Glioblastoma
Glioblastomas also called glioblastoma multiforme (GBM) or grade 4 astrocytoma, is an aggressive and fast-growing type of brain cancer that forms from glial (supportive) tissue of the brain and spinal cord and has cells that look very different from normal cells 1. Glioblastoma forms from cells called astrocytes and these cells support and nourish neurons (nerve cells of the brain) and form scar tissue that helps repair brain damage in response to injury. Glioblastoma can occur at any age, but tends to occur more often in older adults with median age of diagnosis is 64 years 2. Glioblastoma is the most common malignant brain tumor in adults, accounting for 35-45% of malignant brain tumors and affects the brain more often than the spinal cord 3, 4. With a growing and aging US population, the number of glioblastoma cases is expected to increase 5. Based on the 2016 Central Brain Tumor Registry of the United States (CBTRUS) report, the average annual age-adjusted incidence rate of glioblastoma multiforme is 3.22/100,000 population 6. Approximately 14,000 cases of glioblastoma are diagnosed each year in the United States 7. Glioblastomas or glioblastoma multiforme (GBM) are primary tumors, meaning they originate in the brain rather than spreading to the brain from cancer elsewhere in the body 8. Glioblastoma tends to occur in active, otherwise healthy people, and more frequently in males. Men are 50% more likely to be diagnosed with glioblastoma than women 9. Whites have the highest incidence rates for glioblastoma, followed by blacks, Asian/Pacific Islanders and American Indian/Alaska Native 8.
Glioblastoma is uncommon in children accounting only approximately 3% of all brain and spinal cord tumors reported among 0–19 year olds 8.
Glioblastomas are often very aggressive that grow into surrounding brain tissue, but rarely spreads outside of the brain. Glioblastoma multiforme is located mainly in the cerebral hemispheres 10 or subtentorially in the brain stem 11 and cerebellum 12. Glioblastoma is characterized by infiltrating growth; therefore the tumor mass is not clearly distinguishable from the normal tissue 13. Glioblastomas frequently recur (in 75–90%) within 2–3 cm from the borders of the initial lesion and with multiple lesions observed in 5% of cases after treatment 14. A growing glioblastoma tumor causes an increase of intracranial pressure 15 and sometimes it leads to hydrocephaly 16.
Glioblastoma can cause worsening headaches, nausea, vomiting, seizures, weakness on one side of the body, difficulty thinking and speaking, and drowsiness, which may develop when the tumor begins to put excess pressure on the brain. The onset of symptoms can be sudden and acute; however, in some patients, there may be gradual changes, such as problems with language, concentration, or coordination and strength on one side of the body. Affected people may also experience other features depending on the size and location of the brain tumor.
Glioblastoma multiforme metastases by cerebrospinal fluid (CSF) 17 or blood 18 are rare and target the spleen, pleura, lungs, lymph nodes, liver, bones, pancreas and small intestine 19, 20, 21. It has been hypothesized that the low metastatic potential of glioblastoma results from the barrier created by cerebral meninges, but also from the rapid tumor growth and short course of this disease 22. The brain is devoid of lymphatic vessels, so metastases through this pathway are impossible 19. The available literature describes 8 cases of glioblastoma multiforme metastases to the skin – tumors usually developed around post-operative sutures. This suggests implantation of glioblastoma multiforme cells around post-operative wounds during the removal of a primary tumor 23.
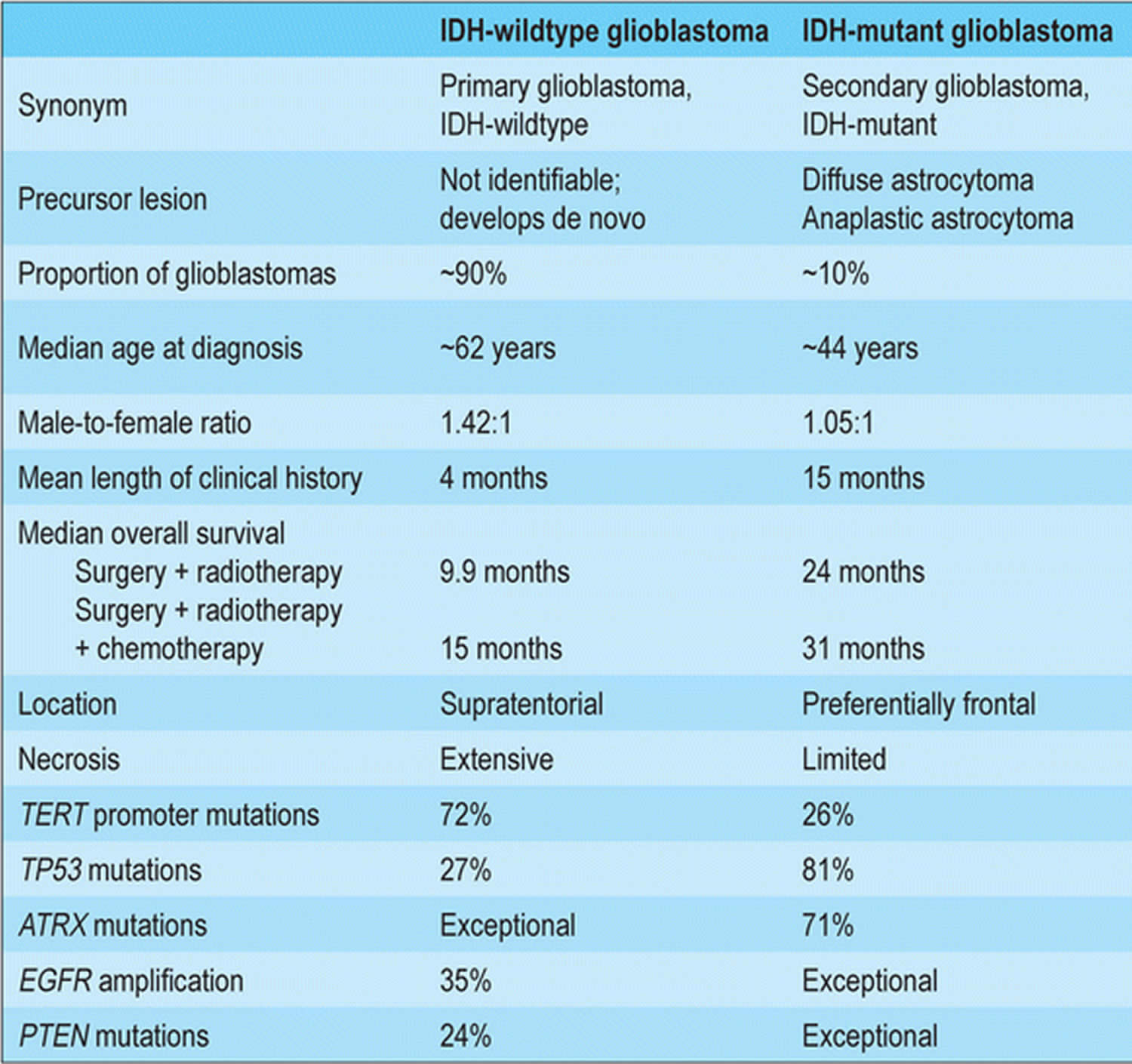
Glioblastoma comprises primary and secondary subtypes that evolve through different genetic pathways affecting patients at different ages and have differences in outcomes 24. Primary glioblastoma account for 80% of glioblastoma and occur in older patients with a mean age of 62 years 4, 25. While secondary glioblastoma develop from lower-grade astrocytoma or oligodendroglioma and occur in younger patients with a mean age of 45 years 26, 27. Secondary glioblastoma are usually located in the frontal lobe, have a lesser degree of necrosis, and carry a better prognosis than primary glioblastoma 25.
In most glioblastoma cases, the exact underlying cause is unknown; however, glioblastoma multiforme can rarely occur in people with certain genetic syndromes such as neurofibromatosis type 1, Turcot syndrome and Li Fraumeni syndrome.
The diagnostic mode of imaging for glioblastoma is contrast-enhanced magnetic resonance imaging. Studies have shown that in the majority of cases, tumor diameter is between 5-10 cm at diagnosis 28, 29, 30. The tumor usually involves corpus callosum and grows into occipital and temporal lobes bilaterally, resulting in a butterfly pattern on imaging, thus the name “butterfly glioma” 31.
Glioblastoma or glioblastoma multiforme (GBM) can be very difficult to treat and a cure is often not possible. Treatments may slow progression of the cancer and reduce signs and symptoms. The standard of treatment for a glioblastoma is surgery, followed by daily radiation and oral chemotherapy for six and a half weeks, then a six-month regimen of oral chemotherapy given five days a month. To start, the neurosurgeon will remove as much of the tumor as possible and may implant medicated carmustine (BCNU)-polymer wafers (Gliadel) right into the brain. These wafers dissolve naturally and gradually release chemotherapy drugs into the tumor area over time. Another chemotherapy drug called temozolomide (Temodar) was approved by the FDA in 2013 and is commonly used to treat glioblastomas and other advanced brain cancers 32. The drug is taken in pill form and works by slowing down tumor growth. Radiation may be used to destroy additional tumor cells and treat tumors in patients who are not well enough for surgery.
Within the last two decades, temozolomide (TMZ) and a non-invasive device called the tumor-treating field (TTF; Optune®) have demonstrated clinical efficacy and achieved improved outcomes 33. Additional treatment options that demonstrated activity include bevacizumab (Avastin), lomustine, carmustine, PCV (combination of procarbazine, lomustine and vincristine), and, more recently, multikinase inhibitor regorafenib, which demonstrated superior outcomes over lomustine in a recent phase 2 trial 34, 35.
Glioblastoma is usually associated with pseudoprogression, which is a sub-acute worsening of MRI findings that occur within three months after the completion of chemoradiotherapy. It is a treatment-related effect. It is important to distinguish between pseudoprogression and the true progression of the glioblastoma cancer to avoid abrupt discontinuation of treatment. The key feature to distinguish is that pseudoprogression is usually asymptomatic. Treatment should be continued if pseudoprogression is suspected unless the patient is symptomatic or worsening of clinical features occur.
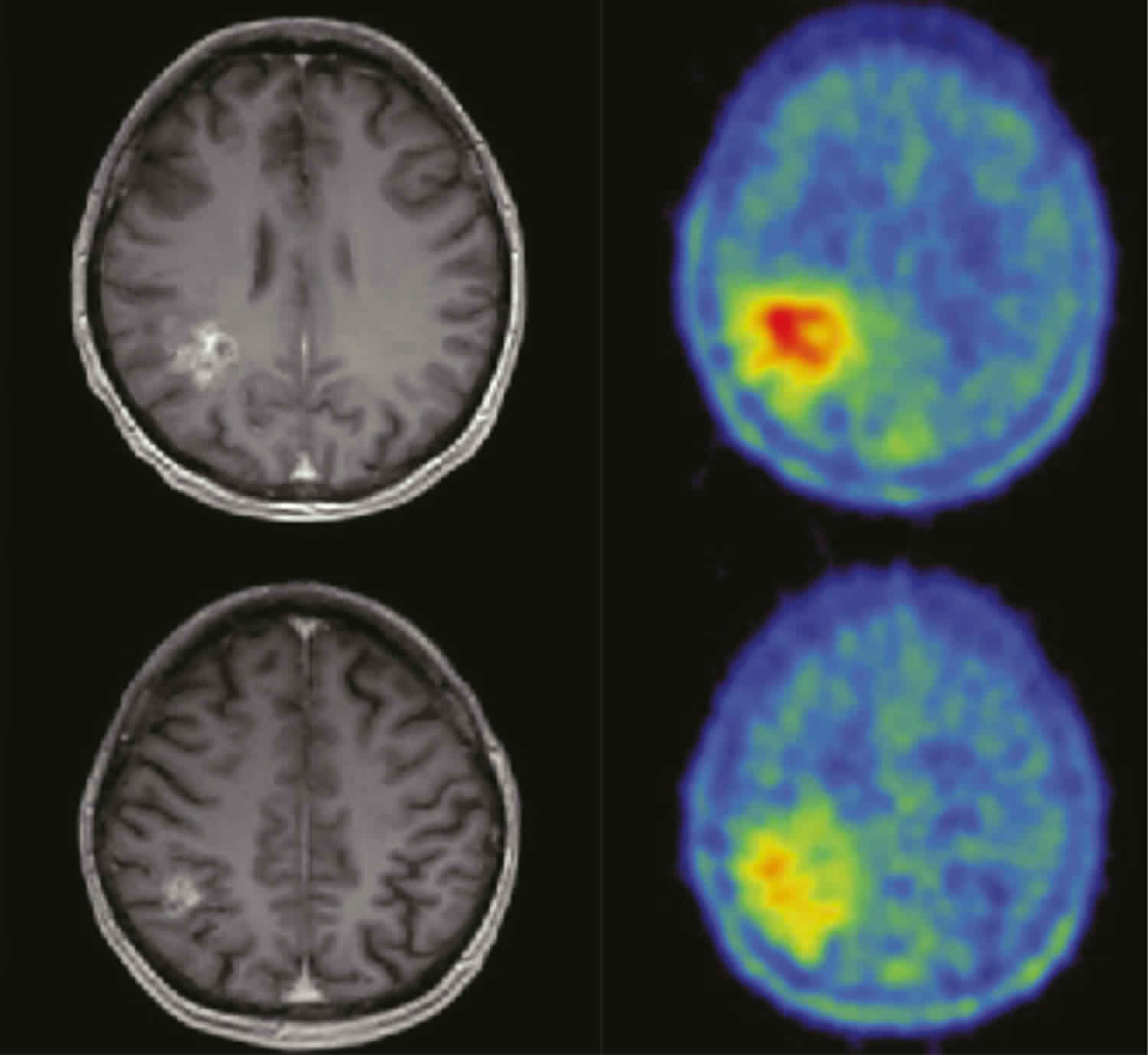
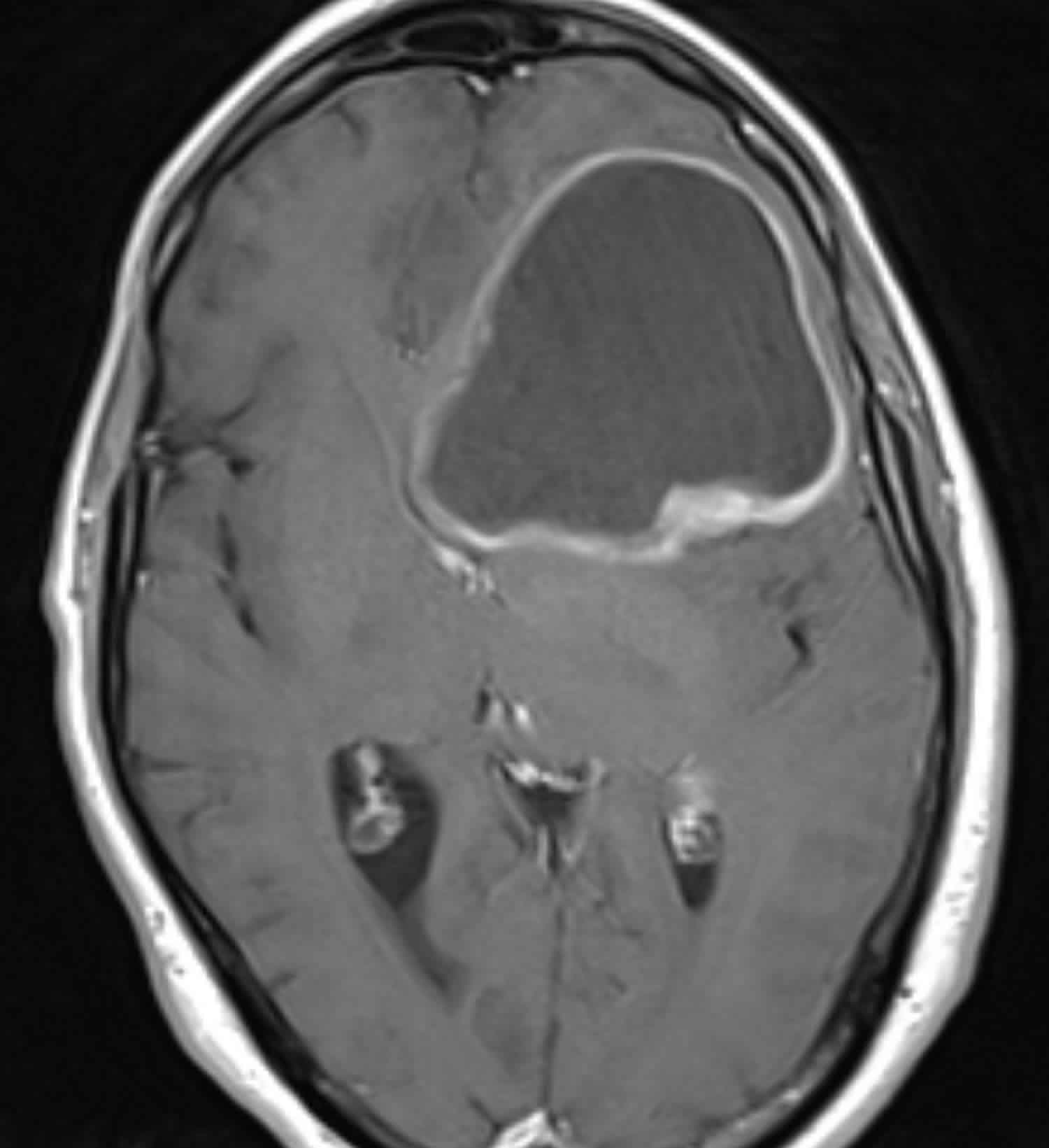
Figure 1. Glioblastoma multiforme (MRI)
What is glioma brain cancer?
Glioma is brain tumor that start in glial cells. These are the supporting cells of the brain and the spinal cord. There are different types of gliomas.
There are 3 types of glial cells:
- Astrocytes – tumor that start in these cells are called astrocytoma or glioblastoma
- Oligodendrocytes – tumor that start in these cells are called oligodendrogliomas
- Ependymal cells – tumor that start in these cells are called ependymomas
About 3 out of 10 of all brain tumors are gliomas. Most fast-growing brain tumors are gliomas. The most common type is called astrocytoma.
What are astrocytomas?
Astrocytomas are tumors that start in glial cells called astrocytes. Astrocytes are star shaped cells. They support the nerve cells (neurones) in the brain. Astrocytomas are the most common type of brain tumors in both adults and children. About 2 out of 10 brain tumors are astrocytomas. Symptoms of astrocytoma depend on where the tumor is in the brain. Common symptoms include headaches and seizures (fits).
Most astrocytomas can spread widely throughout the brain and blend with the normal brain tissue, which can make them very hard to remove with surgery. Sometimes they spread along the cerebrospinal fluid (CSF) pathways. It is very rare for them to spread outside of the brain or spinal cord.
As with other types of brain tumors, astrocytomas are often grouped by grade (according to how quickly they are likely to grow).
- Low-grade (grade 1 or 2) astrocytomas tend to grow slowly. These include:
- Non-infiltrating (grade 1) astrocytomas, which do not usually grow into nearby tissues and tend to have a good prognosis. Examples include pilocytic astrocytomas and subependymal giant cell astrocytomas (SEGAs). These are more common in children than in adults.
