Germinal matrix hemorrhage
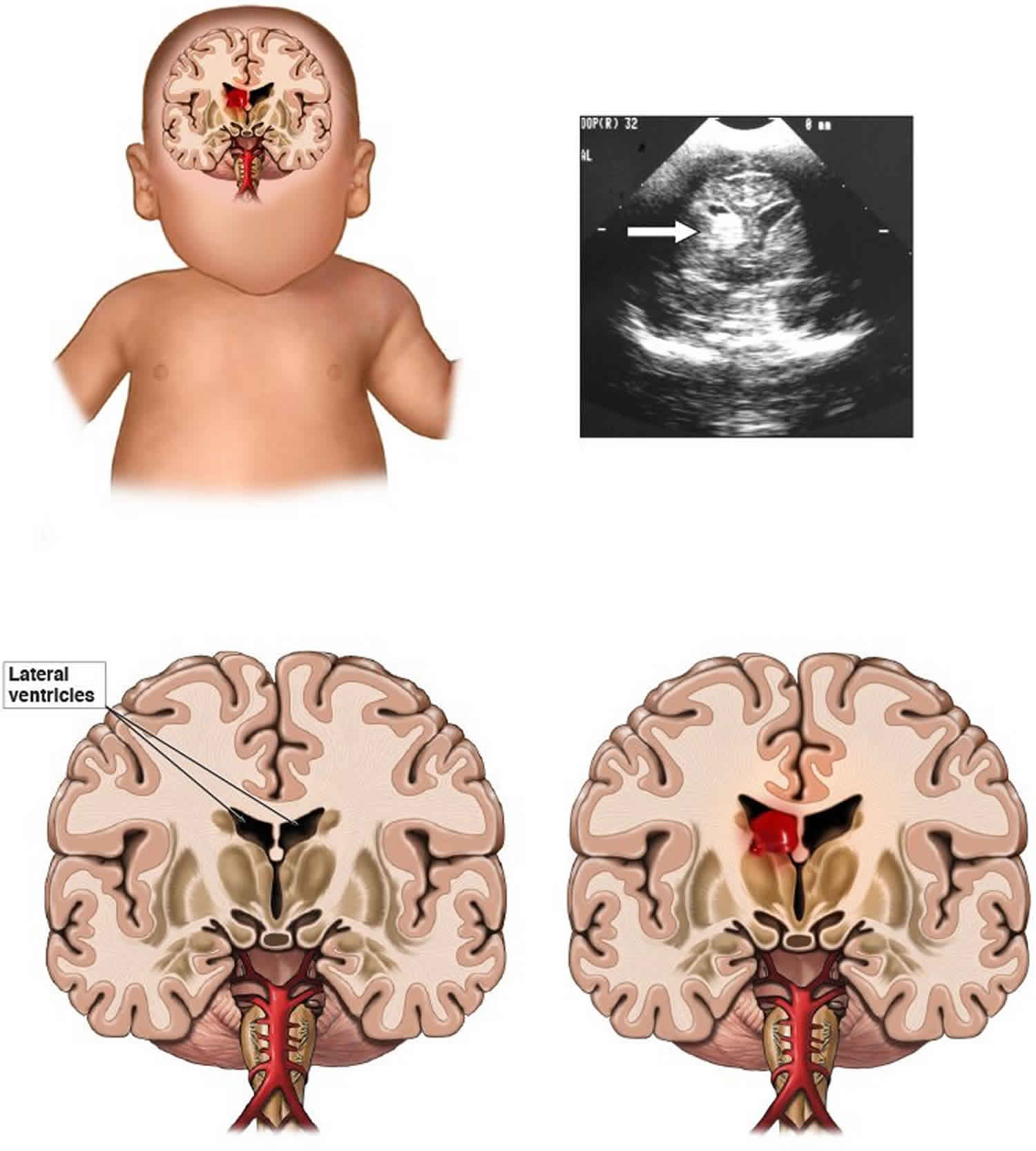
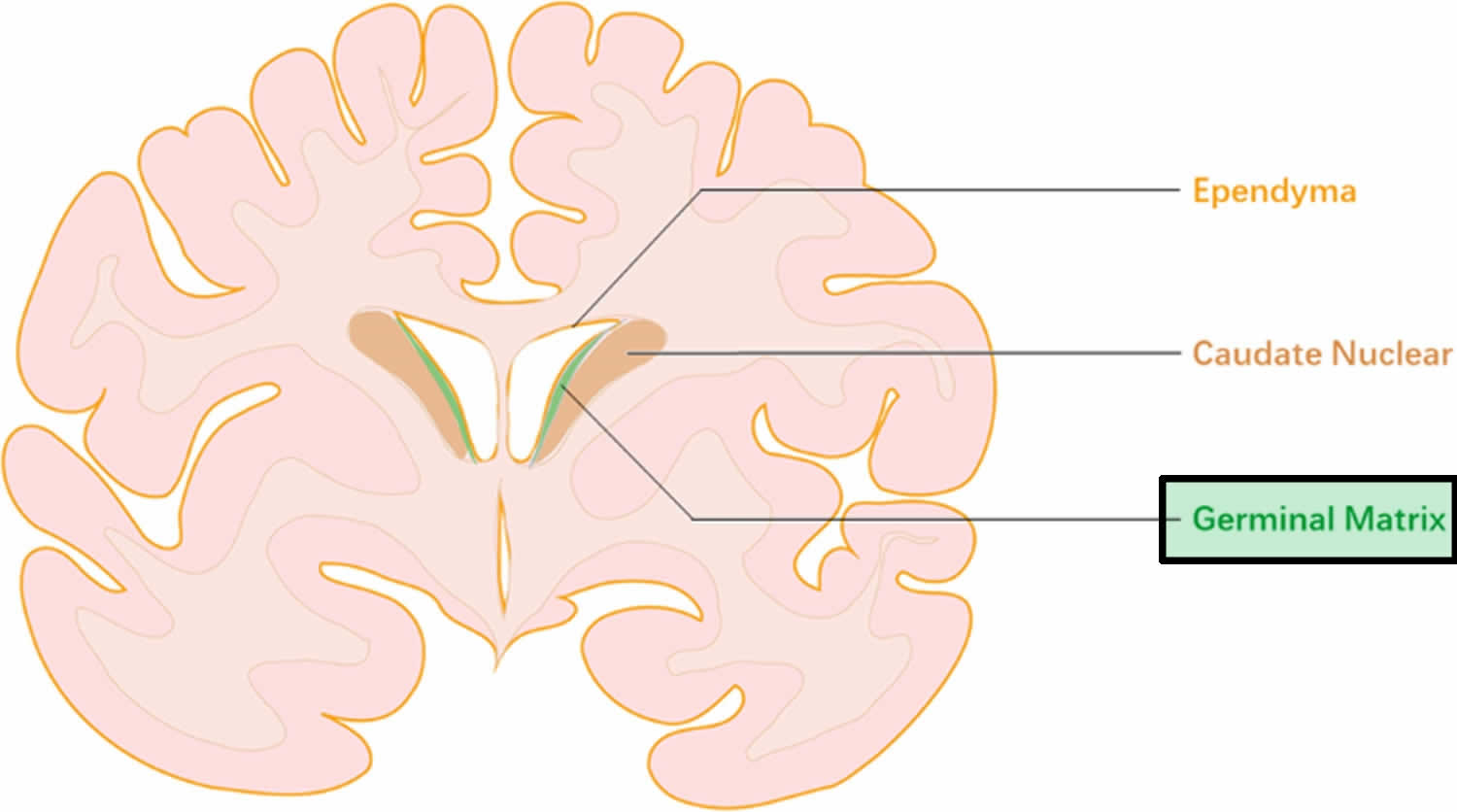
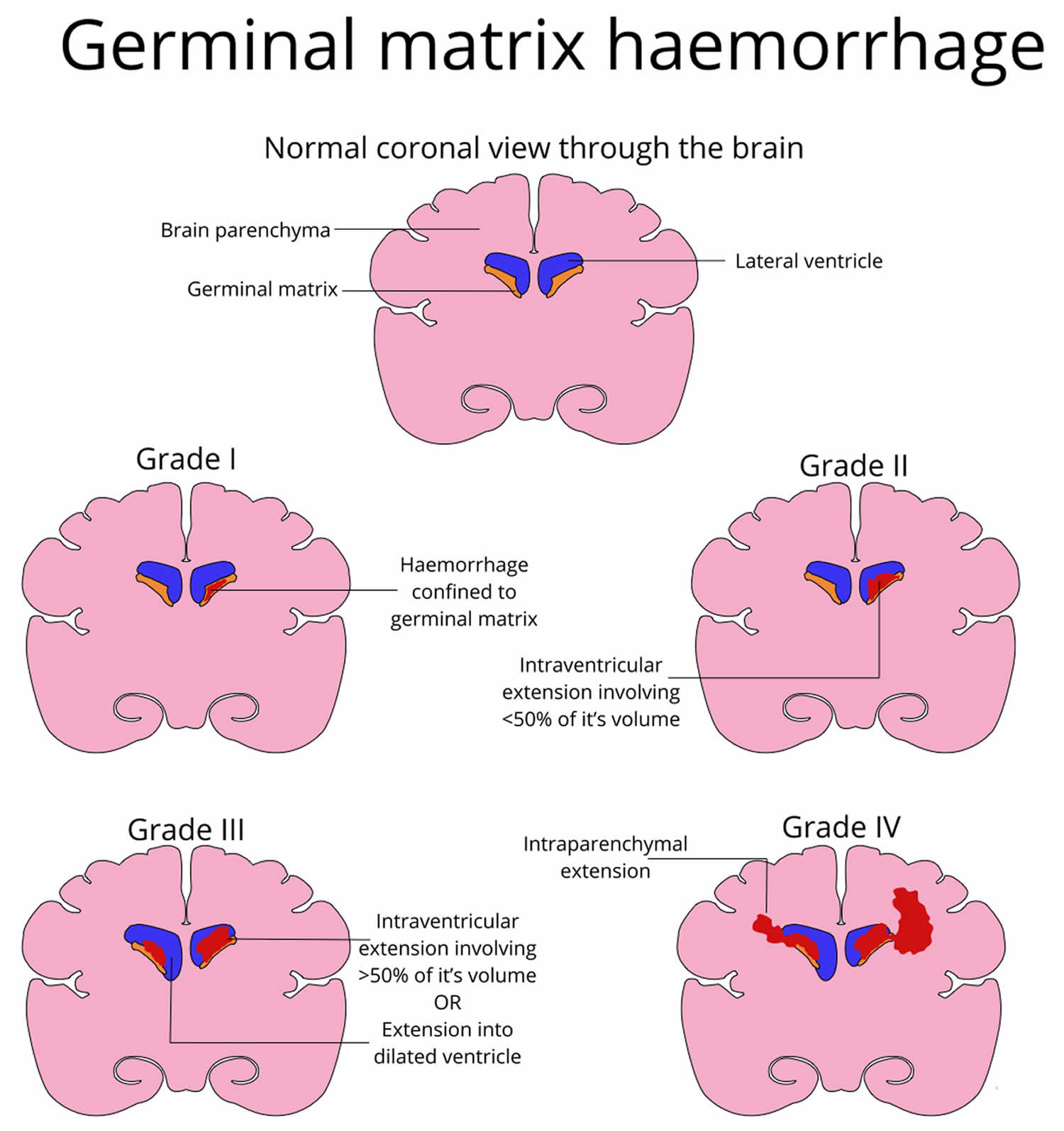
Germinal matrix also called subependymal germinal matrix, is a thick cellular layer of immature cells or neuroblasts (neuronal and glial precursors) under the ependymal lining of the ventricles, which is located above the caudate nucleus in the floor of the lateral ventricle and caudothalamic groove, where neuroblasts migrate from between 10 and 20 weeks, and becomes the source of glioblasts in the developing brain. Usually germinal matrix involutes after 32 weeks of gestation. Germinal matrix is a very vascular structure containing a rich network of fragile thin-walled blood vessels that is sensitive to ischemia which may lead to hemorrhage (see Figure 1). Germinal matrix hemorrhage is most frequent before 35 weeks gestation and is typically seen in very low birth-weight (<1500g) premature infants,because they lack the ability for auto regulation of cerebral blood flow. Increased arterial blood pressure in these blood vessels leads to rupture and hemorrhage into germinal matrix. Germinal matrix hemorrhage starts usually between the thalamus and the caudate nucleus, adjacent to the foramina of Monro, and is frequently bilateral. If it is large, it ruptures into the ventricles, flooding the lateral, third, and fourth ventricles (intraventricular hemorrhage – IVH). Blood then exits through the foramina of Luschka, causing subarachnoid hemorrhage. Thick clots along the ventral aspect of the brain stem may block the foramina of Luschka. Grading of germinal matrix hemorrhage depends on its ventricular or parenchymal extension. Four grades of germinal matrix hemorrhage are distinguished by imaging: grade 1 (confined to the germinal matrix), grade 2 (intraventricular hemorrhage without ventricular dilatatation, grade 3 (intraventricular hemorrhage with ventricular dilatation), and gade 4 (germinal matrix hemorrhage with intraventricular rupture and hemorrhage into the surrounding white matter or intraparenchymal hemorrhage). Large, bilateral intraventricular hemorrhage causes fatal acute distention of the ventricles or exsanguination into the ventricles and subarachnoid space. Patients surviving large intraventricular hemorrhage often develop hydrocephalus due to clots or gliosis of the aqueduct and from obliteration of the foramina of Luschka and subarachnoid space by clots and the fibrous tissue that develops from their organization.
A common lesion that characterizes the neuropathology of germinal matrix hemorrhage-intraventricular hemorrhage is bleeding into the subependymal germinal matrix, with or without subsequent rupture into the lateral ventricle (see the images below). Complications of germinal matrix hemorrhage-intraventricular hemorrhage include germinal matrix destruction, periventricular hemorrhagic infarction with subsequent encephalomalacia, and posthemorrhagic hydrocephalus 1.
