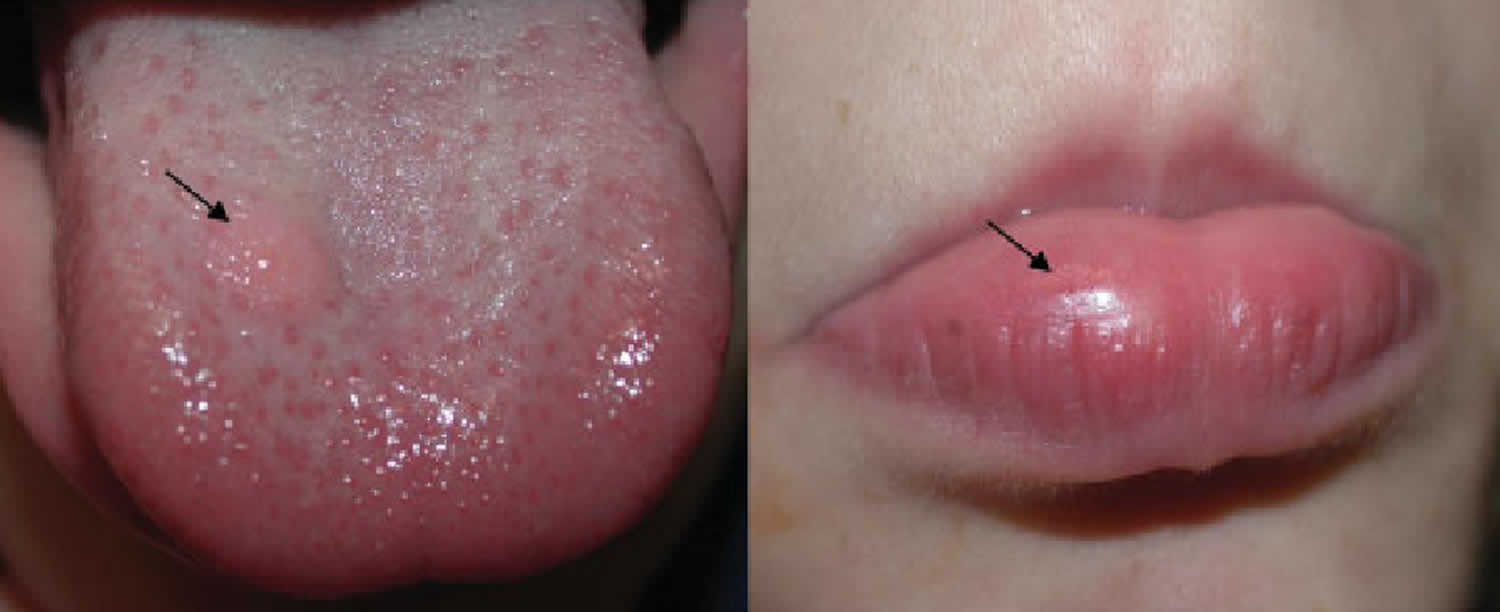
Granular cell tumor
Granular cell tumors also known as Abrikossoff tumors, are rare soft-tissue tumors that can occur anywhere in the body 1. Granular cell tumors are thought to arise from the cells that surround and insulate the nerve cells in our body (Schwann cells). Most granular cell tumors are benign (non-cancerous), although some may be locally aggressive 2. Less than 2% are malignant (cancerous), but these are aggressive and associated with a poor prognosis 3. A tumor 3 cm or less in size can be regarded as benign. However, if the tumor grows rapidly and forms an ulcer, malignancy should be suspected 4.
Granular cell tumors appear to be more common in black persons. Multiplicity of lesions is definitely more common in black persons. Up to 10% of granular cell tumors are multiple (ie, two to four lesions).
Granular cell tumors affect persons of varying ages, and the range is wide. Most patients are middle-aged, with a peak incidence in the fourth through sixth decades of life.
Granular cell tumors affect females more often than males with the female-to-male ratio is estimated at approximately 3:2 5 and usually present as a solitary painless mass. A few individuals have multiple granular cell tumors. In most cases, granular cell tumors are located in the skin of the head and neck, especially inside the mouth (70%), the tongue being the most common site. Granular cell tumor may also occur in the breast, heart, pituitary gland, stomach, esophagus, genitalia, and upper respiratory tract 6. Granular cell tumors may be part of some genetic syndromes, such as LEOPARD syndrome and neurofibromatosis. Complete surgical removal of the tumor is usually curative, but in some cases the tumors may recur 7.
