Gastrointestinal hormones
Gastrointestinal hormones are classified as endocrines, paracrine, or neurocrine based on the method by which the molecule gets delivered to its target cell(s). Gastrointestinal hormones are produced by specialized enteroendocrine cells that are located in the epithelial layer throughout the whole gastrointestinal tract 1. All together, these cells form the bigger endocrine organ of the body and produce the largest number of hormones 2. Enteroendocrine cells are characterized as being either open- or closed-type. Open-type cells display direct contact with the gastrointestinal lumen and are able to sense molecules present in the luminal content, such as nutrients, which play a major regulatory role on hormone secretion. In contrast, closed-type cells lack connection with the lumen because their apical side is enclosed by epithelial cells. Closed-type cells are mainly regulated by molecules coming from the gastrointestinal capillaries or the autonomic activity. Interestingly, some enteroendocrine cells display long cytoplasmic basal processes that are filled with secretory granules and extend beneath the absorptive epithelium in order to facilitate the communication with adjacent cells 1.
Endocrine hormones are secreted from enteroendocrine cells directly into the bloodstream, passing from the portal circulation to the systemic circulation, before being delivered to target cells with receptor-specificity for the hormone. The five gastrointestinal hormones that qualify as endocrines are gastrin, cholecystokinin (CCK), secretin, glucose-dependent insulinotropic peptide (GIP), and motilin. Enteroendocrine cells also secrete paracrine hormones, but they diffuse through the extracellular space to act locally on target tissues and do not enter the systemic circulation. Two examples of paracrine hormones are somatostatin and histamine. Additionally, some hormones may operate via a combination of endocrine and paracrine mechanisms. These “candidate” hormones are glucagon-like peptide-1 (GLP-1), pancreatic polypeptide, and peptide tyrosine-tyrosine (PYY). Lastly, neurocrine hormones get secreted by postganglionic non-cholinergic neurons of the enteric nervous system. Three neurocrine hormones with significant physiologic functions in the gut are vasoactive intestinal peptide (VIP), gastrin release peptide (GRP), and enkephalins 3.
