Hemangiopericytoma
Hemangiopericytoma is a rare type of tumor involving blood vessels and soft tissue. Hemangiopericytoma is a term used to described a group of tumors that are derived from pericytes, the cells normally arranged along specific types of blood vessels called capillaries and venules. Hemangiopericytomas are typically slow-growing, may be either benign (non-cancerous) or malignant (cancerous), and may occur originate anywhere in the body where there are capillaries 1. The most common locations reported are the brain, lower extremities, pelvic area, head, and neck 2. Hemangiopericytomas often are painless masses and may not have any associated symptoms. Malignant hemangiopericytomas can metastasize or spread to other areas in the body, primarily the lungs and bones.
Hemangiopericytomas can be located in the nasal cavity and paranasal sinuses. Though rare, their prognosis is better because they tend to be less aggressive and do not metastasize.
Solitary fibrous tumor has been merged nosologically with hemangiopericytoma into a single entity in the 2013 WHO classification of soft tissue and bone 3. Previously, there was an ongoing debate among sarcoma pathologists about how best to differentiate solitary fibrous tumors and hemangiopericytomas, owing to the frequent misdiagnosis of hemangiopericytomas as solitary fibrous tumors, and vice versa 4. Recent work has shown that the majority of solitary fibrous tumors and hemangiopericytomas in soft tissue share inversions at 12q13, fusing the NAB2 and STAT6 genes 5; thus, the term “hemangiopericytoma” is no longer used and these 2 diseases have been integrated into the single term “solitary fibrous tumor” 6. Accordingly, after many studies on central nervous system (brain and spinal cord) hemangiopericytomas and solitary fibrous tumors, they were integrated into a new entity, “solitary fibrous tumor/hemangiopericytoma” in the 2016 WHO classification of tumors of the central nervous system 7.
In the central nervous system (brain and spinal cord), hemangiopericytomas constitute 2.5% of meningeal tumors and less than 1% of intracranial tumors 8 and are categorized into low-grade (WHO grade I and II) or high-grade (WHO grade III, anaplastic) neoplasms 9. Most radiological features are similar to those typically associated with meningiomas 10, but central nervous system hemangiopericytomas have a high propensity for local recurrence and extracranial metastasis, unlike meningiomas 11.
Hemangiopericytoma causes
The cause of hemangiopericytoma or solitary fibrous tumor is unknown.
Hemangiopericytoma symptoms
Hemangiopericytomas usually are painless masses, often without any associated symptoms. Hemangiopericytomas can remain undetected for long periods of time, due to the fact that they originate in soft tissue that is flexible and easily makes room for the new mass.
When symptoms occur, they are likely to be associated with an enlarging mass. Brain hemangiopericytoma can mimic meningiomas around the brain and skull base, causing neurologic disturbances.
Hemangiopericytoma diagnosis
Your doctor will perform a physical exam and ask you about symptoms you are experiencing.
Imaging and radiology testing for hemangiopericytoma
Imaging studies will reveal the exact location and size of the mass, as well as the extent of spread. Biopsy of the tissue allows for the identification of specific characteristics.
Hemangiopericytoma treatment
Your cancer treatment team may recommend a combination of surgical and non-surgical approaches for hemangiopericytoma treatment.
Minimally invasive surgery
Surgical removal of the tumor is the primary treatment.
Hemangiopericytomas of the skull base may be approached directly using the endoscopic endonasal approach. This state-of-the-art, minimally invasive approach allows surgeons to access the tumor through the natural corridor of the nose, without making an open incision. Surgeons then remove the hemangiopericytoma through the nose and nasal cavities.
Endoscopic endonasal approach offers the benefits of no incisions to heal, no disfigurement, and a faster recovery time.
If you need complementary treatments, such as radiation, those therapies can begin soon after endoscopic endonasal approach surgery.
When evaluating you for treatment, your neurosurgeons will look at your hemangiopericytoma from every direction. Your neurosurgeon will find the surgical path that is least disruptive to your brain, critical nerves, and ability to return to normal functioning.
Gamma knife radiosurgery
Gamma Knife radiosurgery is a painless procedure that uses hundreds of highly focused radiation beams to target tumors and lesions within the brain, with no surgical incision.