Germinal matrix hemorrhage and intraventricular hemorrhage are the most common and most important neurologic injuries in premature infants 2. Germinal matrix hemorrhage is primarily associated with prematurity and intrinsic weakness of germinal matrix vasculature. The brain of a premature infant lacks the ability to autoregulate cerebral blood pressure; thus, fluctuations in cerebral blood pressure and flow can rupture the primitive germinal matrix vessels or lead to infarction of the metabolically active germinal matrix. The damage can extend into the periventricular white matter, resulting in significant neurologic complications 3. Germinal matrix hemorrhage usually develops a few hours after birth, but it can occur at any time, including prenatally.
Approximately 50–75% of preterm survivors with intraventricular hemorrhage develop cerebral palsy, mental retardation, and/or hydrocephalus 4. Approximately, a quarter of non-disabled survivors develop psychiatric disorders and problems with executive function 5. According to the U.S. Census Bureau and the NICHD Neonatal Research Network, over 3600 new cases of mental retardation each year are children who were born premature and suffered intraventricular hemorrhage 6. Hence, intraventricular hemorrhage continues to be a major problem of premature infant in modern neonatal intensive care units worldwide and its resultant neurologic and psychiatric consequences continue to be a major public health concern worldwide.
In the United States, about 12,000 premature infants develop intraventricular hemorrhage every year 7. The incidence of intraventricular hemorrhage in very low birth weight infants (<1500g) has declined from 40–50% in the early 1980s to 20% in the late 1980s 8. However, in the last two decades the occurrence of intraventricular hemorrhage has remained almost stationary 9. In extremely premature infants weighing 500–750g, intraventricular hemorrhage occurs in about 45% of neonates 10.
Antenatal glucocorticoid (steroid) exposure remains the most effective means of preventing germinal matrix hemorrhage. Therapies targeted to enhance the stability of the germinal matrix vasculature and minimize fluctuation in the cerebral blood flow might lead to more effective strategies in preventing germinal matrix hemorrhage.
Germinal matrix hemorrhage key points
- Pathogenesis of germinal matrix hemorrhage or intraventricular hemorrhage is ascribed to the intrinsic weakness of germinal matrix vasculature and to the fluctuation in the cerebral blood flow.
