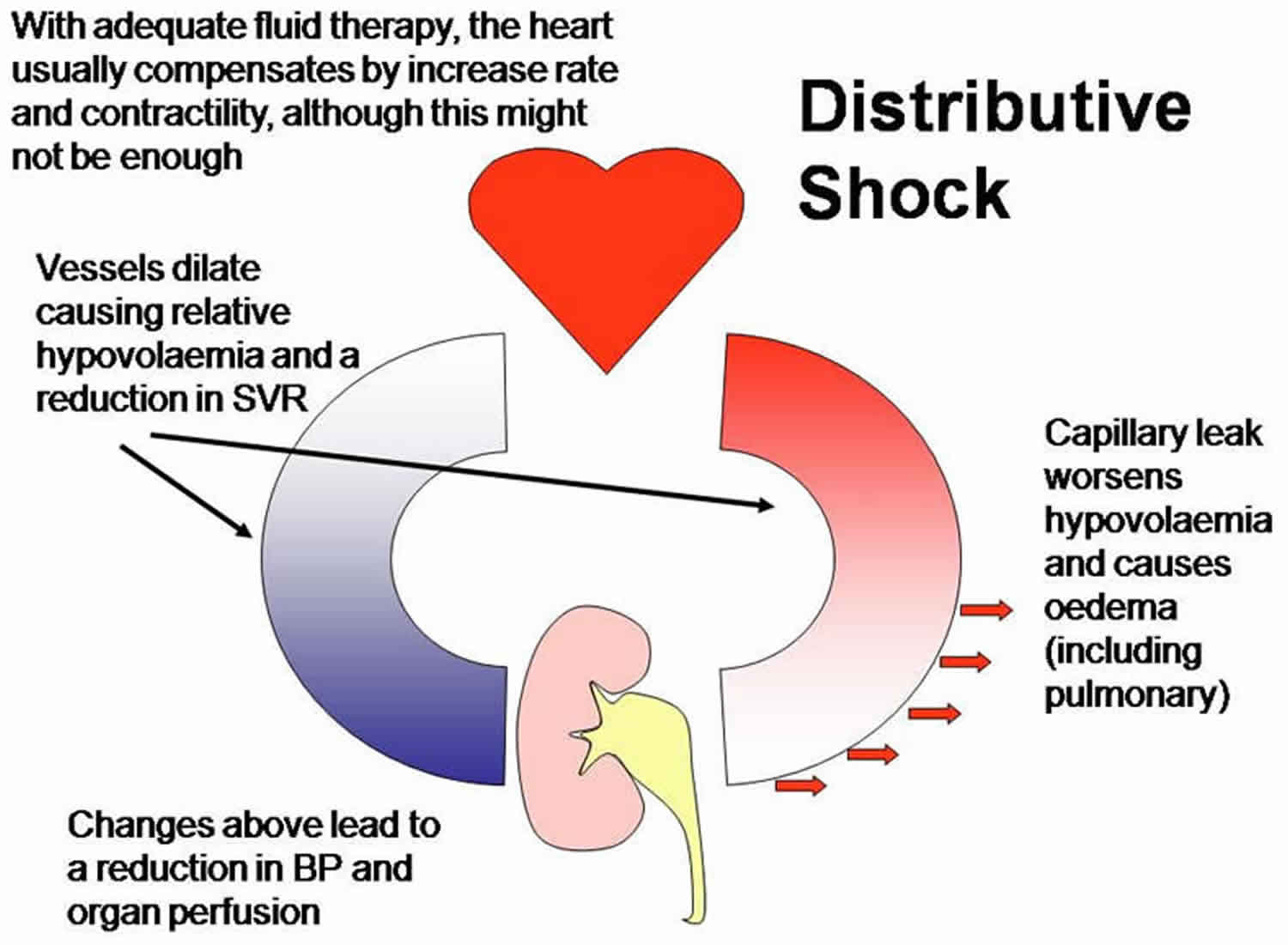
Distributive shock
Distributive shock also known as vasodilatory shock, results from excessive vasodilation and the impaired distribution of blood flow 1. Systemic vasodilation leads to decreased blood flow to the brain, heart, and kidneys causing damage to vital organs. Distributive shock also leads to leakage of fluid from capillaries into the surrounding tissues, further complicating the clinical picture. Due to the complexities of this disease, the causes and treatments for distributive shock are multimodal 2.