Gamma Knife treatment may be an option for people with residual tumor after surgery or for those who show delayed tumor progression despite an initial multipronged treatment. Gamma Knife is used to non-invasively boost the effectiveness of radiation delivered to the hemangiopericytoma tumor.
Radiation therapy and chemotherapy
Surgery may be followed by radiation therapy to prevent recurrence, usually localized to the postoperative site and particularly in cases where the tumor was not totally removed.
Chemotherapy is also effective for treating malignant hemangiopericytomas and is often prescribed after surgery.
Hemangiopericytoma prognosis
For patients with a primary tumor who undergo complete resection, 5-year survival is 89-100%. For patients with solitary fibrous tumors of an extremity, the local recurrence rate is 0-6%, and the distant metastasis rate is 0-19% 12.
The criteria for WHO grade 1 included no nuclear atypia and no high cellularity, depending on the neuropathologist’s determination. WHO grades 2 and 3 were differentiated based on a cutoff of 5 or more mitoses per 10 hpfs. Patients in the solitary fibrous tumor or hemangiopericytoma WHO grade 1 group showed a more benign course. The WHO grade 3 group presented with a disease of a more aggressive nature and shorter overall survival compared with that of the grade 2 group. The overall survival is calculated as the time from the initial surgery to death or the date of the last postoperative follow-up. In the WHO grade 2 group, the extent of resection and adjuvant radiation therapy were significant factors for longer progression-free survival. Progression-free survival was calculated from the time of the first operation to the date of the confirmation of local recurrence and extracranial metastasis. Local recurrence was defined as definite evidence supporting tumor growth in the operative site in follow-up postoperative imaging. Extracranial metastasis was deemed to be present if symptomatic or asymptomatic lesions in extra-central nervous system sites were confirmed using imaging studies such as PET-CT, CT, and MRI. The period to metastasis was defined as the time from the first operation to the confirmation of extracranial metastasis. In addition, aggressive treatment after recurrence is important for patients’ survival. The long-term follow up and periodic systemic evaluation are mandatory to detect delayed recurrence and/or systemic metastasis.
In previous studies, 85%–90% of solitary fibrous tumors showed a benign, indolent clinical course 13. This group became the
solitary fibrous tumor or hemangiopericytoma grade 1 group.
A number of authors have reported a significant correlation between gross-total resection and improved survival. The gross-total resection rates reported in their studies varied between 50% and
83.3% 14. However, these studies did not include WHO grades. In another study 3, the WHO grade 2 group, the gross-total resection rate was 67.5%. The gross-total resection group had a significantly longer progression-free survival compared with that of the subtotal resection group in the WHO grade 2 group. Moreover, the gross-total resection group had a lower recurrence rate than that of the subtotal resection group, although the difference was not significant. However, unlike in the other studies, there was no significant difference in overall survival between the gross-total resection and subtotal resection groups in the WHO grade 2 group. In addition, patients with adjuvant radiation therapy had a significantly longer progression-free survival compared with that of patients without adjuvant radiation therapy in the WHO grade 2 group. Melone et al. 15 and other groups have reported that adjuvant radiation therapy prolonged the time before first recurrence. Another study reported that adjuvant radiation therapy had a significant effect on overall survival 16. However, another study results showed that there was no significant difference in overall survival between the adjuvant and nonadjuvant radiation therapy groups. These results showed the importance of aggressive treatment, including stereotactic radiosurgery and radiation therapy after recurrence. Some authors have reported that stereotactic radiosurgery is effective in disease control after recurrence 17. Another study showed the importance of multimodal treatment for the recurrence of hemangiopericytomas 18. Although the gross-total resection and adjuvant radiation therapy group did not show much benefit for overall survival compared with the subtotal resection and nonadjuvant radiation therapy group, these factors can increase the disease-free period of patients. The physicians tried to remove as much tumor during surgery as possible, even if they performed subtotal resection. It is important to reduce the patient’s tumor burden related to radiation therapy as much as possible
for effective adjuvant treatment results. Even after subtotal resection, adjuvant radiation therapy is meaningful to maintain patient’s overall survival similar to that of the gross-total resection group. Moreover, even after recurrence, active treatment options such as reoperation, repeat Gamma Knife surgery, and repeat radiation therapy are meaningful in maintaining overall survival.