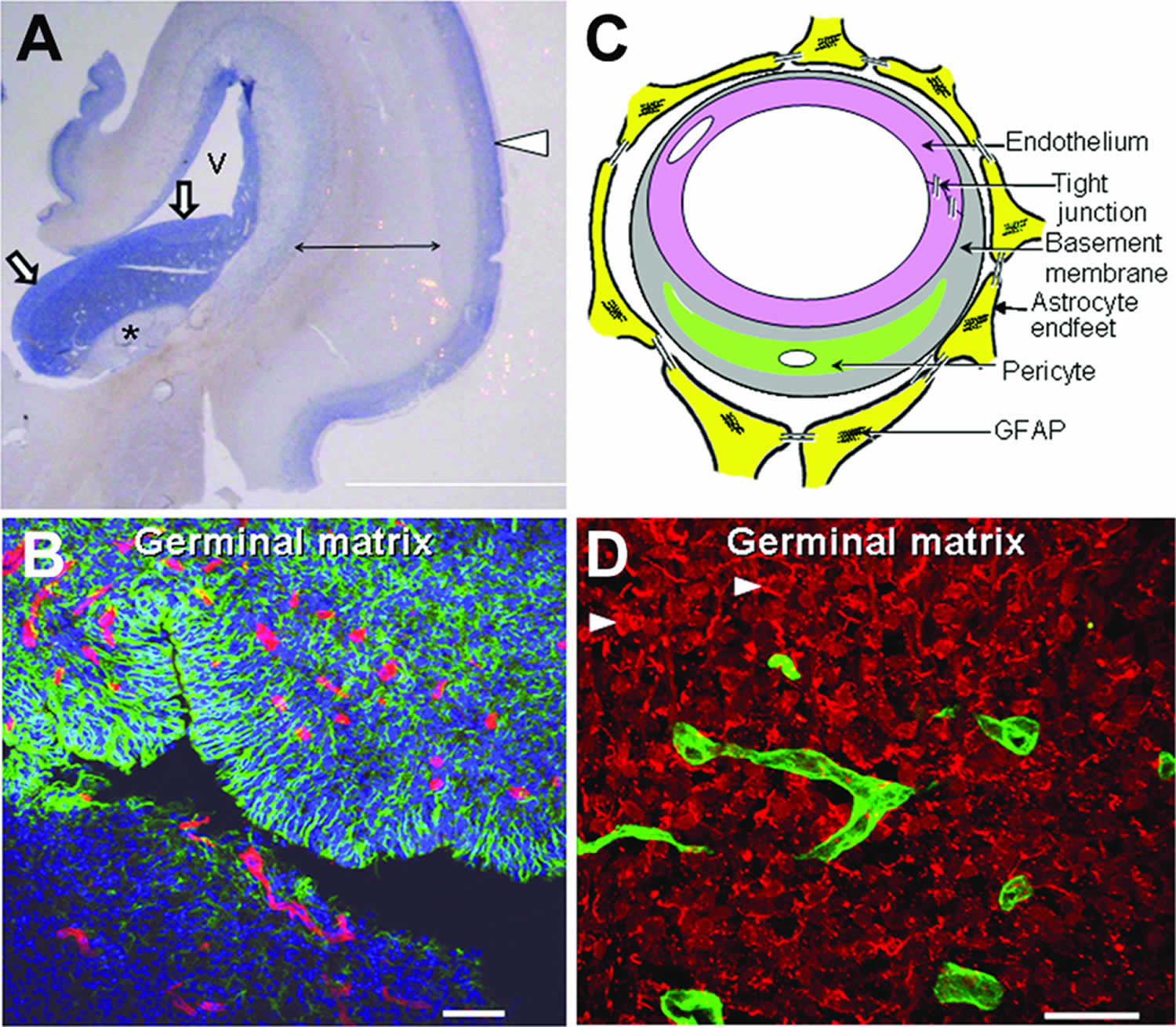
- The germinal matrix displays accelerated angiogenesis that orchestrates formation of nascent vessels that lack pericytes, display immature basal lamina low in fibronectin, and have astrocyte end-feet coverage deficient in glial fibrillary acidic protein. These morphological and molecular factors contribute to the fragility of the germinal matrix vasculature.
- The fluctuations in the cerebral blood flow is attributed to the cardiorespiratory and hemodynamic instability frequently associated with extremely premature infants, including hypotension, hypoxia, pneumothorax, patent ductus arteriosus, and others.
- Prenatal glucocorticoids have emerged as the most effective intervention to prevent intraventricular hemorrhage. Therapies designed to enhance the stability of the germinal matrix vasculature and reduce fluctuation of cerebral blood flow could lead to strategies that are more effective in preventing intraventricular hemorrhage.
Figure 1. Germinal matrix
Figure 2. Morphology of germinal matrix
Footnote: A) Representative cresyl violet staining of coronal section of the right-sided cerebral hemisphere of a 20-week fetus. Note cortical plate (arrowhead), white matter (arrow with 2 pointers), germinal matrix (arrow head), caudate nucleus (asterisk), and lateral ventricle (indicated by “v”). Germinal matrix (violet staining) surrounds the whole ventricle, but is most conspicuous on the head of caudate nucleus. Scale bar, 0.5 cm. B) Representative immunofluorescence of cryosection from germinal matrix of a 24 week premature infant labeled with DAPI (blue), glial fibrillary acidic protein (GFAP) (green) and CD34 (red). Note: germinal matrix is highly vascular (vascular endothelium in red) and enriched with GFAP (+) glial cells (green). C) Coronal brain section was double labeled with doublecortin (red, labels neuronal precursors) and CD34 (green, labels endothelium) specific antibodies. Note doublecortin (+) neuronal precursor cells are abundantly present in the germinal matrix. Scale bar; 50 (B) and 20µm (C). D) Schematic drawing of the blood brain barrier in cross section showing endothelium, endothelial tight junction, basal lamina, pericyte and astrocyte endfeet.
[Source 11 ]Germinal matrix hemorrhage grading
Grading of germinal matrix hemorrhage has taken several forms over the years. The most commonly used system is the sonographic grading system proposed by Burstein, Papile et al. Classification 12
Germinal matrix hemorrhage-intraventricular hemorrhage grading system:
- Grade 1: Germinal matrix hemorrhage only (restricted to subependymal region), which is seen in the caudothalamic groove. Overall good prognosis 13
- Grade 2: Germinal matrix hemorrhage + intraventricular hemorrhage typically filling less than 50% of the volume of the ventricle, normal ventricle size. Overall good prognosis 13
- Grade 3: Germinal matrix hemorrhage + intraventricular hemorrhage + ventricular expansion ~20% mortality
- Grade 4: Germinal matrix hemorrhage-intraventricular hemorrhage + intraparenchymal hemorrhage ~ 90% mortality 13. It was initially postulated that grade 4 intraventricular hemorrhage represented parenchymal extension of ventricular hemorrhage. It is now recognized that grade 4 intraventricular hemorrhage represents parenchymal hemorrhage secondary to venous infarction caused by compression of deep terminal veins by an expanded ventricle filled with blood.
A mnemonic to remember the radiological grading of germinal matrix hemorrhage is:
- CV²P
Mnemonic
It can be read as a central venous pressure
- C: limited to the caudothalamic groove/ germinal matrix (grade 1 germinal matrix hemorrhage)
- V: expansion into ventricles less than 50% (grade 2 germinal matrix hemorrhage)
- V: dilated ventricles (grade 3 germinal matrix hemorrhage)
- P: parenchymal hemorrhage (grade 4 germinal matrix hemorrhage)
Figure 3. Germinal matrix hemorrhage grading
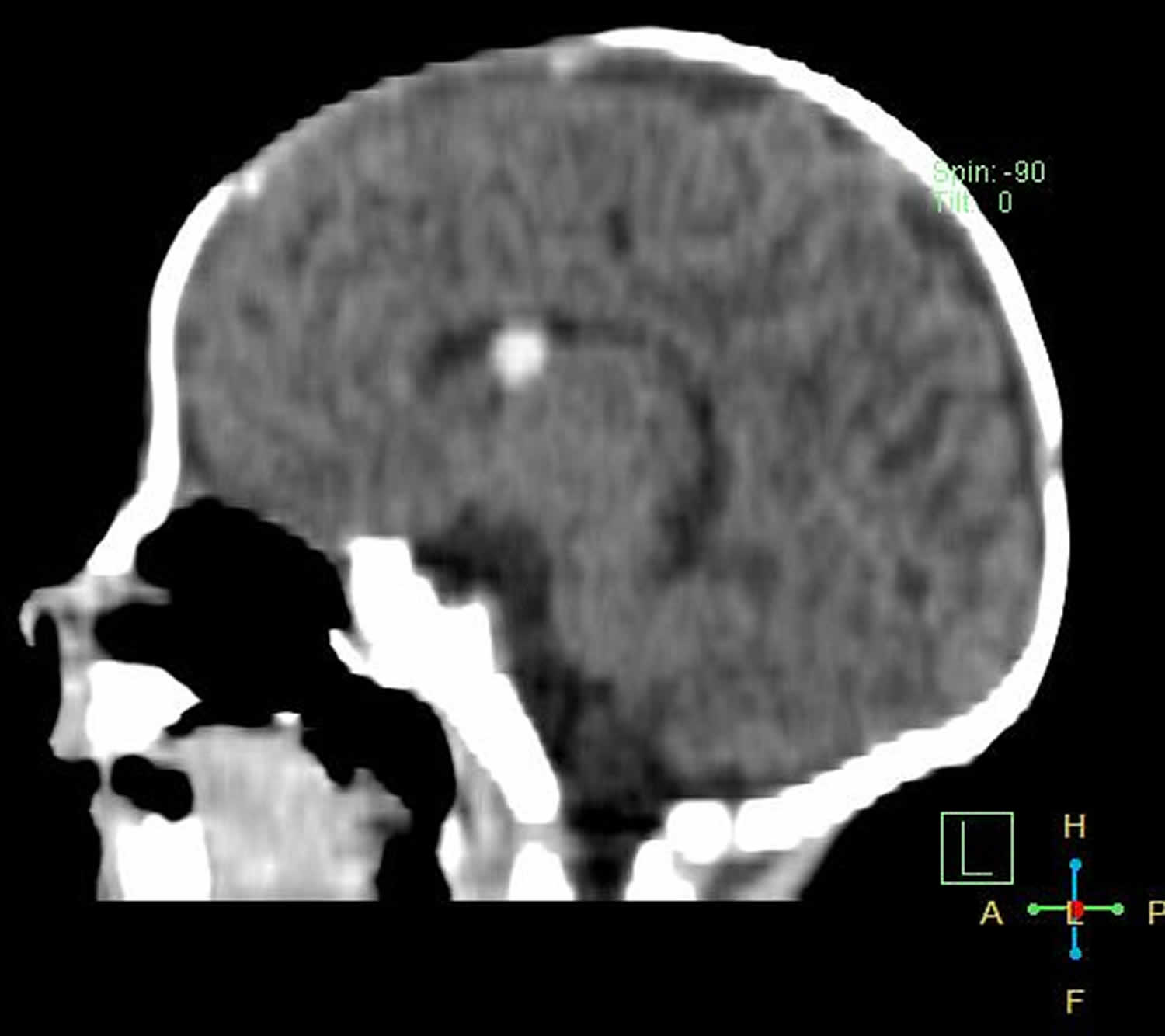
Figure 4. Grade 1 germinal matrix hemorrhage
Footnote: Preterm baby ( 31 weeks gestation ) with poor sucking and weak crying. Left subependymal oval shape hematoma with averagey density of 65 HU seen adjacent to foramen of Monroe. No evidence of ventricular dilatation or intraventricular extension. Another smaller subepedymal hematoma seen at trigone of right lateral ventricle.
