Fetal hydantoin syndrome
Fetal hydantoin syndrome is a disorder that is caused by exposure of a fetus to phenytoin, a drug commonly prescribed for epilepsy 1. Phenytoin crosses the placenta in such a way that the developing fetus receives a much higher dose of the medication than the mother is taking (the drug is metabolized differently by the fetus). This is especially true when the medication is used during the first trimester of pregnancy. Despite these risks, seizure control during pregnancy is very important. Therefore, when a woman with epilepsy is planning a pregnancy, it is important for her to meet with both her neurologist and her obstetrician, before conception, to discuss the specific treatment to be used to control seizures while pregnant.
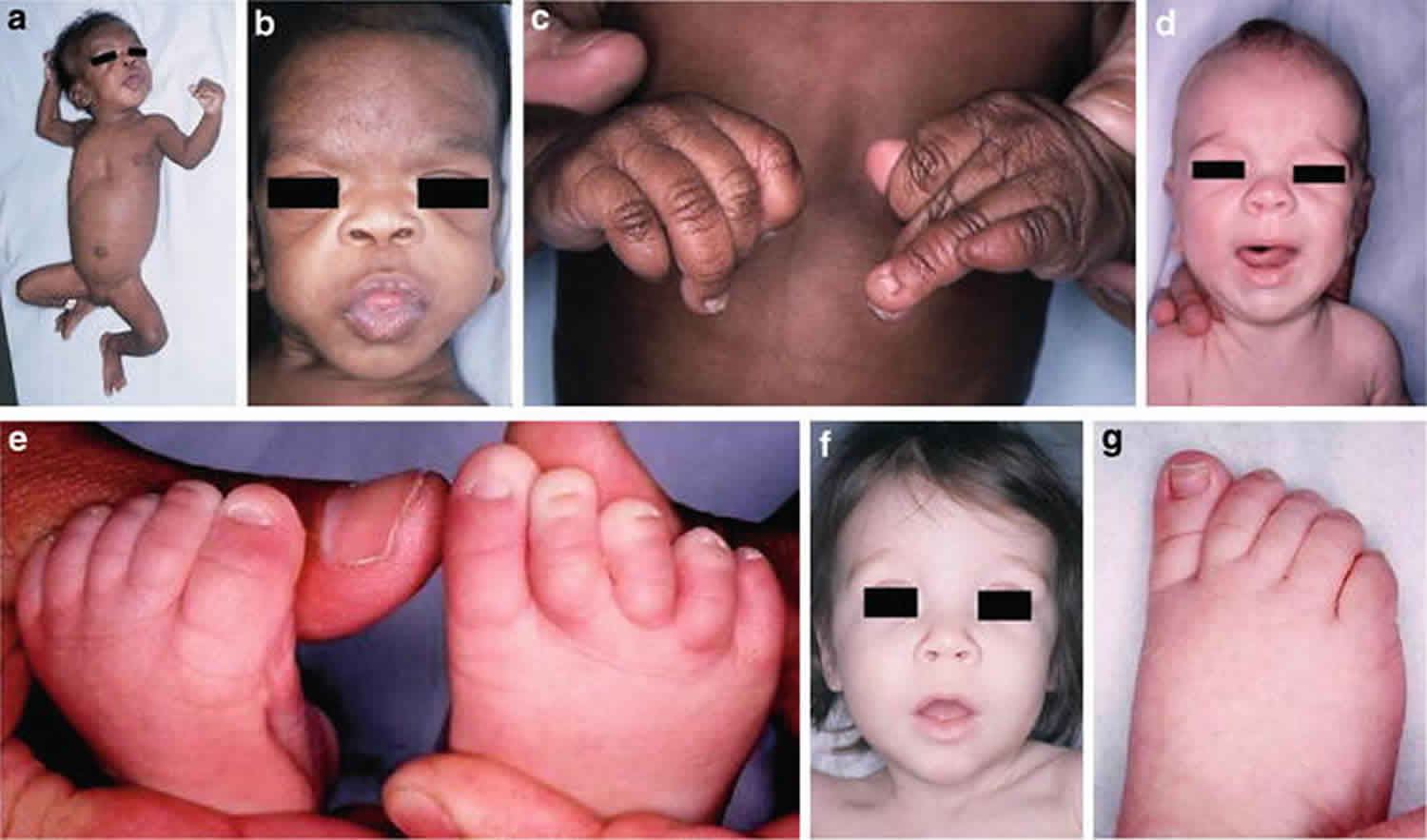
Not all infants exposed to phenytoin will be affected with the disorder. If phenytoin is taken by the mother in the first trimester, there is approximately a 5 to 10 percent chance that the baby could be born with a combination of birth defects known as the fetal hydantoin syndrome. Fetal hydantoin syndrome symptoms in affected individuals may include abnormalities of the skull and facial features, growth deficiencies, underdeveloped nails of the fingers and toes, and/or mild developmental delays. Other findings occasionally associated with this syndrome include cleft lip and palate, having an abnormally small head (microcephaly) and brain malformations with more significant developmental delays 1.