- Grade 2 astrocytomas, such as diffuse astrocytomas and pleomorphic xanthoastrocytomas (PXAs). These tumors tend to be slow growing, but they can grow into nearby areas, which can make them harder to remove with surgery. These tumors can become more aggressive and faster growing over time.
- High-grade (grade 3 or 4) astrocytomas tend to grow quickly and spread into the surrounding normal brain tissue. These include:
- Anaplastic (grade 3) astrocytomas
- Glioblastomas (grade 4) are also called glioblastoma multiforme (GBM), which are the fastest growing. These tumors make up more than half of all gliomas and are the most common malignant brain tumors in adults.
Does glioblastoma affect children?
Yes it can, but glioblastoma is much more common in adults than in children.
Glioblastoma classification
Glioblastomas are divided in the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System into 1:
- Glioblastoma isocitrate dehydrogenase (IDH)-wildtype (about 90 % of cases), which corresponds most frequently with the clinically defined primary glioblastoma or de novo glioblastoma and predominates in patients over 55 years of age 36
- Glioblastoma isocitrate dehydrogenase (IDH)-mutant (about 10 % of cases), which corresponds closely to so-called secondary glioblastoma with a history of prior lower grade diffuse glioma and preferentially arises in younger patients 36
- Glioblastoma NOS (not otherwise specified), a diagnosis that is reserved for those tumors for which full IDH evaluation cannot be performed. The definition of full IDH evaluation can differ for glioblastomas in older patients relative to glioblastomas in younger adults and relative to World Health Organization (WHO) grade 2 and grade 3 diffuse gliomas: in the latter situations, IDH sequencing is highly recommended following negative R132H IDH1 immunohistochemistry, whereas the near absence of non-R132H IDH1 and IDH2 mutations in glioblastomas from patients over about 55 years of age suggests that sequencing may not be needed in the setting of negative R132H IDH1 immunohistochemistry in such patients 37.
One provisional new variant of glioblastoma has been added to the classification is epithelioid glioblastoma. It joins giant cell glioblastoma and gliosarcoma under the umbrella of IDH-wildtype glioblastoma 1. Epithelioid glioblastomas feature large epithelioid cells with abundant eosinophilic cytoplasm, vesicular chromatin, and prominent nucleoli (often resembling melanoma cells), and variably present rhabdoid cells. They have a predilection for children and younger adults, typically present as superficial cerebral or diencephalic masses, and often harbor a BRAF V600E mutation (which can be detected immunohistochemically) 38, 39. In one series, rhabdoid glioblastomas were distinguished from their similarly appearing epithelioid counterparts on the basis of loss of INI1 expression 40. IDH-wildtype epithelioid glioblastomas often lack other molecular features of conventional adult IDH-wildtype glioblastomas, such as EGFR amplification and chromosome 10 losses; instead, there are frequent hemizygous deletions of ODZ3. Such cases may have an associated low-grade precursor, often but not invariably showing features of pleomorphic xanthoastrocytoma 41.
Glioblastoma with primitive neuronal component was added as a pattern in glioblastoma. This pattern, previously referred to in the literature as glioblastoma with PNET-like component, is usually comprised of a diffuse astrocytoma of any grade (or oligodendroglioma in rare cases) that has well-demarcated nodules containing primitive cells that display neuronal differentiation (e.g., Homer Wright rosettes, gain of synaptophysin positivity and loss of GFAP expression) and that sometimes has MYC or MYCN amplification; these tumors have a tendency for craniospinal fluid dissemination 42. About a quarter develop in patients with a previously known lower grade glioma precursor, a subset of which shows R132H IDH1 immunoreactivity in both the glial and primitive neuronal components 43. From a clinical point of view, the recognition of this pattern may prompt evaluation of the craniospinal axis to rule out tumor seeding.
Small cell glioblastoma/astrocytoma and granular cell glioblastoma/astrocytoma remain patterns, the former characterized by uniform, deceptively bland small neoplastic cells often resembling oligodendroglioma and frequently demonstrating EGFR amplification, and the latter by markedly granular to macrophage-like, lysosome-rich tumor cells. In both examples, there is a particularly poor glioblastoma-like prognosis even in the absence of microvascular proliferation or necrosis.
Glioblastoma signs and symptoms
The signs and symptoms of brain tumors differ from person to person. Some people may have symptoms that suggest there is a brain tumor, others have no obvious symptoms. Signs and symptoms depend on where the tumor forms in the brain, what the affected part of the brain controls, and the size of the tumor 11, 44.
Commonly, people with brain tumor experience long-term headaches, seizures or convulsions, difficulty thinking and speaking/finding words, personality changes, tingling or stiffness in one side of the body, a loss of balance, vision changes, nausea, and/or disorientation.
Consult with your doctor if you have any of the following symptoms:
- Headaches that are new or worsening, especially in the morning or when lying down (these are often the first symptom of a brain tumor)
- Frequent nausea or vomiting
- Dizziness, loss of balance and coordination or room spinning
- Vision, hearing, and speech problems
- Difficulty with balance and trouble walking
- Weakness on one side or part of the body
- Unusual sleepiness or change in activity level
- Changes in personality or mood, such as anger, irritability or emotional withdrawal
- Changes to how you think
- Inability to focus or vision loss
- Confusion
- Seizures
- Loss of consciousness
- Impaired sense of smell or taste
- Drowsiness and fatigue
- Endocrine dysfunction (hormone/gland changes)
You may notice other signs, like memory problems or difficulty speaking or remembering words.
Having one or more of the symptoms above does not necessarily mean that you have a brain or spinal cord tumor. All of these symptoms can have other causes. Due to these unspecific symptoms, glioma is often misdiagnosed as infections, inflammatory processes and circulatory and immunological diseases 11. The occurrence of back and leg pain and sciatica may also suggest a herniated lumbar 45. The occurrence of seizures in people who have not been previously diagnosed with epilepsy can also be an indication for neuroimaging because of glioblastoma suspicion 46. Still, if you have any of these symptoms, especially if they don’t go away or get worse over time, see your doctor so the cause can be found and treated, if needed.
Glioblastoma causes
The exact cause of glioblastoma is still not known 47. But there are some factors that may increase your risk of a brain tumor. Like many other cancers, glioblastoma is sporadic, although a study showed a high prevalence (17%) of prior therapeutic radiation therapy among patients with glioblastoma 48. The latency between irradiation and the development of glioblastoma varies from a few years to several decades.
The vast majority of glioblastoma patients do not have a family history of cancer 49. Approximately 5% of all gliomas are familial 50 and there are multiple rare inherited syndromes that involve adult glioma and glioblastoma 51 such as: tuberous sclerosis 52, Turcot syndrome 53, multiple endocrine neoplasia type 2A 54 and neurofibromatosis type 1 (NF1) 55.
Genome-wide association studies of genetic risk factors have validated 25 single nucleotide polymorphisms associated with increased risk for glioma, where 11 are specific to glioblastoma 56. While the biological significance of these associations remains to be elucidated, this genome-wide approach identified loci containing critical glioma genes such as telomerase reverse transcriptase (TERT), RTEL1, epidermal growth factor receptor (EGFR), and cyclin-dependent kinase inhibitor 2B (CDKN2B) 56. The majority of these loci are associated with molecularly defined glioma subtypes 57. Continued improvements in accurate measurement of potential risk factors and advances in technology allowing for discovery of additional germline and tumor molecular features will be critical to future understanding of causes and risk factors for glioblastoma.
Acquired head injuries, which occurred as a result of a brain contusion, may predispose to the onset of glioblastoma 58, 59.
Development of glioblastoma is related to deregulation of the G1/S checkpoint in the cell cycle 60 and occurrence of many genetic disturbances in glioma cells (loss of genetic material within chromosome 10q, amplification of EGFR, FGFR2, IRS2 and AKT3 genes as well as mutations in PTEN, TP16, TP53, PARK2, PTPRD and NF1 genes) 61.
Among women, a higher risk of its occurrence is noted in postmenopausal women, so a hypothesis on the involvement of sex hormones in glioblastoma development was created 62. Incidence of this tumor is also related to height and body mass index (BMI) – high values of these two features increase the risk of glioblastoma incidence 63.
Viruses, such as human cytomegalovirus (CMV), are also believed to be among the etiologic agents for glioma development 28. Human cytomegalovirus (CMV) induces congenital encephalitis and multi-organ changes in immunocompromised adults. Human cytomegalovirus shows tropism for glial cells. The virus encodes proteins (such as IE1, US28, GB), which activate intracellular signaling pathways involved in mitogenesis, mutagenesis, apoptosis, inflammation and angiogenesis. Products of these genes cause dysregulation of the key signaling pathways (including PDGFR, Akt, STAT3), but also cause disturbances in monocyte and glial cell functions 64. It is believed that granulocyte-colony stimulating factor (G-CSF) is involved in the development of glioblastomas – a high level of expression of this glycoprotein and its receptor (G-CSFR) was found in glioblastomas of different grades of malignancy. Granulocyte-colony stimulating factor stimulates proliferation and migration of a commercially available glioblastoma cell line. Blocking of G-CSFR by antibody results in inhibition of the cell growth and mobility in test tube study 65.
The following chemicals are considered as potentially dangerous: pesticides, polycyclic aromatic compounds and solvents. Electromagnetic fields and certain metals are also considered to be involved in glioma development 66. It is believed that the use of a mobile phone does not increase the risk of developing glioblastoma, but the effect of long-term use of mobile phones is still undetermined. Glioblastoma multiforme can be considered as an occupational disease – persons employed in the rubber and petrochemical industry are considered to be at a higher risk of glioma incidence 67.
Some studies showed the risk of glioblastoma with decreased susceptibility to allergy, immune factors, immune genes, and some single nucleotide polymorphisms detected by genome-wide association studies 68. Studies have shown a low risk of gliomas with allergies and atopic diseases 69. Also, in the short term, less than 10 years use of anti-inflammatory medications is associated with a protective effect on glioblastoma 70. There is no substantial evidence of glioblastoma association with lifestyle factors like smoking, alcohol consumption, drug use, or exposure to N-nitroso compounds 71.
Studies have shown that the use of mobile phones doesn’t increase the risk of development of glioblastoma; however, association with long term use needs further confirmation 28.
Table 1. Inherited syndromes associated with adult gliomas and adult glioblastomas
| Gene Symbol (Chromosome Location) | Disorder/Syndrome (Online Mendelian Inheritance in Man ID) | Mode of Inheritance | Phenotypic Features | Associated Brain Tumors |
|---|---|---|---|---|
| APC, MMR (5q21) | Familial adenomatous polyposis (FAP, 175100), Turcots syndrome type 2 | Dominant | Development of multiple adenomatous colon polyps (>100), predisposition to colorectal cancer, and brain tumors | Medulloblastoma, glioma |
| ATM (11q22.3) | Ataxia- telangiectasia (208900) | Autosomal recessive trait | Progressive cerebellar ataxia, susceptibility to infections, predisposition to lymphoma and lymphocytic leukemia. | Astrocytoma and medulloblastoma |
| CDKN2A (9p21.3) | Melanoma-neural system tumor syndrome (155755) | Dominant | Predisposition to malignant melanoma and malignant brain tumors | Glioma |
| IDH1/IDH2 (2q33.3/15q26.1) | Ollier disease | Acquired post-zygotic mosaicism, dominant with reduced penetrance | Development of intraosseous benign cartilaginous tumors, cancer predisposition | Glioma |
| MLH1, PMS2 | Turcots syndrome type 1 | Autosomal recessive trait | Development of multiple adenomatous colon polyps (<100), predisposition to colorectal cancer, and brain tumors | Medulloblastoma, glioma, |
| MSH2,MLH1,MSH6,PMS2 | Lynch syndrome (120435), biallelic mismatch repair deficiency, constitutional MMR deficiency | Dominant | Predisposition to gastrointestinal, endometrial and other cancers | Glioblastoma, other gliomas |
| MSH2,MLH1,MSH6,PMS2 | Mismatch repair deficiency syndrome (276300) | Recessive | Pediatric cancer predisposition; café-au-lait spots; colon polyps | Glioma |
| NF1 (17q11.2) | Neurofibromatosis 1 (NF1) (162200) | Dominant | Neurofibromas, schwannomas, café-au-lait macules | Astrocytoma, schwannomas, optic nerve glioma |
| RB1 (13q14) | Retinoblastoma | Dominant | Development of multiple tumors of the eye, increased risk of some brain tumors | Retinoblastoma, pineoblastoma, malignant glioma |
| TP53 (17p13.1) | Li–Fraumeni syndrome (151623) | Dominant | Predisposition to numerous cancers, especially breast, brain, and soft-tissue sarcoma | Glioblastoma, other gliomas |
| TSC1,TSC2 (9q34.14,16p13.3) | Tuberous sclerosis (TSC) (191100, 613254) | Dominant | Development of multisystem nonmalignant tumors | Giant cell astrocytoma |
Abbreviations: ATM = ataxia telangiectasia; APC = adenomatous polyposis coli; CDKN2A = cyclin-dependent kinase inhibitor 2A; MLH1 = MutL homolog 1, colon cancer, nonpolyposis type 2; MSH2 = MutS protein homolog 2; MSH6 = MutS protein homolog 6; OMIM = Online Mendelian Inheritance in Man; PMS2 = postmeiotic segregation increased homolog 2; RB1 = retinoblastoma transcriptional corepressor 1; TP53 = tumor protein p53.
[Source 72 ]Risk factors for developing glioblastoma
Risk factors for developing glioblastoma include:
- Your age. Your risk of a brain tumor increases as you age. Gliomas are most common in adults between ages 45 and 65 years old. However, a brain tumor can occur at any age. Certain types of gliomas, such as ependymomas and pilocytic astrocytomas, are more common in children and young adults.
- Exposure to radiation. People who have been exposed to a type of radiation called ionizing radiation have an increased risk of brain tumor. Examples of ionizing radiation include radiation therapy used to treat cancer and radiation exposure caused by atomic bombs. More-common forms of radiation, such as electromagnetic fields from power lines and radiofrequency radiation from microwave ovens have not been shown to increase the risk of glioma. It isn’t clear whether cellphone use increases the risk of brain cancer. Some studies have found a possible association between cellphone use and a type of brain cancer called acoustic neuroma. Many other studies have found no association. Because cellphones are a relatively new factor, more long-term research is needed to understand the potential impact on cancer risk. For the time being, if you’re concerned about the possible link between cellphones and cancer, experts recommend limiting your exposure by using a speaker or hands-free device, which keeps the cellphone itself away from your head.
- Family history of glioma. It’s rare for glioma to run in families. But having a family history of glioma can double the risk of developing it. Some genes have been weakly associated with glioma, but more study is needed to confirm a link between these genetic variations and brain tumors.
Glioblastoma diagnosis
If it’s suspected that you have a glioblastoma, your doctor may recommend a number of tests and procedures, including:
- A neurological exam. A neurological exam may include, among other things, checking your vision, hearing, balance, coordination, strength and reflexes. Difficulty in one or more areas may provide clues about the part of your brain that could be affected by a brain tumor.
- Imaging tests. Magnetic resonance imaging (MRI) and computed tomography (CT) scans are used most often to look for brain diseases. These scans will almost always show a brain tumor, if one is present. Doctors can often also get an idea about what type of tumor it might be, based on how it looks on the scan and where it is in the brain.
- Magnetic resonance imaging (MRI) scan. MRI scans use radio waves and strong magnets (instead of x-rays) to make pictures. A contrast material called gadolinium may be injected into a vein before the scan to help see details better. MRI scans are very good for looking at the brain and spinal cord and are considered the best way to look for tumors in these areas. The images they provide are usually more detailed than those from CT scans. But they do not pick up the bones of the skull as well as CT scans and therefore may not show the effects of tumors on the skull.
- Special types of MRI can be useful in some situations:
- Magnetic resonance angiography (MRA) and magnetic resonance venography (MRV): These special types of MRI may be used to look at the blood vessels in the brain. This can be very useful before surgery to help the surgeon plan an operation.
- Magnetic resonance spectroscopy (MRS): This test can be done as part of an MRI. It measures biochemical changes in an area of the brain (displayed in graph-like results called spectra, although basic images can also be created). By comparing the results for a tumor to that of normal brain tissue, it can sometimes help determine the type of tumor (or how quickly it is likely to grow), although a biopsy of the tumor is often still needed to get an accurate diagnosis. Magnetic resonance spectroscopy (MRS) can also be used after treatment to help determine if an area that still looks abnormal on another test is remaining tumor or if it is more likely to be scar tissue.
- Magnetic resonance perfusion: For this test, also known as perfusion MRI, a contrast dye is injected quickly into a vein. A special type of MR image is then obtained to look at the amount of blood going through different parts of the brain and tumor. Tumors often have a bigger blood supply than normal areas of the brain. A faster growing tumor may need more blood. Perfusion MRI can give doctors an idea of the best place to take a biopsy. It can also be used after treatment to help determine if an area that still looks abnormal is remaining tumor or if it is more likely to be scar tissue.
- Functional MRI (fMRI): This test looks for tiny blood flow changes in an active part of the brain. It can be used to determine what part of the brain handles a function such as speech, thought, sensation, or movement. Doctors can use this to help determine which parts of the brain to avoid when planning surgery or radiation therapy. This test is similar to a standard MRI, except that you will be asked to do specific tasks (such as answering simple questions or moving your fingers) while the scans are being done.
- Special types of MRI can be useful in some situations:
- Computed tomography (CT) scan. A CT scan uses x-rays to make detailed cross-sectional images of your brain and spinal cord (or other parts of the body). Unlike a regular x-ray, a CT scan creates detailed images of the soft tissues in the body. CT scans are not used as often as MRI scans when looking at brain or spinal cord tumors, but they can be useful in some cases. They may be used if MRI is not an option (such as in people who are very overweight or people who have a fear of enclosed spaces). CT scans also show greater detail of the bone structures near the tumor. As with MRI, you may get an injection of a contrast dye through an IV (intravenous) line before the scan (although a different dye is used for CT scans). This helps better outline any tumors that are present.
- CT angiography (CTA): For this test, you are injected with a contrast material through an IV line while you are in the CT scanner. The scan creates detailed images of the blood vessels in the brain, which can help doctors plan surgery. CT angiography can provide better details of the blood vessels in and around a tumor than MR angiography in some cases.
- Positron emission tomography (PET) scan. For a PET scan, you are injected with a slightly radioactive substance (usually a type of sugar known as FDG) which collects mainly in tumor cells. A special camera is then used to create a picture of areas of radioactivity in the body. The picture is not as detailed as a CT or MRI scan, but it can provide helpful information about whether abnormal areas seen on other tests (such as MRIs) are likely to be tumors or not. This test is more likely to be helpful for fast-growing (high-grade tumors) than for slower-growing tumors. This test is also useful after treatment to help determine if an area that still looks abnormal on an MRI scan is remaining tumor or if it is more likely to be scar tissue. Remaining tumor might show up on the PET scan, while scar tissue will not.
