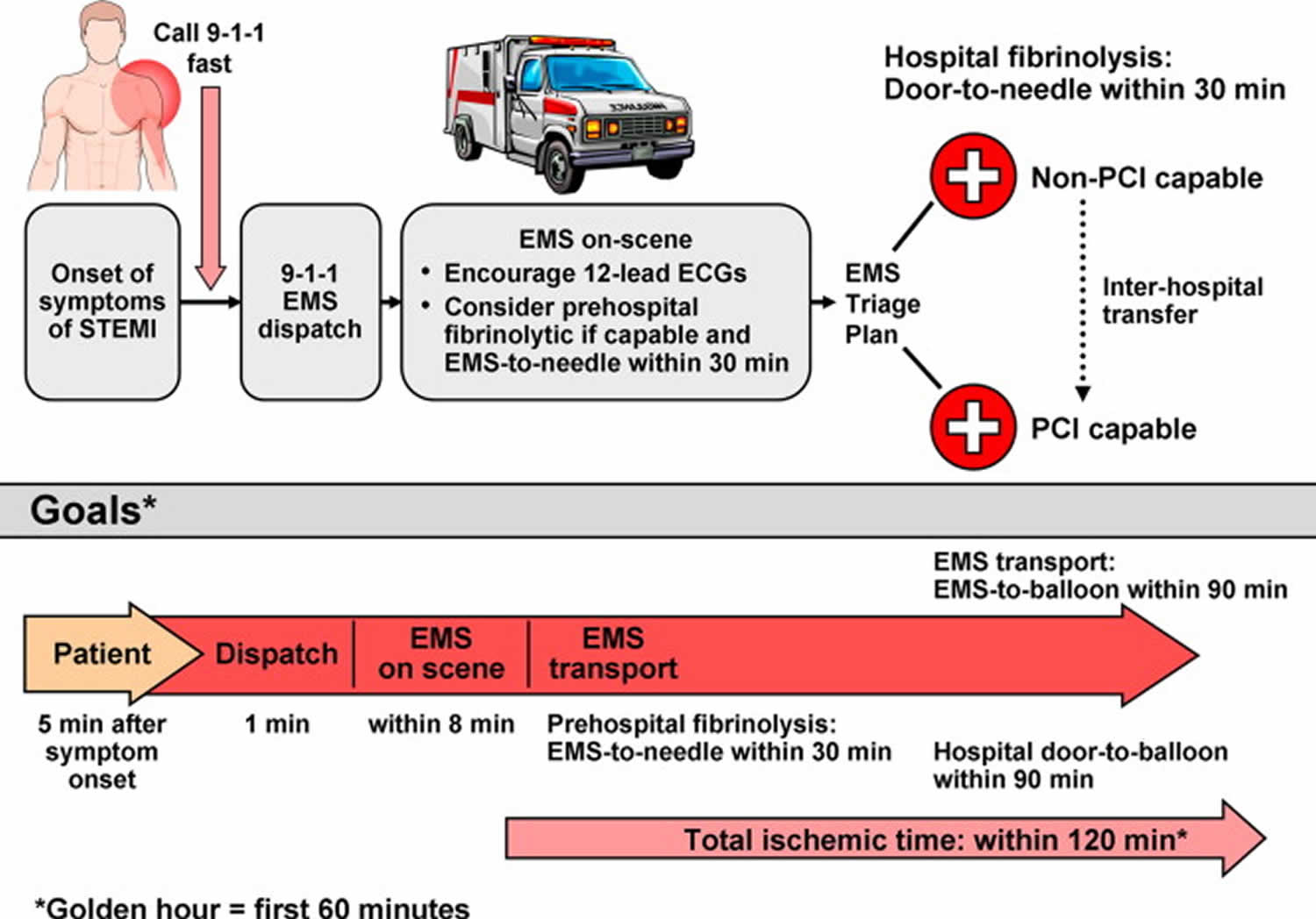
Door to balloon time
Door-to-balloon time is a phrase that denotes the time between the arrival of a patient with ST segment elevation myocardial infarction (STEMI) in the emergency room until the time that a balloon is inflated in the occluded coronary artery in the cardiac catheter lab. In patients with ST‐segment–elevation myocardial infarction, timely reperfusion therapy with door‐to‐balloon time of under 90 minutes is recommended by the current guidelines 1. Shortening door-to-balloon time was significantly associated with survival benefit and the survival benefit of shortening door-to-balloon time was consistently observed, even <60 to 90 minutes 1. A 2018 meta-analysis 2 covering 32 studies involving 299,320 patients with STEMI (ST segment elevation myocardial infarction) who experienced longer (>90 minutes) door-to-balloon delay had a higher risk of short-term mortality and medium-term to long-term mortality. A non-linear time–risk relation was observed. The association between longer door-to-balloon delay and short-term mortality differed between those presented early and late after symptom onset with a stronger relationship among those with shorter prehospital delays 2.