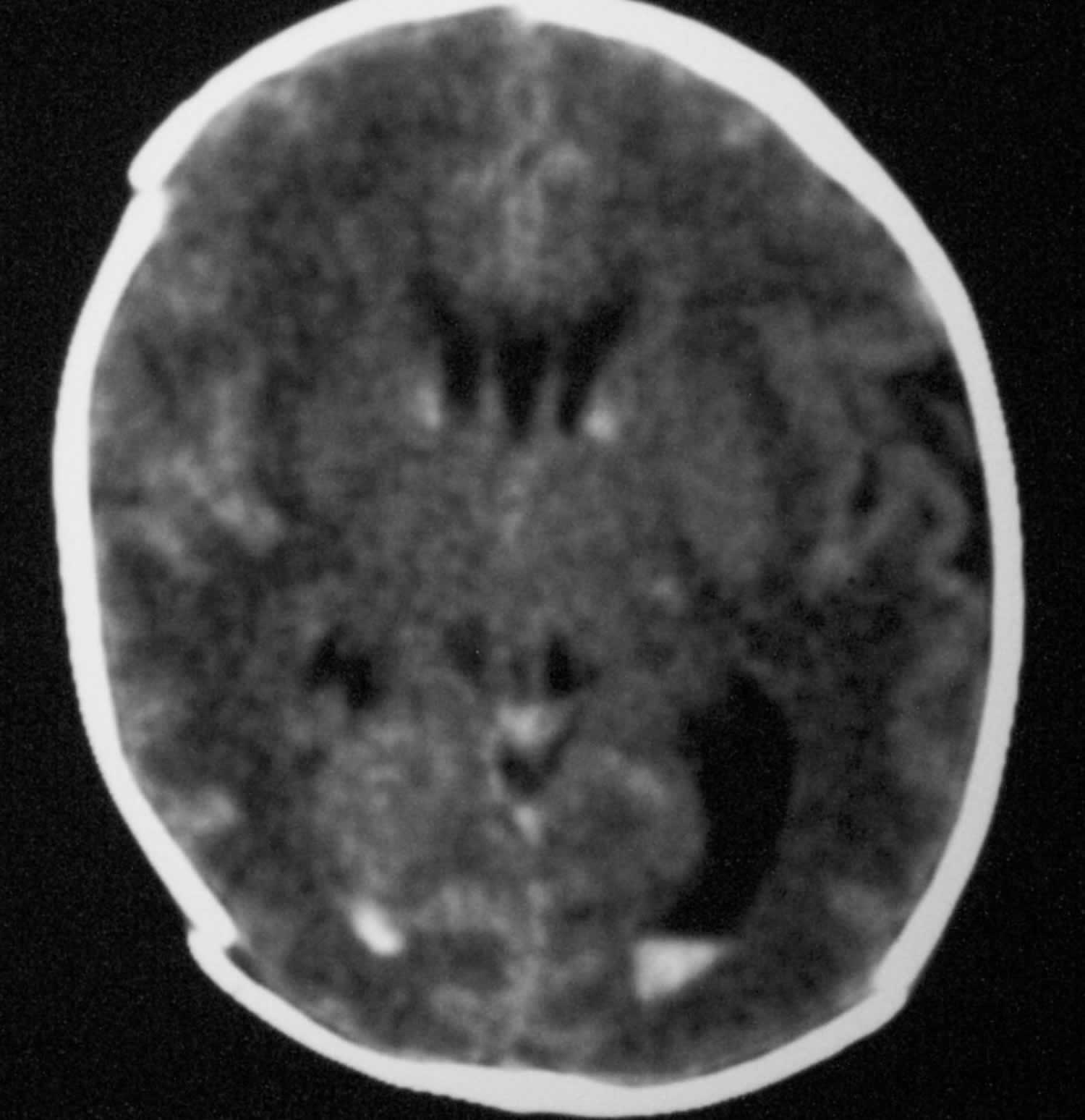
Figure 5. Grade 2 germinal matrix hemorrhage
Footnote: Axial nonenhanced computed tomography scan. The image shows bilateral grade 2 subependymal and intraventricular germinal matrix hemorrhage without hydrocephalus.
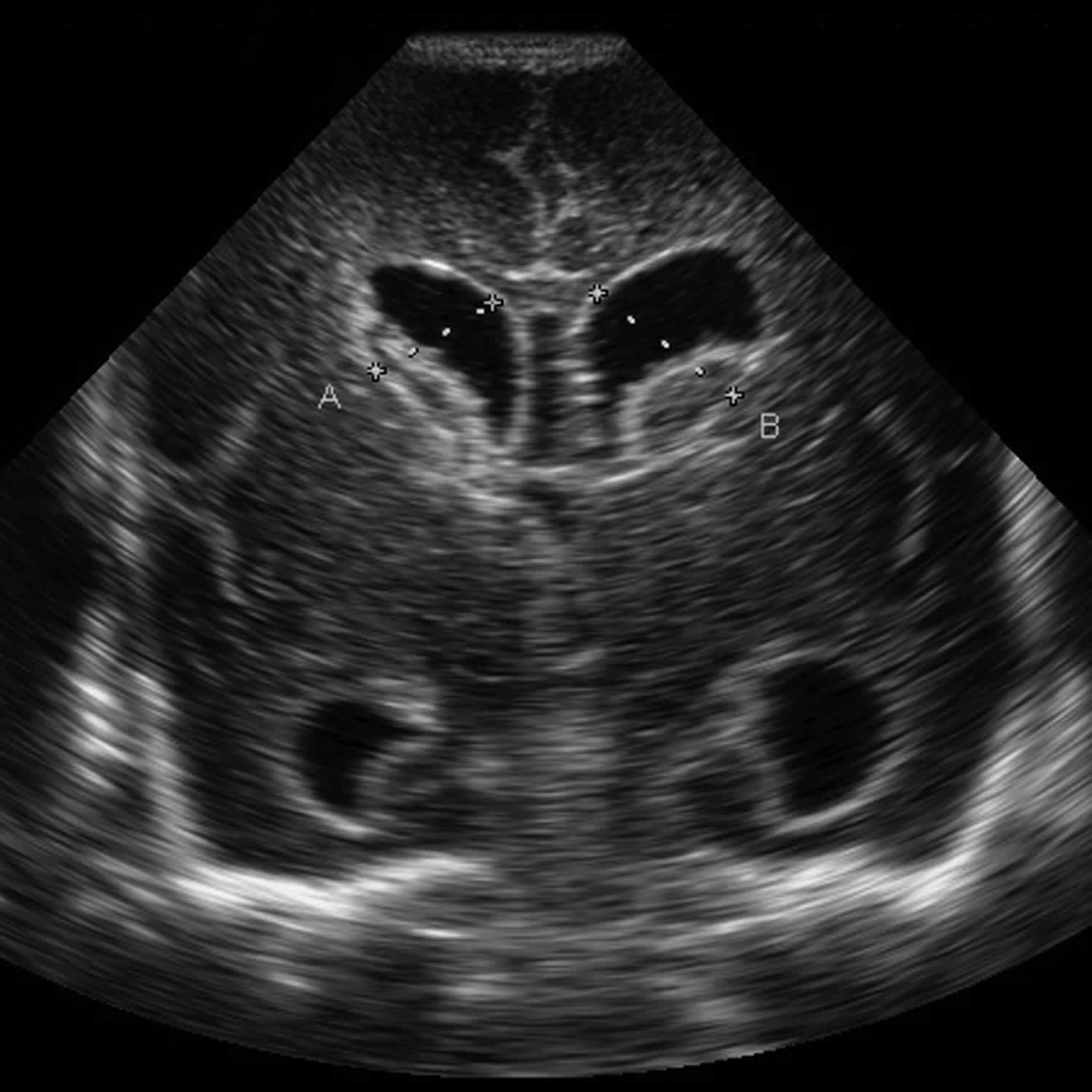
Figure 6. Grade 3 germinal matrix hemorrhage
Footnote: Grade 3 germinal matrix hemorrhage demonstrating marked ventriculomegaly. Note the echogenic material anterior to the caudothalamic groove.