- Chest x-ray. A chest x-ray might be done to look for tumors in the lungs if a tumor is found in the brain. This is because in adults, most tumors in the brain actually have started in another organ (most often the lung) and then spread to the brain. This test can be done in a doctor’s office, in an outpatient radiology center, or in a hospital.
- Magnetic resonance imaging (MRI) scan. MRI scans use radio waves and strong magnets (instead of x-rays) to make pictures. A contrast material called gadolinium may be injected into a vein before the scan to help see details better. MRI scans are very good for looking at the brain and spinal cord and are considered the best way to look for tumors in these areas. The images they provide are usually more detailed than those from CT scans. But they do not pick up the bones of the skull as well as CT scans and therefore may not show the effects of tumors on the skull.
- Brain or spinal cord tumor biopsy. Imaging tests such as MRI and CT scans may show an abnormal area that is likely to be a brain or spinal cord tumor. But these scans can’t always tell exactly what type of tumor it is. Often this can only be done by removing some of the tumor tissue in a procedure called a biopsy. A biopsy may be done as a procedure on its own, or it may be part of surgery to remove the tumor. Sometimes, a tumor may look so characteristically obvious on an MRI scan (for example, clearly looking like an astrocytoma) that a biopsy is not needed, especially if the tumor is in a part of the brain that would make it hard to biopsy (such as the brain stem). In rare cases a PET scan or MR spectroscopy may give enough information so that a biopsy is not needed. The 2 main types of biopsies for brain tumors are:
- Stereotactic (needle) biopsy. Stereotactic (needle) biopsy may be used if, based on imaging tests, surgery to remove the tumor might be too risky (such as with some tumors in vital areas, those deep within the brain, or other tumors that probably can’t be removed safely with surgery) but a sample is still needed to make a diagnosis. The patient may be asleep (under general anesthesia) or awake during the biopsy. If the patient is awake, the neurosurgeon injects a local anesthetic into areas of skin above the skull to numb them. The skull and brain do not feel pain. The biopsy itself can be done in two main ways:
- One approach is to get an MRI or CT, and then use either markers (each about the size of a nickel) placed on different parts of the scalp, or facial and scalp contours, to create a map of the inside of the head. An incision (cut) is then made in the scalp, and a small hole is drilled in the skull. An image-guidance system is then used to direct a hollow needle into the tumor to remove small pieces of tissue.
- In another approach that’s being used less often, a rigid frame is attached to the head. An MRI or CT scan is often used along with the frame to help the neurosurgeon guide a hollow needle into the tumor. This also requires an incision in the scalp and a small hole in the skull.
- The removed tissue is sent to a pathologist (a doctor specializing in diagnosis of diseases by lab tests). Sometimes it might need to be looked at by a neuropathologist, a pathologist who specializes in nervous system diseases. The pathologist looks at it under a microscope (and might do other lab tests) to determine if the tumor is benign or malignant (cancerous) and exactly what type of tumor it is. This is very important in determining a person’s prognosis (outlook) and the best course of treatment. A preliminary diagnosis might be available the same day, although it often takes at least a few days to get a final diagnosis.
- Surgical or open biopsy (craniotomy). If imaging tests show the tumor can likely be treated with surgery, the neurosurgeon may not do a needle biopsy. Instead, an operation called a craniotomy might be done to remove all or most of the tumor. (If removing all of the tumor would likely damage nearby important structures, removing most of the tumor, known as debulking, might be done.) For a preliminary diagnosis, small samples of the tumor are looked at right away by the pathologist while the patient is still in the operating room. This can help guide treatment, including whether further surgery should be done at that time. A final diagnosis is made within a few days in most cases.
- Stereotactic (needle) biopsy. Stereotactic (needle) biopsy may be used if, based on imaging tests, surgery to remove the tumor might be too risky (such as with some tumors in vital areas, those deep within the brain, or other tumors that probably can’t be removed safely with surgery) but a sample is still needed to make a diagnosis. The patient may be asleep (under general anesthesia) or awake during the biopsy. If the patient is awake, the neurosurgeon injects a local anesthetic into areas of skin above the skull to numb them. The skull and brain do not feel pain. The biopsy itself can be done in two main ways:
- Lab tests of biopsy specimens. Finding out which type of tumor someone has is very important in helping to determine their outlook (prognosis) and treatment options. But in recent years, doctors have found that changes in certain genes, chromosomes, or proteins within the cancer cells can also be important. Some tumors are now tested for these types of changes. For example:
- Gliomas that are found to have IDH1 or IDH2 gene mutations tend to have a better outlook than gliomas without these gene mutations.
- In high-grade gliomas, the presence of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation is linked with better outcomes and a higher likelihood of responding to chemotherapy.
- Lab tests looking for other gene or chromosome changes might also be done.
- Lumbar puncture (spinal tap). Lumbar puncture (spinal tap) is used mainly to look for cancer cells in the cerebrospinal fluid (CSF), the liquid that surrounds the brain and spinal cord. For this test, you lie on your side on a bed or exam table with your knees up near your chest. The doctor first numbs an area in the lower part of the back near the spine. A small, hollow needle is then placed between the bones of the spine to withdraw some of the fluid. This fluid is sent to a lab to be looked at for cancer cells. Other tests may be done on the fluid as well. Lumbar punctures are usually very safe, but doctors have to make sure the test does not result in a large drop in fluid pressure inside the skull, which could possibly cause serious problems. For this reason, imaging tests such as CT or MRI scans are done first. Lumbar punctures usually aren’t done to diagnose brain tumors, but they may be done to help determine the extent of a tumor by looking for cancer cells in the CSF. They are often used if a tumor has already been diagnosed as a type that can commonly spread through the CSF, such as an ependymoma. Lumbar punctures are particularly important in people with suspected brain lymphomas because lymphoma cells often spread into the CSF.
- Blood and urine tests. Blood and urine tests rarely are part of the actual diagnosis of brain and spinal cord tumors, but they may be done to check how well the liver, kidneys, and some other organs are working. This is especially important before any planned surgery. If you are getting chemotherapy, blood tests will be done routinely to check blood counts and to see if the treatment is affecting other parts of your body.
If you’re uncertain about your diagnosis, consider seeking a second opinion at a medical center where many brain biopsies are evaluated every year.
Definitive diagnosis is based on histopathological examination of the intraoperatively removed tumor or its parts, using traditional histological, cytologic and histochemical methods 73. When neurosurgical tumor resection is not possible, fine needle aspiration biopsy is performed 74.
Testing to assess for the presence or absence of glial fibrillary acidic protein (GFAP), isocitrate dehydrogenase (IDH) mutation status [IDH1 or IDH2], and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status is usually recommended. MGMT methylation status is useful to predict response to specific chemotherapeutic agents 75. The presence of IDH 1/2 mutation doesn’t guide directly towards specific treatment, but most IDH 1/2 mutant glioblastoma has methylation of MGMT promotor guiding therapy indirectly. Also, IDH 1/2 mutation denotes improved prognosis and eligibility for clinical trials 25.
Verification of a primary diagnosis is performed on the basis of immunohistochemistry for the presence in the glioma cells of glial fibrillary acidic protein (GFAP), which is a major intermediate filament protein of mature astrocytes with the mass of 50 kD 76, 77. The glial fibrillary acidic protein (GFAP) is the most specific marker of astrocytes, both in normal and pathological conditions. It is believed that this protein plays a role in maturation of astrocytes. Increasing malignancy of tumors of astrocytic origin is associated with the loss of GFAP expression 78. Similar effects are observed for glioblastoma multiforme – glioma cells negative for GFAP proliferate faster in comparison to positive cells. Loss of GFAP expression indicates significantly undifferentiated tumor cells, but does not decide about tumor progression and development 76. Sometimes, astrocytes with GFAP and characteristic lipomatous cytoplasm can occur 79. The acid protein S100 present in glial cells is another specific marker for tumors of the central nervous system, but its expression cannot constitute a basic criterion in differential diagnosis 80.
Glioblastoma stage
Staging of the tumors in the brain and spinal cord is based on criteria defined by the World Health Organization (WHO) 1, which includes assessment of their morphology, grade of malignancy (grade I–IV), proliferative index, response to treatment and survival time. Grade 1 includes non-malignant tumors, grade 2 is used for relatively non-malignant tumors, grade 3 includes tumors of low-grade malignancy, while grade 4 denotes the most malignant tumors, with median survival of 6–12 months. Glioblastoma multiforme is classified as grade 4 because they grow rapidly and invade surrounding brain tissue 81.
Completely staging most glioblastoma multiforme is neither practical nor possible because these tumors do not have clearly defined margins 82. Rather, glioblastoma multiforme exhibit well-known tendencies to invade locally and spread along compact white matter pathways, such as the corpus callosum, internal capsule, optic radiation, anterior commissure, fornix, and subependymal regions. Such spread may create the appearance of multiple glioblastomas or multicentric gliomas on imaging studies.
Careful histological analyses have indicated that only 2-7% of glioblastoma multiforme are truly multiple independent tumors rather than distant spread from a primary site. Despite its rapid infiltrative growth, the glioblastoma tends not to invade the subarachnoid space and, consequently, rarely metastasizes via cerebrospinal fluid (CSF). Hematogenous spread to extraneural tissues is very rare in patients who have not had previous surgical intervention, and penetration of the dura, venous sinuses, and bone is exceptional 83.
Glioblastoma treatment
Glioblastoma treatment consists of surgery to safely remove as much of the brain tumor as possible followed by radiation and chemotherapy. The brain surgeon (neurosurgeon) will try to remove as much of the tumor as possible without injuring surrounding normal brain tissue and compromising normal neurological function. Surgery alone is not enough for glioblastoma as these tumors are very invasive in nature. These tumor cells invade surrounding brain tissue, making it nearly impossible to ever remove the tumor entirely. Surgery, however, is an important part of current glioblastoma therapy, because it allows for removal of the solid tumor and any cells that may make the tumor resistant to radiation and chemotherapy, debulking of the tumor, and reduction of intracranial pressure.
Glioblastoma treatment options include:
- Surgery to remove the glioblastoma. Surgery is presently an essential component to prolonging the lives of glioblastoma patients. Your brain surgeon (neurosurgeon) will work to remove the glioblastoma. The goal is to remove as much of the tumor as possible. The surgery for glioblastoma is a craniotomy. The skull is opened to reach the tumor with assistance from intra-operative mapping techniques. The patient can be awake for the surgery. The areas of the brain are mapped with the patient’s assistance. The doctor will then decide which portions of the tumor are safe to remove. But because glioblastoma grows into the normal brain tissue and complete removal isn’t possible. For this reason, most people receive additional treatments after surgery to target the remaining cells.
- Radiation therapy. Radiation therapy uses high-energy beams, such as X-rays or protons, to kill cancer cells. After surgery and once your wound has healed, radiation can begin. The goal of radiation is to kill the remaining tumor cells that have infiltrated the normal brain tissue. During radiation therapy, you lie on a table while a machine moves around you, directing beams to precise points in your brain. Radiation therapy is usually recommended after surgery and may be combined with chemotherapy. For people who can’t undergo surgery, radiation therapy and chemotherapy may be used as a primary treatment. Radiation is administered 5 days/week for 6 weeks. A total of 10 to 30 treatments are given. The use of radiation therapy provides patients with better outcomes than surgery alone.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. In some cases, thin, circular wafers containing chemotherapy medicine may be placed in your brain during surgery. The wafers dissolve slowly, releasing the medicine and killing cancer cells. After surgery, the chemotherapy drug temozolomide (Temodar) — taken as a pill — is often used during and after radiation therapy. The drug is generally administered everyday during radiation therapy, and then for 6-12 cycles after radiation. Each cycle lasts 28 days with temozolomide given the first five days of each cycle followed by 23 days of rest. Temozolomide is only effective for about 20% of patients. Before considering chemotherapy, patients are encouraged to talk to their neuro-oncologists. Other types of chemotherapy may be recommended if your glioblastoma recurs. These other types of chemotherapy are often administered through a vein in your arm.
- Tumor treating fields (TTF) therapy. Tumor treating fields (TTF) therapy uses an electrical field to disrupt the tumor cells’ ability to multiply. Tumor treating fields (TTF) therapy involves applying adhesive pads to your scalp. The pads are connected to a portable device that generates the electrical field. TTF is combined with chemotherapy and may be recommended after radiation therapy.
- Targeted drug therapy. Targeted drugs focus on specific abnormalities in cancer cells that allow them to grow and thrive. The drugs attack those abnormalities, causing the cancer cells to die. Bevacizumab (Avastin) targets the signals that glioblastoma cells send to the body that cause new blood vessels to form and deliver blood and nutrients to cancer cells. Bevacizumab may be an option if your glioblastoma recurs or doesn’t respond to other treatments 84.
- Clinical trials. Clinical trials are studies of new treatments. These studies give you a chance to try the latest treatment options, but the risk of side effects may not be known. Ask your doctor whether you might be eligible to participate in a clinical trial.
- Supportive (palliative) care. Palliative care is specialized medical care that focuses on providing relief from pain and other symptoms of a serious illness. Palliative care specialists work with you, your family and your other doctors to provide an extra layer of support that complements your ongoing care. Palliative care can be used while undergoing other aggressive treatments, such as surgery, chemotherapy or radiation therapy.
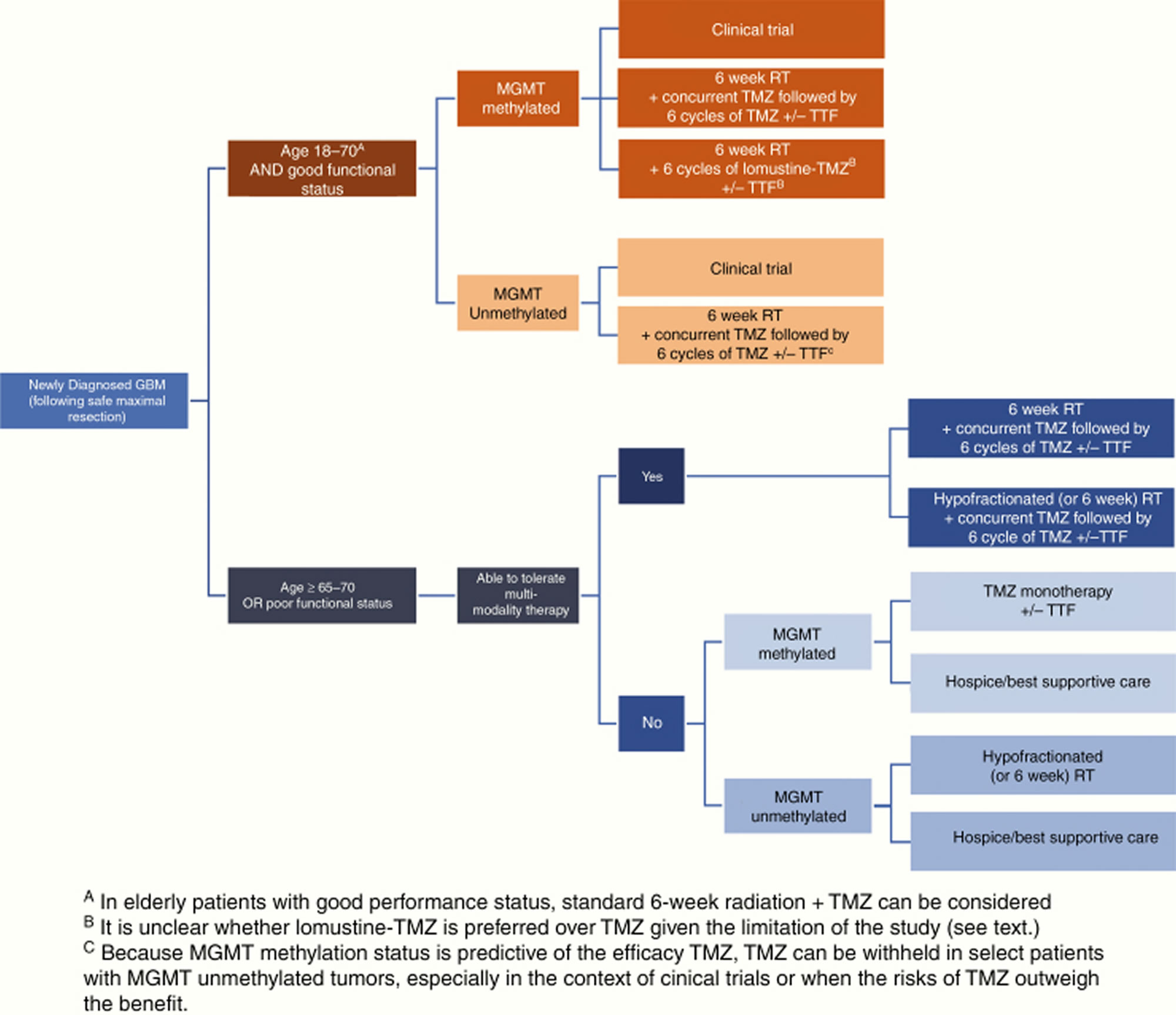
According to the 2020 National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology 34, glioblastoma multiforme treatment options depend on patient age, performance status (performance status) and O6-methylguanine DNA methyltransferase (MGMT) promoter methylation status (methylated vs. unmethylated). Patients aged 70 years or younger with a good performance status, regardless of the tumor’s MGMT methylation status, should receive standard brain radiation therapy plus concurrent and adjuvant temozolomide (TMZ) with alternating electric field therapy 85. Patients older than 70 years with good performance status should receive hypofractionated or standard brain radiation therapy plus concurrent and adjuvant temozolomide (TMZ) and alternating electric field therapy (TTF).
Figure 2. Newly diagnosed glioblastoma treatment algorithm
Abbreviations: GBM = glioblastoma multiforme; TMZ = temozolomide; RT = radiation therapy; MGMT = O6-methylguanine-DNA methyltransferase; TTF = tumor-treating fields.