Septic shock is the most common cause of distributive shock seen in the emergency department and is characterized by considerable mortality (treated, around 30%; untreated, probably >80%) 3. The number of patients admitted with severe sepsis now approaches one million per year with mortality rates extending 50%. It’s crucial to note that nearly 50% of septic patients that present with some degree of end-organ damage will have a cryptic shock, meaning they have inadequate tissue perfusion despite a normal blood pressure reading. In the United States, septic shock is the leading cause of noncardiac death in intensive care units (ICUs).
Anaphylaxis, likely the second leading cause of distributive shock, can occur at any age regardless of prior history. Nut allergy and history of asthma have been identified as independent predictors of mortality in patients with anaphylaxis, and great care should be taken when monitoring this subset of patients 4. Other causes of distributive shock include systemic inflammatory response syndrome (SIRS) due to noninfectious inflammatory conditions such as burns and pancreatitis; toxic shock syndrome (TSS); reactions to drugs or toxins, including insect bites, transfusion reaction, and heavy metal poisoning; addisonian crisis; hepatic insufficiency; and neurogenic shock due to brain or spinal cord injury 3.
Types of shock
Shock is a clinical syndrome characterized by inadequate tissue perfusion that results in end-organ dysfunction. It can be divided into the following four categories 3:
- Distributive shock (vasodilation), which is a hyperdynamic process
- Cardiogenic shock (pump failure)
- Hypovolemic shock (intravascular volume loss)
- Obstructive shock (physical obstruction of blood circulation and inadequate blood oxygenation)
Causes of distributive shock
The most common causes of distributive shock in the emergency department are sepsis and anaphylaxis. In cases of trauma, the neurogenic shock should also be on the differential. Other less common causes of distributive shock include adrenal insufficiency and capillary leak syndrome. Drug overdose or toxicity should always be considered, particularly potent vasodilators such as calcium channel blockers and hydralazine 5.
Distributive shock as a result of sepsis occurs due to a dysregulated immune response to infection that leads to systemic cytokine release and resultant vasodilation and fluid leak from capillaries. These inflammatory cytokines can also cause some cardiac dysfunction, called septic cardiomyopathy, which can contribute to the shock state.
A common cause is the systemic inflammatory response syndrome due to noninfectious causes such as pancreatitis and burns.
In anaphylaxis, the patient typically has a history of previous exposure to an antigen, although this is not required, with resulting IgE formation to that antigen. These IgE molecules then attach to the surface of mast cells in the tissues and basophils in blood. Consequent exposure to the same antigen results in the IgE-mediated release of histamine from mast cells and basophils, leading to systemic vasodilation and capillary fluid leak.
Toxic shock syndrome should be considered in distributive shock. This disease is caused by Staphylococcus aureus and group A streptococci exotoxins that stimulate systemic cytokine release with resulting vasodilation and capillary leak. Historically, this is associated with both vaginal and nasal tampon use.
Neurogenic shock classically occurs in cases of trauma involving the cervical spinal cord. The sympathetic nervous system is damaged resulting in a decreased adrenergic input to the blood vessels and heart, causing vasodilation with resultant hypotension and paradoxical bradycardia.
The distributive shock from adrenal insufficiency occurs due to decreased alpha-1 receptor expression on arterioles secondary to cortisol deficiency, which results in vasodilation. This is seen in patients on chronic steroids that are stopped suddenly.
Capillary leak syndrome, while rare, should be considered in the edematous patient with distributive shock. It occurs due to low blood albumin. Decreased oncotic pressure leads to fluid loss from the blood into the interstitium 6.
Distributive shock pathophysiology
In most cases, inflammatory mediators play a major role in the development of distributive shock. Inflammatory cytokines released in both sepsis and toxic shock syndrome induce systemic vasodilation and capillary leak, as well as cardiomyopathy. The systemic release of histamine in anaphylaxis results in similar effects.
