers and pressure ulcers.
Venous ulcers occur primarily in the legs of elderly patients and are caused by problems with blood circulation due to dysfunctional blood valves.
Diabetic ulcers often start as small scratches or bruises which patients with diabetes fail to notice due to nerve damage and limited sensitivity. Compromised immune systems and damaged capillaries lead to these formerly small and benign wounds becoming dangerously infected.
Pressure ulcers primarily afflict patients who are bedridden or of limited mobility. The constant pressure on the tissue over powers the pressure of the capillaries, affecting blood flow. Areas at the greatest risk for pressure ulcers are the sacrum, shoulder blades and heels. Correctly identifying the cause of a chronic wound as well as the local and systemic factors that may be contributing to poor wound healing is critical to successful wound treatment.
Chronic wounds pathophysiology
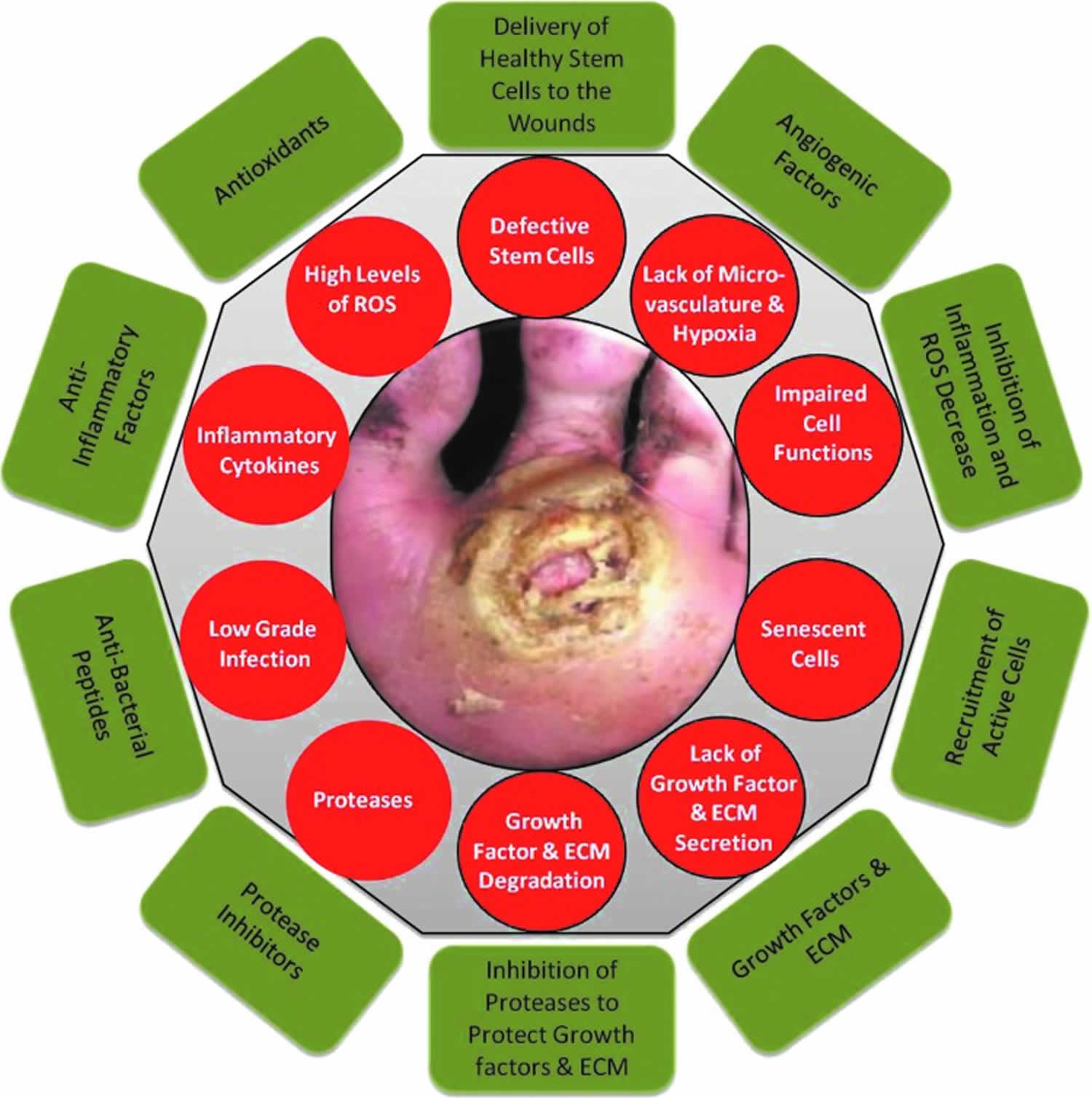
Chronic wounds are defined as wounds that fail to proceed through the normal phases of wound healing in an orderly and timely manner. Often, chronic wounds stall in the inflammation phase of healing. Despite differences in etiology at the molecular level, chronic wounds share certain common features, including excessive levels of proinflammatory cytokines, proteases, reactive oxygen species (ROS), and senescent cells, as well as the existence of persistent infection, and a deficiency of stem cells that are often also dysfunctional (Figure 1).
Due to repeated tissue injury, microorganisms and platelet-derived factors, such as transforming growth factor-β (TGF-β) or extracellular matrix fragment molecules, stimulate the constant influx of immune cells; the proinflammatory cytokine cascade therefore becomes amplified and persists for a prolonged time, leading to elevated levels of proteases. In acute wounds, proteases are tightly regulated by their inhibitors. In chronic wounds, protease levels exceed that of their respective inhibitors, leading to destruction of extracellular matrix and degradation of growth factors and their receptors. The proteolytic destruction of extracellular matrix not only prevents the wound from moving forward into the proliferative phase but also attracts more inflammatory cells, thus amplifying the inflammation cycle 16.
Immune cells produce reactive oxygen species, which in low concentrations provides defense against microorganisms. In chronic wounds, however, the predominant hypoxic and inflammatory environment increases reactive oxygen species production, which damages extracellular matrix proteins and causes cell damage. This sequence of events leads to an enhanced stimulation of proteases and inflammatory cytokines 17. It has been suggested in an animal model that application of strong antioxidants reduces reactive oxygen species to normal levels, which results in the reverse of the chronicity of wounds and improves healing 18.
Furthermore, chronic wounds are characterized by senescent cell populations with impaired proliferative and secretory capacities, rendering them unresponsive to typical wound healing signals 19. It has been reported that fibroblasts from venous and pressure ulcers are senescent and have a diminished ability to proliferate. This diminished proliferative capacity is directly correlated with the failure of a wound to heal 20. Accumulated data also indicate that chronic wounds contain senescent keratinocytes, endothelial cells, fibroblasts, and macrophages 21. The senescent phenotype of cells in chronic wounds is attributed to oxidative stress that leads to DNA damage-related cell cycle arrest or to abnormal metabolic changes in diabetic patients, which results in defects in intracellular biochemical pathways such as the GSK-3β/Fyn/Nrf2 pathway 22.
In recent years, mesenchymal stem cells (mesenchymal stem cells) have been shown to play an important role in wound healing 23. These cells can be recruited into the circulation in response to injury. Subsequently, they are found to engraft into the remodeling microvasculature. Nonetheless, it has also been shown that stem cells in animals and patients with diabetes or chronic wounds are both deficient and defective 24. Thus, these patients may require a direct delivery of healthy donor-derived functional mesenchymal stem cells to overcome this deficiency and achieve wound healing 25.
Nonhealing ulcers and wounds represent a failure to achieve complete reepithelialization in the appropriate temporal sequence of tissue repair 26. Understanding the underlying molecular and physiologic perturbations of nonhealing wounds, one can appreciate the necessity to modify these wounds toward the characteristics of an acute healing wound. The need to restore the proper balance of cytokines, growth factors, proteases, and metabolically competent cells is illustrated in Figure 1.
Figure 1. Chronic wounds pathophysiology
Footnote: Molecular and cellular deficiencies in chronic wounds (red circles) and factors required to overcome them (green rectangles). Nonhealing ulcers and wounds represent a failure to achieve complete reepithelialization in the appropriate temporal sequence of tissue repair. Such wounds are characterized by excessive inflammation (including elevated levels of proteases, reactive oxygen species, and inflammatory cytokines), by senescent cell populations with impaired proliferative and secretory capacities, and by defective mesenchymal stem cells. Excessive inflammation leads to degradation of newly synthesized growth factors and extracellular matrix. There is a need to restore the proper balance of cytokines, growth factors, and proteases, to recruit functional cells (epithelial cells, fibroblasts, and endothelial cells) to the wound area, and to deliver healthy functional mesenchymal stem cells directly to the wound to compensate for the patient’s own dysfunctional stem cells.
Abbreviations: ECM = extracellular matrix; MSCs = mesenchymal stem cells; ROS = reactive oxygen species.
[Source 2 ]Chronic wound symptoms
Common chronic skin and soft tissue wounds include the diabetic foot ulcer, the pressure ulcer, and the venous stasis ulcer 27. Chronic wounds are typically identified by a raised, hyperproliferative, yet non-advancing wound margin. The area around the wound will be inflamed and this inflammation may be affect healing negatively.