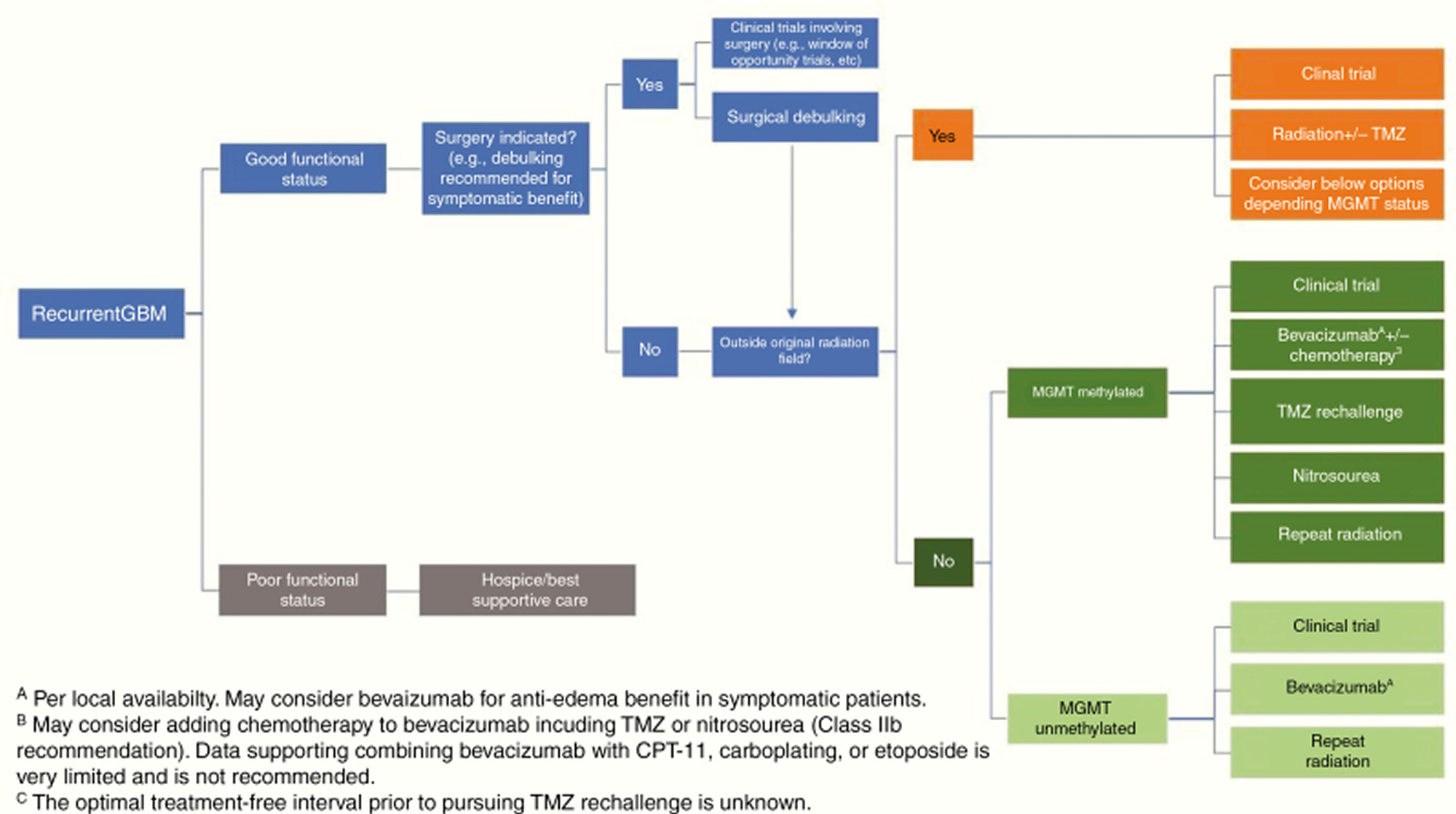
[Source 49 ]Figure 3. Recurrent glioblastoma treatment algorithm
Abbreviations: GBM = glioblastoma multiforme; TMZ = temozolomide; MGMT = O6-methylguanine-DNA methyltransferase; TTF = tumor-treating fields.
[Source 49 ]Surgery
The goal of surgery is the maximum safe resection to preserve neurological function with improved survival. Since glioblastoma infiltrates surrounding tissues, its complete resection is not always possible 28 and radiotherapy not always efficient 13.
Data from Surveillance, Epidemiology, and End Results (SEER) suggest gross total resection and subtotal resection is associated with improved survival compared to biopsy alone or no surgical intervention. The decision regarding subtotal resection vs. stereotactic biopsy vs. palliative surgery depends on location, age, comorbidities, and goals of care.
Most of the time, surgical resection is useful for definitive diagnosis and treatment. Postoperatively a repeat imaging should be done in 24 to 48 hours to assess the extent of resection.
Radiation therapy
The goal of radiotherapy is to deliver radiation to the tumor and to a margin of radiographically normal tissue to limit the recurrence. The radiation dose is 50-60 Gy delivered over a period of six weeks in fractions of 2 Gy 86. The two most common methods of delivering radiotherapy include three-dimensional conformal RT (3D-CRT) and intensity-modulated RT (IMRT).
According to National Comprehensive Cancer Network guidelines, hypofractionated radiotherapy can be recommended in patients with poor performance status or age >70 irrespective of performance status or methylated status of MGMT promoter either with or without chemotherapy.
Clinical trials are ongoing regarding the role of irradiation in patients with recurrent glioblastoma. More evidence is needed regarding dose, frequency, fractionation, and prior treatments, etc 87.
Studies have shown patients who had radiotherapy doses of 50 to 60 Gy had longer median survival than those who received lower postoperative radiation therapy doses 88. Studies have also shown marginal or no benefit of brachytherapy for high-grade gliomas given their infiltrative nature 89. Brachytherapy is a type of radiation therapy in which radioactive material sealed in needles, seeds, wires, or catheters is placed directly into or near a tumor.
Studies testing the efficacy of proton and neutron therapy are underway. Radiation sensitizers are the compounds given along with radiotherapy with an idea to increase its therapeutic effect. None of these compounds is approved for glioblastoma at present.
Side effects of radiation therapy include radiation dermatitis, neurocognitive toxicity, and endocrinopathies in the future.
Patients receiving concurrent chemotherapy with radiotherapy are at increased risk for leucopenia, thrombocytopenia, and hepatotoxicity. Weekly complete blood count (CBC) with differential and liver function tests are recommended. Severe thrombocytopenia can result in withholding of radiotherapy until blood counts are stabilized. Due to lymphopenia especially affecting CD4 count, pneumocystis pneumonia prophylaxis should be administered to all patients receiving daily chemotherapy while on radiotherapy.
Chemotherapy
According to National Comprehensive Cancer Network guidelines, patients with newly diagnosed MGMT-methylated glioblastoma, age 70 years or younger, should be treated with temozolomide and radiotherapy. Clinical trials have shown increased overall survival at 2 and 5 years on continued follow up in a group who received radiotherapy with concurrent daily temozolomide followed by six monthly cycles of adjuvant temozolomide. Results are similar in patients with age >60 and poor prognostic factors 90. Trials were conducted with a combined regimen of temozolomide and lomustine with radiation therapy as an alternative option in MGMT-methylated glioblastoma, which resulted in inconclusive results 91.
Treatment is similar in MGMT-unmethylated glioblastoma, age 70 years or younger; however, these patients derive less benefit from temozolomide when compared to the methylated group. Standard therapy with temozolomide and radiotherapy is recommended if MGMT status is unknown due to benefit from temozolomide with tolerable side effect profile along with the lack of available alternatives for unmethylated tumors.
In patients with age 70 years or older with good performance status, temozolomide and radiotherapy are recommended, But a hypofractionated radiation course can be done rather than the standard course. Twelve cycles of adjuvant temozolomide are recommended with hypofractionated radiotherapy rather than six cycles in such patients 92. In patients with age 70 years or older with poor performance status, single modality, either temozolomide or radiotherapy, can be considered to avoid side effects and toxicities. In such cases, MGMT methylation status can be helpful in deciding between chemo and radiotherapy 93.
No strong evidence exists for alternatives to temozolomide for MGMT- unmethylated glioblastomas, although trials have been conducted using a combination of bevacizumab/irinotecan in the past 94.
Temozolomide is given orally daily during radiation therapy. Adjuvant treatment starts four weeks after radiotherapy, and it is given for six cycles, daily for five days in a 28-day cycle. Side effects include leucopenia, thrombocytopenia, hepatotoxicity, nausea, constipation, fatigue, etc. Regular complete blood count (CBC) should be done, and therapy should be held if absolute neutrophil count (ANC) falls below 1500/microL or platelets fall below 100,000/microL.
Prophylaxis for Pneumocystis pneumonia (PCP) should be given to all patients receiving concomitant chemoradiotherapy due to the high risk of CD4 T cell depletion by temozolomide.
Magnetic resonance imaging (MRI) with contrast is recommended within one month after completing radiotherapy and then frequently every two months during adjuvant temozolomide to assess the disease status. Further recommendations, according to National Comprehensive Cancer Network, includes MRI every two to four months for two to three years, and less frequently after that.
Treatment recommendation guidelines:
- Age> 70 – radiotherapy plus concomitant therapy with temozolomide followed by adjuvant temozolomide with six cycles.
- Age< 65-70 – hypofractionated radiotherapy plus concomitant therapy with temozolomide followed by adjuvant temozolomide with 12 cycles.
Targeted drug therapy
Targeted therapy is a type of cancer treatment that targets proteins that control how cancer cells grow, divide, and spread. As researchers learn more about the DNA changes and proteins that drive cancer, they are better able to design treatments that target these proteins. Targeted cancer drugs attack those abnormalities, causing the cancer cells to die. Bevacizumab (Avastin) targets the signals that glioblastoma cells send to the body that cause new blood vessels to form and deliver blood and nutrients to cancer cells. Bevacizumab may be an option if your glioblastoma recurs or doesn’t respond to other treatments 84. Multiple studies of the humanized vascular endothelial growth factor (VEGF) antibody bevacizumab for glioblastoma have failed to demonstrate a survival benefit 95. However, bevacizumab is often effective in reducing peritumoral edema and related clinical symptoms and signs 96. Bevacizumab (Avastin) is approved in the United States and some other countries, but not in the European Union, for use in recurrent glioblastoma due to improvement in progression-free survival and reduction in corticosteroid use 95. Continuation of bevacizumab post progression did not improve outcome in a small study 97. Patients with recurrent glioblastoma should ideally be considered for clinical trials before receiving bevacizumab, as most trials exclude prior use of bevacizumab. Bevacizumab has also been proven to be effective in radiation-induced necrosis, although the doses used are lower than standard dosing for recurrent glioblastoma (typically 7.5 mg/kg every 3 week for a maximum of 4 treatments) 98.
Electric-field therapy
The Optune device also known as the NovoTTF-100A System, uses low-intensity, intermediate-frequency, alternating electric fields (tumor- treating fields) to target dividing cells in glioblastoma multiforme while generally not harming normal cells 99. The tumor-treating fields are generated via electrodes placed directly on the scalp. To target the tumor, array placement is based on the individual patient’s magnetic resonance imaging results.
Optune was initially approved in 2011 for use in glioblastoma multiforme that had recurred or progressed after treatment. In October 2015, the FDA expanded approval to include use of the device in conjunction with temozolomide chemotherapy in the first-line setting. Approval was based on an open-label, randomized phase 3 trial in 700 patients, in which median overall survival was 19.4 months with use of the device plus temozolomide, versus 16.6 months with chemotherapy only 99.
In a randomized, open-label trial in 695 patients with glioblastoma, the addition of tumor-treating fields to treatment with temozolomide improved median progression-free survival from 4.0 months to 6.7 months (hazard ratio 0.63). Median overall survival improved from 16.0 months to 20.9 months (hazard ratio 0.63) 100.
Corticosteroids
Corticosteroid drugs, preferably dexamethasone (in conjunction with gastric protection if used at high doses) are often given to reduce swelling around brain tumors 101. Dexamethasone alleviates neurologic deficits and signs of increased intracranial pressure such as headache and drowsiness. Low doses (eg, 4 mg/day given in 1–2 doses) are effective in most clinically symptomatic patients without signs of herniation 102. There is no need to give dexamethasone 4 times a day 102. Side effects of dexamethasone worsen with increased dose and duration of treatment 103. There is also growing evidence that corticosteroids may have an adverse effect on patient outcome, so they should be avoided if patients are not symptomatic 104. Patients on chronic corticosteroids (≥20 mg prednisone equivalents daily for ≥1 month) should be considered for prophylaxis for osteoporosis and pneumocystis jerovecii pneumonia 105.
Anti-seizure drugs (anticonvulsants)
Seizures affect 23% of glioblastoma patients at presentation and an additional 20% later in the disease course 106. While patients with seizures require anti-epileptic drugs (anticonvulsants), studies have not clearly shown a benefit of prolonged primary anti-epileptic drug prophylaxis in patients who have never had a seizure 107. Current guidelines recommend tapering anti-epileptic drugs 1–2 weeks after surgery and avoiding long-term prophylaxis 108. There is no role for primary perioperative prophylaxis (ie, in patients who have never had a seizure). A meta-analysis of 6 studies 109, a Cochrane systematic review 110 and a subsequent randomized trial of phenytoin versus no prophylaxis 111 have all shown no significant benefit from primary anti-epileptic drug prophylaxis. When anti-epileptic drugs are used, newer agents including levetiracetam and lacosamide are preferred over older drugs because of generally more favorable side effect profiles, reduced laboratory monitoring requirements, and lack of drug-drug interactions.110 Emerging data suggesting that neurons and glioma cells form synapses via AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors raises the possibility that anti-epileptic drugs that inhibit these receptors, such as perampanel, may be beneficial not only in controlling seizures, but also through possible antiglioma activity 112. However, a prior trial with another glutamate inhibitor, talampanel, was ultimately interpreted to be negative 113.
Anticoagulants
Venous thromboembolism (venous blood clots) risk is high in the perioperative period and persists well beyond, with one-year incidence of approximately 20% 114, mandating a low threshold for pursuing diagnostic studies 115. Most 116, 117, though not all 118, studies suggest that the risk of precipitating intratumoral hemorrhage with anticoagulants is acceptably low, even in patients receiving bevacizumab 119. The preferred anticoagulant is not well studied in brain tumors; in systemic cancer, low molecular weight heparin (LMWH) is preferred over warfarin 120. Direct oral anticoagulants (factor Xa and thrombin inhibitors) have been reported to be safe in patients with brain tumors 121. However, no randomized data are available for glioma patients and randomized trials on secondary prophylaxis of venous thromboembolism with direct oral anticoagulants enrolling cancer patients have generally shown a similar or slightly higher efficacy than low molecular weight heparin (LMWH) but with a slightly higher risk of bleeding 122.
A high incidence of recurrent venous thromboembolism with inferior vena cava (IVC) filters limits their use to patients with recent intracranial surgery, intratumoral hemorrhage, or absolute contraindications to anticoagulation 123. Prophylaxis with anticoagulation outside of the perioperative setting has not been definitively studied, as the only trial addressing this issue was prematurely terminated for slow accrual 124. A meta-analysis of pooled randomized clinical trial data indicated no survival benefit from anticoagulation in glioblastoma patients, but rather suggested that venous thromboembolism should be treated more vigorously in this patient population 125.
Other symptomatic treatments
Cognitive deficits, personality changes, and mood disturbances are major comorbidities for glioblastoma patients 101. Before treatment, up to 91% of brain tumor patients have cognitive deficits, with only moderate correlation with cognitive complaints 126. The frequent presence of fatigue and sleep disturbance contributes to cognitive impairment 127. Medical treatments with acetylcholinesterase inhibitors (donepezil) or psychostimulants (methylphenidate, modafinil) to prevent cognitive decline and fatigue after radiation therapy in patients with brain tumors (<50% were glioblastoma) have been unsuccessful 128. Although the 6-month prevalence of clinical depression is about 20% in brain tumor patients 129, randomized studies on medical treatment are lacking.
Regular exercise 130, adoption of a healthy diet, avoidance of hyperglycemia 131, early discussion of goals of care, and involvement of palliative care should be considered. Despite extensive interest in ketogenic diets and cannabinoids, there are currently no clinical data supporting their routine use.
Recurrent glioblastoma treatment
Glioblastoma patients invariably recur after a median interval of less than 7 months 132 and there is no clear standard-of-care salvage therapy (Figure 3). The National Comprehensive Cancer Network guidelines list clinical trials as the preferred option for eligible patients 133. Surgery may have a role for symptomatic and/or large lesions. However, only patients who undergo complete resections have any survival benefit 134. Other options include systemic therapy such as temozolomide rechallenge, nitrosoureas, bevacizumab, re-irradiation, and Tumor Treatment Fields (NovoTTF-100A system or Optune device in the US) 135, none of which have been shown to prolong survival in randomized trials in this setting, or palliative care for patients with poor performance status.
Although other chemotherapeutic agents such as irinotecan, carboplatin, procarbazine, and etoposide are sometimes used for patients with recurrent glioblastomas, there are no data suggesting that they are beneficial 136. A recent randomized phase 2 trial suggested that regorafenib, a VEGF receptor 2 and multikinase inhibitor, increased survival in patients with recurrent glioblastoma compared with lomustine 137.
Repeat radiation therapy in the form of radiosurgery or hypofractionated radiotherapy (30–35 Gy in 5–15 fractions) is increasingly used for recurrent glioblastoma, although there is currently no definitive data regarding benefit 138, 139. A secondary analysis of the NRG Oncology/RTOG 0525 trial showed no significant survival benefit of re-irradiation over systemic therapy after tumor progression 140. Preliminary results of the NRG phase 2 trial comparing bevacizumab alone versus bevacizumab with re-irradiation in patients with recurrent glioblastomas showed that the addition of re-irradiation improved progression-free survival (7.1 months with the combination vs 3.8 months with bevacizumab alone) but not overall survival 141.
Glioblastoma new treatment
Given the poor outcomes with current treatments, there is great interest in various experimental approaches under investigation 142.
Targeted Molecular (Precision) Therapies
Despite advances in understanding the molecular pathogenesis of glioblastoma, there has been only modest progress in developing effective targeted molecular therapies 143. Challenges include the paucity of agents that effectively cross the blood-brain barrier (BBB) 144, the relative lack of “easy” targets such as BRAFV600E mutations, redundant signaling pathways 145 and tumor heterogeneity 146.
The 2016 update of the WHO classification incorporated molecular parameters into the definition of certain brain tumors 1. Several of these markers are easily assessed by immunohistochemistry, including IDH1-R132H and histone H3 K27M, while other point mutations can be determined by sequencing. BRAFV600E mutation status, while challenging by immunohistochemistry is easily assessed by sequencing and has therapeutic implications for a subset of glioblastoma patients. For more comprehensive profiling, targeted NGS panels have proven to be useful, while some centers also have the capacity to perform whole exome sequencing or whole genome sequencing. Microsatellite instability can be readily assessed by either genome-wide or medium-sized panel approaches, and is relevant given the tumor-agnostic approval by the FDA for pembrolizumab for cancers with high microsatellite instability. Copy number variations—for example, the aforementioned chromosomal +7/−10 pattern—are relevant for glioma diagnosis and possibly treatment. Fusion detection, to identify a potentially relevant and druggable group of alterations (for example, fusions of neurotrophic-tropomyosin RTK [NTRK fusions]), requires specific coverage by either DNA-based approaches or alternatively mRNA-based analyses. Routine examination of these (and potentially additional) alterations will be critical if scientists are to make substantial steps forward for precision glioblastoma therapies 147.
Examples of putative treatment-predictive biomarkers exist. The most often investigated biomarkers, high level EGFR amplification and EGFRvIII mutation, have been targeted with and without tumor pretesting, with the aim of suppressing pathway activation with EGFR inhibitors such as erlotinib 148, targeting the heterogeneously expressed EGFRvIII neoantigen by vaccination with a peptide vaccine, rindopepimut 149 or using the conformational change for specific binding of an antibody-drug conjugate, depatuxizumab mafodotin (ABT414) 150, 151 without clinical activity 152. Targeting BRAFV600E mutations showed responses to monotherapy with Raf inhibitors such as vemurafenib 153 or dual therapy with combined BRAF/MEK inhibition with trametinib and dabrafenib 154, but these mutations are rare in glioblastoma except for epithelioid glioblastoma 155, a somewhat controversial entity likely to be often confused with pleomorphic xanthoastrocytoma. Other potentially targetable mutations, such as NTRK fusions 156, H3K27M mutations 157 and FGFR mutations and FGFR3-TACC3 fusions 158, are all uncommon in glioblastoma. Of note, mutations in the telomerase reverse transcriptase (TERT) promoter are found in up to 85% of glioblastomas 159, although to date this mutation has been challenging to target.