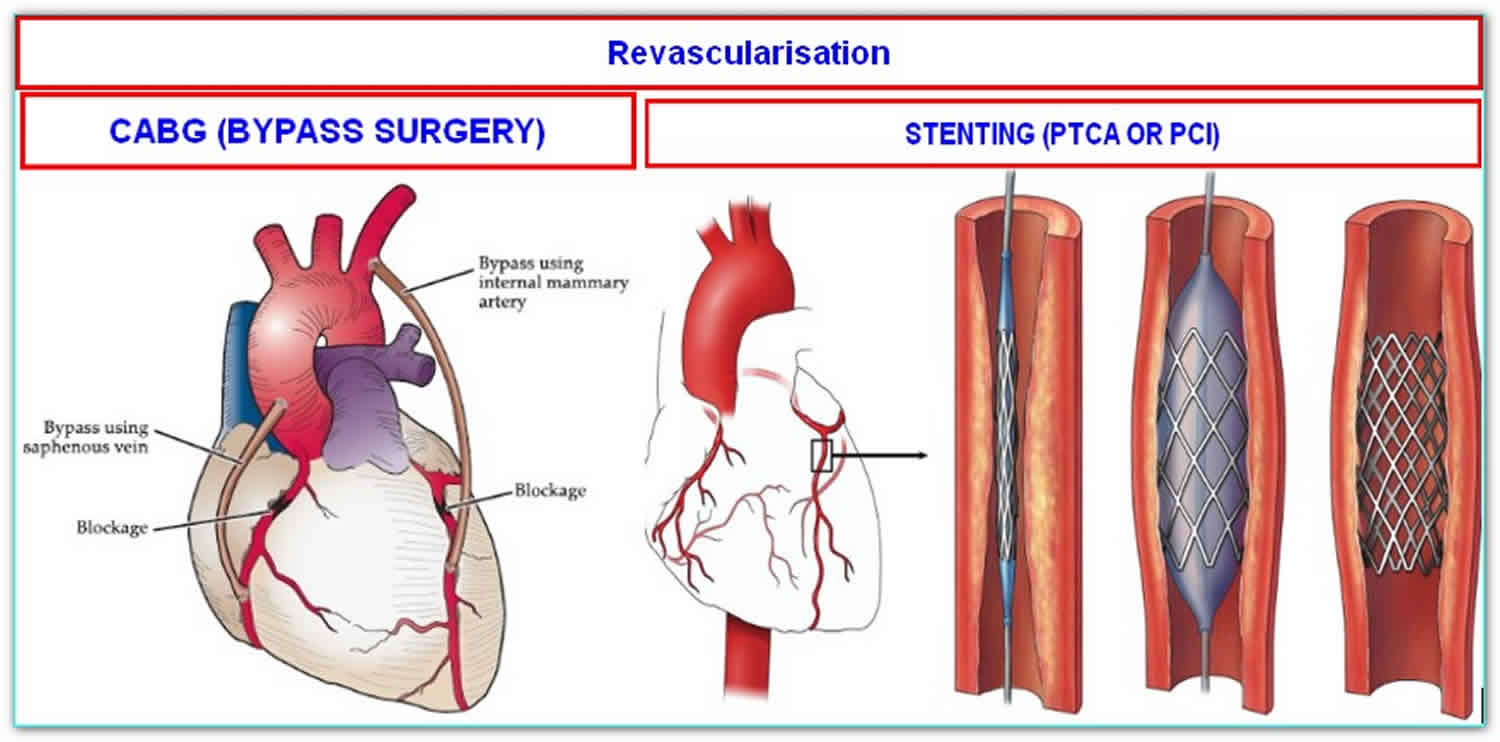
Primary percutaneous coronary intervention (PCI) is the preferred reperfusion strategy in patients with STEMI within 12 hours of symptom onset, provided it can be performed expeditiously (i.e. 120 minutes from STEMI diagnosis) by an experienced team 3. An experienced team includes not only interventional cardiologists but also skilled support staff. Lower mortality rates among patients undergoing primary percutaneous coronary intervention are observed in centers with a high volume of percutaneous coronary intervention procedures 4. Real-life data confirm that primary percutaneous coronary intervention is performed faster and results in lower mortality if performed in high-volume centers 5. Randomized clinical trials in high-volume, experienced centers have repeatedly shown that, if delay to treatment is similar, primary percutaneous coronary intervention is superior to fibrinolysis in reducing mortality, reinfarction, or stroke 6. However, in some circumstances, primary percutaneous coronary intervention is not an immediate option and fibrinolysis could be initiated expeditiously. The extent to which the percutaneous coronary intervention-related time delay diminishes the advantages of percutaneous coronary intervention over fibrinolysis has been widely debated. Because no specifically designed study has addressed this issue, caution is needed when interpreting available data from post hoc analyses. A percutaneous coronary intervention-related time delay potentially mitigating the benefits of percutaneous coronary intervention has been calculated as 60 minutes 7, 110 minutes 8 and 120 minutes 9 in different studies. Registry data estimated this time limit as 114 minutes for in-hospital patients 10 and 120 minutes in patients presenting in a non-percutaneous coronary intervention center 11. All these data are old and patients undergoing fibrinolysis did not undergo routine early angiography, which improves outcomes in patients receiving fibrinolysis. The recent STrategic Reperfusion Early After Myocardial infarction (STREAM) trial randomized early STEMI presenters without the possibility of immediate percutaneous coronary intervention to immediate fibrinolysis (followed by routine early angiography) or transfer to primary percutaneous coronary intervention 12. The median percutaneous coronary intervention-related delay in this trial was 78 minutes, and there were no differences in clinical outcomes. The European Society of Cardiology Task Force recognizes the lack of contemporaneous data to set the limit to choose percutaneous coronary intervention over fibrinolysis. For simplicity, an absolute time from STEMI diagnosis to percutaneous coronary intervention-mediated reperfusion [i.e. wire crossing of the infarct-related artery] rather than a relative percutaneous coronary intervention-related delay over fibrinolysis has been chosen. This limit is set to 120 minutes. Given the maximum limit of 10 minutes from STEMI diagnosis to bolus of fibrinolytics, the 120 minutes absolute time would correspond to a percutaneous coronary intervention-related delay in the range of 110–120 minutes, being in the range of the times identified in old studies and registries as the limit delay to choose percutaneous coronary intervention 11.
