Granulosa cell tumor
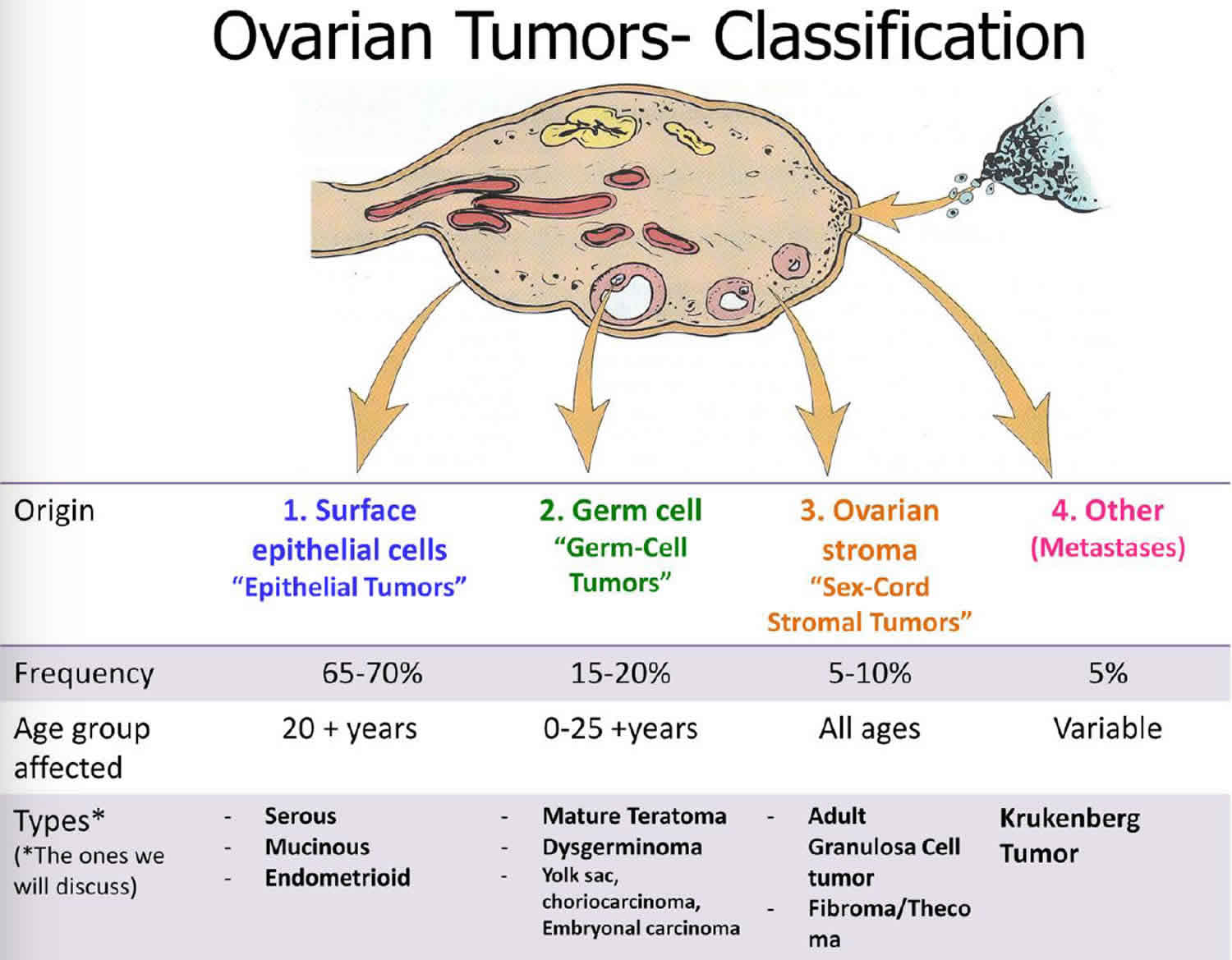
Granulosa cell tumor also called granulosa theca cell tumor, is a very rare type of slow-growing tumor with malignant potential that usually affects the ovary, accounting for approximately 2% of all ovarian tumors 1. The majority of patients with granulosa cell tumor of the ovary are diagnosed at early stage and have a relatively favorable prognosis 2. There are three main types of ovarian cancer: sex cord-stromal (8%), epithelial (>70%), and germ cell (20%) 3. Sex cord-stromal tumors develop from gonadal stromal component (composed of granulosa cells, theca cells, and fibroblasts in varying degrees and combinations) and represent about 8% of all ovarian tumors 4. Among them, 70% are granulosa cell tumors 5, characterized by indolent growth with malignant potential 6 and late recurrence, even after more than a decade 7. Granulosa cell tumors are considered stromal tumors and include those composed of granulosa cells, theca cells, and fibroblasts. Granulosa cell tumors account for approximately 2 percent of all ovarian tumors. Granulosa cell tumors are thought to be tumors of low malignant potential. Most of these tumors follow a benign course, with only a small percentage showing aggressive behavior, perhaps due to early stage at diagnosis. Metastatic disease can involve any organ system, although tumor growth usually is confined to the abdomen and pelvis.