Interactions between catecholamines and adrenergic receptors in the blood vessels are crucial in other causes of distributive shock. Both norepinephrine and epinephrine stimulate alpha-1 receptors on arterioles to cause vasoconstriction and regulate blood pressure. In the case of neurogenic shock, the sympathetic nervous system is compromised, leading to reduced catecholamine delivery to these receptors. Cortisol is a key regulator of the expression of alpha-1 receptors on the arteriolar surface, but this becomes compromised in patients with adrenal insufficiency.
In effect, the factors leading to vasodilation and shock are multimodal and complex. This necessitates a careful history and physical examination to elucidate the underlying cause and a multi-system approach to treatment.
Distributive shock signs and symptoms
Patients with shock frequently present with tachycardia, tachypnea, hypotension, altered mental status changes, and oliguria.
Patients with septic shock or systemic inflammatory response syndrome (SIRS) may have prior symptoms that suggest infection or inflammation of the respiratory tract, urinary tract, or abdominal cavity.
Septic shock occurs frequently in hospitalized patients with risk factors such as indwelling catheters or venous access devices, recent surgery, or immunosuppressive therapy.
Patients with anaphylaxis commonly have recent iatrogenic (drug) or accidental (bee sting) exposure to an allergen and coexisting respiratory symptoms, such as wheezing and dyspnea, pruritus, or urticaria.
Staphylococcal toxic shock syndrome (TSS) is still observed most commonly in women who are menstruating, but it is also associated with recent soft-tissue injury, cutaneous infections, postpartum and cesarean delivery, wound infections, pharyngitis, and focal staphylococcal infections, such as abscess, empyema, pneumonia, and osteomyelitis. Patients often have a history of influenzalike illness (fever, arthralgias, myalgias) and a desquamating rash.
Pancreatitis may be another cause of distributive shock; expect symptoms of abdominal pain that radiate to the back, as well as nausea and vomiting. Burns also have been described as a cause of distributive shock.
Adrenal insufficiency
Adrenal insufficiency as a cause of shock should be considered in any patient with hypotension who lacks signs of infection, cardiovascular disease, or hypovolemia.
Long-term treatment with corticosteroids may result in inadequate response of the adrenal axis to stress, such as infection, surgery, or trauma, and subsequent onset or worsening of shock.
If the clinical picture is consistent with adrenal insufficiency in a person without this diagnosis, consider that this could be the first presentation of this disorder.
There is a high incidence of adrenal insufficiency in critically ill patients infected with the human immunodeficiency virus (HIV), although this incidence varies with the criteria used to diagnose adrenal insufficiency 7.
Distributive shock diagnosis
Medical history and physical examination
If possible, a careful and directed history should be taken directly from the patient. Often this is not possible, and information should be collected from the emergency management service, family members, or other witnesses of the inciting event. Symptoms of infection, like shortness of breath, cough, fever, chills, nausea, vomiting, abdominal pain, and dysuria, as well as an immunocompromised status and recent hospitalizations, should be noted as this information may point to sepsis. Additionally, identifying known allergies and a history of anaphylaxis as well as possible.
Exposures to known allergens can aid the identification of the cause of the patient’s presentation. Review the patient’s medications, particularly steroids and anti-hypertensives, and illicit drug use to determine if overdose or intoxication could be contributing to the clinical picture.
While the physical exam is unreliable in determining the source of shock, some findings can be suggestive of underlying etiology. Warm extremities can point to vasodilation as the cause of shock. A careful skin exam should be completed to identify a cutaneous source of infection such as cellulitis, ulcers, or abscess. Urticaria strongly suggests anaphylaxis.
Always consider adrenal insufficiency in a patient with hypotension, no signs of an infection and showing resistance to usual methods of resuscitation.