The lack of success in targeted therapy trials in glioblastoma is likely due to tumor heterogeneity, lack of knowledge of the contribution of genetic alterations to tumor maintenance, targeting subclonal or unstable genetic alterations instead of stable and clonal oncogenic drivers, redundant signaling pathways, use of archival instead of freshly obtained recurrent tumor tissue for biomarker testing, insufficient assessment of drug brain tumor concentrations, failure of target inhibition, and development of rapid secondary resistance and clonal selection.
Currently, most therapeutic strategies and biomarkers are focused on single or multiple biological features that are differentially detected in patient groups responding to a given therapy. In several studies post-hoc exploratory analyses suggested subsets of patients that may have benefited from experimental treatments, but in the absence of validation, these remain only hypothesis generating. For example, the proneural subtype of glioblastoma defined by expression analyses 160 or MRI features 161 may derive benefit from the addition of bevacizumab to standard treatment. Lower levels of carboxypeptidase G2 promoter methylation of cluster of differentiation (CD)95 ligand (CD95L) were correlated with improved overall survival with the CD95 inhibitory treatment asunercept (APG101) in combination with re-irradiation compared with re-irradiation alone 162. Also, based on a retrospective analysis, mammalian target of rapamycin (mTOR) Ser2448 phosphorylation may be a putative predictive biomarker of response to the mTOR inhibitor temsirolimus plus radiation in patients with newly diagnosed glioblastoma lacking MGMT promoter methylation 163. Others have suggested PTEN loss predicts benefit from mTOR inhibitors 164. Without preselection, mTOR inhibition is not only ineffective but may even confer a survival disadvantage compared with the standard of care. For example, the addition of a different mTOR inhibitor, everolimus, resulted in worse outcome in an unselected group of patients with newly diagnosed glioblastoma irrespective of MGMT status (Table 2) 165.
Several clinical trials are based on well-defined molecular characteristics of the tumor, confirmation of adequate drug penetration and biological efficacy (eg, target engagement and modulation in neoadjuvant, “window-of-opportunity” surgery-based trials), as well as necessary retrospective validation of potential biomarkers (Table 3). Several large clinical trials are underway where prospectively assigned biomarkers will enrich predefined patient cohorts for potentially benefiting patients. The National Center for Tumor Diseases‒Heidelberg Neuro Master Match (N2M2) (NCT03158389), a trial of molecularly matched targeted therapies plus radiation therapy in patients with newly diagnosed glioblastoma without MGMT promoter methylation is currently ongoing 166. Similarly, the National Cancer Institute MATCH trial, while designed mainly for extracranial solid tumors, does allow patients with glioblastoma if they meet the eligibility criteria. The Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT) trial evaluating EGFR, mTOR/DNA-PK, and CDK4/6 inhibitors 167 and the GBM Adaptive, Global, Innovative Learning Environment (AGILE) consortium 168 are taking a different approach by enrolling patients into unselected cohorts with given therapies first, assessing potential biomarkers as the trial accrues and integrating this information via adaptive randomization processes to enrich specific arms that may be showing benefit with particular biomarkers (Table 3) 168.
The extensive tumor heterogeneity in glioblastoma suggests that combination therapy may be more effective than treatment with single agents. However, combination studies to date have been associated with little activity and often significant toxicity, and increase the need for assessment of the targets in the tumor 143. Potentially, combinations of more potent selective agents with less off-target effects may be better tolerated. To address the issues of heterogeneity and redundant signaling pathways, there is significant interest in exploiting synthetic lethality (targeting tumor stem cells) 169 or common downstream pathways with agents such as marizomib, a proteasome inhibitor (NCT03345095), and selinexor, an exportin 1 inhibitor 170.
Table 2. Selected completed trials with targeted molecular therapies
| Molecular Target | Signaling Pathway | Therapy | Trial | Trial Concept (examples) | Trial Result |
|---|---|---|---|---|---|
| BRAFV600 mutation | Vemurafenib 153 | NCT01524978 | Basket trial with recurrent glioma arm | Objective response rate 25% overall; 3/6 glioblastoma had stable disease as best response | |
| BRAFV600E mutation | Dabrafenib + Trematenib 155 | NCT02034110 | Phase II basket trial using novel Bayesian hierarchical statistical design | Objective response rate for glioblastoma 29%; 62% for low grade gliomas | |
| EGFR amplification | Depatuxizumab mafodotin (ABT414) 150 | NCT02573324 (Intellance 1) | Randomized phase III trial in newly diagnosed glioblastoma with EGFR amplification comparing radiation therapy + temozolomide ± depatuxizumab mafodotin | 639 patients randomized Ocular toxicity common Depatuxizumab mafodotin median survival 18.9 Placebo: 18.7 Hazard ratio 1.02 | |
| EGFR amplification | Depatuxizumab mafodotin (Depatuxizumab mafodotin) (ABT414)206 | NCT02343406 (Intellance 2) | Randomized phase II in recurrent glioblastoma comparing Depatuxizumab mafodotin, Depatuxizumab mafodotin + temozolomide, or temozolomide alone | 260 patients 25–30% grade 3 or 4 Ocular toxicity Hazard ratio for the combination arm Depatuxizumab mafodotin+temozolomide compared with the temozolomide was 0.71 at initial analysis. On long-term follow-up, hazard ratio for the comparison of the depatuxizumab mafodotin+temozolomide compared with control was 0.66. Efficacy of depatuxizumab mafodotin monotherapy was comparable to that of temozolomide (hazard ratio = 1.04). | |
| Exportin 1 | Important for transport of tumor suppressor proteins and oncoprotein mRNA from nucleus to cytoplasm | Selinexor | NCT01986348 | Multi-arm phase II trial in recurrent glioblastoma | Objective response rate 10% Progression-free survival at 6 months 19% 6 cycle progression-free survival (24 weeks) 30% |
| FGFR mutations and FGFR-TACC gene fusions | Highly oncogenic FGFR mutations and FGFR-TACC gene fusion that confers sensitivity to FGFR inhibitors | AZD4547 | NCT02824133 | Phase I/II study in patients recurrent glioma positive for FGFR fusion | Not available |
| FGFR mutations and FGFR-TACC gene fusions | Highly oncogenic FGFR mutations and FGFR-TACC gene fusion that confers sensitivity to FGFR inhibitors | Infigratinib (BGJ398) 171 | NCT01975701 | Phase II study in recurrent glioblastoma with FGFR1-TACC1, FGFR3-TACC3 fusion and/or activating mutation in FGFR1, 2 or 3 | 26 patients objective response rate 7.7% 4 patients disease control > 1 year (2 FGFR1 mutations, 1 FGFR3 mutation, 1 FGFR3-TACC3 fusion) Progression-free survival at 6 months 16% |
| mTOR | Everolimus 165 | NCT01062399 | Randomized phase II trial of radiotherapy+temozolomide ± everolimus in newly diagnosed glioblastoma | 171 patients No difference in progression-free survival (median progression-free survival 8.2 months for everolimus vs 10.2 months for control) Overall survival for everolimus was inferior to that for control patients (median overall survival: 16.5 vs 21.2 months, respectively) | |
| mTOR | Temsirolimus | NCT01019434 | Randomized phase II of radiation therapy+temozolomide versus radiation therapy + temsirolimus in newly diagnosed unmethylated glioblastoma | 111 patients randomized Not difference in 1 year survival (72.2% in temozolomide arm; 69.6% in the temsirolimus arm (hazard ratio 1.16). Phosphorylation of mTORSer2448 in tumor (hazard ratio 0.13), detected in 37.6%, associated with benefit from temsirolimus | |
| Phosphatidylinositol 3-kinase (PI3K) | PIK3CA or PIK3R1 mutation, loss of PTEN activity through PTEN mutation, homozygous deletion or negative PTEN expression (<10% of tumor cells that stained positive), or positive phosphorylated AKTS473 (pAKTS473) | Buparlisib 172 | NCT01339052 | Multicenter, open-label, multi-arm, phase II trial in patients with PI3K pathway-activated glioblastoma at first or second recurrence | Objective response rate = 0 Progression-free survival at 6 months 8% Median progression-free survival 1.7 month |
| vascular endothelial growth factor | Bevacizumab 173 | NCT0094382 (AVAGlio) | Phase III placebo-controlled trial comparing radiation therapy + temozolomide ± bevacizumab | 921 patients randomized Median progression-free survival longer in the bevacizumab group than in the placebo group (10.6 months vs 6.2 months). Overall survival did not differ between groups. | |
| vascular endothelial growth factor | Bevacizumab 174 | NCT00884741 (RTOG 0825) | Phase III placebo-controlled trial comparing RT + temozolomide ± bevacizumab | 637 patients randomized No difference in overall survival (bevacizumab median, 15.7 months, control 16.1 months (hazard ratio 1.13) progression-free survival was longer in the bevacizumab group (10.7 months vs 7.3 months; hazard ratio, 0.79) | |
| vascular endothelial growth factor | Bevacizumab 95 | NCT01290939 (EORTC 26101) | Phase III trial comparing lomustine to lomustine + bevacizumab in recurrent glioblastoma | 437 patients randomized No survival advantage with addition of bevacizumab Median overall survival 9.1 months with lomustine compared with 8.6 months in combination group Progression-free survival 4.2 months with bevacizumab + lomustine compared with 1.5 month with lomustine alone | |
| vascular endothelial growth factor receptors 1, 2, and 3 and PDGF receptors | No test yet required | Regorafenib 137 | NCT02926222 | Randomized phase II comparing regorafenib with lomustine in patients with relapsed glioblastoma (REGOMA): | 7·4 months in the regorafenib group and 5·6 months in the lomustine group |
| vascular endothelial growth factorR2, cMET, AXL, RET | No testing required | Cabozantinib 175 | NCT00704288 | Single arm phase II in recurrent glioblastoma | 220 patients Bevacizumab naive 14.5–17.6% objective response rate; progression-free survival at 6 months 22.3 to 27.6% Bevacizumab failure 4.3% objective response rate. |
| CD95/CD95ligand | Lower levels of methylation of the CpG2 in the promoter of the CD95 ligand | Asunercept 162 | NCT01071837 NCT03152708 | Re-irradiation ± asunercept in progressive glioblastoma CAN008 biomarker CD95 Ligand and CpG2 methylation in Chinese patients with glioblastoma | Progression-free survival at 6 months rates were 3.8% for reirradiation and 20.7% for reirradiation+APG101. Ongoing |
Abbreviations: mTOR; mammalian target of rapamycin
[Source 49 ]Table 3. Selected ongoing trials with molecularly targeted treatments
| Molecular Target | Therapy | Phase | Design | Tumor Type | Trial |
|---|---|---|---|---|---|
| mouse double minute 2 | AMG-232 | Phase 0/I | • P53 wildtype status • Phase 0/I to measure concentrations in tumor in patients with recurrent glioblastoma and of AMG 232 in combination with radiation therapy in patients with newly diagnosed glioblastoma and unmethylated MGMT promoter | Recurrent and newly diagnosed glioblastoma | NCT03107780 |
| mTORC1/2 | Sapanisertib (MLN0128) | Phase 0 | • No selection • Phase 0 to evaluate tumor pharmacokinetics and pharmacodynamics effects | Recurrent glioblastoma | NCT02133183 |
| CDK4/6 | Abemaciclib | 0/II | • Patients with activation of CDK4/6 pathway and intact retinoblastoma | Recurrent glioblastoma | NCT02981940 |
| CDKs 1, 2, 7, and 9, JAK2 and FLT3 | Zotiraciclib (TG02) with metronomic temozolomide | I/randomized phase II | • Phase I with metronomic temozolomide followed by randomized phase II comparing zoltiraciclib = temozolomide versus temozolomide | Recurrent grade III glioma and glioblastoma | NCT02942264 |
| CDKs 1, 2, 7 and 9, JAK2 and FLT3 | Zotiraciclib (TG02) with metronomic temozolomide | I | • Zotiraciclib + radiation therapy for unmethylated MGMT patients • Zotiraciclib with temozolomide for methylated MGMT patients • Zotiraciclib alone for recurrent patients | Newly diagnosed and recurrent grade III glioma and glioblastoma in elderly population | NCT03224104 |
| H3K27M mutation | ONC201 (dopamine receptor D2 inhibitor and ClpP agonist) 176 | II | • H3K27M mutated gliomas • Non H3K27M mutated midline glioblastoma | Recurrent H3K27M mutated gliomas and other glioblastoma | NCT02525692 NCT03295396 |
| Interleukin-4 (IL-4) receptor | MDNA55 | II | • Convection enhanced delivery of genetically engineered IL-4 linked to a modified version of the Pseudomonas aeruginosa exotoxin A | Recurrent glioblastoma | NCT02858895 |
| hypoxia-inducible factor alpha | PTC2977 | II | • No selection | Recurrent glioblastoma | NCT02974738 |
| Proteasome | Marizomib | III | • No selection | Newly diagnosed glioblastoma | NCT03345095 |
| Platform Trials | |||||
| Alk mouse double minute 2 sonic hedgehog CDK4/6 mTOR | Alectinib Idasanutlin Vismodegib Palbociclib Temsirolimus | Phase II | • Umbrella trial N2M2/NOA-20 • Alk expression • P53wild-type/mouse double minute 2 high • sonic hedgehog activation • CDK4/6 high or codeletion of CDKN2A/B • Phospho mTOR Ser 24448 | Newly diagnosed glioblastoma without MGMT hypermethylation, targeted treatment according to molecular profile | NCT03158389 |
| EGFR mTOR/DNA pharmacokinetics CDK4/6 | Neratinib CC115 Abemaciclib | Bayesian adaptive randomized phase II platform trial | INSIGhT Agnostic (assessment post hoc) | Newly diagnosed unmethylated glioblastoma | NCT02977780 |
| Agnostic (assessment post hoc) | Multiple regimens (regorafenib) | Bayesian adaptive randomized phase II/III platform trial | glioblastoma AGILE Multiple | Newly diagnosed and recurrent glioblastoma | NCT03970447 |
Abbreviations: mTOR; mammalian target of rapamycin
[Source 49 ]Viral therapy and gene therapy
There has been resurgent interest in oncolytic viruses and gene therapy in clinical trials for glioblastoma 177. Oncolytic viruses are either natural viral strains or genetically engineered viruses designed to infect and/or replicate selectively in tumor cells 178. Gene therapy instead utilizes viruses that have been rendered replication incompetent but deliver anticancer cDNAs. With either therapy there is an initial phase of direct cytotoxic activity caused by oncolytic virus replication or the gene therapy-delivered anticancer cDNA. This cytotoxicity may then induce a second phase of innate and adaptive antitumor immunity caused by released tumor antigens 179. There is one oncolytic virus (talimogene laherparepvec) that has been FDA approved for melanoma 180 and several gene therapys have been approved since 2017, such as voretigene neparvovec (Luxturna) for blindness and onasemnogene abeparvovec (Zolgensma) for spinal muscular atrophy.
For glioblastoma, several phase I clinical trials of both oncolytic viruses and gene therapys are in progress or have been completed, usually in the recurrent setting 181. In most, the treatment is delivered by intratumoral injection at the time of surgery. Currently there are several open gene therapy trials that include: (i) injection in the resected recurrent glioblastoma cavity of an adenoviral gene therapy vector that delivers an IL-12 cDNA whose transcription is activated by an oral agent, veledimex (NCT03636477)259; and (ii) injection into the resected newly diagnosed glioblastoma cavity of an adenoviral vector that delivers a thymidine kinase cDNA that leads to cytotoxicity when subjects take the oral drug, valacyclovir, combined with chemoradiation (NCT03576612) 182. Both trials also entail neoadjuvant or adjuvant immune checkpoint inhibition to counteract T-cell dysfunction. The latter trial is also being tested in pediatric brain tumors. Currently open oncolytic virus trials in patients with recurrent glioblastoma include: (i) stereotactic injection of an oncolytic herpes simplex virus type 1 (oHSV) that delivers an IL-12 cDNA (NCT02062827); (ii) stereotactic injection of an oHSV that has been engineered to replicate better in glioblastoma cells that express the stem cell marker, nestin (NCT03152318); and (iii) monthly injections of oHSV G47∆ 183. There are also trials delivering oncolytic virus with stem cells: (i) intra-arterial delivery of allogeneic bone marrow‒derived human mesenchymal stem cells loaded with the oncolytic adenovirus DNX-2401 (BM-hMSCs-DNX2401) (NCT03896568); and (ii) injection of neural stem cells that deliver an oncolytic adenovirus into newly diagnosed glioblastoma (NCT03072134). A general conclusion is that both oncolytic virus and gene therapy treatments have been well tolerated. When post-tissue treatment is available, there has been evidence of increased infiltration of immune cells, including cytotoxic T cells that also may have upregulated inhibitory immune checkpoint signaling 184.
More advanced trials (phase II and beyond) are also ongoing mostly for oncolytic viruses in the recurrent glioblastoma setting. These include: (i) convection-enhanced delivery of an engineered poliovirus (PVSRIPO; NCT02986178) 185; (ii) stereotactic injection of an oncolytic adenovirus with selectivity for glioblastoma cells driven by the p16/retinoblastoma (RB) pathway and integrin expression (tasadenoturev; DNX-2401) in combination with pembrolizumab (NCT02798406) 186; and (iii) intracavitary injection after glioblastoma resection of a retrovirus that delivers a cytosine deaminase cDNA that provides chemosensitivity to 5-fluorocytosine (Toca 511; NCT02414165) 187. However, the phase III trial of Toca 511 was recently reported to show no survival benefit compared with standard of care in patients with recurrent glioblastoma 188. A phase III trial of another viral therapy, ofranergene obadenovec (VB-111), which targets tumor endothelium, also failed to show a survival advantage in combination with bevacizumab compared with bevacizumab alone 189, although it is possible that the simultaneous administration of bevacizumab may have impeded the effects of the virus 190. Therefore, while there is currently optimism in the pursuit of novel concepts, this optimism must also be coupled with ongoing efforts to find molecular and immunologic variables and targets that may be associated with benefit.
Glioblastoma vaccines
Several experimental therapeutic vaccines have been developed over the past decades to treat glioblastoma multiforme. Dendritic cell vaccines have been able to modify the immune response in patients with malignant neoplasms and induce anti-tumor immunity 191. Recent advances in vaccination with dendritic cells have achieved encouraging results in clinical trials and may improve the survival of patients with glioblastoma multiforme 191. A recent double-blind, randomized phase II trial 192 evaluated the efficacy of the ICT-107 vaccine based on autologous dendritic cells loaded with six epitopes targeting glioblastoma multiforme-associated antigens: melanoma antigen gene 1, human epidermal growth factor receptor 2 (HER-2), absent in melanoma 2, tyrosi-nase-related protein-2, gp100 (also known as premelanosome protein) and interleukin-13 receptor subunit α-2 in patients with newly diagnosed glioblastoma multiforme, whose major histocompatibility complex serotype was human leukocyte antigen (HLA)-A1+ and/or HLA-A2+. The vaccine increased progression-free survival by 2.2 months but did not increase overall survival (17 months for the treatment group and 15 for the control group).
