Glanzmann thrombasthenia
Glanzmann thrombasthenia also called Glanzmann disease, is a rare bleeding disorder that is characterized by prolonged or spontaneous bleeding starting from birth (neonates) or during infancy, caused impaired function of platelets that are essential for proper blood clotting. People with Glanzmann thrombasthenia tend to bruise easily, have frequent nosebleeds (epistaxis), and may bleed from the gums. They may also develop red or purple spots on the skin caused by bleeding underneath the skin (petechiae) or swelling caused by bleeding within tissues (hematoma). Glanzmann thrombasthenia can also cause prolonged bleeding following injury, trauma, or surgery (including dental work). Women with this condition can have prolonged and sometimes abnormally heavy menstrual bleeding. Affected women also have an increased risk of excessive blood loss during pregnancy and childbirth.
About a quarter of individuals with Glanzmann thrombasthenia have bleeding in the gastrointestinal tract, which often occurs later in life. Rarely, affected individuals have bleeding inside the skull (intracranial hemorrhage) or joints (hemarthrosis).
The severity and frequency of the bleeding episodes in Glanzmann thrombasthenia can vary greatly among affected individuals, even in the same family. Spontaneous bleeding tends to become less frequent with age.
Glanzmann thrombasthenia is estimated to affect 1 in one million individuals worldwide, but may be more common in certain groups, including those of Romani ethnicity, particularly people within the French Manouche community. Glanzmann thrombasthenia affects males and females in equal numbers. Approximately 500 cases have been reported, but many cases have probably not been reported. Glanzmann thrombasthenia occurs with greater frequency in populations in which intermarriage within a group (consanguinity) is more prevalent such as in some regions of the Middle East, India, and France.
Glanzmann thrombasthenia causes
Mutations in the ITGA2B or ITGB3 gene cause Glanzmann thrombasthenia. These genes provide instructions for making the two parts (subunits) of a receptor protein called integrin alphaIIb/beta3 (αIIbβ3). This protein is abundant on the surface of platelets. Platelets are small cell fragments that circulate in blood and are an essential component of blood clots. During clot formation, integrin αIIbβ3 helps platelets bind together. Blood clots protect the body after injury by sealing off damaged blood vessels and preventing further blood loss.
ITGA2B or ITGB3 gene mutations result in a shortage (deficiency) of functional integrin αIIbβ3. As a result, platelets cannot clump together to form a blood clot, leading to prolonged bleeding.
Three types of Glanzmann thrombasthenia have been classified according to the amount of integrin αIIbβ3 that is available. People with type I (the most common type) have less than 5 percent of normal integrin αIIbβ3 levels, people with type II have between 5 and 20 percent of normal integrin αIIbβ3 levels, and people with the variant type have adequate integrin αIIbβ3 levels but produce only nonfunctional integrin.
Some people with Glanzmann thrombasthenia do not have an identified mutation in either the ITGA2B or ITGB3 gene; the cause of the disorder in these individuals is unknown.
Glanzmann thrombasthenia inheritance pattern
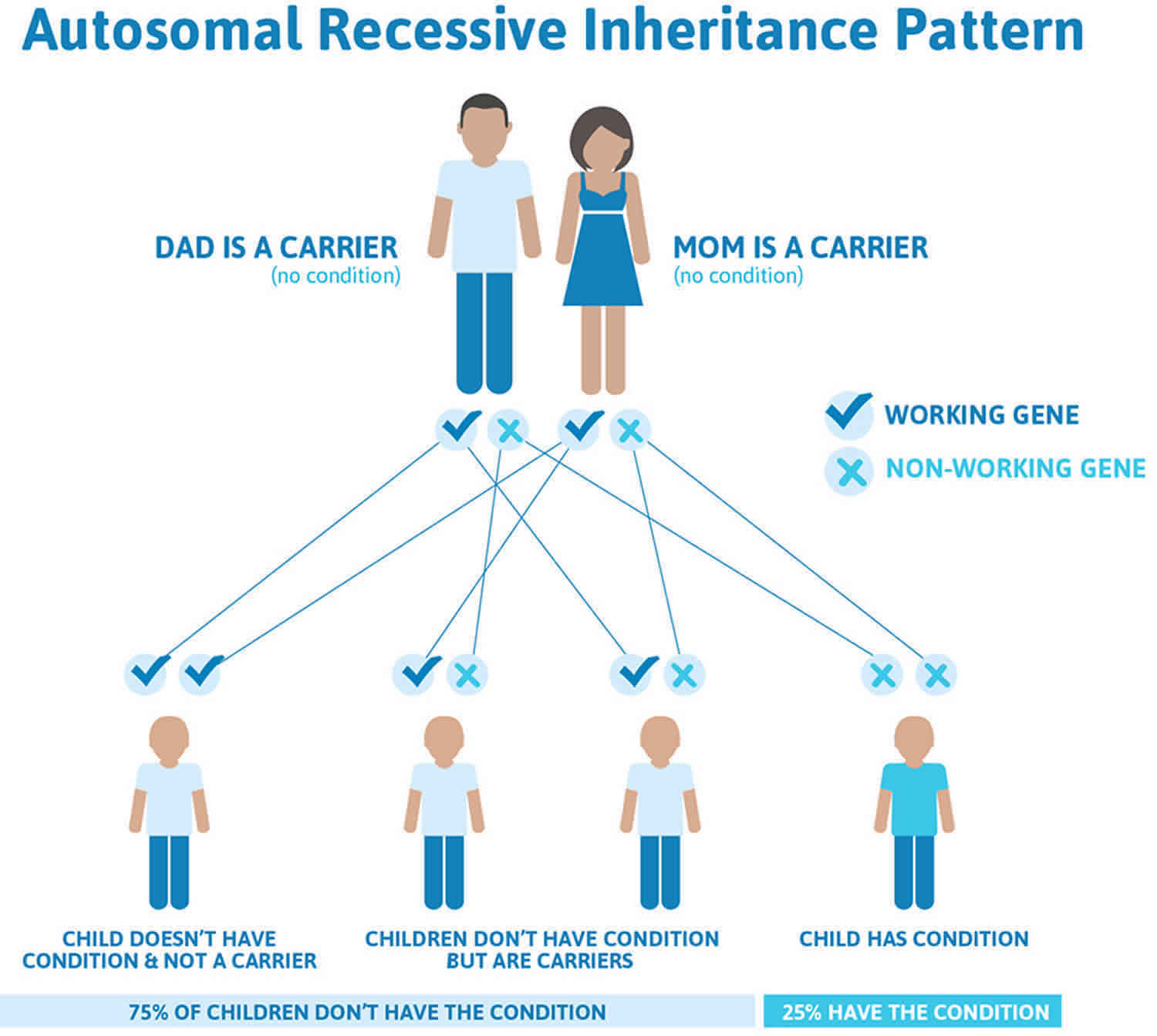
Glanzmann thrombasthenia is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Glanzmann thrombasthenia autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Glanzmann thrombasthenia symptoms