The physical exam will reveal:
- Altered mental status
- Tachycardia: Heart rate greater than 90 beats per minute (note that heart rate elevation is not evident if the patient is on a beta blocker)
- Tachypnea: Respiratory rate greater than 20 breaths per minute
- Hypotension: Systolic blood pressure less than 90 mm Hg or a reduction of 40 mm Hg from baseline
- Warm extremities with bounding pulses and increased pulse pressure (systolic minus diastolic blood pressure) in early shock; late shock may present as critical organ dysfunction
- Hypothermia: Core body temperate less than 96.8°F (36°C)
- Hyperthermia: Core body temperature greater than 101°F (38.3°C)
- Decreased urine output
- Low oxygen saturation (hypoxemia)
Clinical symptoms of the underlying infections found in distributive shock include the following:
- Pneumonia – Dullness to percussion, rhonchi, crackles, bronchial breath sounds
- Urinary tract infection – Costovertebral angle tenderness, suprapubic tenderness, dysuria and polyuria
- Intra-abdominal infection or acute abdomen – Focal or diffuse tenderness to palpation, diminished or absent bowel sounds, rebound tenderness
- Gangrene or soft-tissue infection – Pain out of proportion to lesion, skin discoloration and ulceration, desquamating rash, areas of subcutaneous necrosis
Anaphylaxis is characterized by the following clinical symptoms:
- Respiratory distress
- Wheezing
- Urticarial rash
- Angioedema
Toxic shock syndrome is characterized by the following clinical symptoms:
- High fever
- Diffuse rash with desquamation on the palms and soles over a subsequent 1-2 weeks
- Hypotension (may be orthostatic) and evidence of involvement of 3 other organ systems
Streptococcal toxic shock syndrome more frequently presents with focal soft-tissue inflammation and is less commonly associated with diffuse rash. Occasionally, it can progress explosively within hours.
Adrenal insufficiency is characterized by the following clinical symptoms:
- Hyperpigmentation of skin, oral, vaginal, and anal mucosal membranes may be present in chronic adrenal insufficiency.
- In acute or acute-on-chronic adrenal insufficiency brought on by physiologic stress, hypotension may be the only physical sign.
Distributive shock treatment
Regardless of the type of shock, the majority of patients will tolerate and benefit from an initial fluid bolus of 250-500 mL. Patients with distributive shock are significantly more likely to require vasopressor support. The ultimate goal is to achieve adequate tissue perfusion utilizing fluid resuscitation and vasopressors. This can be achieved by targeting a mean arterial pressure of greater than 65 mmHg, which is the approximate critical perfusion pressure for both the heart and kidneys. Adequacy of tissue perfusion can be monitored with multiple modalities, including physical exam, Scv02 greater than or equal to 70%, and laboratory parameters including lactate and base deficit. The pressor choice will vary depending on the suspected etiology of distributive shock 8.
In septic shock, the initial pressor of choice is norepinephrine (2 mcg/min to 20 mcg/min), as this offers both alpha-1 and beta-1 stimulation, which will increase peripheral vasoconstriction without significantly compromising cardiac output. Vasopressin (0.03 U/min to 0.04 U/min) is a second-line agent which acts primarily as a vasoconstrictor by binding to V1 receptors. Attention should also be given to aggressive fluid resuscitation (Per the surviving sepsis campaign, 30 mL/kg in the first 3 hours), early antibiotic administration, and source control.
For cases of anaphylactic shock, epinephrine is the pressor of choice, as it offers alpha-1 and beta-1 stimulation similar to norepinephrine but also provides beta-2 stimulation, which stimulates bronchodilation and stabilization of mast cells and basophils. Adjunct interventions will include both H1 and H2 antihistamines, steroids, albuterol, fluids, and potentially glucagon for those using beta-blockers.
The shock that is unresponsive to both fluids and vasopressors may indicate adrenal insufficiency. In such cases, steroids can be given to the increased arteriolar expression of alpha-1 receptors. Hydrocortisone 100 mg is the typical treatment.
In cases where vasopressor drips are not immediately available, and the patient has critically low perfusion pressure (particularly mean arterial pressure less than 50 mmHg, the critical perfusion pressure to the brain), push dose vasopressors can be utilized. Epinephrine and phenylephrine are common agents of choice for this purpose.
Soon after resuscitation consider a diet. Some patients may require tube feedings and others may require parenteral feeding.
Resuscitation
Early, efficient resuscitation is the key. The duration of hypotension before antibiotic treatment has been found to be a critical factor in determining mortality 9. Rivers et al 10 found a significant decrease in in-hospital mortality when patients were treated with early, goal-directed therapy. This protocol-driven resuscitation strategy focused on optimizing hemodynamic parameters and reversing hypoperfusion beginning in the emergency department; these protocols have been successfully implemented not only in research centers, but also in community-based settings 11.