Modified polio vaccine therapy
The poliovirus receptor CD155 is broadly upregulated on the surface of malignant solid tumors, and a preliminary study of intratumoral infusion of a modified poliovirus vaccine has demonstrated benefit in some cases of recurrent malignant glioma. In a dose-finding and toxicity study, 61 patients with recurrent supratentorial WHO grade 4 malignant glioma received seven doses of a live attenuated poliovirus type 1 vaccine with its cognate internal ribosome entry site replaced with that of human rhinovirus type 2. The recombinant nonpathogenic polio–rhinovirus chimera was infused into the glioma via an implanted catheter 185.
In contrast to overall survival rates in a historical control group, which declined steadily to 14% at 24 months and 4% at 36 months, overall survival in the study patients stabilized at 21% at 24 months, remaining at that rate through 36 months. Adverse events that affected more than 20% of the study patients in the dose-expansion phase included headache (52%), hemiparesis (50%), seizure (45%), dysphasia (28%), and cognitive disturbance (25%) 185.
CAR T-cell therapy
CAR T-cell therapy is a type of immunotherapy. You might also hear it called a type of adoptive cell transfer. CAR T-cell therapy is a very complex and specialist treatment. With this treatment, a specialist collects and makes a small change to your T cells. After a few weeks, you have a drip containing these cells back into your bloodstream. The CAR T-cells then recognize and attack the cancer cells.
There is a growing interest in CAR T cells, and more recently, CAR-transduced natural killer cells. CAR T cells have been engineered to express CAR molecules on their surface, which allow them to recognize and bind to specific antigens on tumor cells, leading to target cell killing in an HLA-independent manner 193. Although one patient with leptomeningeal spread of glioblastoma responded to treatment with CAR T cells against IL-13 receptor subunit alpha-2 194, the experience with CAR T cells to date for glioblastoma has been generally disappointing 193. Current efforts are focused on developing next generation CAR T cells directed against multiple antigens, designing them to induce epitope spreading, combining them with checkpoint inhibitors to help overcome immunosuppression or with conventional therapies such as radiotherapy, and delivering them directly into the tumor 195, 196.
Other therapies
Overall, almost 100 therapies are under evaluation for glioblastomas. In addition to the ones listed above, other treatments include cytotoxic agents such as Val 083 (NCT02717962), BAL101553 (NCT03250299), and agents that may augment the activity of temozolomide, such as ibudilast (NCT03782415).
Glioblastoma prognosis
Although glioblastoma does not spread to other parts of the body, it is a very aggressive, grade 4 brain cancer that grows and spreads quickly within the brain and, thus, has a poor prognosis. At the present time scientists haven’t yet to find a cure for glioblastoma multiforme. In the mid-1990s, the median length of survival for glioblastoma was only 8–10 months, but it has almost doubled to 15–18 months now 197, 198 , 199, 174. The current standard glioblastoma multiforme treatment is effective and has resulted in more people living two, three, four years and longer. In addition, in the mid-1990s, essentially no patients with glioblastoma survived 5 years after their diagnosis; now 5.5% to 15% of patients survive 5 years post-diagnosis 200. Once glioblastomas recur, median overall survival is estimated to be 24–44 weeks 201.
Various clinical factors are shown to affect survival glioblastoma like age, performance status, MGMT methylation status, and IDH1/2 mutation status. Young age, good performance status, MGMT methylation, IDH 1/2 mutant variety confers to have improved survival compared to their counterparts 202.
Figure 4. Glioblastoma 5-year relative survival
[Source 49 ]Glioblastoma life expectancy
Patients treated with optimal therapy, including surgical resection, radiation therapy, and chemotherapy, have a median survival of approximately 15–18 months, with fewer than 25% of patients surviving up to 2 years and fewer than 10% of patients surviving up to 5 years 14.
References- Louis, D.N., Perry, A., Reifenberger, G. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131, 803–820 (2016). https://doi.org/10.1007/s00401-016-1545-1
- Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014 Oct;23(10):1985-96. doi: 10.1158/1055-9965
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006 Apr 15;20(4):E1. doi: 10.3171/foc.2006.20.4.E1
- Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014 Oct;23(10):1985-96. doi: 10.1158/1055-9965.EPI-14-0275
- Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012 Mar;14(3):351-9. doi: 10.1093/neuonc/nor218
- Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019 Nov 1;21(Suppl 5):v1-v100. doi: 10.1093/neuonc/noz150
- Glioblastoma—Unraveling the Threads: A Q&A with Drs. Mark Gilbert and Terri Armstrong of the NIH Neuro-Oncology Branch. https://www.cancer.gov/news-events/cancer-currents-blog/2017/glioblastoma-research-making-progress
- Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013 Nov;15 Suppl 2(Suppl 2):ii1-56. doi: 10.1093/neuonc/not151. Erratum in: Neuro Oncol. 2014 May;16(5):760.
- Information about Glioblastoma. https://glioblastomafoundation.org/patients/glioblastoma-brain-tumor-information
- Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G; German Glioma Network. Long-term survival with glioblastoma multiforme. Brain. 2007 Oct;130(Pt 10):2596-606. doi: 10.1093/brain/awm204
- Lakhan SE, Harle L. Difficult diagnosis of brainstem glioblastoma multiforme in a woman: a case report and review of the literature. J Med Case Rep. 2009 Oct 30;3:87. doi: 10.1186/1752-1947-3-87
- Hur H, Jung S, Jung TY, Kim IY. Cerebellar glioblastoma multiforme in an adult. J Korean Neurosurg Soc. 2008 Apr;43(4):194-7. doi: 10.3340/jkns.2008.43.4.194
- Karcher S, Steiner HH, Ahmadi R, Zoubaa S, Vasvari G, Bauer H, Unterberg A, Herold-Mende C. Different angiogenic phenotypes in primary and secondary glioblastomas. Int J Cancer. 2006 May 1;118(9):2182-9. doi: 10.1002/ijc.21648
- Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci. 2018 Aug;54:7-13. doi: 10.1016/j.jocn.2018.05.002
- de Castro-Costa CM, de Araújo RW, de Arruda MA, de Araújo PM, de Figueiredo EG. Increased intracranial pressure in a case of spinal cervical glioblastoma multiforme. Analysis of these two rare conditions. Arq Neuropsiquiatr. 1994 Mar;52(1):64-8. doi: 10.1590/s0004-282×1994000100011
- Jung WH, Choi S, Oh KK, Chi JG. Congenital glioblastoma multiforme–report of an autopsy case. J Korean Med Sci. 1990 Dec;5(4):225-31. doi: 10.3346/jkms.1990.5.4.225
- Birbilis TA, Matis GK, Eleftheriadis SG, Theodoropoulou EN, Sivridis E. Spinal metastasis of glioblastoma multiforme: an uncommon suspect? Spine (Phila Pa 1976). 2010 Apr 1;35(7):E264-9. doi: 10.1097/BRS.0b013e3181c11748
- Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol. 2011 Nov;105(2):261-73. doi: 10.1007/s11060-011-0575-8
- Robert M, Wastie M. Glioblastoma multiforme: a rare manifestation of extensive liver and bone metastases. Biomed Imaging Interv J. 2008 Jan;4(1):e3. doi: 10.2349/biij.4.1.e3
- Mujic A, Hunn A, Taylor AB, Lowenthal RM. Extracranial metastases of a glioblastoma multiforme to the pleura, small bowel and pancreas. J Clin Neurosci. 2006 Jul;13(6):677-81. doi: 10.1016/j.jocn.2005.08.016
- al-Rikabi AC, al-Sohaibani MO, Jamjoom A, al-Rayess MM. Metastatic deposits of a high-grade malignant glioma in cervical lymph nodes diagnosed by fine needle aspiration (FNA) cytology–case report and literature review. Cytopathology. 1997 Dec;8(6):421-7. doi: 10.1111/j.1365-2303.1997.tb00573.x
- Tysnes BB, Mahesparan R. Biological mechanisms of glioma invasion and potential therapeutic targets. J Neurooncol. 2001 Jun;53(2):129-47. doi: 10.1023/a:1012249216117
- Mentrikoski M, Johnson MD, Korones DN, Scott GA. Glioblastoma multiforme in skin: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008 Aug;30(4):381-4. doi: 10.1097/DAD.0b013e31817532c4
- Kleihues P, Ohgaki H. Phenotype vs genotype in the evolution of astrocytic brain tumors. Toxicol Pathol. 2000 Jan-Feb;28(1):164-70. doi: 10.1177/019262330002800121
- Kanderi T, Gupta V. Glioblastoma Multiforme. [Updated 2021 Nov 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558954
- Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009 Dec;100(12):2235-41. doi: 10.1111/j.1349-7006.2009.01308.x
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007 May;170(5):1445-53. doi: 10.2353/ajpath.2007.070011
- Urbańska K, Sokołowska J, Szmidt M, Sysa P. Glioblastoma multiforme – an overview. Contemp Oncol (Pozn). 2014;18(5):307-12. doi: 10.5114/wo.2014.40559
- Ulutin C, Fayda M, Aksu G, Cetinayak O, Kuzhan O, Ors F, Beyzadeoglu M. Primary glioblastoma multiforme in younger patients: a single-institution experience. Tumori. 2006 Sep-Oct;92(5):407-11.
- Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993 May 20;26(2):239-44. doi: 10.1016/0360-3016(93)90203-8
- Agrawal A. Butterfly glioma of the corpus callosum. J Cancer Res Ther. 2009 Jan-Mar;5(1):43-5. doi: 10.4103/0973-1482.48769
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352(10):987-96. https://www.nejm.org/doi/10.1056/NEJMoa043330
- Khaddour K, Johanns TM, Ansstas G. The Landscape of Novel Therapeutics and Challenges in Glioblastoma Multiforme: Contemporary State and Future Directions. Pharmaceuticals (Basel). 2020 Nov 14;13(11):389. doi: 10.3390/ph13110389
- Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S, Butowski N, Campian JL, Clark SW, Fabiano AJ, Forsyth P, Hattangadi-Gluth J, Holdhoff M, Horbinski C, Junck L, Kaley T, Kumthekar P, Loeffler JS, Mrugala MM, Nagpal S, Pandey M, Parney I, Peters K, Puduvalli VK, Robins I, Rockhill J, Rusthoven C, Shonka N, Shrieve DC, Swinnen LJ, Weiss S, Wen PY, Willmarth NE, Bergman MA, Darlow SD. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020 Nov 2;18(11):1537-1570. https://doi.org/10.6004/jnccn.2020.0052
- Grothey A, Blay JY, Pavlakis N, Yoshino T, Bruix J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat Rev. 2020 Jun;86:101993. doi: 10.1016/j.ctrv.2020.101993
- Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013 Feb 15;19(4):764-72. doi: 10.1158/1078-0432.CCR-12-3002
- Chen L, Voronovich Z, Clark K, Hands I, Mannas J, Walsh M, Nikiforova MN, Durbin EB, Weiss H, Horbinski C (2014) Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neurooncology 16:1478–1483. doi:10.1093/neuonc/nou097
- Broniscer A, Tatevossian RG, Sabin ND, Klimo P Jr, Dalton J, Lee R, Gajjar A, Ellison DW (2014) Clinical, radiological, histological and molecular characteristics of paediatric epithelioid glioblastoma. Neuropathol Appl Neurobiol 40:327–336. doi:10.1111/nan.12093
- Kleinschmidt-DeMasters BK, Aisner DL, Foreman NK (2015) BRAF VE1 immunoreactivity patterns in epithelioid glioblastomas positive for BRAF V600E mutation. Am J Surg Pathol 39:528–540. doi:10.1097/PAS.0000000000000363
- Kleinschmidt-DeMasters BK, Alassiri AH, Birks DK, Newell KL, Moore W, Lillehei KO (2010) Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI-1 but not claudin 6. Am J Surg Pathol 34:341–354. doi:10.1097/PAS.0b013e3181ce107b
- Alexandrescu S, Korshunov A, Lai SH, Dabiri S, Patil S, Li R, Shih CS, Bonnin JM, Baker JA, Du E et al (2015) Epithelioid glioblastomas and anaplastic epithelioid pleomorphic xanthoastrocytomas—same entity or first cousins? Brain Pathol. doi:10.1111/bpa.12295
- Perry A, Miller CR, Gujrati M, Scheithauer BW, Zambrano SC, Jost SC, Raghavan R, Qian J, Cochran EJ, Huse JT et al (2009) Malignant gliomas with primitive neuroectodermal tumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol 19:81–90. doi:10.1111/j.1750-3639.2008.00167.x
- Joseph NM, Phillips J, Dahiya S, M Felicella M, Tihan T, Brat DJ, Perry A (2013) Diagnostic implications of IDH1-R132H and OLIG2 expression patterns in rare and challenging glioblastoma variants. Mod Pathol. 26:315–326. doi:10.1038/modpathol.2012.173
- Levine SA, McKeever PE, Greenberg HS. Primary cerebellar glioblastoma multiforme. J Neurooncol. 1987;5(3):231-6. doi: 10.1007/BF00151226
- Gee TS, Ghani AR, Idris B, Awang MS. Case report: a rare case of pediatric conus medularis glioblastoma multiforme. Med J Malaysia. 2012 Aug;67(4):438-41.
- Sanli AM, Turkoglu E, Dolgun H, Sekerci Z. Unusual manifestations of primary Glioblastoma Multiforme: A report of three cases. Surg Neurol Int. 2010 Dec 22;1:87. doi: 10.4103/2152-7806.74146
- Shafi O, Siddiqui G. Tracing the origins of glioblastoma by investigating the role of gliogenic and related neurogenic genes/signaling pathways in GBM development: a systematic review. World J Surg Oncol. 2022 May 10;20(1):146. doi: 10.1186/s12957-022-02602-5
- Hodges LC, Smith JL, Garrett A, Tate S. Prevalence of glioblastoma multiforme in subjects with prior therapeutic radiation. J Neurosci Nurs. 1992 Apr;24(2):79-83. doi: 10.1097/01376517-199204000-00005
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, Lim M, Mehta MP, Mellinghoff IK, Minniti G, Nathanson D, Platten M, Preusser M, Roth P, Sanson M, Schiff D, Short SC, Taphoorn MJB, Tonn JC, Tsang J, Verhaak RGW, von Deimling A, Wick W, Zadeh G, Reardon DA, Aldape KD, van den Bent MJ. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020 Aug 17;22(8):1073-1113. doi: 10.1093/neuonc/noaa106
- Ranger AM, Patel YK, Chaudhary N, Anantha RV. Familial syndromes associated with intracranial tumours: a review. Childs Nerv Syst. 2014 Jan;30(1):47-64. doi: 10.1007/s00381-013-2309-z
- Vijapura C, Saad Aldin E, Capizzano AA, Policeni B, Sato Y, Moritani T. Genetic Syndromes Associated with Central Nervous System Tumors. Radiographics. 2017 Jan-Feb;37(1):258-280. doi: 10.1148/rg.2017160057
- Padmalatha C, Harruff RC, Ganick D, Hafez GB. Glioblastoma multiforme with tuberous sclerosis. Report of a case. Arch Pathol Lab Med. 1980 Dec;104(12):649-50.
- Grips E, Wentzensen N, Sutter C, Sedlaczek O, Gebert J, Weigel R, Schwartz A, von Knebel-Doeberitz M, Hennerici M. Glioblastoma multiforme als Manifestation des Turcot-Syndroms [Glioblastoma multiforme as a manifestation of Turcot syndrome]. Nervenarzt. 2002 Feb;73(2):177-82. German. doi: 10.1007/s00115-001-1233-8
- Sánchez-Ortiga R, Boix Carreño E, Moreno-Pérez O, Picó Alfonso A. Glioblastoma multiforme y neoplasia endocrina múltiple tipo 2 A [Glioblastoma multiforme and multiple endocrine neoplasic type 2 A]. Med Clin (Barc). 2009 Jul 4;133(5):196-7. Spanish. doi: 10.1016/j.medcli.2008.06.021
- Broekman ML, Risselada R, Engelen-Lee J, Spliet WG, Verweij BH. Glioblastoma multiforme in the posterior cranial fossa in a patient with neurofibromatosis type I. Case Rep Med. 2009;2009:757898. doi: 10.1155/2009/757898
- Melin BS, Barnholtz-Sloan JS, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017 May;49(5):789-794. doi: 10.1038/ng.3823
- Labreche K, Kinnersley B, Berzero G, Di Stefano AL, Rahimian A, Detrait I, Marie Y, Grenier-Boley B, Hoang-Xuan K, Delattre JY, Idbaih A, Houlston RS, Sanson M. Diffuse gliomas classified by 1p/19q co-deletion, TERT promoter and IDH mutation status are associated with specific genetic risk loci. Acta Neuropathol. 2018 May;135(5):743-755. doi: 10.1007/s00401-018-1825-z
- Zhen L, Yufeng C, Zhenyu S, Lei X. Multiple extracranial metastases from secondary glioblastoma multiforme: a case report and review of the literature. J Neurooncol. 2010 May;97(3):451-7. doi: 10.1007/s11060-009-0044-9
- Moorthy RK, Rajshekhar V. Development of glioblastoma multiforme following traumatic cerebral contusion: case report and review of literature. Surg Neurol. 2004 Feb;61(2):180-4; discussion 184. doi: 10.1016/s0090-3019(03)00423-3
- Lam PY, Di Tomaso E, Ng HK, Pang JC, Roussel MF, Hjelm NM. Expression of p19INK4d, CDK4, CDK6 in glioblastoma multiforme. Br J Neurosurg. 2000 Feb;14(1):28-32. doi: 10.1080/02688690042870
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008 Oct 23;455(7216):1061-8. doi: 10.1038/nature07385. Epub 2008 Sep 4. Erratum in: Nature. 2013 Feb 28;494(7438):506.