Approximately 25,000 new cases of ovarian cancer are diagnosed in the United States each year. This disease accounts for more than 14,000 deaths in the United States annually and is the leading cause of death from gynecologic malignancies. Because sex cord–stromal tumors account for only 5% of all ovarian tumors and approximately 8% of all malignant ovarian neoplasms, each year only 1500-2000 new cases of granulosa cell tumors are diagnosed in the United States.
There are two distinct forms of granulosa cell tumor, adult and juvenile, based primarily on clinical presentations and histopathologic characteristics 8.
- Adult granulosa cell tumor comprises about 95% of granulosa cell tumors and frequently presents in postmenopausal women with uterine bleeding 9.
- Juvenile granulosa cell tumor is much more rare, comprising only about 5% of granulosa cell tumors, and affects young women in their first 3 decades of life 9.
Both subtypes commonly produce estrogen, and estrogen production often is the reason for early diagnosis 10. However, while adult granulosa cell tumors usually occur in postmenopausal women and have late recurrences, most juvenile granulosa cell tumors develop in individuals younger than 30 years and often recur within the first 3 years. Theca cell tumors almost always are benign and carry an excellent prognosis. The rare malignant thecoma likely represents a tumor with a small admixture of granulosa cells.
Recognition of the signs and symptoms of abnormal hormone production and consideration of granulosa cell tumors in the differential diagnosis of an adnexal mass can allow for early identification, timely surgical management, and excellent cure rates. Despite the good overall prognosis, long-term follow-up always is required in patients with granulosa cell tumors 11.
Recent studies have revealed that FOXL2 gene 402C > G (C134W) mutation plays a key role in the pathogenesis of adult granulosa cell tumor 7.
Treatment consists of surgery to remove the granulosa cell tumor and additional treatments may also be used depending on the extent of the tumor. Surgical management of granulosa tumors is based on the stage of the granulosa cell tumor as well as age of the patient 12. Premenarchal women or patients presenting in the reproductive years with early stage granulosa cell tumor are often managed with unilateral salpingo-oophorectomy and appropriate surgical staging in an attempt to preserve fertility. In postmenopausal women and those who have completed childbearing, surgery consists of a total abdominal hysterectomy and bilateral salpingo-oophorectomy, along with standard surgical staging 12.
Adult granulosa cell tumors and juvenile granulosa cell tumors have very good cure rates due to the early stage of disease at diagnosis. More than 90% of adult granulosa cell tumors and juvenile granulosa cell tumors are diagnosed before spread occurs outside the ovary. Five-year survival rates usually are 90-95% for stage 1 tumors compared to 25-50% for patients presenting with advanced-stage disease. Although 5-year survival rates are quite good, adult granulosa cell tumors have a propensity for late recurrence, some occurring as many as 37 years after diagnosis. Mean survival after the diagnosis of a recurrence is 5 years. Approximately 20% of patients diagnosed with granulosa cell tumors die of their disease over the course of their lifetime.
Ovarian cancer is a surgically staged disease. Stage is the most important prognostic factor, with 10-year survivals of 84-95% for stage 1 granulosa cell tumors, decreasing to 50-65% for stage 2 granulosa cell tumors, and to 17-33% for stages 3 and 4 13. Patients with low risk stage 1 granulosa cell tumor should be kept on observation 13. Patients with high risk stage 1 granulosa cell tumor associated with large tumor size (≥10-15 cm), stage 1C, poorly differentiated tumor, high mitotic index, or tumor rupture might be considered for adjuvant chemotherapy in view of increased risk of relapse 14. The prognostic significance of these factors and benefit of chemotherapy is still uncertain 12. For patients with stage 2-4 granulosa cell tumors, postoperative treatment is recommended 15, but the survival benefit is still not known due to rarity of these tumors and lack of randomized trials 12.
Morbidity related to granulosa cell tumors primarily is due to endocrine manifestations of the granulosa cell tumor. Physical changes brought on by high estrogen levels from the granulosa cell tumor usually regress upon removal of the tumor. However, a small group of patients present with symptoms of androgen excess from the granulosa cell tumor. Changes caused by androgen excess may be permanent or may only partially regress over time.
Serious estrogen effects can occur in various end organs. Unopposed estrogen production by these tumors has been shown to cause stimulation of the endometrium. Anywhere from 30-50% of patients develop endometrial hyperplasia and another 8-33% have endometrial adenocarcinoma. Patients also may be at an increased risk for breast cancer, although a direct correlation has been difficult to prove.
Granulosa cell tumor causes
The exact cause and molecular pathogenesis of granulosa cell tumors are unknown 16. Granulosa cell tumors have morphologic and molecular features that are similar to those of normal granulosa cells, including the expression of follicle-stimulating hormone receptor and inhibin 17. Cytogenetic studies have shown that granulosa cell tumors have greater genomic stability than the more common epithelial ovarian cancers 16.
Two theories exist to explain the cause of sex cord–stromal tumors. The first proposes that these neoplasms are derived from the mesenchyme of the developing genital ridge. The second purports that sex cord and stromal cells of the mature ovary are derived from precursors found within the mesonephric and coelomic epithelium.
Reports of extraovarian granulosa cell tumors can be found in the literature and may lend support to the derivation of this class of tumors from epithelium of the coelom and mesonephric duct.
