Exosomes
Exosomes are specialized membranous extracellular nano-sized vesicles (∼30–100 nm in diameter) derived from endocytic compartments that are released by many cell types 1 and are implicated in cell–cell communication and the transmission of disease states, and explored as a means of drug discovery 2. Exosomes are capable of regulating cell function by delivering proteins, lipids, and nucleic acids 3. Exosomes are best defined as extracellular vesicles that are released from cells upon fusion of an intermediate endocytic compartment, the multivesicular body (MVB), with the plasma membrane. This liberates intraluminal vesicles (ILVs) into the extracellular milieu and the vesicles thereby released are what is known as exosomes (Figure 1). Despite 20 years of research, the very basics of exosome biology are in their infancy and scientists know little of the part they play in normal cellular physiology 2.
Over the last few years, exosomes have emerged as a major mediator of cell therapy derived therapeutic benefits, personalized targeted drug delivery vehicles, as well as a biomarker and promising treatment option for several neurological diseases 4.
Microvesicles are distinctive from exosomes in that they are produced by shedding of the plasmamembrane and usually larger in size (>1 µm). Exosome biogenesis involves the tightly controlled process of inward budding from the limiting membrane of multivesicular bodies (MVBs). This results in numerous intraluminal vesicles in the lumen of multivesicular bodies that contain distinct protein repertoires. It has been suggested that microvesicles shed by certain tumor cells hold functional messenger RNA (mRNA) that may promote tumor progression.
There are other types of microvesicle, including apoptotic bodies and ectosomes, which are derived from cells undergoing apoptosis and plasma membrane shedding, respectively. Although apoptotic bodies, ectosomes and exosomes are all roughly the same size (typically 40–100 nm) and all also contain ‘gulps’ of cytosol, they are different species of vesicles and understanding differences between them is of paramount importance but has too often been overlooked.
There are many proposed functions for exosomes, the best-established being in immune responses 2. Exosomes isolated from B lymphocytes and bearing MHC class II molecules were shown in early experiments 5 to activate T lymphocytes in vitro, suggesting that they were communicating with the T lymphocytes in just the way that the parent B cells did. Later work by the same group, who showed that exosomes derived from dendritic cells, which are specialized to activate T cells in the initiation of immune responses, could promote antitumour immune responses in mice 6, exciting interest in the possibility of practical applications. Or, as with follicular dendritic cells, exosome-associated MHC II can be found on the surface of cell types that neither express MHC II nor secrete exosomes, indicating that exosomes are delivered from one cell type to another 7.
However, exosomes may have roles other than in immune responses as several non-immune cells secrete exosomes. The only physiological role so far reported for non-immune cells is in keratinocyte-derived exosomes, which have been shown to modulate melanin synthesis by increasing the expression and activity of proteins within the melanosomes that modulate skin pigmentation 8.
Mesenchymal stem cells (MSC) are a self‐replicating multipotent stromal cell isolated from mesenchymal tissues such as bone marrow (BMSC) 9, adipose 10, dental pulp 11 and umbilical cord blood 12 as well as other tissues. Mesenchymal stem cells have demonstrated therapeutic efficacy at promoting the protection and regeneration of central nervous system (CNS) neurons, which lack the capacity to regenerate, or be replaced following loss 13.
Exosomes key points 14
- Exosomes are involved in many aspects of normal brain physiology and facilitate communication between brain cells and between the brain and the periphery.
- Increasing evidence suggests that exosomes from mesenchymal stromal cells mediate the beneficial effects of cell therapy for stroke and traumatic brain injury (TBI).
- The effects of mesenchymal stromal cell-derived exosomes alone have the potential to improve neurological outcomes in animal models of stroke, traumatic brain injury and other neurological diseases.
- Of the cargo in exosomes, microRNA (miRNA) is of prime importance in mediating the therapeutic effects.
- Compared with naive mesenchymal stromal cell-derived exosomes, engineered mesenchymal stromal cell-derived exosomes that contain selected miRNA have more potent therapeutic effects in stroke and traumatic brain injuries.