As soon as hypoperfusion is detected, continue fluid over the first 6 hours using protocol-defined goals for central venous pressure, mean arterial pressure, urine output, and/or mixed venous oxygen saturation. If central venous oxygen saturation of more than 70% is not achieved in the first 6 hours, Surviving Sepsis Campaign recommendations suggest (based on clinical assessment) transfusing packed red blood cells, targeting a hematocrit at greater than or equal to 30% or treatment with dobutamine 12. However, two major studies, ARISE [Australasian Resuscitation in Sepsis Evaluation] 13 and ProCESS [Protocolized Care for Early Septic Shock] 14 have not shown improved survival with protocol-based therapy, as compared with nonprotocol “usual” therapy. Furthermore, a major study on a perioperative, cardiac output–guided hemodynamic therapy algorithm, with the use of inotropic medication for patients with major gastrointestinal surgery, also did not show increased survival 15. The impact of these three studies on protocol-based therapy is currently controversial.
The choice of fluid for resuscitation has been a matter of ongoing debate. The Surviving Sepsis Campaign recommends the use of either colloids or crystalloids, finding inadequate evidence to recommend one over the other. The Saline Versus Albumin Fluid Evaluation (SAFE) trial found crystalloid and colloid to be equally safe and effective for ICU patients. In contrast to prior studies, it also found no difference or increased mortality among patients receiving albumin 16.
Owing to the findings of various trials such as ARISE 13, ProCESS 14 and OPTIMISE (Optimisation of Cardiovascular Management to Improve Surgical Outcome) 15, the 2016 Surviving Sepsis Campaign guidelines now recommend frequent clinical reassessment of patients after initial fluid resuscitation 17. A plausible way to do so is with point-of-care ultrasound. Monitoring of fluid status via ultrasound assessment of the inferior vena cava 18 and the lungs 19 has been studied to assess fluid responsiveness in sepsis/septic shock.
A meta-analysis revealed that passive leg raising-induced changes in cardiac output (PLR-cCO) can reliably predict fluid responsiveness, regardless of ventilation mode and cardiac rhythm 20. Passive leg raising-induced changes in cardiac output (PLR-cCO) has a significantly higher predictive value than arterial pulse pressure.
A retrospective cohort study of trauma patients who received allogeneic packed red blood cells sought to determine the association between infection or death and blood storage duration. Results showed that patients who received 7 units or more of older blood had a higher risk of complicated sepsis compared with patients who received 1 or fewer units. The effects of allogeneic blood is best reduced by avoiding unnecessary transfusions, but it may also be important to avoid transfusions of multiple units of older blood 21.
In the Multicenter Randomized Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, comparing hydroxyethyl starch (HES) to Ringer’s lactate, the hydroxyethyl starch group had higher rates of renal failure and more days on renal replacement therapy. Additional investigation is required to fully appreciate the risks versus benefits of this intervention 22.
Vasoactive drugs
In patients with hypotension due to sustained septic shock in whom fluid resuscitation does not reverse hypotension, the use of systemic vasopressors is indicated to restore blood flow to pressure-dependent vascular beds (eg, the heart and brain). Either norepinephrine or dopamine should be used as first-line treatment; no evidence suggests the use of one over the other. Several vasopressor agents are available.
If dopamine is tried first and fails to increase mean arterial pressure to more than 60 mm Hg or if excessive tachycardia or tachyarrhythmias develop, norepinephrine (Levophed) should be used. As a second-line treatment, phenylephrine (Neo-Synephrine) may be added to or substituted for dopamine. Dobutamine may be added to the therapeutic regimen when cardiac output is low, recognizing that this drug acts primarily as a positive inotropic agent and may further decrease systemic vascular resistance (SVR).