Various theories propose explanations for the differentiation of normal granulosa and/or stromal cells into neoplastic entities. To date, no clear etiologic process has been identified. However, the most recent molecular data regarding these tumors have linked a missense point mutation (C402G) in the FOXL2 gene to granulosa cell tumors 18. Using whole transcriptome sequencing of 4 adult granulosa cell tumors, the mutation in FOXL2 was identified. They confirmed this mutation was present in an additional 86 of 89 adult granulosa cell tumors, 3 of 14 thecomas, and 1 in 10 juvenile-type granulosa cell tumors. Moreover, the mutation was not found in any of 49 sex cord stromal tumors of other types or in epithelial ovarian tumors. This suggests a potential pathogenic mutation and raises the possibility of identifying novel targeted therapies 19.
Granulosa cell tumor symptoms
Granulosa cell tumors have bimodal age distribution but the peak incidence is in postmenopausal period with median age of diagnosis around 50-55 years 13. These patients may present with abdominal pain, abdominal distention, abdominal mass, and menstrual disturbances 20. Most patients have a palpable mass found during examination. Abdominal symptoms may be due to enlargement of the mass but also can be due to the production of ascites, which occurs in approximately 10% of patients. Increasing size of the mass also can lead to symptoms associated with compression of adjacent structures, such as abdominal pain, dysuria, urinary frequency, and constipation.
Pelvic mass is the most consistent finding on pelvic and rectal examination in patients of all ages with granulosa cell tumor. A palpable mass can be found in 85-97% of patients. A bimanual examination and a rectovaginal examination should be performed to evaluate the pelvis and lower abdomen for masses, the posterior cul-de-sac for nodularity, and any other areas associated with tenderness.
Other findings generally relate to endocrine manifestations of hyperestrogenic and/or hyperandrogenic states.
The symptoms of excess estrogen depend on the woman’s menstrual status; the most common symptoms include early puberty for affected young girls, an increase in abdomen size or irregularities of menstrual cycles in premenopausal women, and abnormal uterine bleeding in postmenopausal women.
Acute onset of abdominal pain also can occur, although rarely. Acute abdominal or pelvic pain may be observed in combination with nausea, vomiting, dizziness, and shoulder pain. These symptoms may be due to adnexal torsion, rupture of a partially cystic granulosa cell tumor, or hemorrhage either within the tumor or into the peritoneum.
For patients presenting with acute abdominal pain, a careful speculum examination should be performed to help rule out infectious etiologies. Wet preparation and testing for Neisseria gonorrhoeae and Chlamydia trachomatis should be considered. Gram stain for gram-negative diplococci can be helpful if other findings are consistent with a diagnosis of pelvic inflammatory disease and/or cervicitis.
Prepubertal girls
Juvenile type account for 5% of the granulosa cell tumors that occur in prepubertal girls and in women younger than 30 years of age 21. Patients usually present with precocious pseudopuberty (70-80%) and have secondary sex characteristics at a very early age. These may include increased linear growth, breast enlargement, clitoral enlargement, pubic hair development, increased vaginal secretions, and vaginal bleeding.
In a few instances, patients present with virilizing symptoms as a result of testosterone production by the tumor cells. Many of these hormone-induced symptoms abate following resection of the granulosa cell tumor.
Concomitant endometrial pathology in form of hyperplasia is a common finding in granulosa cell tumor, occurring in 25-50%, due to excess of estrogen produced by these tumors 22. Endometrial adenocarcinoma can be found simultaneously in 5-10% of patients with granulosa cell tumor and are often well-differentiated and in an early stage with a favorable prognosis 22.
Premenopausal women
Increasing abdominal girth and other symptoms related to an enlarging adnexal mass may be seen in this group of patients. Menstrual irregularities such as oligomenorrhea, menorrhagia, and secondary amenorrhea tend to be the hallmark of granulosa cell tumors in reproductive-aged women.
Postmenopausal women
The most common endocrine manifestation of granulosa cell tumors in postmenopausal women is abnormal uterine bleeding. This is caused by resumption of endometrial proliferation due to estrogen production by the tumor. For this reason, endometrial hyperplasia and/or endometrial adenocarcinoma may be a concomitant finding in women with granulosa cell tumor.
Patients also can have breast tenderness and increased vaginal secretions from estrogenic stimulation of the breast and vaginal tissues, respectively.
Rarely, a patient may present with virilizing symptoms such as acne, hirsutism, deepening of the voice, and clitoral enlargement. This is due to testosterone and/or androstenedione production in a minority of granulosa cell tumors.
During pregnancy
Ovarian tumors occur or can be found during pregnancy. Because only 2% of masses in pregnant women are malignant, these masses can be followed expectantly if diagnosed in the first trimester because most masses resolve spontaneously. Tumors that persist into the second trimester, especially if complex or larger than 6 cm, should be managed surgically.