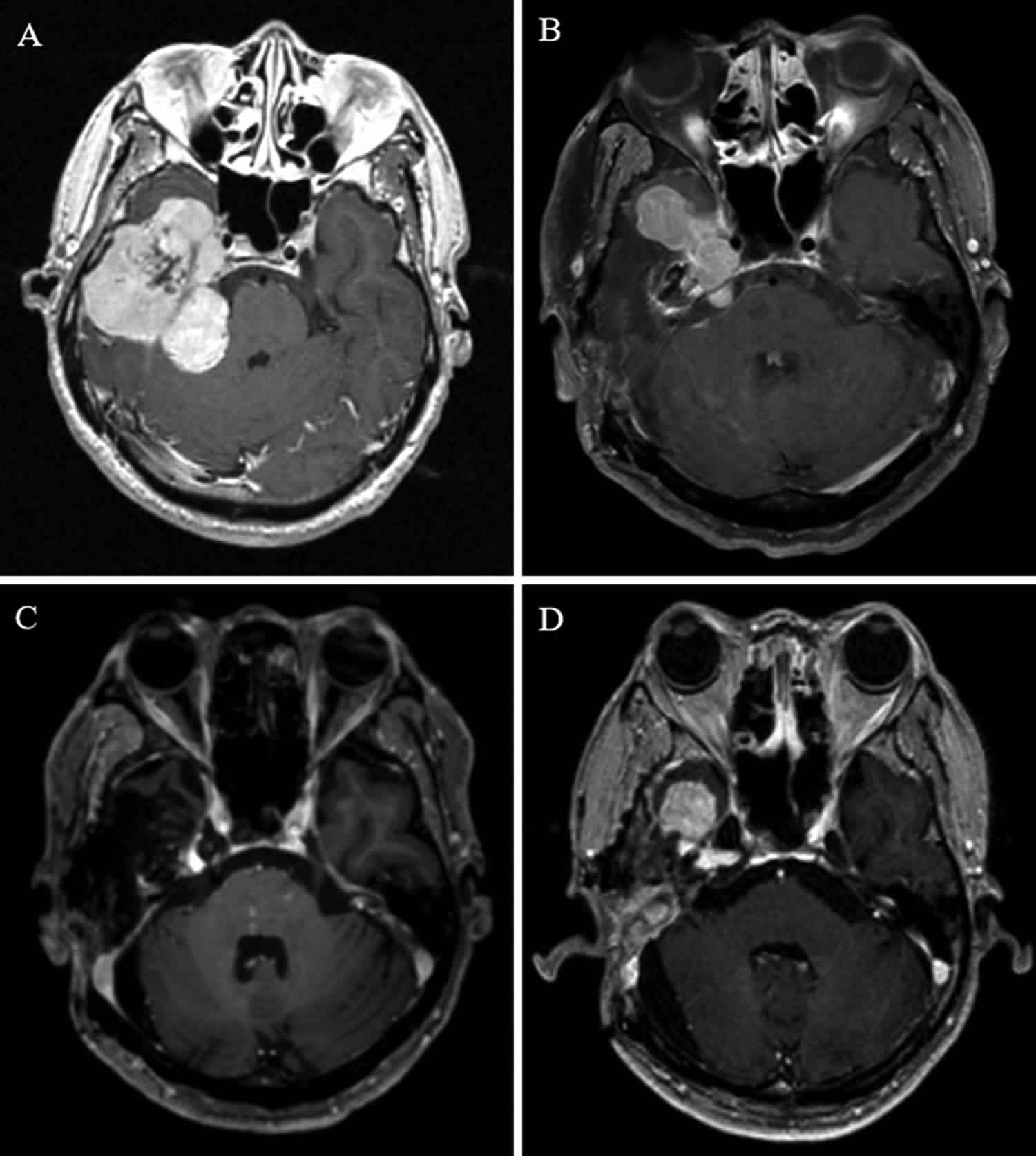
For example, a 51-year-old man had a large tumor in his right-side temporal lobe, petrous bone, and cerebellopontine angle region. The intended second-stage operation and adjuvant Gamma Knife surgery for the remnant lesion in the cavernous sinus were performed. The pathologic results indicated an solitary fibrous tumor; thus, it was classified as WHO grade 2. The patient presented with tumor recurrence at 35 months after the initial treatment. After the second Gamma Knife surgery, the patient showed recurrences at 24, 29, 7, and 28 months after each active
treatment, respectively. The patient underwent a total of 4 Gamma Knife surgery procedures, 1 radiation therapy, and 1 reoperation. The patient remains alive 11.6 years after the initial operation.