Fetal hydantoin syndrome affects males and females in equal numbers. The exact incidence and prevalence of the disorder is unknown.
Fetal hydantoin syndrome treatment may include surgery for cleft lip and palate and special education and related services for children with learning delays. Other treatment is symptomatic and supportive 1.
Fetal hydantoin syndrome causes
Maternal use of anti-seizure medications such as phenytoin, which is often used to treat epileptic seizures, can result in multiple effects on the developing embryo and fetus, including fetal hydantoin syndrome. The specific amount of phenytoin ingestion required to cause the disorder has not been determined. Phenytoin is often given with other anti-seizure drugs and other (adjunct) medications that may influence development of the disorder. Fetal hydantoin syndrome may be caused by a combination of specific genetic and environmental factors.
It is not known whether the adverse effects of phenytoin during fetal development are due to the drug itself or if they are caused by one of phenytoin’s by-products (metabolites). In addition, the potential role of other factors remains unclear, such as genetic influences that affect phenytoin metabolism or additional environmental factors (e.g. smoking). There have been reports in the medical literature that women with mutations in the methylenetetrahydrofolate reductase (MTHFR) gene are at an increased risk of having an infant with fetal hydantoin syndrome. Researchers believe that the protein product of this gene plays a role in the proper breakdown (metabolism) of phenytoin or one of its metabolites.
Another theory speculates that intermediate metabolites of phenytoin are responsible for its teratogenesis. These intermediate metabolites are free radicals that bind to DNA, proteins and lipids adversely affecting neurodevelopment. Genetic differences in formation of these free radicals, drug clearance, and repair mechanisms may explain different susceptibility across individuals.
Determining the precise, underlying reasons why fetal hydantoin syndrome develops, requires further research to discover the specific genetic and environmental factors that play a role in the development of the disorder.
Fetal hydantoin syndrome prevention
It is recommended that women be treated with a single anticonvulsant prior to conception and throughout pregnancy, since it appears that children exposed to multiple anticonvulsants may be at a greater risk for significant birth defects.
Also, it is recommended that women taking phenytoin take folic acid supplements, both before conception and during pregnancy as a preventive measure against malformations.
Counseling is recommended for women to discuss the risks that seizures prevent for both the developing fetus and the expectant mother as well as the risks to the developing fetus involved with taking anti-seizure medications during pregnancy.
Fetal hydantoin syndrome symptoms
There is a wide range in the nature and severity of characteristics associated with fetal hydantoin syndrome. Babies with fetal hydantoin syndrome may be born with some of the following health problems:
- Growth deficiency
- Developmental delay
- Cleft palate
- Certain facial characteristics
- Heart defects
- Genitourinary abnormalities
- Abnormalities of the fingers and nails
The specific symptoms and physical features associated with fetal hydantoin syndrome can vary greatly from one infant to another. Symptoms may not be noticeable at birth (congenital), but will become apparent as an affected child grows older.
Of infants born to women who used phenytoin during pregnancy, 10-30% are reported to show some of the characteristics associated with this syndrome. Few infants exposed only to phenytoin have all of the characteristic that have been reported 2.
Children with fetal hydantoin syndrome may be small at birth due to growth deficiency during development (prenatal growth deficiency), with increased hair on the body and face, and with poorly developed fingernails and toenails 1. Growth deficiency may be mild to moderate in severity and can continue during the newborn period (postnatal growth deficiency). They may also have poor muscle tone 1. Facial features that may be present with fetal hydantoin syndrome include a flat broad bridge of the nose; a short nose; an underdeveloped vertical groove in the center of the upper lip (philtrum); a large wide mouth; malformed ears and microcephaly. Features specific to the eyes may include down-slanted eyes; widely spaced eyes (hypertelorism); crossed eyes (strabismus); drooping eyelids (ptosis); and/or epicanthal folds (skin folds of the eyelid covering the inner corner of the eye). Affected infants often have a gap or cleft of missing tissue of the upper lip (cleft lip) and/or a gap or cleft of missing tissue on the roof of the mouth (cleft palate). Other features that have been reported include a short or webbed neck (pterygium colli) and low-set hair line 3. Growth deficiencies may include underdeveloped fingers and/or toes, , toes that resembled fingers (digitalized toes) and malformed, underdeveloped fingernails and toenails, as well as finger-like thumbs 1. An increased number of fingerprint arches have also been noted.