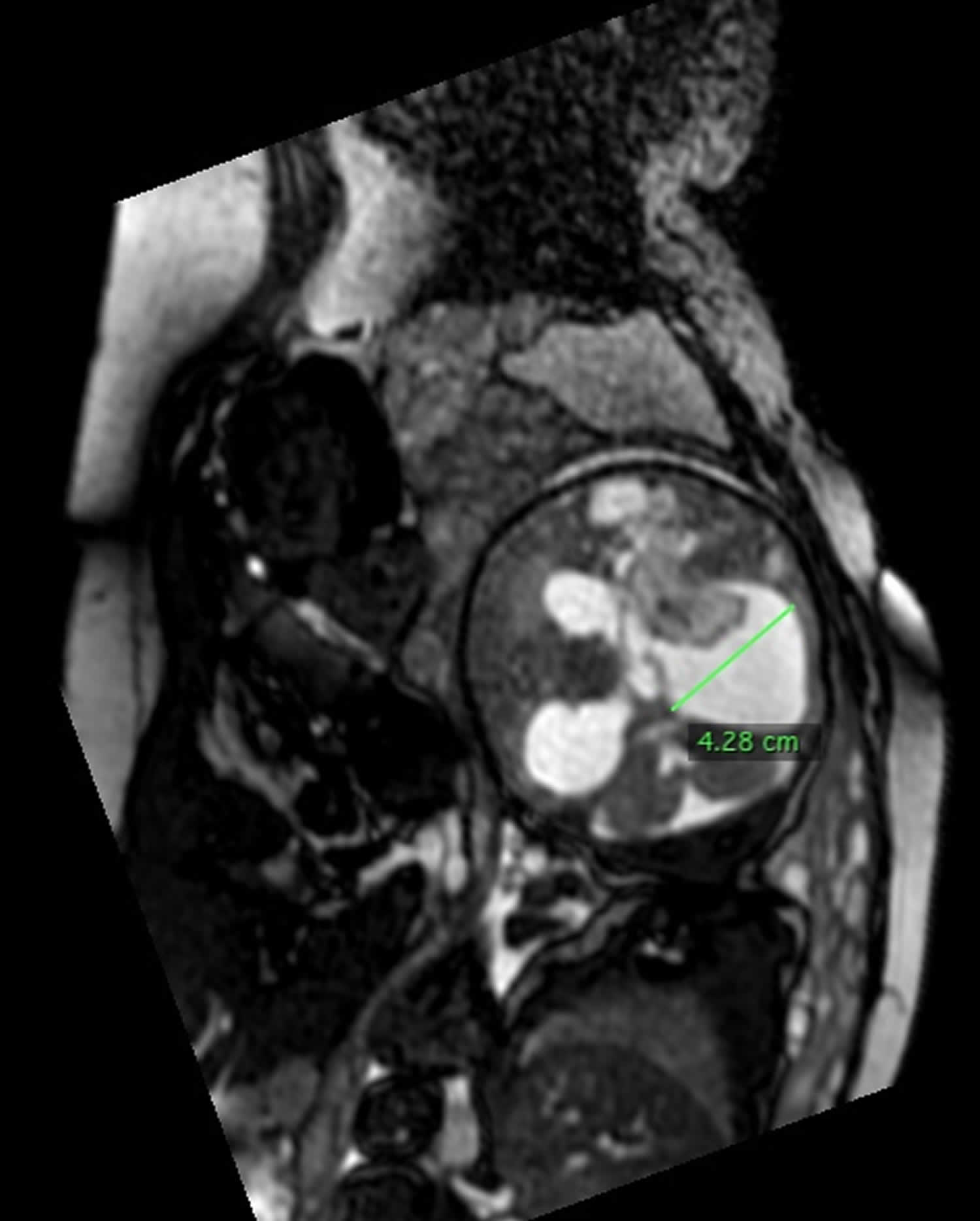
Figure 7. Grade 4 germinal matrix hemorrhage
Footnote: 36 weeks of gestation pregnant, ultrasound revealed ventriculomegaly. The brain shows supratentorial (4th ventricle is normal) hydrocephalic changes reaching about 4 cm at the level of right atrium with markedly dilated both occipital horns (colpocephaly). The right choroid plexus is enlarged (measuring 4.2 x 1.2 cm), shows cystic changes, extending to the frontal horn and cerebral parenchyma in the fronto-pareital regions. It elicits rim of bright signal in T1WI, intermediate in T2WI with evident restricted diffusion. Normal left choroid plexus. Agenesis of corpus callosum manifested by colpocephaly, parallel arrangement of lateral ventricles and absent precentral gyrus. Normal posterior fossa structures. Findings are suggestive of intraventricular hemorrhage with extension to the adjacent brain parenchyma and secondary hydrocephalic changes ( grade IV germinal matrix hemorrhage).
Germinal matrix hemorrhage causes
The causes of germinal matrix hemorrhage is multifactorial, complex, and heterogeneous 14. Local anatomical factors include the high vascularity of the germinal matrix, its immature fragile capillary bed with poor stromal support, and the sharp U-turn the thalamostriate veins take at that point, which makes the germinal matrix prone to congestion. Systemic factors include capillary damage from hypoxia, loss of vascular autoregulation, fluctuations in blood flow velocity, and venous congestion. The pathogenesis of germinal matrix hemorrhage probably involves an interaction between the environmental factors listed above and genetic variations affecting inflammatory pathways, coagulation, and vacular structure. Muscle paralysis eliminates fluctuations of cerebral blood flow velocity and reduces the incidence of intraventricular hemorrhage.
An inherent fragility of the germinal matrix vasculature sets the ground for hemorrhage and fluctuation in the cerebral blood flow induces the rupture of vasculature 14. Germinal matrix exhibits rapid angiogenesis orchestrating formation of immature vessels. The angiogenic vessels lack pericytes, display immature basal lamina low in fibronectin, and have astrocyte end-feet coverage deficient in glial fibrillary acidic protein. These factors contribute to the fragility of the germinal matrix vasculature. Fluctuation in the cerebral blood flow results from a wide range of respiratory and hemodynamic instability associated with the preterm infants. If there are associated platelet or coagulation disorders, the homeostasis mechanisms are impaired which might accentuate the hemorrhage. Vaginal delivery, low Apgar score, severe respiratory distress syndrome, pneumothorax, hypoxia, hypercapnia, seizures, patent ductus arteriosus, infection, and others seem to increase primarily the fluctuations in the cerebral blood flow and thus, represent important risk factors to the development of intraventricular hemorrhage 14.
Pathogenesis of germinal matrix vasculature 14:
- Fragility of germinal matrix vasculature
- Fluctuation in the cerebral blood flow
- Platelet and coagulation disorder
Intraventricular hemorrhage typically initiates in the periventricular germinal matrix 15. This brain region is known to developmental neurobiologists as the ganglionic eminence (see Figure 2A). The germinal matrix consists of neuronal and glial precursor cells (Figure 2B and 2C) and is most prominent on the head of caudate nucleus. The subependymal germinal matrix is highly vascular and is selectively vulnerable to hemorrhage. After 24 gestational weeks, thickness of the germinal matrix decreases, and it almost disappears by 36–37 gestational weeks. When hemorrhage in the germinal matrix is substantial, the underlying ependyma breaks and germinal matrix hemorrhage progresses to intraventricular hemorrhage, as blood fills the lateral cerebral ventricle.
Mutations in the type IV procollagen gene, COL4A1, are associated with intraventricular hemorrhage in dizygotic preterm twins 16. Since inflammatory mediators and coagulation factors might have a role in the development of intraventricular hemorrhage, polymorphisms of pro-inflammatory cytokines and mutations in the coagulation factors have been evaluated as candidate genes that modify the severity and risk of intraventricular hemorrhage. Mutations in factor V Leiden, prothrombin G20210A, and IL1β have been implicated in the development of grade I and II intraventricular hemorrhage 17. Polymorphisms of IL-6 and TNFα are proposed as genetic modifiers of intraventricular hemorrhage risk 18. In addition, polymorphism of methylene tetrahydrofolate reductase (MTHFR) gene has been reported in infants with intraventricular hemorrhage. C677T polymorphism in MTHFR enzyme is associated with high plasma homocysteine levels, which is a known risk factor for vascular disease 19. Together, mutations in coagulation, thrombophilia, and inflammation related genes might contribute to the development of intraventricular hemorrhage.