Hemangiopericytoma survival rate
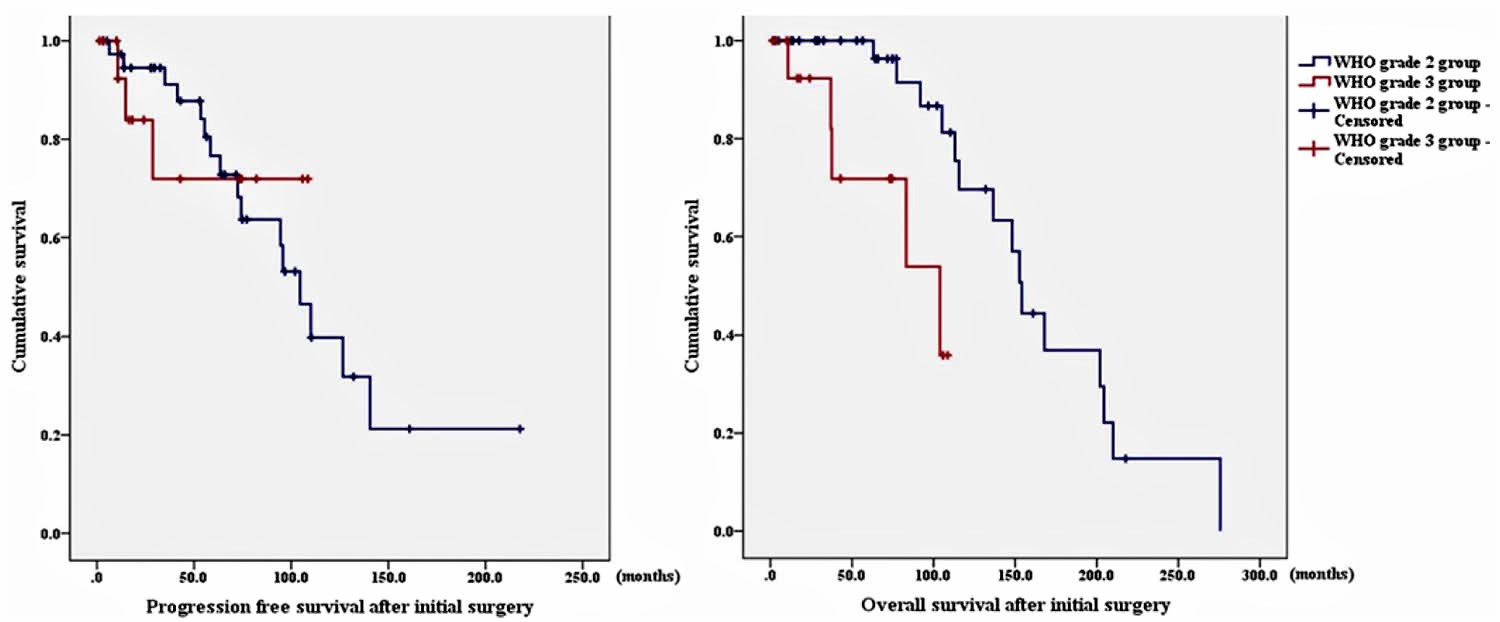
The 2007 WHO classification of central nervous system introduced anaplastic hemangiopericytomas. Since the introduction of WHO grade 3, recent studies have examined the differences in outcomes between the 2 grades of hemangiopericytoma. Some authors reported that grade 3 had a higher recurrence rate compared with that of grade 2 16. Damodaran et al. 19 reported that the grade 2 group had significantly improved survival compared with the grade 3 group (216 vs 142 months). However, in their study, the grade 2 group showed no significant difference in recurrence. In this study 3, 14 (87.5%) of 16 WHO grade 3 patients underwent adjuvant radiation therapy. In comparison, 25 (62.5%) of 40 WHO grade 2 patients underwent adjuvant radiation therapy. The study authors 3 believe that their treatment strategy for grade 3 disease resulted in the relatively lower recurrence rate in this group. However, analysis of overall survival revealed that the WHO grade 3 group had a more aggressive disease compared with that in the WHO grade 2 group, with higher disease-related mortality and morbidity; longer follow-up periods are also necessary to detect recurrence in patients in the WHO grade 3 group.