Gastrointestinal hormones undergo synthesis in specialized cells of the gastrointestinal tract mucosa known as enteroendocrine cells. Enteroendocrine cells are specialized endoderm-derived epithelial cells that originate from stem cells located at the base of intestinal crypts. These cells are dispersed throughout the gastrointestinal mucosa, sprinkled in between epithelial cells from the stomach all the way through to the colon. Also, these enteroendocrine cells possess hormone-containing granules concentrated at the basolateral membrane, adjacent to capillaries, that secrete their hormones via exocytosis in response to a wide range of stimuli related to food intake. These stimuli include small peptides, amino acids, fatty acids, oral glucose, distension of an organ, and vagal stimulation 4.
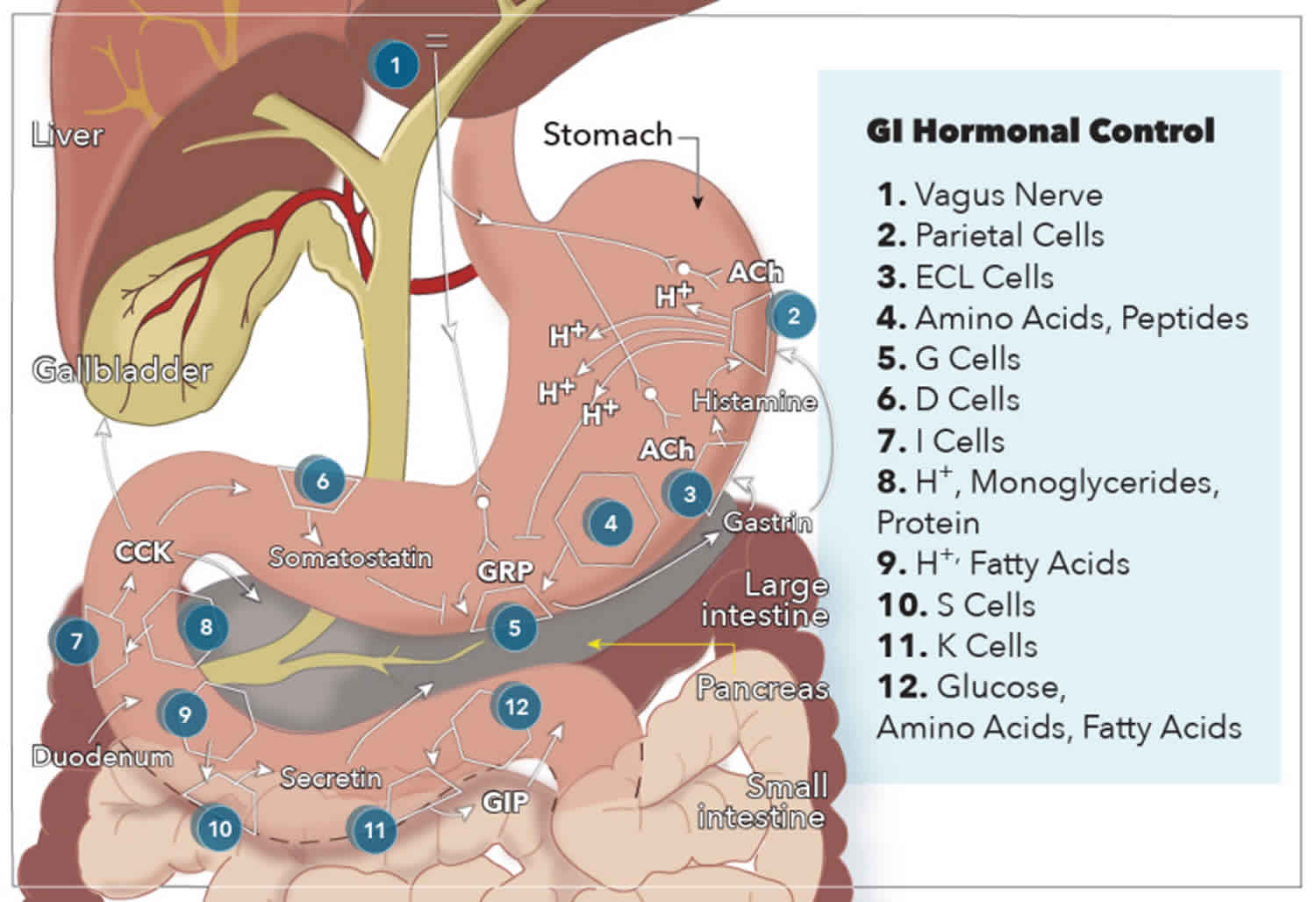
G cells secrete gastrin in the antrum of the stomach and the duodenum in response to the presence of breakdown products of protein digestion (such as amino acids and small peptides), distention by food, and vagal nerve stimulation via GRP. More specifically, phenylalanine and tryptophan are the most potent stimulators of gastrin secretion among the protein digestion products. The vagal nerve stimulation of gastrin secretion is unique because gastrin and motilin are the only hormones released directly by neural stimulation.
Cholecystokinin (CCK) is secreted from I cells in the duodenum and jejunum in response to acids and monoglycerides (but not triglycerides), as well as the presence of protein digestion products.
Secretin is secreted from S cells in the duodenum in response to H+ and fatty acids in the lumen. Specifically, a pH less than 4.5 signals arrival of gastric contents, which initiates the release of secretin.
Glucose-dependent insulinotropic peptide (GIP) is secreted by K cells in the duodenum and jejunum in response to glucose, amino acids, and fatty acids. Glucose-dependent insulinotropic peptide (GIP) is the only gastrointestinal hormone with a response to all three macronutrient types, and newer studies suggest that changes in intraluminal osmolarity may be what stimulates GIP secretion 5.
Glucagon-like peptide-1 (GLP-1) is also produced in the small intestine and secreted from L cells. The presence of hexose and fat stimulate its release. Pancreatic polypeptide and peptide YY are secreted by protein and fat, respectively, although their functions are still relatively unknown.