Risk factors for germinal matrix hemorrhage
Intraventricular hemorrhages occur most frequently in babies who are born at less than 32 weeks of gestation or have a birth weight of less than 1500 grams and are not properly managed 20. The numbers are higher in infants born extremely premature, with intraventricular hemorrhages occuring in 45% of infants born weighing 500-750 g. Other risk factors for intraventricular hemorrhage include hypoxic-ischemic encephalopathy, birth trauma, lack of prenatal steroid (betamethasone) therapy in babies who are expected to be born preterm, prolonged neonatal resuscitation, and respiratory distress.
Intraventricular hemorrhages is not usually present at birth, but rather found within three or four days following birth 21. It can be caused by a lack of oxygen to the baby’s brain and brain trauma. Oxygen deprivation can cause bleeding because when the brain receives insufficient oxygen, cells start to degrade. When the cells that make up the blood vessel walls start to break down, the vessels become fragile and can rupture very easily.
Traumatic head injury can also result in ruptured blood vessels. Head trauma is often caused by the use of forceps and vacuum extractors. These devices are placed directly on the baby’s head to help ease them out of the birth canal. Unfortunately, physicians often apply excessive pressure or misuse these devices, resulting in a brain bleed. The main causes of intraventricular hemorrhage are 22:
- Prematurity: The germinal matrix is more fragile and less structurally sound in infants that are born prematurely.
- Hypoxic-ischemic encephalopathy (HIE): Babies with hypoxic-ischemic encephalopathy are more likely to have intraventricular hemorrhage as well. Prolonged labor, labor-enhancing drugs such as Pitocin or Cytotec, macrosomia (a condition in which the baby is large for gestational age and cannot easily fit through the birth canal), cephalopelvic disproportion (when there is a size mismatch between the baby’s head and the mother’s pelvis) and other conditions increase the risk of a baby having birth asphyxia and hypoxic-ischemic encephalopathy.
- Trauma during delivery
- Intrapartum hypoxia: The “hypoxic” part of hypoxic-ischemic encephalopathy, wherein the baby experiences a lack of oxygen.
- Abnormal changes in fetal blood pressure
These are just a few examples of conditions and complications that may result in intraventricular hemorrhage.
Table 1. Neonatal risk factors in the pathogenesis of intraventricular hemorrhage
| Major Pathogenic mechanism | Putative mechanisms* | Risk factors | Preventive measures | |
|---|---|---|---|---|
| 1. | Disturbance in cerebral blood flow | 1. Fluctuation in cerebral blood flow |
|
|
|
| |||
|
| |||
| 2. High cerebral venous pressure |
|
| ||
| ||||
| 3. Abnormal blood pressure |
|
| ||
| 4. Pressure passive circulation | Extreme prematurity and low birth weight (<1000g) Clinically unstable resulting from respiratory compromise, sepsis or other reasons |
| ||
| 2 | inherent fragility of germinal matrix vasculature | Might be worsened by an inflammatory injury to the blood brain barrier | Hypoxic ischemic insult Sepsis | Prenatal glucocorticoids stabilizes the microvasculature by increasing:
|
| 3. | Platelet and coagulation disturbances | Hemostatic failure | Thrombocytopenia Disseminated intravascular coagulopathy | Replacement of blood products |
Footnote: *Correlation of mechanisms with the risk factors and preventive measures is based on available evidence and author’s speculations.
Germinal matrix hemorrhage prevention
Since germinal matrix hemorrhage-intraventricular hemorrhage is primarily attributed to increased vascular fragility and disturbance in cerebral blood flow, strategies are directed to strengthening the germinal matrix vasculature and to stabilizing the cerebral blood flow. Germinal matrix has a subset of vessels that are angiogenic, immature, and lack pericytes; and they thrive because of high levels of vascular endothelial growth factor (VEGF) and angiopoietin 2 23. These blood vessels exhibit high fragility and propensity to bleed. It appears that the immature neovasculature are pruned within a few days of premature delivery, thus stabilizing the germinal matrix microvasculature. This is because oxygen concentration increases above intrauterine level shortly after birth, which possibly downregulates the VEGF levels in the germinal matrix. Indeed, preterm infants become relatively immune to the development of intraventricular hemorrhage after postnatal day 3. Prenatal glucocorticoids or antenatal celecoxib also downregulates VEGF levels, which leads to apoptosis of naked endothelial cells, lacking pericyte coverage 24. Hence, prenatal use of angiogenic inhibitors—glucocorticoids or celecoxib–trims the nascent vessels, but not the functional vessels protected by pericytes, thereby orchestrating a vascular network consisting of stable blood vessels 24. Fluctuations in the cerebral blood flow is related to routine procedures performed in neonatal units, such as suctioning, handling, placing intravenous lines, and common problem associated with prematurity, including respiratory distress syndrome, patent ductus arteriosus, apneic episodes, seizures, hypoxemia, hypercarbia and others (see Table 1). Hence, fluctuations in the cerebral blood flow can be minimized by reducing the stimulation to the infant and appropriately managing the common complications of prematurity. Overall preventive approach is listed in Table 2.
Table 2. Prevention of intraventricular hemorrhage
| A. Prenatal interventions: |
|
| B. Care during infant delivery: | Optimize obstetric care and prevent prolonged labor |
| C. Postnatal interventions: |
|
Prenatal pharmacological treatments to prevent intraventricular hemorrhage
Glucocorticoids
In the United States, the preterm birth rate is about 12.5% and approximately 75% women in premature labor with gestational age of less than 34 weeks are treated with either betamethasone or dexamethasone 25. A number of studies have confirmed that prenatal glucocorticoid reduces both severity and incidence of intraventricular hemorrhage 26. The beneficial effect of prenatal glucocorticoid is attributed to stabilization of the microvasculature of the germinal matrix and alleviation of disturbance in the cerebral blood flow. Prenatal glucocorticoid, suppresses angiogenesis in the germinal matrix microvasculature and thus trims the nascent and fragile vasculature, which are vulnerable to hemorrhage. Thus, glucocorticoid exposure stabilizes the blood brain barrier (BBB) of the germinal matrix; and infants treated with prenatal glucocorticoid exhibit greater pericyte coverage, higher fibronectin levels and more glial fibrillary acidic protein (GFAP) in the astrocyte end-feet of the blood vessels of the germinal matrix compared to untreated infants 19. Moreover, it reduces the incidence and severity of respiratory distress syndrome, which might minimize fluctuation in the cerebral blood flow. Postnatal betamethasone (0.1 mg/kg) also reduces cerebral flow velocity possibly by exerting a vasoconstrictor effect on cerebral vessels in preterm infants 27. Similarly, prenatal betamethasone has been shown to reduce cerebral blood flow by increasing cerebrovascular resistance in fetal sheep model 28.
The optimal effects of prenatal glucocorticoid have been observed after a complete course of 2 doses of betamethasone or 4 doses of dexamethasone when administered within a week of delivery of the premature newborn 29. However, benefits have also been noted with incomplete courses of glucocorticoids 30. Comparison of the two glucocorticoids—betamethasone and dexamethasone–has not conclusively shown superiority of one over the other and clinicians should choose whatever is available 30. Betamethasone exposed infants exhibit less severe respiratory distress syndrome, but more intraventricular hemorrhage, compared to prenatal dexamethasone treated infants 29. There has been concern that prenatal dexamethasone might increase the incidence of periventricular leukomalacia 31. However, a subsequent study on a larger population clearly showed that there is no difference in the incidence of cystic periventricular leukomalacia between dexamethasone and betamethasone exposed infants 32. This study also noted that both glucocorticoids are equally efficacious in preventing severe intraventricular hemorrhage, however there is a trend toward better efficacy for dexamethasone compared to betamethasone 32. Importantly, prenatal betamethasone is associated with a reduced risk for neonatal death compared with dexamethasone 32. Together, there is no recommendation for the use of one glucocorticoid over the other, despite multiple clinical trials undertaken. Another key issue related to the use of prenatal steroid is single versus repeated course. Unfortunately, there is no agreement among the experts on the advantage of single vs. multiple course of glucocorticoids 30. There are concerns that multiple course of prenatal glucocorticoid might have adverse effects on brain and other organ systems.
Phenobarbital and magnesium sulfate
As etiopathogenesis of intraventricular hemorrhage was more mysterious in 80s than now, a number of agents without a concrete rationale were tried to prevent intraventricular hemorrhage. Phenobarbital and vitamin K are important to mention here, as these medications attracted the attention of investigators. Initial studies showed some protective effect of phenobarbital, however, subsequent clinical trials failed to confirm the protective effect of phenobarbital in preventing intraventricular hemorrhage 33. Maternal treatment of vitamin K or magnesium sulfate to prevent intraventricular hemorrhage did not demonstrate any benefit either 34.