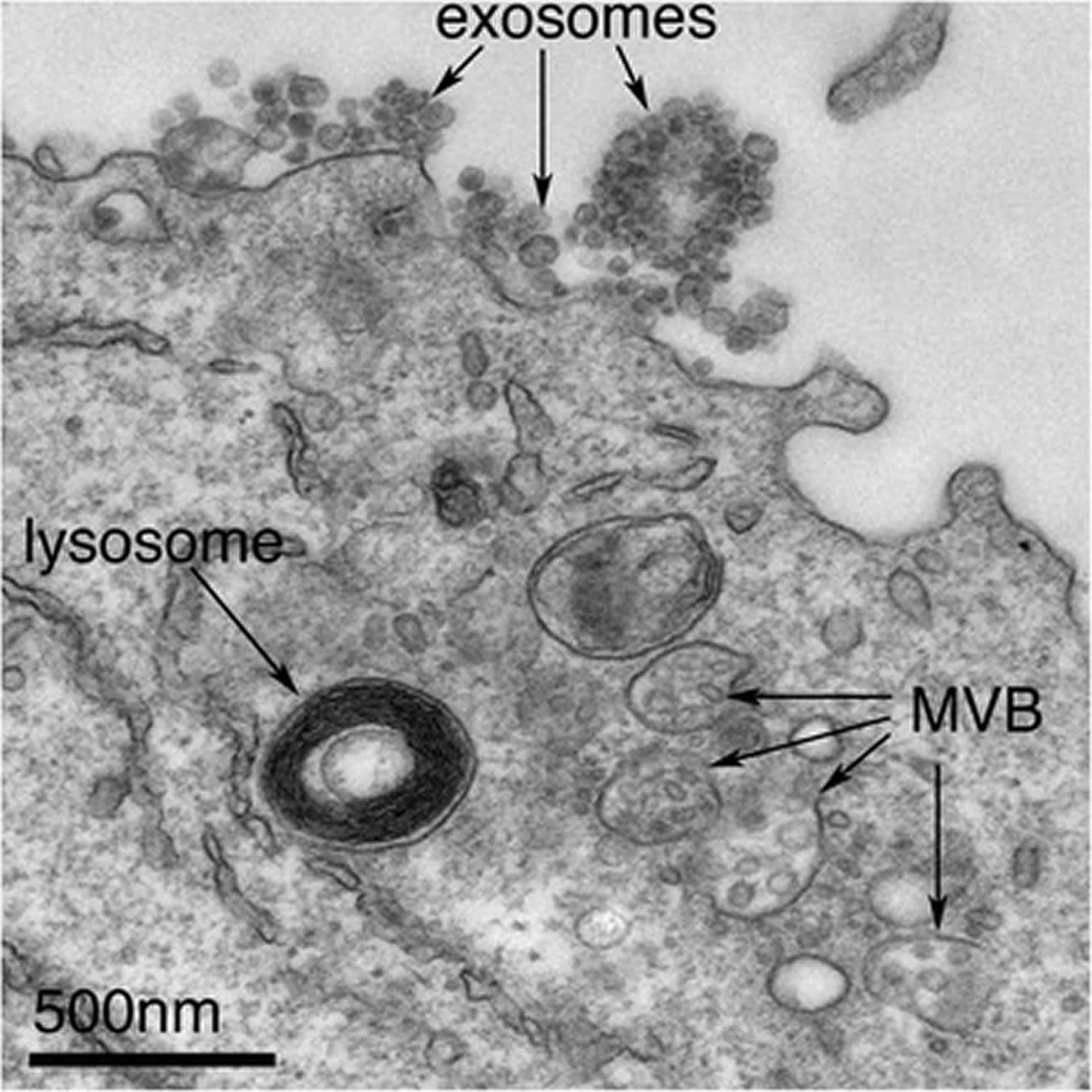
Figure 1. Exosomes
Footnote: Exosomes correspond to intraluminal vesicles of multivesicular bodies. A transmission electron micrograph of an Epstein–Barr virus-transformed B cell displaying newly expelled exosomes at the plasma membrane. Multivesicular bodies (MVB) can be seen which can deliver content to lysosomes for degradation or can fuse with the cell surface to release intraluminal vesicles as exosomes, indicated by the arrows at the top of the picture.