Chronic wound patients often report pain as dominant in their lives 28. It is recommended that healthcare providers handle the pain related to chronic wounds as one of the main priorities in chronic wound management (together with addressing the cause). Six out of ten venous leg ulcer patients experience pain with their ulcer 29 and similar trends are observed for other chronic wounds.
Persistent pain (at night, at rest, and with activity) is the main problem for patients with chronic ulcers 30. Frustrations regarding ineffective analgesics and plans of care that they were unable to adhere to were also identified.
Chronic wound treatment
The treatment of chronic wounds varies based on the type of wound. Often, underlying causes must be addressed first before wound healing can progress. Individuals with diabetes will need to improve their nutrition and vascular health and both diabetic and pressure ulcers will require offloading for the affected area. Arterial ulcers will require revascularization while venous ulcers will benefit from compression therapy. The Wound Healing Society advocates use of the acronym TIME to remember factors that contribute to poor wound healing:
- T: Tissue, such as the presences of necrotic tissue in a wound
- I: Inflammation or infection.
- M: Moisture, i.e. whether the wound is macerated or dessicated.
- E: (Wound) Edge – whether reepithelializing or nonadvancing.
The care for chronic wounds therefore relies upon basic tenets that aim to not only remove or ameliorate the etiologic causes but also to address underlying systemic and metabolic perturbations such as infection or peripheral arterial disease 2. Proper care of the wound is facilitated initially by employing thorough patient and wound assessment. Factors contributing to the development or recalcitrance of the wounds are then addressed accordingly. Concurrent with the management of associated complications or etiologic factors, wound bed preparation plays a key role in encouraging the proper environment in which tissue repair can take place.
Basic tenets of wound care 2:
- Patient assessment
- Medical comorbidities/history
- Diabetes, chronic kidney disease, coronary artery disease, congestive heart failure, peripheral arterial disease, alcohol, etc.
- Obesity, functional status, smoking
- Medications—steroids, warfarin, antibiotics, etc.
- Laboratory parameters/vital signs
- Glucose, hemoglobin A1c, creatinine, complete blood count, albumin, erythrocyte sedimentation rate, C-reactive protein, etc.
- Nutrition
- Reliability
- Medical comorbidities/history
- Wound assessment
- Wound diagnosis—diabetic foot ulcer, venous leg ulcer, pressure ulcer, postsurgical, etc.
- Etiology
- Shoes, high plantar pressure, injury
- Depth, extent, area, location, appearance, temp., odor
- Neurological—10 G monofilament, deep tendon reflexes, vibration perception threshold
- Vascular—pulses (ABI, toe blood pressure, transcutaneous oximetry, arteriography as necessary)
- Infection—probe for depth and abscess, tissue culture as necessary
- Classify, determine presence of osteomyelitis
- X-ray (MRI, scans, computed tomography, as necessary)
- Structural deformities
- Charcot, hammertoes, bunions, prior amps
- Treatment
- Medical management
- Vascular—revascularization (endovascular vs. BPG) restore pulse. Hyperbaric O2. Topical O2.
- Infection—drain abscesses, debride osteomyelitis, antibiotics
- Wound care:
- Debridement
- Wound bed preparation
- Offloading/compression
- Therapeutic agents
- Surgery
Treatment of chronic wounds
Managing chronic wounds, although often times challenging, need not be considered a daunting task if basic principles of care are routinely followed. A thorough assessment of the patient and wound will guide subsequent treatment by elucidating underlying areas of concern that need to be specifically addressed. Therefore, a systematic approach to both assessment and treatment should most often lead to favorable outcomes. Due to the frequent complexity of patients and wounds, a multidisciplinary approach to management has been proven highly successful and is widely recommended 31.
Medical/holistic management of the patient must commence concurrently with wound management. Diabetic patients frequently need improved control of their hyperglycemia, renal insufficiency, nutrition, and other associated medical comorbidities that may adversely affect the healing of their wound(s). Patients with a venous leg ulcer might often have hemodynamic perturbations requiring improved medical management, while pressure ulcer patients, often bedridden from intercurrent illness, will have significant nutritional deficits that need to be corrected to optimize tissue repair 32.
The vascular examination performed during the wound assessment will have determined the need for necessary interventions 33. Since many diabetic foot ulcer s have a component of vascular insufficiency, referral to a vascular surgeon or vascular interventionist for arterial imaging (angiography, duplex scanning, etc.) and subsequent revascularization need to take place early in the course of treatment. The exact roles of endovascular and open bypass procedures are still evolving, but are primarily determined by arterial anatomy, wound severity, and patient comorbidities 34. The ultimate goal is to restore a palpable pulse in the affected foot. In some limited cases where revascularization has failed or is not feasible, hyperbaric oxygen therapy (HBOT) might be indicated.69,70 Topical oxygen therapy, long criticized as having no role in this regard, is emerging again as an adjunctive measure to improve tissue oxygenation 35. In contrast, venous insufficiency must be addressed initially with adequate compression wrapping with or without intermittent pneumatic compression to counteract the detrimental effects of the venous hypertension causing associated venous leg ulcers 36. Where compression therapy is ineffective or for recurrent venous leg ulcers due to significant venous disease, surgical intervention on the superficial, deep, and/or incompetent perforators is indicated 36. Again, care must be taken to identify mixed arterial and venous disease in such circumstances since the associated arterial insufficiency complicates customary treatment protocols for venous ulcerations.
Infection is similarly an important risk factor for wound healing failure and, in the case of diabetic foot ulcer s, for subsequent lower extremity amputation 37. Even excessive bioburden can inhibit normal progression to wound healing 38. While acutely infected wounds are easily diagnosed, neuropathy can mask the presence of deep infections or abscess. Hence, clinical suspicion must remain high when insensate patients complain of pain or flu-like symptoms. Once diagnosed, infection complicating chronic wounds must be treated aggressively. This includes thorough debridement, surgical drainage of abscesses, debridement of infected bone, and tissue culture-guided antimicrobial therapy. As previously mentioned, clinically noninfected ulcers should not be cultured nor treated with systemic antimicrobial therapy 39. However, if osteomyelitis is suspected, bone culture, followed by specific antimicrobial therapy (and perhaps surgery), is warranted for this recalcitrant infection 40.
Specific wound care or wound bed preparation commences concurrently with the aforementioned interventions when feasible 41. Revascularization, however, often follows control of infection and initiation of wound care procedures. The acronym TIME has been used over the last decade or so to facilitate an organized approach to wound bed preparation and has been summarized nicely by Leaper et al 42. This acronym refers to Tissue assessment and management, Infection/Inflammation management, Moisture imbalance management, and Edge of wound observation and management. The TIME principles are an integral, although incomplete, part of this discussion and incorporate the basic tenets of wound care that are critical for managing chronic wounds.
TIME principles of wound bed preparation 42:
- Tissue: assessment and debridement of nonviable or foreign material (including host necrotic tissue, adherent dressing material, multiple organism-related biofilm, or slough, exudate, and debris) on the surface of the wound.
- Infection/inflammation: assessment of the etiology of each wound, need for topical antiseptic and/or systemic antibiotic use to control infection, and management of inappropriate inflammation unrelated to infection.
- Moisture imbalance: assessment of the etiology and management of wound exudate.
- Edge of wound: assessment of nonadvancing or undermined wound edges (and state of the surrounding skin).
Debridement has long been recognized as a critical component for wound care and has been shown by several investigators to expedite healing 43. Sharp debridement removes nonviable tissue and slough along with bacterial biofilms that prolong the inflammatory response in the chronic wound 38. In effect, thorough debridement converts the chronic wound from one that is excessively inflamed, as previously described, to more of an acute profile that can jump-start the wound toward a healing trajectory 41. While sharp debridement is considered to be the most efficient way to debride a wound (with scalpel, curette, tissue nippers, etc.), hydrosurgical or ultrasonic debridement can also be used in this regard 41. The term ulcerectomy has been used to denote complete excision of ulcers down to healthy bleeding tissue, resulting in expedited healing of diabetic foot ulcers 44. Maintenance debridement with enzymes (collagenase) is frequently used between clinic visits to gently remove slough or to enzymatically debride thick crusts (especially in neuroischemic wounds) 45. Biodebridement with maggots has been used for many years in patients not suitable for surgical debridement and has shown some promise in removing slough and necrotic tissue while promoting granulation tissue development 46. Simple hydrogels or hydrocolloid dressings can provide for slow autolytic debridement of slough and dried crusts, especially in ischemic patients or those who cannot undergo surgical debridement. Regardless of the method used, effective debridement of chronic wounds is accepted as an essential component of care throughout the wound healing continuum 47. Nonetheless, healing can be delayed if debridement is performed too frequently and/or extensively. Development of diagnostic tools, including biomarker analysis and noninvasive imaging, is necessary to better distinguish viable from nonviable tissues in the wound and guide debridement practices 48.