Approximately 10% of granulosa cell tumors occur in pregnant patients. These masses do not resolve with expectant management, and surgical therapy should be carried out as follows:
- The optimal time for abdominal exploration is approximately 16-18 weeks of gestation. The incidence of fetal loss, preterm labor, and maternal morbidity appears to be lower at this gestational age. A high vertical incision should be employed because the adnexa are out of the pelvis at this point in pregnancy. Because most tumors are stage Ia (see Staging), a unilateral salpingo-oophorectomy is sufficient treatment for most patients. In cases in which spread outside of the ovary already has occurred, treatment recommendations become unclear. Total abdominal hysterectomy and bilateral salpingo-oophorectomy, with removal of the fetus and placenta in toto, is another option to be considered. Some patients have been treated with unilateral salpingo-oophorectomy, resection of all visible tumor, and adjuvant chemotherapy.
- The decision to begin chemotherapy during pregnancy rests with the patient and physician because all chemotherapy agents are potential teratogens. Reports of chemotherapeutic agent use in the second and third trimester for other ovarian tumors exist, but caution should be taken because little is known about the long-term effects on the developing fetus. Delaying adjunct therapy until after delivery is not well studied but could be considered because many of these tumors exhibit indolent growth.
A higher propensity for torsion exists in pregnant patients because the adnexa become abdominal structures early in the second trimester. In pregnant patients presenting with acute abdominal pain and a palpable mass, sonographic evaluation of the adnexa can help confirm the presence of an adnexal mass.
Granulosa cell tumor diagnosis
Laboratory Studies
Order a pregnancy test in all reproductive-aged patients (even at the extremes of reproductive age) who present with abdominopelvic symptoms.
The standard workup for a patient with an adnexal mass varies depending on patient age, as follows:
- In patients who are prepubertal or younger than 30 years, especially if the mass has solid components present, obtain blood to check for beta–human chorionic gonadotropin (bhCG), alpha-fetoprotein (AFP), lactate dehydrogenase (LDH), and cancer antigen 125 (CA125). Each of these may be elevated in women with ovarian malignancies and in patients with a normal or abnormal pregnancy. CA125 levels in menstruating women can be slightly to moderately elevated due to a host of benign disorders including, but not limited to, endometriosis, uterine leiomyoma, appendicitis, pancreatitis, and inflammatory bowel disease. In this group of patients, CA125 level is not as useful as a diagnostic test, but it may be helpful in monitoring patients long term if they are found to have a tumor that causes elevation of this tumor marker.
- In reproductive-aged women older than 30 years, CA125 level should be checked; remember that this can be elevated in benign disorders in women who still are menstruating. Serum inhibin levels are now clinically available for work-up of masses suspicious for granulosa cell tumor and should be considered in such patients (see below). This is now the most specific marker for granulosa cell tumors that is currently clinically available 23. Serum levels for estrogen, testosterone, and dehydroepiandrosterone can be drawn if elevation of these hormones is suggested based on clinical findings. Abrupt onset or rapid progression of endocrinologic manifestations should heighten the suspicion for a neoplastic process.
- In postmenopausal women, obtain blood for a CA125 test. A CA125 level higher than 60 U/mL in a postmenopausal woman has a good positive predictive value for malignancy. Again, serum sex hormone levels can be ordered based on clinical findings consistent with excess hormone production.
Other ancillary laboratory studies that may be useful in narrowing the differential include stool guaiac testing, CBC count with differential, blood chemistries, urinalysis, and cervical cultures for gonorrhea and chlamydia.
Several other tumor markers have been evaluated in patients with granulosa cell tumors.
Inhibin
Inhibin has been studied in women with granulosa cell tumors. It is a peptide hormone produced by ovarian granulosa cells that plays a role in regulation of FSH secretion by the pituitary. It is composed of an alpha subunit and 1 of 2 beta subunits (BA or BB). Although inhibin A and inhibin B levels can both be elevated in patients with granulosa cell tumors, inhibin B level is usually elevated in a higher proportion of these tumors. Furthermore, this increase in serum levels from baseline is often higher as well. Typical cutoffs for normal inhibin levels in postmenopausal or oophorectomized women are less than or equal to 5 ng/L and 15 ng/L for inhibin A and B, respectively.
Studies of inhibin in patients with granulosa cell tumors have shown that levels are elevated preoperatively and return to the reference range postoperatively in both adult and juvenile types of tumors. In a 2002 study by Robertson et al, total serum inhibin level was elevated in 100% of granulosa cell tumors and 100% of thecomas 24. Additionally, 84% of patients with mucinous ovarian carcinomas had elevated inhibin levels. However, only 18% of patients with serous and 54% of patients with endometrioid ovarian cancers had elevation of total serum inhibin level. Current inhibin assays allow us to distinguish between inhibin A and inhibin B.
Mom et al evaluated the use of serum inhibin levels in 30 women with granulosa cell tumors. The sensitivities and specificities for inhibin A were 67 and 100% and for inhibin B were 89 and 100%, respectively. They also noted that inhibin A level was elevated before or at the time of first clinical recurrence in 58% of patients while inhibin B level was elevated in 85%. Lead time from elevation of inhibin levels to clinical recurrence was estimated to be 11 months. Inhibin A and B levels were not elevated in any of the 17 patients who were postoperatively disease free. Serum inhibin levels are currently available for diagnosis and clinical follow-up of women with granulosa cell tumors of the ovary 25.