Germinal matrix hemorrhage signs and symptoms
Babies with intraventricular hemorrhage may not present with symptoms. In fact, between 25-50% of intraventricular hemorrhage cases present without symptoms 22. It is important that screening cranial ultrasounds be administered if it is suspected. Listed below are some signs that the baby may have intraventricular hemorrhage 35:
- The baby is limp (hypotonic) and/or weak
- The baby has decreased movement
- The baby’s breathing is abnormal – in severe cases, the baby is not breathing enough, has irregular breathing, and/or has periods in which they stop breathing (apnea)
- Changes in eye positioning and movement
- The baby has pale or blue coloring
- The baby has seizures
- The baby’s heart rate is slow and/or their blood pressure is low
- The baby has high-pitched crying
- The baby is sleeping for abnormally long periods
- The baby has slow or decreased reflexes
In severe cases, intraventricular hemorrhage can involve more severe symptoms, including, but not limited to 22:
- Flaccid weakness
- Seizures
- Coma or stupor
- Apnea or hypoventilation
- Pupils fixed to light
- Bulging anterior fontanelle
- Bradycardia
- Hypotension
Germinal matrix hemorrhage diagnosis
Head (cranial) ultrasound is the preferred screening and diagnostic tool for germinal matrix hemorrhage 36. The portability of this modality allows imaging in the nursery with minimal disturbance of the infant. Physicians use the ultrasound to determine the location and extent of the intraventricular hemorrhage. All babies born prematurely should be given a cranial ultrasound. Ultrasonography also depicts germinal matrix hemorrhages that are larger than 5 mm, with a sensitivity of nearly 100% and specificity of 91%. Smaller germinal matrix hemorrhages, however, are more difficult to identify. Power and pulsed-wave Doppler ultrasonography can be used to identify preterm neonates who are at risk for germinal matrix hemorrhage and intraventricular hemorrhage during their first week of life. Using this modality, clinicians can detect autoregulatory fluctuations in the preterm neonate’s cerebral blood flow with examination of the lenticulostriate arteries; measurements of the peak velocity, resistive index, and coronal vascular cross-sectional area; and product of the peak velocity and vascular cross-sectional area 37.
CT scanning and MRI are also used and have better interobserver agreement 38. Because these modalities more readily depict small germinal matrix hemorrhages, CT scanning and MRI have a higher sensitivity than that of ultrasonography. However, these 2 imaging modalities require that the infant be moved from the nursery; there is also the possibility that sedation would be required 39.
Limitations of techniques
All imaging modalities have relatively low negative predictive values. In a 2000 study, Blankenberg et al 40 found negative predictive values of 53% at 2-month follow-up and 59% at 2-year follow-up, irrespective of the modality. However, the absence of neuroimaging abnormalities in the infant does not exclude the possibility of later neurodevelopmental problems.
Blankenberg et al 40 also found that CT scanning had nearly twice the sensitivity of ultrasonography in the detection of germinal matrix hemorrhage and intraventricular hemorrhage; interobserver agreement with this modality was also improved relative to ultrasonography.
Special concerns
Normal imaging findings must be viewed with caution. Ultrasonography, CT scanning, and MRI all have low negative predictive values of approximately 60%.
With fetal ultrasonography and fetal MRI, germinal matrix hemorrhage/intraventricular hemorrhage can be identified in utero, remote in time from delivery 41.
Germinal matrix hemorrhage treatment
Postnatal pharmacological treatment to prevent intraventricular hemorrhage
Indomethacin
Indomethacin, commonly used in premature neonates to close patent ductus arteriosus, has been shown to prevent intraventricular hemorrhage in several clinical trials. Indomethacin, a non-selective cyclo-oxygenase (COX) inhibitor, suppresses both COX1 and COX2 isoforms to reduce prostaglandin synthesis. It attenuates cerebral vascular hyperemic responses induced by hypoxia, hypercapnia, hypertension, and asphyxia 42. It reduces alterations in the BBB permeability after cerebral ischemia and promotes maturation of basement membrane by increasing the expression of laminin and collagen V 43. The maturation of basal lamina upon indomethacin treatment can be attributed to COX2 inhibition, which suppresses angiogenesis and matures the germinal matrix vasculature 23.
In a number of clinical trials, indomethacin treatment has shown short-term benefit of reducing the incidence of intraventricular hemorrhage 44. Secondary analyses of a multicenter study based on gender have shown that indomethacin treatment reduces the rate of intraventricular hemorrhage in male infants, but not in female infants 45. However, another study on a larger population of preterm infants showed just a weak differential effect of indomethacin by sex.104 Since indomethacin reduces the occurrence of intraventricular hemorrhage, it was anticipated that this treatment would improve the neurodevelopment outcome of the infants. However, indomethacin treatment failed to reduce the rate of cerebral palsy, deafness and blindness on long term follow up 46. A meta-analyses of 19 clinical trials also did not show any improvement in the long term outcome of indomethacin treated infants 47. Together, indomethacin prophylaxis has immediate benefits of reduction in symptomatic patent ductus arteriosus, and severe intraventricular hemorrhage; however, this does not impact long term neurodevelopmental outcomes. Hence, indomethacin is not recommended for routine prophylaxis against intraventricular hemorrhage. However, indomethacin is still being used in some neonatal units depending on clinical circumstances and personal preferences.
Ibuprofen is another non-selective COX inhibitor that has shown promise in closing patent ductus arteriosus. This compound does not reduce cerebral blood flow, in contrast to indomethacin. More importantly, ibuprofen does not prevent intraventricular hemorrhage in premature infants 48.
Other clinical trials of unproven benefit
Postnatal phenobarbital has been used in a number of clinical trials to prevent intraventricular hemorrhage in 1980s, based on the premise that it might reduce abrupt changes in the cerebral blood flow during tracheal suctioning, handling, and motor activity 49. However, phenobarbital did not reduce the incidence of intraventricular hemorrhage significantly. Another agent that drew the attention of investigators in 1980s was etamsylate. This compound reduces prostaglandin synthesis and promotes platelet adhesiveness. In addition, etamsylate induces polymerization of hyaluronic acid in the vascular basement membrane which might promote homeostasis and minimize bleeding. However, large clinical trials showed that etamsylate neither reduced the incidence of intraventricular hemorrhage nor enhanced the neurodevelopmental outcome of premature infants 50. Pathogenesis of intraventricular hemorrhage has always puzzled the investigators and thus, a role of free radicals in the etiology of intraventricular hemorrhage cannot be totally excluded. Hence, vitamin E—a potent antioxidant–has been tried to prevent intraventricular hemorrhage in preterm infants without appreciable benefits 51. To address the need to minimize asynchrony between infant and ventilator breath, pancuronium has been tried in 1980s and was found to reduce fluctuation in cerebral blood flow and also the incidence of intraventricular hemorrhage 52. A relatively recent meta-analysis identified 6 clinical trials in which the use of neuromuscular blocking agent during mechanical ventilation was compared to no paralysis in newborn infants 53. It was concluded that neuromuscular paralyses with pancuronium reduced the rate of intraventricular hemorrhage. However, owing to complications associated with neuromuscular paralysis, routine use of pancuronium in extremely premature infants has not been recommended. The role of activated recombinant factor VII (rVIIa) in promoting coagulation is promising and it has been hypothesized that early (prophylactic) administration of recombinant factor VII (rVIIa) to extremely premature infants would reduce the incidence of intraventricular hemorrhage. However, intraventricular hemorrhage is not primarily a coagulation disorder and this hypothesis does not seem to be logical.
Optimizing care of fetuses and premature newborns.
Prenatal interventions
Inter-hospital transport of extremely premature infants is associated with increased incidence and severity of intraventricular hemorrhage. This correlation has remained constant in recent years 54. Hence, pregnant mothers in preterm labor should be transported to a tertiary care center specializing in high-risk delivery. Prolonged labor might increase the risk of intraventricular hemorrhage and should be managed appropriately. Data on the incidence of intraventricular hemorrhage in Cesarean section vs. vaginal delivery is inconsistent 55 and thus, infants are delivered based on the decisions made by obstetricians.
Postnatal interventions
There is no specific recommendation about neonatal resuscitation of premature infants to prevent intraventricular hemorrhage. However, restoration of normal oxygenation and ventilation immediately at birth is important as hypoxemia and hypercarbia can cause fluctuation in the cerebral blood flow which might contribute to intraventricular hemorrhage. While preventing metabolic acidosis and accomplishing normal perfusion is important, a rapid sodium bicarbonate infusion might add to the risk of intraventricular hemorrhage. After infants have been transferred to the neonatal unit, gentle and synchronized ventilation, prompt closure of ductus arteriosus, and maintenance of normal O2 and CO2 levels in the arterial blood are important preventive measures. Preventing pneumothorax, apneic episodes, seizures, as well as minimizing tracheal suctioning and handling will prevent disturbances in the cerebral blood flow. Reducing stimulation and gentle caretaking decreases the incidence of intraventricular hemorrhage 56. Effect of surfactant treatment on the development of intraventricular hemorrhage is unclear. Use of high frequency ventilators does not increase the risk of intraventricular hemorrhage 57 and therefore, an individualized approach in selection of appropriate ventilator should be pursued.