If the reperfusion strategy is fibrinolysis, the goal is to inject the bolus of fibrinolytics within 10 minutes from STEMI diagnosis. This time is selected based on the median time from randomization to bolus recorded in the STrategic Reperfusion Early After Myocardial infarction (STREAM) trial, which was 9 minutes 12. In previous European Society of Cardiology STEMI guidelines 13, the target time was 30 minutes, but this was calculated from first medical contact (as opposed to STEMI diagnosis). STEMI diagnosis should occur within 10 minutes from first medical contact.
To shorten time to treatment, fibrinolysis should be administered in the pre-hospital setting if possible 12. Patients should be transferred to a percutaneous coronary intervention-capable facility as soon as possible after bolus of lytics administration. Rescue percutaneous coronary intervention is indicated in the case of failed fibrinolysis (i.e. ST-segment resolution < 50% within 60–90 minutes of fibrinolytic administration), or in the presence of hemodynamic or electrical instability, worsening ischemia, or persistent chest pain 12, while a routine early percutaneous coronary intervention strategy is indicated after successful fibrinolysis (preferably 2–24 hours after fibrinolysis) 14.
Patients with a clinical presentation compatible with acute myocardial infarction and a non-interpretable ST-segment on the ECG, such as those with bundle branch block or ventricular pacing, should undergo a primary percutaneous coronary intervention strategy 15.
There is general agreement that a primary percutaneous coronary intervention strategy should also be followed for patients with symptoms lasting >12 hours in the presence of: (1) ECG evidence of ongoing ischaemia; (2) ongoing or recurrent pain and dynamic ECG changes; and (3) ongoing or recurrent pain, symptoms, and signs of heart failure, shock, or malignant arrhythmias. However, there is no consensus as to whether percutaneous coronary intervention is also beneficial in patients presenting >12 hours from symptom onset in the absence of clinical and/or electrocardiographic evidence of ongoing ischemia. In asymptomatic patients without persistent symptoms 12–48 hours after symptom onset, a small (n = 347) randomized study showed improved myocardial salvage and 4 year survival in patients treated with primary percutaneous coronary intervention compared with conservative treatment alone 16. However, in stable patients with persistent occlusion of the infarct-related artery 3–28 days after myocardial infarction, the large (n = 2166) Occluded Artery Trial (OAT) revealed no clinical benefit from routine coronary intervention with medical management, beyond that from medical management alone 17. A meta-analysis of trials testing whether late recanalization of an occluded infarct-related artery is beneficial showed no benefit of reperfusion 18. Therefore, routine percutaneous coronary intervention of an occluded infarct-related artery in asymptomatic patients >48 hours after onset of symptoms is not indicated. These patients should be managed like all patients with chronic total occlusion, in which revascularization should be considered in the presence of symptoms or objective evidence of viability/ischemia in the territory of the occluded artery 19.