Antimüllerian hormone (AMH) or Müllerian-inhibiting substance (MIS)
This hormone is produced exclusively by granulosa cells in postnatal females and both prenatally and postnatally by the Sertoli cells in the male testis. This hormone functions in male fetuses to induce regression of the mullerian system. Normally, müllerian-inhibiting substance (MIS) or antimüllerian hormone (AMH) is found in low levels in reproductive-aged females and functions as a paracrine inhibitory factor decreasing resting ovarian follicle response to follicle stimulating hormone (FSH). This insures the emergence of a single dominant follicle. Serum müllerian-inhibiting substance (MIS) or antimüllerian hormone (AMH) may be a marker of ovarian reserve and typically disappears from the serum after menopause or bilateral oophorectomy. However, in patients with granulosa cell tumors, levels have been shown to parallel the extent of disease 26.
Lane et al 27 found that 76% of patients with granulosa cell tumors had elevated müllerian-inhibiting substance (MIS) or antimüllerian hormone (AMH) levels preoperatively. No patient with levels within the reference range postoperatively experienced recurrence, whereas 6 of 15 patients with elevated levels had a recurrence. On average, elevated levels were detected 3 months before clinical evidence of recurrence was found. In 2000, Long et al 28 used an ultrasensitive ELISA and found that AMH levels became undetectable in 15 of 16 women treated for granulosa cell tumors and were elevated in 14 of 15 women (sensitivity 93%) with recurrent granulosa cell tumors.
Anttonen et al 29 reported that müllerian-inhibiting substance (MIS) gene expression was significantly decreased in 87% of tumors greater than 10 cm. This inverse relationship between müllerian-inhibiting substance (MIS) expression and tumor size raised concerns that müllerian-inhibiting substance (MIS) or antimüllerian hormone (AMH) may not be a useful marker in advanced cases of granulosa cell tumor.
Serum müllerian-inhibiting substance (MIS) or antimüllerian hormone (AMH) levels correlate well with tumor presence in patients with granulosa cell tumors. This marker is highly specific for granulosa cell tumor in postmenopausal or oophorectomized women. It may also be elevated in women with Sertoli-Leydig cell tumors of the ovary, but is not typically produced by other gonadal or extragonadal tumors. This is in sharp contrast to inhibin and estradiol levels, both of which may be elevated in a variety of other extraovarian disorders. This makes müllerian-inhibiting substance (MIS) or antimüllerian hormone (AMH) attractive as a marker for diagnosis and prospective follow-up of patients with granulosa cell tumors. However, studies have been limited to retrospective trials. With widespread clinical availability of antimüllerian hormone (AMH) testing, this marker may gain ground in the management of women with granulosa cell tumors and perhaps could be a molecular target in the future.
Granulosa cell tumor testing
Granulosa cell tumors are the most common estrogen-producing neoplasms in females and are found to produce estradiol in approximately 40-60% of patients. This estradiol production is dependent on stimulation by testosterone secreted by the theca cells. However, not all granulosa cell tumors are hormonally active or have theca cells that secrete testosterone, and this type of testing lacks sensitivity and specificity.
Imaging studies
Ultrasonography
Transvaginal ultrasound is by far the best primary modality for imaging pelvic structures. This may allow for delineation between ovarian, tubal, uterine, and other pelvic masses. If an adnexal mass is identified, the presence of cystic or solid components should be noted and remarks on the internal architecture of cystic structures (eg, septations, excrescences) should be made. Free pelvic fluid also can be identified readily on transvaginal sonography images. The presence of solid, complex, cystic, or bilateral masses, with or without free fluid, increases the possibility of malignancy.
Granulosa cell tumors have a heterogeneous appearance on both sonographic and CT imaging, depending on the histologic pattern. Most commonly, they appear as round-to-ovoid masses that are multicystic, sometimes with solid components at the center or periphery. Fewer cases appear as unilocular simple or complex cysts or even homogeneous solid masses. The average size of these tumors is 12 cm, but they can range from 2-50 cm.
X-ray
Chest radiography is useful in helping exclude pulmonary spread of malignant diseases of the ovary. Abdominopelvic CT scanning or MRI may help in diagnosing intraperitoneal spread or involvement of other organ systems prior to surgery. Abdominopelvic imaging also can be used in follow-up evaluations to confirm the presence of recurrent tumor identified after clinical examination.
Abdominal radiography, intravenous pyelography (IVP), barium enemas, and upper GI series also can be useful adjuncts in patients with symptoms involving the GI or genitourinary tracts.
Perform a barium enema or colonoscopy in any patient with a pelvic mass prior to surgical intervention to help rule out colonic involvement, colon cancer, or both as the primary tumor in women older than 40 years. However, if abdominopelvic CT scanning with oral and intravenous contrast already has been performed, IVP, colonoscopy, and barium enema are not required.
Mammography
The preoperative workup also should include mammography for women older than 40 years who have not had one in the preceding 6-12 months. This is especially important in women with estrogen-producing tumors because these may increase the risk of breast malignancies.
Additionally, breast cancers can metastasize to the ovaries and are often bilateral. Mammography can help rule out the possibility of a nongynecologic primary neoplasm in the breast.
Granulosa cell tumor staging
Once diagnosed with ovarian cancer, the stage of a tumor can be determined during surgery, when the doctor can tell if the cancer has spread outside the ovaries. There are four stages of ovarian cancer – Stage I (early disease) to Stage IV (advanced disease). The treatment plan and prognosis (the probable course and outcome of the disease) will be determined by the stage of the cancer.