These features are often associated with growth delay and varying degrees of developmental delay such as learning to sit up or crawl (developmental delays). As affected children grow older, the developmental delays improve, but studies suggest that children may remain slightly behind unexposed siblings. The risk for an affected child to be neurologically impaired is estimated at 1 to 11 % (two to three times higher than for the general population). The risk of cleft lip and/or palate and heart defects is estimated to be about five times higher among exposed infants. Some case reports have suggested an increased risk for the occurrence of benign (noncancerous) or malignant (cancerous) tumors, such as neuroblastoma or other neonatal tumors (ependymoma, ectodermal tumors, Wilms tumor), but such an increased risk is not proven 3.
The neurological effects of phenytoin exposure in utero have not been clearly established by studies, which have demonstrated conflicting results. Borderline to mild intellectual disability has been reported in some cases, and some studies have suggested that children exposed to phenytoin in the womb have a greater risk of developing learning disabilities, particularly in verbal skills. Reports in the medical literature disagree as to the likelihood of infants with fetal hydantoin syndrome experiencing developmental delays or intellectual disability. The exact risk of these findings in these children is not fully understood. More research is necessary to determine the specific long-term risks in neurological development of infants and children exposed to phenytoin in utero.
Additional symptoms occurring with varying frequencies have been reported in the medical literature including congenital heart defects, cardiac rhythm disturbances, behavioral abnormalities such as attention deficit hyperactivity disorder, ocular defects including nearsightedness (myopia), joint laxity, kidney abnormalities, and inguinal and umbilical hernia. Inguinal hernia is when a portion of the intestines pushes through the muscular layers of the abdominal wall. An umbilical hernia is when the intestines or fatty tissue pushes through the area near the bellybutton.
How might fetal hydantoin syndrome affect individuals in adulthood?
As children with fetal hydantoin syndrome grow older, the developmental delays typically improve but studies suggest that they may remain slightly behind siblings that have not been exposed. The growth delays remain through life, as does the tendency to have increased body hair (hirsutism) 4.
Fetal hydantoin syndrome diagnosis
There is no diagnostic testing that can identify fetal hydantoin syndrome. A diagnosis is made clinically based upon identification of characteristic symptoms in an affected infant in conjunction with a history of phenytoin exposure during gestation. It is important to note that the majority of infants born to women who take phenytoin during pregnancy will not develop fetal hydantoin syndrome.
Fetal hydantoin syndrome treatment
The treatment of fetal hydantoin syndrome is directed toward the specific symptoms that are apparent in each individual. Treatment may require the coordinated efforts of a team of specialists. Pediatricians, oral surgeons, plastic surgeons, neurologists, psychologists, and other healthcare professionals may need to systematically and comprehensively plan an affect child’s treatment.
Infants with fetal hydantoin syndrome can benefit from early developmental intervention to ensure that affected children reach their potential. Affected children may benefit from occupational, physical and speech therapy. Various methods of rehabilitative and behavioral therapy may be beneficial. Additional medical, social and/or vocational services may be necessary. Psychosocial support for the entire family is essential as well.
When cleft lip and/or palate are present, the coordinated efforts of a team of specialists may be used to plan an affected child’s treatment and rehabilitation. Cleft lip may be surgically corrected. Generally surgeons repair the lip when the child is still an infant. A second surgery is sometimes necessary for cosmetic purposes when the child is older. Cleft palate may be repaired by surgery or covered by an artificial device (prosthesis) that closes or blocks the opening. Surgical repair can be carried out in stages or in a single operation, according to the nature and severity of the defect. The first palate surgery is usually scheduled during the toddler period.
References- Fetal Hydantoin Syndrome. https://rarediseases.org/rare-diseases/fetal-hydantoin-syndrome
- Seizure Disorders in Pregnancy. Fetal Congenital Abnormalities and Adverse Outcomes. https://emedicine.medscape.com/article/272050-overview#a2
- Fetal hydantoin syndrome. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=1912
- Fetal Hydantoin Syndrome. https://rarediseases.org/rare-diseases/fetal-hydantoin-syndrome/