[Source 2 ]How exactly would exosomes from one cell influence the expression and activity of proteins in an acceptor cell?
Exosomes transfer not only protein and lipids but mRNA and microRNA into acceptor cells and these RNAs have been shown in experiments in vitro to have functional effects in recipient cells. For example, exosomes from mice can be transferred to human cells and mRNA can be translated into mouse protein 15. Similarly, microRNAs—double-stranded RNA fragments that can regulate specific sets of mRNA (and so protein levels)—can act functionally in acceptor cells. The mode of action of exosomes has been a focus of special interest in cancer biology. Exosomes from breast cancer cell lines, for example, have been shown to be enriched for miRNAs relative to nontumorigenic breast cell lines and exposure of normal cells to exosomes derived from breast cancer cell lines increased both cell survival and proliferation, accompanied by loss of expression of some tumour-suppressor proteins 16. Exosome levels are elevated in the serum of some cancer patients versus controls. However, whether these vesicles are exosomes or other forms of extracellular vesicle, or a mix, is unclear 2.
Can exosomes also contribute to disease?
Yes. As exosomes provide a means of intercellular communication, they may also act as vehicles for ‘bad’ communication or spread 2. As well as miRNAs in the case of cancer, exosomes have been shown to contain numerous disease-associated cargos—for example, neurodegenerative-associated peptides, such as Aβ in Alzheimer’s disease 17, tau (in numerous neurodegenerative diseases) 18, prions (in transmissible spongiform encephalopathies) 19, alpha-synuclein (in synucleinopathies, including Parkinson’s disease) 20 and superoxide dismutase 1 (in amyotrophic lateral sclerosis) 21. Exosomes have thus been suggested to be propagators of neurodegenerative protein spread, although some cargos are easier to envisage than others.
Of the neurodegenerative-associated proteins, only some are integral membrane proteins, that is, proteins inserted into lipid bilayers, rather than cytosolic. Sorting of proteins into intraluminal vesicles and thus exosomes is easier to envisage for membrane proteins, where tags such as ubiquitin regulate where they end up. So far, the presence of both Aβ 22 and PrPc 19 has in fact been shown in intraluminal vesicles, though this has not been demonstrated for other membrane proteins, such as alpha-synuclein and tau.
The mechanism whereby cytosolic proteins may be sorted to intraluminal vesicles/exosomes, however, is not clear. In order for cytosolic proteins to become concentrated in intraluminal vesicles, they would require positive incorporation and sorting, possibly by membrane-associated components on endosomes. All scientists can say is that there is evidence that this does in fact happen; cytosolic factors such as miRNAs are enriched in exosomes relative to cytosol, indicating that sorting must occur whereby certain miRNAs are concentrated and others are not 23.
The means by which disease-associated factors spread between cells remains poorly understood and exosomes would provide a means for such transmission. The presence of exosomal proteins, such as Alix, in association with Alzheimer’s senile plaques strengthens the circumstantial case for exosomes as a mediator in such spread. The hope is that having a means to regulate exosome release and spread may be useful in combatting some of these diseases but much more basic biology needs to be established before then.
Exosome therapy for stroke
Approximately, 16 million strokes occur worldwide each year, causing 6 million deaths and considerable disability, implying an enormous social, individual health, and economic burden 24. Due to this high incidence, strategies to promote stroke recovery are urgently needed. Neurorestorative therapies using stem cell based and stem cell derived exosomes hold promise as either stand‐alone or as combination treatments with pharmacological agents to improve stroke outcome in non‐diabetes mellitus and diabetes mellitus patients 3. Cell based and stem cell derived exosome therapy also has therapeutic benefits in other neurological diseases such as traumatic brain injury, which has been reviewed previously 25. Since diabetes mellitus stroke induces extensive neural and vascular damage, it is critical for therapeutic interventions to promote remodeling of the neurovascular unit, which fundamentally describes the structural and functional interactions between neurons, capillaries and glia in the brain. Pre‐clinical studies in animal models of stroke and diabetes mellitus stroke have shown that cell therapies have long treatment time windows ranging from several hours to days after stroke onset and improve neurological functional outcome by amplifying endogenous brain repair mechanisms such as neurovascular remodeling, white matter remodeling, and attenuating local and systemic inflammatory and immune responses 26.