The current staging classification system is based on 1987 International Federation of Gynecology and Obstetrics (FIGO) nomenclature.
- Stage 1: Tumor is confined to the ovaries
- Stage 1a: Tumor is limited to one ovary with an intact capsule. No tumor is present on the external surface of the capsule, and no ascites containing malignant cells are present.
- Stage 1b: Tumor involves both ovaries, with intact capsules. No tumor is present on the external surface of the capsule, and no ascites containing malignant cells are present.
- Stage 1c: Tumor is stage 1a or 1b with tumor on the external surface of one or both ovaries, or ruptured capsule(s) or malignant cells are present in ascitic fluid or peritoneal washings.
- Stage 2: Tumor involves one or both ovaries, with pelvic extension
- Stage 2a: Extension and/or metastases to the uterus and/or fallopian tubes are present.
- Stage 2b: Extension to the bladder, rectum, or other pelvic tissues occurs.
- Stage 2c: Tumor is stage 2a or 2b, with tumor on the external surface of one or both ovaries or ruptured capsule(s), or malignant cells are present in ascitic fluid or peritoneal washings.
- Stage 3: Tumor involves one or both ovaries, with peritoneal implants outside of the pelvis and/or positive retroperitoneal lymph nodes. Superficial liver metastases also are included in stage 3.
- Stage 3a: Tumor is grossly confined to the pelvis but with microscopic seeding of the abdominal peritoneal surfaces. Lymph nodes are negative.
- Stage 3b: Tumor implants are present on the abdominal peritoneum, none larger than 2 cm in diameter. Lymph nodes are negative.
- Stage 3c: Tumor implants on the abdominal peritoneum 2 cm or more are present, and/or retroperitoneal or inguinal nodes are positive.
- Stage 4: Distant metastases are present. Pleural effusions must be confirmed cytologically to classify a case as stage 4. Metastases to the liver parenchyma also are included in stage 4.
Grades of ovarian cancer
Tumor grade is not the same as the cancer stage. Grade (G), describes how healthy the cancer cells look when viewed under a microscope. The doctor compares the cancerous tissue with healthy tissue. Healthy tissue is made up of many types of cells grouped together. If the cancer looks like the healthy tissue with different cell groupings, it is called differentiated or a low-grade tumor. If the tissue looks very different, it is called poorly differentiated or a high-grade tumor. The cancer’s grade may help the doctor predict how quickly the cancer will spread. It also helps determine a treatment plan decision.
The following is a description of tumor grades:
- GX: The grade cannot be evaluated
- GB: The tissue is considered borderline cancerous. This is commonly called low malignant potential (LPM).
- G1: The tissue is well-differentiated (contains many healthy looking cells)
- G2: The tissue is moderately differentiated (more cells appear abnormal than healthy)
- G3 to G4: The tissue is poorly differentiated or undifferentiated (more cells appear abnormal, and lack normal tissue structures)
Granulosa cell tumor treatment
Surgery is the first step of treating a granulosa cell tumor of the ovary and aims to remove as much of the tumor as possible. Additional treatments – such as radiation therapy, chemotherapy, or hormone therapy – may follow surgery depending on the severity and extent of the original tumor, or if the tumor regrows after surgery (a recurrence). These treatments may improve survival or increase the length of disease-free time before a recurrence. After treatments are complete, individuals should continue to have regular visits with their physicians to check for recurrences. These visits should include updating the medical history, a pelvic examination, and possibly blood testing to look for chemicals produced by cancer cells (tumor markers). If there are any suspicious findings during these visits, an imaging test such as a CT scan may be done 10.
Radiotherapy for patients with advanced or recurrent granulosa cell tumors has been studied and appears to have limited efficacy.
In a 1999 study by Wolf et al 30 at the MD Anderson Cancer Center, 6 of 14 patients with measurable disease had complete clinical responses to pelvic radiation and 3 patients were without evidence of disease 10-21 years after radiation. However, 3 patients experienced a recurrence 4-5 years after radiation. Eight of 14 had no response to treatment and had a median survival of 12.3 months overall 30.
A more recent study by Hauspy et al 31 reviewed 45 years of granulosa cell tumor treatment at Princess Margaret Hospital. Thirty-one of 103 women received abdominal and/or pelvic radiation as adjuvant therapy. Multivariate analysis showed that adjuvant radiation significantly improved survival and that stage 3 disease was independently predictive of a poor response. They concluded that patients receiving radiation had better disease-free survival (251 months vs 114 months for those not receiving radiation). However, 86% of those receiving radiation were stage 1 versus only 52% of those who did not receive radiation. Moreover, only 2 of the 103 patients received chemotherapy 31.
Currently, radiation is considered an option for advanced-stage patients and in patients with pelvic recurrence, radiotherapy should be considered because a clinical response occurs in almost half of patients treated with radiation therapy.
Adjuvant therapy for granulosa cell tumors has typically been carried out using chemotherapy. There is also data available regarding hormonal manipulation of these tumors using gonadotropin-releasing hormone (GnRH) analogues and aromatase inhibitors.