The importance of offloading the chronic wound cannot be overemphasized 49. In fact, when this component of wound care is neglected, the chances of a successful outcome are extremely low. When one recognizes that most wounds, especially diabetic foot ulcer s, have excessive pressure as their proximate cause, it is quite understandable that the high pressures must be ameliorated before healing can take place. For nonplantar wounds caused by tight shoes, it is imperative that the source of offending pressures be eliminated. A number of studies and reviews have confirmed the essential role of offloading in this regard 50. For diabetic foot ulcer s, the total contact cast has long been considered as the gold standard for offloading by virtue of its pressure redistribution properties as well as irremovability. Numerous additional offloading modalities have been reported for diabetic foot ulcer s, including braces, removable cast walkers, irremovable cast walkers (often referred to as instant total contact casts), half shoes, modified surgical shoes, foot casts, and various felt or foam dressings 51. While each device has its own advantages for any given patient, almost any offloading modality is superior to no offloading for the management of diabetic foot ulcers.
Along the same lines, compression therapy for chronic venous leg ulcers is equally important. Since venous hypertension is at the source of these lesions, hydrostatic pressure into the skin and subcutaneous tissues underlying venous ulcers must be mitigated by external compression 36. Different modalities, such as the classic Unna’s boot, three- or four-layer compression bandages, and short stretch compression bandages, have long been used in this setting 52. For ulcerated patients with significant venous insufficiency and associated chronic lymphedema, intermittent pneumatic compression pump therapy can also be recommended. While not supplanting the need for directly applied compression wraps, pump therapy will augment their effect and assist in maintaining long-term control of peripheral edema and lymphedema 53.
Surgical offloading is a term that (as its name implies) refers to the surgical management of foot deformities causing high pressures resulting in chronic ulcerations 54. While not generally considered a primary treatment for most neuropathic diabetic foot ulcers, those that prove to be recalcitrant to standard wound bed preparation and effective offloading should be considered for surgical internal decompression. Many such procedures have been reported as effective over the last several decades 55. Metatarsal head osteotomies or resections, sesamoidectomies, hammertoe repair, bunionectomies, first metatarsal phalangeal joint arthroplasties, plantar exostectomies, arthrodeses, and partial calcanectomies have all been described for the management of noninfected as well as infected chronic foot ulcers. Soft tissue tendon balancing procedures, such as tenotomies, tendon transfers, and lengthening procedures, including tendo-Achilles lengthening and gastrocnemius-soleus recession, can be done as isolated procedures or in concert with osseous procedures to reduce deformities and high forefoot plantar pressures 56. Several authors have proposed a validated classification scheme for such operations based on the presence or absence of wounds as well as their acuity 57. Elective procedures are done in the absence of neuropathy, while Prophylactic operations are performed in neuropathic individuals to prevent initial ulceration over deformities or to prevent their recurrence. Curative procedures are undertaken to internally decompress chronic wounds, reduce high plantar pressures, or to remove foci of bone infection/osteomyelitis to engender final healing. Emergent operations, often including amputations, must be performed to control acute infection as limb or life-threatening measures. As has been validated, this classification trends toward an increasing need for hospitalization and frequency of amputation in the progression from elective through emergent procedures 57. Residual postoperative wounds in these patients must be treated with the same tenets of wound care as discussed above until final healing occurs 58.
Topical wound therapies and dressings
While there are a myriad of topical therapies/antimicrobials and dressings available to the clinician, very few have prospective data to support their effectiveness in promoting wound repair. Nonetheless, many therapies can indeed be useful, despite reliance on anecdotal experience. Hence, clinicians tend to use what they are accustomed to or what seems to be effective based on personal experience. Topically applied agents for wounds run the gamut from sterile saline or hydrogel to povidone–iodine solutions, cadexomer iodine, hypochlorous acid, honey, and collagenase. One topical antimicrobial agent, superoxidized solution, has recently been formally studied for efficacy in healing diabetic foot ulcers and was found to be effective in this regard 59. Similarly, the inexhaustible availability of dressings can make selection of the appropriate wound covering somewhat daunting. While standard cotton gauze dressings have long been considered standard of care (even in clinical trials), many other primary and secondary dressings are commercially available. Highly absorbent and moisture-retaining foam dressings, acrylics, alginates, hydrofibers, hydrocolloids, honey alginates, oxidized regenerated cellulose, micronized collagen, and many others can be considered as circumstances warrant. Many of the aforementioned products also are available with silver for control of bacterial overburden. While the use of silver-containing products is widespread, misuse of this antibacterial element for prolonged periods is also common. While its primary use is for the reduction of bacterial colonization, there are little data to support its efficacy as a wound healing agent 60. It cannot be emphasized enough that standard dressings and topical therapies never supplant the need for debridement, effective offloading, and appropriate management of infection and ischemia.
Advanced therapies
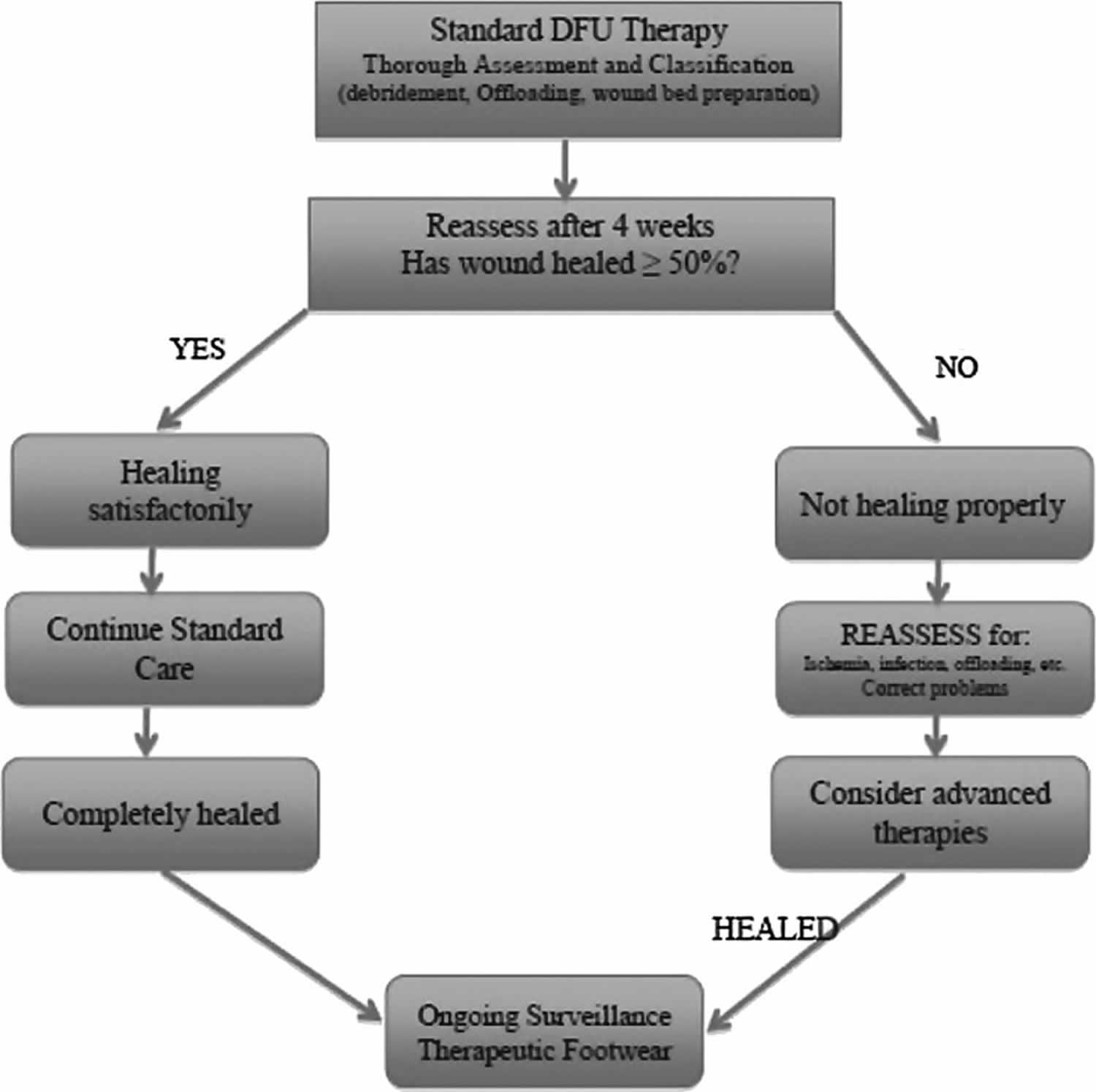
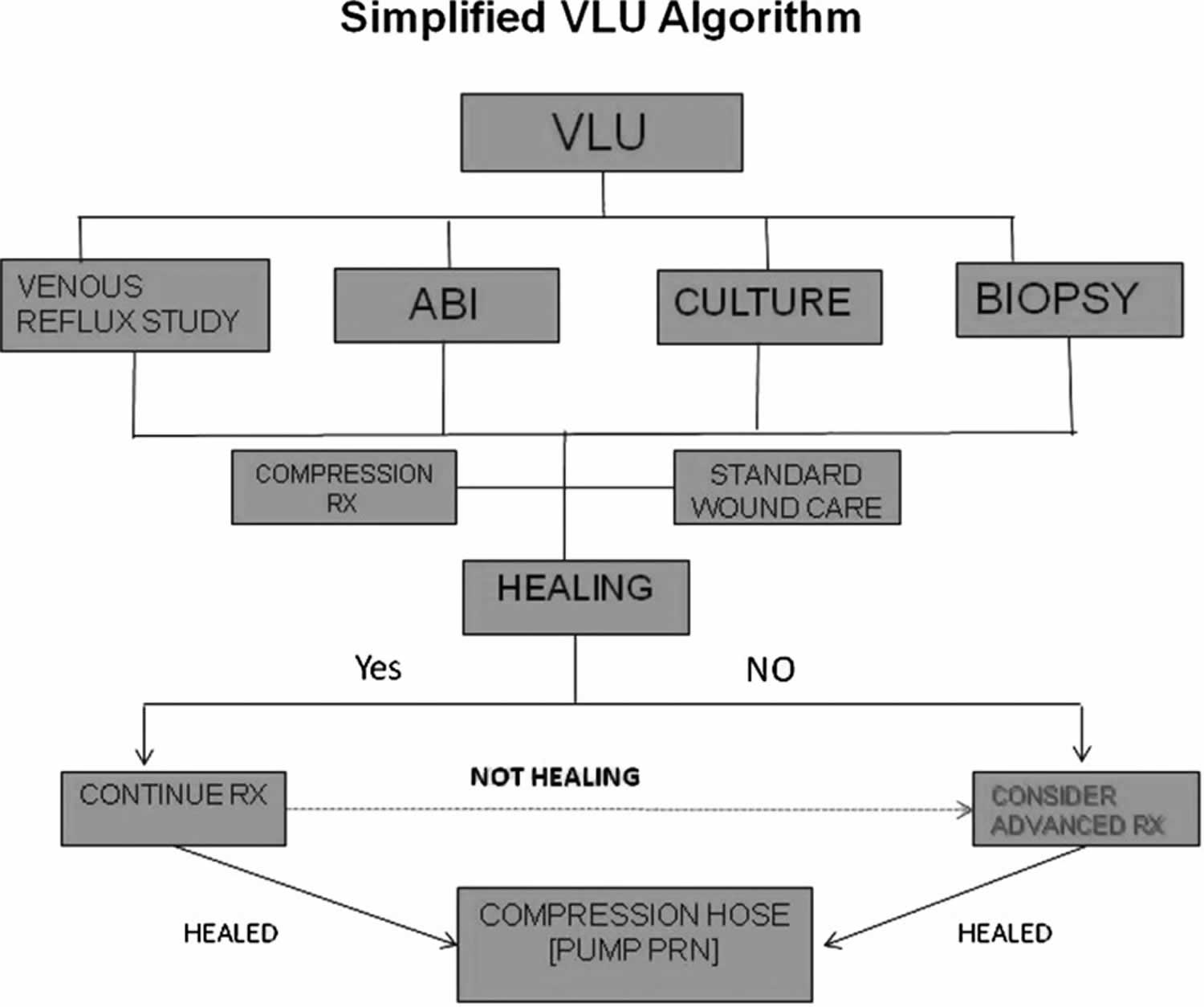
All currently published guidelines and consensus reviews on the management of chronic diabetic foot ulcers, venous leg ulcers, and pressure ulcers support the belief that all such wounds should initially be treated with standard wound care principles as have been discussed. In most cases, these basic tenets of wound care should be carried out before consideration of the use of more advanced therapies. Most wound care protocols now advocate the use of such standard measures for an initial period of 4 weeks, after which an assessment of wound area reduction should be made. While the 1999 American Diabetes Association publication was one of the first consensus documents on diabetic foot ulcer assessment and treatment, it only mentioned that wounds failing to heal by 4 weeks were associated with worse outcomes, including amputation 61. In 2003, however, Sheehan et al. published the often quoted article supporting the ability of the 4-week healing rate to predict complete healing by 12 weeks 62. Using data from another large, multicenter randomized controlled trial, it was determined that the midpoint (median) percent wound area reduction from baseline at 4 weeks between those that healed and those that remained unhealed at 12 weeks was 53% 63. Those who exceeded this midpoint healed in 58% of cases by 12 weeks. In contrast, those that did not achieve 53% wound area reduction by 4 weeks only healed in 9% of cases. Even more striking, the mean 4-week percent change in ulcer area was 82% in healers versus 25% in the nonhealing group regardless of the treatment arm. Subsequently, the 4-week 50% wound area reduction has been widely adopted and confirmed as a robust indicator for predicting healing at 3 months 64. Consistent with this premise, wounds failing to achieve a 50% area reduction at this time point need to be reassessed and subsequently considered for advanced therapies in the absence of underlying disease or nonadherence to prescribed basic treatment 31. Figure 2 illustrates a fairly common algorithm for diabetic foot ulcer treatment incorporating these principles. Figure 3 similarly illustrates an algorithm for venous leg ulcer treatment.