- Kabat GC, Etgen AM, Rohan TE. Do steroid hormones play a role in the etiology of glioma? Cancer Epidemiol Biomarkers Prev. 2010 Oct;19(10):2421-7. doi: 10.1158/1055-9965.EPI-10-0658
- Benson VS, Pirie K, Green J, Casabonne D, Beral V; Million Women Study Collaborators. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer. 2008 Jul 8;99(1):185-90. doi: 10.1038/sj.bjc.6604445
- Cobbs CS. Evolving evidence implicates cytomegalovirus as a promoter of malignant glioma pathogenesis. Herpesviridae. 2011 Oct 26;2(1):10. doi: 10.1186/2042-4280-2-10
- Wang J, Yao L, Zhao S, Zhang X, Yin J, Zhang Y, Chen X, Gao M, Ling EA, Hao A, Li G. Granulocyte-colony stimulating factor promotes proliferation, migration and invasion in glioma cells. Cancer Biol Ther. 2012 Apr;13(6):389-400. doi: 10.4161/cbt.19237
- Spinelli V, Chinot O, Cabaniols C, Giorgi R, Alla P, Lehucher-Michel MP. Occupational and environmental risk factors for brain cancer: a pilot case-control study in France. Presse Med. 2010 Feb;39(2):e35-44. doi: 10.1016/j.lpm.2009.06.020
- Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002 Oct;4(4):278-99. doi: 10.1093/neuonc/4.4.278
- Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, Ballman KV, Berger M, Buckner JC, Chang S, Giannini C, Halder C, Kollmeyer TM, Kosel ML, LaChance DH, McCoy L, O’Neill BP, Patoka J, Pico AR, Prados M, Quesenberry C, Rice T, Rynearson AL, Smirnov I, Tihan T, Wiemels J, Yang P, Wiencke JK. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009 Aug;41(8):905-8. doi: 10.1038/ng.408
- Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM, Inskip PD. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002 May 10;99(2):252-9. doi: 10.1002/ijc.10320
- Scheurer ME, Amirian ES, Davlin SL, Rice T, Wrensch M, Bondy ML. Effects of antihistamine and anti-inflammatory medication use on risk of specific glioma histologies. Int J Cancer. 2011 Nov 1;129(9):2290-6. doi: 10.1002/ijc.25883
- Hochberg F, Toniolo P, Cole P, Salcman M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990 Feb;8(1):55-60. doi: 10.1007/BF00182087. Erratum in: J Neurooncol 1990 Aug;9(1):90.
- Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME, Bondy ML. Risk factors for childhood and adult primary brain tumors. Neuro Oncol. 2019 Nov 4;21(11):1357-1375. doi: 10.1093/neuonc/noz123
- Katsetos CD, Dráberová E, Legido A, Dumontet C, Dráber P. Tubulin targets in the pathobiology and therapy of glioblastoma multiforme. I. Class III beta-tubulin. J Cell Physiol. 2009 Dec;221(3):505-13. doi: 10.1002/jcp.21870
- Schultz S, Pinsky GS, Wu NC, Chamberlain MC, Rodrigo AS, Martin SE. Fine needle aspiration diagnosis of extracranial glioblastoma multiforme: Case report and review of the literature. Cytojournal. 2005 Nov 14;2:19. doi: 10.1186/1742-6413-2-19
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005 Mar 10;352(10):997-1003. doi: 10.1056/NEJMoa043331
- Wilhelmsson U, Eliasson C, Bjerkvig R, Pekny M. Loss of GFAP expression in high-grade astrocytomas does not contribute to tumor development or progression. Oncogene. 2003 May 29;22(22):3407-11. doi: 10.1038/sj.onc.1206372
- Messing A, Brenner M. GFAP: functional implications gleaned from studies of genetically engineered mice. Glia. 2003 Jul;43(1):87-90. doi: 10.1002/glia.10219
- Rutka JT, Murakami M, Dirks PB, Hubbard SL, Becker LE, Fukuyama K, Jung S, Tsugu A, Matsuzawa K. Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg. 1997 Sep;87(3):420-30. doi: 10.3171/jns.1997.87.3.0420
- Rickert CH, Riemenschneider MJ, Schachenmayr W, Richter HP, Bockhorn J, Reifenberger G, Paulus W. Glioblastoma with adipocyte-like tumor cell differentiation–histological and molecular features of a rare differentiation pattern. Brain Pathol. 2009 Jul;19(3):431-8. doi: 10.1111/j.1750-3639.2008.00199.x
- Dohan FC Jr, Kornblith PL, Wellum GR, Pfeiffer SE, Levine L. S-100 protein and 2′,3′-cyclic nucleotide 3′-phosphohydrolase in human brain tumors. Acta Neuropathol. 1977 Oct 10;40(2):123-8. doi: 10.1007/BF00688700
- Kleihues P, Soylemezoglu F, Schäuble B, Scheithauer BW, Burger PC. Histopathology, classification, and grading of gliomas. Glia. 1995 Nov;15(3):211-21. doi: 10.1002/glia.440150303
- Glioblastoma Multiforme Workup. https://emedicine.medscape.com/article/283252-workup#c7
- Lampl Y, Eshel Y, Gilad R, Sarova-Pinchas I. Glioblastoma multiforme with bone metastase and cauda equina syndrome. J Neurooncol. 1990 Apr. 8(2):167-72.
- Reardon DA, Herndon JE 2nd, Peters KB, Desjardins A, Coan A, Lou E, Sumrall AL, Turner S, Lipp ES, Sathornsumetee S, Rich JN, Sampson JH, Friedman AH, Boulton ST, Bigner DD, Friedman HS, Vredenburgh JJ. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer. 2012 Oct 23;107(9):1481-7. doi: 10.1038/bjc.2012.415
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017 Dec 19;318(23):2306-2316. doi: 10.1001/jama.2017.18718. Erratum in: JAMA. 2018 May 1;319(17):1824.
- Cabrera AR, Kirkpatrick JP, Fiveash JB, Shih HA, Koay EJ, Lutz S, Petit J, Chao ST, Brown PD, Vogelbaum M, Reardon DA, Chakravarti A, Wen PY, Chang E. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol. 2016 Jul-Aug;6(4):217-225. doi: 10.1016/j.prro.2016.03.007
- Sneed PK, Lamborn KR, Larson DA, Prados MD, Malec MK, McDermott MW, Weaver KA, Phillips TL, Wara WM, Gutin PH. Demonstration of brachytherapy boost dose-response relationships in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1996 Apr 1;35(1):37-44. doi: 10.1016/s0360-3016(96)85009-7
- Chang CH, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, Weinstein A, Nelson JS, Tsukada Y. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983 Sep 15;52(6):997-1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2
- Koot RW, Maarouf M, Hulshof MC, Voges J, Treuer H, Koedooder C, Sturm V, Bosch DA. Brachytherapy: Results of two different therapy strategies for patients with primary glioblastoma multiforme. Cancer. 2000 Jun 15;88(12):2796-802.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009 May;10(5):459-66. doi: 10.1016/S1470-2045(09)70025-7
- Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann RD, Krex D, Grauer O, Goldbrunner R, Schnell O, Bähr O, Uhl M, Seidel C, Tabatabai G, Kowalski T, Ringel F, Schmidt-Graf F, Suchorska B, Brehmer S, Weyerbrock A, Renovanz M, Bullinger L, Galldiks N, Vajkoczy P, Misch M, Vatter H, Stuplich M, Schäfer N, Kebir S, Weller J, Schaub C, Stummer W, Tonn JC, Simon M, Keil VC, Nelles M, Urbach H, Coenen M, Wick W, Weller M, Fimmers R, Schmid M, Hattingen E, Pietsch T, Coch C, Glas M; Neurooncology Working Group of the German Cancer Society. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019 Feb 16;393(10172):678-688. doi: 10.1016/S0140-6736(18)31791-4
- Martínez-Garcia M, Álvarez-Linera J, Carrato C, Ley L, Luque R, Maldonado X, Martínez-Aguillo M, Navarro LM, Vaz-Salgado MA, Gil-Gil M. SEOM clinical guidelines for diagnosis and treatment of glioblastoma (2017). Clin Transl Oncol. 2018 Jan;20(1):22-28. doi: 10.1007/s12094-017-1763-6
- Zarnett OJ, Sahgal A, Gosio J, Perry J, Berger MS, Chang S, Das S. Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol. 2015 May;72(5):589-96. doi: 10.1001/jamaneurol.2014.3739
- Herrlinger U, Schäfer N, Steinbach JP, Weyerbrock A, Hau P, Goldbrunner R, Friedrich F, Rohde V, Ringel F, Schlegel U, Sabel M, Ronellenfitsch MW, Uhl M, Maciaczyk J, Grau S, Schnell O, Hänel M, Krex D, Vajkoczy P, Gerlach R, Kortmann RD, Mehdorn M, Tüttenberg J, Mayer-Steinacker R, Fietkau R, Brehmer S, Mack F, Stuplich M, Kebir S, Kohnen R, Dunkl E, Leutgeb B, Proescholdt M, Pietsch T, Urbach H, Belka C, Stummer W, Glas M. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine-DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J Clin Oncol. 2016 May 10;34(14):1611-9. doi: 10.1200/JCO.2015.63.4691
- Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Le Rhun E, Dubois F, Weller M, von Deimling A, Golfinopoulos V, Bromberg JC, Platten M, Klein M, van den Bent MJ. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017 Nov 16;377(20):1954-1963. doi: 10.1056/NEJMoa1707358
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009 Oct 1;27(28):4733-40. doi: 10.1200/JCO.2008.19.8721
- Hovey EJ, Field KM, Rosenthal MA, Barnes EH, Cher L, Nowak AK, Wheeler H, Sawkins K, Livingstone A, Phal P, Goh C, Simes J; CABARET/COGNO investigators. Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: an exploratory randomized phase II trial. Neurooncol Pract. 2017 Sep;4(3):171-181. doi: 10.1093/nop/npw025
- Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011 Apr 1;79(5):1487-95. doi: 10.1016/j.ijrobp.2009.12.061. Erratum in: Int J Radiat Oncol Biol Phys. 2012 Sep 1;84(1):6. Grewal, Jai [added].
- FDA Expands Indication for Optune Device in Glioblastoma. https://www.medscape.com/viewarticle/852196
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017 Dec 19. 318 (23):2306-2316.
- Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Rudà R, Marosi C, Le Rhun E, Grant R, Oliver K, Oberg I, Bulbeck HJ, Rooney AG, Henriksson R, Pasman HRW, Oberndorfer S, Weller M, Taphoorn MJB; European Association of Neuro-Oncology palliative care task force. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017 Jun;18(6):e330-e340. doi: 10.1016/S1470-2045(17)30345-5
- Lim-Fat MJ, Bi WL, Lo J, Lee EQ, Ahluwalia MS, Batchelor TT, Chang SM, Chiocca EA, Chukwueke U, Cloughesy TF, Colman H, Deangelis LM, Galanis E, Gilbert MR, De Groot JF, Lassman AB, Liau LM, Mason W, McFaline-Figueroa JR, Mehta MP, Mellinghoff IK, Nabors LB, Nayak L, Reardon DA, Wen PY. Letter: When Less is More: Dexamethasone Dosing for Brain Tumors. Neurosurgery. 2019 Sep 1;85(3):E607-E608. doi: 10.1093/neuros/nyz186
- Ly KI, Wen PY. Clinical Relevance of Steroid Use in Neuro-Oncology. Curr Neurol Neurosci Rep. 2017 Jan;17(1):5. doi: 10.1007/s11910-017-0713-6
- Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M, Chan TA, Beal K, Bishop AJ, Barker CA, Jones TS, Hentschel B, Gorlia T, Schlegel U, Stupp R, Weller M, Holland EC, Hambardzumyan D. Corticosteroids compromise survival in glioblastoma. Brain. 2016 May;139(Pt 5):1458-71. doi: 10.1093/brain/aww046
- Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, Langston AA, Nastoupil LJ, Rajotte M, Rolston KV, Strasfeld L, Flowers CR. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018 Oct 20;36(30):3043-3054. doi: 10.1200/JCO.18.00374
- Chang SM, Parney IF, Huang W, Anderson FA Jr, Asher AL, Bernstein M, Lillehei KO, Brem H, Berger MS, Laws ER; Glioma Outcomes Project Investigators. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005 Feb 2;293(5):557-64. doi: 10.1001/jama.293.5.557
- Forsyth PA, Weaver S, Fulton D, Brasher PM, Sutherland G, Stewart D, Hagen NA, Barnes P, Cairncross JG, DeAngelis LM. Prophylactic anticonvulsants in patients with brain tumour. Can J Neurol Sci. 2003 May;30(2):106-12. doi: 10.1017/s0317167100053361
- Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, Grossman SA, Cairncross JG. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000 May 23;54(10):1886-93. doi: 10.1212/wnl.54.10.1886
- Kuijlen JM, Teernstra OP, Kessels AG, Herpers MJ, Beuls EA. Effectiveness of antiepileptic prophylaxis used with supratentorial craniotomies: a meta-analysis. Seizure. 1996 Dec;5(4):291-8. doi: 10.1016/s1059-1311(96)80023-9
- Pulman J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post-craniotomy seizures. Cochrane Database Syst Rev. 2013 Feb 28;(2):CD007286. doi: 10.1002/14651858.CD007286.pub2. Update in: Cochrane Database Syst Rev. 2015;(3):CD007286
- Wu AS, Trinh VT, Suki D, Graham S, Forman A, Weinberg JS, McCutcheon IE, Prabhu SS, Heimberger AB, Sawaya R, Wang X, Qiao W, Hess K, Lang FF. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013 Apr;118(4):873-883. doi: 10.3171/2012.12.JNS111970
- Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, Woo PJ, Taylor KR, Agarwal A, Regev A, Brang D, Vogel H, Hervey-Jumper S, Bergles DE, Suvà ML, Malenka RC, Monje M. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019 Sep;573(7775):539-545. doi: 10.1038/s41586-019-1563-y
- Grossman SA, Ye X, Chamberlain M, Mikkelsen T, Batchelor T, Desideri S, Piantadosi S, Fisher J, Fine HA. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009 Sep 1;27(25):4155-61. doi: 10.1200/JCO.2008.21.6895
- Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S; Glioma Outcomes Investigators. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003 Sep;99(3):467-73. doi: 10.3171/jns.2003.99.3.0467
- Czap AL, Becker A, Wen PY. Thrombotic Complications in Gliomas. Semin Thromb Hemost. 2019 Jun;45(4):326-333. doi: 10.1055/s-0039-1687892
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology. 1993 Jun;43(6):1111-4. doi: 10.1212/wnl.43.6.1111
- Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006 Mar 10;24(8):1310-8. doi: 10.1200/JCO.2005.04.6656. Erratum in: J Clin Oncol. 2006 May 1;24(13):2133.
- Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017 Jun 22;129(25):3379-3385. doi: 10.1182/blood-2017-02-767285
- Norden AD, Bartolomeo J, Tanaka S, Drappatz J, Ciampa AS, Doherty LM, Lafrankie DC, Ruland S, Quant EC, Beroukhim R, Wen PY. Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol. 2012 Jan;106(1):121-5. doi: 10.1007/s11060-011-0642-1
- Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003 Jul 10;349(2):146-53. doi: 10.1056/NEJMoa025313
- Carney BJ, Uhlmann EJ, Puligandla M, Mantia C, Weber GM, Neuberg DS, Zwicker JI. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019 Jan;17(1):72-76. doi: 10.1111/jth.14336
- Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR; Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018 Feb 15;378(7):615-624. doi: 10.1056/NEJMoa1711948
- Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. 2015 Apr;17(4):488-504. doi: 10.1093/neuonc/nou304
- Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, Malkin MG, Sawaya R, Baker R, Falanga A, Parpia S, Finch T, Levine MN. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010 Sep;8(9):1959-65. doi: 10.1111/j.1538-7836.2010.03973.x
- Le Rhun E, Genbrugge E, Stupp R, Chinot OL, Nabors LB, Cloughesy T, Reardon DA, Wick W, Gorlia T, Weller M. Associations of anticoagulant use with outcome in newly diagnosed glioblastoma. Eur J Cancer. 2018 Sep;101:95-104. doi: 10.1016/j.ejca.2018.06.029
- Gehring K, Taphoorn MJ, Sitskoorn MM, Aaronson NK. Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neurooncol Pract. 2015 Mar;2(1):20-31. doi: 10.1093/nop/npu035
- Armstrong TS, Shade MY, Breton G, Gilbert MR, Mahajan A, Scheurer ME, Vera E, Berger AM. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. 2017 Mar 1;19(3):323-335. doi: 10.1093/neuonc/now119
- Lee EQ, Muzikansky A, Drappatz J, Kesari S, Wong ET, Fadul CE, Reardon DA, Norden AD, Nayak L, Rinne ML, Alexander BM, Arvold ND, Doherty L, Stefanik J, LaFrankie D, Ruland SF, Pulverenti J, Smith KH, Gaffey SC, Hammond S, Wen PY. A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol. 2016 Jun;18(6):849-54. doi: 10.1093/neuonc/now007
- Rooney AG, McNamara S, Mackinnon M, Fraser M, Rampling R, Carson A, Grant R. Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. J Clin Oncol. 2011 Nov 10;29(32):4307-12. doi: 10.1200/JCO.2011.34.8466
- Ruden E, Reardon DA, Coan AD, Herndon JE 2nd, Hornsby WE, West M, Fels DR, Desjardins A, Vredenburgh JJ, Waner E, Friedman AH, Friedman HS, Peters KB, Jones LW. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011 Jul 20;29(21):2918-23. doi: 10.1200/JCO.2011.34.9852
- Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009 Mar 1;27(7):1082-6. doi: 10.1200/JCO.2008.19.1098
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352(10):987-96. doi: 10.1056/NEJMoa043330
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Central Nervus System Cancers. Version 3.2019-October 18, 2019. NCCN; 2019. NCCN.org.
- Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, Herrlinger U, Ketter R, Schlegel U, Marosi C, Reifenberger G, Wick W, Tonn JC, Wirsching HG. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016 Apr;18(4):549-56. doi: 10.1093/neuonc/nov326
- Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbalý V, Ram Z, Villano JL, Rainov N, Weinberg U, Schiff D, Kunschner L, Raizer J, Honnorat J, Sloan A, Malkin M, Landolfi JC, Payer F, Mehdorn M, Weil RJ, Pannullo SC, Westphal M, Smrcka M, Chin L, Kostron H, Hofer S, Bruce J, Cosgrove R, Paleologous N, Palti Y, Gutin PH. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012 Sep;48(14):2192-202. doi: 10.1016/j.ejca.2012.04.011
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Central Nervus System Cancers. Version 3.2019-October 18, 2019. NCCN; 2019. NCCN.org
- Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, Lolli I, Pace A, Daniele B, Pasqualetti F, Rizzato S, Bellu L, Pambuku A, Farina M, Magni G, Indraccolo S, Gardiman MP, Soffietti R, Zagonel V. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019 Jan;20(1):110-119. doi: 10.1016/S1470-2045(18)30675-2
- Straube C, Kessel KA, Zimmer C, Schmidt-Graf F, Schlegel J, Gempt J, Meyer B, Combs SE. A Second Course of Radiotherapy in Patients with Recurrent Malignant Gliomas: Clinical Data on Re-irradiation, Prognostic Factors, and Usefulness of Digital Biomarkers. Curr Treat Options Oncol. 2019 Jul 19;20(9):71. doi: 10.1007/s11864-019-0673-
- Scoccianti S, Francolini G, Carta GA, Greto D, Detti B, Simontacchi G, Visani L, Baki M, Poggesi L, Bonomo P, Mangoni M, Desideri I, Pallotta S, Livi L. Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol. 2018 Jun;126:80-91. doi: 10.1016/j.critrevonc.2018.03.024
- Shi W, Scannell Bryan M, Gilbert MR, Mehta MP, Blumenthal DT, Brown PD, Valeinis E, Hopkins K, Souhami L, Andrews DW, Tzuk-Shina T, Howard SP, Youssef EF, Lessard N, Dignam JJ, Werner-Wasik M. Investigating the Effect of Reirradiation or Systemic Therapy in Patients With Glioblastoma After Tumor Progression: A Secondary Analysis of NRG Oncology/Radiation Therapy Oncology Group Trial 0525. Int J Radiat Oncol Biol Phys. 2018 Jan 1;100(1):38-44. doi: 10.1016/j.ijrobp.2017.08.038
- Tsien C, Pugh S, Dicker A, et al.. ACTR-232. NRG Oncology RTOG 1205: randomized phase II trial of concurrent bevacizumab and re-irradiation versus bevacizumab alone as treatment for recurrent glioblastoma. Neuro Oncol. 2019;21:vi 20.