In December 2017, synthetic human angiotensin II (Giapreza) was approved by the US Food and Drug Administration for adults with septic or other distributive shock. Approval was based on the ATHOS-3 clinical trial (n = 321) in patients with vasodilatory shock and critically low blood pressure. Eligible patients had vasodilatory shock despite intravenous volume resuscitation with at least 25 mL/kg over the previous 24 hours and the administration of high-dose vasopressors. Significantly more patients responded to treatment with the angiotensin II injection added to conventional therapy compared with those on conventional therapy plus placebo. At 48 hours, the mean improvement in the cardiovascular Sequential Organ Failure Assessment (SOFA) score (scores range from 0 to 4, with higher scores indicating more severe dysfunction) was greater in the angiotensin II group than in the placebo group 23.
Important to note, because severe sepsis is usually associated with some degree of myocardial depression, the use of an unopposed alpha stimulant to increase vasomotor tone without a concomitant increase in inotropy decreases cardiac output. This was the universal finding when nitric oxide synthase inhibitors were used to treat the hypotension of septic shock in a large prospective, clinical trial. The doses and cardiovascular characteristics of commonly used vasoactive drugs for shock are summarized in Table 1, below.
Table 1. Vasoactive drugs in sepsis and the usual hemodynamic responses
| Drug | Dose | Principal Mechanism | Cardiac Output | Blood Pressure | Systemic vascular resistance (SVR) |
| Inotropic agents | |||||
| Dobutamine | 2-20 mcg/kg/min | Beta 1 | ++ | + | + |
| Dopamine (low dose) | 5-10 mcg/kg/min | Beta 1, dopamine | ++ | + | + |
| Epinephrine (low dose) | 0.06-0.20 mcg/kg/min | Beta 1, beta 2 >alpha | ++ | + | + |
| Inotropic agents and vasoconstrictors | |||||
| Dopamine (high dose) | >10 mcg/kg/min | Alpha, beta 1, dopamine | ++ | ++ | + |
| Epinephrine (high dose) | 0.21-0.42 mcg/kg/min | Alpha >beta 1, beta 2 | ++ | ++ | + |
| Norepinephrine | 0.02-0.25 mcg/kg/min | Alpha >beta 1, beta 2 | + | ++ | ++ |
| Vasoconstrictors | |||||
| Synthetic human angiotensin II | 20 ng/kg/min; up to 80 ng/kg/min during first 3 h of therapy Maintenance: 1.25-40 ng/kg/min | Renin-angiotensin-aldosterone system (RAAS) | +/- | ++ | +/- |
| Phenylephrine | 0.2-2.5 mcg/kg/min | Alpha | + | ++ | ++ |
| Vasopressin | 0.10-0.40 U/min | V1 receptor | + | + | ++ |
| Vasodilators | |||||
| Dopamine (very low dose) | 1-4 mcg/kg/min | Dopamine | +/- | +/- | – |
| Milrinone | 0.4-0.6 mcg/kg/min after loading dose; 50 mcg/kg bolus over 5 min | Phosphodiesterase inhibitor | + | +/- | – |
Footnotes:
Alpha and beta refer to agonist activity at these adrenergic receptor sites.
Beta 1-adrenergic effects are inotropic and increase contractility.
Beta 2-adrenergic effects are chronotropic 24.
Vasopressin
As a second-line treatment, vasopressin may be helpful to increase mean arterial pressure and systemic vascular resistance (SVR) and may be considered in patients who are refractory to inotropic agents and have a cardiac output that is already more than 3.5 L/min/m². Endogenous vasopressin is released from the pituitary gland as part of the physiologic response to shock, acting on V1 receptors of vascular smooth muscle to induce vasoconstriction. As shock continues, endogenous vasopressin levels may be depressed, perhaps due to depletion of the stores or impaired hypophyseal function in the setting of infection. This contributes to refractory hypotension 25.
In this setting of hypotension, treatment with exogenous vasopressin has a role. Vasopressin treatment carries the risk of acidosis by causing splanchnic vasodilation and resultant ischemia. Myocardial ischemia is also possible, given increased afterload and coronary vasoconstriction. Although current treatment guidelines support vasopressin’s use, a randomized trial in which vasopressin was added to ongoing norepinephrine treatment did not find a benefit to the use of vasopressin over norepinephrine, suggesting that additional investigation will be required to define vasopressin’s role 25. In a 2012 meta-analysis, Serpa et al 26 found that vasopressin treatment in patients with vasodilatory shock was safe and was associated with reduced mortality.