Figure 2. Algorithm for diabetic foot ulcer treatment
Footnote: Simplified algorithm for diabetic foot ulcer (DFU) treatment.
[Source 2 ]Figure 3. Algorithm for venous leg ulcer treatment
Footnote: Simplified algorithm for venous leg ulcer (VLU) treatment. ABI = ankle–brachial indices.
[Source 2 ]Once it is determined that the patient might benefit from an advanced therapeutic agent, there are a number of options currently available 65. While there have been several key randomized controlled trials published on advanced wound care agents for chronic wounds, most such products do not have the benefit of high-level evidence or even nonrandomized prospective studies to attest to their efficacy. Advanced wound therapies can best be discussed by broadly categorizing them according to their specific technologies or engineering, tissue types, cell types, or protein content (i.e., growth factors). Even this scheme will not capture the myriad products, proteins, and molecules that have undergone trials and failed to demonstrate superiority over standard of care treatment. While not exhaustive, the list below provides a listing of the more common wound care technologies currently in use in the United States and abroad.
Wound care technologies 2:
Negative pressure wound therapy
- Standard electrically powered—VAC®
- Mechanically powered—SNaP®
Hyperbaric oxygen therapy (HBOT)
- Topical oxygen therapy
Biophysical
- Electrical stimulation, diathermy, pulsed electromagnetic fields
- Pulsed radiofrequency energy
- Low-frequency noncontact ultrasound—MIST®
- Extracorporeal shock wave therapy–DermaPACE®
Growth factors
- Becaplermin—platelet-derived growth factor—Regranex®
- Fibroblast growth factor (Japan)
- Epidermal growth factor (Cuba)
- Platelet-rich plasma
Acellular matrix tissues
- Xenograft dermis
- Primatrix®—bovine neonatal dermis
- Integra®—bovine collagen
- Matriderm®—bovine dermis
- Xenograft acellular matrices
- Oasis®—small intestine submucosa
- Matristem®— porcine urinary bladder matrix
- Ovine forestomach—Endoform®
- Equine pericardium
- Human dermis
- Graftjacket®
- D-cell®
- DermACELL®
- Theraskin®
- Human pericardium
- Placental tissues
- Amniotic tissues/amniotic fluid
- Umbilical cord
- Dehydrated human amnion/chorion membrane (dHACM)—Epifix®
Bioengineered allogeneic cellular therapies
- Bilayered skin equivalent—Apligraf®
- Dermal replacement therapy—Dermagraft®
Stem cell therapies
- Autogenous—bone marrow-derived stem cells
- Allogeneic—amniotic matrix with mesenchymal stem cells—Grafix®
Miscellaneous
- Hyalomatrix® (Hyaluronan)
Negative pressure wound therapy
Since its introduction in the mid 1990s, negative pressure therapy has assumed a major role in the management of traumatic, acute, and chronic wounds, as well as for stabilizing skin grafts, flaps, and surgical incisions 66. Since the early studies of Morykwas et al. 67 and Argenta and Morykwas 68 that demonstrated the numerous attributes of negative pressure wound therapy, a very large body of evidence has been published supporting the clinical efficacy of this very important biophysical modality. Clinical trials have been conducted in the ensuing years that have proven the superiority of negative pressure wound therapy over standard therapy for managing open amputation wounds, diabetic foot ulcers, venous leg ulcers, and other wounds 69. As an adjunct to standard chronic wound care, negative pressure wound therapy very efficiently manages wound drainage and can provide expedited granulation tissue development, wound area contraction/reduction, preparation for delayed closure or grafting, or primary healing 70. Negative pressure wound therapy is also quite useful as a bolster to enhance the incorporation of skin grafts onto recipient wound beds 71. Application techniques are important, however, since inappropriate placement of tubing can potentially cause skin pressure lesions. Therefore, a bridging method that provides for suction tubing placement away from plantar surfaces has become an important application technique. More recently, these electrically powered devices have added the ability to instill saline or other antimicrobial agents to assist in the cleansing of the wounds concurrent with providing the aforementioned benefits of negative pressure wound therapy 72. A mechanically powered, ultralight, and portable negative pressure wound therapy device has also been introduced in recent years 71. Two articles comparing this device with the traditional electrically powered device in a prospective randomized controlled trial have shown equivalent diabetic foot ulcer healing outcomes with faster application times and a high degree of patient satisfaction 73. Clinicians have also combined negative pressure wound therapy with other advanced therapies such as acellular and cellular matrices 74. Such multimodal therapies can be administered concurrently or sequentially as wound characteristics change to provide the optimum therapy for any given wound.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) has been advocated as being beneficial for a wide variety of chronic wounds for over two decades 75. While there are numerous retrospective and prospective studies, case series, cohort studies, and several trials indicating the efficacy of HBOT for the treatment of diabetic foot ulcers and venous leg ulcers, the general quality of these studies is not robust since inclusion criteria and outcomes are highly variable 75. While the recent Cochrane review in 2012 indicated significant short-term improvement for healing diabetic foot ulcers over controls at 6 weeks (relative risk [RR] 5.20), this benefit was not evident at 1 year or longer 76. While there was a suggestion that hyperbaric oxygen therapy (HBOT) may decrease the major amputation rate in diabetic foot ulcer patients, pooled analysis of the data did not yield a significant estimate for this association (RR 0.36). The 2010 randomized controlled trial by Londahl et al. reported favorable outcomes at 1 year for hyperbaric oxygen therapy in those patients with Wagner grade 2, 3, or 4 diabetic foot ulcers failing prior standard interventions for >3 months (52% vs. 29%) 77. Unfortunately, the heterogeneity of the patients and time to reported outcomes (9 months or 1 year) make comparisons with other diabetic foot ulcer trials difficult. A more recent review of hyperbaric oxygen therapy in a large observational cohort study by Margolis 78 found that the use of hyperbaric oxygen therapy neither improved the likelihood that a wound would heal nor prevented amputation in patients with adequate lower limb arterial perfusion. This review and the aforementioned Cochrane review both called for a reevaluation of hyperbaric oxygen therapy (HBOT) with rigorously designed adequately powered trials to assess efficacy in healing chronic wounds.