What is STEMI
An ST-elevation myocardial infarction or STEMI is an event in which transmural myocardial ischemia results in myocardial injury or necrosis 20. STEMI occurs when one or more of the coronary arteries that supply the heart with blood is blocked 21. The cause of this abrupt disruption of blood flow is usually plaque rupture, erosion, fissuring or dissection of coronary arteries that results in an obstructing thrombus 21. The major risk factors for ST-elevation myocardial infarction (STEMI) are dyslipidemia, diabetes mellitus, hypertension, smoking, and family history of coronary artery disease 22. Approximately 38% of patients who present to the hospital with acute coronary syndrome have an ST-elevation myocardial infarction (STEMI) 23.
The current 2018 clinical definition of myocardial infarction (MI) requires the confirmation of the myocardial ischemic injury with abnormal cardiac biomarkers 24. It is a clinical syndrome involving myocardial ischemia, ECG (EKG) changes and chest pain.
Myocardial infarction (MI) in general can be classified from Type 1 to Type 5 myocardial infarction based on the etiology and pathogenesis 25.
- Type 1 myocardial infarction is due to acute coronary atherothrombotic myocardial injury with plaque rupture. Most patients with ST-segment elevation myocardial infarction (STEMI) and many with non-ST-segment elevation myocardial infarction (NSTEMI) comprise this category.
- Type 2 myocardial infarction is the most common type of myocardial infarction encountered in clinical settings in which is there is demand-supply mismatch resulting in myocardial ischemia. This demand supply mismatch can be due to multiple reasons including but not limited to presence of a fixed stable coronary obstruction, tachycardia, hypoxia or stress. However, the presence of fixed coronary obstruction is not necessary. Other potential causes include coronary asospasm, coronary embolus, and spontaneous coronary artery dissection ( SCAD).
- Sudden cardiac death patients who succumb before any troponin elevation comprise Type 3 myocardial infarction.
- Types 4 and 5 myocardial infarctions are related to coronary revascularization procedures like Percutaneous Coronry Intervention (PCI) or Coronary Artery Bypass Grfting ( CABG).
For an acute thrombotic coronary event to cause ST-segment elevation on a surface ECG, there needs to be a complete and persistent occlusion of a coronary artery blood flow by blood clot (thrombus). Coronary athersclerosis and presence of high risk thin cap fibroatheroma can result in sudden onset plaque rupture 26. This results in changes in vascular endothelium resulting in cascade of platelet adhesion, activation and aggregartion resulting in thrombosis formation 27.
As soon as the coronary blood supply is interrupted, myocardial damage begins and the longer the blood supply is occluded the greater the amount of heart muscle lost. In animal models of experimental coronary artery occlusion a “ischemic wavefront” of myocardial injury spreads from the inner layer of heart muscle (sub-endocardial myocardium) to the outermost layer (sub-epicardial myocardium) resulting in a transmural infarction that appears as an ST elevation on surface ECG, whereupon the infarction is then said to be ‘full thickness’ 28. In those who survive STEMI, the infarcted muscle is gradually replaced by scar tissue (fibrosis), and the extent of damage will determine the overall pumping ability of the heart, and is a determinant of ‘heart failure’ 29 and longer-term survival. Because there are also some less common pathophysiological explanations for myocardial infarction an international definition of myocardial infarction has been agreed 30.
Nearly half of potentially salvageable myocardium is lost within 1 hour of the coronary artery being occluded, and two-thirds is lost within 3 hours 31. The extent of myocardial damage may be modulated by the presence of any collateral supply to the ischemic territory from other coronary arteries. It was demonstrated that complete coronary occlusion was the cause of STEMI more than 30 years ago using coronary angiography 32 and this quickly resulted in clinical trials of ‘clot-busting’ (thrombolytic, fibrinolytic) and other drugs 33 being undertaken in an attempt to reopen thrombosed coronary arteries and thereby limit myocardial damage.