Antimicrobial treatment
In all patients with suspected sepsis, blood and urine cultures should be collected prior to empiric antibiotic therapy, provided that this does not cause a significant delay in treatment. At least 2 blood cultures should be collected and should be drawn percutaneously, as well as from any vascular access site. Cultures such as respiratory tract secretions and cerebrospinal fluid should be collected if infection at these sites is suspected clinically.
Patients who receive prompt effective antimicrobial therapy are more likely to survive than are patients whose antibiotic therapy is delayed; measurable increases in mortality occur for each hour’s delay in antibiotic treatment. Because initial therapy must be empiric, antimicrobial coverage should be broad and should have good penetration to all suspected sites of infection.
The choice of agent should be guided by history, suspected site of infection, comorbid diseases, and pathogen susceptibility patterns in the hospital and community. Avoid antibiotics recently received by the patient. Treatment of fungal infection should be considered and selection of an antifungal agent should be guided by the local prevalence of Candida species. Recommended empiric antibiotic regimens based on the suspected site are outlined in Table 2, below.
Antimicrobial regimens should be tailored once the causative pathogen and its susceptibility are identified because narrow-spectrum treatment decreases the risk of superinfection with resistant organisms. The duration of therapy varies based on clinical context, but the SSC guidelines suggest that the typical duration will be 7-10 days, with adjustments made for factors such as underlying immune status and undrainable foci of infection.
Consider the removal of any devices, such as intravenous or urinary catheters and prostheses. Surgical drainage or debridement should be performed promptly, when appropriate (eg, intra-abdominal abscess, necrotizing fasciitis).
Table 2. Empiric antimicrobial therapy in septic shock based on suspected site of infection
| Suspected Source | Recommended Antibiotic Therapy | Alternative Therapy |
| No source evident in a healthy host | Third-generation cephalosporin, eg, ceftriaxone 2 g IV q12h, ceftizoxime, ceftazidime | Nafcillin and aminoglycoside, imipenem, piperacillin/tazobactam |
| No source evident in an immunocompromised host | Ceftazidime 2 g IV q8h plus aminoglycoside | Imipenem or piperacillin/tazobactam plus aminoglycoside |
| No source evident in a user of intravenous drugs | Nafcillin 2 g IV q4h plus aminoglycoside | Vancomycin plus aminoglycoside, ceftazidime, imipenem, or piperacillin/tazobactam |
| Bacterial pneumonia, community acquired | Ceftriaxone 2 g IV q12-24 h plus macrolide | Levofloxacin 750mg IV q24h, cotrimoxazole or imipenem plus macrolide |
| Bacterial pneumonia, hospital acquired | Piperacillin/tazobactam 4.5 g IV q6h plus aminoglycoside, plus levofloxacin 750 mg IV q24h | Imipenem plus aminoglycoside, plus macrolide |
| Urinary tract infection | Ampicillin 2 g IV q4h plus aminoglycoside | Fluoroquinolone or third-generation cephalosporin plus aminoglycoside |
| Mixed aerobic and anaerobic abdominal sepsis, aspiration pneumonia, pelvic infection, and necrotizing cellulitis | Third-generation cephalosporin or ampicillin 2 g IV q4h plus aminoglycoside plus clindamycin 600 mg IV q8h or metronidazole 500 mg IV q6h | Fluoroquinolone plus clindamycin, imipenem, piperacillin/tazobactam |
| Meningitis | Ceftriaxone 2 g IV q12h plus vancomycin | Meropenem plus vancomycin, chloramphenicol plus cotrimoxazole plus vancomycin |
| Cellulitis/erysipelas | Nafcillin 2 g IV q4h | Cefazolin, vancomycin, clindamycin |
| Toxic shock syndrome (TSS) or streptococcal necrotizing fasciitis | Clindamycin 600 mg IV q8h | Cephalosporin, vancomycin, nafcillin |
Monitoring
All patients with distributive shock should be admitted to an intensive care unit (ICU). Vital signs and fluid intake and output should be measured and charted on an hourly basis. Daily weights should be obtained, and adequate intravascular access should be secured. A central venous access device should be considered if vasoactive drug support is required. Placement of pulmonary artery (PA) and arterial catheters should be considered. Most patients should have an indwelling urinary catheter.