Since the very poorly conducted 14-day study by Leslie et al. 79 in 1988, the use of topical wound oxygen for diabetic foot ulcers and venous leg ulcers has been considered as highly controversial, especially by advocates of hyperbaric oxygen therapy. The lack of a formal study in this regard has been offset by several reviews, case series, and experimental studies on the effect of topically administered oxygen at the wound surface that seem to indicate a benefit in promoting wound repair 80. While several randomized controlled trials with different devices have been ongoing, there have been no publications to date comparing 12-week healing rates with those of standard care.
Biophysical modalities
Electrical stimulation has been the most studied biophysical device for healing chronic wounds to date, primarily utilized by physical therapists and physiatrists 81. An abundance of studies advocates the beneficial healing effect of electrical stimulation at various modes and frequencies for a variety of chronic wounds 82. Other nonthermal forms of electromagnetic energy have also been used for wound healing, including pulsed radiofrequency energy (PRFE), pulsed shortwave diathermy, and pulsed electromagnetic fields 83. One in vitro study 84 of pulsed radiofrequency energy effects on cultured dermal fibroblasts and keratinocytes found this electromagnetic field to upregulate expression of a variety of genes involved in modulating the inflammatory stage of wound healing. In general, by interacting with endogenous bioelectric currents, electromagnetic fields indirectly upregulate the production of nitric oxide and multiple growth factors, resulting in cellular mobilization, angiogenesis, and expedited wound repair 83.
Ultrasound, most frequently used for diagnostic and musculoskeletal therapy purposes, has also assumed a role in wound management. Several lower frequency devices are currently available for debridement that use the delivery of sound waves to generate cavitation at the wound bed 83. Wounds with thick fibrinous slough and necrosis can thereby be very aggressively debrided with low-frequency ultrasound (LFU) devices, although trials to show improved healing rates have not been conclusive 85. Noncontact low-frequency ultrasound using a saline droplet carrier to the wound surface has been proposed as another low-frequency ultrasound device that can improve healing rates of chronic ulcers, including those complicated by ischemia 86. While this noncontact low-frequency ultrasound modality can assist with wound bed preparation, it cannot aggressively debride wounds as well as the aforementioned units. Several retrospective observational studies and one low quality randomized controlled trial suggested improvements in healing rates over standard of care comparators, but confirmatory prospective studies have not been forthcoming 87.
Extracorporeal shock wave therapy (ESWT) has been used for a number of years for a variety of musculoskeletal conditions and has recently been adapted for the treatment of cutaneous wounds. Extracorporeal shock wave therapy is defined as a series of high-energy acoustic pulses delivered to tissues by electrohydraulic, electromagnetic, and piezoelectric sources 88. The pressure pulses generated promote a cascade of cytokine and growth factor upregulation leading to enhanced neovascularization, anti-inflammatory response, and tissue regeneration 89. Several recent reviews and one study comparing extracorporeal shock wave therapy with hyperbaric oxygen therapy (HBOT) on diabetic foot ulcer healing support its potential role in expediting wound repair 88. Nonetheless, randomized controlled trials are still required to evaluate the clinical efficacy of this modality in healing chronic wounds of different etiologies.
Biological and bioengineered therapies
Concurrent with the explosive growth of negative pressure therapies and hyperbaric oxygen therapy for the management of chronic wounds in the last two decades, there has been an enormous amount of research and interest in advanced biological therapies. In this regard, biological therapies refer to tissue-based treatments (acellular and cellular), autologous platelet-rich plasma (PRP), as well as recombinant human growth factor therapies. While the latter can be considered as a category unto themselves due to the enormous amount of preclinical research on cytokine and growth factor-mediated wound repair, there are currently only several such therapies commercially available to clinicians—recombinant human platelet-derived growth factor (rhPDGF), recombinant human fibroblast growth factor (rhFGF), and recombinant human epidermal growth factor (rhEGF). Numerous other growth factors have been isolated and investigated, such as vascular endothelial growth factor (VEGF), keratinocyte growth factor-2, TGF-β, and granulocyte-macrophage colony-stimulating factor, but these currently are not approved for use in wound care 90.
Platelet-rich plasma and growth factors
The clinical interest in growth factor therapies over the last 25 years actually stemmed from the early work of Knighton et al. 91 on autologous platelet-rich plasma and the cascade of growth factors released from activated platelets during the centrifugation of whole blood. Several small trials and retrospective reviews have affirmed the potential efficacy of topically applied, activated, autologous platelet supernatants for expedited healing of chronic lower extremity wounds 92. After recognizing the potential benefit of topically applied platelet-derived growth factor to chronic wounds, becaplermin gel (rhPDGF) was studied in the setting of chronic neuropathic diabetic foot ulcers and became the first commercially available advanced therapy for the management of these difficult wounds (and the only growth factor approved for use in the United States) 93. In the 20-week phase III clinical trial by Wieman leading to its approval, topically applied recombinant human platelet-derived growth factor (rhPDGF) gel was found to significantly increase the incidence of complete wound closure by 43% and decrease the time to healing by 32% over placebo-controlled standard wound care 94. The efficacy of the single topically applied growth factor was corroborated as being beneficial to the healing of chronic nonischemic foot ulcers when combined with good standard wound care in several other studies pooling data from multiple sources 95.
Recombinant humanrh epidermal growth factor (rhEGF) is perhaps the best studied growth factor for cutaneous ulcers and wounds, but is not available in the United States 96. Applied as a topical cream, or more commonly, by intralesional injections, epidermal growth factor has been found also to expedite the healing of a variety of types of cutaneous wounds. Several small trials have been published from Asia and Cuba attesting to the healing benefits of epidermal growth factor compared with controls 97. However, the heterogeneity of wounds, applications, patient management protocols, and outcomes has not lead to clinical trials in the United States nor to widespread global adoption of this therapy.
Fibroblast growth factor (FGF) has been studied primarily in Asia for a variety of chronic wounds, including diabetic foot ulcers, venous leg ulcers, and pressure ulcers, and is approved in Japan for this use 98. Fibroblast growth factor, considered a potent angiogenic growth factor, also has an isoform (FGF-10) commonly known as keratinocyte growth factor-2 or repifermin 99. This agent has been studied in the United States for chronic venous leg ulcers as a topical spray. Repifermin was shown to accelerate wound healing with significantly more patients achieving 75% wound closure with repifermin than with placebo after 12 weeks. The treatment effect appeared more marked for a subgroup of patients with initial wound areas ≤15 cm2 and wound ages of ≤18 months 99. Unfortunately, there were no significant differences between the study and control groups for the primary outcome (healing at 12 weeks), likely due to methodological issues in the study protocol. Neither repifermin nor fibroblast growth factor (FGF) has been approved in the United States for wound management.
Acellular therapies
The most common types of advanced biological therapies for chronic wounds can generally be classified as acellular therapies—those dermal, amniotic, or collagen-based tissues (human or animal) that have been decellularized during their processing. Often referred to as acellular and/or extracellular matrixs, these biological products serve as substrates into which cells can migrate and initiate angiogenesis, thereby promoting granulation tissue development and tissue regeneration 100. Once considered only as inert structural collagen tissue providing a scaffold for cellular ingrowth, extracellular matrixs are now known to play an active part in tissue regeneration through a dynamic interaction with growth factors and host cells 101. Extracellular matrices contain not only structural collagen but also glycosaminoglycans (including hyaluronan), proteoglycans, and glycoproteins—all essential components to replace the defective extracellular matrix of injured tissues 102. Currently available nonhuman extracellular matrix products with clinical data to support their efficacy include porcine-derived small intestinal submucosa, porcine urinary bladder matrix, bovine dermis, equine pericardium, and sheep (ovine) bladder 103. Another matrix dressing (Hyalomatrix®; Anika Therapeutics, Inc., Bedford, MA) comprising primarily hyaluronan, one of the main constituents of extracellular matrix, has also been studied in Europe as a dermal substitute for use in burns, venous leg ulcers, and diabetic foot ulcers 104. Integra® bilayer wound matrix (Integra LifeSciences, Plainsboro, NJ) is a dermal regeneration template in this category (bovine collagen, glycosaminoglycans, and silicone layer) that has primarily been used in burns for a number of years, but is being more frequently used in lower extremity chronic wounds and foot ulcers 105. Although there are few published prospective studies to support their efficacy, most of these products are commercially available in the United States at relatively low cost and are approved for use in multiple chronic wound types.