Granular cell tumor causes
Pareja et al 8 identified loss-of-function mutations in ATP6AP1 or ATP6AP2 in 72% of granular cell tumors. Silencing of these genes in vitro results in impaired vesicle acidification, redistribution of endosomal compartments, and accumulation of intracytoplasmic granules, which are cardinal phenotypic characteristics of granular cell tumors.
Granular cell tumors are typically solitary and smaller than 3 cm. They are usually located in the dermis or subcutis and less frequently in the submucosa, smooth muscle, or striated muscle. Granular cell tumors are also found in the internal organs, particularly in the upper aerodigestive tract. Benign and malignant counterparts are known; the latter are rare, comprising fewer than 2% of all granular cell tumors 5.
Granular cell tumor symptoms
Benign lesions manifest as nonulcerated and usually painless nodules with an insidious onset and slow growth rate. They are rarely larger than 3 cm and usually have been noted for less than 6 months.
Granular cell tumors may occur at any site, but easily noticed surface lesions (ie, head, neck, trunk, extremities) are far more common than visceral lesions. Other aspects of location are as follows:
- The tongue is affected in approximately 25% of cases; breast involvement is also common
- All together, parenchyma, subcutaneous tissue, and dermis account for approximately 15% of cases; a third of those are in the parenchyma
- Origin in skeletal muscle is rare
- The lesions usually involve small- to medium-sized cranial or spinal nerves, although neurologic deficit is rare
- Visceral involvement is encountered as mucosal or submucosal nodules in the esophagus 9, stomach, small and large intestines, larynx, bronchi, gallbladder, and biliary tract
- The gastrointestinal tract harbors approximately 5% of all granular cell tumors
- Central nervous system granular cell tumors are extremely rare 10
- Lesions can be incidental findings, or they may give rise to obstructive or pressure symptoms when large enough and in a critical location
Nodules are usually solitary but may be multiple in up to 10% of cases (patients with 26 and 52 tumors are on record). Multiple granular cell tumors have been reported in patients with LEOPARD (lentigenes, electrocardiographic conduction anomalies, ocular hypertelorism, pulmonary stenosis, abnormalities of genitalia, retardation of growth, deafness) syndrome (Noonan syndrome), a rare autosomal dominant, disorder caused by a mutation in the protein tyrosine phosphatase, nonreceptor type 11 gene (PTPN11) 1.
An association between multiple cutaneous granular cell tumors and neurofibromatosis has been suggested, but remains unproven 11.
Malignant lesions
Malignant granular cell tumors are rare. By convention, granular cell tumors are classified as malignant when their constituent cells show cytologic features of malignancy or when a morphologically benign granular cell tumor metastasizes to regional lymph nodes or distant sites or otherwise causes death.
Malignancy is encountered more often in deep-seated lesions in adults, with a mean patient age of 50 years. Sex and race distribution of malignant tumors mirrors that of benign lesions. A history of long clinical duration and rapid recent growth has been observed in some cases, suggesting a possibility of malignant transformation from a preexisting benign granular cell tumor.
The lesions are usually larger (ie, 4-15 cm) and may be locally destructive, thus causing symptoms (eg, pressure, obstruction, hemorrhage, ulceration, secondary infection) depending on the site. Metastases to regional lymph nodes and/or distant organs (most common site is the lungs) and concomitant symptoms may be present.
Granular cell tumor diagnosis
The diagnosis of granular cell tumors centers on analysis of biopsy specimens.
Microscopic features of benign granular cell tumors are remarkably uniform, regardless of the site. Granular cell tumors are sometimes located near a nerve twig, usually within the perineurium, and are variably circumscribed at the periphery. Approximately half of all granular cell tumors have poorly defined or infiltrative margins. The nodules are composed of large polyhedral cells arranged in sheets, nests, lobules, or trabeculae and are surrounded by variable stroma. A reticulin framework may be around individual cells or small groups of cells. Occasionally, granular cell tumors are extensively collagenized.
The tumor cells have abundant granular eosinophilic cytoplasm with centrally located vesicular or pyknotic nuclei. Markedly enlarged lysosomes in tumor cells may be observed as eosinophilic globules surrounded by a clear halo; some are extruded from cells and may be phagocytosed by histiocytes. In such cases, they are termed angulate bodies. Usually, the granules stain positive with periodic acid-Schiff (PAS) staining and are resistant to diastase. They also stain with Sudan black B and are magenta in trichrome preparations. Multinucleation, plentiful mitotic activity, nuclear pleomorphism, and prominent nucleoli are uncommon features. Squamous epithelium overlying the peripheral superficial lesions exhibits acanthosis and pseudoepitheliomatous hyperplasia.