Peptide YY (PYY) is produced by L cells, which can also co-secrete CCK, GLP-1, glicentin, oxyntomodulin, and GIP to a varying extent 6. L cells are located in the distal intestine, mainly in the rectum, fol-lowed by the colon and ileum 7. Additionally, PYY is expressed in the endocrine pancreas and in some neurons of the central nervous system (CNS) 8.
Oxyntomodulin (OXM) is a 37-residue peptide derived from the post-translational processing of the pro-glucagon precursor. Oxyntomodulin is produced by L cells of the small intestine, and its distribution along the gastrointestinal tract mirrors that of GLP-1 9. Similarly to GLP-1, oxyntomodulin is also produced in the pancreas and the brain 10. Oxyntomodulin binds to both GLP1 receptor and glucagon receptors, although its affinity is almost two orders of magnitude lower than their native ligands 8. Furthermore, oxyntomodulin elicits a biased GLP1 receptor response as compared with GLP-1, as it has less preference toward the Gαs pathway as compared to the ERK pathway 11.
Pancreatic polypeptide (PP) is mainly produced in specialized pancreatic islets cells, called F cells, and to a lesser extent in the exocrine pancreas, colon, and rectum 12. As all members of the PP-fold family, PP binds to Y receptor (YR) showing the highest affinity for Y4R but also binding to Y5R and Y1R, all of which are expressed in the central nervous system (CNS) 13. The PP production is under vagal control 14, and its effects are also mainly mediated by the vagus nerve 15.
Somatostatin is a cyclic peptide that was first identified in 1973 in hypothalamic extracts 16. Somatostatin displays a widespread distribution in the body, being produced in the gastrointestinal tract, as well as in the brain, peripheral nerves, the pancreas, and the retina 17. Most of the circulating somatostatin derives from the gastrointestinal, where it is released from D cells as well as from intrinsic neurons located in the stomach, intestines, and pancreas 18. Interestingly, prosomatostatin can yield two bioactive products: a short 14-residue form (somatostatin-14) and a longer 28- residue form (somatostatin-28) that contain an extension at the N-terminus 17. Both somatostatin forms present a disulfide bond between cysteine residues at positions 1 and 12. Somatostatin-28 predominates in the intestinal mucosal cells, while somatostatin-14 predominates in the pancreas, the stomach, and neural tissues. Somatostatin binds to five G protein-coupled receptors, named SSTR1–5. SSTRs 1 to 4 bind somatostatin-14 and somatostatin-28 with similar affinity, while SSTR5 binds somatostatin-28 with five- to tenfold higher affinity 19. All SSTRs are coupled to the Gαi pathway and widely distributed in the body, although SSTR2 and SSTR5 are predominantly expressed in the peripheral tissues 18. Somatostatin secretion is increased by a combination of nutritional, hormonal, and neural signals after meals 18, and somatostatin levels can remain elevated postprandially for few hours, despite somatostatin in plasma displays a very short half-life 19.
Ghrelin is a 28-residue octanoylated peptide hor-mone discovered by Kojima in 1999 20. Ghrelin is the only known secreted peptide modified by an O-octanoylation. Ghrelin is predominantly synthesized by closed-type enteroendocrine ghrelin cells located within the gastric oxyntic fundic mucosa, previously referred to as P/D1-type cells in humans and A like-type cells in rodents 21. The synthesis of ghrelin is atypical as it involves the enzyme ghrelin O-acyltransferase (GOAT), which catalyzes ghrelin octanoylation. GOAT is present in the endoplasmic reticulum and acylates proghrelin before being translocated to the Golgi 22. In the Golgi complex, the octanoylated proghrelin is cleaved by PC1/3. Since the 28 residues of ghrelin sequence immediately follow the signal peptide, the N-terminal fragment generated by cleavage of proghrelin produces bioactive ghrelin, whereas the C-terminal fragment further yields a peptide named obestatin 23. Initial reports indicated that obestatin had anorexigenic effects; however, further studies could not confirm such observations, and its role on energy balance is still under debate 24. Ghrelin is also secreted as a des-octanoylated version, named des-acyl ghrelin which circulates even at higher levels than ghrelin because it is also produced in plasma by ghrelin deacylation 25. Some studies suggest that des-acyl ghrelin displays some actions in a growth hormone secretagogue receptor type 1a-independent manner; however, no specific receptor for des-acyl ghrelin has yet been reported, and, as a consequence, the role of this peptide remains uncertain 26.