Human dermal allografts have become increasingly popular in recent years for augmenting tissue regeneration in chronic lower extremity wounds and have been formally studied in diabetic foot ulcers and venous leg ulcers. These are defined by the Food and Drug Administration as human cellular and tissue-based products. The allografts are harvested from screened donors, and each is prepared with proprietary processes to decellularize and cryopreserve the dermis while maintaining the natural structure of the collagen and extracellular matrix 106. As with the aforementioned extracellular matrix products, the dermal matrices serve as scaffolds for cellular repopulation and angiogenesis, with varying degrees of incorporation into the recipient wound bed 107. One of these cryopreserved dermal allografts, referred to as a human skin allograft, claims that both the extracellular and cellular components are preserved during the minimal processing 106. Two of the available dermal regenerative matrices have successfully undergone small randomized controlled trials (Graftjacket®; KCI, San Antonio, TX and TheraSkin®; Soluble Systems, Newport News, VA), while a third is currently undergoing a clinical trial for diabetic foot ulcers (dCell®; Tissue Regenix, San Antonio, TX) 106. Available data suggest that acellular dermal matrices, in addition to basic wound care principles, may provide an effective technique for tissue regeneration in deep and cutaneous extremity wounds 107.
Amniotic membranes and umbilical cord tissues have been used for many years for corneal ulcers and were actually the earliest reported biomaterials used for wound repair. In recent years, there has been a greatly expanded interest in these tissues for chronic wounds, likely due to the wide availability of placental tissues after cesarean deliveries. Due to their rich cellular content in the native state, amniotic membranes contain a number of cytokines and growth factors bound to the extracellular matrix after decellularization and preparation that remain available to augment angiogenesis and tissue repair when implanted into chronic wounds 108. amniotic membranes are available in the cryopreserved state or as dehydrated products for direct implantation 109. Several products are also available in a micronized formulation that can be applied topically or hydrated for injection into wounds or other inflamed tissues (tendonitis, plantar fasciitis, etc.) to augment healing 110. Amniotic fluid with granulized amniotic matrix is also available for the management of chronic wounds 111. While several retrospective and prospective studies have supported the benefit of amniotic matrix products for chronic wound repair, the only published randomized and comparative studies to date have utilized the dehydrated human amnion/chorion membrane (dHACM) 112. In the initial small randomized controlled trial of just 25 diabetic foot ulcer patients in a single center, Zelen et al. 112 reported a 92% healing rate after 6 weeks in the dehydrated human amnion/chorion membrane (Epifix®; Mimedix Group, Marietta, GA) group compared with 8% in the standard of care group. In a crossover study of unhealed patients in the control arm of the randomized controlled trial, 91% healed by 12 weeks with biweekly dehydrated human amniotic membrane application 113. Subsequent studies ascertained that weekly applications of this allograft provided more rapid healing of diabetic foot ulcers than biweekly application and that healing frequency with the amnion/chorion product was significantly higher than patients assigned to either a bilayered skin substitute or to standard of care treatment in another comparative trial 114. Many of the aforementioned studies, however, were sponsored by a single company with a single product and the same investigative group. Since a number of other amniotic membrane products are now commercially available, further studies should be forthcoming to determine if there is a distinction between different amniotic matrices pertaining to efficacy in wound repair.
Bioengineered cellular therapies
For more than a decade, two allogeneic bioengineered skin replacement therapies utilizing neonatal expanded cells have been available in the United States market to address chronic wounds. Both products underwent formal, controlled clinical trials before their approval for use. The first cellular product (Apligraf®; Organogenesis, Canton, MA) is a bilayered construct consisting of a bovine collagen matrix seeded with living human neonatal fibroblasts and a neonatal keratinocyte neoepidermis. It is approved for both chronic venous leg ulcers as well as diabetic foot ulcers. This living skin equivalent not only addresses the deficient extracellular matrix of chronic wounds by adding a collagen matrix but also introduces immune-privileged living cells that proliferate and actively synthesize growth factors, cytokines, and extracellular matrix products 115. In the pivotal, multicenter, 12-week clinical trial, patients randomized to the Apligraf group achieved complete healing in 56% of cases compared with 38% in the control group 116. With an average of four applications, the bilayered skin equivalent also healed the chronic diabetic foot ulcers significantly faster than those patients treated with standard care (65 vs. 90 days). Other subsequent investigations found similar efficacy of this bioengineered product for healing chronic diabetic foot wounds as well as venous leg ulcers 117.
Dermagraft® (Organogenesis, Canton, MA) is a human fibroblast-derived dermal substitute comprising a cryopreserved, absorbable, three-dimensional polyglactin mesh substrate seeded with living neonatal dermal fibroblasts 118. Similar to the bilayered skin replacement, these cells secrete a host of growth factors, cytokines, matrix proteins, and glycosaminoglycans that induce tissue regeneration through the development of granulation tissue and ingrowth of host fibroblasts and keratinocytes 119. This dermal substitute was proven effective in healing chronic diabetic foot ulcers in the pivotal trial by Marston et al. in 2003 120. In this multicenter clinical trial, those patients assigned to the human fibroblast-derived dermal substitute group healed significantly faster after 12 weeks compared with the standard wound care group (30% vs. 18.3%). Despite the ostensibly low overall healing rate, the study group achieved a 64% increased healing compared with the standard care group. This was a greater margin of effect than reported in any previous trial. Despite the failure to achieve superiority in its primary outcome of complete healing in a large venous leg ulcer trial, significant differences in complete healing were achieved for a subgroup of ulcers ≤12 months in duration 121. Other authors have reported success with the dermal substitute in a variety of lower extremity wounds, often used in concert with other wound healing modalities 122. A recent study investigated the incidence of amputations and bone resections within the two arms of the diabetic foot ulcer pivotal trial and found a decreased incidence of these complications in the human fibroblast-derived dermal substitute group, likely related to a lower incidence of infection and faster healing in the investigational treatment group 123.
Stem cell therapies
Perhaps the most recent advancements for wound care therapies are that of stem cell therapies, primarily bone marrow derived, and most recently, placental-derived stem cells. Both sources are considered as adult stem cells, and the cell lineage of interest are the mesenchymal stem cells. Briefly, mesenchymal stem cells are multipotent progenitor cells that can directly differentiate into mesenchymal tissues, such as bone, tendon, and cartilage. Their ability to affect cutaneous repair, however, is through an indirect paracrine function (trophic activity), whereby they synthesize essential growth factors and cytokines that affect cell migration, proliferation, and metabolic activity of host cells and tissues 124. In this manner, mesenchymal stem cells play an active role in the inflammatory, proliferative, and remodeling phases of wound repair.281 Interestingly, mesenchymal stem cells can respond to the host environment by upregulating anti-inflammatory cytokines in the presence of inflammation and respond to hypoxic environments by upregulating the release of VEGF to induce angiogenesis. During the remodeling phase, mesenchymal stem cells produce growth factors, such as TGF-β3, to limit excessive scarring as well as modulate the balance between matrix metalloproteinases and tissue inhibitors of metalloproteinases while regulating collagen deposition. Important for allogeneic implantation, mesenchymal stem cells are characterized as being immune privileged since they lack cell surface antigens that would typically engender a foreign body reaction 125.
Bone marrow-derived stem cells have been of interest for some time now and studies have indicated their ability to augment repair or regeneration of numerous tissues, including cardiac, bone, cartilage, blood vessels, and skin 126. Although much interest has focused on orthopedic and critical limb ischemia applications, Bmesenchymal stem cells are increasingly being studied for use in enhancing chronic wound and cutaneous repair 127. Several articles and clinical studies have specifically focused on diabetic foot ulcer management 128. One of the earlier studies by Yamaguchi et al. 129 took a novel approach by decorticating exposed bone at the base of the ulcer to locally release bone marrow cells directly into the wound. This was followed several weeks later by application of autologous epidermal grafts, resulting in significantly improved healing compared with standard wound care. In another small case series by Rogers et al. 130, bone mesenchymal stem cells were harvested from the ipsilateral distal tibial metaphysis, and the aspirate was applied topically or by peripheral injection under the debrided wounds with good results. Large randomized studies have not yet been published comparing wounds treated with bone mesenchymal stem cells with standard care. Another issue for consideration in such trials will be standardizing methods for obtaining and processing the marrow aspirates, as well as potential complications associated with this surgical procedure.