Immunohistochemical findings
Granular cell tumors have an uncertain histogenesis. Many immunohistochemical and ultrastructural studies suggest a Schwann cell origin 12.
The tumor cells stain positively for S-100 protein, neuron-specific enolase, and NK1-C3 in almost all cases. Positivity with stains for myelin-associated P0 and P2 proteins, myelin basic protein, and Leu-7 is less consistent 13.
The tumor cells are nonimmunoreactive for epithelial, muscle, endothelial, and glial cell markers. This is useful for differentiating a granular cell tumor from other diagnostic possibilities.
Ultrastructural findings
Ultrastructural findings with granular cell tumors are highly characteristic. Pleomorphic secondary lysosomes are observed within the cytoplasm of tumor cells 14.
Features indicating neural derivation of granular cell tumors (eg, myelin residues, long-spacing collagen, arrays of neuritic processes among tumor cells) may be observed.
Variants
Gingival granular cell tumor of newborns is an extremely rare variant and manifests as a polypoid swelling situated exclusively over the lateral alveolar ridge, especially of the maxilla. More than 90% of patients are girls 15. These lesions are likely to be reactive rather than neoplastic in nature, and, ultimately, they may be segregated from granular cell tumors. Lesions, noticed soon after birth, show the usual histopathologic features of granular cell tumors; however, the following differences from the adult counterpart are noted:
- The lesions do not grow, and some regress
- Recurrence has not been noted, even after incomplete resection
- The lesions do not have a malignant counterpart
- The lesions have a prominent plexiform network of capillaries and scattered inflammatory cells
- Occasionally, lesions show entrapped odontogenic epithelium
- Pseudoepitheliomatous hyperplasia of overlying squamous epithelium is less conspicuous or may be absent
- The cells do not stain positively for the S-100 protein
- Ultrastructurally, the lesional cells show a few histiocytic features, and giant lysosomes (globules/angulate bodies) are not observed.
The primitive polypoid granular cell tumor is another rare subset, which manifests as exophytic polypoid skin lesions at any site or in a person of any age. The lesions are characterized by nuclear pleomorphism, frequent mitoses, and poor immunohistochemical reactions. They are not aggressive tumors. Most likely, they represent a nonimmunoreactive phenotype of granular cell tumor 16. In 2005, Lazar and Fletcher published a series of similar cases. Only one of 13 cases gave rise to a local lymph node metastasis. In this case, the patient had no recurrence and is currently disease-free 70 months after lymphadenectomy 17.
Gross and microscopic features of malignant granular cell tumors Histopathologic features of malignancy are unmistakable in some patients and do not pose any diagnostic difficulty. Some malignancies are identical to their benign counterparts (ie, small size, no local destruction, no infiltrative activity at the edge, bland cytology) and yet demonstrate malignant potential by way of metastases 5.
Therefore, with granular cell tumors larger than 3 cm, malignancy may be indicated by the following:
- Locally destructive changes (eg, ulceration, necrosis, hemorrhage)
- Infiltrative activity at the edges
- Frequent mitoses
- Vesicular nuclei with prominent nucleoli
Imaging studies
Imaging studies may be necessary to detect deep-seated visceral lesions.
Procedures
Kobara et al 18 reported that endoscopic imaging under direct view has potential diagnostic value for submucosal tumors in the gastrointestinal (GI) tract. The two granular cell tumors in this study were both white, cloudy, round, and elastic, with no visible tumor coating. Final pathological diagnosis was obtained by core biopsy using the submucosal endoscopy with mucosal flap method.
Staging
Universally recommended and accepted staging schemes specific for granular cell tumors do not exist. A general staging scheme developed by the American Joint Committee on Cancer for use with other soft tissue tumors may be followed.
Granular cell tumor treatment
Granular cell tumor treatment is with surgical resection. With benign granular cell tumors, local surgical excision is curative, if complete resection is achieved; however, recurrence is possible even withclear margins. Wide en bloc excision is recommended for malignant lesions.
Radiation and chemotherapy are not needed for benign lesions and are not effective for malignant lesions. However, case reports describe response to pazopanib in patients with metastatic disease 19.
The main complication of benign lesions is recurrence. With malignant granular cell tumors, the main complications are recurrence, metastases, or both.
Granular cell tumor prognosis
Prognosis for patients with granular cell tumors is as follows 5:
- In benign lesions, recurrence rates are 2-8%, even when the resection margins are deemed free of tumor infiltration; they are around 20% when the margins are positive for tumor.