Functions of gastrointestinal hormones
The two gastrointestinal hormone families discussed above are responsible for most of the regulation of gastrointestinal function. The main actions of the gastrin-CCK family and the secretin family of hormones are listed below.
Gastrin
- Stimulates H+ (acid) secretion by parietal cells in the stomach
- Trophic (growth) effects on the mucosa of the small intestine, colon, and stomach
- Inhibits the actions of secretin and glucose-dependent insulinotropic peptide (GIP)
- Inhibited by H+
Cholecystokinin (CCK)
- Contraction of the gallbladder with simultaneous relaxation of the sphincter of Oddi
- Inhibits gastric emptying
- Stimulates secretion of pancreatic enzymes: lipases, amylase, and proteases
- Secretion of bicarbonate from the pancreas
- Trophic effects on the exocrine pancreas and gallbladder
Secretin
- Inhibits gastrin, H+ secretion, and growth of stomach mucosa
- Stimulates biliary secretion of bicarbonate and fluid
- Secretion of bicarbonate from the pancreas
- Trophic effect on the exocrine pancreas
Glucose-dependent insulinotropic peptide (GIP)
- Stimulation of insulin secretion
- Induces satiety
- In large doses, decreases gastric acid secretion
- In large doses, decreases the motor activity of the stomach and therefore slows gastric emptying when the upper small intestine is already full of food products.
- Stimulates the activity of lipoprotein lipase in adipocytes
- Protects beta-cells of the pancreas from destruction by apoptosis
Glucagon-like peptide-1 (GLP-1)
- Decreases gastric emptying
- Induces satiety
- Increases sensitivity of pancreatic beta-cells to glucose.
Motilin
- Increases gastrointestinal motility by stimulating the “migrating motility” or “myoelectric complex” that moves through the fasting stomach and small intestines every 90 minutes. This cyclical release and action get inhibited by the ingestion of food. Not much is known about this peptide, except for this essential function.
Peptide tyrosine-tyrosine (PYY)
- Peptide YY (PYY) also known as peptide tyrosine tyrosine exerts its action through NPY receptors; it inhibits gastric motility and increases water and electrolyte absorption in the colon 27. PYY reduces gastric emptying and acid secretion, increases ileal absorption, and delays gall-bladder and pancreatic secretion 8. Thus, PYY is part of the feedback mechanism known as the “ileal break,” which is elicited by the presence of unabsorbed dietary components in the distal gastrointestinal tract and is aimed to slow proximal gastrointestinal motility in order to facilitate efficient digestion and uptake of nutrients 8. PYY may also act as a satiety signal 28 since PYY3–36 administration reduces food intake in both rodents and humans 28. This anorexigenic effect seems to occur via Y2R and involves the inhibition of both orexigenic ARC NPY neurons and vagal afferents fibers 29. PYY administration affects glycemia, but the physiological relevance of endogenous PYY on glucose homeostasis in humans is still a matter of debate 6.
Oxyntomodulin
- Oxyntomodulin inhibits gastric secretion, pancreatic exocrine secretion, and gastric emptying 30. Oxyntomodulin also inhibits food intake, in part, due to the suppression of ghrelin levels (Wren and Bloom 2007). Oxyntomodulin might be involved in long-term energy balance in humans since its repeated administration reduces body weight as a result of both reduction in food intake and increase in energy expenditure 30. Despite oxyntomodulin binds to both GLP1 receptor and glucagon receptors, it mainly inhibits appetite via the GLP1 receptor since co-administration of oxyntomodulin and a GLP1 receptor antagonist blocks the anorectic actions of oxyntomodulin 11. In contrast to GLP-1, oxyntomodulin is thought to inhibit food intake by acting directly at the ARC level 31. This possibility together with the additional effect of oxyntomodulin on glucagon receptors may explain the reason why this gastrointestinal hormone is as potent as GLP-1 to reduce food intake despite its lower affinity for the GLP1 receptor 7. Oxyntomodulin also exhibits incretin activity albeit modest compared to that of GLP-1 32.