Apart from resuscitation from any cardiac arrest, the highest priority in the management of STEMI is to restore an adequate coronary blood flow as quickly as possible. During the 1980s and 1990s the best means of achieving restoration of flow was to administer a fibrinolytic drug. These were initially given by direct intracoronary injection, but later administration intravenously was shown to be at least as effective and had the advantage of being much more easily administered, offering the possibility of being given by trained ambulance crews. However, whilst shown to be much more effective than placebo, fibrinolysis was not without its imperfections: some people were unsuitable for its use (for instance because of bleeding complications), in around 20%–30% it failed to result in coronary reperfusion, and in a few (1.0%) it caused hemorrhagic stroke. In an attempt to improve outcomes attention turned to mechanical techniques as a means of restoring coronary flow (coronary angioplasty, thrombus extraction catheters, stenting), that are grouped under the overarching term ‘primary percutaneous coronary intervention’ (PPCI).
STEMI diagnosis
A working diagnosis of STEMI must first be made. This is usually based on symptoms consistent with myocardial ischemia (i.e. persistent chest pain) and signs [i.e. 12-lead electrocardiogram (ECG)]. Important clues are a history of coronary artery disease and radiation of pain to the neck, lower jaw, or left arm. Some patients present with less-typical symptoms such as shortness of breath, nausea/vomiting, fatigue, palpitations, or syncope 34. A reduction in chest pain after nitroglycerin (glyceryl trinitrate) administration can be misleading and is not recommended as a diagnostic manoeuver 35. In cases of symptom relief after nitroglycerin administration, another 12-lead ECG must be obtained. A complete normalization of the ST-segment elevation after nitroglycerin administration, along with complete relief of symptoms, is suggestive of coronary spasm, with or without associated myocardial infarction. In these cases, an early coronary angiography (within 24 hours) is recommended. In cases of recurrent episodes of ST-segment elevation or chest pain, immediate angiography is required.
It is recommended to initiate ECG monitoring as soon as possible in all patients with suspected STEMI in order to detect life-threatening arrhythmias and allow prompt defibrillation if indicated. When a STEMI is suspected, a 12-lead ECG must be acquired and interpreted as soon as possible at the time of first medical contact to facilitate early STEMI diagnosis and triage 36. If the ECG is equivocal or does not show evidence to support the clinical suspicion of myocardial infarction, ECGs should be repeated and, when possible compared with previous recordings. If interpretation of pre-hospital ECG is not possible on-site, field transmission of the ECG is recommended 37.
The American College of Cardiology, American Heart Association, European Society of Cardiology, and the World Heart Federation committee established the following ECG criteria for ST-elevation myocardial infarction (STEMI) 38:
- New ST-segment elevation at the J point in 2 contiguous leads with the cutoff point as greater than 0.1 mV in all leads other than V2 or V3
- In leads V2-V3 the cutoff point is greater than 0.2 mV in men older than 40 years old and greater than 0.25 in men younger than 40 years old, or greater than 0.15 mV in women
ECG criteria are based on changes of electrical currents of the heart (measured in millivolts or mV). Standard calibration of the ECG is 10mm/mV. Therefore 0.1 mV equals to 1 mm square on the vertical axis. For simplicity, in this document ECG deviations are expressed in mm following the standard calibration.
In the proper clinical context, ST-segment elevation (measured at the J-point) is considered suggestive of ongoing coronary artery acute occlusion in the following cases: at least two contiguous leads with ST-segment elevation ≥ 2.5 mm in men < 40 years, ≥2 mm in men ≥ 40 years, or ≥ 1.5 mm in women in leads V2–V3 and/or ≥ 1 mm in the other leads [in the absence of left ventricular (LV) hypertrophy or left bundle branch block LBBB)] 39. In patients with inferior myocardial infarction, it is recommended to record right precordial leads (V3R and V4R) seeking ST-segment elevation, to identify concomitant right ventricular (RV) infarction 39. Likewise, ST-segment depression in leads V1–V3 suggests myocardial ischaemia, especially when the terminal T-wave is positive (ST-segment elevation equivalent), and confirmation by concomitant ST-segment elevation ≥ 0.5 mm recorded in leads V7–V9 should be considered as a means to identify posterior myocardial infarction 39. The presence of a Q-wave on the ECG should not necessarily change the reperfusion strategy decision.