- Malignant lesions are aggressive and difficult to eradicate with surgery. Local recurrences are as high as 32%, and metastases were reported in half of the patients. Metastases are usually detected within 2 years.
- Of patients with malignant granular cell tumors, 39% die of the disease within 3 years after detection of the primary tumor.
- Ki-67 immunoreactivity of 10% or more tumor cells is an adverse prognostic factor.
- Richmond AM, La Rosa FG, Said S. Granular cell tumor presenting in the scrotum of a pediatric patient: a case report and review of the literature. J Med Case Rep. 2016 Jun 4. 10 (1):161.
- Yilmaz AD, Unlu RE, Orbay H, et al. Recurrent granular cell tumor: how to treat. J Craniofac Surg. 2007 Sep. 18(5):1187-9.
- Mirza FN, Tuggle CT, Zogg CK, Mirza HN, Narayan D. Epidemiology of malignant cutaneous granular cell tumors: A US population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database. J Am Acad Dermatol. 2018 Mar. 78 (3):490-497.e1.
- Aoyama, K., Kamio, T., Hirano, A. et al. Granular cell tumors: a report of six cases. World J Surg Onc 10, 204 (2012). https://doi.org/10.1186/1477-7819-10-204
- Fanburg-Smith JC, Meis-Kindblom JM, et al. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998 Jul. 22(7):779-94.
- Thumallapally N, Ibrahim U, Kesavan M, Chang Q, Opitz L, Dhar M & Andrawes S. Esophageal Granular Cell Tumor: A Case Report and Review of Literature. Cureus. September 14, 2016; 8(9):e782. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5065346
- Richmond AM, La Rosa FG & Said S. Granular cell tumor presenting in the scrotum of a pediatric patient: a case report and review of the literature. J Med Case Rep. June 4, 2016; 10(1):161. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4893259
- Pareja F, Brandes AH, Basili T, Selenica P, Geyer FC, et al. Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nat Commun. 2018 Aug 30. 9 (1):3533.
- Nie L, Xu G, Wu H, Huang Q, Sun Q, Fan X. Granular cell tumor of the esophagus: a clinicopathological study of 31 cases. Int J Clin Exp Pathol. 2014. 7(7):4000-7.
- Li P, Yang Z, Wang Z, Zhou Q, Li S, Wang X, et al. Granular cell tumors in the central nervous system: a report on eight cases and a literature review. Br J Neurosurg. 2016 May 18. 1-8.
- Sahn EE, Dunlavey ES, Parsons JL. Multiple cutaneous granular cell tumors in a child with possible neurofibromatosis. J Am Acad Dermatol. 1997 Feb. 36 (2 Pt 2):327-30.
- Bellezza G, Colella R, Sidoni A, Del Sordo R, Ferri I, Cioccoloni C, et al. Immunohistochemical expression of Galectin-3 and HBME-1 in granular cell tumors: a new finding. Histol Histopathol. 2008 Sep. 23(9):1127-30.
- Mazur MT, Shultz JJ, Myers JL. Granular cell tumor. Immunohistochemical analysis of 21 benign tumors and one malignant tumor. Arch Pathol Lab Med. 1990 Jul. 114(7):692-6.
- Seo IS, Azzarelli B, Warner TF, et al. Multiple visceral and cutaneous granular cell tumors. Ultrastructural and immunocytochemical evidence of Schwann cell origin. Cancer. 1984 May 15. 53(10):2104-10.
- Fletcher CD. Peripheral neuroectodermal tumors. Fletcher CD, ed. Diagnostic Histopathology of Tumors. London, UK: Churchill Livingstone; 1995. Vol 2: 1233-5.
- LeBoit PE, Barr RJ, Metcalf JS. Primitive polypoid granular-cell tumor and other cutaneous granular-cell neoplasms of apparent non-neural origin. Am J Surg Pathol. 1991. Jan;15(1):48-58.
- Lazar AJ, Fletcher CD. Primitive nonneural granular cell tumors of skin: clinicopathologic analysis of 13 cases. Am J Surg Pathol. 2005. Jul;29(7):927-34.
- Kobara H, Mori H, Rafiq K, Matsunaga T, Fujihara S, Nishiyama N, et al. Evaluation of gastric submucosal tumors using endoscopically visualized features with submucosal endoscopy. Oncol Lett. 2014 Jul. 8(1):161-168.
- Wei L, Liu S, Conroy J, Wang J, Papanicolau-Sengos A, et al. Whole-genome sequencing of a malignant granular cell tumor with metabolic response to pazopanib. Cold Spring Harb Mol Case Stud. 2015 Oct. 1 (1):a000380.