Pancreatic polypeptide (PP)
- Pancreatic polypeptide (PP) is released after meals, in an amount proportional to the calories ingested, and in response to hypoglycemia, exercise, gastric distension, and elevations in gastrin, secretin, and motilin 30. Similarly to PYY, PP has a short half-life but its levels remain elevated for several hours after meals 15. In humans, peripheral administration of PP inhibits gastric emptying, exocrine pancreatic secretion, and gallbladder motility and acutely decreases food intake 14. In rodents, peripheral administration of PP also reduces food intake and gastric emptying, and these effects seem to occur via hypothalamic actions but also require the integrity of the vagal system 12. PP inhibits glucagon release, through the activation of Y1R in pancreatic α cells, and delays the postprandial rise in insulin 33.
Somatostatin
- Well known for its inhibitory physiological actions in multiple targets, somatostatin can function via endocrine, paracrine, or neurocrine pathways. Somatostatin is involved in a variety of effects, and it is unclear whether its effects on the energy balance are direct or indirect, as somatostatin inhibits the release of numerous hormones 19. Somatostatin inhibits the secretion of some gastrointestinal hormones (CCK, ghrelin, GLP-1, GIP, secretin), gastric emptying, and gastrointestinal motility while also inhibiting insulin and glucagon secretion from the pancreas 19. The effect of somatostatin on food intake is unclear as studies in rodents have been found inconsistent results 19. Still, the relevance of somatostatin in the regulation of energy balance remains uncertain 6.
Ghrelin
- Ghrelin levels display a surge before meals, decline after meals, and then increase gradually until the next preprandial peak 34. In addition, ghrelin levels are elevated in energy deficit conditions, such as fasting, malnutrition, anorexia nervosa, or cachexia 34. Ghrelin is the only peptide hormone known to increase food intake 21. In contrast to other gastrointestinal hormones that mainly impact on meal size and frequency, ghrelin seems also relevant for the long-term control of energy intake and body weight regulation as its plasma levels are inversely related to body adiposity in healthy-weight, obese, and weight-reduced humans 35. Ghrelin regulates both homeo-static and hedonic aspects of eating 35. Ghrelin may also contribute to glycemic control and, to a lesser extent, to the regulation of the gastrointestinal motility 6.
- Ghrelin administration increases gastric emptying; however, it is uncertain if endogenous patterns of ghrelin levels are sufficient to affect gastric emptying in humans 6. Pre-meal ghrelin levels correlate with hunger sensations and meal size, and administration of ghrelin to humans strongly increases food intake; still, the causal role of ghrelin in hunger remains unclear 34. Studies in rodents have shown that ghrelin plays a key role in the regulation of food intake 35. The main target of the orexigenic actions of ghrelin are the neuropeptide Y (NPY)-producing neurons of the arcuate nucleus (ARC) that express high levels of growth hormone secretagogue receptor type 1a 36. In contrast to rodents, intact vagal afferents seem to be required for ghrelin-induced food intake in humans 37. Additionally, ghrelin affects rewarding aspects of eating by enhancing the hedonic value of some foods via regulation located in several nuclei of the mesolimbic pathway 35. By acting at the central level, ghrelin also promotes adiposity 38. On the other hand, ghrelin affects the glycemic control by activating a variety of mechanisms that tend to increase glucose levels. Intravenous infusion of ghrelin reduces insulin levels in response to glucose infusion and increases growth hormone and cortisol levels, without any effect on glucagon or epinephrine levels 39. Studies in mice indicate that the insulin-inhibitory and glucagon-stimulatory effects of ghrelin involve a direct pancreatic action 40.