Patients with a pre-existing left bundle branch block can be further evaluated using Sgarbossa’s criteria 40:
- ST-segment elevation of 1 mm or more that is concordant with (in the same direction as) the QRS complex
- ST-segment depression of 1 mm or more in lead V1, V2, or V3
- ST-segment elevation of 5 mm or more that is discordant with (in the opposite direction) the QRS complex
STEMI treatment
After making the diagnosis of acute ST-elevation myocardial infarction (STEMI), intravenous access should be obtained, and cardiac monitoring started. Patients that are hypoxemic or at risk for hypoxemia benefit from oxygen therapy; however, recent studies show possible deleterious effects in normoxic patients 41. Patients should undergo percutaneous coronary intervention (PCI) within 90 minutes of presentation at a percutaneous coronary intervention capable hospital or within 120 minutes if transfer to a percutaneous coronary intervention capable hospital is required 42. If percutaneous coronary intervention (PCI) is not possible within the first 120 minutes of first medical contact, fibrinolytic therapy should be initiated within 30 minutes of patient arrival at the hospital 42. It is important to rule out conditions that can mimic an acute coronary syndrome like acute aortic dissection or acute pulmonary embolism.
All patients with an acute myocardial infarction should be started on a beta blocker, high intensity statin, aspirin, and a P2Y12 inhibitor as soon as possible, with certain exceptions. Nitroglycerin administration can reduce anginal pain however it should be avoided in patients who have used phosphodiesterase inhibiting medication within the last 24 hours and in cases of right ventricular infarction. Further pain relief with morphine can be given for patients that continue report discomfort after nitroglycerin administration however judicious use is not recommended as it may adversely affect outcomes 43. P2Y inhibiting antiplatelet medication choice depends on whether the patient underwent PCI or fibrinolytic therapy. Ticagrelor and prasugrel are preferred to clopidogrel in patients who undergo PCI due to recent trials showing superiority 44. Patients undergoing fibrinolytic therapy should be started on clopidogrel 45. It is important to be careful about relative contraindications of P2Y12 inhibitors. Prasugrel is contraindicated in pateints with history of Transient Ischemic attack and stroke.
Anticoagulation should also be started aongside with unfractionated heparin, low-molecular-weight heparin, bivalirudin, or fondaparinux 46.
STEMI complications
There are 3 life-threatening mechanical complications of myocardial infarction:
- ventricular free wall rupture,
- interventricular septum rupture, and
- acute mitral regurgitation.
Ventricular free wall rupture occurs within 5 days in half the cases and within 2 weeks in 90% of cases with an overall mortality rate of greater than 80% 47. Rupture of the interventricular septum is reported about half as often as free wall rupture and typically occurs 3 to 5 days with an overall mortality rate greater than 70% 48. Prompt surgery reduces the mortality rate in both conditions. Acute mitral regurgitation following a myocardial infarction is most commonly due to ischemic papillary muscle displacement, left ventricular dilatation, or rupture of the papillary muscle of chordae 49. In ST-elevation myocardial infarction (STEMI), the degree of mitral regurgitation is usually severe and associated with a 30-day survival of 24% 49.
STEMI prognosis
Mortality rates at 30 days for patients presenting with ST-elevation myocardial infarction are between 2.5% to 10% 50. The most commonly used scoring system for 30-day mortality is the Thrombolysis in Myocardial Infarction (TIMI) risk score 51. The TIMI scoring system considers:
- Age older than 75 years (3 points); Age 64 to 74 (2 points)
- Diabetes, hypertension, or history of angina (1 point)
- Systolic blood pressure less than 100 mm Hg (3 points)
- Heart rate greater than 100 beats per minute (2 points)
- Killip class II to IV (2 points)
- Body weight less than 150 lbs (1 point).
- Prognostic Implications of Door‐to‐Balloon Time and Onset‐to‐Door Time on Mortality in Patients With ST‐Segment–Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. Journal of the American Heart Association. May 7, 2019 Vol 8, Issue 9 https://doi.org/10.1161/JAHA.119.012188
- Foo CY, Bonsu KO, Nallamothu BK, et al. Coronary intervention door-to-balloon time and outcomes in ST-elevation myocardial infarction: a meta-analysis. Heart 2018;104:1362-1369.