Granulosa cell tumor prognosis
The prognosis of granulosa cell tumor is dependent on the severity and extent (stage) of the tumor, age, tumor size, tumor rupture, mitotic index, presence of residual disease, and grade has been shown to be of prognostic significance in various studies 32. The majority of cases (80-90%) of granulosa cell tumor of the ovary are stage 1 at diagnosis (cancer is still contained within the ovary and has not spread). Stage 1 granulosa cell tumors of the ovary have been found to have a good prognosis with a 5 year survival rate ranging from 90-100% and a 10 year survival rate ranging from 84-95% 33. Patients with stage 1 granulosa cell tumor had better overall survival as compared to advanced stage (stages 2-4) which was statistically significant. The recurrence rates are also related to the stage 20. In a study by Evans et al. 34, only 9% of women with stage 1A had tumor recurrence as compared to 30% or more for higher stage disease. For stage 2 granulosa cell tumors, reported 5-year survival rates vary widely, from 55% to 95%, perhaps indicating inconsistent diagnostic criteria for granulosa cell tumors 35. In a study by Ayhan et al. 36, recurrence rates were 5.4, 21, and 40% for stages 1, 2, and 3, respectively. Mangili et al. 32, found that 20% of patients with stage 1 granulosa cell tumor recurred after 20 years from diagnosis, thus emphasizing the need of long-term follow-up in patients of granulosa cell tumors.
Granulosa cell tumors greater than 10-15 cm in diameter have been associated with high recurrence rates and shorter progression-free survival, independent of stage 37. However, in some series, granulosa cell tumor size was not found to have any prognostic significance when adjusted for stage 38. High mitotic index has been found to be associated with high recurrence rates in few studies 39. In this study 12, patients with mitotic activity less than 5/10 HPF had better survival rate as compared to those with more than 5/10 HPF (100 vs 72% at 5 years). In a study by Miller et al. 40, 19 patients with recurrent disease were compared to 51 patients without disease. The mitotic rate was higher in the group with recurrence. In a study by Lauszus et al. 41, there was no difference between women with relapse and women with no relapse with respect to mitotic count. Survival was significantly worse in patients with residual disease as compared to patients without residual disease in this report 12. In various studies, presence of residual disease was associated with poor survival and outcome 32.
References- Types & Stages of Ovarian Cancer. http://www.ovarian.org/about-ovarian-cancer/what-is-ovarian-cancer/types-a-stages
- Khosla D, Dimri K, Pandey AK, Mahajan R, Trehan R. Ovarian granulosa cell tumor: clinical features, treatment, outcome, and prognostic factors. N Am J Med Sci. 2014;6(3):133–138. doi:10.4103/1947-2714.128475 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3978936/
- Scully RE. Classification of human ovarian tumors. Environ Health Perspect. 1987;73:15–25. doi: 10.1289/ehp.877315.
- Outwater EK, Wagner BJ, Mannion C, McLarney JK, Kim B. Sex cord-stromal and steroid cell tumors of the ovary. Radiographics. 1998;18:1523–1546. doi: 10.1148/radiographics.18.6.9821198
- Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019
- Geetha P, Nair MK. Granulosa cell tumours of the ovary. Aust N Z J Obstet Gynaecol. 2010;50:216–220. doi: 10.1111/j.1479-828X.2010.01154.x
- Mancari R, Portuesi R, Colombo N. Adult granulosa cell tumours of the ovary. Curr Opin Oncol. 2014;26:536–541. doi: 10.1097/CCO.0000000000000106
- Jamieson S, Fuller PJ. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev. 2012;33:109–144. doi: 10.1210/er.2011-0014
- Kavuri S, Kulkarni R, Reid-Nicholson M. Granulosa cell tumor of the ovary: cytologic findings. Acta Cytol. 2010;54:551–559
- Granulosa-Theca Cell Tumors. https://emedicine.medscape.com/article/254489-overview
- Kottarathil VD, Antony MA, Nair IR, Pavithran K. Recent Advances in Granulosa Cell Tumor Ovary: A Review. Indian J Surg Oncol. 2013 Mar. 4(1):37-47.
- Khosla D, Dimri K, Pandey AK, Mahajan R, Trehan R. Ovarian granulosa cell tumor: clinical features, treatment, outcome, and prognostic factors. N Am J Med Sci. 2014;6(3):133–138. doi:10.4103/1947-2714.128475 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3978936
- Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–9.
- Schneider DT, Calaminus G, Wessalowski R, Pathmanathan R, Selle B, Sternschulte W, et al. Ovarian sex cord-stromal tumors in children and adolescents. J Clin Oncol. 2003;21:2357–63.
- Pautier P, Gutierrez-Bonnaire M, Rey A, Sillet-Bach I, Chevreau C, Kerbrat P, et al. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors. Int J Gynecol Cancer. 2008;18:446–52.
- Mayr D, Hirschmann A, Marlow S, Horvath C, Diebold J. Analysis of selected oncogenes (AKT1, FOS, BCL2L2, TGFbeta) on chromosome 14 in granulosa cell tumors (GCTs): a comprehensive study on 30 GCTs combining comparative genomic hybridization (CGH) and fluorescence-in situ-hybridization (FISH). Pathol Res Pract 2008;204:823-830
- Fuller PJ, Chu S. Signalling pathways in the molecular pathogenesis of ovarian granulosa cell tumours. Trends Endocrinol Metab 2004;15:122-128
- Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009 Jun 25. 360(26):2719-29.