The release of gastrointestinal hormones is in response to input from G-protein-coupled receptors that detect changes in luminal contents. Some of these receptors only respond to selective luminal substances and subsequently release gastrointestinal hormones from their respective enteroendocrine cells through unknown mechanisms. Overall, gastrointestinal hormones manage a diverse set of actions in the body including:
- Contraction and relaxation of smooth muscle wall and sphincters
- Secretion of enzymes for digestion
- Secretion of fluid and electrolytes
- Trophic (growth) effects on tissues of gastrointestinal tract
- Regulating secretion of other gastrointestinal peptides (i.e., somatostatin inhibits secretion of all gastrointestinal hormones)
To better understand how these actions are carried out by gastrointestinal hormones, it is best to use gastrin’s functions as an example. Gastrin is an interesting hormone because it acts through two mechanisms that ultimately increase the secretion of gastric acid (hydrogen ions) into the stomach. The first mechanism involves gastrin binding to CCK-2 receptors on parietal cells, causing increased expression of K/H ATPase enzymes that are directly responsible for increased hydrogen ion secretion into the stomach. The second mechanism is mediated by enterochromaffin-like cells, which secrete histamine in response to activation by gastrin. Histamine then binds H2 receptors on nearby parietal cells, which further stimulates secretion of hydrogen ions. In addition to stimulating enterochromaffin-like cells to produce acid, gastrin also stimulates these parietal cells and enterochromaffin-like cells to proliferate.
Gastrin levels are routinely measured to diagnose Zollinger-Ellison syndrome, which is characterized by gastrin-producing tumors in the duodenum or pancreas that result in recurrent, refractory peptic ulcers and diarrhea. A gastrin level 10 times the upper limit of normal (approximately 1000 pg/mL) plus a gastric pH below 2.0 is diagnostic of Zollinger-Ellison syndrome 41.
Pentagastrin injection is used to diagnose carcinoid syndrome because administration will induce symptoms in patients who present with minimal or inconsistent symptoms. Additionally, the pentagastrin-stimulated calcitonin test for patients with normal calcitonin levels who have symptoms suspicious for medullary thyroid carcinoma.
Cholecystokinin is used for diagnostic radiography examinations of the gallbladder, called biliary scintigraphy, for patients with suspected gallbladder disease, and also has utility in manometry for patients with sphincter of Oddi dysfunction.
Secretin is used as an adjunct with magnetic resonance imaging (MRI) for diagnostic testing of pancreatic secretion and potential ductal obstruction.
References- Andreoli, María & De Francesco, Nicolas & Perello, Mario. (2018). Gastrointestinal Hormones Controlling Energy Homeostasis and Their Potential Role in Obesity. 10.1007/978-3-319-89506-2_7.
- Valle, J. D. (2014). Gastrointestinal hormones in the regulation of gut function in health and dis-ease. In Gastrointestinal anatomy and physiology(pp. 15–32). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781118833001.ch2
- Fothergill LJ, Furness JB. Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem. Cell Biol. 2018 Dec;150(6):693-702.
- Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016 May;28(5):620-30.
- Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010 Apr 22;1(1-2):8-23.
- Steinert, R. E., Feinle-Bisset, C., Asarian, L., Horowitz, M., Beglinger, C., & Geary, N. (2017). Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physi-ological roles in eating and Glycemia in health, obesity, and after RYGB. Physiological Reviews, 97(1), 411–463. https://doi.org/10.1152/physrev.00031.2014
- Wynne, K., & Bloom, S. R. (2006). The role of oxynto-modulin and peptide tyrosine-tyrosine (PYY) in appe-tite control. Nature Clinical Practice. Endocrinology & Metabolism, 2(11), 612–620. https://doi.org/10.1038/ncpendmet0318
- Spreckley, E., & Murphy, K. G. (2015). The L-cell in nutritional sensing and the regulation of appetite. Frontiers in Nutrition, 2, 23. https://doi.org/10.3389/fnut.2015.00023