- Borja Ibanez, Stefan James, Stefan Agewall, Manuel J Antunes, Chiara Bucciarelli-Ducci, Héctor Bueno, Alida L P Caforio, Filippo Crea, John A Goudevenos, Sigrun Halvorsen, Gerhard Hindricks, Adnan Kastrati, Mattie J Lenzen, Eva Prescott, Marco Roffi, Marco Valgimigli, Christoph Varenhorst, Pascal Vranckx, Petr Widimský, ESC Scientific Document Group, 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC), European Heart Journal, Volume 39, Issue 2, 07 January 2018, Pages 119–177, https://doi.org/10.1093/eurheartj/ehx393
- Thiemann DR, Coresh J, Oetgen WJ, Powe NR. The association between hospital volume and survival after acute myocardial infarction in elderly patients. N Engl J Med 1999;340(21):1640–1648.
- West RM, Cattle BA, Bouyssie M, Squire I, de Belder M, Fox KA, Boyle R, McLenachan JM, Batin PD, Greenwood DC, Gale CP. Impact of hospital proportion and volume on primary percutaneous coronary intervention performance in England and Wales. Eur Heart J 2011;32(6):706–711.
- Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, Villadsen AB, Krusell LR, Haghfelt T, Lomholt P, Husted SE, Vigholt E, Kjaergard HK, Mortensen LS, DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003;349(8):733–742.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003;92(7):824–826.
- Betriu A, Masotti M. Comparison of mortality rates in acute myocardial infarction treated by percutaneous coronary intervention versus fibrinolysis. Am J Cardiol 2005;95(1):100–101.
- Boersma E, Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006;27(7):779–788.
- Pinto DS, Kirtane AJ, Nallamothu BK, Murphy SA, Cohen DJ, Laham RJ, Cutlip DE, Bates ER, Frederick PD, Miller DP, Carrozza JP, Antman EM, Cannon CP, Gibson CM. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006;114(19):2019–2025.
- Pinto DS, Frederick PD, Chakrabarti AK, Kirtane AJ, Ullman E, Dejam A, Miller DP, Henry TD, Gibson CM, National Registry of Myocardial Infarction Investigators. Benefit of transferring ST-segment-elevation myocardial infarction patients for percutaneous coronary intervention compared with administration of onsite fibrinolytic declines as delays increase. Circulation 2011;124(23):2512–2521.
- Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell Ortiz F, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F, STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 2013;368(15):1379–1387.
- Task Force on the management of ST-segment elevationsacute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van ‘t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33(20):2569–2619.
- Madan M, Halvorsen S, Di Mario C, Tan M, Westerhout CM, Cantor WJ, Le May MR, Borgia F, Piscione F, Scheller B, Armstrong PW, Fernandez-Aviles F, Sanchez PL, Graham JJ, Yan AT, Goodman SG. Relationship between time to invasive assessment and clinical outcomes of patients undergoing an early invasive strategy after fibrinolysis for ST-segment elevation myocardial infarction: a patient-level analysis of the randomized early routine invasive clinical trials. JACC Cardiovasc Interv 2015;8(1 Pt B):166–174.
- Liakopoulos V, Kellerth T, Christensen K. Left bundle branch block and suspected myocardial infarction: does chronicity of the branch block matter? Eur Heart J Acute Cardiovasc Care 2013;2(2):182–189.
- Ndrepepa G, Kastrati A, Mehilli J, Antoniucci D, Schomig A. Mechanical reperfusion and long-term mortality in patients with acute myocardial infarction presenting 12 to 48 hours from onset of symptoms. JAMA 2009;301(5):487–488.
- Menon V, Pearte CA, Buller CE, Steg PG, Forman SA, White HD, Marino PN, Katritsis DG, Caramori P, Lasevitch R, Loboz-Grudzien K, Zurakowski A, Lamas GA, Hochman JS. Lack of benefit from percutaneous intervention of persistently occluded infarct arteries after the acute phase of myocardial infarction is time independent: insights from Occluded Artery Trial. Eur Heart J 2009;30(2):183–191.
- Ioannidis JP, Katritsis DG. Percutaneous coronary intervention for late reperfusion after myocardial infarction in stable patients. Am Heart J 2007;154(6):1065–1071.
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35(37):2541–2619.
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J. Am. Coll. Cardiol. 2000 Sep;36(3):959-69.
- Akbar H, Foth C, Kahloon RA, et al. Acute Myocardial Infarction ST Elevation (STEMI) [Updated 2020 Jun 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532281
- Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV, Ornato JP, Zalenski RJ, Penney J, Tiefenbrunn AJ, Greenland P., NRMI Investigators. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011 Nov 16;306(19):2120-7.
- Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB., American Heart Association Statistics Committee. Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26;133(4):e38-360.
- Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am. J. Hypertens. 1994 Jul;7(7 Pt 2):7S-12S.