There has been controversy about extracranial metastasis in solitary fibrous tumor or hemangiopericytoma. The overall reported extracranial metastasis rates vary from 2.6% to 56% 11. Guthrie et al. 20 reported that the development of extracranial metastasis results in a significantly reduced survival period. In another study 10, extracranial metastasis was the cause of death in 3 of the 6 patients. The earliest and longest reported periods to metastasis were 7 months and 20 years, respectively. Moreover, some authors have reported that a higher grade is associated with an increased risk of extracranial metastasis 21. However, others have shown that a higher grade and adjuvant radiation therapy are not correlated with extracranial metastasis 15. In the current study, extracranial metastasis was not associated with extent of resection, adjuvant radiation therapy, and intracranial local recurrence. These are unpredictable characteristics. Therefore, long-term follow-up and careful clinical examinations to detect extracranial metastasis and to identify its clinical characteristics must be performed at regular intervals. Some authors recommend regular chest radiographs at 6- to 12-month intervals as an inexpensive option, with follow-up CT scans for lung metastasis 21. In the current study, 6 patients presented with extracranial
metastasis. The metastatic lesion was confirmed by pathological examination in 5 patients. One patient underwent follow-up CT scans at regular intervals because of a very small lesion in the lung, which was first detected through PET-CT.
Figure 1. Hemangiopericytoma survival rate
Footnote: Kaplan-Meier survival curves showing a comparison between the WHO grade II and III groups. Left: The progression-free survival between the 2 groups showed no statistically significant difference. Right: The overall survival of the WHO grade 3 group was significantly shorter than that of the WHO grade 2 group.
[Source 3 ] References- Kumar: Robbins and Cotran: Pathologic Basis of Disease, 7th ed.. Saunders, An Imprint of Elsevier; 2005
- Hemangiopericytoma Tumors. https://www.upmc.com/services/neurosurgery/brain/conditions/brain-tumors/hemangiopericytoma
- Solitary fibrous tumor/hemangiopericytoma: treatment results based on the 2016 WHO classification. J Neurosurg 130:418–425, 2019 https://doi.org/10.3171/2017.9.JNS171057
- Park MS, Araujo DM: New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol 21:327–331, 2009
- Fritchie KJ, Jin L, Rubin BP, Burger PC, Jenkins SM, Barthelmeß S, et al: NAB2-STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J Neuropathol Exp Neurol 75:263–271, 2016
- Fletcher CDM: The evolving classification of soft tissue tumours – an update based on the new 2013 WHO classification. Histopathology 64:2–11, 2014
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820, 2016
- Ghose A, Guha G, Kundu R, Tew J, Chaudhary R: CNS hemangiopericytoma: a systematic review of 523 patients. Am J Clin Oncol 40:223–227, 2017
- Malignant hemangiopericytoma: Treatment patterns and survival. Rolando Barjas and David Eric Piccioni. Journal of Clinical Oncology 2017 35:15_suppl, e13520-e13520
- Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G: Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci 21:1310–1314, 2014
- Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N: Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg 114:747–755, 2011
- Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012 Sep. 25 (9):1298-306.
- Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR: The central nervous system solitary fibrous tumor: a review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg 113:703–710, 2011
- Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, et al: Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118:1628–1636, 2012
- Melone AG, D’Elia A, Santoro F, Salvati M, Delfini R, Cantore G, et al: Intracranial hemangiopericytoma—our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg 81:556–562, 2014
- Sonabend AM, Zacharia BE, Goldstein H, Bruce SS, Hershman D, Neugut AI, et al: The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a Surveillance, Epidemiology, and End Results analysis. J Neurosurg 120:300–308, 2014
- Kim BS, Kong DS, Seol HJ, Nam DH, Lee JI: Gamma knife radiosurgery for residual or recurrent intracranial hemangiopericytomas. J Clin Neurosci 35:35–41, 2017
- Rutkowski MJ, Bloch O, Jian BJ, Chen C, Sughrue ME, Tihan T, et al: Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci 18:1500–1504, 2011
- Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G: Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci 21:1310–1314,2014
- Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG: Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery 25:514–522, 1989
- Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE: Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol 22:84–91, 1991