Several types of stem/progenitor cells from different sources have been investigated in preclinical studies to test feasibility, efficacy, and mechanisms of therapeutic effects in stroke. There are a number of sources for stem cells such as mesenchymal stromal cells, human umbilical cord blood cells, induced pluripotent stem cells, neural stem cells and embryonic stem cells, with the advantages and disadvantages of each discussed elsewhere 27.
Transplanted stem cells and exosomes stimulate host brain parenchymal cells to generate a plethora of cytokines, growth factors and trophic factors which promote endogenous brain repair mechanisms while suppressing apoptotic signaling and inflammatory responses 28, as summarized in Figure 2. As a result, functional recovery following cell based and exosome therapy is often observed as early as several days after treatment. Employing exosomes as therapeutic agents has several advantages over cell therapy. Exosomes have no vascular obstructive effect, low risk of secondary microvascular thrombosis and have a low risk of tumor formation. Favoring clinical translation, a large quantity of exosomes can be derived from a small quantity of cells; exosomes are stable and can be stored; exosomes can pass the blood brain barrier (BBB); and exosomes do not elicit immune rejection. Exosomes mediate benefit by transferring genetic instructions, often via microRNA to concurrently stimulate and activate multiple restorative pathways 4. Therefore, by modifying microRNA content, exosomes can be programmed to target specific restorative and protective pathways within recipient and target cells and systemic administration of exosomes may be a means to deliver designer genetic instructions as well as the active components of cell‐based therapy to the central nervous system 28.
Research into new therapeutic approaches for stroke has determined that intravenous administration of mesenchymal stem cells (MSCs) is a good strategy to improve recovery by amplifying mechanisms implicated in brain plasticity. Recent studies have demonstrated the efficacy of mesenchymal stem cells in stroke, with no need for them to reach the area of brain injury. Although the mechanisms by which they mediate restorative effects are still unknown, the evidence suggests that mesenchymal stem cells might use specialised communication by sending and receiving biological information included in elements called exosomes. Exosomes are nanosized extracellular vesicles released into physical environments, and they have recently been suggested to mediate restorative stem cell effects. Moreover, after stroke, exosomes can also be synthesised and released from brain cells, passing through the blood-brain barrier (BBB), and can be detected in peripheral blood or in cerebrospinal fluid. Thus, exosomes could possibly be biomarkers that reflect pathological progress and promote stroke recovery.
Figure 2. Exosome therapy
Footnote: Mechanisms of cell‐based and exosome therapy induced neurorestorative effects after stroke in diabetic rodents.
Abbreviations: BBB = blood brain barrier; EC = endothelial cell; HUCBC = human umbilical cord blood cells; MSC = mesenchymal stromal cell; OL = oligodendrocyte.
[Source 3 ]Summary
Cells‐based and exosome therapies are powerful tools to promote endogenous brain repair mechanisms after stroke. Cell and exosome therapy using bone marrow mesenchymal stromal cells and human umbilical cord blood cells are promising treatment options for diabetes mellitus stroke with low ethical barriers, wide treatment time frames, and high translational feasibility. Some of the challenges of cell therapy such as finding matching donor, low yield and adverse effects such as thrombosis, may be overcome by employing exosomes for stroke treatment. Largely, exosome therapy appears to be safe without adverse effects; however, exosomes may facilitate intercellular membrane exchange and the spread of infectious agents like prions 29.
The content of exosomes consisting primarily of proteins, noncoding RNAs, and lipids vary depending on the donor cells and cell culturing and exosome harvesting conditions. Since the therapeutic efficacy of exosomes depend on the intercellular transfer of their content, and microRNAs appear to play an important role in mediating exosome function, optimization of donor cell cultures and characterization of exosome content, particularly that of the key microRNAs mediating therapeutic benefits are warranted. The effects of exosome therapy on immune system need to be fully understood. To facilitate clinical translation, high purity, low cost, and large scale exosome isolation techniques need to be developed.