Oxygen should be administered immediately by mask. In patients with altered mental status, respiratory distress, or severe hypotension, elective endotracheal intubation and mechanical ventilation should be considered; these avoid emergent intubation in the event of subsequent respiratory arrest. Mechanical ventilation can also aid in hemodynamic stabilization, by decreasing the demands posed by the respiratory muscles on the circulation (as much as 40% of the cardiac output during respiratory distress).
Prophylaxis
All patients should be treated prophylactically against thromboembolic disease, gastric stress ulceration, and pressure ulcers.
Distributive shock prognosis
Patients who do respond to a fluid challenge and have no organ failure have good outcomes. Patients with multiple organ dysfunction and the need for high doses of vasopressors usually have a poor outcome 27.
The mortality from distributive shock varies on the cause and can range from 20-80%. Early recognition is the key to improved survival. Higher mortality rates are linked to:
- Positive blood cultures
- Advanced age
- Elevated serum lactate or failure to clear lactate when labs are repeated
- Infection due to pseudomonas aeruginosa
- Alcohol use
- Immunocompromised state
- Poor functional status prior to the event.
- Smith N, Lopez RA, Silberman M. Distributive Shock. [Updated 2019 Nov 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470316
- Rahulkumar HH, Bhavin PR, Shreyas KP, Krunalkumar HP, Atulkumar S, Bansari C. Utility of Point-of-Care Ultrasound in Differentiating Causes of Shock in Resource-Limited Setup. J Emerg Trauma Shock. 2019 Jan-Mar;12(1):10-17.
- Distributive Shock. https://emedicine.medscape.com/article/168689-overview
- Hooper N, Armstrong TJ. Hemorrhagic Shock. [Updated 2019 May 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470382
- Gabbay U, Carmi D, Birk E, Dagan D, Shatz A, Kidron D. The Sudden Infant Death Syndrome mechanism of death may be a non-septic hyper-dynamic shock. Med. Hypotheses. 2019 Jan;122:35-40.
- Casey JD, Brown RM, Semler MW. Resuscitation fluids. Curr Opin Crit Care. 2018 Dec;24(6):512-518.
- Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002 Jun. 30(6):1267-73.
- Vincent JL, Leone M. Optimum treatment of vasopressor-dependent distributive shock. Expert Rev Anti Infect Ther. 2017 Jan;15(1):5-10.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun. 34(6):1589-96.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8. 345(19):1368-77.
- Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007 Aug. 132(2):425-32.
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008 Jan. 34(1):17-60.
- ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014 Oct 16. 371 (16):1496-506.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014 May 1. 370 (18):1683-93.
- Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014 Jun 4. 311 (21):2181-90.
- Finfer S, Myburgh J, Bellomo R. Albumin supplementation and organ function. Crit Care Med. 2007 Mar. 35(3):987-8.
- [Guideline] Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017 Mar. 43 (3):304-377.
- Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004 Sep. 30 (9):1834-7.
- Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014 Aug. 121 (2):320-7.
- Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010 Sep. 36(9):1475-83.
- Hassan M, Pham TN, Cuschieri J, Warner KJ, Nester T, Maier RV, et al. The association between the transfusion of older blood and outcomes after trauma. Shock. 2011 Jan. 35(1):3-8.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008 Jan 10. 358(2):125-39.
- Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017 Aug 3. 377 (5):419-430.
- Holmes CL. Vasoactive drugs in the intensive care unit. Curr Opin Crit Care. 2005 Oct. 11(5):413-7.
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008 Feb 28. 358(9):877-87.
- Serpa Neto A, Nassar Junior AP, Cardoso SO, Manettta JA, Pereira VG, Esposito DC, et al. Vasopressin and terlipressin in adult vasodilatory shock: a systematic review and meta-analysis of nine randomized controlled trials. Crit Care. 2012 Aug 14. 16(4):R154.
- Roumpf SK, Hunter BR. Does the Addition of Vasopressin to Catecholamine Vasopressors Affect Outcomes in Patients With Distributive Shock? Ann Emerg Med. 2019 Jul;74(1):153-155.