- Holst, J. J. (2007). The physiology of glucagon-like pep-tide 1. Physiological Reviews, 87(4), 1409–1439. https://doi.org/10.1152/physrev.00034.2006
- Stanley, S., Wynne, K., & Bloom, S. (2004). Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. American Journal of Physiology Gastrointestinal and Liver Physiology, 286(5), G693–G697. https://doi.org/10.1152/ajpgi.00536.2003
- Pocai, A. (2014). Action and therapeutic potential of oxyntomodulin. Molecular Metabolism, 3(3), 241–251. https://doi.org/10.1016/j.molmet.2013.12.001
- Khandekar, N., Berning, B. A., Sainsbury, A., & Lin, S. (2015). The role of pancreatic polypeptide in the regulation of energy homeostasis. Molecular and Cellular Endocrinology, 418(Pt 1), 33–41. https://doi.org/10.1016/j.mce.2015.06.028
- Huda, M. S. B., Wilding, J. P. H., & Pinkney, J. H. (2006). Gut peptides and the regulation of appe-tite. Obesity Reviews, 7(2), 163–182. https://doi.org/10.1111/j.1467-789X.2006.00245.x
- Lean, M. E., & Malkova, D. (2016). Altered gut and adi-pose tissue hormones in overweight and obese indi-viduals: Cause or consequence? International Journal of Obesity, 40(4), 622–632. https://doi.org/10.1038/ijo.2015.220
- Choudhury, S. M., Tan, T. M., & Bloom, S. R. (2016). Gastrointestinal hormones and their role in obe-sity. Current Opinion in Endocrinology, Diabetes, and Obesity, 23(1), 18–22. https://doi.org/10.1097/MED.0000000000000216
- Brazeau, P., Vale, W., Burgus, R., Ling, N., Butcher, M., Rivier, J., & Guillemin, R. (1973). Hypothalamic polypeptide that inhibits the secretion of immunore-active pituitary growth hormone. Science, 179(4068), 77–79.
- Kumar, U., & Grant, M. (2010). Somatostatin and somatostatin receptors. Results and Problems in Cell Differentiation, 50, 137–184. https://doi.org/10.1007/400_2009_29
- Low, M. J. (2004). Clinical endocrinology and metabo-lism. The somatostatin neuroendocrine system: Physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Practice & Research Clinical Endocrinology & Metabolism, 18(4), 607–622. https://doi.org/10.1016/j.beem.2004.08.005
- Rai, U., Thrimawithana, T. R., Valery, C., & Young, S. A. (2015). Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacology & Therapeutics, 152, 98–110. https://doi.org/10.1016/j.pharmthera.2015.05.007
- Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., & Kangawa, K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature, 402(6762), 656–660. https://doi.org/10.1038/45230
- Kojima, M., & Kangawa, K. (2010). Ghrelin: More than endogenous growth hormone secretagogue. Annals of the New York Academy of Sciences, 1200, 140–148. https://doi.org/10.1111/j.1749-6632.2010.05516.x
- Gutierrez, J. A., Solenberg, P. J., Perkins, D. R., Willency, J. A., Knierman, M. D., Jin, Z., Witcher, D. R., Luo, S., Onyia, J. E., & Hale, J. E. (2008). Ghrelin octanoylation mediated by an orphan lipid transferase. Proceedings of the National Academy of Sciences of the United States of America, 105(17), 6320–6325. https://doi.org/10.1073/pnas.0800708105
- Takahashi, T., Ida, T., Sato, T., Nakashima, Y., Nakamura, Y., Tsuji, A., & Kojima, M. (2009). Production of n-octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghre-lin O-acyltransferase, as well as n-octanoic acid. Journal of Biochemistry, 146(5), 675–682. https://doi.org/10.1093/jb/mvp112
- Gourcerol, G., Coskun, T., Craft, L. S., Mayer, J. P., Heiman, M. L., Wang, L., Million, M., St-Pierre, D. H., & Tache, Y. (2007). Preproghrelin-derived pep-tide, obestatin, fails to influence food intake in lean or obese rodents. Obesity (Silver Spring), 15(11), 2643–2652. https://doi.org/10.1038/oby.2007.316
- De Vriese, C., Gregoire, F., Lema-Kisoka, R., Waelbroeck, M., Robberecht, P., & Delporte, C. (2004). Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology, 145(11), 4997–5005. https://doi.org/10.1210/en.2004-0569
- Callaghan, B., & Furness, J. B. (2014). Novel and conven-tional receptors for ghrelin, desacyl-ghrelin, and phar-macologically related compounds. Pharmacological Reviews, 66(4), 984–1001. https://doi.org/10.1124/pr.113.008433
- Liu CD, Aloia T, Adrian TE, et al. Peptide YY: a potential proabsorptive hormone for the treatment of malabsorptive disorders. Am Surg. 1996;62(3):232-236.