- Alexander BM, Cloughesy TF. Adult Glioblastoma. J Clin Oncol. 2017 Jul 20;35(21):2402-2409. doi: 10.1200/JCO.2017.73.0119
- Le Rhun E, Preusser M, Roth P, Reardon DA, van den Bent M, Wen P, Reifenberger G, Weller M. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019 Nov;80:101896. doi: 10.1016/j.ctrv.2019.101896
- Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020 Jan;20(1):26-41. doi: 10.1038/s41568-019-0205-x
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L; TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013 Oct 10;155(2):462-77. doi: 10.1016/j.cell.2013.09.034. Erratum in: Cell. 2014 Apr 24;157(3):753.
- Draaisma K, Chatzipli A, Taphoorn M, Kerkhof M, Weyerbrock A, Sanson M, Hoeben A, Lukacova S, Lombardi G, Leenstra S, Hanse M, Fleischeuer R, Watts C, McAbee J, Angelopoulos N, Gorlia T, Golfinopoulos V, Kros JM, Verhaak RGW, Bours V, van den Bent MJ, McDermott U, Robe PA, French PJ. Molecular Evolution of IDH Wild-Type Glioblastomas Treated With Standard of Care Affects Survival and Design of Precision Medicine Trials: A Report From the EORTC 1542 Study. J Clin Oncol. 2020 Jan 1;38(1):81-99. doi: 10.1200/JCO.19.00367
- Horbinski C, Ligon KL, Brastianos P, Huse JT, Venere M, Chang S, Buckner J, Cloughesy T, Jenkins RB, Giannini C, Stupp R, Nabors LB, Wen PY, Aldape KJ, Lukas RV, Galanis E, Eberhart CG, Brat DJ, Sarkaria JN. The medical necessity of advanced molecular testing in the diagnosis and treatment of brain tumor patients. Neuro Oncol. 2019 Dec 17;21(12):1498-1508. doi: 10.1093/neuonc/noz119
- van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, Clement PM, Frenay M, Campone M, Baurain JF, Armand JP, Taphoorn MJ, Tosoni A, Kletzl H, Klughammer B, Lacombe D, Gorlia T. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009 Mar 10;27(8):1268-74. doi: 10.1200/JCO.2008.17.5984
- Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, Drappatz J, O’Rourke DM, Wong M, Hamilton MG, Finocchiaro G, Perry J, Wick W, Green J, He Y, Turner CD, Yellin MJ, Keler T, Davis TA, Stupp R, Sampson JH; ACT IV trial investigators. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017 Oct;18(10):1373-1385. doi: 10.1016/S1470-2045(17)30517-X
- Lassman A, Pugh S, Wang A, et al.. A randomized, double-blind, placebo-controlled phase 3 trial of depatuxizumab mafodotin (ABT-414) in Epidermal Growth Factor Receptor (EGFR) Amplified (Amp) Newly Diagnosed Glioblastoma (nGBM). Neuro Oncol. 2019;21:vi17.
- Van Den Bent M, Eoli M, Sepulveda JM, Smits M, Walenkamp A, Frenel JS, Franceschi E, Clement PM, Chinot O, De Vos F, Whenham N, Sanghera P, Weller M, Dubbink HJ, French P, Looman J, Dey J, Krause S, Ansell P, Nuyens S, Spruyt M, Brilhante J, Coens C, Gorlia T, Golfinopoulos V. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020 May 15;22(5):684-693. doi: 10.1093/neuonc/noz222. Erratum in: Neuro Oncol. 2021 Aug 2;23(8):1415.
- Reardon DA, Wen PY, Mellinghoff IK. Targeted molecular therapies against epidermal growth factor receptor: past experiences and challenges. Neuro Oncol. 2014 Oct;16 Suppl 8(Suppl 8):viii7-13. doi: 10.1093/neuonc/nou232
- Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, Keedy V, Bielle F, Hofheinz RD, Joly F, Blay JY, Chau I, Puzanov I, Raje NS, Wolf J, DeAngelis LM, Makrutzki M, Riehl T, Pitcher B, Baselga J, Hyman DM. BRAF Inhibition in BRAFV600-Mutant Gliomas: Results From the VE-BASKET Study. J Clin Oncol. 2018 Dec 10;36(35):3477-3484. doi: 10.1200/JCO.2018.78.9990
- Wen PY, Stein A, Van Den Bent M, et al.. Updated efficacy and safety of dabrafenib plus trametinib in patients with recurrent/refractory BRAF V600E–mutated high-grade glioma (HGG) and low-grade glioma (LGG). Neuro Oncol. 2019;21(suppl 6;abstr ACTR-30).
- Behling F, Barrantes-Freer A, Skardelly M, Nieser M, Christians A, Stockhammer F, Rohde V, Tatagiba M, Hartmann C, Stadelmann C, Schittenhelm J. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016 Jun 27;11(1):55. doi: 10.1186/s13000-016-0506-2
- Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019 Nov 1;30(Suppl_8):viii23-viii30. doi: 10.1093/annonc/mdz282
- Chi AS, Tarapore RS, Hall MD, Shonka N, Gardner S, Umemura Y, Sumrall A, Khatib Z, Mueller S, Kline C, Zaky W, Khatua S, Weathers SP, Odia Y, Niazi TN, Daghistani D, Cherrick I, Korones D, Karajannis MA, Kong XT, Minturn J, Waanders A, Arillaga-Romany I, Batchelor T, Wen PY, Merdinger K, Schalop L, Stogniew M, Allen JE, Oster W, Mehta MP. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019 Oct;145(1):97-105. doi: 10.1007/s11060-019-03271-3
- Lasorella A, Sanson M, Iavarone A. FGFR-TACC gene fusions in human glioma. Neuro Oncol. 2017 Apr 1;19(4):475-483. doi: 10.1093/neuonc/now240
- Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih IeM, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):6021-6. doi: 10.1073/pnas.1303607110
- Wirsching HG, Tabatabai G, Roelcke U, Hottinger AF, Jörger F, Schmid A, Plasswilm L, Schrimpf D, Mancao C, Capper D, Conen K, Hundsberger T, Caparrotti F, von Moos R, Riklin C, Felsberg J, Roth P, Jones DTW, Pfister S, Rushing EJ, Abrey L, Reifenberger G, Held L, von Deimling A, Ochsenbein A, Weller M. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: the randomized, open-label, phase II ARTE trial. Ann Oncol. 2018 Jun 1;29(6):1423-1430. doi: 10.1093/annonc/mdy120
- Ellingson BM, Gerstner ER, Smits M, Huang RY, Colen R, Abrey LE, Aftab DT, Schwab GM, Hessel C, Harris RJ, Chakhoyan A, Gahrmann R, Pope WB, Leu K, Raymond C, Woodworth DC, de Groot J, Wen PY, Batchelor TT, van den Bent MJ, Cloughesy TF. Diffusion MRI Phenotypes Predict Overall Survival Benefit from Anti-VEGF Monotherapy in Recurrent Glioblastoma: Converging Evidence from Phase II Trials. Clin Cancer Res. 2017 Oct 1;23(19):5745-5756. doi: 10.1158/1078-0432.CCR-16-2844
- Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, Wiestler B, Schliesser MG, von Deimling A, Pichler J, Vetlova E, Harting I, Debus J, Hartmann C, Kunz C, Platten M, Bendszus M, Combs SE. A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014 Dec 15;20(24):6304-13. doi: 10.1158/1078-0432.CCR-14-0951-T
- Wick W, Gorlia T, Bady P, Platten M, van den Bent MJ, Taphoorn MJ, Steuve J, Brandes AA, Hamou MF, Wick A, Kosch M, Weller M, Stupp R, Roth P, Golfinopoulos V, Frenel JS, Campone M, Ricard D, Marosi C, Villa S, Weyerbrock A, Hopkins K, Homicsko K, Lhermitte B, Pesce G, Hegi ME. Phase II Study of Radiotherapy and Temsirolimus versus Radiochemotherapy with Temozolomide in Patients with Newly Diagnosed Glioblastoma without MGMT Promoter Hypermethylation (EORTC 26082). Clin Cancer Res. 2016 Oct 1;22(19):4797-4806. doi: 10.1158/1078-0432.CCR-15-3153
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008 Jan 22;5(1):e8. doi: 10.1371/journal.pmed.0050008
- Chinnaiyan P, Won M, Wen PY, Rojiani AM, Werner-Wasik M, Shih HA, Ashby LS, Michael Yu HH, Stieber VW, Malone SC, Fiveash JB, Mohile NA, Ahluwalia MS, Wendland MM, Stella PJ, Kee AY, Mehta MP. A randomized phase II study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: results of NRG Oncology RTOG 0913. Neuro Oncol. 2018 Apr 9;20(5):666-673. doi: 10.1093/neuonc/nox209
- Wick W, Dettmer S, Berberich A, Kessler T, Karapanagiotou-Schenkel I, Wick A, Winkler F, Pfaff E, Brors B, Debus J, Unterberg A, Bendszus M, Herold-Mende C, Eisenmenger A, von Deimling A, Jones DTW, Pfister SM, Sahm F, Platten M. N2M2 (NOA-20) phase I/II trial of molecularly matched targeted therapies plus radiotherapy in patients with newly diagnosed non-MGMT hypermethylated glioblastoma. Neuro Oncol. 2019 Jan 1;21(1):95-105. doi: 10.1093/neuonc/noy161
- Alexander BM, Trippa L, Gaffey S, Arrillaga-Romany IC, Lee EQ, Rinne ML, Ahluwalia MS, Colman H, Fell G, Galanis E, de Groot J, Drappatz J, Lassman AB, Meredith DM, Nabors LB, Santagata S, Schiff D, Welch MR, Ligon KL, Wen PY. Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT): A Bayesian Adaptive Platform Trial to Develop Precision Medicines for Patients With Glioblastoma. JCO Precis Oncol. 2019 Mar 27;3:PO.18.00071. doi: 10.1200/PO.18.00071
- Alexander BM, Ba S, Berger MS, Berry DA, Cavenee WK, Chang SM, Cloughesy TF, Jiang T, Khasraw M, Li W, Mittman R, Poste GH, Wen PY, Yung WKA, Barker AD; GBM AGILE Network. Adaptive Global Innovative Learning Environment for Glioblastoma: GBM AGILE. Clin Cancer Res. 2018 Feb 15;24(4):737-743. doi: 10.1158/1078-0432.CCR-17-0764
- Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017 Feb 1;127(2):415-426. doi: 10.1172/JCI89587
- Lassman AB, Wen PY, van den Bent M, et al.. Efficacy and safety of selinexor in recurrent glioblastoma. Neuro Oncol. 2019;21:iii3–iii4.
- Lassman AB, Sepúlveda-Sánchez JM, Cloughesy T, et al.. Infigratinib (BGJ398) in FGFR altered recurrent malignant glioma: a multicenter phase II study. Neuro Oncology. 2019;21:vi20.
- Wen PY, Touat M, Alexander BM, Mellinghoff IK, Ramkissoon S, McCluskey CS, Pelton K, Haidar S, Basu SS, Gaffey SC, Brown LE, Martinez-Ledesma JE, Wu S, Kim J, Wei W, Park MA, Huse JT, Kuhn JG, Rinne ML, Colman H, Agar NYR, Omuro AM, DeAngelis LM, Gilbert MR, de Groot JF, Cloughesy TF, Chi AS, Roberts TM, Zhao JJ, Lee EQ, Nayak L, Heath JR, Horky LL, Batchelor TT, Beroukhim R, Chang SM, Ligon AH, Dunn IF, Koul D, Young GS, Prados MD, Reardon DA, Yung WKA, Ligon KL. Buparlisib in Patients With Recurrent Glioblastoma Harboring Phosphatidylinositol 3-Kinase Pathway Activation: An Open-Label, Multicenter, Multi-Arm, Phase II Trial. J Clin Oncol. 2019 Mar 20;37(9):741-750. doi: 10.1200/JCO.18.01207
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014 Feb 20;370(8):709-22. doi: 10.1056/NEJMoa1308345
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014 Feb 20;370(8):699-708. doi: 10.1056/NEJMoa1308573
- Wen PY, Drappatz J, de Groot J, Prados MD, Reardon DA, Schiff D, Chamberlain M, Mikkelsen T, Desjardins A, Holland J, Ping J, Weitzman R, Cloughesy TF. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018 Jan 22;20(2):249-258. doi: 10.1093/neuonc/nox154
- Arrillaga-Romany I, Kurz S, Sumrall A, et al.. Single agent ONC201 in previously-treated, progressive adult H3 K27M-mutant glioma. Neuro Oncol. 2019;21(Suppl 6):vi20–vi21. [Abstr.]
- Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017 Jun 1;3(6):841-849. doi: 10.1001/jamaoncol.2016.2064
- Kaufmann JK, Chiocca EA. Glioma virus therapies between bench and bedside. Neuro Oncol. 2014 Mar;16(3):334-51. doi: 10.1093/neuonc/not310
- Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014 Apr;2(4):295-300. doi: 10.1158/2326-6066.CIR-14-0015. Erratum in: Cancer Immunol Res. 2014 Jul;2(7):699.
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015 Sep 1;33(25):2780-8. doi: 10.1200/JCO.2014.58.3377
- Chiocca EA, Nassiri F, Wang J, Peruzzi P, Zadeh G. Viral and other therapies for recurrent glioblastoma: is a 24-month durable response unusual? Neuro Oncol. 2019 Jan 1;21(1):14-25. doi: 10.1093/neuonc/noy170
- Chiocca EA, Yu JS, Lukas RV, Solomon IH, Ligon KL, Nakashima H, Triggs DA, Reardon DA, Wen P, Stopa BM, Naik A, Rudnick J, Hu JL, Kumthekar P, Yamini B, Buck JY, Demars N, Barrett JA, Gelb AB, Zhou J, Lebel F, Cooper LJN. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci Transl Med. 2019 Aug 14;11(505):eaaw5680. doi: 10.1126/scitranslmed.aaw5680
- Todo T. Results of phase II clinical trial of oncolytic Herpes virus G47Δ In patients with glioblastoma. Neuro Oncol. 2019;21:vi4 [Abstr.].
- Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017 Sep 7;170(6):1109-1119.e10. doi: 10.1016/j.cell.2017.08.027. Erratum in: Cell. 2018 Aug 9;174(4):1031-1032.
- Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med. 2018 Jul 12;379(2):150-161. doi: 10.1056/NEJMoa1716435
- Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, Gumin J, Vence LM, Wistuba I, Rodriguez-Canales J, Villalobos PA, Dirven CMF, Tejada S, Valle RD, Alonso MM, Ewald B, Peterkin JJ, Tufaro F, Fueyo J. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol. 2018 May 10;36(14):1419-1427. doi: 10.1200/JCO.2017.75.8219
- Cloughesy TF, Landolfi J, Vogelbaum MA, Ostertag D, Elder JB, Bloomfield S, Carter B, Chen CC, Kalkanis SN, Kesari S, Lai A, Lee IY, Liau LM, Mikkelsen T, Nghiemphu P, Piccioni D, Accomando W, Diago OR, Hogan DJ, Gammon D, Kasahara N, Kheoh T, Jolly DJ, Gruber HE, Das A, Walbert T. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018 Sep 3;20(10):1383-1392. doi: 10.1093/neuonc/noy075
- Cloughesy T, Petrecca K, Walbert T, et al.. TOCA 5: Toca 511 & Toca FC versus standard of care in patients with recurrent high grade glioma Late Breaking Abstract Society For Neuro-Oncology 2019. meeting.
- Cloughesy TF, Brenner A, de Groot JF, Butowski NA, Zach L, Campian JL, Ellingson BM, Freedman LS, Cohen YC, Lowenton-Spier N, Rachmilewitz Minei T, Fain Shmueli S; GLOBE Study Investigators, Wen PY. A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2020 May 15;22(5):705-717. doi: 10.1093/neuonc/noz232
- Brenner AJ, Peters KB, Vredenburgh J, Bokstein F, Blumenthal DT, Yust-Katz S, Peretz I, Oberman B, Freedman LS, Ellingson BM, Cloughesy TF, Sher N, Cohen YC, Lowenton-Spier N, Rachmilewitz Minei T, Yakov N, Mendel I, Breitbart E, Wen PY. Safety and efficacy of VB-111, an anticancer gene therapy, in patients with recurrent glioblastoma: results of a phase I/II study. Neuro Oncol. 2020 May 15;22(5):694-704. doi: 10.1093/neuonc/noz231
- Srivastava S, Jackson C, Kim T, Choi J, Lim M. A Characterization of Dendritic Cells and Their Role in Immunotherapy in Glioblastoma: From Preclinical Studies to Clinical Trials. Cancers (Basel). 2019 Apr 15;11(4):537. doi: 10.3390/cancers11040537
- Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, Curry WT, Zhu JJ, Glantz M, Peereboom DM, Markert JM, LaRocca R, O’Rourke DM, Fink K, Kim L, Gruber M, Lesser GJ, Pan E, Kesari S, Muzikansky A, Pinilla C, Santos RG, Yu JS. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin Cancer Res. 2019 Oct 1;25(19):5799-5807. doi: 10.1158/1078-0432.CCR-19-0261
- Bagley SJ, Desai AS, Linette GP, June CH, O’Rourke DM. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018 Oct 9;20(11):1429-1438. doi: 10.1093/neuonc/noy032
- Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016 Dec 29;375(26):2561-9. doi: 10.1056/NEJMoa1610497
- Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020 Jan;20(1):12-25. doi: 10.1038/s41568-019-0224-7
- Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacol Ther. 2020 Jan;205:107419. doi: 10.1016/j.pharmthera.2019.107419
- Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, Weichselbaum RR, McCarthy BJ. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012 Mar;107(1):207-12. doi: 10.1007/s11060-011-0738-7
- Tran B, Rosenthal MA. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010 Apr;17(4):417-21. doi: 10.1016/j.jocn.2009.09.004
- Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr, Mehta MP. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013 Nov 10;31(32):4085-91. doi: 10.1200/JCO.2013.49.6968
- Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro Oncol. 2016 Oct 1;18(suppl_5):v1-v75. doi: 10.1093/neuonc/now207
- Clarke JL, Ennis MM, Yung WK, Chang SM, Wen PY, Cloughesy TF, Deangelis LM, Robins HI, Lieberman FS, Fine HA, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Lamborn KR, Prados MD; North American Brain Tumor Consortium. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011 Oct;13(10):1118-24. doi: 10.1093/neuonc/nor110
- Rao AM, Quddusi A, Shamim MS. The significance of MGMT methylation in Glioblastoma Multiforme prognosis. J Pak Med Assoc. 2018 Jul;68(7):1137-1139.