- Hartikainen TS, Sörensen NA, Haller PM, Goßling A, Lehmacher J, Zeller T, Blankenberg S, Westermann D, Neumann JT. Clinical application of the 4th Universal Definition of Myocardial Infarction. Eur. Heart J. 2020 Jun 14;41(23):2209-2216.
- Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, Finn AV, Virmani R. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001 Sep;16(5):285-92.
- Scharf RE. Platelet Signaling in Primary Haemostasis and Arterial Thrombus Formation: Part 1. Hamostaseologie. 2018 Nov;38(4):203-210.
- Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab. Invest. 1979 Jun;40(6):633-44.
- Chronic heart failure in adults: diagnosis and management. NICE guideline [NG106] Published date: 12 September 2018 https://www.nice.org.uk/guidance/ng106
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. European Heart Journal, Volume 33, Issue 20, October 2012, Pages 2551–2567, https://doi.org/10.1093/eurheartj/ehs184
- Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56(5):786-794. doi:10.1161/01.cir.56.5.786
- DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303(16):897-902. doi:10.1056/NEJM198010163031601
- Rentrop KP, Blanke H, Karsch KR, et al. Acute myocardial infarction: intracoronary application of nitroglycerin and streptokinase. Clin Cardiol. 1979;2(5):354-363. doi:10.1002/clc.4960020507
- de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, Stricker BH, Hofman A, Witteman JC. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J 2006;27(6):729–736.
- Henrikson CA, Howell EE, Bush DE, Miles JS, Meininger GR, Friedlander T, Bushnell AC, Chandra-Strobos N. Chest pain relief by nitroglycerin does not predict active coronary artery disease. Ann Intern Med 2003;139(12):979–986.
- Diercks DB, Peacock WF, Hiestand BC, Chen AY, Pollack CVJr, Kirk JD, Smith SCJr, Gibler WB, Ohman EM, Blomkalns AL, Newby LK, Hochman JS, Peterson ED, Roe MT. Frequency and consequences of recording an electrocardiogram >10 minutes after arrival in an emergency room in non-ST-segment elevation acute coronary syndromes (from the CRUSADE Initiative). Am J Cardiol 2006;97(4):437–442.
- Dhruva VN, Abdelhadi SI, Anis A, Gluckman W, Hom D, Dougan W, Kaluski E, Haider B, Klapholz M. ST-Segment Analysis Using Wireless Technology in Acute Myocardial Infarction (STAT-MI) trial. J Am Coll Cardiol 2007;50(6):509–513.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012 Oct 16;126(16):2020-35.
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HDWriting Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial InfarctionThygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, ESC Committee for Practice Guidelines. Third universal definition of myocardial infarction. Eur Heart J 2012;33(20):2551–2567.
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012 Dec;60(6):766-76.
- Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, Arefalk G, Frick M, Alfredsson J, Nilsson L, Ravn-Fischer A, Omerovic E, Kellerth T, Sparv D, Ekelund U, Linder R, Ekström M, Lauermann J, Haaga U, Pernow J, Östlund O, Herlitz J, Svensson L., DETO2X–SWEDEHEART Investigators. Oxygen Therapy in Suspected Acute Myocardial Infarction. N. Engl. J. Med. 2017 Sep 28;377(13):1240-1249.
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW., American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
- Meine TJ, Roe MT, Chen AY, Patel MR, Washam JB, Ohman EM, Peacock WF, Pollack CV, Gibler WB, Peterson ED., CRUSADE Investigators. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am. Heart J. 2005 Jun;149(6):1043-9.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, PLATO Investigators. Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009 Sep 10;361(11):1045-57.
- Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, McCabe CH, Braunwald E., CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N. Engl. J. Med. 2005 Mar 24;352(12):1179-89.
- Braun M, Kassop D. Acute Coronary Syndrome: Management. FP Essent. 2020 Mar;490:20-28.
- Yip HK, Wu CJ, Chang HW, Wang CP, Cheng CI, Chua S, Chen MC. Cardiac rupture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era. Chest. 2003 Aug;124(2):565-71.
- Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, Vahanian A, Califf RM, Topol EJ. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation. 2000 Jan 4-11;101(1):27-32.
- Tcheng JE, Jackman JD, Nelson CL, Gardner LH, Smith LR, Rankin JS, Califf RM, Stack RS. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann. Intern. Med. 1992 Jul 01;117(1):18-24.
- Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation. 2012 Apr 17;125(15):1848-57.
- Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000 Oct 24;102(17):2031-7.