- Manning, S., & Batterham, R. L. (2014). The role of gut hormone peptide YY in energy and glucose homeostasis: Twelve years on. Annual Review of Physiology, 76, 585–608. https://doi.org/10.1146/annurev-physiol-021113-170404
- Stadlbauer, U., Woods, S. C., Langhans, W., & Meyer, U. (2015). PYY3-36: Beyond food intake. Frontiers in Neuroendocrinology, 38, 1–11. https://doi.org/10.1016/j.yfrne.2014.12.003
- Field, B. C. T., Wren, A. M., Cooke, D., & Bloom, S. R. (2008). Gut hormones as potential new targets for appetite regulation and the treatment of obesity. Drugs, 68(2), 147–163.
- Wren, A. M., & Bloom, S. R. (2007). Gut hormones and appetite control. Gastroenterology, 132(6), 2116–2130. https://doi.org/10.1053/j.gastro.2007.03.048
- Du, X., Kosinski, J. R., Lao, J., Shen, X., Petrov, A., Chicchi, G. G., Eiermann, G. J., & Pocai, A. (2012). Differential effects of oxyntomodulin and GLP-1 on glucose metabolism. American Journal of Physiology. Endocrinology and Metabolism, 303(2), E265–E271. https://doi.org/10.1152/ajpendo.00142.2012
- Aragon, F., Karaca, M., Novials, A., Maldonado, R., Maechler, P., & Rubi, B. (2015). Pancreatic poly-peptide regulates glucagon release through PPYR1 receptors expressed in mouse and human alpha-cells. Biochimica et Biophysica Acta, 1850(2), 343–351. https://doi.org/10.1016/j.bbagen.2014.11.005
- Cummings, D. E. (2006). Ghrelin and the short- and long-term regulation of appetite and body weight. Physiology & Behavior, 89(1), 71–84. https://doi.org/10.1016/j.physbeh.2006.05.022
- Perello, M., & Zigman, J. M. (2012). The role of ghre-lin in reward-based eating. Biological Psychiatry, 72(5), 347–353. https://doi.org/10.1016/j.biopsych.2012.02.016
- Cabral, A., Portiansky, E., Sanchez-Jaramillo, E., Zigman, J. M., & Perello, M. (2016). Ghrelin acti-vates hypophysiotropic corticotropin-releasing fac-tor neurons independently of the arcuate nucleus. Psychoneuroendocrinology, 67, 27–39. https://doi.org/10.1016/j.psyneuen.2016.01.027
- Arnold, M., Mura, A., Langhans, W., & Geary, N. (2006). Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(43), 11052–11060. https://doi.org/10.1523/JNEUROSCI.2606-06.2006
- Al Massadi, O., Lopez, M., Tschop, M., Dieguez, C., & Nogueiras, R. (2017). Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends in Neurosciences.https://doi.org/10.1016/j.tins.2016.12.003
- Tong, J., Prigeon, R. L., Davis, H. W., Bidlingmaier, M., Tschop, M. H., & D’Alessio, D. (2013). Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. The Journal of Clinical Endocrinology and Metabolism, 98(6), 2536–2543. https://doi.org/10.1210/jc.2012-4162
- Kurashina, T., Dezaki, K., Yoshida, M., Sukma Rita, R., Ito, K., Taguchi, M., Miura, R., Tominaga, M., Ishibashi, S., Kakei, M., & Yada, T. (2015). The beta-cell GHSR and downstream cAMP/TRPM2 signal-ing account for insulinostatic and glycemic effects of ghrelin. Scientific Reports, 5, 14041. https://doi.org/10.1038/srep14041
- Metz DC, Cadiot G, Poitras P, Ito T, Jensen RT. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int J Endocr Oncol. 2017;4(4):167-185.