- Anttonen M, Pihlajoki M, Andersson N, et al. FOXL2, GATA4, and SMAD3 co-operatively modulate gene expression, cell viability and apoptosis in ovarian granulosa cell tumor cells. PLoS One. 2014. 9(1):e85545.
- Uygun K, Aydiner A, Saip P, Basaran M, Tas F, Kocak Z, et al. Granulosa cell tumor of the ovary: retrospective analysis of 45 cases. Am J Clin Oncol. 2003;26:517–21.
- Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984;8:575–96.
- Kanthan R, Senger JL, Kanthan S. The multifaceted granulosa cell tumours-myths and realities: A review. ISRN Obstet Gynecol 2012. 2012 878635.
- Lappohn RE, Burger HG, Bouma J, Bangah M, Krans M, de Bruijn HW. Inhibin as a marker for granulosa-cell tumors. N Engl J Med. 1989 Sep 21. 321(12):790-3.
- Robertson DM, Stephenson T, Pruysers E. Characterization of inhibin forms and their measurement by an inhibin alpha-subunit ELISA in serum from postmenopausal women with ovarian cancer. J Clin Endocrinol Metab. 2002 Feb. 87(2):816-24.
- Miller BE, Barron BA, Wan JY. Prognostic factors in adult granulosa cell tumor of the ovary. Cancer. 1997 May 15. 79(10):1951-5.
- Ali Hel-S, Kitahara G, Nibe K, Yamaguchi R, Horii Y, Zaabel S, et al. Plasma anti-Müllerian hormone as a biomarker for bovine granulosa-theca cell tumors: comparison with immunoreactive inhibin and ovarian steroid concentrations. Theriogenology. 2013 Nov. 80(8):940-9.
- Lane AH, Lee MM, Fuller AF Jr. Diagnostic utility of Mullerian inhibiting substance determination in patients with primary and recurrent granulosa cell tumors. Gynecol Oncol. 1999 Apr. 73(1):51-5.
- Long WQ, Ranchin V, Pautier P. Detection of minimal levels of serum anti-Mullerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. J Clin Endocrinol Metab. 2000 Feb. 85(2):540-4.
- Anttonen M, Unkila-Kallio L, Leminen A, Butzow R, Heikinheimo M. High GATA-4 expression associates with aggressive behavior, whereas low anti-Müllerian hormone expression associates with growth potential of ovarian granulosa cell tumors. J Clin Endocrinol Metab. 2005 Dec. 90(12):6529-35.
- Wolf JK, Mullen J, Eifel PJ. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecol Oncol. 1999 Apr. 73(1):35-41.
- Hauspy J, Beiner ME, Harley I, Rosen B, Murphy J, Chapman W. Role of adjuvant radiotherapy in granulosa cell tumors of the ovary. Int J Radiat Oncol Biol Phys. 2011 Mar 1. 79(3):770-4.
- Mangili G, Ottolina J, Gadducci A, Giorda G, Breda E, Savarese A, et al. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer. 2013;109:29–34.
- Jessica E. Stine, Stuart Pierce, John T. Soper,. A Comprehensive Review of Diagnostic and Treatment Options for Granulosa Cell Tumors of the Ovary. Obstet Gynecol Surv. 2014 Jan;69(1):29-38. doi: 10.1097/OGX.0000000000000010
- Evans AT, 3rd, Gaffey TA, Malkasian GD, Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55:231–8.
- Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol 2003;21:1180-1189
- Ayhan A, Salman MC, Velipasaoglu M, Sakinci M, Yuce K. Prognostic factors in adult granulose cell tumors of the ovary: A retrospective analysis of 80 cases. J Gynecol Oncol. 2009;20:158–63.
- Chan JK, Zhang M, Kaleb V, Loizzi V, Benjamin J, Vasilev S, et al. Prognostic factors responsible for survival in sex cord stromal tumors of the ovary–a multivariate analysis. Gynecol Oncol. 2005;96:204–9.
- Zhang M, Cheung MK, Shin JY, Kapp DS, Husain A, Teng NN, et al. Prognostic factors responsible for survival in sex cord stromal tumors of the ovary–an analysis of 376 women. Gynecol Oncol. 2007;104:396–400.
- King LA, Okagaki T, Gallup DG, Twiggs LB, Messing MJ, Carson LF. Mitotic count, nuclear atypia, and immunohistochemical determination of Ki-67, c-myc, p21- ras, c-erbB2, and p53 expression in granulosa cell tumors of the ovary: mitotic count and Ki-67 are indicators of poor prognosis. Gynecol Oncol. 1996;61:227–32.
- Miller BE, Barron BA, Wan JY, Delmore JE, Silva EG, Gershenson DM. Prognostic factors in adult granulosa cell tumor of the ovary. Cancer. 1997;79:1951–5.
- Lauszus FF, Petersen AC, Greisen J, Jakobsen A. Granulosa cell tumor of the ovary: A population-based study of 37 women with stage I disease. Gynecol Oncol. 2001;81:456–60.