Together, the incidence of intraventricular hemorrhage has remained unchanged over the last couple of decades, despite advances in care of newborns. Use of prenatal glucocorticoids remains the most effective strategy in the prevention of intraventricular hemorrhage.
Germinal matrix hemorrhage prognosis
Germinal matrix hemorrhage-intraventricular hemorrhage reduces the survival of premature infants and enhances the risk of a number of neurological complications 11. A higher mortality rate in premature infants with severe intraventricular hemorrhage has been reported compared to infants without intraventricular hemorrhage 58. Premature infants with moderate to severe intraventricular hemorrhage (grade 3–4) are at high risk of post-hemorrhagic hydrocephalus, cerebral palsy and mental retardation, while infants with mild intraventricular hemorrhage (grade 1–2) are at risk of developmental disabilities 59. About 45–85% of premature infants with moderate-to-severe intraventricular hemorrhage develop major cognitive deficits and approximately 75% of these infants need special education in school 60. A recent study has shown better functional outcome of surviving preterm infants with perventricular hemorrhagic infarction at school age than previously thought 61.
References- Roze E, Kerstjens JM, Maathuis CG, ter Horst HJ, Bos AF. Risk factors for adverse outcome in preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2008 Jul. 122(1):e46-52.
- Germinal Matrix Hemorrhage Imaging. https://emedicine.medscape.com/article/408862-overview
- Sherlock RL, Anderson PJ, Doyle LW, et al. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81(11):909–916.
- Luu TM, Ment LR, Schneider KC, et al. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123(3):1037–1044.
- Whitaker AH, Feldman JF, Lorenz JM, et al. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch Gen Psychiatry. 2011;68(7):742–752.
- McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35(4):777–792. vii.
- Jain NJ, Kruse LK, Demissie K, et al. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22(6):491–500.
- Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics. 1989;84:797–801.
- Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009;22:491–500.
- Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003.
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1-8. doi:10.1203/PDR.0b013e3181c1b176 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2799187
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. doi:10.1016/s0022-3476(78)80282-0
- Castillo M. Neuroradiology Companion, Methods, Guidelines, And Imaging Fundamentals. Lippincott Williams & Wilkins. (2006) ISBN:0781779499
- Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41(1):47-67. doi:10.1016/j.clp.2013.09.007 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3925310
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8.
- Bilguvar K, DiLuna ML, Bizzarro MJ, et al. COL4A1 mutation in preterm intraventricular hemorrhage. J Pediatr. 2009;155(5):743–745.
- Ryckman KK, Feenstra B, Shaffer JR, et al. Replication of a genome-wide association study of birth weight in preterm neonates. J Pediatr. 2012;160(1):19–24. e14.
- Gopel W, Hartel C, Ahrens P, et al. Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes Immun. 2006;7(1):65–68.
- Harteman JC, Groenendaal F, van Haastert IC, et al. Atypical timing and presentation of periventricular haemorrhagic infarction in preterm infants: the role of thrombophilia. Developmental medicine and child neurology. 2012;54(2):140–147.
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1-8. doi:10.1203/PDR.0b013e3181c1b176
- Intraventricular Hemorrhage (IVH). https://americanpregnancy.org/birth-defects/intraventricular-hemorrhage-26152
- Germinal matrix hemorrhage and intraventricular hemorrhage (GMH-IVH) in the newborn: Pathogenesis, clinical presentation, and diagnosis. https://www.uptodate.com/contents/germinal-matrix-hemorrhage-and-intraventricular-hemorrhage-gmh-ivh-in-the-newborn-pathogenesis-clinical-presentation-and-diagnosis
- Ballabh P, Xu H, Hu F, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13(4):477–485.
- Vinukonda G, Dummula K, Malik S, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41(8):1766–1773.
- Meadow WL, Bell A, Sunstein CR. Statistics, not memories: what was the standard of care for administering antenatal steroids to women in preterm labor between 1985 and 2000? Obstet Gynecol. 2003;102(2):356–362.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454
- Cambonie G, Mesnage R, Milesi C, et al. Betamethasone impairs cerebral blood flow velocities in very premature infants with severe chronic lung disease. J Pediatr. 2008;152(2):270–275.
- Lohle M, Muller T, Wicher C, et al. Betamethasone effects on fetal sheep cerebral blood flow are not dependent on maturation of cerebrovascular system and pituitary-adrenal axis. The Journal of physiology. 2005;564(Pt 2):575–588.
- Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008;(4):CD006764
- Wapner R, Jobe AH. Controversy: antenatal steroids. Clin Perinatol. 2011;38(3):529–545.
- Baud O, Foix-L’Helias L, Kaminski M, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341(16):1190–1196.
- Lee BH, Stoll BJ, McDonald SA, et al. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006;117(5):1503–1510.
- Thorp JA, Ferrette-Smith D, Gaston LA, et al. Combined antenatal vitamin K and phenobarbital therapy for preventing intracranial hemorrhage in newborns less than 34 weeks’ gestation. Obstet Gynecol. 1995;86(1):1–8.
- Basu SK, Chickajajur V, Lopez V, et al. Immediate clinical outcomes in preterm neonates receiving antenatal magnesium for neuroprotection. J Perinat Med. 2012;40(2):185–189.
- Ballabh P. (2010). Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research, 67(1), 1–8. doi:10.1203/PDR.0b013e3181c1b176
- Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, et al. Low-grade intraventricular hemorrhage: is ultrasound good enough?. J Matern Fetal Neonatal Med. 2013 Aug 23.
- Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, et al. Low-grade intraventricular hemorrhage: is ultrasound good enough?. J Matern Fetal Neonatal Med. 2015 Nov. 28 Suppl 1:2261-4.
- Fumagalli M, Bassi L, Sirgiovanni I, Mosca F, Sannia A, Ramenghi LA. From germinal matrix to cerebellar haemorrhage. J Matern Fetal Neonatal Med. 2013 Aug 23.
- Fumagalli M, Bassi L, Sirgiovanni I, Mosca F, Sannia A, Ramenghi LA. From germinal matrix to cerebellar haemorrhage. J Matern Fetal Neonatal Med. 2015 Nov. 28 Suppl 1:2280-5.
- Blankenberg FG, Loh NN, Bracci P, et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. AJNR Am J Neuroradiol. 2000 Jan. 21(1):213-8.
- Morioka T, Hashiguchi K, Nagata S, et al. Fetal germinal matrix and intraventricular hemorrhage. Pediatr Neurosurg. 2006. 42(6):354-61.
- Coyle MG, Oh W, Petersson KH, et al. Effects of indomethacin on brain blood flow, cerebral metabolism, and sagittal sinus prostanoids after hypoxia. Am J Physiol. 1995;269(4 Pt 2):H1450–1459.
- Zuckerman SL, Mirro R, Armstead WM, et al. Indomethacin reduces ischemia-induced alteration of blood-brain barrier transport in piglets. Am J Physiol. 1994;266(6 Pt 2):H2198–2203.
- Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F464–466.
- Ment LR, Vohr BR, Makuch RW, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145(6):832–834.
- Ment LR, Vohr B, Allan W, et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2000;105(3 Pt 1):485–491.
- Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;(7):CD000174
- Dani C, Bertini G, Pezzati M, et al. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics. 2005;115(6):1529–1535.
- Anwar M, Kadam S, Hiatt IM, et al. Phenobarbitone prophylaxis of intraventricular haemorrhage. Arch Dis Child. 1986;61(2):196–197.
- Benson JW, Drayton MR, Hayward C, et al. Multicentre trial of ethamsylate for prevention of periventricular haemorrhage in very low birthweight infants. Lancet. 1986;2(8519):1297–1300.
- Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665
- Perlman JM, Goodman S, Kreusser KL, et al. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312(21):1353–1357.
- Cools F, Offringa M. Neuromuscular paralysis for newborn infants receiving mechanical ventilation. Cochrane Database Syst Rev. 2005;(2):CD002773
- Mohamed MA, Aly H. Transport of premature infants is associated with increased risk for intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F403–407.
- Wadhawan R, Vohr BR, Fanaroff AA, et al. Does labor influence neonatal and neurodevelopmental outcomes of extremely-low-birth-weight infants who are born by cesarean delivery? Am J Obstet Gynecol. 2003;189(2):501–506.
- Szymonowicz W, Yu VY, Walker A, et al. Reduction in periventricular haemorrhage in preterm infants. Arch Dis Child. 1986;61(7):661–665.
- Henderson-Smart DJ, Cools F, Bhuta T, et al. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2007;(3):CD000104
- Whitelaw A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol. 2001;6:135–146.
- Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–F41.
- Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–e346.
- Roze E, Van Braeckel KN, van der Veere CN, Maathuis CG, Martijn A, Bos AF. Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2009;123:1493–1500.