Two clinical trials have reported therapeutic benefits of administering culture‐expanded autologous mesenchymal stromal cells in patients with ischemic stroke compared to placebo control group 30. Another clinical trial reports that stereotactic placement of modified mesenchymal stromal cells (SB623) at the margin of stroke in patients with chronic motor deficits at >6 months after their initial stroke was safe and improved clinical outcome at 12 months after stroke 31. However, the study groups of these trials were small and large clinical trials to evaluate the safety and efficacy of mesenchymal stromal cells (NCT01922908), adipose tissue derived mesenchymal stromal cells (NCT01678534), human umbilical cord blood cells (NCT02580019, NCT02397018, NCT03004976), mesenchymal stromal cell‐exosomes enriched by miR‐124 (NCT03384433) for treatment of stroke and intracerebral hemorrhage (NCT03371329) are still at a preliminary stage.
References- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3(5):447–450. doi:10.4161/cib.3.5.12339 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2974077
- Edgar JR. Q&A: What are exosomes, exactly?. BMC Biol. 2016;14:46. Published 2016 Jun 13. doi:10.1186/s12915-016-0268-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4906597
- Venkat P, Chopp M, Chen J. Cell-Based and Exosome Therapy in Diabetic Stroke. Stem Cells Transl Med. 2018;7(6):451–455. doi:10.1002/sctm.18-0014 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5980126
- Xin H, Wang F, Li Y et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b‐overexpressing multipotent mesenchymal stromal cells. Cell Transplant 2017;26:243–257.
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. doi: 10.1084/jem.183.3.1161
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594
- Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165(3):1259–65. doi: 10.4049/jimmunol.165.3.1259
- Lo Cicero A, Delevoye C, Gilles-Marsens F, Loew D, Dingli F, Guere C, et al. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun. 2015;6:7506. doi: 10.1038/ncomms8506
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea‐pig bone marrow and spleen cells. Cell Tissue Kinet 1970;3:393–403.
- Zuk PA, M Zhu, H Mizuno et al. Multilineage cells from human adipose tissue: Implications for cell‐based therapies. Tissue Eng 2001;7:211–228.
- Gronthos S, M Mankani, J Brahim et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2000;97:13625–13630.
- Kogler G, S Sensken, JA Airey et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 2004;200:123–135.
- Mead B, Tomarev S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl Med. 2017;6(4):1273–1285. doi:10.1002/sctm.16-0428 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5442835
- Zhang, Z.G., Buller, B. & Chopp, M. Exosomes — beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol 15, 193–203 (2019). https://doi.org/10.1038/s41582-018-0126-4
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596
- Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–21. doi: 10.1016/j.ccell.2014.09.005
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103(30):11172–7. doi: 10.1073/pnas.0603838103
- Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287(6):3842–9. doi: 10.1074/jbc.M111.277061
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101(26):9683–8. doi: 10.1073/pnas.0308413101
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–51. doi: 10.1523/JNEUROSCI.5699-09.2010
- Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett. 2007;428(1):43–6. doi: 10.1016/j.neulet.2007.09.024
- Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357
- Edgar JR, Willen K, Gouras GK, Futter CE. ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-beta accumulation. J Cell Sci. 2015;128(14):2520–8. doi: 10.1242/jcs.170233
- Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl Stroke Res. 2019 Jun;10(3):241-249. doi: 10.1007/s12975-018-0654-7. Epub 2018 Aug 13. https://doi.org/10.1007/s12975-018-0654-7
- Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res 2017;12:19–22.
- Chen J, Ning R, Zacharek A et al. MiR‐126 contributes to human umbilical cord blood cell‐induced neurorestorative effects after stroke in type‐2 diabetic mice. Stem Cells 2016;34:102–113.
- Venkat P, Shen Y, Chopp M et al. Cell‐based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology 2017. [Epub ahead of print].
- Xin H, Li Y, Cui Y et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 2013;33:1711–1715.
- Porto‐Carreiro I, Fevrier B, Paquet S et al. Prions and exosomes: From PrPc trafficking to PrPsc propagation. Blood Cells Mol Dis 2005;35:143–148.
- Lee JS, Hong JM, Moon GJ et al. A long‐term follow‐up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–1106.
- Steinberg GK, Kondziolka D, Wechsler LR et al. Clinical outcomes of transplanted modified bone marrow‐derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 2016;47:1817–1824.