The symptoms of Glanzmann thrombasthenia usually begin at birth or shortly thereafter and include the tendency to bruise and bleed easily and sometimes profusely, especially after surgical procedures. Other symptoms may include susceptibility to easy bruising, nosebleeds (epistaxis), bleeding from the gums (gingival), intermittent gastrointestinal bleeding, and/or variably large red or purple colored spots on the skin that are caused by bleeding in the skin (purpura). Women with Glanzmann thrombasthenia often also have unusually heavy menstrual bleeding, irregular uterine bleeding, and excess bleeding in childbirth. Rarely, internal bleeding and blood in the urine (hematuria) can occur. The severity of the symptoms varies greatly. Some affected individuals have mild bruising and others have severe hemorrhages that can be life threatening.
Glanzmann thrombasthenia diagnosis
Most individuals affected with Glanzmann thrombasthenia have a normal number of platelets but have a prolonged bleeding time, which means it takes longer than usual for a standardized cut to stop bleeding. Platelet aggregation studies are abnormal and show that platelets are not able to clump together when stimulated as they should to form platelet aggregates. Glanzmann thrombasthenia is definitively diagnosed by tests that determine if there is a deficiency of the aIIbβ3 (GPIIb/GPIIIa) receptor. These tests usually involve monoclonal antibodies and flow cytometry. Genetic tests can identify the DNA mutations responsible for the disorder.
Carrier and prenatal testing by DNA analysis is possible if the specific gene abnormality has been identified in an affected family member. Otherwise prenatal testing can be performed based on analyzing the fetus’s platelet integrin alphaIIb/beta3 (αIIbβ3).
Glanzmann thrombasthenia treatment
Some individuals with Glanzmann thrombasthenia may require blood platelet transfusions. Since transfusions may continue to be necessary throughout life, affected individuals may benefit from transfusions from HLA matched donors. Some patients develop antibodies to transfused platelets and these antibodies may diminish the benefit from subsequent platelet transfusions 1. To reduce the potential for platelet alloimmunization, patients should receive leukocyte-depleted blood products. Leukocyte depletion can be accomplished with mechanical filtration. Only filtered blood products should be given.
In 2014, NovoSeven RT, a recombinant coagulation factor VIIa product, was approved to treat Glanzmann thrombasthenia. This medication is indicated to treat bleeding episodes and perioperative management when platelet transfusions are not effective. Treatment is usually given prior to most surgical procedures or should be available if needed. Platelet transfusions are usually necessary prior to delivery. Analysis of data from the prospective Glanzmann’s Thrombasthenia Registry (829 bleeds and 206 procedures in 218 Glanzmann thrombasthenia patients) found that recombinant coagulation factor VIIa (NovoSeven RT) was frequently used in nonsurgical and surgical bleeds, with high efficacy rates and good safety profile, irrespective of platelet antibodies/refractoriness status 2.
Nosebleeds can usually be treated with nasal packing or application of foam soaked in thrombin. Regular dental care is important to prevent bleeding from the gums.
Hormonal therapy can be used to suppress menstrual periods.
Other treatment of Glanzmann thrombasthenia includes preventive measures such as avoidance of antiplatelet agents (e.g., aspirin and NSAIDs), use of antifibrinolytic agents, iron or folate supplementation for anemia, and vaccination for hepatitis B due to the infectious risks associated with multiple transfusions.
Genetic counseling is recommended for people with Glanzmann thrombasthenia and their families.
References- Chitlur M, Rajpurkar M, Recht M, Tarantino MD, Yee DL, Cooper DL, et al. Recognition and management of platelet-refractory bleeding in patients with Glanzmann’s thrombasthenia and other severe platelet function disorders. Int J Gen Med. 2017. 10:95-99.
- Poon MC, Di Minno G, d’Oiron R, Zotz R. New Insights Into the Treatment of Glanzmann Thrombasthenia. Transfus Med Rev. 2016 Apr. 30 (2):92-9.