Placental tissues—including the umbilical cord, the amnion, and the chorion—are a rich source of mesenchymal stem cells and are readily available without the ethical concerns of embryonic stem cells 128. Furthermore, the mesenchymal stem cells in these tissues do not suffer from the age-related effects nor decreased cell counts as found in mesenchymal stem cells harvested from adult patients with comorbid diseases 131. Placental-derived mesenchymal stem cells show minimal differences from those obtained from different sites, and yet retain all of the metabolic, paracrine, and immunomodulative properties previously described while maintaining their immune-privileged status 125. Traditionally, placental or amniotic tissues used for wound repair were prepared from fresh cesarean section donors. One small modern study investigated the efficacy of fresh amniotic membrane on the healing of chronic venous leg ulcers and found a positive effect on pain reduction and a significant clinical response (20% completely healed) within the 3-month follow-up period 132. Overcoming the difficulties of fresh transplantation, a new cryopreserved amniotic membrane product (Grafix®; Osiris Therapeutics, Inc., Columbia, MD) has become commercially available in recent years. This human MSC-rich wound matrix contains viable cells, including fibroblasts and epithelial cells, in addition to mesenchymal stem cells and a natural extracellular matrix 125. In a single-center retrospective study of 67 chronic lower extremity wounds, including venous leg ulcers and diabetic foot ulcers, this viable wound matrix in association with good standard wound care was found to close 76.1% of wounds at 12 weeks with a mean time to healing of 5.8 weeks 133. A subsequent 12-week randomized controlled trial comparing the efficacy of Grafix to standard of care for the healing of diabetic foot ulcers was published in 2014.297 In this pivotal trial where the primary outcome was complete wound healing at 12 weeks, those patients assigned to weekly applications of the viable human matrix healed significantly more diabetic foot ulcers than those in the control group (62% vs. 21%). The median time to healing was also significantly faster in the study group (42 vs. 69.5 days). Of particular interest, those unhealed patients in the control group were allowed to crossover to receive up to 12 weeks of viable stem cell matrix therapy. The probability for closure in these patients was 67.8% with a mean time to closure of 42 days 134 for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 2014;11:554–560)). The results of this study represent a margin of effect between active and control groups of 191%, higher than any other published study to date.
References- Chronic Wounds. https://www.woundsource.com/patientcondition/chronic-wounds
- Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4(9):560–582. doi:10.1089/wound.2015.0635 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4528992
- Psychosomatic Aspects of Chronic Wounds. Dermatology and Psychosomatics. 4: 5–13. doi:10.1159/000070529 https://doi.org/10.1159/000070529
- Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 2014;7:1205–1213
- Woo K, Ayello EA, Sibbald RG. The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care 2007;20:99–117; quiz 118–119
- Brownrigg JR, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg 2013;45:673–681
- Richmond NA, Maderal AD, Vivas AC. Evidence-based management of common chronic lower extremity ulcers. Dermatol Ther 2013;26:187–196
- Canadian Agency for Drugs and Technologies in Health. Optimal Care of Chronic, Non-Healing, Lower Extremity Wounds: A Review of Clinical Evidence and Guidelines. Ottawa, ON, Canada, 2013
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2014;37:651–658
- Shankaran V, Brooks M, Mostow E. Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol Ther 2013;26:215–221
- Heng MC. Wound healing in adult skin: aiming for perfect regeneration. Int. J. Dermatol. 2011 Sep;50(9):1058-66.
- Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35-43.
- Broughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast. Reconstr. Surg. 2006 Jun;117(7 Suppl):12S-34S.
- Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002 Jan-Feb;10(1):16-25.
- Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011 Aug;19(3):441-53.
- McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care 2013;2:438–447
- Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol 2010;163:257–268
- Dhall S, Do DC, Garcia M, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014;2014:562625
- Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11 Suppl 1:S1–S28
- Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int 2013;2013:385641
- Bourguignon LY. Matrix hyaluronan-activated CD44 signaling promotes keratinocyte activities and improves abnormal epidermal functions. Am J Pathol 2014;184:1912–1919
- Bitar MS. The GSK-3beta/Fyn/Nrf2 pathway in fibroblasts and wounds of type 2 diabetes: on the road to an evidence-based therapy of non-healing wounds. Adipocyte 2012;1:161–163
- Ennis WJ, Sui A, Bartholomew A. Stem cells and healing: impact on inflammation. Adv Wound Care 2013;2:369–378
- Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen 2013;21:545–553
- Shin L, Peterson DA. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med 2013;2:33–42
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130:489–493
- Markova A, Mostow EN. US Skin Disease Assessment: Ulcer and Wound Care. Dermatol Clin. 2012 Jan. 30(1):107-11.
- Krasner, D (1998). “Painful venous ulcers: Themes and stories about living with the pain and suffering”. Journal of Wound, Ostomy, and Continence Nursing. 25 (3): 158–68. doi:10.1097/00152192-199805000-00008
- Hofman, D; Ryan, TJ; Arnold, F; Cherry, GW; Lindholm, C; Bjellerup, M; Glynn, C (1997). “Pain in venous leg ulcers”. Journal of Wound Care. 6 (5): 222–4. doi:10.12968/jowc.1997.6.5.222
- Walshe, Catherine (2006). “Living with a venous leg ulcer: A descriptive study of patients’experiences”. Journal of Advanced Nursing. 22 (6): 1092–100. doi:10.1111/j.1365-2648.1995.tb03110.x
- Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45:S1–S66
- Molnar JA, Underdown MJ, Clark WA. Nutrition and chronic wounds. Adv Wound Care 2014;3:663–681
- Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31:1679–1685
- Mills JL, Sr., Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–234.e221–e222
- Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds 2009;8:105–111
- O’Donnell TF, Jr., Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery (R) and the American Venous Forum. J Vasc Surg 2014;60:3S–59S
- Faglia E, Clerici G, Caminiti M, Quarantiello A, Gino M, Morabito A. The role of early surgical debridement and revascularization in patients with diabetes and deep foot space abscess: retrospective review of 106 patients with diabetes. J Foot Ankle Surg 2006;45:220–226
- Panuncialman J, Falanga V. The science of wound bed preparation. Clin Plast Surg 2007;34:621–632
- Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–e173
- Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care 2014;37:789–795
- Granick M, Boykin J, Gamelli R, Schultz G, Tenenhaus M. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen 2006;14 Suppl 1:S1–S10
- Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years?. Int Wound J 2012;9 Suppl 2:1–19
- Cardinal M, Eisenbud DE, Armstrong DG, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen 2009;17:306–311
- Piaggesi A, Schipani E, Campi F, et al. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med 1998;15:412–417
- Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA. Maintenance debridement in the treatment of difficult-to-heal chronic wounds. Recommendations of an expert panel. Ostomy Wound Manage 2008;Suppl:2–13; quiz 14–15
- Tian X, Liang XM, Song GM, Zhao Y, Yang XL. Maggot debridement therapy for the treatment of diabetic foot ulcers: a meta-analysis. J Wound Care 2013;22:462–469
- Steed DL, Attinger C, Colaizzi T, et al. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 2006;14:680–692
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–1222
- Snyder RJ, Frykberg RG, Rogers LC, et al. The management of diabetic foot ulcers through optimal off-loading building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc 2014;104:555–567
- Lewis J, Lipp A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev 2013;1:CD002302
- Katz IA, Harlan A, Miranda-Palma B, et al. A randomized trial of two irremovable off-loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care 2005;28:555–559
- Dolibog P, Franek A, Taradaj J, et al. A randomized, controlled clinical pilot study comparing three types of compression therapy to treat venous leg ulcers in patients with superficial and/or segmental deep venous reflux. Ostomy Wound Manage 2013;59:22–30
- Kimmel H, Robin A. An evidence-based algorithm for treating venous leg ulcers utilizing the cochrane database of systematic reviews. Wounds 2013;25:242–250
- La Fontaine J, Lavery LA, Hunt NA, Murdoch DP. The role of surgical off-loading to prevent recurrent ulcerations. Int J Low Extrem Wounds 2014;13:320–334
- Blume PA, Donegan R, Schmidt BM. The role of plastic surgery for soft tissue coverage of the diabetic foot and ankle. Clin Podiatr Med Surg 2014;31:127–150
- Laborde JM. Treatment of diabetic foot ulcers with tendon lengthening. Am Fam Physician 2009;80:1351; author reply 1351
- Armstrong DG, Lavery LA, Frykberg RG, Wu SC, Boulton AJ. Validation of a diabetic foot surgery classification. Int Wound J 2006;3:240–246
- Frykberg RG, Bevilacqua NJ, Habershaw G. Surgical off-loading of the diabetic foot. J Vasc Surg 2010;52:44S–58S
- Piaggesi A, Goretti C, Mazzurco S, et al. A randomized controlled trial to examine the efficacy and safety of a new super-oxidized solution for the management of wide postsurgical lesions of the diabetic foot. Int J Low Extrem Wounds 2010;9:10–15
- Carter MJ, Tingley-Kelley K, Warriner RA., 3rd Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: a systematic review and meta-analysis. J Am Acad Dermatol 2010;63:668–679
- American Diabetes Association. Consensus development conference on diabetic foot wound care. Diabetes Care 1999;22:1354
- Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003;26:1879–1882
- Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–827
- Snyder RJ, Cardinal M, Dauphinee DM, Stavosky J. A post-hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44–50
- Snyder RJ, Kirsner RS, Warriner RA, 3rd, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010;56:S1–S24
- Smith APS, Whittington K, Frykberg RG, DeLeon J. Negative pressure wound therapy. In: Krasner DL, editor; , Rodeheaver GT, editor; , Sibbald RG, editor; , Woo KY, editor. , eds. Chronic Wound Care 5: A Clinical Source Book for Healthcare Professionals. Malvern, PA: HMP Communications, 2012:271–299
- Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117:121S–126S
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–576; discussion 577
- Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–636
- Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 2008;122:786–797
- Isaac AL, Rose J, Armstrong DG. Mechanically powered negative pressure wound therapy as a bolster for skin grafting. Plast Reconstr Surg Glob Open 2014;2:e103
- Bollero D, Degano K, Gangemi EN, Aloj D, Malvasio V, Stella M. Long-term follow-up of negative pressure wound therapy with instillation: a limb salvage procedure? Int Wound J 2014. [Epub ahead of print]; DOI: 10.1111/iwj.12373
- Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen 2012;20:332–341
- Neiderer K, Martin B, Hoffman S, Jolley D, Dancho J. A mechanically powered negative pressure device used in conjunction with a bioengineered cell-based product for the treatment of pyoderma gangrenosum: a case report. Ostomy Wound Manage 2012;58:44–48
- Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg 2014;47:647–655
- Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2012;4:CD004123
- Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care 2010;33:998–1003
- Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care 2013;36:1961–1966
- Leslie CA, Sapico FL, Ginunas VJ, Adkins RH. Randomized controlled trial of topical hyperbaric oxygen for treatment of diabetic foot ulcers. Diabetes Care 1988;11:111–115
- Tawfick WA, Sultan S. Technical and clinical outcome of topical wound oxygen in comparison to conventional compression dressings in the management of refractory nonhealing venous ulcers. Vasc Endovascular Surg 2013;47:30–37
- Graebert JK, Henzel MK, Honda KS, Bogie KM. Systemic evaluation of electrical stimulation for ischemic wound therapy in a preclinical model. Adv Wound Care 2014;3:428–437
- Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005;4:23–44
- Houghton PE, Campbell KE. Therapeutic modalities in the treatment of chronic recalcitrant wounds. In: Krasner DL, editor; , Rodeheaver GT, editor; , Sibbald RG, editor; , Woo KY, editor. , eds. Chronic Wound Care 5: A Clinical Source Book for Healthcare Professionals. Malvern, PA: HMP Communications, 2012:253–270
- Moffett J, Griffin NE, Ritz MC, George FR. Pulsed radio frequency energy field treatment of cells in culture results in increased expression of genes involved in the inflammation phase of lower extremity diabetic wound healing. J Diabet Foot Complications 2010;2:57–64
- Amini S, Shojaeefard A, Annabestani Z, et al. Low-frequency ultrasound debridement in patients with diabetic foot ulcers and osteomyelitis. Wounds 2013;25:193–198
- Kavros SJ, Miller JL, Hanna SW. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: the Mayo clinic experience, 2004–2006. Adv Skin Wound Care 2007;20:221–226
- Kavros SJ, Liedl DA, Boon AJ, Miller JL, Hobbs JA, Andrews KL. Expedited wound healing with noncontact, low-frequency ultrasound therapy in chronic wounds: a retrospective analysis. Adv Skin Wound Care 2008;21:416–423
- Dymarek R, Halski T, Ptaszkowski K, Slupska L, Rosinczuk J, Taradaj J. Extracorporeal shock wave therapy as an adjunct wound treatment: a systematic review of the literature. Ostomy Wound Manage 2014;60:26–39
- Mittermayr R, Antonic V, Hartinger J, et al. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair Regen 2012;20:456–465
- Li VW, Kung EF, Li WW. Molecular therapy for wounds: modalities for stimulating angiogenesis and granulation. In: Yee BY, editor. , ed. The Wound Management Manual. New York, NY: McGraw Hill, 2005:17–43
- Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surg Gynecol Obstet 1990;170:56–60
- Ramos-Torrecillas J, Luna-Bertos E, Garcia-Martinez O, Ruiz C. Clinical utility of growth factors and platelet-rich plasma in tissue regeneration: a review. Wounds 2014;26:2017–2213
- Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 1999;7:335–346
- Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998;21:822–827
- Margolis DJ, Bartus C, Hoffstad O, Malay S, Berlin JA. Effectiveness of recombinant human platelet-derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005;13:531–536
- Gomez-Villa R, Aguilar-Rebolledo F, Lozano-Platonoff A, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial. Wound Repair Regen 2014;22:497–503
- Fernandez-Montequin JI, Valenzuela-Silva CM, Diaz OG, et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo-controlled, double-blind study. Int Wound J 2009;6:432–443
- Morimoto N, Yoshimura K, Niimi M, et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng Part A 2013;19:1931–1940
- Robson MC, Phillips TJ, Falanga V, et al. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair Regen 2001;9:347–352
- Mulder G, Tenenhaus M, D’Souza GF. Reduction of diabetic foot ulcer healing times through use of advanced treatment modalities. Int J Low Extrem Wounds 2014;13:335–346
- Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen 2013;21:194–210
- Mulder G, Harding K, Kirsner R, Lee D, Serena T. International consensus. Acellular Matrices for the Treatment of Wounds. An expert working group review. London: Wounds International, 2010.
- Kavros SJ, Dutra T, Gonzalez-Cruz R, et al. The use of PriMatrix, a fetal bovine acellular dermal matrix, in healing chronic diabetic foot ulcers: a prospective multicenter study. Adv Skin Wound Care 2014;27:356–362
- Coban YK. Combination of negative pressure wound therapy and hyalomatrix application for soft tissue defect of the great toe. Int J Low Extrem Wounds 2012;11:155–156
- Yao M, Attalla K, Ren Y, French MA, Driver VR. Ease of use, safety, and efficacy of integra bilayer wound matrix in the treatment of diabetic foot ulcers in an outpatient clinical setting: a prospective pilot study. J Am Podiatr Med Assoc 2013;103:274–280
- Sanders L, Landsman AS, Landsman A, et al. A prospective, multicenter, randomized, controlled clinical trial comparing a bioengineered skin substitute to a human skin allograft. Ostomy Wound Manage 2014;60:26–38
- Iorio ML, Shuck J, Attinger CE. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast Reconstr Surg 2012;130:232S–241S
- Litwiniuk M, Grzela T. Amniotic membrane: new concepts for an old dressing. Wound Repair Regen 2014;22:451–456
- Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J 2013;10:493–500
- Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis—a feasibility study. Foot Ankle Int 2013;34:1332–1339
- Werber B, Martin E. A prospective study of 20 foot and ankle wounds treated with cryopreserved amniotic membrane and fluid allograft. J Foot Ankle Surg 2013;52:615–621
- Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J 2014;11:122–128
- Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care 2013;22:347–348, 350–341
- Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J 2014. [Epub ahead of print]; DOI: 10.1111/iwj.12395
- Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg 1995;21:839–843
- Veves A, Falanga V, Armstrong DG, Sabolinski ML; Apligraf Diabetic Foot Ulcer S. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–295
- Marston WA, Sabolinski ML, Parsons NB, Kirsner RS. Comparative effectiveness of a bilayered living cellular construct and a porcine collagen wound dressing in the treatment of venous leg ulcers. Wound Repair Regen 2014;22:334–340
- Mansbridge J, Liu K, Patch R, Symons K, Pinney E. Three-dimensional fibroblast culture implant for the treatment of diabetic foot ulcers: metabolic activity and therapeutic range. Tissue Eng 1998;4:403–414
- Roberts C, Mansbridge J. The scientific basis and differentiating features of Dermagraft. Can J Plast Surg 2002;10:6A–13A
- Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–1705
- Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J 2013;10:132–137
- Martin E, Tierney E, Tallis A, Frykberg RG. Use of human fibroblast derived dermal substitute (HFDDS) to close a complex chronic wound in the presence of peripheral arterial disease. J Diabet Foot Complications 2013;5:39–43
- Frykberg RG, Marston WA, Cardinal M. The incidence of lower-extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast-derived dermal substitute. Adv Skin Wound Care 2015;28:17–20
- Wu Y, Huang S, Enhe J, Fu X. Insights into bone marrow-derived mesenchymal stem cells safety for cutaneous repair and regeneration. Int Wound J 2012;9:586–594
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–149
- Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Reg Med 2008;2:169–183
- Murray IR, Corselli M, Petrigliano FA, Soo C, Peault B. Recent insights into the identity of mesenchymal stem cells: implications for orthopaedic applications. Bone Joint J 2014;96-B:291–298
- Gu C, Huang S, Gao D, et al. Angiogenic effect of mesenchymal stem cells as a therapeutic target for enhancing diabetic wound healing. Int J Low Extrem Wounds 2014;13:88–93
- Yamaguchi Y, Yoshida S, Sumikawa Y, et al. Rapid healing of intractable diabetic foot ulcers with exposed bones following a novel therapy of exposing bone marrow cells and then grafting epidermal sheets. Br J Dermatol 2004;151:1019–1028
- Rogers LC, Bevilacqua NJ, Armstrong DG. The use of marrow-derived stem cells to accelerate healing in chronic wounds. Int Wound J 2008;5:20–25
- Peng Y, Huang S, Cheng B, et al. Mesenchymal stem cells: a revolution in therapeutic strategies of age-related diseases. Ageing Res Rev 2013;12:103–115
- Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen 2007;15:459–464
- Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage 2013;59:38–43
- Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix((R